Abstract
Most mammals prefer the sweet taste of sugars, which is mediated by the heterodimeric T1R2+T1R3 taste receptor. Sugar appetite is also enhanced by the post-oral reinforcing actions of the nutrient in the gut. Here, we examined the contribution of gut T1R3 (either alone or as part of the T1R3+T1R3 receptor) to post-oral sugar reinforcement using a flavor-conditioning paradigm. We trained mice to associate consumption of a flavored solution (CS+) with intragastric (IG) infusions of a sweetener, and a different flavored solution (CS-) with IG infusions of water (23 h/day); then, we measured preference in a CS+ vs. CS- choice test. In experiment 1, we predicted that if activation of gut T1R3 mediates sugar reinforcement, then IG infusions of a nutritive (sucrose) or nonnutritive (sucralose) ligand for this receptor should condition a preference for the CS+ in B6 wild-type (WT) mice. While the mice that received IG sucrose infusions developed a strong preference for the CS+, those that received IG sucralose infusions developed a weak avoidance of the CS+. In experiment 2, we used T1R3 knockout (KO) mice to examine the necessity of gut T1R2+T1R3 receptors for conditioned flavor preferences. If intact gut T1R3 (or T1R2+T1R3) receptors are necessary for flavor-sugar conditioning, then T1R3 KO mice should not develop a sugar-conditioned flavor preference. We found that T1R3 KO mice, like WT mice, acquired a strong preference for the CS+ paired with IG sucrose infusions. The KO mice were also like WT mice in avoiding a CS+ flavor paired with IG sucralose infusions These findings provide clear evidence that gut T1R3 receptors are not necessary for sugar-conditioned flavor preferences or sucralose-induced flavor avoidance in mice.
Keywords: flavor learning, sucralose, gastric infusions, T1R3 knockout mouse
most mammals are attracted to the sweet taste of sugars, i.e., have a “sweet tooth” (29). The capacity to sense sweeteners is thought to be mediated by a single heterodimeric taste receptor, T1R2+T1R3. This inference is based on studies showing that T1R2+T1R3 binds selectively to compounds that humans describe as sweet (3) and that when T1R2+T1R3 (or one of its subunits) is absent, mammals show attenuated taste-mediated responses to sugars and nonnutritive sweeteners (7, 41, 44, 45). The attraction to sugars is not determined solely by taste, however (13). Post-oral nutritive actions of sugars can also stimulate intake and enhance flavor preference (32). For instance, rodents acquire strong preferences for flavored solutions that have been paired with concurrent intragastric (IG) infusions of sugars or maltodextrins (1, 32, 37). Recent evidence indicates that the upper intestinal tract is a critical site of action for sugar-conditioned flavor preferences (2, 9), but the identity of the intestinal sugar sensor is not known. The recent discovery of T1R2, T1R3, and other taste signaling proteins (gustducin, TRPM5) in the gastrointestinal tract (5, 10, 15, 16) raises the possibility that ingested sugars stimulate feeding and promote flavor conditioning by activating a “sweet taste” signaling pathway in the gut.
The “sweet taste” receptor in the gut is thought to contribute to post-oral sugar processing in two ways. It binds to sweeteners and activates signaling pathways that upregulate expression of two glucose transporters (SGLT1 and GLUT2) in the intestinal epithelium; this has the effect of increasing sugar absorption during a meal (19, 23, 25, 26, 39), but see Refs. 11, 22, and 28. Importantly, the upregulation of sugar transporters can be stimulated by IG infusions of both nutritive and nonnutritive sugars (25, 39). Gut sweetener receptors may also mediate the release of incretin hormones (i.e., GLP-1 and GIP) in mice. This inference is based on the observation that T1R3 knockout (KO) mice show impaired GLP-1 release from a surgically isolated duodenum preparation following glucose infusions (18).
The present study asked whether gut T1R3 receptors acting alone, potentially as a homodimer (44), or as part of the T1R2+T1R3 heterodimer contribute to sugar-conditioned flavor preferences in mice. In experiment 1, we compared the conditioning effects of IG infusions of sucrose with those of the nonnutritive sweetener sucralose in normal C57BL/6 mice. This was of interest because sucralose is highly preferred by mice (4), binds to both the T1R2+T1R3 receptor (20) and its component subunits (27), and promotes expression of sugar transporters (SGLT1 and GLUT2) in the intestinal epithelium of B6 mice (23, 25). We previously reported robust flavor conditioning with IG sucrose in mice (37), but the conditioning effects of nonnutritive sweetener infusions have not been investigated. In experiment 2, we used T1R3 KO mice to examine the necessity of gut T1R3 receptors for sugar-conditioned flavor preferences. T1R3 KO mice show greatly attenuated preferences for orally consumed nutritive and nonnutritive sweeteners (7, 41, 44, 45) and, as noted above, have diminished post-oral responses to sugars (18, 25). If gut T1R2+T1R3 signaling or T1R3 signaling alone is necessary for flavor-nutrient conditioning, then T1R3 KO mice should not develop a preference for a flavored solution paired with IG infusions of nutritive or nonnutritive sweeteners.
MATERIALS AND METHODS
Subjects
The C57BL/6J wild-type (B6 WT) mice were derived from individuals obtained from the Jackson Laboratories (Bar Harbor, ME), while the T1R3 KO mice were derived from individuals that were produced by homologous recombination in C57BL/6J embryonic stem cells and maintained on this background (7). Male mice from each strain were 9 wk of age at testing. They were housed singly in plastic tub cages with ad libitum access to chow (5001, PMI Nutrition International, Brentwood, MO) and tap water. The mice were kept in a room maintained at 22°C with a 12:12-h light-dark cycle. Experimental protocols were approved by the Institutional Animal Care and Use Committee at Brooklyn College and were performed in accordance with the National Institutes of Health's Guidelines for the Care and Use of Laboratory Animals.
Gastric Surgery
The mice were anesthetized with isoflurane (2%) inhalation and fitted with a gastric catheter, as described previously (37). In brief, the catheter was inserted into the stomach through a small incision in the greater curvature and secured with a purse-string suture and polypropylene mesh. The distal end of the catheter was inserted through an incision in the abdominal muscle, routed under the skin to the back of the neck, and extended 2 cm through a hole in the skin. The tip of the catheter was heat-sealed at the time of surgery. The abdominal incision was closed with Nexaband adhesive (Veterinary Products Laboratories, Phoenix, AZ), and the skin incision was sutured closed (5–0 silk) and treated with triple antibiotic ointment. Afterward, the mice were returned to their tub cages. To facilitate recovery, the animals were given rations of a palatable liquid diet, both 2 days before and 3 days after surgery (3 ml/day, Chocolate Ensure, Abbott Laboratories, Abbott Park, IL), in addition to their chow diet. A week after surgery, the animals were transferred to the infusion test cages described below. Three days later, the mice were briefly (5 min) anesthetized with 2% isoflurane, and the gastric catheter was extended with a 27-cm length of microrenathane tubing. The tubing passed through an infusion harness with a spring tether (CIH62; Instech Laboratories, Plymouth Meeting, PA), which was attached to each mouse's harness. The mice were then returned to the test cages, and the tether was attached to an infusion swivel mounted on a counterbalanced lever (Instech Laboratories) positioned at the top of the cage.
Testing Apparatus
Testing occurred in infusion cages (15 × 15 × 32 cm high) described previously (37). Fluid was available from one or two stainless-steel sipper spouts attached to 50-ml plastic centrifuge tubes. The sipper spouts were interfaced to a microcomputer via electronic lickometers (Med Electronics, St. Albans, VT) and the computer operated a syringe pump (A-99; Razel Scientific, Stamford, CT) that infused liquid into the gastric catheters as the animals drank. The pump rate was nominally 0.5 ml/min, but the animal controlled the overall infusion rate and volume by its licking response; oral intake-to-infusion ratio was maintained at 1:1 by computer software. In two-bottle tests, two infusion pumps were attached via a 20-gauge Y-connector to the gastric catheters. The volume of the infusion tube from the Y-connector to the gastric outlet was 0.06 ml. A limitation of the Y-connector system is that when two flavors are paired with different infusates in two-bottle tests and the animal drinks one flavor followed sometime later by the second flavor, the intake of the second flavor will be initially paired with a small amount (0.06 ml) of the first flavor's infusate. Daily intakes were measured to the nearest 0.1 g, and IG infusions were recorded to the nearest 0.5 ml.
RESULTS
Experiment 1: Flavor Conditioning in B6 Mice by IG Infusions of Sucrose or Sucralose
This experiment determined whether B6 WT mice would learn to prefer a flavored solution (the conditioned stimulus, CS+) when its intake was paired with IG infusions of 1.6% sucralose. As a positive control, we also tested for flavor-nutrient conditioning with IG infusions of 16% sucrose (37). Note that the infused concentrations of sucralose and sucrose were diluted in the stomach to 0.8% and 8%, respectively, by the orally consumed CS+ solution. In 1-min and 48-h two-bottle tests, naive B6 mice ingested equal volumes of 8% sucrose and 0.8% sucralose solutions, indicating that the two solutions are initially isopreferred when orally consumed (A. Sclafani, unpublished data).
We initially trained the animals with unsweetened CS solutions, which were effective in a prior study (37). Then, we repeated the conditioning experiment with saccharin-sweetened CS solutions to stimulate higher training intakes and enhance conditioning. Once the experiments involving IG infusions were completed, the same mice were given a series of two-bottle tests with 8% sucrose and 0.8% sucralose to assess the relative palatability of these sweeteners.
Experiment 1A.
The mice were given ad libitum access to chow and fluid in the infusion cages 23 h/day; the cages were serviced during the remaining hour. The mice were infused with water as they drank water from the sipper tube for 3 days prior to the onset of flavor conditioning. The mice were then divided randomly into Sucrose (n = 9) and Sucralose (n = 11) groups, which were equated for water intake and body weight.
The CS solutions contained 0.05% (wt/wt) cherry or grape unsweetened Kool-Aid mix (General Foods, White Plains, NY) in tap water. These citric acid-based flavors are equally unpreferred relative to plain water by the B6 mice (A. Sclafani, unpublished findings). The IG infusates were water, 16% (467 mM) sucrose (Domino Sugar, Yonkers, NY), or 1.6% (40.2 mM) sucralose (Tate & Lyle, Dayton, OH) in water. For five mice in each group, cherry was the CS+ flavor paired with IG sweetener infusion, and grape was the CS- flavor paired with IG water infusion; for the remaining mice in each group, the flavor-infusate pairs were reversed.
The mice were given six one-bottle training days. The CS- solution was offered on days 1, 3, and 5; and the CS+ solution on days 2, 4, and 6. Intake of the CS- solution was paired with IG infusions of water, while intake of the CS+ solution was paired with IG infusions of 16% sucrose and 1.6% sucralose in the Sucrose and Sucralose groups, respectively, over the 23-h test sessions. A two-bottle test was then conducted for 2 days with the CS+ vs. CS- solution. Intake of each CS+ solution was paired with IG infusions of the same sweetener that was used during training. Once this experiment was completed, the same mice were used in experiments 1B and 1C.
Total fluid intake (oral intake plus IG infusate) during each of the 6 training days and 2 test days was calculated. The training data were evaluated with a three-way ANOVA, using group (Sucrose or Sucralose) as a between factor and training day and CS solution (CS+ or CS-) as within factors. The two-bottle test data were averaged over the 2 test days and analyzed with two-way ANOVA, using group as a between factor and CS solution as a within factor. The two-bottle intakes of the individual mice were also expressed as percent CS+ intakes (CS+ intake/total intake × 100). These data were analyzed with a Student's t-test. In all statistical comparisons, the null hypothesis was rejected when P < 0.05.
Experiment 1B.
The design of this experiment was identical to that described in experiment 1A, except that the CS+ and CS- solutions were both sweetened with 0.2% sodium saccharin (Sigma Chemical, St. Louis, MO). The mice were kept in the same respective IG infusion and flavor-pairing groups. The data were analyzed as in experiment 1A.
Experiment 1C.
The mice were run through four sequential two-bottle tests without IG infusions (23 h/day). Because two mice died during this experiment, the final sample size was 9 per group. The solutions used in each two-bottle test were 0.8% sucralose vs. 8% sucrose (test 1), 0.8% sucralose vs. water (test 2), 8% sucrose vs. water (test 3), and 0.8% sucralose vs. 8% sucrose (test 4). Each test was conducted over 2 days with the left-right position of the fluid choices counter-balanced across each day. Intakes by mice that received the sucrose or sucralose infusions in the prior experiment were initially analyzed separately with a three-way ANOVA, using IG infusate group as a between factor and test number and CS solution as within factors. Overall, the mice in the Sucrose group consumed more fluid than did those in the Sucralose group [11.4 vs. 8.6 g/day; F (1,16) = 4.74, P < 0.05], but the relative intakes of each solution did not significantly differ across groups. Therefore, the data from the two groups were subsequently combined in two additional two-way ANOVAs. The first ANOVA included results from tests 1 and 4 and asked whether relative intakes of sucralose and sucrose changed across the two tests. The second ANOVA included results from tests 2 and 3 and compared the preference for each sweetener over water.
Experiment 1: Results
Experiment 1A.
Figure 1 presents total daily intakes by B6 WT mice in the Sucrose and Sucralose groups across the 6 days of one-bottle training and the two-bottle tests, using unsweetened CS solutions. Irrespective of their different infusates, the mice in the Sucrose and Sucralose groups consumed similar amounts of the CS+ and CS- solutions during training. In the two-bottle test, overall CS+ intakes exceeded CS- intakes [F (1,18) = 7.4, P < 0.05]. While the Group × CS interaction was not significant, post hoc tests indicated that only the sucrose-infused mice consumed significantly more CS+ than CS- [5.8 vs. 3.5 g/day, P < 0.05]. However, the preference for the CS+ in the sucrose-infused mice was weak and did not exceed that of the sucralose-infused mice, 59% vs. 54%.
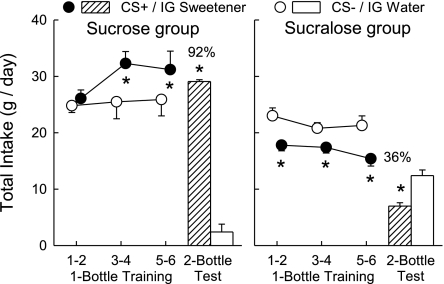
Fig. 1.
Experiment 1A. Flavor conditioning with unsweetened conditioned stimulus (CS) solutions in B6 WT mice. Intake of the CS+ solution was paired with IG infusions of either 16% sucrose (left) or 1.6% sucralose (right). Intake of the CS- solution was paired with IG infusions of water (both panels). We show total daily fluid intake (oral CS intake plus IG infusate) across each of the six one-bottle training days. The CS-solution was offered on days 1, 3, and 5, and the CS+ solution was offered on days 2, 4, and 6. Following training, the mice were offered a choice between the CS+ and CS- solutions over two consecutive days. Values are mean (± S.E.) total daily intakes. Numbers atop bars represent mean percent preference for the CS+ solutions. Significant differences (*P < 0.05) between CS+ and CS- intakes during 2-bottle test are shown.
Experiment 1B.
When the CS solutions were sweetened with saccharin, intakes increased dramatically (Fig. 2). Overall, training intakes by the sucrose-infused mice exceeded those of the sucralose-infused mice [F (1,18) = 18.66, P < 0.001]. Note that the groups consumed similar amounts of CS- on the first training day, but thereafter, CS intakes increased in the sucrose-infused mice and decreased in the sucralose-infused mice [F (2,36) = 4.15, P < 0.05]. Furthermore, whereas the sucrose-infused mice consumed more [P < 0.01] CS+ than CS-, the sucralose-infused mice consumed more [P < 0.01] CS- than CS+ [CS × group interaction, F (1,18) = 41.18, P < 0.001] during training. The groups also differed in their CS intakes during the two-bottle test [F (1,18) = 18.14, P < 0.001]. The Sucrose mice consumed substantially more [P < 0.001] CS+ than CS-, whereas the Sucralose mice consumed more CS- than CS+ [CS × Group interaction, F (1,18) = 80.3, P < 0.001]. Consequently, the percent CS+ intakes differed significantly between the sucrose- and sucralose-infused mice [92 vs. 36%, respectively; t = 11.35, df = 18, P < 0.001].
Fig. 2.
Experiment 1B. Flavor conditioning with saccharin-sweetened CS solutions in B6 WT mice. Intake of the CS+ solution was paired with IG infusions of either 16% sucrose (left) or 1.6% sucralose (right). Intake of the CS- solution was paired with IG infusions of water (both panels). We show total daily fluid intake (oral CS intake plus IG infusate) across each of the six one-bottle training days. The CS- solution was offered on days 1, 3, and 5, and the CS+ solution was offered on days 2, 4, and 6. Following training, the mice were offered a choice between the CS+ and CS- solutions over two consecutive days. Values are expressed as means ± SE total daily intakes. Numbers atop bars represent mean percent preference for the CS+ solutions. Significant differences (*P < 0.05) between CS+ and CS- intakes during 1-bottle training or 2-bottle test are shown.
Experiment 1C.
The relative sweetener intakes differed significantly between tests 1 and 4 [F (1,17) = 40.34, P < 0.001] (Fig. 3). In test 1, the mice consumed slightly (but not significantly) more sucralose than sucrose solution; in test 4, the mice consumed significantly more sucrose than sucralose solution [P < 0.001]. The percent sucralose intakes in tests 1 and 4 also significantly differed [71% vs. 24%, t = 7.13, df = 17, P < 0.001]. In tests 2 and 3, intakes of both sucrose and sucralose exceeded intakes of water [F (1,17) = 243.92, P < 0.001], and daily intake of sucrose (in test 3) was significantly greater [P < 0.001] than that of sucralose (in test 2).
Fig. 3.
Experiment 1C. Oral sweetener two-bottle preference tests. The same B6 WT mice were run in the four consecutive tests. In test 1, the mice received 0.8% sucralose vs. 8% sucrose; in test 2, 0.8% sucralose vs. water; in test 3, 8% sucrose vs. water; and in test 4, 0.8% sucralose vs. 8% sucrose. There were no IG infusions during the tests. Values are expressed as means + SE daily intakes averaged across each two-day test. Numbers atop bars represent mean percent preference for that solution. Significant intake difference (*P < 0.05) within a two-bottle test is shown.
Experiment 2. Flavor Conditioning in T1R3 KO Mice by IG Infusions of Sucrose or Sucralose
This experiment was designed to test whether the T1R3 receptor is necessary for development of the sweetener-conditioned flavor preference using T1R3 KO and B6 mice. In experiment 1 and prior studies (33, 36), flavor preference conditioning by IG sugar infusions was enhanced when mice were trained with saccharin-sweetened CS solutions. T1R3 KO mice, however, are not attracted to saccharin solutions (7, 45). Therefore, to enhance the intake of the training solutions in the present experiment, the CS+ and CS- solutions both contained 1% Intralipid. Prior work established that this oil emulsion stimulates high daily intake in both B6 and T1R3 KO mice (45).
Experiment 2A.
The CS solutions contained 0.05% (wt/wt) cherry or grape unsweetened Kool-Aid mix in a 1% Intralipid solution prepared by diluting 20% Intralipid (Baxter, Deerfield, IL) with water. Intake of the CS+ and CS- solutions were paired with IG infusions of 16% sucrose and water, respectively. Unlike the saccharin-sweetened CS solutions used in experiment 1, the Intralipid-containing CS+ and CS- solutions in this experiment contained some energy (0.1 kcal/g), but much less than that provided by the IG infusions of 16% sucrose (0.64 kcal/g).
The mice were adapted to the test cages as in experiment 1A. The B6 (n = 11) and T1R3 KO (n = 9) mice were given six one-bottle training days. The CS- solution was offered on days 1, 3, and 5; and the CS+ solution on days 2, 4, and 6. A two-bottle choice test was then conducted with the CS+ vs. CS- solution; the test was extended to 4 days because of problems with some infusion lines on day 2 of testing. The data analysis described for experiment 1A was used in this and the next experiment. Note that we used the same mice in experiments 2A–C.
Experiment 2B.
The mice from experiment 2A were trained and tested as described above, except that new flavored CS+ and CS- solutions were paired with IG 1.6% sucralose infusions and water, respectively. The Intralipid-containing CS solutions were flavored with orange and lemon-lime Kool-Aid. Because two B6 mice died during this experiment, the sample size in this and the next experiment was 9 per strain.
Experiment 2C.
This final experiment asked whether the IG infusions of sucralose diminished the palatability of the CS Intralipid solutions relative to water. To this end, we subjected the mice from each strain to two preference tests. In test 1, the B6 and T1R3 KO mice were given the choice between the CS+ (paired with IG sucralose infusions) and plain water (paired with IG water infusions) for 2 days. In test 2, the same mice were given the choice between the CS- (paired with IG water) and plain water (paired with IG water) for 2 days. The CS solutions used in experiment 2B were used here. A separate two-way ANOVA was run for each strain, with test (1 or 2) and CS solution serving as within factors.
Experiment 2: Results
Experiment 2A.
One-bottle training intakes of the T1R3 KO and B6 WT mice did not differ (Fig. 4). Overall, the mice consumed more CS- than CS+ [F (1,18) = 66.48, P < 0.001], but CS intakes varied over days [F (2,36) = 9.34, P < 0.001]. In both groups, CS+ intakes were lowest during the first training day and then increased over successive days; CS- intakes, on the other hand, did not change. The low CS+ intakes during the first training session presumably reflect a satiating effect of the infused sucrose combined with the orally consumed oil emulsion. The increased CS+ intakes in subsequent training days may reflect an adaptation to the satiating effect combined with the preference conditioning action of the infused sucrose. In the two-bottle test, both strains consumed substantially more CS+ than CS- [F (1,18) = 51.38, P < 0.001], and they did not differ in their overall intakes. The percent CS+ preferences of the T1R3 KO and B6 WT groups also did not differ significantly (92 vs. 87%).
Fig. 4.
Experiment 2A. Flavor conditioning by IG sucrose in T1R3 KO (left) and B6 WT (right) mice. Intake of the CS+ solution was paired with IG infusions of 16% sucrose, while intake of the CS- solution was paired with IG infusions of water. The flavored CS solutions contained 1% Intralipid to stimulate intake. We show total daily fluid intake (oral intake plus IG infusate) across each of the six one-bottle training days. The CS- solution was offered on days 1, 3, and 5; and the CS+ solution was offered on days 2, 4, and 6. Following training, the mice were offered a choice between the CS+ and CS- solutions over four consecutive days. Values are expressed as mean ± SE total daily intakes. Numbers atop bars represent mean percent preference for the CS+ solutions. Significant differences (*P < 0.05) between CS+ and CS- intakes during 1-bottle training or 2-bottle test are shown.
Experiment 2B.
The mice from both strains consumed a substantial amount of the CS- solution on the first training day, but their intakes decreased significantly the next day when given the CS+ paired with IG sucralose infusions (Fig. 5). CS+ intake remained relatively stable over the subsequent training days, whereas CS- intakes declined to the level of the CS+. The decline in CS- intake over training may represent a generalization of the learned inhibitory response to the CS+ flavor to the CS- flavor. Overall, the training intakes of the T1R3 KO and B6 WT mice did not differ, and both groups consumed more CS- than CS+ [F (1,16) = 25.06, P < 0.001], although intakes changed over days [F (2,32) = 11.90, P < 0.001]. In the two-bottle test, the T1R3 KO and B6 WT mice consumed more CS- than CS+, although this difference was not quite significant [F (1,16) = 4.07, P = 0.061]. The T1R3 KO and B6 WT mice did not differ in their relative avoidance of the CS+ flavor (36 vs. 43%).
Fig. 5.
Experiment 2B. Flavor conditioning by IG sucralose in T1R3 KO (left) and B6 WT (right) mice. Intake of the CS+ solution was paired with IG infusions of 1.6% sucralose, while intake of the CS- solution was paired with IG infusions of water. The flavored CS solutions contained 1% Intralipid to stimulate intake. We show total daily fluid intake (oral intake plus IG infusate) of the CS+ and CS- across each of the six one-bottle training days. The CS- solution was offered on days 1, 3, and 5; and the CS+ solution on days 2, 4, and 6. Following training, the mice were offered a choice between the CS+ and CS- solutions over four consecutive days. Values are expressed as means ± SE total daily intakes. Numbers atop bars represent mean percent preference for the CS+ solutions. Significant differences (*P < 0.05) between CS+ and CS- intakes during 1-bottle training or 2-bottle test are shown.
Experiment 2C.
Figure 6 shows the results of tests in which mice from both strains were offered a choice between the CS+ vs. water and the CS- vs. water. The CS+ remained paired with IG sucralose and the CS- and water were paired with IG water in these tests. Both strains consumed more CS+ than water in test 1 [F (1,16) = 25.04, P < 0.001], and more CS- than water in the test 2 [F (1,16) = 34.40, P < 0.001]. Overall, the groups did not differ in their CS intakes or CS preferences. Thus, while the CS+ was less preferred than the CS- in experiment 2B, the CS+ and CS- were strongly preferred over plain water in the present experiment. This indicates that the IG sucralose infusions did not diminish the palatability of the flavored oil emulsion relative to water.
Fig. 6.
Experiment 2C. CS vs. water two-bottle preference tests with T1R3 KO mice (left) and B6 WT (right). In test 1, the CS+ was the flavored Intralipid emulsion paired with IG sucralose infusions. In test 2, the CS- was the flavored Intralipid emulsion paired with IG water infusions. In both tests, unflavored water was paired with IG water infusions. We show total daily fluid intake (oral intake plus IG infusate) of the CS+ and water during two-bottle test 1, or the CS- and water during test 2. Values are expressed as means ± SE total daily intakes averaged across each 2-day test. Numbers atop bars represent mean percent preference for that solution. Significant differences (*P < 0.05) between CS solution and water are shown.
DISCUSSION
Does Post-oral Sucralose Condition a Flavor Preference in Normal Mice?
Experiment 1 was designed to test whether IG infusions of sucralose, a nonnutritive sweetener that binds to T1R2+T1R3 (and its respective monomers) (27), would condition a flavor preference in B6 WT mice. As a positive control, we subjected a second group of mice to the same conditioning procedure but infused sucrose instead of sucralose. In experiment 1A, the mice in both the sucralose and sucrose infusion groups ingested small quantities of the unsweetened CS solutions during training, and as a result, they had limited opportunities to associate the CS solutions with the IG infusions. Although we previously obtained sucrose-conditioned preferences in B6 mice with unsweetened CS solutions (37), we also observed that preference conditioning is related to how much of the CS solutions they consume during training (36).
By adding saccharin to the CS solutions in experiment 1B, we enhanced daily intakes of the mice in the Sucralose and Sucrose groups. However, the extent of feeding stimulation was disproportionately enhanced in the sucrose-infused mice. The sucrose-infused mice not only consumed more CS+ than CS- during training, but they also displayed a strong preference for the CS+ (93%) during the two-bottle test. This result confirms our prior findings (36, 37) and demonstrates the potent reinforcing action of IG infusions of 16% sucrose. In contrast, the sucralose-infused mice consumed more CS- than CS+ during training, and they avoided the CS+ during the two-bottle test (36%). The finding that mice in the Sucralose group consumed significantly less CS+ than CS- in the choice test suggests that the IG sucralose infusions had an inhibitory post-oral effect (see below).
Does Post-oral Sucrose Condition Flavor Preference in T1R3 KO Mice?
In experiment 2A, we examined the contribution of gut T1R3 receptors to post-oral sugar conditioning by comparing the ability of IG sucrose infusions to condition flavor preferences in T1R3 KO and B6 WT mice. There was no apparent effect of knocking out the T1R3 receptor on the behavioral responses to the IG sucrose solutions. The KO and WT mice consumed comparable amounts of the CS+ solution paired with IG sugar infusions during one-bottle training, and both strains exhibited strong preferences for the CS+ flavor (92 and 87%) in the two-bottle test.
The IG conditioning results are consistent with prior studies demonstrating that T1R3 KO mice develop strong preferences for concentrated sucrose solutions when they are given a choice between the sugar solutions and water during 48-h tests (7, 44, 45). In particular, we observed that T1R3 KO mice were indifferent to 0.5–8% solutions but preferred 16–32% sucrose to water (45). Yet, brief access taste tests (1-min two-bottle) and gustatory nerve recordings indicate that T1R3 KO show little or no response to concentrated sucrose solutions (44, 45). The preference of the T1R3 KO mice for concentrated sucrose solutions in 48-h tests was attributed to a post-oral conditioned preference for orosensory features of the sugar solutions (i.e., odor, texture, and/or T1R2-mediated taste) (41, 45, 46). The present findings confirm the ability of T1R3 KO mice to learn a flavor preference, in this case for a grape or cherry flavor that was associated with IG infusions of sucrose. It is notable, however, that even though the T1R3 knockout mice develop a strong preference for 16% sucrose in 48-h tests, their absolute intake of the sugar solution was less than that of the B6 WT mice (45, 46). On the other hand, the T1R3 KO mice in experiment 2 consumed as much of the CS+ flavored solution paired with IG infusions of 16% sucrose as did the WT mice. This discrepancy can be explained by the presence of Intralipid in the CS+ flavor, which is attractive to T1R3 KO and B6 WT mice alike (45).
The significant CS+ preference conditioned by IG sucrose in T1R3 KO mice indicates that intestinal sweetener receptors do not mediate post-oral sugar conditioning. Other sweet taste signaling elements found in the gut are also not required for post-oral sugar conditioning. TRPM5 KO mice, which show little or no preference for sugar solutions in oral tests (6, 43), learned preferences for cues paired with IG glucose infusions (8, 12). Similarly, gustducin KO mice (42) learned to prefer a CS+ flavor paired with IG sucrose infusion (A. Sclafani unpublished data).
Why Did IG Sucralose Suppress CS+ Intake and Preference?
A novel finding of the present study is that the IG sucralose infusions suppressed intake of the CS+ and conditioned a weak avoidance of the CS+. Recent findings indicate that sucralose stimulates the release of GLP-1 from GLUTag cells (a mouse enteroendocrine cell line) by acting on sweet receptors located in the cell (25). Given that GLP-1 inhibits feeding, increases satiety, delays gastric emptying, and induces taste aversions (24, 27), it would be logical to infer that sucralose-induced GLP-1 release mediated the inhibitory actions of IG sucralose on CS+ intake and preference. However, if sucralose promoted GLP-1 release by activating T1R3 receptors, then the T1R3 KO mice should have been resistant to the inhibitory actions of IG sucralose. This was not the case, and thus argues against a role of GLP-1 release in the flavor avoidance conditioned by of IG sucralose infusions.
IG sucralose may have suppressed intake and preference for the CS+ by acting on T2R bitter receptors in the gut. In 48-h choice tests, B6 mice prefer sucralose to water at concentrations up to 30 mM (1.2%) (4, 7), whereas T1R3 KO mice were indifferent to sucralose up to 3 mM (0.12%) and avoided higher concentrations (7). These data suggest that sucralose has a bitter taste at concentrations >3 mM, but that the bitter taste is masked in B6 WT mice by the sweet taste of sucralose. Given that T2R bitter receptors are expressed in intestinal cells (30), and that IG infusions of bitter compounds can condition flavor aversion (14), it may be that IG sucralose infusions suppressed CS+ intake and preference by activating gut T2R receptors. This remains speculative, however, and the post-oral inhibitory effect of sucralose and the concentration range over which it operates require further study.
While the IG sucralose infusions conditioned a weak avoidance to the CS+ (relative to the CS-) in experiment 1B, the B6 mice significantly preferred the 0.8% sucralose solution to plain water in experiment 1C. In fact, they tended to consume more sucralose than sucrose during test 1 of experiment 1C, but this weak preference for sucralose did not persist. By test 4 of experiment 1C, the mice consumed substantially more sucrose than sucralose. We attribute the dramatic shift in sweetener preference from tests 1 to 4 to the mice learning (during tests 2 and 3) to associate the unique flavor profiles of the two sweeteners to their differential post-oral consequences; that is, with the inhibitory post-oral actions of sucralose and stimulatory post-oral actions of sucrose. Such learning would have been difficult during test 1 if the mice consumed the two sweeteners in close temporal proximity.
Experiment 2C revealed that while the mice avoided the sucralose-paired CS+ flavor to the water-paired CS- flavor, they significantly preferred the sucralose-paired CS+ to plain water. Taken together, experiments 1C and 2C show that the post-oral inhibitory action of sucralose is sufficient to shift the animals' preference from one preferred solution to another (i.e., CS+ to CS-) but does not diminish the palatability of the sweetener itself or a sweetener-paired oil emulsion (Intralipid) relative to a neutral alternative (water). Thus, sucralose infusions did not have toxic or strongly aversive effects like lithium chloride, which condition strong aversions to sweet and fatty flavors (38).
As noted previously, we selected a sucralose infusion concentration of 1.6% (which was diluted to 0.8% in the stomach) on the basis of our preliminary findings that 0.8% sucralose and 8% sucrose are isopreferred by naïve B6 mice. This is a high concentration for humans, however, who rate 0.02% sucralose to have a sweetness intensity similar to that 8.5% sucrose (31). Consequently, the current finding that orally consumed 0.8% or IG infused 1.6% sucralose inhibits fluid intake and preference in mice may not be relevant to the sweetener's action in humans at the concentrations used in commercial beverages.
Perspectives and Significance
It is now established that taste receptors are expressed in the mouth, gastrointestinal tract, and other organ systems (5, 10, 15, 17). It is also known that sugar appetite is mediated by T1R2+T1R3 taste receptors in the mouth (7, 44) and by as yet unidentified sugar receptors in the gut (36, 37, 45). The present study asked whether activation of the gut T1R3 receptors acting alone, potentially as a homodimer (44), or as part of the T1R2+T1R3 heterodimers contributes to the post-oral reinforcement provided by sugars. Our results provide clear evidence that these receptors are not essential for post-oral sugar reinforcement. T1R3 KO mice, which are unresponsive to sugars in the mouth (7, 41, 44, 45), were fully responsive to the positive conditioning effects of sugar in the gut. Also, the artificial sweetener, sucralose, which activates T1Rs in both the mouth and gut (7, 23, 25), did not reinforce flavor preferences when infused into the gut. Rather, IG sucralose had an unexpected inhibitory effect on flavor solution intakes in normal and T1R3 KO mice alike. Consistent with the differential conditioning actions of sucrose and sucralose, recent data indicate that sucrose, but not sucralose, can act post-orally to promote dopamine release in the nucleus accumbens (8), a critical site for flavor-nutrient conditioning (40).
Additional studies are needed to identify the sensor and signaling pathway responsible for the post-oral sugar conditioning process. A glucose-specific sensor is suggested by the finding that glucose is much more potent that fructose in supporting flavor conditioning (35). An intestinal site of action is suggested by the findings that intestinal glucose infusions are more effective than hepatic-portal infusions (2, 9). Finally, a humoral pathway is suggested by the failure of visceral deafferentiation to prevent post-oral glucose conditioning (21, 34).
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-031135 (to A. Sclafani) and National Institute of Deafness and Other Communications Disorders Grants DC-03055 and DC-03155 (to R. F. Margolskee).
DISCLOSURES
R. F. Margolskee has a personal financial interest in the form of stock ownership in the Redpoint Bio and is an inventor on patents and patent applications that have been licensed to the Redpoint Bio Company.
ACKNOWLEDGMENTS
The authors thank Kwame McCartney and Martin Zartarian for their expert technical assistance and Karen Ackroff for her helpful comments on this manuscript.
REFERENCES
- 1.Ackroff K, Dym C, Yiin YM, Sclafani A. Rapid acquisition of conditioned flavor preferences in rats. Physiol Behav 97: 406–413, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackroff K, Yiin YM, Sclafani A. Post-oral infusion sites that support glucose-conditioned flavor preferences in rats. Physiol Behav 99: 402–411, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmanov AA, Beauchamp GK. Taste receptor genes. Annu Rev Nutr 27: 389–414, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses 26: 905–913, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bezençon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses 32: 41–49, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Perez CA, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses 31: 253–264, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301: 850–853, 2003 [DOI] [PubMed] [Google Scholar]
- 8.De Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MAL, Simon SA. Food reward in the absence of taste receptor signaling. Neuron 57: 930–941, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Drucker DB, Sclafani A. The role of gastric and postgastric sites in glucose-conditioned flavor preferences in rats. Physiol Behav 61: 351–358, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans 33: 302–305, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Fujita Y, Wideman RD, Speck M, Asadi A, King DS, Webber TD, Haneda M, Kieffer TJ. Incretin release from gut is acutely enhanced by sugar but not by sweeteners in vivo. Am J Physiol Endocrinol Metab 296: E473–E479, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Glass DS, Margolskee RF, Sclafani A. Glucose-conditioned preferences in taste-impaired TRPM5 knockout mice (Abstract). Appetite 52: 833, 2009 [Google Scholar]
- 13.Glendinning JI, Beltran F, Benton L, Cheng S, Gieseke J, Gillman J, Spain HN. Taste does not determine daily intake of dilute sugar solutions in mice. Am J Physiol Regul Integr Comp Physiol 299: R1333–R1341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glendinning JI, Yiin YM, Ackroff K, Sclafani A. Intragastric infusion of denatonium conditions flavor aversions and delays gastric emptying in rodents. Physiol Behav 93: 757–765, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hass N, Schwarzenbacher K, Breer H. T1R3 is expressed in brush cells and ghrelin-producing cells of murine stomach. Cell Tissue Res 339: 493–504, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Höfer D, Püschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci USA 93: 6631–6634, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kokrashvili Z, Jiang P, Mosinger B, Margolskee RF. Roles of taste signaling molecules in endocrine cells in pancreas and tongue (Abstract). Chem Senses 35: A12, 2010 [Google Scholar]
- 18.Kokrashvili Z, Mosinger B, Margolskee RF. T1r3 and alpha-gustducin in gut regulate secretion of glucagon-like peptide-1. Ann NY Acad Sci 1170: 91–94, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Le Gall M, Tobin V, Stolarczyk E, Dalet V, Leturque A, Brot-Laroche E. Sugar sensing by enterocytes combines polarity, membrane bound detectors and sugar metabolism. J Cell Physiol 213: 834–843, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA 99: 4692–4696, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucas F, Sclafani A. Capsaicin attenuates feeding suppression but not reinforcement by intestinal nutrients. Am J Physiol Regul Integr Comp Physiol 270: R1059–R1064, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Ma J, Bellon M, Wishart JM, Young RL, Blackshaw LA, Jones KL, Horowitz M, Rayner CK. Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol 296: G735–G739, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mace OJ, Affleck JA, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 582: 379–392, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maljaars PWJ, Peters HPF, Mela DJ, Masclee AAM. Ileal brake: A sensible food target for appetite control. A review. Physiol Behav 95: 271–281, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KSH, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 104: 15075–15080, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moran AW, Al Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Daly K, Ionescu C, Bravo D, Shirazi-Beechey SP. Expression of Na+/glucose co-transporter 1 (SGLT1) is enhanced by supplementation of the diet of weaning piglets with artificial sweeteners. Brit J Nutr 1–10, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr Biol 15: 1948–1952, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Parker HE, Habib AM, Rogers GJ, Gribble FM, Reimann F. Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 52: 289–298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirez I. Why do sugars taste good? Neurosci Biobehav Rev 14: 125–134, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Rozengurt E. Bitter taste receptors and alpha-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol 291: G171–G177, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Schiffman SS, Booth BJ, Losee ML, Pecore SD, Warwick ZS. Bitterness of sweeteners as a function of concentration. Brain Res Bull 36: 505–513, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Sclafani A. Oral and postoral determinants of food reward. Physiol Behav 81: 773–779, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Sclafani A. Sucrose motivation in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice measured by progressive ratio licking. Physiol Behav 87: 734–744, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Sclafani A, Ackroff K, Schwartz GJ. Selective effects of vagal deafferentation and celiac-superior mesenteric ganglionectomy on the reinforcing and satiating action of intestinal nutrients. Physiol Behav 78: 285–294, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Sclafani A, Cardieri C, Tucker K, Blusk D, Ackroff K. Intragastric glucose but not fructose conditions robust flavor preferences in rats. Am J Physiol Regul Integr Comp Physiol 265: R320–R325, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Sclafani A, Glendinning JI. Flavor preferences conditioned in C57BL/6 mice by intragastric carbohydrate self-infusion. Physiol Behav 79: 783–788, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: Oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol 289: R712–R720, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Smith JC, Fisher EM, Maleszewski V, McClain B. Orosensory factors in the ingestion of corn oil/sucrose mixtures by the rat. Physiol Behav 69: 135–146, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Stearns AT, Balakrishnan A, Rhoads DB, Tavakkolizadeh A. Rapid upregulation of sodium-glucose transporter SGLT1 in response to intestinal sweet taste stimulation. Ann Surg 251: 865–871, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Touzani K, Bodnar RJ, Sclafani A. Activation of dopamine D1 receptors in the nucleus accumbens is critical for the acquisition, but not the expression, of flavor preference conditioned by intragastric glucose in rats. Eur J Neurosci 27: 1525–1533, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Treesukosol Y, Blonde G, Spector AC. The T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to Polycose: Implications for saccharide taste receptors in mice. Am J Physiol Regul Integr Comp Physiol 296: R855–R865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature 381: 796–800, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJP. Coding of sweet, bitter, and umami tastes: Different receptor cells sharing similar signaling pathways. Cell 112: 293–301, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJP, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 115: 255–266, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not Polycose taste. Am J Physiol Regul Integr Comp Physiol 296: R866–R876, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zukerman S, Touzani K, Margolskee RF, Sclafani A. Role of olfaction in the conditioned sucrose preference of sweet-ageusic T1R3 knockout mice. Chem Senses 34: 685–694, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]