Abstract
In the title compound, [CuMoO4(C8H20N4)]·H2O, the CuII atom is coordinated by four N atoms of the 1,4,7,10-tetraazacyclododecane (cyclen) ligand and one O atom of the molybdate unit in a distorted square-pyramidal environment. The water molecules are linked to the complex unit to form centrosymmetric dimers [R 4 4(12) and R 4 4(16)] and discrete D 3 2(9), D 3 3(11) and D 3 3(13) chains by O—H⋯O and N—H⋯O interactions. Additionally, the complex molecules are linked into C 4 4(18) chain motifs by N—H⋯O interactions. As a result [(cyclen)CuMoO4] units and water molecules are linked to layers that are oriented parallel to the ac plane. The stacking of the layers in the b-axis direction is supported by weak C—H⋯O hydrogen bridges.
Related literature
For inorganic–organic hybrid materials based on copper complexes with bridging molybdate ligands, see, for example: Rarig et al. (2002 ▶); Hagrman et al. (1998 ▶). For copper complexes with the cyclen ligand, see: Clay et al. (1979 ▶); Lu et al. (1997 ▶); Yeung et al. (2000 ▶); Guo et al. (2008 ▶). For related literature, see: Bernstein et al. (1995 ▶); Choi et al. (2004 ▶).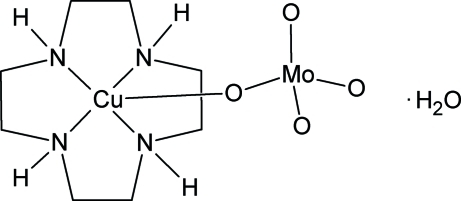
Experimental
Crystal data
[CuMoO4(C8H20N4)]·H2O
M r = 413.78
Triclinic,
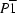
a = 8.6985 (6) Å
b = 8.9784 (6) Å
c = 9.0055 (6) Å
α = 90.358 (6)°
β = 91.949 (6)°
γ = 100.742 (5)°
V = 690.54 (8) Å3
Z = 2
Mo Kα radiation
μ = 2.47 mm−1
T = 200 K
0.22 × 0.21 × 0.13 mm
Data collection
Stoe IPDS 2T diffractometer
Absorption correction: numerical (X-RED; Stoe & Cie, 2009 ▶) T min = 0.612, T max = 0.737
9700 measured reflections
3017 independent reflections
2788 reflections with I > 2σ(I)
R int = 0.037
Refinement
R[F 2 > 2σ(F 2)] = 0.024
wR(F 2) = 0.063
S = 1.04
3017 reflections
196 parameters
6 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.48 e Å−3
Δρmin = −1.05 e Å−3
Data collection: X-AREA (Stoe & Cie, 2009 ▶); cell refinement: X-AREA; data reduction: X-RED (Stoe & Cie, 2009 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: DIAMOND (Brandenburg, 2009 ▶); software used to prepare material for publication: SHELXL97 and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810026000/bx2287sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810026000/bx2287Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O5—H5⋯O4i | 0.83 (2) | 1.95 (2) | 2.770 (2) | 169 (3) |
| N1—H1⋯O5ii | 0.86 (2) | 2.35 (2) | 3.156 (3) | 156 (3) |
| N2—H2⋯O1ii | 0.89 (2) | 2.19 (2) | 2.949 (2) | 143 (3) |
| N3—H3⋯O4i | 0.88 (2) | 2.28 (3) | 2.955 (3) | 133 (3) |
| N4—H4⋯O5iii | 0.88 (2) | 2.10 (2) | 2.928 (3) | 157 (3) |
| O5—H6⋯O2iv | 0.84 (2) | 1.85 (2) | 2.666 (3) | 163 (4) |
| C3—H3B⋯O3v | 0.99 | 2.36 | 3.345 (3) | 175 |
Symmetry codes: (i) 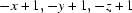 ; (ii)
; (ii)  ; (iii)
; (iii) 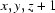 ; (iv)
; (iv) 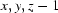 ; (v)
; (v)  .
.
supplementary crystallographic information
Comment
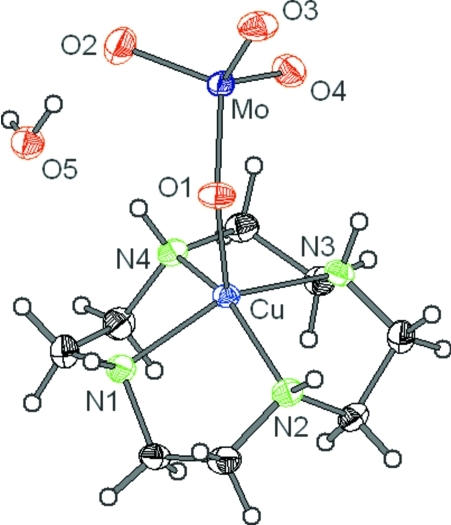
In the title compound (I) the central copper atom is coordinated by four nitrogen atoms of the cyclen ligand and one oxygen atom of the molydate group. This leads to a distorted square pyramidal coordination environment with the four N atoms in the basal positions and the molybdate oxygen atom at the apex. The arrangement of the four nitrogen atoms is nearly co-planar within a maximum deviation of 0.015 Å and the copper atom lies 0.569 Å above this plane. The Cu—N distances ranging from 2.033 (2) to 2.049 (2) Å which is comparable to the Cu—N bond lengths oberserved in other cyclen copper complexes, like [(cyclen)Cu(NO3)]NO3 (Clay et al., 1979), [(cyclen)Cu(SCN)]2[Ca(NCS)6] H2O (Lu et al., 1997), [(cyclen)Cu(Ag(CN)2)][Ag(CN)2] (Yeung et al., 2000) and [(cyclen)Cu(MnN(CN)5)] (Guo et al., 2008). The molybdate unit coordinates as a monodentate ligand with a Cu—O distance of 2.104 (2) Å. The Mo—O distances ranging from 1.749 to 1.765 Å for the terminal oxygen atoms. In the case of the µ-bridging O atom O1 the Mo—O distance is slightly enlarged to 1.796 (2) Å. A similar monodentate coordination of molybdate has been observed in the copper complex [LCuMoO4]2 5H2O (L = 1,3,10, 12,16,19-hexaazatetracyclo[17,3,1,112.16,04.9]tetracosane with Cu—O distances of 2.320 (12) and 2.418 (11) Å and Mo—O distances ranging from 1.621 to 1.849 Å (Choi et al., 2004).
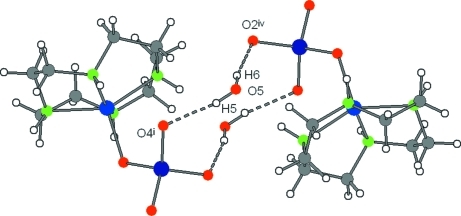
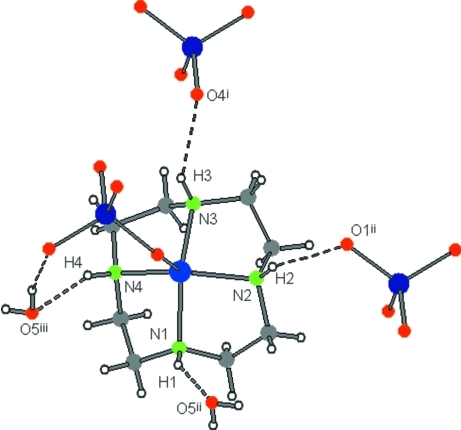
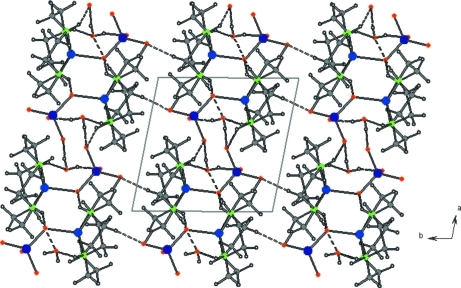
Compound (I) contains a water molecule which is involved into two strong hydrogen bonds to the molybdate O2 and O4 oxygen atoms . This leads to [(cyclen)CuMoO4]2(H2O)2 dimers containing a centrosymmetric Mo2O6H4 ring of the type R44(12) (Fig. 2) (Bernstein et al., 1995). Additionally the NH groups of the cyclen ligand are also involved in hydrogen bonds either to water molecules (N1H1 and N4H4) or to molybdate groups (N2H2 and N3H3). As a resuslt of these hydrogen bonds [(cyclen)CuMoO4] units and water are interlinked to layers which are oriented parallel to the crystallographic a-c plane. The stacking of the layers in direction of the crystallographic b axis is supported by weak C—H···O hydrogen bonds (C···O 3.35 Å) between cyclen ligands and molydate units.
Experimental
To a stirred suspension of 0.440 mg (2.0 mmol) CuMoO4 in 70 ml of aqueous methanol (90%) 0.350 mg (2.0 mmol) cyclen was added. After four hours the blue suspension was filtered and the filtrate evaporated to dryness.Yield. 420 mg (50%). The residue was dissolved in 3 ml of methanol and then the solution was layered with 2-propanol. After one week single crystals of (I) were formed at the methanol/2-propanol interface. IR(cm-1): 3172(s), 2960(w), 2937(w), 2920(w), 2869(w), 1631(w), 1592(w), 824(s); Elemental Analysis [Cu(cyclen)MoO4] H2O (413.78), C 23.0 (calc. 23.2), H 5.4 (5.4), N 13.1 (13.5) %.
Refinement
C-bound H atoms of the cyclen ligand were positioned geometrically and refinded using a riding model with Uiso(H) = 1.2 Ueq(C) [(C-H) = 0.99 Å]. H atoms attached to N and O were located from difference fourier maps and refined with N-H distances fixed in a range of 0.87 (2) to 0.89 (2) Å and O-H distances fixed at 0.83 (2) and 0.84 (2) Å. The corresponding Uiso(H) were refined freely.
Figures
Fig. 1.
Molecular structure of the [(cyclen)CuMoO4] unit. Thermal ellipsoids are drawn at the 50% probability level.
Fig. 2.
[(cyclen)CuMoO4]2(H2O)2 dimers formed by hydrogen bonds. Symmetry codes: (i) 1 - x, 1 - y, 1 - z; (iv) x, y, -1 + z
Fig. 3.
N—H···O hydrogen bonds around the [(cyclen)CuMoO4] unit. Symmetry codes (i) 1 - x, 1 - y, 1 - z; (ii) 2 - x, 1 - y, 1 - z; (iii) x, y, 1 + z
Fig. 4.
Stacking of the [(cyclen)CuMoO4]H2O layers along the crystallographic b axis.
Crystal data
| [CuMoO4(C8H20N4)]·H2O | Z = 2 |
| Mr = 413.78 | F(000) = 418 |
| Triclinic, P1 | Dx = 1.990 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.6985 (6) Å | Cell parameters from 16326 reflections |
| b = 8.9784 (6) Å | θ = 3.0–29.6° |
| c = 9.0055 (6) Å | µ = 2.47 mm−1 |
| α = 90.358 (6)° | T = 200 K |
| β = 91.949 (6)° | Plate, blue |
| γ = 100.742 (5)° | 0.22 × 0.21 × 0.13 mm |
| V = 690.54 (8) Å3 |
Data collection
| Stoe IPDS 2T diffractometer | 3017 independent reflections |
| Radiation source: fine-focus sealed tube | 2788 reflections with I > 2σ(I) |
| graphite | Rint = 0.037 |
| Detector resolution: 6.67 pixels mm-1 | θmax = 27.0°, θmin = 3.0° |
| rotation method scans | h = −11→11 |
| Absorption correction: numerical (X-RED; Stoe & Cie, 2009) | k = −11→11 |
| Tmin = 0.612, Tmax = 0.737 | l = −11→10 |
| 9700 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.024 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.063 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0403P)2 + 0.2037P] where P = (Fo2 + 2Fc2)/3 |
| 3017 reflections | (Δ/σ)max = 0.002 |
| 196 parameters | Δρmax = 0.48 e Å−3 |
| 6 restraints | Δρmin = −1.05 e Å−3 |
| 0 constraints |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 1.0572 (3) | 0.1738 (3) | 0.7826 (3) | 0.0250 (4) | |
| H1B | 0.9796 | 0.0794 | 0.7636 | 0.030* | |
| H1A | 1.1463 | 0.1485 | 0.8422 | 0.030* | |
| C2 | 1.1135 (3) | 0.2435 (3) | 0.6371 (3) | 0.0262 (5) | |
| H2B | 1.2008 | 0.3300 | 0.6565 | 0.031* | |
| H2A | 1.1523 | 0.1675 | 0.5757 | 0.031* | |
| C3 | 0.8796 (3) | 0.1769 (2) | 0.4653 (2) | 0.0226 (4) | |
| H3B | 0.8488 | 0.0845 | 0.5248 | 0.027* | |
| H3A | 0.9349 | 0.1504 | 0.3775 | 0.027* | |
| C4 | 0.7365 (3) | 0.2401 (3) | 0.4169 (3) | 0.0264 (5) | |
| H4B | 0.7672 | 0.3273 | 0.3506 | 0.032* | |
| H4A | 0.6610 | 0.1614 | 0.3615 | 0.032* | |
| C5 | 0.5518 (3) | 0.1652 (2) | 0.6197 (3) | 0.0227 (4) | |
| H5B | 0.5989 | 0.0732 | 0.6275 | 0.027* | |
| H5A | 0.4539 | 0.1403 | 0.5579 | 0.027* | |
| C6 | 0.5167 (3) | 0.2183 (3) | 0.7734 (3) | 0.0246 (5) | |
| H6B | 0.4571 | 0.3019 | 0.7645 | 0.030* | |
| H6A | 0.4521 | 0.1338 | 0.8261 | 0.030* | |
| C7 | 0.7290 (3) | 0.1527 (3) | 0.9414 (3) | 0.0272 (5) | |
| H7B | 0.7284 | 0.0635 | 0.8761 | 0.033* | |
| H7A | 0.6640 | 0.1198 | 1.0277 | 0.033* | |
| C8 | 0.8955 (3) | 0.2202 (3) | 0.9938 (3) | 0.0289 (5) | |
| H8B | 0.8945 | 0.3004 | 1.0695 | 0.035* | |
| H8A | 0.9460 | 0.1405 | 1.0393 | 0.035* | |
| N1 | 0.9846 (2) | 0.2852 (2) | 0.8642 (2) | 0.0224 (4) | |
| H1 | 1.060 (3) | 0.356 (3) | 0.895 (3) | 0.027 (7)* | |
| N2 | 0.9827 (2) | 0.2966 (2) | 0.5561 (2) | 0.0214 (4) | |
| H2 | 1.023 (3) | 0.370 (3) | 0.494 (3) | 0.028 (7)* | |
| N3 | 0.6625 (2) | 0.2895 (2) | 0.5510 (2) | 0.0216 (4) | |
| H3 | 0.616 (3) | 0.365 (3) | 0.526 (4) | 0.036 (8)* | |
| N4 | 0.6657 (2) | 0.2715 (2) | 0.8587 (2) | 0.0218 (4) | |
| H4 | 0.650 (4) | 0.339 (3) | 0.924 (3) | 0.032 (8)* | |
| O1 | 0.86208 (19) | 0.58802 (17) | 0.7174 (2) | 0.0254 (3) | |
| O2 | 0.6912 (2) | 0.7196 (2) | 0.9423 (2) | 0.0353 (4) | |
| O3 | 0.7520 (2) | 0.87043 (18) | 0.6678 (2) | 0.0310 (4) | |
| O4 | 0.5312 (2) | 0.58763 (19) | 0.6764 (2) | 0.0290 (4) | |
| Cu | 0.83378 (3) | 0.35012 (3) | 0.71091 (3) | 0.01771 (8) | |
| Mo | 0.70937 (2) | 0.692556 (18) | 0.750742 (19) | 0.01796 (7) | |
| O5 | 0.6967 (2) | 0.4797 (2) | 0.1142 (2) | 0.0292 (4) | |
| H5 | 0.621 (3) | 0.452 (3) | 0.168 (3) | 0.033 (8)* | |
| H6 | 0.678 (5) | 0.556 (3) | 0.069 (4) | 0.058 (12)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0261 (11) | 0.0227 (10) | 0.0282 (11) | 0.0109 (9) | −0.0037 (9) | −0.0040 (9) |
| C2 | 0.0220 (11) | 0.0245 (11) | 0.0330 (12) | 0.0070 (9) | 0.0008 (9) | −0.0045 (9) |
| C3 | 0.0269 (11) | 0.0193 (10) | 0.0219 (10) | 0.0044 (8) | 0.0027 (8) | −0.0037 (8) |
| C4 | 0.0320 (12) | 0.0280 (11) | 0.0195 (10) | 0.0068 (9) | −0.0017 (9) | −0.0009 (8) |
| C5 | 0.0204 (10) | 0.0188 (10) | 0.0281 (11) | 0.0021 (8) | −0.0020 (9) | −0.0031 (8) |
| C6 | 0.0189 (10) | 0.0226 (10) | 0.0319 (12) | 0.0029 (8) | 0.0021 (9) | −0.0027 (9) |
| C7 | 0.0331 (13) | 0.0239 (11) | 0.0247 (11) | 0.0047 (9) | 0.0038 (9) | 0.0039 (9) |
| C8 | 0.0357 (13) | 0.0306 (12) | 0.0215 (11) | 0.0099 (10) | −0.0027 (9) | −0.0001 (9) |
| N1 | 0.0219 (9) | 0.0202 (9) | 0.0251 (9) | 0.0053 (7) | −0.0039 (7) | −0.0042 (7) |
| N2 | 0.0231 (9) | 0.0162 (8) | 0.0247 (9) | 0.0028 (7) | 0.0027 (7) | −0.0005 (7) |
| N3 | 0.0233 (9) | 0.0183 (8) | 0.0245 (9) | 0.0078 (7) | −0.0026 (7) | 0.0005 (7) |
| N4 | 0.0255 (9) | 0.0164 (8) | 0.0238 (9) | 0.0048 (7) | 0.0005 (7) | −0.0032 (7) |
| O1 | 0.0254 (8) | 0.0135 (7) | 0.0378 (9) | 0.0048 (6) | 0.0036 (7) | 0.0007 (6) |
| O2 | 0.0475 (11) | 0.0360 (10) | 0.0237 (9) | 0.0112 (8) | 0.0024 (8) | −0.0024 (7) |
| O3 | 0.0388 (10) | 0.0194 (8) | 0.0370 (10) | 0.0095 (7) | 0.0086 (8) | 0.0038 (7) |
| O4 | 0.0268 (9) | 0.0279 (8) | 0.0325 (9) | 0.0062 (7) | −0.0039 (7) | −0.0001 (7) |
| Cu | 0.01851 (14) | 0.01362 (13) | 0.02126 (14) | 0.00388 (10) | −0.00018 (10) | −0.00115 (10) |
| Mo | 0.02191 (11) | 0.01353 (10) | 0.01901 (11) | 0.00478 (7) | 0.00084 (7) | 0.00004 (7) |
| O5 | 0.0301 (9) | 0.0259 (8) | 0.0324 (9) | 0.0067 (7) | 0.0048 (7) | −0.0017 (7) |
Geometric parameters (Å, °)
| C1—N1 | 1.482 (3) | C7—C8 | 1.520 (4) |
| C1—C2 | 1.513 (3) | C7—H7B | 0.9900 |
| C1—H1B | 0.9900 | C7—H7A | 0.9900 |
| C1—H1A | 0.9900 | C8—N1 | 1.484 (3) |
| C2—N2 | 1.484 (3) | C8—H8B | 0.9900 |
| C2—H2B | 0.9900 | C8—H8A | 0.9900 |
| C2—H2A | 0.9900 | N1—Cu | 2.034 (2) |
| C3—N2 | 1.486 (3) | N1—H1 | 0.864 (17) |
| C3—C4 | 1.513 (3) | N2—Cu | 2.0493 (19) |
| C3—H3B | 0.9900 | N2—H2 | 0.890 (17) |
| C3—H3A | 0.9900 | N3—Cu | 2.033 (2) |
| C4—N3 | 1.491 (3) | N3—H3 | 0.881 (18) |
| C4—H4B | 0.9900 | N4—Cu | 2.0413 (19) |
| C4—H4A | 0.9900 | N4—H4 | 0.875 (17) |
| C5—N3 | 1.485 (3) | O1—Mo | 1.7955 (16) |
| C5—C6 | 1.520 (3) | O1—Cu | 2.1043 (15) |
| C5—H5B | 0.9900 | O2—Mo | 1.7571 (18) |
| C5—H5A | 0.9900 | O3—Mo | 1.7490 (16) |
| C6—N4 | 1.482 (3) | O4—Mo | 1.7652 (17) |
| C6—H6B | 0.9900 | O5—H5 | 0.829 (18) |
| C6—H6A | 0.9900 | O5—H6 | 0.838 (19) |
| C7—N4 | 1.482 (3) | ||
| N1—C1—C2 | 108.15 (18) | N1—C8—H8A | 109.9 |
| N1—C1—H1B | 110.1 | C7—C8—H8A | 109.9 |
| C2—C1—H1B | 110.1 | H8B—C8—H8A | 108.3 |
| N1—C1—H1A | 110.1 | C1—N1—C8 | 113.52 (18) |
| C2—C1—H1A | 110.1 | C1—N1—Cu | 103.80 (14) |
| H1B—C1—H1A | 108.4 | C8—N1—Cu | 109.08 (15) |
| N2—C2—C1 | 109.64 (18) | C1—N1—H1 | 107 (2) |
| N2—C2—H2B | 109.7 | C8—N1—H1 | 109 (2) |
| C1—C2—H2B | 109.7 | Cu—N1—H1 | 115 (2) |
| N2—C2—H2A | 109.7 | C2—N2—C3 | 114.06 (17) |
| C1—C2—H2A | 109.7 | C2—N2—Cu | 107.64 (14) |
| H2B—C2—H2A | 108.2 | C3—N2—Cu | 102.43 (13) |
| N2—C3—C4 | 107.08 (18) | C2—N2—H2 | 108.6 (19) |
| N2—C3—H3B | 110.3 | C3—N2—H2 | 107.2 (19) |
| C4—C3—H3B | 110.3 | Cu—N2—H2 | 117 (2) |
| N2—C3—H3A | 110.3 | C5—N3—C4 | 113.35 (17) |
| C4—C3—H3A | 110.3 | C5—N3—Cu | 103.78 (14) |
| H3B—C3—H3A | 108.6 | C4—N3—Cu | 107.78 (14) |
| N3—C4—C3 | 109.07 (18) | C5—N3—H3 | 111 (2) |
| N3—C4—H4B | 109.9 | C4—N3—H3 | 109 (2) |
| C3—C4—H4B | 109.9 | Cu—N3—H3 | 112 (2) |
| N3—C4—H4A | 109.9 | C6—N4—C7 | 115.36 (18) |
| C3—C4—H4A | 109.9 | C6—N4—Cu | 107.91 (14) |
| H4B—C4—H4A | 108.3 | C7—N4—Cu | 104.25 (14) |
| N3—C5—C6 | 108.09 (17) | C6—N4—H4 | 108 (2) |
| N3—C5—H5B | 110.1 | C7—N4—H4 | 107 (2) |
| C6—C5—H5B | 110.1 | Cu—N4—H4 | 114.3 (19) |
| N3—C5—H5A | 110.1 | Mo—O1—Cu | 125.18 (8) |
| C6—C5—H5A | 110.1 | N3—Cu—N1 | 148.36 (7) |
| H5B—C5—H5A | 108.4 | N3—Cu—N4 | 85.93 (8) |
| N4—C6—C5 | 109.38 (18) | N1—Cu—N4 | 84.96 (8) |
| N4—C6—H6B | 109.8 | N3—Cu—N2 | 85.57 (8) |
| C5—C6—H6B | 109.8 | N1—Cu—N2 | 85.70 (8) |
| N4—C6—H6A | 109.8 | N4—Cu—N2 | 146.84 (7) |
| C5—C6—H6A | 109.8 | N3—Cu—O1 | 102.97 (7) |
| H6B—C6—H6A | 108.2 | N1—Cu—O1 | 108.66 (7) |
| N4—C7—C8 | 107.65 (18) | N4—Cu—O1 | 106.23 (7) |
| N4—C7—H7B | 110.2 | N2—Cu—O1 | 106.91 (7) |
| C8—C7—H7B | 110.2 | O3—Mo—O2 | 108.43 (9) |
| N4—C7—H7A | 110.2 | O3—Mo—O4 | 110.51 (9) |
| C8—C7—H7A | 110.2 | O2—Mo—O4 | 108.75 (9) |
| H7B—C7—H7A | 108.5 | O3—Mo—O1 | 110.04 (8) |
| N1—C8—C7 | 108.83 (19) | O2—Mo—O1 | 110.65 (9) |
| N1—C8—H8B | 109.9 | O4—Mo—O1 | 108.45 (8) |
| C7—C8—H8B | 109.9 | H5—O5—H6 | 106 (4) |
| N1—C1—C2—N2 | −53.5 (2) | C1—N1—Cu—N4 | 121.02 (14) |
| N2—C3—C4—N3 | −55.8 (2) | C8—N1—Cu—N4 | −0.30 (15) |
| N3—C5—C6—N4 | −53.4 (2) | C1—N1—Cu—N2 | −27.13 (14) |
| N4—C7—C8—N1 | −53.0 (2) | C8—N1—Cu—N2 | −148.44 (15) |
| C2—C1—N1—C8 | 168.05 (19) | C1—N1—Cu—O1 | −133.53 (13) |
| C2—C1—N1—Cu | 49.76 (19) | C8—N1—Cu—O1 | 105.15 (15) |
| C7—C8—N1—C1 | −87.1 (2) | C6—N4—Cu—N3 | −1.03 (14) |
| C7—C8—N1—Cu | 28.1 (2) | C7—N4—Cu—N3 | 122.10 (15) |
| C1—C2—N2—C3 | −84.7 (2) | C6—N4—Cu—N1 | −150.70 (15) |
| C1—C2—N2—Cu | 28.2 (2) | C7—N4—Cu—N1 | −27.57 (14) |
| C4—C3—N2—C2 | 168.62 (18) | C6—N4—Cu—N2 | −76.5 (2) |
| C4—C3—N2—Cu | 52.62 (18) | C7—N4—Cu—N2 | 46.6 (2) |
| C6—C5—N3—C4 | 165.92 (19) | C6—N4—Cu—O1 | 101.31 (14) |
| C6—C5—N3—Cu | 49.29 (19) | C7—N4—Cu—O1 | −135.56 (14) |
| C3—C4—N3—C5 | −85.7 (2) | C2—N2—Cu—N3 | −150.00 (14) |
| C3—C4—N3—Cu | 28.5 (2) | C3—N2—Cu—N3 | −29.46 (13) |
| C5—C6—N4—C7 | −87.4 (2) | C2—N2—Cu—N1 | −0.44 (13) |
| C5—C6—N4—Cu | 28.7 (2) | C3—N2—Cu—N1 | 120.10 (14) |
| C8—C7—N4—C6 | 168.22 (19) | C2—N2—Cu—N4 | −74.40 (19) |
| C8—C7—N4—Cu | 50.08 (19) | C3—N2—Cu—N4 | 46.1 (2) |
| C5—N3—Cu—N1 | 46.9 (2) | C2—N2—Cu—O1 | 107.77 (13) |
| C4—N3—Cu—N1 | −73.6 (2) | C3—N2—Cu—O1 | −131.69 (13) |
| C5—N3—Cu—N4 | −26.66 (13) | Mo—O1—Cu—N3 | 56.76 (13) |
| C4—N3—Cu—N4 | −147.15 (14) | Mo—O1—Cu—N1 | −122.84 (11) |
| C5—N3—Cu—N2 | 121.25 (14) | Mo—O1—Cu—N4 | −32.74 (13) |
| C4—N3—Cu—N2 | 0.77 (14) | Mo—O1—Cu—N2 | 146.02 (11) |
| C5—N3—Cu—O1 | −132.40 (13) | Cu—O1—Mo—O3 | −153.37 (11) |
| C4—N3—Cu—O1 | 107.12 (14) | Cu—O1—Mo—O2 | 86.82 (12) |
| C1—N1—Cu—N3 | 47.2 (2) | Cu—O1—Mo—O4 | −32.38 (13) |
| C8—N1—Cu—N3 | −74.1 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O5—H5···O4i | 0.83 (2) | 1.95 (2) | 2.770 (2) | 169 (3) |
| N1—H1···O5ii | 0.86 (2) | 2.35 (2) | 3.156 (3) | 156 (3) |
| N2—H2···O1ii | 0.89 (2) | 2.19 (2) | 2.949 (2) | 143 (3) |
| N3—H3···O4i | 0.88 (2) | 2.28 (3) | 2.955 (3) | 133 (3) |
| N4—H4···O5iii | 0.88 (2) | 2.10 (2) | 2.928 (3) | 157 (3) |
| O5—H6···O2iv | 0.84 (2) | 1.85 (2) | 2.666 (3) | 163 (4) |
| C3—H3B···O3v | 0.99 | 2.36 | 3.345 (3) | 175 |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) −x+2, −y+1, −z+1; (iii) x, y, z+1; (iv) x, y, z−1; (v) x, y−1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BX2287).
References
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
- Brandenburg, K. (2009). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Choi, K.-Y., Whang, M.-A., Lee, H.-H., Lee, K.-C. & Kim, M.-J. (2004). Transition Met. Chem.29, 792–796.
- Clay, R., Murray-Rust, P. & Murray-Rust, J. (1979). Acta Cryst. B35, 1894–1895.
- Guo, J.-F., Yeung, W.-F., Gao, S., Lee, G.-H., Peng, S.-M., Lam, M. H.-W. & Lau, T.-C. (2008). Eur. J. Inorg. Chem. pp. 158–163.
- Hagrman, D., Warren, C. J., Haushalter, R. C., Seip, C., O’Connor, C. J., Rarig, R. S., Johnson, K. M., LaDuca, R. L. & Zubieta, J. (1998). Chem. Mater.10, 3294–3297.
- Lu, T.-H., Lin, J.-L., Lan, W.-J. & Chung, C.-S. (1997). Acta Cryst. C53, 1598–1600.
- Rarig, R. S., Lam, R., Zavalij, P. Y., Ngala, J. K., LaDuca, R. L., Greedan, J. E. & Zubieta, J. (2002). Inorg. Chem.41, 2124–2133. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Stoe & Cie (2009). X-AREA and X-RED Stoe & Cie, Darmstadt, Germany.
- Yeung, W.-F., Wong, W.-T., Zuo, J.-L. & Lau, T.-C. (2000). J. Chem. Soc. Dalton Trans. pp. 629–631.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810026000/bx2287sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810026000/bx2287Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report