Abstract
The effects of ethylene on cell division are generally considered inhibitory. In this study, we demonstrate that transient ethylene exposure, while suppressing cytokinesis, stimulates DNA synthesis. We monitored DNA synthesis and cytokinesis in the epidermis of cucumber (Cucumis sativus) hypocotyls, an organ whose post-germination development involves strictly limited cell division. During exposure to ethylene, DNA synthesis, assessed by the incorporation of the thymidine homolog 5-bromo-2′-deoxyuridine, was detected in 20% of the epidermal cells, whereas DNA synthesis was nearly undetectable in normal air. Cytofluorometric analysis of nuclei in affected cells showed an up to 8-fold increase in DNA content. During this time, new cell plate formation was not detected. However, shortly after ethylene was removed, DNA content was rapidly restored to 2C (diploid) levels in all cells, and new cell plate formation dramatically increased. These results demonstrate that ethylene promotes DNA synthesis and its endoreduplication but inhibits cytokinesis, thereby maintaining some cells in G2 phase.
Ethylene regulates a wide variety of developmental processes in plants, from seedling growth to leaf and fruit senescence. In etiolated dicot seedlings, ethylene enhances tightening of the apical hook, suppresses elongation, and stimulates radial expansion of stem and root cells (Abeles et al., 1992). The notion that ethylene inhibits DNA synthesis and cell division is not entirely clear. Inhibition of DNA synthesis by ethylene treatment was first demonstrated in meristematic (Apelbaum and Burg, 1971), apical hook and subhook regions of etiolated pea (Pisum sativum) seedlings (Kang and Burg, 1973). Ethylene was also shown to inhibit DNA synthesis associated with wound-induced cell division of potato (Solanum tuberosum) tuber slices (Sato et al., 1976). In contrast, Lorbiecke and Sauter (1999) demonstrated that ethylene treatment can stimulate mitotic cyclin expression in rice (Oryza sativa) stem cuttings and that this is associated with the growth of adventitious roots.
Abeles et al. (1992) demonstrated that the effects of ethylene on etiolated seedlings of dicots are reversible. When ethylene-treated plants are transferred to ethylene-free air, apical hook cells elongate, and the apical meristem starts producing new cells, which differentiate and elongate, resulting in a stem that is wider at its base. This observation suggests that cell division in the apical meristem is suppressed as long as ethylene is present in ambient air, but that the suppressed cells retain the potential for later cell division, differentiation, and elongation.
We have now reported that transient ethylene exposure dramatically affects division polarity and cell fate in the epidermis of cucumber (Cucumis sativus) seedlings but that these effects are only manifested after the removal of ethylene (H. Kazama, H. Dan, H. Imaseki, and G.O. Wasteneys, unpublished data). During ethylene treatment, the number and positional arrangement of stomata and trichomes remain unchanged. However, removal of ethylene dramatically increases stoma and trichome numbers, generates abnormally oriented stomata, increases the number of cells in trichomes, and causes trichome branching. These results suggest that although cell division is apparently suppressed during ethylene exposure, cell division processes involved in the formation of stomata and trichomes are stimulated. Here, we demonstrate that ethylene exposure causes DNA endoreduplication and that subsequent removal of ethylene triggers rapid cytokinesis.
RESULTS
In this study, we documented DNA synthesis and cell division in the epidermis of cucumber hypocotyls during and after exposure to ethylene. Our previous study demonstrated that the stimulation of cell proliferation by transient exposure to ethylene was specific to stomatal and trichome progenitor cells (H. Kazama, H. Dan, H. Imeseki, and G.O. Wasteneys, unpublished data). We therefore measured relative DNA content and probed for DNA synthesis and cell plate formation using techniques that kept the hypocotyl epidermis intact. For this study, we exposed seedlings to ethylene for 24 to 48 h and then followed development over similar periods of time after the removal of ethylene. The 24- and 48-h treatment and recovery periods generated similar results, but 24-h treatments were preferred because these prevented excessive growth of the etiolated control seedlings, which tended to outgrow the containers.
Ethylene Stimulates DNA Synthesis in Hypocotyl Epidermal Cells
We assessed DNA synthesis by detecting the incorporation of the thymidine analog 5-bromo-2′-deoxyuridine (BrdU) into nuclei. Immunofluorescence microscopy demonstrated that during treatment with ethylene, DNA synthesis took place in a significant number of nuclei, whereas control cells exposed to normal air rarely incorporated BrdU (Fig. 1). Trichome and stomata development is not equally distributed around the surface of the cucumber hypocotyl, which is square in profile, with some areas producing trichomes or stomata in greater abundance than others. Hair-form trichomes, for example, are most abundant at the hypocotyl corners. Careful examination of epidermal strips revealed that DNA synthesis occurred mainly in strips obtained from regions of the hypocotyl that generate stomata and/or trichomes in greatest abundance. The number of BrdU-labeled nuclei increased rapidly over the 24-h period of ethylene exposure (Fig. 2). Initiation of DNA synthesis during ethylene treatment was rapid; after 1 h, 9% of the epidermal cells showed DNA synthesis activity, and the frequency steadily increased until 10 h, when as many as 20% of nuclei had incorporated BrdU. However, even at the end of the 24-h exposure, there was no sign of nuclear division. Figure 2 also shows that in control plants, BrdU incorporation was almost undetectable over the same 24-h period. These results suggest that ethylene stimulated DNA synthesis in those epidermal cells that had retained the potential to divide, but that cytokinesis did not proceed as long as ethylene was present.
Figure 1.
DNA synthesis, as assessed by the incorporation of BrdU, is stimulated by ethylene exposure. BrdU was applied at 200 μm for 24 h. A and B, In an untreated hypocotyl, Hoechst 33258-labeled nuclei (A) show no evidence of DNA synthesis, with no BrdU incorporation detected (anti-BrDU immunofluorescence; B). C and D, After 24 h of exposure to ethylene, many nuclei (Hoechst 33258-labeled; C) have incorporated BrdU (anti-BrdU immunofluorescence; D). Bar = 50 μm.
Figure 2.
The proportion of hypocotyl epidermal cells in S phase increases over 24 h during ethylene exposure. Seedlings were pretreated with BrdU (200 μm) for 1 h before ethylene treatment at a concentration of 100 μLL-1. Each measurement (percentage) consisted of five samples of more than 100 epidermal cells. Bars indicate se.
Ethylene Affects Relative DNA Content
The lack of nuclear division during ethylene exposure suggested that endoreduplication had taken place. To confirm this, we measured relative nuclear DNA content of epidermal cells by the fluorescence intensity of 4′,6-diamino-phenylindole (DAPI)-stained nuclei. Figure 3 shows frequency distribution histograms of relative nuclear DNA contents generated after background fluorescence intensity was normalized. Untreated control cells had 2C to 2.5C levels of relative DNA content (Fig. 3, A and C). During ethylene exposure, cells with 4C to 8C DNA content were observed after 24 h (Fig. 3B), and cells with up to 16C were observed after 48 h (Fig. 3D). After 24 h, 26% of the cells had a relative DNA content over 4C (Fig. 3B), and after 48 h, this increased to 35% (Fig. 3D). These results indicate that DNA synthesis observed during ethylene exposure was a result of endoreduplication and that in some cells, as many as three rounds of DNA duplication had taken place.
Figure 3.
Relative nuclear DNA content increases in the presence of ethylene. DNA contents were assessed by the relative fluorescent intensity of DAPI-labeled nuclei in hypocotyl epidermal cells from 4-d-old (A and B) and 5-d-old (C and D) seedlings. Each data point was from three samples, each measuring at least 100 nuclei. A, Seedlings grown in normal air for 4 d (control). B, Seedlings exposed to 100 μL L-1 ethylene for 24 h on d 3. C, Seedlings grown in normal air for 5 d (control). D, Seedlings exposed to 100 μL L-1 ethylene for 48 h on d 3.
Ethylene Removal Triggers Cytokinesis
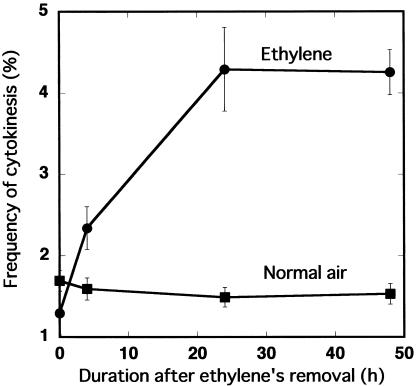
Callose accumulates during early cell plate formation, but levels decrease by late stages of cytokinesis (Samuels et al., 1995; Staehelin and Hepler, 1996). Aniline blue can be used to detect this transient accumulation of callose (Samuels et al., 1995). We used aniline blue fluorescence to identify newly formed cell plates in epidermal strips taken from the hypocotyls of seedlings that had been exposed to ethylene for 24 h and then removed from exogenous ethylene for up to 48 h (Fig. 4). Whereas no cytokinesis was detected during ethylene treatment, many cells with newly formed cell plates were detected soon after removal of ethylene. Figure 4 also shows that cells with intensely stained cell plates were surrounded by clusters of smaller cells, which had also apparently formed after the removal of ethylene. The frequency of cells undergoing cytokinesis increased over time after ethylene removal (Fig. 5). Because aniline blue also stained other cell wall material but only weakly, the number of cells undergoing cytokinesis was determined by counting only those cell plates with an above background level of fluorescent intensity. As shown in Figure 5, shortly after the end of ethylene treatment (0 h), there was no significant difference in the frequency of cytokinesis between control and treated seedlings. The frequency of cytokinesis in ethylene-treated seedlings, however, increased with time after ethylene removal until 24 h and remained constant thereafter at a little over 4% until 48 h. By contrast, no increase in cytokinesis frequency was observed over the same period of time in the control seedlings.
Figure 4.
Cytokinesis is stimulated upon removal of ethylene. Aniline blue detection of callose in newly formed epidermal cell plates was visualized by epifluorescence microscopy. A, New cell plate in a guard mother cell from a 5-d-old seedling grown in normal air. B and C, Multiple cell plates are evident in 5-d-old seedlings exposed to 100 μL L-1 ethylene for 24 h on d 3. These extra cell divisions stimulated by ethylene exposure produce subsidiary cells (B) and larger stomatal complexes (C). Bar = 50 μm.
Figure 5.
Ethylene removal increases cell division frequency. After exposure to 100 μL L-1 ethylene for 24 h, the percentage of newly divided cells, as indicated by high levels of aniline blue fluorescence (Fig. 4), was calculated. At least 10 epidermal strips, each with more than 100 cells, were used for each time point. Bars indicate se.
Cells That Completed Cytokinesis Contain 2C DNA Content
To examine the fate of endoreduplicated nuclei formed during ethylene exposure, we assayed the distribution of DNA contents of epidermal cells 48 h after removal of ethylene. Cells near the base of the hypocotyl that were expanding rapidly when ethylene was applied underwent radial swelling and relatively little elongation during ethylene treatment. They generated the swollen base of the hypocotyl apparent after the removal of ethylene. In contrast, the cells still in the apical hook and sub-cotyledon regions during ethylene exposure elongated after the removal of ethylene so the upper part of the hypocotyl developed a slender morphology. Those cells in the apical and apical hook regions formed the new apical hook and the elongation zones of the hypocotyl, respectively, during the first 24 to 48 h in normal air. DNA content was measured in epidermal strips taken from both the upper, slender region and the lower, swollen region of hypocotyls. Results shown in Figure 6 indicate that although 35% of cells had 4C to 16C DNA content during exposure to ethylene (Fig. 3D), 48 h after the removal of ethylene, all of the epidermal cell nuclei throughout the length of the hypocotyls had a 2C level of DNA content.
Figure 6.
After removal of ethylene, nuclear DNA content in epidermal cells decreases. Seedlings were exposed to 100 μL L-1 ethylene for 48 h on d 3 followed by 48 h of incubation in normal air. Measurements were from three replicates for each treatment, each sample including at least 100 nuclei. DNA contents are expressed as a relative value of fluorescent intensity of DAPI staining. A, Apical region of hypocotyl. B, Radial swelling region of hypocotyl.
DISCUSSION
The present study dealt with the effects of ethylene on nuclear DNA synthesis and cytokinesis, using cell biological techniques. The results clearly indicate that DNA synthesis was induced during ethylene exposure in a subset of epidermal cells from etiolated cucumber seedlings and that the frequency of DNA synthesizing cells in the epidermal cell population increased during ethylene treatment. Ethylene-stimulated DNA synthesis apparently results in endoreduplication because nuclear DNA content increased to as high as 16C after 48 h, and nuclear division during ethylene exposure was never detected despite extensive microscopical examination.
Earlier work demonstrated that ethylene suppresses cell division and mitotic DNA synthesis in apical meristems (Apelbaum and Burg, 1972). More recently, however, it has been shown that ethylene promotes the enlargement of light-grown Arabidopsis hypocotyls (Smalle et al., 1997) and stimulates endoreduplication (Gendreau et al., 1999). Gendreau et al. (1999) used flow cytometry to measure nuclear content, however, and so were not able to identify which cells were affected. Endoreduplication is thought to assist cell enlargement by increasing transcription potential (Kondorosi et al., 2000), but one study demonstrates that it can precede amitotic-based cell division (Valente et al., 1998).
Our results imply that ethylene stimulates the transition from G1 to S phase in the cell cycle, but arrests the cell cycle at G2. In some cells, multiple endoreduplication by a short circuit between G2 and S phases apparently occurred during ethylene exposure. The affected cells mostly differentiated into either stomata (including stoma subsidiary cells) or trichomes, and their number dramatically increased after the removal of ethylene (H. Kazama, H. Dan, H. Imaseki, G.O. Wasteneys, unpublished data). It is therefore clear that some of the non-specialized epidermal cells that would not normally form stomata and trichome precursors must also be stimulated to reduplicate DNA, eventually to divide and differentiate into stomata and/or trichomes.
The results reported here differ from those reported for pea seedlings (Burg et al., 1971), in which tritiated thymidine incorporation into the apical meristem was markedly inhibited by ethylene. Hypocotyls lack meristems, so our study is not directly comparable with the effects of ethylene on pea meristems. Our data suggest that all regions of the hypocotyl contain cells in which endoreduplication is stimulated by ethylene. Because it was technically unfeasible to prepare single-layer epidermal strips from the apical hook region, we could not confirm that nuclear DNA synthesis occurred during ethylene exposure in this region. It remains possible that epidermal cells in the apical hook do not synthesize DNA during ethylene exposure but instead are stimulated to enter S phase followed by M phase as soon as the cells elongate upon the removal of ethylene. Nevertheless, the rapid proliferation of these cells immediately after removing ethylene suggests that these cells underwent endoreduplication in the same manner as the more basally situated cells.
The continued cell division documented after removing ethylene indicates that the ethylene-affected cells acquire a capacity for further DNA synthesis well beyond the period of ethylene treatment. Cells that had apparently completed cytokinesis were often observed in cell clusters (Fig. 4B). Because multiply endoreduplicated cells are quickly reduced to 2C DNA levels after ethylene removal, cytokinesis of those cells probably occurred in rapid succession. However, the frequency of multiply endoreduplicated nuclei appears to be less than the frequency of cell clusters. Thus it appears that cells formed by cytokinesis of endoreduplicated (or G2 arrested) cells retain a capacity for further rounds of the cell cycle after the removal of ethylene, indicating that ethylene also has a residual effect on the regulatory mechanism of transition from G1 to S phase. In another article, we show that transient ethylene exposure also induced formation of multicellular protuberances terminating in a single stoma (H. Kazama, H. Dan, H. Imaseki, G.O. Wasteneys, unpublished data). The main body of these protuberances may contain thousands of smaller cells that apparently originate from stomatal subsidiary cells.
The results presented here demonstrate that ethylene can have a regulatory role on the cell cycle progression in progenitor cells for stomatal complexes and trichomes. In these progenitor cells, ethylene induces the transition from G1 to S phase, and inhibits the progression of G2 phase to M phase. Knowledge of factors involved in the regulation of the cell cycle in plants is accumulating, and a model of the plant cell cycle has recently been presented (Meijer and Murray, 2001). Understanding the molecular mechanisms of the dual effect of ethylene on the epidermal cell division cycle will be the goal of future studies.
MATERIALS AND METHODS
Plant Materials and Ethylene Treatment
Seeds of cucumber (Cucumis sativus L. cv Aonagajibai, purchased from Takii Seed [Kyoto]) were placed on 0.35% (w/v) agar medium (Bacto-agar, Difco, Detroit) and grown, four seedlings per 825-mL glass container, as described (H. Kazama, H. Dan, H. Imaseki, and G.O. Wasteneys, unpublished data). Seedlings were grown in the dark at 25°C ± 1°C in a growth cabinet (Koitotron KG206, Koito Industries, Tokyo; Sherer CEL15 cabinet, Sherer-Pennant, Seven Hill, NSW, Australia).
To stimulate stoma and trichome differentiation in hypocotyls, 2-d-old seedlings were irradiated with red light filtered through a Rohm and Haas no. 2444 filter (4.5 × 10-5 J cm-2 s-1 at the plant level) for 30 min (Kazama and Mineyuki, 1997) and grown for an additional 24 h. Ethylene was injected into the culture container to a concentration of 100 μL L-1. After 24 h (or 48 h) of ethylene treatment, the seedlings were transferred to ethylene-free air and grown for another 24 to 48 h in normal air. Except for the red-light irradiation, all handling, including the BrDU application to seedlings, was performed under total darkness using Noctovision (Hamatsu Photonics Co., Hamamatsu, Japan). The same procedures were applied to control plants without ethylene treatment. Epidermal peels were collected from hypocotyls immediately after and 24 or 48 h after the ethylene treatment.
Detection of DNA Synthesis
Nuclei in the process of DNA synthesis were visualized by their incorporation of BrdU and its fluorescent immunochemical staining (Gratzner, 1982). Seedlings were carefully removed from the agar medium 24 h after red light irradiation, and their roots were immersed in 1 mL of 200 μm BrdU and allowed to absorb the chemical for 1 h. The seedlings, while immersed in BrdU, were returned to the airtight containers and incubated for 24 h in ethylene-containing air. Ten-millimeter-long hypocotyl sections starting 5 mm below the cotyledonary node were excised immediately after the ethylene/BrdU treatment. These sections were therefore taken from the region of hypocotyl that had undergone maximum swelling. Two thin epidermal strips with a few cortex cell layers attached were sliced longitudinally with a razor blade from each hypocotyl section and were immediately fixed in 4% (w/v) paraformaldehyde in 50 mm PIPES, 1 mm MgSO4, and 5 mm EGTA, pH 6.4 (PME buffer) for 6 h at 25°C under gentle shaking (Bio-Shaker Br-30L, Taitec, Saitama, Japan). The fixed epidermal strips were washed at least three times with PME buffer and affixed to coverslips coated with 0.2% (w/v) polyethyleneimine (Sigma-Aldrich, St. Louis) with the epidermal side facing down. Single-layered epidermal strip preparations were made according to the procedures described by Kazama and Mineyuki (1997) with some modifications. The epidermal strips were treated with a cell wall-digesting solution that contained 1% (w/v) Pectolyase Y-23 (Seishin Pharmaceutical Co., Tokyo) and 2% (w/v) Driselase (Sigma-Aldrich) for 10 to 20 min at room temperature. Overlying cortex cells were mechanically removed by gentle stroking with an eyelash attached at the end of a thin rod. Debris from released cortex cells was washed away with phosphate-buffered saline (PBS; 0.075 mm KH2PO4, 0.3 mm NaHPO4, 7.0 mm NaCl, 0.135 mm KCl, and 0.02% [w/v] NaN3), and the epidermal layers attached to coverslips were immersed in 100% (w/v) methanol at -20°C for 10 min to stop the enzyme activity. The epidermal layers were rinsed with PBS and treated with 1.5 m HCl for 30 min to render anti-BrdU antibody accessible to cellular nuclei (Levi et al., 1987; Gunning and Sammut, 1990). They were washed with PBS three times each for 10 min, then treated with 1% (w/v) Nonidet P-40 (Sigma-Aldrich) for 15 min, and washed with PBS containing 0.1 m Gly for 15 min. Each specimen was overlayered with 10 μL of 10-fold diluted anti-BrdU mouse monoclonal antibody (Roche Diagnostics, Mannheim, Germany) and subsequently incubated at 4°C ± 1°C overnight in a dark moist chamber. After rinsing three times with PBS containing 0.1 m Gly for 20 min each, the specimens were incubated with the secondary antibody for 90 min at 37°C. The secondary antibody used was fluorescein isothiocyanate-conjugated sheep anti-mouse IgG antibody (Sigma-Aldrich) diluted 100-fold with PBS containing 1% (w/v) bovine serum albumin. The specimens were rinsed three times with PBS for 10 min each, treated with 100 ng mL-1 Hoechst 33258 for 10 min, and finally mounted in 50 mm Tris buffer with 50% (w/v) glycerol and 0.1% (w/v) p-phenylenediamine, pH 9.0, onto glass slides.
Microscopic observation was made with a New Vanox microscope (AHB S-F, Olympus, Tokyo) with Epifluorescence Optics with a standard filter set and a D Plan Apo UV 20× objective lens. Excitation of fluorescein isothiocyanate and Hoechst 33258 was made with blue and UV filters, respectively, and photographic images were taken. Hoechst 33258-stained and BrdU-stained nuclei were separately counted on the photographic images that covered more than 100 nuclei. Measurement was made with at least five epidermal strips taken from different hypocotyls for each treatment. Frequency of DNA synthesis was calculated by the following equation: Frequency of DNA synthesis (%) = [(no. of BrdU-stained nuclei)/(Hoechst-stained total nuclei)] × 100.
Quantification of Endoreduplicated Nuclei
Epidermal strips were treated with 30 to 50 μL of 100 ng mL-1 DAPI solution (Hamada and Fujita, 1983; Mineyuki et al., 1988) for 30 min at room temperature in a dark moist chamber. After removing DAPI solution, the specimens were mounted in 100 mm Tris buffer at pH 9.0 that contained 1 mm EDTA, 0.25 m Suc, and 1% (w/v) 2-mercaptoethanol. The fluorescence intensity of DAPI-stained nuclei was measured by an Olympus Epifluorescence Quantification System attached to a New Vanox microscope, and a combination of filters, Ex = 365 nm, Dm = 400 nm, and Em = 450 nm, was used for measurement. The photomultiplier voltage, the period of measurement, and the pinhole number were adjusted to 600 to 602 mV, 0.5 s, and number 5, respectively, throughout measurements, and data were recorded by computer. More than 100 nuclei were included in each sample, and at least five samples taken from different hypocotyls were measured.
Detection of Cytokinesis
Cells under cytokinesis were identified by the staining of newly formed cell plates with aniline blue, a specific dye for β-1,3-glucan (callose), which is abundant in cell plates (Evans et al., 1984; Stone et al., 1984). Five-millimeter-long hypocotyl sections were obtained from two regions; the zone 5 mm below the cotyledonary node (the upper region that had elongated after ethylene removal) and the region of maximum swelling further down the hypocotyl. Epidermal strips were peeled from the hypocotyl sections with fine forceps and stained with 0.05% (w/v) aniline blue (Schmidt GmbH, Könegen, Germany) in PBS buffer, pH 8.5, for 5 min in a dark moist chamber and finally mounted in PBS onto glass slides. Cells were observed with a New Vanox microscope (AHB S-F, Olympus) equipped with a D Plan Apo UV 20× objective lens excited by violet light. At least 10 epidermal strips, each having more than 100 cells, were taken from different seedlings. Images were photographed with Fujicolor Superia film ASA 400 (Fuji Film, Tokyo). The frequency of cytokinesis (percentage) was expressed as [(no. of cells with dye-stained cell plates)/(total no. of cells)] × 100.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Acknowledgments
We thank Dr. P.C.L. John (The Australian National University) for valuable discussion.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.025783.
This work was supported by the Ministry of Education of Japan (grant-in-aid 11874120 to H.K.) and by the Australian Research Council Discovery Project (grant no.DP0208872 to G.O.W.).
References
- Abeles FB, Morgan PW, Saltveit ME Jr (1992) Ethylene in Plant Biology, Ed 2. Academic Press, San Diego
- Apelbaum A, Burg SP (1971) Altered cell microfibrillar orientation in ethylene-treated Pisum sativum stems. Plant Physiol 48: 648-652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelbaum A, Burg SP (1972) Effect of ethylene on cell division and deoxyribonucleic acid synthesis in Pisum sativum. Plant Physiol 50: 117-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg SP, Apelbaum A, Eisinger W, Kang BG (1971) Physiology and mode of action of ethylene. Hortscience 6: 359-364 [Google Scholar]
- Evans NA, Hoyne PA, Stone BA (1984) Characteristics and specificity of the interaction of a fluorochrome from aniline blue (sirofluor) with polysaccharides. Carbohydr Polym 4: 215-230 [Google Scholar]
- Gendreau E, Orbovic V, Höfte H, Traas J (1999) Gibberellin and ethylene control endoreduplication levels in the Arabidopsis thaliana hypocotyl. Planta 209: 513-516 [DOI] [PubMed] [Google Scholar]
- Gratzner HG (1982) Monoclonal antibody to 5-bromo- and 5-iodo-deoxyuridine: a new reagent for detection of DNA replication. Science 218: 474-475 [DOI] [PubMed] [Google Scholar]
- Gunning BES, Sammut M (1990) Rearrangements of microtubules involved in establishing cell division planes start immediately after DNA synthesis and are completed just before mitosis. Plant Cell 2: 1273-1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S, Fujita S (1983) DAPI staining improved for quantitative cytofluorometry. Histochemistry 79: 219-226 [DOI] [PubMed] [Google Scholar]
- Kang BG, Burg SP (1973) Influence of ethylene on nucleic acid synthesis in etiolated Pisum sativum. Plant Cell Physiol 14: 981-988 [Google Scholar]
- Kazama H, Mineyuki Y (1997) Alteration of division polarity and preprophase band orientation in stomatogenesis by light. J Plant Res 110: 489-493 [Google Scholar]
- Kondorosi E, Roudier F, Gendreau E (2000) Plant cell-size control: growing by ploidy? Curr Opin Plant Biol 3: 488-492 [DOI] [PubMed] [Google Scholar]
- Levi M, Sparvoli E, Sgorbati S, Chiatante D (1987) Rapid immunofluorescent determination of cells in the S phase in pea root meristems: an alternative to autoradiography. Physiol Plant 71: 68-72 [Google Scholar]
- Lorbiecke R, Sauter M (1999) Adentitious root growth and cell-cycle induction in deepwater rice. Plant Physiol 119: 21-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer M, Murray JA (2001) Cell cycle control and the development of plant form. Curr Opin Plant Biol 4: 44-49 [DOI] [PubMed] [Google Scholar]
- Mineyuki Y, Wick SM, Gunning BES (1988) Preprophase bands of microtubules and the cell cycle: kinetics and experimental uncoupling of their formation from the nuclear cycle in onion root-tip cells. Planta 174: 518-526 [DOI] [PubMed] [Google Scholar]
- Samuels AL, Giddings TH Jr, Staehelin LA (1995) Cytokinesis in tobacco BY-2 and root tip cells: a new model of cell plate formation in higher plants. J Cell Biol 130: 1345-1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Watanabe A, Imaseki H (1976) Effect of ethylene on DNA synthesis in potato tuber discs. Plant Cell Physiol 17: 1255-1262 [Google Scholar]
- Smalle J, Haegman M, Kurepa J, van Montagu M, van der Straeten D (1997) Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA 94: 2756-2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin LA, Hepler PK (1996) Cytokinesis in higher plants. Cell 6: 821-824 [DOI] [PubMed] [Google Scholar]
- Stone BA, Evans NA, Bonig I, Clarke AE (1984) The application of Sirofluorm a chemically defined fluorochrome from aniline blue for the histochemical detection of callose. Protoplasma 122: 191-195 [Google Scholar]
- Valente P, Tao WH, Verbelen JP (1998) Auxins and cytokinins control DNA endoreduplication and deduplication in single cells of tobacco. Plant Sci 134: 207-215 [Google Scholar]