Abstract
A complete ferredoxin (Fd) cDNA clone was isolated from potato (Solanum tuberosum L. cv Desiree) leaves. By molecular and immunoblot analysis, the gene was identified as the leaf-specific Fd isoform I. Transgenic potato plants were constructed by introducing the homologous potato fed 1 cDNA clone as an antisense construct under the control of the constitutive cauliflower mosaic virus 35S promoter. Stable antisense lines with Fd contents between 40% and 80% of the wild-type level were selected by northern- and western-blot analysis. In short-term experiments, the distribution of electrons toward their stromal acceptors was altered in the mutant plants. Cyclic electron transport, as determined by the quantum yields of photosystems I and II, was enhanced. The CO2 assimilation rate was decreased, but depending on the remaining Fd content, some lines showed photoinhibition. The leaf protein content remained largely constant, but the antisense plants had a lower total chlorophyll content per unit leaf area and an increased chlorophyll a/b ratio. In the antisense plants, the redox state of the quinone acceptor A in photosystem II (QA) was more reduced than that of the wild-type plants under all experimental conditions. Because the plants with lower Fd amounts reacted as if they were grown under a higher light intensity, the possibility that the altered chloroplast redox state affects light acclimation is discussed.
Ferredoxins (Fds) are small, iron- and sulfur-containing proteins that act as low-potential one-electron carriers. In chloroplasts, Fd distributes the electrons from PSI onto the various electron-consuming reactions in the chloroplast stroma (Arnon, 1988). During nitrogen (N) and sulfur (S) assimilation, reduced Fd is directly used by nitrite reductase, Gln synthase, sulfite reductase (Knaff and Hirasawa, 1991), and by enzymes of secondary metabolism, such as choline monooxygenase (Brouquisse et al., 1989). Moreover, Fd supplies electrons via Ferredoxin-NADP+-Reductase for NADP reduction (Knaff and Hirasawa, 1991). The generated NADPH serves as a soluble reductant in the chloroplast stroma, mainly for the reduction of 1,3bisphosphoglycerate by NAD(P)-dependent glyceraldehyde 3-phosphate dehydrogenase during CO2 assimilation (Leegood, 1996). Fd is involved in the regulation of redox-modulated chloroplast enzymes via electron flow toward ferrodoxin-thioredoxin-reductase (FTR) and thioredoxins (Knaff and Hirasawa, 1991). Thioredoxins reduce their target enzymes and, thus, in interplay with specific metabolic effectors, adjust the enzyme activation states to the actual demand (Scheibe, 1991). Moreover, the redox state of thioredoxins also acts on chloroplast gene expression by regulating transcription and translation of several chloroplast proteins (Kim and Mayfield, 1997; Link, 2001).
Fd is furthermore involved in nonassimilatory electron fluxes that act to adjust the stromal ATP/2e- ratio. Electrons are often generated in excess to the amount required for CO2 fixation or photorespiration (Backhausen et al., 1994). This would lead to overreduction of the electron transport chains, if no other electron acceptors were present. Redox poising can be achieved in three ways: (a) During the operation of the malate valve, oxaloacetate is reduced by NADP-dependent malate dehydrogenase (NADP-MDH) to malate with consumption of NADPH. This reaction is controlled by the activation state of NADP-MDH (Backhausen et al., 1994). (b) Cyclic electron flow around PSI possibly follows two pathways that differ in their demand for reduced Fd and in their sensitivity toward antimycin A inhibition (Bendall and Manasse, 1995; Scheller, 1996). (c) The reduction of O2 leads to the formation of oxygen radicals and hydrogen peroxide (Polle, 1996). Fdred is required as electron source for all antioxidative pathways, i.e. for monodehydroascorbate reduction (Miyake and Asada, 1994), dehydroascorbate reductase (Mano et al., 1997), and NADPH-glutathione reductase (Foyer and Halliwell, 1976).
Thus, chloroplast Fd is an enzyme with at least eight different substrates, and there is evidence that the different electron acceptors in the stroma are organized in a hierarchical manner (Backhausen et al., 2000). In isolated chloroplasts, this allows that, in addition to CO2 assimilation, additional assimilatory electron fluxes, e.g. toward nitrite reduction, can proceed without competition for electrons. Any excess of electrons is at first taken over by the malate valve. Only when this pathway is unavailable or saturated does cyclic electron flow occur. O2 reduction seems to require a much higher electron pressure, i.e. the accumulation of reduced electron donors in PSI or in the stroma, than other pathways. Electron flow to these alternative sinks maintains the ΔpH at the required level and prevents the occurrence of uncontrolled overreduced states in the electron transport chains and in the stroma. Although the structural basis for this hierarchy remains unknown, it can be expected that an alteration in the leaf Fd content will affect some electron acceptors more than others.
At least six different Fd isoforms from various green and nongreen tissues have been described so far. In most plant species, Fd I is present as an intronless single-copy gene (Elliott et al., 1989). The isoproteins Fd I and Fd II have been found in leaves, but they also occur in green pericarp of tomato (Lycopersicon esculentum) fruits. In ripe pericarp only, Fd II prevails, and the new isoform Fd IV appears (Kamide et al., 1995). Maize (Zea mays) leaves contain two Fd isoforms that are very similar to each other. Fd I catalyzes linear electron flow in mesophyll cells, whereas Fd II catalyzes cyclic electron flow in bundle sheath cells (Matsumura et al., 1999; Kimata-Ariga et al., 2000). Interestingly, the difference between Fd I and Fd II varies among species. In some species, only two amino acids are different, whereas in others, as many as 18 amino acids are different (Kamide et al., 1995).
Fds from heterotrophic sources are different in both amino acid sequences and biochemical characteristics from the Fds in photosynthetic tissues (Onda et al., 2000). In non-photosynthetic plastids, e.g. from spinach (Spinacia oleracea) roots (Morigasaki et al., 1990), radish (Raphanus sativus) roots (Wada et al., 1989), etiolated seedlings (Kimata and Hase, 1989), and red tomato fruits (Green et al., 1991), Fd catalyzes electron transfer from stromal NADPH via FNR to Fd-dependent target enzymes. Fd III has been isolated from maize seedlings and tomato roots (Kimata and Hase, 1989; Green et al., 1991). In maize roots treated with nitrate, Fd VI is induced (Matsumura et al., 1997). An additional cDNA clone, pFD5, was identified, but to our knowledge, the respective isoprotein has not been found yet (Hase et al., 1991).
Because Fd plays such a central role in electron distribution and maintenance of the stromal redox state, any change in Fd availability should alter electron distribution toward the stromal acceptors in a typical way, according to the model developed for isolated spinach chloroplasts (Backhausen et al., 2000). In this work, potato (Solanum tuberosum L. cv Desiree) cDNA clones encoding Fd I were isolated, characterized, and used to generate transgenic plants with reduced Fd I protein levels. However, in addition to alterations in electron distribution, the altered redox state in the thylakoid membranes interfered with light acclimation of the transgenic plants.
RESULTS
Isolation and Characterization of Potato fed 1 cDNA Clones and Fd I Protein
First strand cDNA was prepared via reverse transcription from poly(A+) RNA of mature potato leaves that had been illuminated for 6 h. The cDNA was used as a template in PCR reactions with degenerate oligonucleotide primers, derived from conserved regions of known fed 1 genes. A 200-bp fragment of fed 1 was obtained and used to screen a λ-ZAP II cDNA library from potato leaf tissue. Four positive clones were selected due to insert sizes of about 600 bp. Sequence analysis showed that all inserts were identical and that the longest clone (575 bp) encoded the entire precursor Fd I polypeptide. The open reading frame ends with a stop codon at nucleotide position 432, corresponding to a polypeptide of 144 amino acid residues (Table I). The cleavage site for the chloroplast targeting presequence probably lies between Cys-46 and Met-47. The deduced amino acid sequence of the mature protein (97 amino acids) has extensive similarity to other leaf Fd I sequences, whereas the alignments with Fd from non-photosynthetic tissues revealed less identity (Table II).
Table I.
Comparison of the amino acid sequences of various Fds
The amino acid sequence of the potato Fd I was compared with Fd sequences from other organisms: tomato (Fd I; John et al., 1997), tomato (Fd II; Kamide et al., 1995), Arabidopsis (Fd I; Somers et al., 1990), spinach (Fd I; Wedel et al., 1988), pea (Pisum sativum; Fd I, Dobres et al., 1987), maize (Fd I and II; Hase et al., 1991), spinach (Fd II; Takahashi et al., 1983), and Fd III from maize roots (Hase et al., 1991). The new potato Fd I sequence has been submitted to the National Center for Biotechnology Information GenBank (accession no. AJ307031). Asterisks, Identical amino acids; double points, highly conserved amino acids with double points; single points, conserved amino acids with single points. The alignments were performed with ClustalW (http://www2.ebi.uk/clustalw). Transit peptides, as far as available, and the mature proteins are compared.
Table II.
Percentage identity of Fd isoforms
The sequence of potato Fd I was compared with the sequences listed in Table I. The determination of the identities of the various Fd I preproteins, mature proteins, and transit peptides was performed with LALIGN (http://www.ch.embnet.org/software/LALINGNform.html).
| Fd Isoform | Preprotein | Mature Protein | Transit Peptide |
|---|---|---|---|
| Potato | 100 | 100 | 100 |
| Tomato I | 96 | 95 | 97 |
| Tomato II | — | 79 | — |
| Arabidopsis | 62 | 72 | 43 |
| Spinach I | 64 | 75 | 42 |
| Spinach II | — | 71 | — |
| Pea I | 64 | 82 | 29 |
| Maize I | 66 | 74 | 21 |
| Maize II | 39 | 69 | 20 |
| Maize III | 43 | 64 | 10 |
Southern blots with genomic DNA were probed with randomly primed [32P]-labeled potato fed 1 cDNA fragments. Hybridization revealed one BamHI (12 kb), one EcoRI (12 kb), one HincII (0.6 kb), one HindIII (1.9 kb), one PstI (10 kb), one XhoI (14 kb), two ScaI (11 and 1.9 kb), and two XbaI (11 and 9 kb) fragments (Fig. 1). This indicates that fed 1 in potato is most likely encoded by a single-copy gene.
Figure 1.
Southern-blot analysis of genomic potato DNA. Genomic DNA (approximately 20 μg) was digested with BamHI, EcoRI, HincII, PstI, ScaI, XbaI, and XhoI (lanes 1-7). The blot was hybridized with [32P]-labeled potato fed1-cDNA fragment. PstI-digested λDNA was used as a marker.
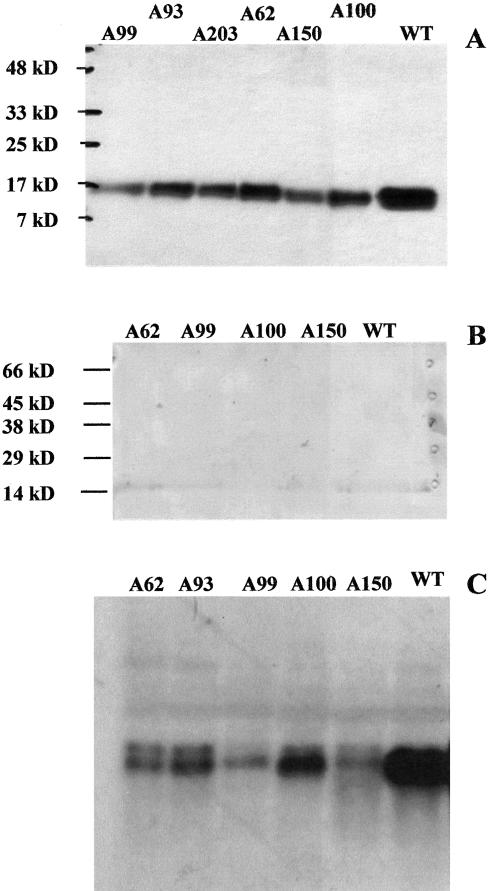
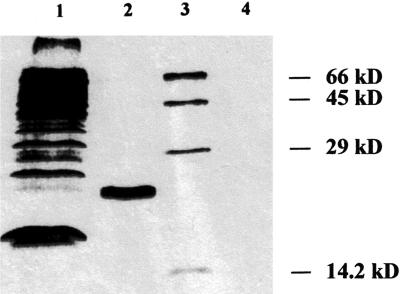
For the detection of Fd I protein in potato leaves, polyclonal antiserum raised against purified spinach Fd was used. Western blots conducted with identical protein amounts from spinach and potato leaves revealed a similar cross-reaction with Fd from both sources (data not shown). Most Fds migrate as a broad band with an apparent molecular mass of about 18 kD (Fig. 2A), as observed earlier with purified tomato Fd I (Green et al., 1991) and recombinant Fd I from spinach (Wedel et al., 1988), but an additional band with an apparent molecular mass of 22 kD was frequently detected in leaf extracts. The size of this additional band corresponds to unprocessed Fd I. In fact, in vitro translation of the mRNA in the presence of [35S]-Met, generated from the complete cDNA clone, resulted in labeling of a 22-kD protein (Fig. 3). On western blots conducted with crude extracts of potato tubers, only a weak signal of around 18 kD was detected (Fig. 2B), whereas in potato root extracts, no signal was found (data not shown). This is in agreement with Kimata and Hase (1989) who also could not detect non-photosynthetic Fd III in maize extracts with an antibody raised against Fd I. In contrast to potato roots, we observed a weak signal in the range of 20 to 22 kD in spinach root extracts (data not shown). This is consistent with the findings of Green et al. (1991), who reported that Fd III from tomato roots migrates slower on gels, and the purified tomato Fd could be detected by an antiserum raised against spinach Fd I.
Figure 2.
Western- and northern-blot analysis of Fd expression. A, Leaf extracts of potato plants (100 μg of soluble protein per lane) were subjected to SDS-PAGE and transferred to nitrocellulose. Immunodetection was performed with an antiserum raised against spinach Fd I. Samples were prepared from wild type (WT) and from transgenic lines A62, A93, A99, A100, and A150. B, Aliquots of 100 μg of soluble protein from tubers of the same plants as in A were probed with the potato Fd I antiserum. C, Abundance of fed 1 mRNA in potato leaves was analyzed with a northern blot. Total leaf mRNA (30 μg lane-1) was subjected to denaturating agarose gel electrophoresis, blotted to nylon membranes, and probed with randomly primed [32P]-labeled fed1 cDNA fragments from potato.
Figure 3.
In vitro translation of the full-length potato fed 1 cDNA clone. The fed 1 cDNA was transcribed and translated in the presence of [35S]-Met in a cell-free rabbit-reticulocyte lysate according to the manufacturer's instructions. The translation products were separated by gel electrophoresis, and the dried gel was exposed to x-ray film. Lane 1, Luciferase was used as a positive control; lane 2, translation product of fed 1; lane 3, molecular mass standard; lane 4, control sample without fed 1 cDNA.
Generation of Transgenic Plants
A homologous antisense-mRNA approach was chosen to reduce the endogenous fed 1 levels in transgenic potato plants. Because potato fed 1 differs significantly from other Fd isoforms (Table II), this approach should specifically affect Fd 1. The entire fed 1 cDNA, including the 5′- and 3′-untranslated regions, was introduced in reverse orientation into a plant expression vector under control of the cauliflower mosaic virus 35S promoter. From 47 independent antisense lines, a high number of 43 lines (91.5% of the tested transformants) showed a marked reduction in Fd I protein amount on western blots. The remaining Fd I was around 50% in antisense line A99; between 50% to 60% in A150, and between 60% to 70% in A100, A93, and A62 (Fig. 2A). The reduction of fed 1 was confirmed at the level of mRNA expression. From 25 randomly selected transgenic antisense plants, only two lines showed transcript levels equivalent to the WT. In lines A62, A93, A99, A100, and A150, the amount of fed 1 transcripts ranged from 5% to 10% of the WT (Fig. 2C).
Phenotypic Differences
Phenotypic differences were observed in most of the transgenic plants. Antisense lines with Fd contents between 80% and 100% resembled potato WT plants in most properties, but visible differences appeared in the antisense lines with 50% to 80% of the Fd contents found in the WT (e.g. A99 and A150). The leaves of those transformants turned pale green and sometimes even yellowish within 6 weeks. These differences were most pronounced when the primary transformants were grown in a greenhouse during the summer. This loss of chlorophyll (Chl) was a dynamic process, as shown by the example of a 10-week-old A99 plant in Figure 4. Initially, the leaves looked like those of the WT plants and grew to the same size. However, with increasing age, they turned yellowish (Fig. 4, bottom line).
Figure 4.
Potato plants with altered Fd I content. The WT and A99 plants were grown in soil in a greenhouse for 6 weeks. Top, Youngest four leaves of a WT plant are shown. Bottom, Same leaf sequence from a strong antisense line (A99), where the continuous loss of Chl becomes visible.
To obtain defined plant material, the plants intended for experimental use were grown from tubers under controlled conditions in a growth chamber. Although the light intensity was around 350 μmol quanta m-2 s-1 for 10 h per day, the phenotypic differences were smaller. Fully expanded WT leaves contained around 0.41 g Chl m-2, and some of the antisense lines showed only 75% of this value (Table III). Changes in Chl content especially affected Chl b. The Chl a/b ratio was increased up to 4.1 in the antisense lines, as compared with 3.4 in WT (Table III). However, leaf protein contents of the leaves showed only small differences. A typical leaf of a WT plant contained 5.6 g protein m-2, and a slight tendency (up to 15%) toward higher protein contents appeared in the leaves of the underexpressing plants (Table III). This suggests that the Chl loss did not arise because of an earlier onset of senescence.
Table III.
Protein and chlorophyll contents of the leaves.
Plants from the indicated transgenic lines were grown for 6 weeks in the growth chamber. For all measurements, the terminal leaflets of fully developed potato leaves were used. The values are means (±SD) of at least three independent experiments.
| Plant | Chl content | Chl a/b ratio | Protein content |
|---|---|---|---|
| g m−2 | g m−2 | ||
| WT | 0.41 ± 0.15 | 3.40 ± 0.14 | 5.6 ± 0.57 |
| A100 | 0.38 ± 0.16 | 3.85 ± 0.12 | 5.58 ± 0.51 |
| A93 | 0.34 ± 0.20 | 4.24 ± 0.11 | 5.93 ± 0.45 |
| A62 | 0.33 ± 0.18 | 3.74 ± 0.08 | 5.22 ± 0.57 |
| A150 | 0.31 ± 0.15 | 3.96 ± 0.12 | 5.75 ± 0.50 |
| A99 | 0.33 ± 0.20 | 4.10 ± 0.14 | 5.41 ± 0.68 |
Photosynthetic Properties of the Transgenic Plants
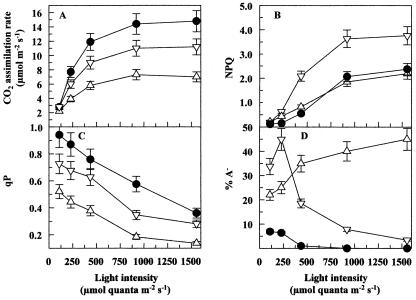
Measurements of assimilation, Chl fluorescence and P700 indicated that the altered Fd I content influenced the photosynthetic properties of the transgenic plants massively. Figure 5 summarizes the differences in gas exchange, Chl fluorescence, and ΔA830 between WT plants, the moderate antisense line A100 (which was similar to A93 and A63), and the stronger antisense line A99 (similar to A150).
Figure 5.
Gas exchange, Chl fluorescence, and ΔA830. Plants were grown in a growth chamber as described in “Materials and Methods”. The data obtained with WT plants (•) and antisense plants of lines A93 (▿) and A99 (▵) are shown. The measurements were started at the lowest light intensity. After steady-state photosynthesis was reached (15-20 min), the light intensity was increased to the next higher level. Each experiment was repeated at least three times, and the values did not vary more than 10% (represented by the error bars). A, Rate of CO2 assimilation; B, NPQ, non-photochemical quenching; C, photochemical quenching (qP); D, amount of reduced or missing PSI acceptor (PSI acceptor limitation [A-]).
In the WT plants, CO2 assimilation was saturated at a light intensity of about 900 μmol quanta m-2 s-1. The rate of CO2 assimilation was reduced in the antisense lines, and there were no indications that an altered behavior of the stomata occurred in the mutants that would in turn influence gas exchange and Chl fluorescence measurements. Interestingly, the level of decrease in the CO2 assimilation rate revealed a good correlation with the remaining Fd content. Both were around 40% in A99 and around 70% in A150 in high light (Fig. 5A). Even at limiting light, the rate of CO2 assimilation was below the WT level, consistent with the reduced maximum photochemical efficiency of PSII in the dark-adapted state (Fv/Fm-ratio) in low light (see below). Measurements of NPQ point to clear differences within the antisense lines, depending on the remaining Fd amount. The stronger antisense lines A150 and A99 had only a slightly decreased NPQ, indicating a slightly lower ΔpH in the thylakoid lumen. However, for the antisense line A93 (Fig. 5B) and for A62 and A100 (not shown), much higher NPQ values (up to 200% of WT) were obtained. It should be noted that different degrees of photo-inhibition (deduced from the dark-adapted Fv/Fm ratios in their leaves) were measured for A99 and A100 (data not shown). The high NPQ values were found only for the lines with Fv/Fm ratios that are similar to WT plants (between 3.6 and 5.0 in A100, A62, or A93), whereas in lines A99 and A150 (which had slightly decreased NPQ values), the Fv/Fm ratio in the dark was between 2.5 and 3.3.
The qP reflects the electron pressure at the QA site of PSII. In the antisense plants, the lower values indicated that the decreased Fd content caused higher reduction states of QA (Fig. 5C), probably due to reduced or (in the case of severely Fdunderexpressing plants) missing electron acceptor. A similar situation was determined for PSI. In WT plants, the P700-redox state was around 60% (at 900 μmol quanta m-2 s-1), whereas in the antisense lines, between 70% and 90% of P700 was reduced (data not shown). Another difference between WT and antisense plants became apparent from measurements of the acceptor side of PSI. WT plants show significant amounts of A- only in low light. The A- in the moderate antisense plants showed the same light dependence as the WT, but the values increased up to 45%. The stronger antisense lines such as A99 exhibited permanently high A- values, indicating that 20% to 40% of P700 was without electron acceptor (Fig. 5D). In the case of the antisense plants, A- does not necessarily indicate reduced PSI acceptors, and the lack of Fd should have the same effect. It must be noted that in some samples, the calculation of ΔA830-derived parameters was obstructed by a decrease in the amount of oxidizable P700 in the stronger antisense lines. The amplitude of the total P700 signal, which represents the difference between reduced and oxidized P700, remained well below the WT level. Illumination with white or far-red light could bring the amplitude above 30% to 40% of the WT level. Such decrease occurred especially in the primary transformants during growth in the greenhouse. However, in the plants used for the measurements shown in Figure 6, the difference between P700red and P700ox remained above 70% of the WT. This phenomenon will be analyzed in more detail in a subsequent paper.
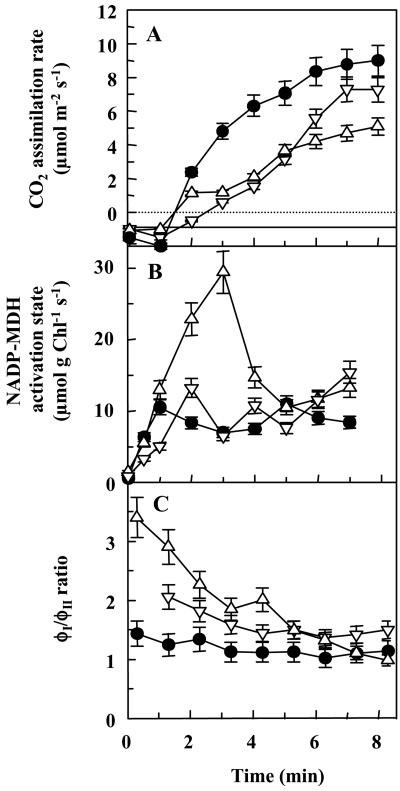
Figure 6.
Electron distribution during photosynthetic induction. Time-courses of changes in the rate of CO2 assimilation (A), NADP-MDH activation state (B), and the ratio between electron fluxes through PSI and PSII (ϕI/ϕII; C) are shown. The measurements were performed with dark-adapted leaves. The data obtained with WT plants (•) and antisense plants of lines 93 (▿) and 99 (▵) are shown. Each experiment was repeated at least three times, and the values did not vary by more than 10% (represented by the error bars).
Effects of Altered Fd Levels on Photosynthetic Electron Distribution
The distribution of light-generated electrons between CO2 assimilation on the one hand and malate valve and cyclic electron flow on the other hand was analyzed in short-term experiments. The measurements were performed within the first minutes of illumination, using leaves that had been predarkened for 1 h. In WT leaves, the rate of CO2 assimilation had a lag phase of 1 to 2 min; afterward the rate increased steadily. Steady-state photosynthesis was reached after 20 to 25 min. The lag phase lasted longer in the antisense plants, and the subsequent assimilation rate was lower (Fig. 6A), indicating that less electrons could be used for CO2 fixation.
The activation state of NADP-MDH (Fig. 6B) is usually around 20% to 30% in WT potato plants during steady-state photosynthesis but increases when an excess of electrons is produced and the NADPH/NADP ratio of the stroma increases. Such a transient increase of NADP-MDH activation state (up to 35% in WT) occurred during the first 2 min of illumination; afterward, the activation state decreased to about 20% to 25%. This transient increase started later in the antisense plants, but it lasted longer, and the NADP-MDH activation state increased to 45% in A100 and up to 90% in A99. However, this increase was only temporary. During steady-state photosynthesis, the NADP-MDH activation state decreased to values that were sometimes even below those in WT leaves (data not shown).
The occurrence of cyclic electron flow can be determined by comparing the electron fluxes through PSI and PSII. A relative increase in the electron flow through PSI indicates the operation of cyclic electron flow in leaves. It is evident from the time course of the ϕI/ϕII ratio (Fig. 6C) that in the WT leaves, only little deviation from the 1:1 ratio occurred. Only the data points collected within the first 2 min of illumination had approximately 1.3-fold higher ϕI than ϕII values. However, cyclic electron flow increased to significant rates in the antisense plants. The ϕI/ϕII ratio in A99 was above 3 when illumination started and decreased afterward. However, values above the WT level (usually around 1.3) were maintained even during steady-state photosynthesis (data not shown). This points to higher rates of cyclic electron flow in the antisense plants.
DISCUSSION
Fd I of Potato Leaves
Four independent fed-cDNA clones were isolated from a potato leaf cDNA library, one of them coding for the entire Fd polypeptide. The isolated potato Fd isoform comprises 144 amino acids, including an N-terminal presequence of 46 amino acids and a mature polypeptide of 97 amino acids. The transit peptide is quite similar to other Fd I transit peptides, which are usually between 46 and 54 amino acids in size. It also starts with Met-Ala, has a high content of Ser and Thr residues, displays an overall positive net charge, and contains an Arg residue in the region of -6 to -10, counted from the assumed cleavage site. The mature Fd I protein is, with the exception of one base, identical to expressed sequence tag TC66090 in The Institute for Genomic Research database (http://www.tigr.org/tdb/tgi/stgi), and reveals extensive identity with other Fd I sequences from higher plants, e.g. shows 95% identity with tomato Fd I (Table I). The shared identical positions with Fd II isoforms are lower (69%-79%), and the identity with Fd III from maize roots is only 64%. This indicates that the isolated fed cDNA codes for the leaf-specific Fd I isoform.
Southern-blot analysis conducted with genomic DNA from potato indicated that fed 1 probably occurs as a single-copy gene in the potato genome. Furthermore, the selectivity of the Southern-blot experiments is a good indication that the antisense construct will not easily hybridize to any of the other endogenous fed mRNA species in the transgenic plants. For the chosen antisense approach, this is an important prerequisite and ensures that the Fd content decreases only in leaves without affecting Fd-dependent metabolism in roots.
In contrast to heterologous antisense approaches that often fail to decrease endogenous mRNA or target protein content (e.g. Faske et al., 1997), the homologous antisense construct using the entire potato fed 1 cDNA was quite successful. Both fed 1 mRNA and Fd I protein contents in leaves were significantly decreased, and antisense phenotypes occurred with the high frequency of 91.5%. However, there was a clear discrepancy between the determined protein levels and the corresponding mRNA levels. In the selected antisense lines, the protein levels ranged between 50% and 80% of the WT, whereas the remaining mRNA levels were only about 5% to 10%. Such a lack of relationship between protein and mRNA content was reported earlier, e.g. for tobacco (Nicotiana tabacum) plants with decreased levels of the Rieske-FeS protein, a nuclear-encoded subunit of the thylakoid cytochrome b6/f complex (Price et al., 1995). In these plants, the protein levels varied between 14% and 40%, with a concomittant reduction in mRNA to less than 10%. The authors concluded that posttranscriptional adjustment functions over a finite range and that a reduction in protein content will only occur when mRNA levels fall below this threshold.
Effects of Decreased Fd Content on Photosynthesis and Electron Distribution
In the various antisense lines, the Fd I protein content varied between 50% and 100% of the WT level. Terashima and Inoue (1985) determined that palisade tissue of spinach leaves contains 2 to 3 times more Fd than PSI. Assuming similar values for WT potato leaves, the ratio between PSI and Fd was decreased to a ratio close to one in transgenic lines A99 and A150. Because we did not obtain viable plants with Fd contents below 40% of the WT amount, it can be concluded that when the Fd content is less than that of PSI, this constitutes a lethal condition. Probably, this was the case for some primary transformants that, upon transfer from tissue culture to soil, failed to grow autotrophically.
Even in the presence of stoichiometric amounts (of perhaps 1 mol Fd per 1 mol PSI), the electron transport chain showed an increased reduction state. The low qP values indicate a more reduced QA in PSII (i.e. increased PSII excitation pressure) and a higher reduction state of the plastoquinone pool. P700 accumulated in the reduced state, and (especially in line A99) there was a decrease in availability of oxidized PSI acceptors (Fig. 5). Furthermore, the alterations in the Fd content influenced photosynthetic electron transport and electron distribution. The amount of Fd correlated with the rate of CO2 fixation in the antisense lines, and changes in the proportion of nonassimilatory electron fluxes were detected. The activation state of NADP-MDH, which controls the electron flux through the malate valve, was temporarily increased in the antisense plants (Fig. 6B) but fell below the WT value during steady-state photosynthesis.
Figure 6C indicates a higher electron flux through PSI than through PSII in the antisense lines, suggesting that the contribution of cyclic electron flow was higher in these plants. Evidence for cyclic electron transport in WT plants is rare and was previously only observed under extreme conditions, e.g. upon removal of O2 and/or CO2 (Harbinson and Foyer, 1991; Backhausen et al., 1998). The low rates of CO2 fixation, the course of the NADP-MDH activation state, and the high fraction of cyclic electron flow are consistent with the hierarchical organization of electron acceptors that was previously deduced from isolated intact spinach chloroplasts (Backhausen et al., 2000). Both the malate valve and CO2 assimilation use NADPH, a soluble electron donor in the stroma. Therefore, the decreased Fd contents in the stronger antisense lines do not seem to be sufficient to maintain the required rate of electron flow into the stroma. As suggested earlier, any excess of electrons that are not consumed by metabolic electron acceptors or cannot be used by the malate valve are mainly used for cyclic electron flow, as seems to be the case in the Fd antisense plants. Although cyclic electron flow does not really remove electrons, it can correct overreduction by down-regulation of PSII (Heber and Walker, 1992; Backhausen et al., 2000).
The light use efficiency of both PSII and PSI was down-regulated in the Fd antisense plants, and this may provide some protection against oxidative stress. Here, one might argue that different strategies are used between the moderately suppressed lines A100, A93, and A62, and the stronger antisense lines A99 and A150. The moderate lines had nearly unchanged Fv/Fm ratios, but they developed a very high NPQ (Fig. 5B), indicating a strong reversible down-regulation of PSII. This would be caused by the high Δ pH developed as a result of cyclic electron transport around PSI, together with an increase in NPQ capacity that may arise from acclimation to the increased excitation pressure on PSII (see below). The situation in A99 and A150 was different. Both lines showed nearly unchanged NPQ but decreased Fv/Fm ratios. However, the consequence was the same as in the other antisense lines: less functional PSII and decreased quantum yields. In both cases, these strategies seem to prevent very efficiently the onset of O2 reduction. This is in agreement with the results of Ruuska et al. (2000), who found for transgenic tobacco plants with decreased Rubisco contents that the electron transport rate is always adjusted to the stromal demand, without any concomitant electron transport toward O2.
Acclimation Effects in the Mutant Plants
In addition to the short-term changes in electron transport, specific changes in the Chl contents occurred which suggest that the antisense plants have acclimated to the reduced Fd contents by alterations of the chloroplast composition. Although the protein contents remained more or less unchanged, the total Chl content decreased by 25%. Furthermore, the Chl a/b ratio was generally higher, reaching up to 4.1 in A99, as compared with 3.4 in WT plants. The Chl a/b ratio is considered as a good indicator for light acclimation (Anderson and Osmond, 1987). Because the majority of Chl b is present in LHCII, the loss in Chl b most likely reflects a decreased amount of LHCII. Such acclimation occurs under natural conditions upon a transfer from low- to high-light conditions. It seems that the transgenic potato plants with decreased Fd react as if they were grown under a much higher light intensity. Consistent with this view is the increase in NPQ capacity in some of the antisense lines; plants grown in high light usually developed an increased NPQ capacity, and this has been associated with an increase in the content of xanthophyll cycle carotenoids and in the PsbS protein.
There is good evidence that the redox states of photosynthetic electron carriers and/or some stromal compounds release signals that are responsible for acclimation. Walters et al. (1999) reported that light-intensity dependent acclimation occurs even in Arabidopsis mutants that lack functional photoreceptors. One possible site for redox sensing would be the electron carriers between PSII and PSI (Allen et al., 1995; Pfannschmidt et al., 1999) and requires the presence of the cyt b/f complex (Anderson et al., 1997). This type of redox sensing would be expected to detect the redox pressure of PSII and, in cases of permanently increased redox states, to down-regulate the expression of LHCII. There is also evidence of redox sensing by the acceptor side of PSI. The thioredoxin-mediated reduction of regulatory disulfide bridges within a specific activator protein controls translation and transcription of several chloroplast-encoded proteins (Kim and Mayfield, 1997). Other environmental changes that concern nuclear-encoded genes are sensed via accumulation of hydrogen peroxide (Willekens et al., 1997) or by the redox state of glutathione (Karpinski et al., 1997). However, in the case of the Fd antisense plants, it is unlikely that any of the latter pathways is involved in redox signaling. Because electron transport into the stroma was obstructed, we conclude that in the antisense plants, the reduction state of thioredoxins may be lower, not higher. Because there was a good correlation in the different antisense lines between qP (which measures the excitation pressure of PSII) and the respective Chl a/b ratio, we conclude that the decrease in the expression of LHCII in the antisense plants is controlled by the redox state between PSII and PSI.
MATERIALS AND METHODS
Isolation of Fd I-cDNA Clones
A specific fed I fragment was amplified from isolated potato (Solanum tuberosum L. cv Desiree) cDNA in PCR reactions using degenerated oligonucleotide primers. The primers were synthesized based on known amino acid sequences from spinach (Spinacia oleracea; Wedel et al., 1988), pea (Pisum sativum; Elliott et al., 1989), and Arabidopsis (Somers et al., 1990). Whole-leaf RNA was isolated as described by Logemann et al. (1987), and poly (A+)-mRNA was selected using the Oligotex dT mRNA Kit (Qiagen, Hilden, Germany). Reverse transcription of poly (A+) mRNA followed by PCR was carried out with a cDNA synthesis kit from Pharmacia LKB (Freiburg, Germany). The amplified 200-bp fragments were cloned into EcoRV-digested pBluescript SK (Stratagene, San Diego), transformed in Escherichia coli XL1 blue (Stratagene), and insert-carrying clones were identified by blue/white selection. Sequencing of plasmid DNA was done by the dideoxynucleotide chain termination method (Sanger et al., 1977) on both strands employing 17-mer T3 and T7 sequencing primers (Sequenase Sequencing kit, USB, Amersham, Braunschweig, Germany). Fragments of fed 1 with high homology to known sequences were excised, purified by gel electrophoresis, and labeled in PCR reactions using a DIG-dNTP-labeled nucleotide mixture (Boehringer Mannheim, Germany). Using these fragments, a potato leaf λZAP II cDNA library (kind gift from the group of Uwe Sonnewald, Institut für Pflanzengenetik und Kulturpflanzenforschung (IPK), Gatersleben, Germany) was screened using these fragments. In vivo excision of positive clones was performed as described in the Stratagene protocol. After sequencing, comparison of the deduced nucleotide and amino acid sequences with those from other higher plants showed that one of the clones codes for a full-length Fd I protein.
Construction of fed 1 Antisense Potato Plants
For generation of the fed 1 antisense construct, the 639-bp cDNA fragment, including the transit peptide, was released with NotI, and the ends were filled with T4-DNA polymerase and subcloned into the SmaI site of the plant expression vector pA35S (Höfte et al., 1991), flanked by the constitutive cauliflower mosaic virus-35S promoter and octopine synthase termination sequences. A 1,550-bp PvuII fragment containing the fed 1 cDNA in antisense orientation (verified by HindIII digestion) was excised and introduced into the SmaI-site in the T-DNA region of binary vector pDE 1001 (Denecke et al., 1990). Orientation of this fragment relative to the NEO transcription unit was determined by NcoI digestion. For Agrobacterium tumefaciens transformation, a plasmid was chosen in which the fed 1 insert was transcribed in the same orientation as the NPT-II gene (conferring kanamycin resistance).
Potato Transformation and Regeneration of Transgenic Plants
A. tumefaciens-mediated transformation of potato leaf discs followed the procedure described by Dietze et al. (1995). For callus induction, the infected leaf discs were transferred to solidified (0.8% [w/v] agar) Murashige and Skoog medium containing 1.6% (w/v) Glc supplemented with 5 mg L-1 naphthalene acetic acid and 0.1 mg L-1 benzylaminopurine. After 1 week, the leaf discs were placed on shoot induction medium containing 1.6% (w/v) Glc, 2 mg L-1 zeatin-Rib, 20 μg L-1 naphthalene acetic acid, and 20 μg L-1 GA3. For selection of transformed shoots, 500 mg L-1 claforan and 500 mg L-1 kanamycin were added to both media. After 6 to 8 weeks, emerging shoots were placed on kanamycin-supplemented Murashige and Skoog media. Shoot cuttings that formed roots were planted in soil and screened by western-blot analyses. As a control, potato WT plants were regenerated in parallel by omitting kanamycin selection and maintained under identical conditions as the transformed plants.
Western-Blot Analysis
Leaf discs were immediately frozen in liquid N and stored at -80°C. For western blots, the leaf discs were crushed in liquid N in the presence of precooled polyvinylpolypyrrolidone, and soluble proteins were extracted in 50 mm HEPES-NaOH (pH 7.5), 0.1% (w/v) SDS, 2 mm sodium bisulfite, 0.01% (w/v) bovine serum albumin, and 1/1,000 (w/v) protease inhibitor cocktail (Sigma-Aldrich, Taufkirchen, Germany). Equal protein amounts were loaded on 15% or 20% discontinuous SDS-polyacrylamide gels using a vertical minigel system (Mini-Protean II, Bio-Rad Laboratories, Hercules, CA). After separation, the proteins were blotted onto polyvinylidene difluoride membranes. Immunodetection was performed essentially as described by Graeve et al. (1994). Polyclonal antibodies, raised against spinach Fd I, showed specific cross-reaction with potato Fd I in 1:1,000 (v/v) dilution; therefore, they could be used to screen for antisense transformants. For the detection of the second antibody, Roti-Lumin (Roth, Karlsruhe, Germany) was used as a substrate. The quantitation of the band intensity was performed with scanned images, using the Optimas 5.2 software (Media Cybernetics, Gleichen, Germany).
Northern- and Southern-Blot Analysis
Total RNA was isolated as described by von Schaewen et al. (1995). Isolation of genomic DNA was as described by Dellaporta et al. (1983). DNA- and RNA-blot analysis was conducted using standard techniques (Sambrook et al., 1989). Hybridization and washing of the membranes followed methods described by Church and Gilbert (1984). Probes were purified from agarose gels using the QIAex-II kit (Qiagen). The DNA fragments were radiolabeled according to the manufacturer's instructions (Pharmacia). Removal of nonincorporated nucleotides was as described by von Schaewen et al. (1995). For quantitation of relative mRNA amounts, dot-blot filters with dilutions from 1 ng to 0.1 pg of plasmid DNA either from spinach or from potato fed 1 cDNA clone were made as described previously (von Schaewen et al., 1995). For reprobing, the blots were stripped by incubating the membranes in 50 mm Tris (pH 8.0), 50% (v/v) formamide, and 1% (w/v) SDS at 68°C for 1 h (Sambrook et al., 1989).
In Vitro Translation
The fed 1-cDNA fragment was transcribed and translated in the presence of [35S]-Met in a cell-free rabbit reticulocyte lysate according to the manufacturer's instructions (TNT Coupled Reticulocyte Lysate System, Promega, Madison, WI). The translation products were subjected to a discontinuous 20% SDS-PAGE and stained with Coomassie Brilliant Blue G250. The gel was dried and exposed to x-ray film (Kodak XAR-5, Eastman-Kodak, Rochester, NY) for 3 d.
Plant Material and Growth Conditions
Potato plants growing in tissue culture were a kind gift from the Institut für Genbiologische Forschung (Berlin). After A. tumefaciens-mediated leaf-disc transformation, transgenic potato plants were regenerated according to Dietze et al. (1995). Plants in tissue culture were grown in a growth chamber (16 h of light at 24°C and 8 h of dark at 20°C) at 70 μmol quanta m-2 s-1 on solidified Murashige and Skoog medium. After 3 to 4 weeks, the plants were transferred into a commercially available soil mixture (10% [w/v] sand, 10% [w/v] pumice, 10% [w/v] loam, 35% [w/v] compost, 35% [w/v] peat, additionally supplemented with 1 kg of horn chips, 1 kg of carbonic lime, and 1 kg of PoliCrisal [14% {w/v} N, 10% {w/v} K, 6% {w/v} P, 0.4% {w/v} Mg, 0.1% {w/v} Mn, 0.052% {w/v} B, 0.041% {w/v} Cu, 0.023% {w/v} Zn, 0.0037% {w/v} Mo, and 0.0001% {w/v} Co] per m3 soil), and subsequently grown in a greenhouse. The plants were watered daily except 2 weeks before tuber harvest. When the plants were completely senescent, the tubers were harvested, washed, and stored at 6°C until shoots started to develop.
Tubers of similar size were placed in pots of 14 cm size in diameter (1.3 L). After 2 weeks, when green shoot tips emerged, the plants were transferred to their final growth regime in the growth chamber. As light sources, SON-T AGRO 400 lamps (Philips, Eindhoven, The Netherlands) were used. The light intensity was 350 μmol quanta m2 s-1, measured at leaf height. Relative humidity was 75% for a daily period of 10 h of light (22°C) and 14 h of darkness (18°C). For all experiments, tuber-grown plants were used. Leaf samples were taken only from the end lobes of fully expanded leaves that were directly light-exposed, i.e. from the third to the fifth leaves counted from the top of the plant.
Gas Exchange, Chl Fluorescence, and P700 Measurements in Leaves
The measurements of gas exchange, Chl fluorescence, and P700-redox state were done simultaneously. Gas exchange was measured either with an ADC-LCA 4 system (ADC, Hoddesdon, UK) or with a Ciras-1 (PP-Systems, Hitchin, UK), using ambient oxygen and CO2 concentrations. Chl fluorescence quenching and ΔA830 were measured with two pulse-amplitude modulated fluorimeters in parallel (Walz, Effeltrich, Germany). The ϕII and the quenching coefficients qP and NPQ were determined by the methods cited by Backhausen et al. (1998).
The redox state of P700 (percentage P700 oxidation), A-, and ϕI were calculated from ΔA830 as described by Backhausen et al. (1998). The amplitude of full P700 oxidation was measured in the dark for each leaf before the illumination was started. In darkness, P700 is in its reduced state, and full oxidation of P700 was achieved by illumination with far-red light (>700 nm), which excites only PSI. The oxidation of P700 was monitored by the change in the A830. During illumination, the same amount of oxidizable P700 should be available, unless the PSI electron acceptors are already in their reduced state and cannot accept more electrons. During illumination, the fraction of reduced or PSI acceptor (A-) is determined by short saturating light pulses, which give full oxidation of P700, followed by a “dark pulse,” which yields fully reduced P700. The difference between the P700 amplitude in the light and the far-red-induced amplitude determined in the dark-adapted leaf must be attributed to A-. All measurements were repeated at least three times using two to seven different plants.
NADP-MDH Measurements in Leaf Samples
The samples used for the estimation of the in vivo NADP-MDH activities were obtained using the freeze-clamp method. To obtain samples, an LCA4 gas exchange system (ADC) with a PLC2 leaf chamber, modified to our demands (Feinmechanische Werkstatt, Universität Osnabrück, Germany) was used. The activation states were determined according to Scheibe and Stitt (1988). The maximum activity of NADP-MDH was determined after exhaustive reduction by reduced dithiothreitol, and the subsequent activity assay was as described by Scheibe and Stitt (1988).
Determination of Chl and Protein Content
Chl a and b contents of the leaves were determined by measuring the absorbance of acetone extracts according to Porra et al. (1989). Protein was determined according to Bradford (1976), with bovine serum albumin as a standard.
Acknowledgments
The authors are grateful to Norbert Wedel (Christian-Albrechts-Universität, Kiel, Germany) for providing the spinach fed 1-cDNA clones. The authors thank Susanne Klocke and Sabrina Jung for their help in performing the experiments. Further thanks are due to Kirsten Jäger, Alexandra Lohstroh, and Heike Wolf-Wibbelmann (Pflanzenphysiologie, Fachbereich Biologie/Chemie, Universität Osnabrück, Germany) for excellently growing the plant material. Potato tissue in sterile culture was a kind gift of the Institut für Genbiologische Forschung (Berlin). The potato leaf λZAP II cDNA library was a kind gift from the group of U. Sonnewald (Gatersleben, Germany).
This work was supported by the Deutsche Forschungsgemeinschaft (grant nos. Ba 1864 and FOR 387,TP1) and by the British Council (Academic Research Collaboration).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.026013.
References
- Allen JF, Alexciev K, Hakansson G (1995) Regulation by redox signaling. Current Biol 5: 869-872 [DOI] [PubMed] [Google Scholar]
- Anderson JM, Osmond CB (1987) Shade-sun responses compromises between acclimation and photoinhibition. In DJ Kyle DJ, CB Osmond, CJ Arntzen, eds, Photoinhibition. Elsevier, Amsterdam, pp 2-36
- Anderson JM, Price GD, Chow WS, Hope AB, Badger MR (1997) Reduced levels of cytochrome bf complex in transgenic tobacco leads to marked photochemical reduction of the plastoquinone pool, without significant change in acclimation to irradiance. Photosynth Res 53: 215-227 [Google Scholar]
- Arnon DI (1988) The discovery of ferredoxins: the photosynthetic path. Trends Biochem Sci 13: 30-33 [DOI] [PubMed] [Google Scholar]
- Backhausen JE, Emmerlich A, Holtgrefe S, Horton P, Rogers JJM, Müller- Röber B, Scheibe R (1998) Transgenic potato plants with altered expression levels of chloroplast NADP-malate dehydrogenase: interactions between photosynthetic electron transport and malate metabolism in leaves and in isolated intact chloroplasts. Planta 207: 104-114 [Google Scholar]
- Backhausen JE, Kitzmann C, Horton P, Scheibe R (2000) Electron acceptors in isolated intact spinach chloroplasts act hierarchally to prevent overreduction and competition for electrons. Photosynth Res 64: 1-13 [DOI] [PubMed] [Google Scholar]
- Backhausen JE, Kitzmann C, Scheibe R (1994) Competition between electron acceptors in photosynthesis: regulation of the malate valve during CO2 fixation and nitrite reduction. Photosynth Res 42: 75-86 [DOI] [PubMed] [Google Scholar]
- Bendall DS, Manasse RS (1995) Cyclic photophosphorylation and electron transport. Biochim Biophys Acta 1229: 23-38 [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254 [DOI] [PubMed] [Google Scholar]
- Brouquisse R, Weigel P, Rhodes D, Yocum CF, Hanson AD (1989) Evidence for a ferredoxin-dependent choline monooxygenase from spinach chloroplast stroma. Plant Physiol 90: 322-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci USA 78: 3595-3599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation, version two. Plant Mol Biol Rep 1: 19-21 [Google Scholar]
- Denecke J, Bottermann J, Deblaere L (1990) Protein secretion in plant cells can occur via a default pathway. Plant Cell 2: 51-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietze J, Blau A, Willmitzer L (1995) Agrobacterium-mediated transformation of potato (Solanum tuberosum). In I Potrykus, G Spangenberg, eds, Gene Transfer to Plants. Springer Lab Manual. Springer, Berlin, pp 24-29
- Dobres MS, Elliott RC, Watson JC, Thompson WF (1987) A phytochrome regulated pea transcript encodes ferredoxin I. Plant Mol Biol 8: 53-59 [DOI] [PubMed] [Google Scholar]
- Elliott RC, Dickey LF, White MJ, Thompson WF (1989) Cis-acting elements for light regulation of pea ferredoxin 1 gene expression are located within transcribed sequences. Plant Cell 1: 691-698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faske M, Backhausen JE, Sendker M, Singer-Bayerle M, Scheibe R, von Schaewen A (1997) Transgenic tobacco plants expressing a heterologous pea chloroplast Nmdh cDNA in sense and antisense orientation Effect on NADP-malate dehydrogenase level, stability of transformants and plant growth. Plant Physiol 115: 705-717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133: 21-25 [DOI] [PubMed] [Google Scholar]
- Graeve K, von Schaewen A, Scheibe R (1994) Purification, characterization, and cDNA sequence of glucose-6-phosphate dehydrogenase from potato (Solanum tuberosum L.). Plant J 5: 353-361 [DOI] [PubMed] [Google Scholar]
- Green LS, Yee BC, Buchanan BB, Kamide K, Sanada Y, Wada K (1991) Ferredoxin and ferredoxin-NADP reductase from photosynthetic and nonphotosynthetic tissues of tomato. Plant Physiol 96: 1207-1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbinson J, Foyer HC (1991) Relationships between the efficiencies of photosystems I and II and stromal redox state in CO2-free air. Plant Physiol 97: 41-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase T, Kimata Y, Yonekura K, Matsumara T, Sakakibara H (1991) Molecular cloning and differential expression of the maize ferredoxin gene family. Plant Physiol 96: 77-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U, Walker DA (1992) Concerning a dual function of coupled cyclic electron transport in leaves. Plant Physiol 100: 1621-1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfte H, Faye L, Dickinson C, Herman EM, Chrispeels MJ (1991) The protein body proteins phytohemaglutinin and tonoplast intrinsic protein are targeted to vacuoles in leaves of transgenic tobacco. Planta 184: 431-437 [DOI] [PubMed] [Google Scholar]
- John I, Hackett R, Cooper W, Drake R, Farrell A, Gierson D (1997) Cloning and characterization of tomato leaf senescence-related cDNAs. Plant Mol Biol 33: 641-651 [DOI] [PubMed] [Google Scholar]
- Kamide K, Sakai H, Aoki K, Sanada Y, Wada K, Green LS, Yee BC, Buchanan BB (1995) Amino acid sequences of heterotrophic and photosynthetic ferredoxins from the tomato plant (Lycopersicon esculentum Mill.). Photosynth Res 46: 301-308 [DOI] [PubMed] [Google Scholar]
- Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux PM (1997) Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during light stress. Plant Cell 9: 627-640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Mayfield SP (1997) Protein disulphide isomerase as a regulator of chloroplast translational activation. Science 278: 1954-1957 [DOI] [PubMed] [Google Scholar]
- Kimata Y, Hase T (1989) Localization of ferredoxin isoproteins in mesophyll and bundle sheath cells in maize leaf. Plant Physiol 89: 1193-1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata-Ariga Y, Matsumura T, Kada S, Fujimoto H, Fujita Y, Endo T, Mano J, Sato T, Hase T (2000) Differential electron floe around photosystem I by two C4-photsynthetic-cell-specific ferredoxins. EMBO J 19: 5041-5050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaff DB, Hirasawa M (1991) Ferredoxin-dependent chloroplast enzymes. Biochim Biophys Acta 1056: 93-125 [DOI] [PubMed] [Google Scholar]
- Leegood RC (1996) Primary photosynthate production: physiology and metabolism. In E Zamski, A Schaffer, eds, Photoassimilate Distribution in Plants and Crops: Source-Sink Relationships. Marcel Dekker Inc., New York, pp 21-41
- Link G (2001) Redox regulation of photosynthetic genes. In EM Aro, B Andersson, eds, Regulation of Photosynthesis, Kluwer, Dordrecht, The Netherlands, pp 85-107
- Mano J, Ushimaru T, Asada K (1997) Ascorbate in thylakoid lumen as an endogenous electron donor to photosystem II: protection of thylakoids from photoinhibition and regeneration of ascorbate in stroma by dehydroascorbate reductase. Photosynth Res 53: 197-204 [Google Scholar]
- Matsumura T, Kimata-Ariga Y, Sakakibara H, Sugijama T, Murata H, Takao T, Shimonishi Y, Hase T (1999) Complementary DNA cloning and characterization of ferredoxin localized in bundle-sheath cells of maize leaves. Plant Physiol 119: 481-488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumara T, Sakakibara H, Nakano R, Kimata Y, Sugiyama T, Hase T (1997) A nitrate-inducible ferredoxin in maize roots. Plant Physiol 114: 653-660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake C, Asada K (1994) Ferredoxin-dependent photoreduction of the monodehydroascorbate radical in spinach thylakoids. Plant Cell Physiol 35: 539-549 [Google Scholar]
- Morigasaki S, Takada K, Sanada Y Wada K, Yee BC, Shin S, Buchanan BB (1990) Novel forms of ferredoxin and ferredoxin-NADP+ reductase from spinach roots. Arch Biochem Biophys 283: 75-80 [DOI] [PubMed] [Google Scholar]
- Onda Y, Matsumura T, Kimata-Ariga Y, Sakakibara H, Sugiyama T, Hase T (2000) Differential interaction of maize root ferredoxin:NADP+ oxidoreductase with photosynthetic and non-photosynthetic ferredoxin isoproteins. Plant Physiol 123: 1037-1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannschmidt T, Nilsson A, Allen JF (1999) Photosynthetic control of chloroplast gene expression. Nature 397: 625-628 [Google Scholar]
- Polle A (1996) Mehler reaction: friend or foe in photosynthesis. Bot Acta 109: 84-89 [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of the accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents. Biochim Biophys Acta 975: 384-394 [Google Scholar]
- Price GD, Yu JW, von Caemmerer S, Evans JR, Chow WS, Anderson JM, Hurry V, Badger MR (1995) Chloroplast cytochrome b6/f and ATP synthase complexes in tobacco. Transformation with antisense RNA against nuclear-encoded transcripts for the Rieske FeS and ATPδ polypeptides. Aust J Plant Physiol 22: 285-297 [Google Scholar]
- Ruuska SA, Badger MR, Andrews TJ, von Caemmerer S (2000) Photosynthetic electron sinks in transgenic tobacco with reduced amounts of Rubisco: little evidence for significant Mehler reaction. J Exp Bot 51: 357-368 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, NY
- Sanger F, Fritsch EF, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463-5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe R (1991) Redox-modulation of chloroplast enzymes. Plant Physiol 96: 1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe R, Stitt M (1988) Comparison of NADP-malate dehydrogenase activation, QA reduction and O2 evolution in spinach leaves. Plant Physiol Biochem 26: 473-481 [Google Scholar]
- Scheller HV (1996) In vitro cyclic electron transport in barley thylakoids follows two independent pathways. Plant Physiol 110: 187-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers DE, Caspar T, Quail PH (1990) Isolation and characterization of a ferredoxin gene from Arabidopsis thaliana. Plant Physiol 93: 572-577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Hase T, Wada K, Matsubara H (1983) Ferredoxin in developing spinach cotyledons The presence of two molecular species. Plant Cell Physiol 24: 189-198 [Google Scholar]
- Terashima I, Inoue Y (1985) Palisade tissue chloroplasts and spongy tissue chloroplasts in spinach Biochemical and ultrastructural differences. Plant Cell Physiol 26: 63-75 [Google Scholar]
- von Schaewen A, Langenkämper G, Graeve K, Wenderoth I, Scheibe R (1995) Molecular characterization of the plastidic glucose-6-phosphate dehydrogenase from potato in comparison to its cytosolic counterpart. Plant Physiol 109: 1327-1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K, Onda M, Matsubara H (1989) Amino acid sequences of ferredoxin isoproteins from radish roots. J Biochem 105: 619-625 [DOI] [PubMed] [Google Scholar]
- Walters RG, Rogers JJM, Shephard F, Horton P (1999) Acclimation of Arabidopsis thaliana to the light environment: the role of photoreceptors. Planta 209: 517-527 [DOI] [PubMed] [Google Scholar]
- Wedel N, Bartling D, Herrmann RG (1988) Analysis of cDNA clones encoding for the entire ferredoxin I precursor polypeptide from spinach. Bot Acta 101: 295-300 [Google Scholar]
- Willekens H, Chamnongpol S, Davey M, Schraudner M, Langebartels C, van Montagu M, Inze D, van Camp W (1997) Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J 16: 4806-4816 [DOI] [PMC free article] [PubMed] [Google Scholar]