Abstract
The elongation of rice (Oryza sativa) coleoptiles is inhibited by light, and this photoinhibition was used to screen for mutants with impaired light response. In one of the isolated mutants, hebiba, coleoptile elongation was stimulated in the presence of red light, but inhibited in the dark. Light responses of endogenous indolyl-3-acetic acid and abscisic acid were identical between the wild type and the mutant. In contrast, the wild type showed a dramatic increase of jasmonate heralded by corresponding increases in the content of its precursor o-phytodienoic acid, whereas both compounds were not detectable in the mutant. The jasmonate response to wounding was also blocked in the mutant. The mutant phenotype was rescued by addition of exogenous methyl jasmonate and o-phytodienoic acid. Moreover, the expression of O. sativa 12-oxophytodienoic acid reductase, an early gene of jasmonic acid-synthesis, is induced by red light in the wild type, but not in the mutant. This evidence suggests a novel role for jasmonates in the light response of growth, and we discuss a cross-talk between jasmonate and auxin signaling. In addition, hebiba represents the first rice mutant in which the induction of the jasmonate pathway is impaired providing a valuable tool to study the role of jasmonates in Graminean development.
Plant growth and development have to be tuned with the environment. This requires an intensive interaction between signaling triggered by external stimuli and endogenous growth regulators. As a consequence of their photosynthetic lifestyle, plants have evolved a very efficient cross-talk between light signaling and growth. Changes in the content of plant hormones have been frequently invoked as effector system for the light responses. For instance, local depletion from auxins in response to blue light (Went, 1928), inhibition of the terminal steps in GA biosynthesis triggered by red light (Campell and Bonner, 1986), or a light-induced block of brassinosteroid synthesis (Li et al., 1996) are classical examples for light-triggered responses in synthesis or distribution of growth regulators. For the cross-talk of light with GAs as well as with brassinosteroids, potential targets have been recently identified in pea (Pisum sativum). For instance, light induced the down-regulation of a small G protein, Pra2, that is required for the activation of a cytochrome P450 C-2 hydroxylase involved in brassinolide synthesis (for review, see Clouse, 2001), whereas at the same time through a different pathway, light stimulates the 2β-hydroxylation of GA1 to GA8, thus removing the active GA from the elongating tissue (O'Neill et al., 2000).
The rice (Oryza sativa) coleoptile represents a useful system to study the interaction between light, hormonal signaling, and a photomorphogenetic response: Aerially grown coleoptiles respond to light by an inhibition of growth. The response is swift and exclusively based on an inhibition of cell elongation concomitant with a block of basipetal auxin transport from the perceptive site in the coleoptile tip to the major site of cell elongation in the basal region (Furuya et al., 1969). The light signal is perceived almost exclusively through the phytochrome system (Pjon and Furuya, 1967; Takano et al., 2001), whereas blue-light receptors are of negligible importance in rice coleoptiles (Neumann and Iino, 1997). The only hormonal inducer of cell growth seems to be auxin; GAs and brassinosteroids are not effective in coleoptiles (Toyomasu et al., 1994; Sekimata et al., 2001). As negative regulators of coleoptile growth, abscisic acid (ABA; Hoffmann-Benning and Kende, 1992; Gianì et al., 1998) and jasmonate (Ueda et al., 1994) have been identified. Using this model system, we ventured to isolate mutants with aberrant light responses of growth. Under saturating red light that caused a maximal repression of coleoptile elongation, it became possible to select mutants with long coleoptiles. These mutants were presumably affected either in the perception or in the processing of the phytochrome signal. The mutant hebiba was obtained from this screen and displayed during early development a light phenotype that clearly represented a phenocopy of wild-type seedlings that were grown in complete darkness. The hebiba mutant thus resembled a “red-blind” photoreceptor mutant. However, it exhibited a peculiar dark phenotype and therefore was analyzed as a potential candidate for a mutant, where the hormonal response to light was altered. We find that in this mutant, in contrast to the wild type, the jasmonate pathway can be induced neither by light nor by wounding. We describe the rescue of the mutant phenotype by exogenous jasmonate and its precursor o-phytodienoic acid (OPDA).
RESULTS
The Response to Light Is Inversed in hebiba Coleoptiles
In etiolated seedlings of the wild type, the coleoptile is long and the mesocotyl is short (Fig. 1A). Under the red-light treatment used for the screen, the coleoptiles are short and pierced by the expanding primary leaves. In contrast, in irradiated seedlings homozygous for the mutated hebiba allele, the coleoptile was long, and the primary leaves were still hidden inside reminiscent of etiolated wild-type seedlings (Fig. 1A). However, etiolated seedlings of the mutant displayed a characteristic phenotype with short, bent coleoptiles and prolonged mesocotyls.
Figure 1.
Phenotype of the hebiba mutant. A, Seedlings of wild type (WT) and hebiba (hb) that have been raised for 6 d either in complete darkness (D) or under the red light used for the screen (RL). White arrows indicate the position of the node separating coleoptile and mesocotyl, white arrowheads the coleoptile tip pierced by the primary leaves. Size bar = 10 mm. B, Appearance of adult plants of wild type and hebiba. C, Leaf base of adult wild-type and hebiba plants. Note the light green color of the leaves in the mutant.
Adult mutant plants were characterized by excessive leaf growth (Fig. 1B). The leaves were not erect but tended to hang downwards and even creep along the soil provoking the name “hebiba” (Japanese for “snake leaf”) for this mutant. The leaves, although growing vigorously, were light green in color in contrast to the dark-green leaves of the wild-type plants raised in the same phytochamber (Fig. 1C). This change in color was accompanied by a 4-fold reduction in the ratio of chlorophyll a to chlorophyll b (data not shown). The mutant flowered earlier than the wild type: After transfer to inducing short day, flowering in the mutant initiated after 25.3 ± 0.6 d (n = 20) as compared with 31.8 ± 0.8 d (n = 20) in the wild type.
Although the mutant plants flowered, the seeds were not filled and therefore were not viable. So far, through seven generations raised under a broad range of climatic conditions, not a single fertile plant homozygous for the mutant allele could be observed. As shown by Table I, reciprocal crosses with wild-type plants show that fertility can be restored by cross-pollination with wild-type pollen indicating male sterility. When seeds from heterozygotes are propagated in the field, the mutation is conveyed to about two-thirds of the following generation matching the predicted frequency under the assumption that the viability of heterozygous embryos is not impaired. The offspring of selfed heterozygotes segregates into about a quarter of seedlings where the mutant phenotype is manifest and three-quarters that are indistinguishable from the wild type. These data are matching with the predicted outcome under monogenic Mendelian inheritance with complete dominance of the wild-type allele over the mutant allele under complete male sterility of homozygous mutant plants.
Table I.
Inheritance of the hebiba mutation upon selfing or cross-pollination with pollen from the wild type
The segregation pattern is compatible with complete dominance of the HB over the hb-allele and male sterility of the homozygous hb/hb genotype. The cross-pollination of homozygous hebiba plants with wild-type pollen restores fertility. U means complete loss of seed viability. P indicates the probability that the observed frequencies correspond to the expected frequencies under the assumption that hb/hb is male sterile, but fertile, if pollen acceptor. n indicates the no. of parents used in each experiment; frequencies are given in percentages. WT, Wild type.
| Phenotype in F1 Generation
|
||||||
|---|---|---|---|---|---|---|
| n | Maternal | Paternal | WT | Mutant | c2 | P |
| % | ||||||
| 5 | HB/HB | HB/HB | 100 | 0 | 0 | >0.99 |
| 49 | hb/hb | hb/hb | U | U | — | — |
| 56 | HB/hb | HB/hb | 77 | 23 | 0.003 | >0.99 |
| 14 | hb/hb | HB/HB | 100 | 0 | 0 | >0.99 |
| 9 | HB/HB | hb/hb | 100 | 0 | 0 | >0.99 |
Red Light Triggers the Jasmonate Pathway in Rice Coleoptiles
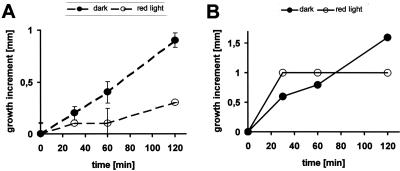
Mutant coleoptiles are short in the dark and long in the light (Fig. 1A). This means that the light response of growth is reversed in hebiba. The presence of a clear dark phenotype suggests that hebiba is not a photoreceptor mutant or a photomorphogenetic mutant in sensu strictu—a “blind” mutant should not have a phenotype in the absence of light. Thus, it is more probable that the mutant is impaired in the light-triggered effector system. Classical studies (Furuya et al., 1969) have linked the perception of light by the phytochrome system to auxin transport as the effector system responsible for the regulation of growth. We therefore asked whether light altered auxin content and whether the mutant behaved differently in this context. To obtain a complete view on a potential hormonal cross-talk, we followed the light responses of those hormones that can regulate growth in rice coleoptiles. We therefore monitored the behavior of ABA, jasmonic acid (JA), and its precursor OPDA during the first 2 h of continuous red light. In an extensive physiological study that will be published elsewhere, we had observed that the center of growth activity is situated in the basal regions of the coleoptile. To take this into account, we determined the hormonal content separately for the basal and apical halves of coleoptiles. We determined the level of hormones for a developmental stage in which the etiolated coleoptile elongates with a velocity of about 0.5 mm h-1 (Fig. 2A). Interestingly, growth was not steady, but occurred in periods of fast growth that were interspersed with periods of slow growth. Upon irradiation with red light, from 30 min after transfer to light, growth was observed to become inhibited. At this time point, around one-third of the coleoptiles was found to elongate roughly to the same extent as in the dark, whereas in two-thirds of the coleoptiles, the growth increment had already dropped below the detection limit. At 60 min, only a small fraction of coleoptiles was still in the growing phase. At later time points, no further growth could be detected; even over long time intervals (24 h), the coleoptiles maintained the same length. It thus appears that growth responds to light in an all-or-none type pattern with a lag phase (where growth rate is essentially the same as in the dark) and a rapid decline of growth. The timing of the lag phase shows variability over the population. From the cumulative frequency distribution of growth inhibition versus time (data not shown), one can estimate the average time for the lag phase to be around 25 min.
Figure 2.
A, Length increment of coleoptiles that had been precultivated for 6 d and had been either kept in ongoing darkness (black symbols) or transferred to red light (white symbols). The dashed lines give the mean values over the whole population. B, Two individual coleoptiles that had been kept in ongoing darkness (black symbols) or transferred to red light (white symbols). Note the discontinuous growth of the dark-grown coleoptiles and the complete inhibition of de-etiolated coleoptiles after a lag phase that can vary among different individuals (data not shown).
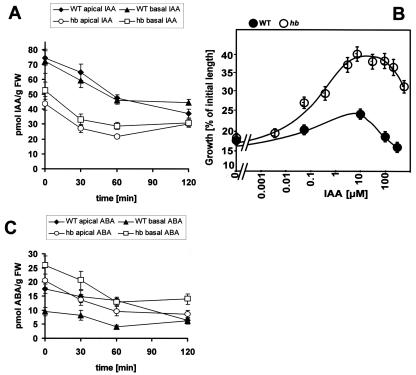
As compared with the wild type, etiolated mutant coleoptiles are characterized by a slightly reduced content (by about one-third as compared with the wild type) of indolyl-3-acetic acid (IAA). In both wild type and mutant, IAA decreases somewhat during irradiation with red light at a comparable time course (Fig. 3A). After 2 h of red light, about 40 to 50 pmol g-1 fresh weight was reached, irrespective of whether the samples originated from wild type or mutant or from the basal or the apical halves of the coleoptile. Interestingly, no significant auxin gradient could be detected between apical and basal halves either in the wild type or in the mutant, either in etiolated or in irradiated coleoptiles.
Figure 3.
A, Response of IAA to red light in coleoptiles from wild type (WT) and hebiba (hb) that had been precultivated for 6 d and then irradiated with red light for 0, 30, 60, and 120 min, respectively. The data represent averages from at least 14 independent experimental series comprising 700 to 1,300 individual coleoptiles. B, Dose response curve of elongation growth in response to exogenous IAA. Segments of decapitated coleoptiles from wild type (WT) and hebiba (hb) were predepleted from endogenous auxin for 1 h and then incubated in solutions of IAA of different concentrations for 1 h. Mean values for 29 to 62 individual segments from at least two independent experimental series are shown. C, Response of ABA to red light, determined in the same coleoptiles as those in A. FW, Fresh weight.
To understand the growth stimulation in the mutant despite a reduced content of auxin, we assayed the auxin sensitivity and responsiveness of growth in a classical segment assay (Fig. 3B). When the dose response curve of auxin-dependent coleoptile growth was determined, the amplitude of the response was found to be dramatically elevated. This was especially impressive for superoptimal concentrations of auxin when growth in the wild type was not induced, whereas it proceeded at almost the maximal velocity in the mutant. In contrast, the threshold and the maximum of the curve were reached at the same concentrations of auxin as in the wild type. Thus, there are no indications for an increase of auxin sensitivity in the mutant, whereas the responsiveness of the auxin response is amplified.
When the content of ABA was measured (Fig. 3C), the apical halves of wild type and mutant were found to be more or less identical (around 20 pmol g-1 fresh weight). During irradiation, the ABA content decreased to less than one-half of the original value, but this decrease was almost identical between wild type and mutant. The situation was different when the basal halves (the center of growth activity) were analyzed: The ABA content was found to be 2.5 times higher in the basal regions of etiolated mutant coleoptiles as compared with the corresponding wild-type sample. Again, ABA content decreased in response to red light, which was more pronounced in the mutant. However, the relative difference (with more than 2-fold increased ABA in the basal region of the mutant) was maintained.
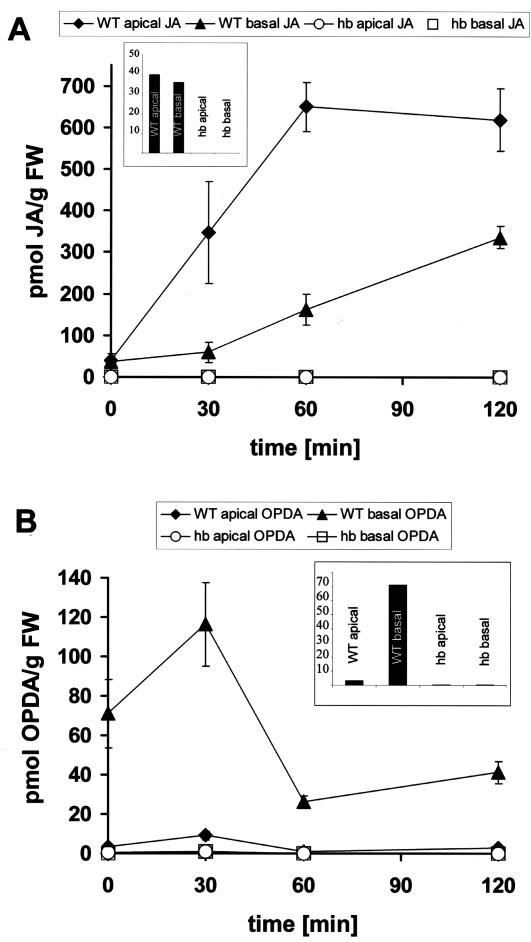
The decreased content of auxin and, more strikingly, the increased content of ABA in the growth zone correlates well with the repressed growth activity of mutant coleoptiles that are kept in the dark. The light-induced decrease of auxin qualitatively correlates with the reduced growth in the wild type (although a reduction by 30% is certainly not sufficient to account for the dramatic inhibition of growth). However, it can obviously not be responsible for the stimulation of growth in the mutant. On the other hand, the pronounced decrease of ABA (an inhibitor of coleoptile growth) in the basal zone of the mutant would be consistent with the stimulation of growth by light. In contrast, the decrease of ABA observed in the wild type cannot account for the observed inhibition of growth in response to red light. Whereas the light responses of auxin and ABA were found to be of relatively minor amplitude, the analysis of JA and its precursor OPDA revealed dramatic responses in the wild type that were completely absent in the mutant. In the wild type, JA was substantially and rapidly stimulated by a factor of 10 to 20 (Fig. 4A). The increase was faster and more pronounced in the apical region of wild-type coleoptiles and was somewhat slower in the basal region. Interestingly, OPDA, a precursor of JA, was found to increase transiently before the strong increase in JA content (Fig. 4B). The peak of OPDA content was observed at 30 min, i.e. concomitant with the inhibition of growth. In the basal region, where growth activity is centered, the OPDA peak was more conspicuous and disappeared concomitantly with the increase of JA content. In the apical region, the OPDA peak became only weakly manifest and the temporal sequence between (transient) OPDA increase and (stable) JA increase was somewhat smaller than in the basal region.
Figure 4.
Response of JA (A) and OPDA (B) to red light in coleoptiles from wild type (WT) and hebiba (hb). For details, refer to the legend of Figure 3. The insets show basal levels of both, JA (A) and OPDA (B) present in etiolated coleoptiles. The data represent averages from at least five independent experimental series comprising 500 to 600 individual coleoptiles for A and at least 17 independent experimental series comprising 850 to 1,300 individual coleoptiles for B. FW, Fresh weight.
Irrespective of coleoptile region or time of irradiation, neither OPDA nor JA could be detected in coleoptiles of the hebiba mutant (Fig. 4, A and B). Interestingly, already before irradiation, certain basal levels of OPDA and jasmonate were clearly present in the wild type, whereas they could not be detected in the mutant (Figs. 4, A and B, insets).
These findings can be summarized in three statements: (a) Red light triggers the jasmonate pathway—the amplitude of this response is in the range of about 1 order of magnitude and thus conspicuously exceeds the fluctuations in the content of other hormones, such as IAA or ABA. (b) The light response of the jasmonate pathway is completely absent in the hebiba mutant. In contrast, the other hormones tested exhibit a light response that is fairly similar to the wild type. (c) The hebiba mutant not only lacks a response of the jasmonate pathway, it does not contain either JA or its precursor, OPDA.
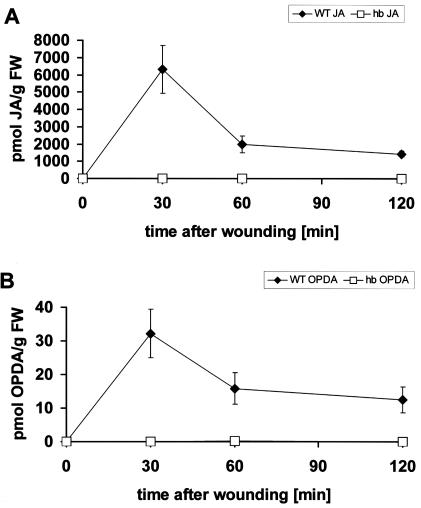
Is It Possible to Trigger the JA Pathway by Wounding in hebiba?
The light response of the jasmonate pathway could be due to a light-specific defect in the signaling toward jasmonate synthesis (i.e. very upstream), or it could be caused by impaired expression or activity of enzymes involved in JA synthesis (i.e. downstream). To distinguish between these possibilities, we tried to trigger the JA pathway by a standardized wounding treatment. In the wild type, this treatment led to a rapid, extreme induction of JA (to more than 6,000 pmol g-1 fresh weight; Fig. 5A) and a 30-fold increase of OPDA (Fig. 5B) in the wild type. In hebiba coleoptiles, neither OPDA nor JA could be detected. This indicates that the mutation affects the general response of the JA pathway and is therefore not specific for light signaling.
Figure 5.
Response of JA (A) and OPDA (B) to wounding in coleoptiles from wild type (WT) and hebiba (hb). Data from 300 to 450 (A) and 300 to 600 (B) individual seedlings from at least six independent experimental series, respectively.
The hebiba Phenotype Can Be Rescued by Exogenous Methyl Jasmonate
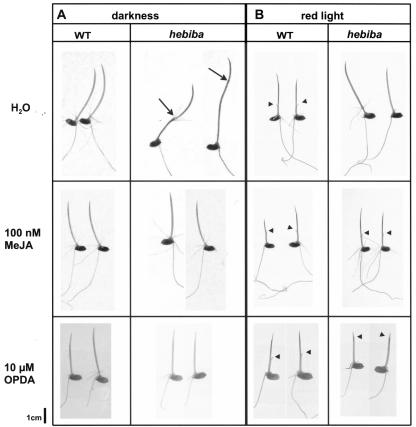
The hebiba mutant cannot trigger the jasmonate pathway in response to light or wounding (Figs. 4 and 5). It exhibits an inverse growth response to light (Fig. 1A), and male sterility (Table I). To understand whether this phenotype is related to the blocked jasmonate induction, we tested whether the mutant can be rescued by exogenous methyl jasmonate. Coleoptiles of wild type and mutant were cultivated on various concentrations of methyl jasmonate. In one experimental set, the coleoptiles were kept in complete darkness (Fig. 6A). Alternatively, they were irradiated with red light between d 4 and 5 after sowing and then returned to the dark (Fig. 6B).
Figure 6.
Rescue of the hebiba phenotype by exogenous application of methyl jasmonate (MeJA) and OPDA. Rice seedlings were grown for 6 d in darkness (A) or darkness interrupted by 24 h of red-light irradiation from d 4 to 5 (B) in water or solutions of 100 nm MeJA or 10 μm OPDA. Black arrows indicate the position of the node separating coleoptile and mesocotyl, black arrowheads the coleoptile tip pierced by the primary leaves. Note that it was not possible to reach the complete inhibition of growth that was observed in the wild type for red-light-irradiated seedlings grown in 10 μm OPDA.
In the dark and in the absence of methyl jasmonate, the mutant showed the characteristic inhibition of coleoptile growth that was accompanied by hyper-trophic elongation of the mesocotyl (Fig. 6A). Upon increasing concentrations of methyl jasmonate, the mutant phenotype progressively disappeared (data not shown). At 100 nm of methyl jasmonate, mutant and wild-type seedlings were found to be of identical morphology with a fully elongated coleoptile and a stunted mesocotyl.
After irradiation and in the absence of methyl jasmonate, the mutant phenotype was most conspicuous with fully elongated coleoptiles of about 30 mm length that were still covering the primary leaves (Fig. 6B), whereas in the wild type, the coleoptiles were short (less than 10 mm) and pierced by the primary leaves. The mutant phenotype was more persistent to lower concentrations of methyl jasmonate as compared with growth in complete darkness. However, at 100 nm of methyl jasmonate, mutant and wild type had become indistinguishable with short, stunted coleoptiles that had been opened by the primary leaves.
To test, whether the phenotype could be rescued by precursors of jasmonate, seedlings of wild type and mutant were fed with exogenous OPDA and linolenic acid. We were able to obtain a complete rescue of the mutant phenotype in the dark by 10 μm of OPDA (Fig. 6A). In the case of red light, the mutant phenotype disappeared in a dose-dependent manner: With an increasing concentration of OPDA, coleoptiles shortened progressively, and the primary leaves penetrated the coleoptile, but it was not possible to reach the complete inhibition of growth that was observed in the wild type (Fig. 6B).
In our experimental setup, it was not possible to rescue the phenotype by addition of exogenous linolenic acid. Although we observed a slight decrease of coleoptile length for 100 μm of linolenic acid in irradiated mutants, but not in the wild type, we failed to approximate the wild-type phenotype by raising the concentration of linolenic acid (we tested 200 μm, 500 μm, and 1 mm) due to a general inhibition of growth in both wild type and mutant.
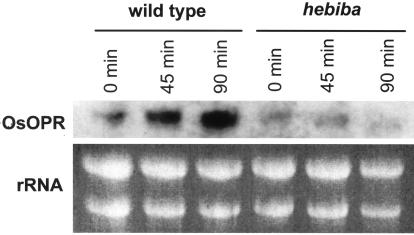
To test the induction of the jasmonate pathway by red light in the hebiba mutant on the level of gene expression, we investigated the red-light response of O. sativa 12-oxophytodienoic acid reductase (OsOPR), an early gene of jasmonate synthesis (Sobajima et al., 2003). As shown in Figure 7, the OsOPR transcripts were induced in the wild type already after 45 min, whereas they remained suppressed in the mutant. The induction was even stronger after 90 min in the wild type, i.e. the time when also the level of JA had reached a maximum (Fig. 4A).
Figure 7.
Expression of OsOPR transcripts in response to red light. Seedlings of wild type and mutant were irradiated for the indicated time intervals with red light, and subsequently the RNA was isolated and examined by northern blotting for the abundance of OsOPR mRNA. The transcripts were induced in the wild type upon irradiation, whereas they were not induced in the mutant. Note the low level of OsOPR expression in hebiba even before irradiation.
To test, whether the male sterility of the mutant could be rescued by exogenous jasmonate, flowering was triggered by transfer of adult plants to inductive short days, and 5 μm methyl jasmonate was administered through the roots at different days after the transfer. Negative controls that were treated by water did not develop a single viable seed. The same was observed when methyl jasmonate was administered later than d 7 after the transfer to inductive short days. In contrast, fertility could be restored when methyl jasmonate was added from the day of transfer to inductive short days.
Summarizing, we observe that the mutant fails to induce jasmonate synthesis, irrespective of the inducing stimulus (light or wounding). The light response of mutant coleoptiles can be rescued with respect to normal photomorphogenesis by exogenous methyl jasmonate and partially by OPDA in a dose-dependent manner. Moreover, the fertility of adult mutant plants can be restored by exogenous methyl jasmonate.
DISCUSSION
The rice mutant hebiba was isolated from a screen for plants defective in photomorphogenesis as a putative “red-blind” candidate. This assumption was made because in this mutant coleoptile, elongation was not inhibited by irradiation with red light. However, growth of mutant coleoptiles was reduced in darkness. Thus, the phenotype cannot be explained in terms of a photoreceptor mutation. Compared with the wild type, the mutant exhibits a mirrored light response (blocked elongation in the dark, stimulated elongation in the light). This situation allows investigating the role of hormonal changes for the growth response to light. We therefore analyzed the response of auxin, the major player in the control of coleoptile elongation, and ABA, an important negative regulator of coleoptile elongation (Hoffmann-Benning and Kende, 1992; Gianì et al., 1998). We measured the content of these hormones separately in the basal and apical halves to see whether transport or gradients of these hormones exist and are disturbed in hebiba. From the observed inversion of growth regulation by light, we expected to find high levels of auxin (accompanied by low levels of ABA) in the mutant under conditions when it is low in the wild type and vice versa.
To our surprise, neither the observed changes in the content of IAA nor those of ABA were consistent with the growth phenotype of hebiba. The level of IAA in the mutant was reduced in etiolated seedlings, but it did not increase after red-light irradiation as would be expected from the stimulation of growth. On the contrary, IAA decreased even further, which was quite similar to the response in the wild type (Fig. 3A). Intriguingly, the amount of ABA was regulated down after red-light irradiation in both the apical and basal regions in hebiba, but this response was observed in the wild type as well (Fig. 3C). Thus the light response of IAA and ABA cannot account for the stimulation of growth that is observed in the mutant.
Because auxin-induced growth of coleoptile segments can be inhibited by exogenous jasmonate (Ueda et al., 1994), we asked for the light response of jasmonate levels. To our surprise, we could detect neither JA nor its precursor OPDA in hebiba, whereas in the wild type, we found a strong induction of JA (and OPDA) after red-light irradiation (Fig. 4, A and B). The jasmonate response is substantial (more than 1 order of magnitude) and represents a qualitative difference between wild type and mutant. Moreover, a normal light response could be restored in mutant coleoptiles by the exogenous application of jasmonate and OPDA (Fig. 6). Therefore, the hebiba mutation must affect a gene upstream of OPDA in the JA biosynthesis. To test whether the mutation affects the biosynthesis of JA downstream of linolenic acid, we tested whether the phenotype could be rescued by the addition of linolenic acid. However, due to experimental difficulties (linolenic acid had to be present for 6 d at 25°C and was probably disactivated by oxidation; higher concentrations caused a general inhibition of growth hiding any potential difference between wild type and mutant), it was not possible to get a clear result. Therefore we cannot rule out that the mutation acts upstream of linolenic acid or even in a step regulating the JA biosynthesis as an entity.
Our result that the biosynthesis of jasmonate is triggered by red light in wild-type rice was confirmed by the finding that the expression of OsOPR mRNA was induced in the wild type upon irradiation with red light, whereas it was not induced in hebiba (Fig. 7). Moreover, OsOPR mRNA is expressed on a low level in the mutant. Nevertheless, the phenotype can be rescued by OPDA, suggesting that the gene product, although expressed at a low level, is functional and can convert its substrate, given that it is provided by the enzymes acting upstream. This supports the conclusion from the complementation assays with jasmonate and OPDA that the HEBIBA gene must be located either upstream of OPR in the biosynthetic pathway or in a component regulating the entire biosynthesis of jasmonate. We do not think that OPR itself is impaired in its activity—it is expressed (although at a low level). The low expression of OPR in the mutant and the impaired induction by red light speaks preferentially for a general defect in the regulation of the pathway rather than for a mutation that inactivates one of the synthesizing enzymes. However, it cannot be excluded that the low expression of OPR originates from feedback mechanisms of the product to the synthesizing machinery that is secondarily impaired in consequence of the very low level of jasmonate in this mutant. To approach this problem further avoiding artifacts caused by potential differences of penetration in feeding assays, we launched a broad-scale analysis, where the fatty-acid profiles are compared between wild type and mutant.
The male sterility of hebiba, a typical symptom of deficient jasmonate biosynthesis or signaling (Xie et al., 1998; Ishiguro et al., 2001), could be rescued by the addition of 5 μm of methyl jasmonate to flowering plants. Interestingly, another rice mutant called coleoptile photomorphogenesis 1, which is impaired in red-light-mediated inhibition of coleoptile growth, is also affected in anthesis (Biswas et al., 2003).
The failure of hebiba mutants to trigger the jasmonate pathway in response to wounding (Fig. 5) along with the finding that even a basic level of jasmonate and OPDA can be observed in the wild type before irradiation (Fig. 4, A and B, insets) suggest that the mutant is not only impaired in the pathway conveying the light signal to jasmonate biosynthesis but possibly in the biosynthetic pathway itself (or in a regulative event relatively close to biosynthesis). We are presently investigating whether enzymes of the jasmonate pathway are impaired in the mutant before irradiation.
Thus through the hebiba mutant, we uncovered a novel role of jasmonate in photomorphogenesis complementing the classical functions of this hormonal pathway such as wounding (for review, see León et al., 2001), pathogen response (Xie et al., 1998; for review, see Reymond and Farmer, 1998; Liechti and Farmer, 2002), and plant fertility (Xie et al., 1998; Ishiguro et al., 2001). Indications for a cross-talk between light and pathogen signaling were already derived from the phenotype of the Arabidopsis mutant psi2 that was found to be hypersensitive to red light and at the same time shows activation of a pathogen response without actually being challenged by a pathogen (Genoud et al., 1998; Genoud and Métraux, 1999). Moreover, the recent report that O. sativa allene oxide synthase, a key enzyme of the biosynthesis of JA, is induced by irradiation with white light (Agrawal et al., 2002) supports our observation of a light-induced induction of jasmonate synthesis.
How could the jasmonate pathway feed into the regulation of growth and cause a down-regulation of cell elongation in response to red light? In a classical segment elongation assay, Ueda et al. (1994) were able to demonstrate an inhibitory effect of JA on the auxin-induced growth in oat (Avena sativa) coleoptiles. Intriguingly, this inhibition of auxin-induced growth seems to be specific for monocotyledonous plants (for review, see Miyamoto et al., 1997). In studies performed with Graminean coleoptiles, the effect of jasmonate was attributed to inhibited synthesis of cell wall polysaccharides (Ueda et al., 1994, 1995) and to a shift in the intracellular pH (Irving et al., 1999). Both processes are well known to be promoted by auxin (Brummer et al., 1985; Felle et al., 1986; Kutschera and Schopfer, 1986; Kutschera and Briggs, 1987; Gehring et al., 1990; Frohnmeyer et al., 1998). A third target for jasmonate might be the modulation of potassium channels (Evans, 2003) that are essential for auxin-induced growth (Philippar et al., 1999). In summary, the growth inhibition by jasmonate is related to a block of typical effectors for auxin-induced cell elongation.
This suggests a scenario, where red light through the jasmonate pathway would down-regulate the auxin responsiveness of growth. In fact, when a dose response curve is measured for etiolated segments of wild type and hebiba (Fig. 3B), it reveals a dramatically elevated amplitude, whereas threshold and peak of the curve are found at the same concentrations as in the wild type. Thus, the complete absence of jasmonate in the mutant (in contrast to a certain basic level in the wild type; see Fig. 4, A and B, insets) is correlated with an increased responsiveness of auxin-induced growth (at a basically identical auxin sensitivity). The observation that before irradiation, the level of IAA was reduced in hebiba, whereas at the same time, the amount of ABA in the basal one-half of mutant coleoptiles was increased (Fig. 3, A and C) indicates that the jasmonate constitutively produced in the wild type acts in a negative feedback loop on the synthesis of ABA and probably indirectly slightly stimulates auxin synthesis.
Is there a mechanism that could explain a reduced responsiveness to auxin after activation of the jasmonate pathway? Recent findings about ubiquitin-related processes in phytohormonal signal transduction (for review, see Frugis and Chua, 2002) suggest that both signaling pathways are working via the 26S proteasome: Auxin responses are mediated by interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIR1 (Schwechheimer et al., 2001), and according to latest results, a similar system acts in jasmonate signaling with the F-box protein COI1 acting as the corresponding E3-type ubiquitin ligase (Devoto et al., 2002). Both ubiquitin-ligase-related pathways interact through a process called neddylation that activates several E3 ubiquitin ligases of the SCF-type. AXR1, a subunit of the complex responsible for this neddylation, is required for multiple E3-mediated processes (Schwechheimer et al., 2002), and the axr1 mutant in Arabidopsis, which originally was isolated as an auxin response mutant, has been shown to be defective in jasmonate response as well (Tyryaki and Staswick, 2002). Both auxin and jasmonate signaling involve protein degradation through the 26S proteasome and thus may interfere in this mechanism in a way that has to be elucidated in future research.
Using the hebiba mutant as a tool, we want to study the cross-talk and antagonism of auxin and jasmonate signaling in other auxin-mediated processes as well and to uncover the mechanism responsible for this antagonism. We recently launched the identification of the HEBIBA gene to get further insight into the cross-talk between auxin and jasmonate. At the same time, our mutant is a powerful tool to analyze signaling events triggered by jasmonate in rice such as pathogen attack.
MATERIALS AND METHODS
Plant Material
The rice (Oryza sativa) hebiba mutant was obtained in a japonica background (rice cv Nihonmasari) and has been propagated independently in two sites in northern Italy (Almo Semi, Mortara) and Japan (Hokuriku Experimental Station, Niigata). The mutant is male sterile, such that it has to be maintained through the heterozygotes. For each plant, small seed aliquots are checked separately to define the genotype of the population. An isogenic sister line homozygous for the wild-type allele was used as reference throughout the study.
Light Sources and Plant Cultivation
The screen was performed under 52 μmol m-2 s-1 of red light (660 nm) originating from fluorescent tubes (FL-20S, Re66, Toshiba, Tokyo) and isolated by a color filter (3 mm thickness; acrylight K5-102, Mitsubishi Rayon, Tokyo) combined with a dispersion filter (3 mm thickness; acrylight K5-001E, Mitsubishi Rayon) installed in a phytotron (Koitotron, Koito, Tokyo). The light sources for red light (660 nm) and green safelight (550 nm) used in the photobiological studies are described in detail by Mohr et al. (1964). All light measurements were performed using a photoradiometer (J16, Tektronix, Beaverton, OR). The seedlings were raised at 25°C in photobiological darkness (using black boxes, black cloth, and isolated dark chambers) on floating meshes as described by Nick et al. (1994). Under these conditions, germination was higher than 97%, and seedling length among the population varied by less than 5%.
Screen for Red-Blind Rice Mutants
Starting material for the screen was a collection of independent mutant lines in a japonica background (rice cv Nihonmasari) that were screened for impaired growth inhibition under continuous red light. From each mutant line, aliquots of 20 seeds were cultivated for 3.5 d at 25°C according to Nick et al. (1994) and then transferred to red light for 3.5 d more. Under these conditions, the coleoptiles of the wild type were 7.2 ± 0.02 mm in length (Toyomasu et al., 1994). Seedlings with long (20 mm or more) coleoptiles were considered as potential mutants, and the respective sample was raised to maturity and subjected to a second round of the screen. This procedure was repeated to exclude false-positive lines. From 6,593 independent mutant lines, 69 potential red-blind mutants were recovered during the first screen. Among those, 11 lines could be confirmed in the second and third screen. Among these 11 lines, the phenotype of the hebiba mutant was the most conspicuous. In three of the lines, among these the hebiba mutant, putative homozygotes flowered but did not produce seeds, such that these lines had to be maintained through the heterozygotes. After the screens, plants were transferred on clay-containing soil and raised to maturity under 8,500 lx m-2 of day light complemented by artificial daylight under a long-day regime (16 h light, 8 h dark). Once a week, they were treated with Yoshida micro- and macroelements (Yoshida, 1976) to ensure optimal nutrition. After 2 months, flowering was induced by 25 consecutive short days (8 h light, 16 h dark). To rescue the male sterility of the mutant by exogenous jasmonate, flowering was triggered by transfer of adult plants to inductive short days, and 5 μm methyl jasmonate (Sigma-Aldrich, Neu-Ulm, Germany) was administered through the roots at different days after the transfer.
Treatment of Seedlings
In the time-course experiment for red-light irradiation, 6-d-old seedlings were transferred into a red-light field (3.4 mmol m-2 s-1) at 25°C for 0, 30, 60, or 120 min, respectively. In the wounding experiment, 6-d-old seedlings were wounded by squeezing them every 0.5 mm along the axis of the coleoptile with a ribbed forceps. Before harvest, they were kept in darkness for 30, 60, and 120 min, respectively. After the respective treatment, the coleoptiles were harvested immediately in green safelight (550 nm).
For the cultivation of seedlings in solutions of different concentrations of methyl jasmonate (Sigma-Aldrich), OPDA (synthesized by Axel Müller according to Laudert et al. [1997]), or linolenic acid (Sigma-Aldrich), five seeds were fixed with B-400 Secure2 adhesive (Factor II Inc., Lakeside, AZ) in a row onto a glass slide. The glass slides were placed in a conventional staining tray vertically such that the row was horizontal, and the tray was filled with the methyl jasmonate solutions until the seed row and the seeds incubated under the same conditions as described above.
For the auxin dose response curve, decapitated coleoptile segments of 5.5 mm were incubated in distilled water for 1 h to deplete them from internal auxin. After the depletion, they were incubated in IAA solutions of different concentrations (wild type: 0 nm, 50 nm, 5 μm, 10 μm, 100 μm, and 200 μm; hebiba: 0, 5 nm, 50 nm, 500 nm, 5 μm, 10 μm, 20 μm, 100 μm, and 500 μm) for 1 h. The growth increment was plotted against the concentration of IAA. The whole procedure was performed in green safelight; during depletion and incubation, the segments were rotated on a topover-shaker.
Growth Measurements
To determine the time course of growth inhibition by light, the growth rate of intact coleoptiles that had been precultivated in complete darkness for 6 d was determined either in continuing darkness or after transfer to red light. To measure the growth rate of dark-grown coleoptiles, they were monitored at different time intervals up to 4 h with an infrared-sensitive video camera (Grundig Electronic, Grundig, Nürnberg, Germany) in front of an infrared light source. By means of a time-lapse control, the infrared source was switched on only during the 1-min interval, when the image was recorded. A long-term experiment showed that the growth of these infrared-irradiated coleoptiles was identical to that of coleoptiles that were cultivated in continuous darkness. In a parallel experiment, dark-grown coleoptiles were transferred to the red light used for the hormonal measurement, and the length increment was determined optically at 0, 30, 60, and 120 min after the transfer.
Extraction of Plant Hormones
For each sample, about 150 to 300 mg of plant material was analyzed. The material was transferred to a glass tube and immediately covered with 1 mL of methanol containing 30 pmol of H2-labeled IAA, ABA, and JA, and 10 pmol of H2-labeled OPDA as standard. After the addition of 1 mL of diethylether, the sample was incubated for 30 min at 50°C in a water bath. Afterward, the solvents were completely evaporated under vacuum.
Quantification of Plant Hormones
Levels of the different hormones were determined as described by Müller et al. (2002).
Northern-Blot Analysis
For the time course of OsOPR induction in response to red-light irradiation, etiolated seedlings grown for 6 d were transferred into the red-light field used for the phytohormonal analysis for the indicated time intervals. Coleoptiles were directly transferred to liquid nitrogen during harvest, and total RNA was extracted with the RNeasy kit (Qiagen, Hilden, Germany) according to the protocol of the producer. Ten micrograms of total RNA of each sample was loaded on a 1.2% (w/v) agarose gel. After electrophoresis, RNA was blotted onto a positively charged nylon membrane following standard protocols (Sambrook et al., 1989) and hybridized with 32P-labeled OsOPR1 cDNA kindly provided by Hisakazu Yamane (Sobajima et al., 2003).
Acknowledgments
We thank Prof. Hisakazu Yamane (University of Tokyo) for kindly providing OsOPR cDNA and Dr. Osamu Yatou (Hokuriku National Agricultural Experiment Station, Niigata, Japan) for providing seed material.
This work was partially supported by the Forschungsschwerpunkt “Molekulare Analyse der Phytohormonwirkung” of the Deutsch Forschungsgemeinschaft (to A.M., E.W., and P.N.), by Hitachi Advanced Research Laboratory (grant no. B2023), by the Japanese Program for Promotion of Basic Research Activities for Innovative Biosciences (to M.F.), and by the Volkswagen-Foundation Nachwuchsgruppen-Programme (to P.N. and M.R.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.027490.
References
- Agrawal GK, Rakwal R, Jwa N-S, Han K-S, Agrawal VP (2002) Molecular cloning and mRNA expression analysis of the first rice jasmonate biosynthetic pathway gene allene oxide synthase. Plant Physiol Biochem 40: 771-782 [Google Scholar]
- Biswas KK, Neumann R, Haga K, Yatoh O, Iino M (2003) Photomorphogenesis of rice seedlings: a mutant impaired in phytochrome-mediated inhibition of coleoptile growth. Plant Cell Physiol 44: 242-254 [DOI] [PubMed] [Google Scholar]
- Brummer B, Bertl A, Potrykus I, Felle H, Parish RW (1985) Evidence that fusicoccon and indole-3-acetic acid induce cytosolic acidification of Zea mays cells. FEBS Lett 189: 109-114 [Google Scholar]
- Campell BR, Bonner BA (1986) Evidence for phytochrome regulation of gibberellin A20 3β-hydroxylation in shoots of dwarf (lele) Pisum sativum L. Plant Physiol 82: 909-915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD (2001) Integration of light and brassinosteroid signals in etiolated seedling growth. Trends Plant Sci 6: 443-445 [DOI] [PubMed] [Google Scholar]
- Devoto A, Nieto-Rostro M, Xie D, Ellis C, Harmston R, Patrick E, Davies J, Sherratt L, Coleman M, Turner JG (2002) COI1 links jasmonate signalling and fertility to the SCF ubiquitin-ligase complex in Arabidopsis. Plant J 32: 457-466 [DOI] [PubMed] [Google Scholar]
- Evans NH (2003) Modulation of guard cell plasma membrane potassium currents by methyl jasmonate. Plant Physiol 131: 8-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H, Brummer B, Bertl A, Parish RW (1986) Indole-3-acetic acid and fusicoccin cause cytosolic acidification of corn coleoptile cells. Proc Natl Acad Sci USA 83: 8992-8995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohnmeyer H, Grabov A, Blatt MR (1998) A role for the vacuole in auxin-mediated control of cytosolic pH by Vicia mesophyll and guard cells. Plant J 13: 109-116 [Google Scholar]
- Frugis G, Chua N-M (2002) Ubiquitin-mediated proteolysis in plant hormone signal transduction. Trends Cell Biol 12: 308-311 [DOI] [PubMed] [Google Scholar]
- Furuya M, Pjon CJ, Fujii T, Ito M (1969) Phytochrome action in Oryza sativa L.: III. The separation of photoperceptive site and growing zone in coleoptiles, and auxin transport as effector system. Dev Growth Differ 11: 62-76 [DOI] [PubMed] [Google Scholar]
- Gehring CA, Irving HR, Parish RW (1990) Effects of auxin and abscisic acid on cytosolic calcium and pH in plat cells. Proc Natl Acad Sci USA 87: 9645-9649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genoud T, Métraux J-P (1999) Crosstalk in plant cell signalling: structure and function of the genetic network. Trends Plant Sci 4: 503-507 [DOI] [PubMed] [Google Scholar]
- Genoud T, Millar AJ, Nishizawa N, Kay SA, Schäfer E, Nagatani A, Chua N-H (1998) An Arabidopsis mutant hypersensitive to red and far-red light signals. Plant Cell 10: 889-904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianì S, Qin X, Faono F, Breviario D (1998) In rice, oryzalin and abscisic acid differentially affect tubulin mRNA and protein level. Planta 205: 334-341 [DOI] [PubMed] [Google Scholar]
- Hoffmann-Benning S, Kende H (1992) On the role of abscisic acid and gibberellin in the regulation of growth in rice. Plant Physiol 99: 1156-1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving HR, Dyson G, McConchie R, Parish RW, Gehring CA (1999) Effects of exogenously applied jasmonates on growth and intracellular pH in maize coleoptile segments. J Plant Growth Regul 18: 93-100 [DOI] [PubMed] [Google Scholar]
- Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K (2001) The DEFEC TIVE IN ANTHER DEHISCENCE 1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13: 2191-2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera U, Briggs WR (1987) Rapid auxin-induced stimulation of cell wall synthesis in pea internodes. Proc Natl Acad Sci USA 84: 2747-2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera U, Schopfer P (1986) Effect of auxin and abscisic acid on cell wall extensibility in maize coleoptiles. Planta 167: 527-535 [DOI] [PubMed] [Google Scholar]
- Laudert D, Hennig P, Stelmach BA, Müller A, Andert L, Weiler EW (1997) Analysis of 12-oxo-phytodienoic acid enantiomers in biological samples by capillary gas chromatography-mass spectrometry using cyclodextrin stationary phases. Anal Biochem 246: 211-217 [DOI] [PubMed] [Google Scholar]
- León J, Rojo E, Sánchez-Serrano JJ (2001) Wound signalling in plants. J Exp Bot 52: 1-9 [DOI] [PubMed] [Google Scholar]
- Li J, Nagpal P, Vitart V, McMorris TC, Chory J (1996) A role for brassinosteroids in light-dependent development of Arabidopsis. Science 272: 398-401 [DOI] [PubMed] [Google Scholar]
- Liechti R, Farmer EE (2002) The jasmonate pathway. Science 296: 1649-1650 [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Oka M, Ueda J (1997) Update on the possible mode of action of jasmonates: focus on the metabolism of cell wall polysaccharides in relation to growth and development. Physiol Plant 100: 631-638 [Google Scholar]
- Mohr H, Meyer U, Hartmann K (1964) Die Beeinflussung der Farnsporenkeimung (Osmunda cinnamomea [L.] und O. claytoniana [L.]) über das Phytochromsystem und die Photosynthese. Planta 60: 483-496 [Google Scholar]
- Müller A, Düchting P, Weiler EW (2002) A multiplex GC-MS/MS technique for the sensitive and quantitative single-run analysis of acidic phytohormones and related compounds, and its application to Arabidopsis thaliana. Planta 216: 44-56 [DOI] [PubMed] [Google Scholar]
- Neumann R, Iino M (1997) Phototropism of rice (Oryza sativa L.) coleoptiles: fluence-response relationships, kinetics and photogravitropic equilibrium. Planta 201: 288-292 [DOI] [PubMed] [Google Scholar]
- Nick P, Yatou O, Furuya M, Lambert A-M (1994) Auxin-dependent micro-tubule responses and seedling development are affected in a rice mutant resistant to EPC. Plant J 6: 651-663 [Google Scholar]
- O'Neill DP, Ross JJ, Reid JB (2000) Changes in gibberellin A1 levels and response during de-etiolation of pea seedlings. Plant Physiol 124: 805-812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippar K, Fuchs I, Lüthen H, Hoth S, Bauer CS, Haga K, Thiel G, Ljung K, Sandberg G, Böttger M et al. (1999) Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc Natl Acad Sci USA 96: 12186-12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pjon CJ, Furuya M (1967) Phytochrome action in Oryza sativa L.: I. Growth responses of etiolated coleoptiles to red, far-red and blue light. Plant Cell Physiol 8: 709-718 [Google Scholar]
- Reymond P, Farmer EE (1998) Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol 1: 404-411 [DOI] [PubMed] [Google Scholar]
- Sambrook L, Fitsch E, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Schwechheimer C, Serino G, Callis J, Crosby WL, Lyapina S, Deshaies RJ, Gray WM, Estelle M, Deng XW (2001) Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIR1 in mediating auxin response. Science 292: 1379-1382 [DOI] [PubMed] [Google Scholar]
- Schwechheimer C, Serino G, Deng W-W (2002) Multiple ubiquitin ligase-mediated processes require COP9 signalosome and AXR1 function. Plant Cell 14: 2553-2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimata K, Kimura T, Kaneko I, Nakano T, Yoneyama K, Takeuchi Y, Yoshida S, Asami T (2001) A specific brassinosteroid biosynthesis inhibitor, Brz2001: evaluation of its effects on Arabidopsis, cress, tobacco, and rice. Planta 213: 716-721 [DOI] [PubMed] [Google Scholar]
- Sobajima H, Takeda M, Sugimori M, Kobashi N, Kiribuchi K, Cho E-M, Akimoto C, Yamaguchi T, Minami E, Shibuya N et al. (2003) Cloning and characterization of a jasmonic acid-responsive gene encoding 12-oxophytodienoic acid reductase in suspension-cultured rice cells. Planta 216: 692-698 [DOI] [PubMed] [Google Scholar]
- Takano M, Kanegae H, Shinomura T, Miyao A, Hirochika H, Furuya M (2001) Isolation and characterization of rice phytochrome A mutants. Plant Cell 13: 521-534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyomasu T, Yamane H, Murofushi N, Nick P (1994) Phytochrome inhibits the effectiveness of gibberellins to induce cell elongation in rice. Planta 194: 256-263 [Google Scholar]
- Tyryaki I, Staswick PE (2002) An Arabidopsis mutant defective in jasmonate response is allelic to the auxin-signaling mutant axr1. Plant Physiol 130: 887-894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda J, Miyamoto K, Aoki M (1994) Jasmonic acid inhibits the IAA-induced elongation of oat coleoptile segments: a possible mechanism involving the metabolism of cell wall polysaccharides. Plant Cell Physiol 35: 1065-1070 [Google Scholar]
- Ueda J, Miyamoto K, Kamisaka S (1995) Inhibition of the synthesis of cell wall polysaccharides in oat coleoptile segments by jasmonic acid: relevance to its growth inhibition. J Plant Growth Regul 14: 69-76 [Google Scholar]
- Went FW (1928) Wuchsstoff und Wachstum. Rec Trav Bot Neerl 25: 1-116 [Google Scholar]
- Yoshida S (1976) Routine procedures for growing rice plants in culture solution. In S Yoshida, DA Forno, JH Cock, KA Gomez, eds, Laboratory Manual for Physiological Studies of Rice. International Rice Research Institute (IRRI), Los Baños, Laguna, Philippines, pp 61-66
- Xie D-X, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280: 1091-1094 [DOI] [PubMed] [Google Scholar]