Abstract
The title Schiff base compound, C16H16BrNO3, adopts an E configuration with respect to the C=N bond. The dihedral angle between the two aromatic rings is 64.02 (6)°.
Related literature
For applications of Schiff-base compounds, see: Yildiz et al. (2008 ▶); Hijji et al. (2009 ▶); Karakas et al. (2008 ▶); Hadjoudis et al. (2004 ▶). For related structures, see: Khalaji et al. (2007 ▶, 2008 ▶, 2009 ▶, 2010 ▶); Khalaji & Harrison (2008 ▶); Khalaji & Simpson (2009 ▶). For standard bond lengths, see: Allen et al. (1987 ▶).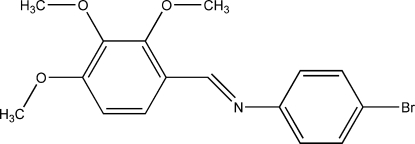
Experimental
Crystal data
C16H16BrNO3
M r = 350.2
Triclinic,
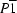
a = 7.9103 (3) Å
b = 9.9902 (4) Å
c = 10.7821 (3) Å
α = 93.068 (3)°
β = 108.568 (3)°
γ = 109.679 (3)°
V = 748.10 (5) Å3
Z = 2
Cu Kα radiation
μ = 3.83 mm−1
T = 120 K
0.49 × 0.38 × 0.25 mm
Data collection
Oxford Diffraction Xcalibur diffractometer with an Atlas (Gemini Ultra Cu) detector
Absorption correction: analytical (CrysAlis PRO; Oxford Diffraction, 2009 ▶) T min = 0.308, T max = 0.631
11571 measured reflections
2546 independent reflections
2485 reflections with I > 3σ(I)
R int = 0.023
Refinement
R[F 2 > 2σ(F 2)] = 0.026
wR(F 2) = 0.093
S = 1.73
2546 reflections
190 parameters
H-atom parameters constrained
Δρmax = 0.29 e Å−3
Δρmin = −0.28 e Å−3
Data collection: CrysAlis PRO (Oxford Diffraction, 2009 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis PRO; program(s) used to solve structure: SIR2002 (Burla et al., 2003 ▶); program(s) used to refine structure: JANA2006 (Petříček et al., 2006 ▶); molecular graphics: DIAMOND (Brandenburg & Putz, 2005 ▶); software used to prepare material for publication: JANA2006.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810028163/fk2021sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810028163/fk2021Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
We acknowledge the Golestan University (GU), the Institutional research plan No. AVOZ1010051 of the Institute of Physics and the Praemium academiae project of the Academy of Sciences of the Czech Republic.
supplementary crystallographic information
Comment
The chemistry of Schiff-bases is very diverse because of a variety of possible substituents with different electron donating and withdrawing groups (Yildiz et al., 2008; Hijji et al., 2009; Karakas et al., 2008). These compounds have been studied for their use as anion sensors (Hijji et al., 2009), antimicrobial activity (Yildiz et al., 2008), photochromism and thermochromism (Hadjoudis et al., 2004) and nonlinear optical properties (Karakas et al., 2008). As a continuation of our work on the synthesis and structural characterization of Schiff-base compounds we report the synthesis and crystal structure of (E)-4-bromo-N-(2,3-dimethoxybenzylidene)aniline (1).
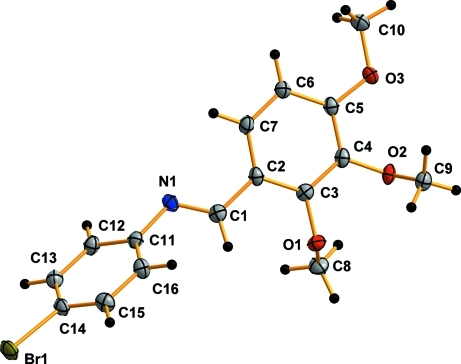
An ORTEP plot, with the atomic numbering scheme is depicted in Fig. 1. Bond lengths in the title compound are normal (Allen et al., 1987). The C1—N1 and C11—N1 bond lengths of 1.286 (3) and 1.415 (3) Å, respectively, conform to the value for a double and single bonds as found in similar Schiff-base compounds (Khalaji et al., 2007; Khalaji & Harrison, 2008; Khalaji et al., 2008; Khalaji & Simpson, 2009; Khalaji et al., 2009; Khalaji et al., 2010). The dihedral angle between the two aromatic rings is 64.02 (6)°, while the plane through the central C1—N1—C1—C2 system is inclined at 21.65 (18)° to the dimethoxyphenyl ring and 42.37 (18)° to the bromobenzene ring. The two methoxy groups attached at C3 and C4 are twisted away from the C2—C7 benzene ring, with corresponding torsion angles C8—O1—C3—C2, C9—O2—C4—C3 of 103.6 (2)°, -88.7 (2)°, respectively. The third methoxy group attached at C5 is almost coplanar with the C2—C7 ring, as shown by the torsion angle C10—O3—C5—C6 of -7.2 (3)°.
Experimental
The title compound was prepared in 83% yield from 2,3,4-trimethoxybenzaldehyde and 4-bromoaniline as reported elsewhere (Khalaji & Harrison, 2008) and recrystallized from chloroform. Anal. Calc. for C16H16BrNO3: C, 54.87; H, 4.60; N, 4.00%. Found: C, 54.66; H, 4.52; N, 4.06%. IR (KBr pellet, cm-1): 2911–2998 (m, C—H aromatic and aliphatic), 2837 (s, –CH=N–); 1615 (s, C=N), 1413–1594 (C=C aromatic).
Refinement
All hydrogen atoms were discernible in difference Fourier maps and could be refined to reasonable geometry. According to common practice the H atoms were placed in geometrically ideal positions and allowed to ride on their respective parent atoms, with C—H distance of 0.96 Å. The isotropic atomic displacement parameters of hydrogen atoms were evaluated as 1.2*Ueq of the parent atom.
Figures
Fig. 1.
The molecular structure of the title compound with atom-labeling scheme. Displacement ellipsoids are drawn at the 50% probability level.
Crystal data
| C16H16BrNO3 | Z = 2 |
| Mr = 350.2 | F(000) = 356 |
| Triclinic, P1 | Dx = 1.554 Mg m−3 |
| Hall symbol: -P 1 | Cu Kα radiation, λ = 1.54184 Å |
| a = 7.9103 (3) Å | Cell parameters from 11782 reflections |
| b = 9.9902 (4) Å | θ = 4.4–66.7° |
| c = 10.7821 (3) Å | µ = 3.83 mm−1 |
| α = 93.068 (3)° | T = 120 K |
| β = 108.568 (3)° | Irregular shape, colourless |
| γ = 109.679 (3)° | 0.49 × 0.38 × 0.25 mm |
| V = 748.10 (5) Å3 |
Data collection
| Oxford Diffraction Xcalibur diffractometer with an Atlas (Gemini Ultra Cu) detector | 2546 independent reflections |
| Radiation source: X-ray tube | 2485 reflections with I > 3σ(I) |
| mirror | Rint = 0.023 |
| Detector resolution: 10.3784 pixels mm-1 | θmax = 65.1°, θmin = 4.4° |
| Rotation method data acquisition using ω scans | h = −9→9 |
| Absorption correction: analytical (CrysAlis PRO; Oxford Diffraction, 2009) | k = −11→11 |
| Tmin = 0.308, Tmax = 0.631 | l = −12→12 |
| 11571 measured reflections |
Refinement
| Refinement on F2 | 64 constraints |
| R[F2 > 2σ(F2)] = 0.026 | H-atom parameters constrained |
| wR(F2) = 0.093 | Weighting scheme based on measured s.u.'s w = 1/[σ2(I) + 0.0025000002I2] |
| S = 1.73 | (Δ/σ)max = 0.010 |
| 2546 reflections | Δρmax = 0.29 e Å−3 |
| 190 parameters | Δρmin = −0.28 e Å−3 |
| 0 restraints |
Special details
| Experimental. CrysAlisPro (Oxford Diffraction, 2009). Analytical numeric absorption correction using a multifaceted crystal model. |
| Refinement. The refinement was carried out against all reflections. The conventional R-factor is always based on F. The goodness of fit as well as the weighted R-factor are based on F and F2 for refinement carried out on F and F2, respectively. The threshold expression is used only for calculating R-factors etc. and it is not relevant to the choice of reflections for refinement.The program used for refinement, Jana2006, uses the weighting scheme based on the experimental expectations, see _refine_ls_weighting_details, that does not force S to be one. Therefore the values of S are usually larger than the ones from the SHELX program. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.74999 (3) | 0.08307 (2) | 0.325568 (16) | 0.02973 (14) | |
| O1 | 0.27449 (16) | 0.17106 (14) | 0.96566 (12) | 0.0209 (5) | |
| O2 | 0.28261 (17) | 0.27191 (14) | 1.21239 (13) | 0.0204 (5) | |
| O3 | 0.58807 (17) | 0.49203 (14) | 1.37866 (12) | 0.0217 (5) | |
| N1 | 0.7545 (2) | 0.28811 (17) | 0.86865 (15) | 0.0205 (6) | |
| C1 | 0.5987 (2) | 0.27332 (18) | 0.88986 (17) | 0.0196 (6) | |
| C2 | 0.6019 (2) | 0.33388 (19) | 1.01757 (17) | 0.0183 (6) | |
| C3 | 0.4387 (2) | 0.27855 (18) | 1.05351 (17) | 0.0179 (6) | |
| C4 | 0.4391 (2) | 0.33244 (18) | 1.17524 (16) | 0.0177 (6) | |
| C5 | 0.6034 (2) | 0.44399 (19) | 1.26324 (17) | 0.0188 (6) | |
| C6 | 0.7671 (2) | 0.50042 (19) | 1.22894 (17) | 0.0205 (7) | |
| C7 | 0.7633 (2) | 0.4451 (2) | 1.10740 (17) | 0.0209 (7) | |
| C8 | 0.2463 (3) | 0.0301 (2) | 1.0001 (2) | 0.0275 (7) | |
| C9 | 0.1411 (2) | 0.3364 (2) | 1.16845 (19) | 0.0225 (7) | |
| C10 | 0.7574 (3) | 0.5963 (2) | 1.4782 (2) | 0.0283 (8) | |
| C11 | 0.7401 (2) | 0.23365 (19) | 0.74029 (17) | 0.0190 (6) | |
| C12 | 0.8644 (2) | 0.16622 (19) | 0.73130 (18) | 0.0214 (7) | |
| C13 | 0.8630 (3) | 0.1162 (2) | 0.60794 (19) | 0.0228 (7) | |
| C14 | 0.7397 (3) | 0.1383 (2) | 0.49439 (18) | 0.0212 (7) | |
| C15 | 0.6134 (3) | 0.2048 (2) | 0.50017 (19) | 0.0232 (7) | |
| C16 | 0.6156 (3) | 0.2530 (2) | 0.62404 (18) | 0.0224 (7) | |
| H1 | 0.477112 | 0.22121 | 0.820107 | 0.0236* | |
| H6 | 0.880465 | 0.576586 | 1.289179 | 0.0246* | |
| H7 | 0.875405 | 0.484421 | 1.084082 | 0.0251* | |
| H8a | 0.117756 | −0.035146 | 0.948638 | 0.0329* | |
| H8b | 0.336604 | −0.004589 | 0.981472 | 0.0329* | |
| H8c | 0.265545 | 0.035921 | 1.093009 | 0.0329* | |
| H9a | 0.039226 | 0.294309 | 1.201348 | 0.0269* | |
| H9b | 0.200061 | 0.438601 | 1.201714 | 0.0269* | |
| H9c | 0.089588 | 0.3194 | 1.073 | 0.0269* | |
| H10a | 0.725742 | 0.625109 | 1.551587 | 0.034* | |
| H10b | 0.852651 | 0.554334 | 1.508865 | 0.034* | |
| H10c | 0.807226 | 0.679308 | 1.440879 | 0.034* | |
| H12 | 0.95208 | 0.154077 | 0.810937 | 0.0256* | |
| H13 | 0.946288 | 0.067032 | 0.60173 | 0.0274* | |
| H15 | 0.526458 | 0.217114 | 0.42029 | 0.0278* | |
| H16 | 0.530309 | 0.300348 | 0.629778 | 0.0268* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.03787 (19) | 0.03187 (19) | 0.02105 (19) | 0.00904 (13) | 0.01747 (12) | 0.00097 (11) |
| O1 | 0.0192 (6) | 0.0209 (7) | 0.0176 (6) | 0.0033 (5) | 0.0045 (5) | 0.0034 (5) |
| O2 | 0.0202 (6) | 0.0239 (7) | 0.0230 (7) | 0.0091 (5) | 0.0132 (5) | 0.0103 (5) |
| O3 | 0.0227 (6) | 0.0259 (7) | 0.0175 (6) | 0.0079 (5) | 0.0099 (5) | 0.0019 (5) |
| N1 | 0.0230 (7) | 0.0245 (8) | 0.0159 (7) | 0.0089 (6) | 0.0090 (6) | 0.0047 (6) |
| C1 | 0.0214 (8) | 0.0192 (8) | 0.0189 (9) | 0.0072 (7) | 0.0078 (7) | 0.0067 (7) |
| C2 | 0.0206 (8) | 0.0211 (8) | 0.0174 (8) | 0.0098 (7) | 0.0094 (7) | 0.0071 (7) |
| C3 | 0.0179 (8) | 0.0183 (8) | 0.0178 (8) | 0.0071 (7) | 0.0059 (6) | 0.0058 (7) |
| C4 | 0.0196 (8) | 0.0197 (8) | 0.0192 (8) | 0.0096 (7) | 0.0107 (7) | 0.0095 (7) |
| C5 | 0.0214 (8) | 0.0216 (9) | 0.0177 (8) | 0.0104 (7) | 0.0093 (7) | 0.0069 (7) |
| C6 | 0.0196 (8) | 0.0213 (9) | 0.0199 (9) | 0.0059 (7) | 0.0082 (7) | 0.0030 (7) |
| C7 | 0.0201 (8) | 0.0238 (9) | 0.0213 (9) | 0.0073 (7) | 0.0114 (7) | 0.0060 (7) |
| C8 | 0.0309 (9) | 0.0197 (9) | 0.0304 (11) | 0.0044 (8) | 0.0147 (8) | 0.0038 (8) |
| C9 | 0.0187 (9) | 0.0268 (9) | 0.0248 (10) | 0.0093 (8) | 0.0105 (7) | 0.0066 (7) |
| C10 | 0.0256 (9) | 0.0369 (11) | 0.0186 (9) | 0.0094 (8) | 0.0066 (7) | −0.0024 (8) |
| C11 | 0.0192 (8) | 0.0185 (8) | 0.0188 (9) | 0.0039 (7) | 0.0091 (7) | 0.0044 (7) |
| C12 | 0.0208 (8) | 0.0256 (9) | 0.0208 (9) | 0.0097 (7) | 0.0098 (7) | 0.0078 (7) |
| C13 | 0.0232 (9) | 0.0227 (9) | 0.0265 (10) | 0.0097 (7) | 0.0126 (7) | 0.0055 (7) |
| C14 | 0.0261 (9) | 0.0205 (9) | 0.0186 (9) | 0.0053 (7) | 0.0140 (7) | 0.0022 (7) |
| C15 | 0.0277 (9) | 0.0249 (9) | 0.0185 (9) | 0.0105 (8) | 0.0088 (7) | 0.0083 (7) |
| C16 | 0.0255 (9) | 0.0241 (9) | 0.0223 (9) | 0.0118 (7) | 0.0116 (7) | 0.0066 (7) |
Geometric parameters (Å, °)
| Br1—C14 | 1.908 (2) | C8—H8a | 0.96 |
| O1—C3 | 1.3805 (16) | C8—H8b | 0.96 |
| O1—C8 | 1.438 (2) | C8—H8c | 0.96 |
| O2—C4 | 1.377 (2) | C9—H9a | 0.96 |
| O2—C9 | 1.440 (3) | C9—H9b | 0.96 |
| O3—C5 | 1.365 (2) | C9—H9c | 0.96 |
| O3—C10 | 1.4342 (19) | C10—H10a | 0.96 |
| N1—C1 | 1.286 (3) | C10—H10b | 0.96 |
| N1—C11 | 1.415 (3) | C10—H10c | 0.96 |
| C1—C2 | 1.463 (3) | C11—C12 | 1.390 (3) |
| C1—H1 | 0.96 | C11—C16 | 1.395 (3) |
| C2—C3 | 1.408 (3) | C12—C13 | 1.391 (3) |
| C2—C7 | 1.3946 (19) | C12—H12 | 0.96 |
| C3—C4 | 1.391 (3) | C13—C14 | 1.383 (3) |
| C4—C5 | 1.3990 (19) | C13—H13 | 0.96 |
| C5—C6 | 1.403 (3) | C14—C15 | 1.387 (3) |
| C6—C7 | 1.382 (3) | C15—C16 | 1.388 (3) |
| C6—H6 | 0.96 | C15—H15 | 0.96 |
| C7—H7 | 0.96 | C16—H16 | 0.96 |
| C3—O1—C8 | 113.46 (13) | O2—C9—H9a | 109.4709 |
| C4—O2—C9 | 112.97 (15) | O2—C9—H9b | 109.4713 |
| C5—O3—C10 | 117.80 (15) | O2—C9—H9c | 109.4711 |
| C1—N1—C11 | 118.43 (13) | H9a—C9—H9b | 109.4714 |
| N1—C1—C2 | 121.70 (13) | H9a—C9—H9c | 109.4709 |
| N1—C1—H1 | 119.1502 | H9b—C9—H9c | 109.4718 |
| C2—C1—H1 | 119.1489 | O3—C10—H10a | 109.4713 |
| C1—C2—C3 | 119.84 (13) | O3—C10—H10b | 109.4716 |
| C1—C2—C7 | 122.20 (18) | O3—C10—H10c | 109.4711 |
| C3—C2—C7 | 117.96 (17) | H10a—C10—H10b | 109.4714 |
| O1—C3—C2 | 119.60 (16) | H10a—C10—H10c | 109.4707 |
| O1—C3—C4 | 119.38 (16) | H10b—C10—H10c | 109.4713 |
| C2—C3—C4 | 121.01 (13) | N1—C11—C12 | 117.91 (16) |
| O2—C4—C3 | 120.43 (12) | N1—C11—C16 | 122.8 (2) |
| O2—C4—C5 | 119.83 (17) | C12—C11—C16 | 119.21 (19) |
| C3—C4—C5 | 119.66 (17) | C11—C12—C13 | 120.60 (17) |
| O3—C5—C4 | 115.31 (17) | C11—C12—H12 | 119.6985 |
| O3—C5—C6 | 124.62 (13) | C13—C12—H12 | 119.6991 |
| C4—C5—C6 | 120.06 (17) | C12—C13—C14 | 118.9 (2) |
| C5—C6—C7 | 119.23 (13) | C12—C13—H13 | 120.5337 |
| C5—C6—H6 | 120.3876 | C14—C13—H13 | 120.5339 |
| C7—C6—H6 | 120.3865 | Br1—C14—C13 | 119.31 (18) |
| C2—C7—C6 | 122.09 (18) | Br1—C14—C15 | 118.89 (14) |
| C2—C7—H7 | 118.9551 | C13—C14—C15 | 121.8 (2) |
| C6—C7—H7 | 118.9554 | C14—C15—C16 | 118.52 (18) |
| O1—C8—H8a | 109.4712 | C14—C15—H15 | 120.7399 |
| O1—C8—H8b | 109.4712 | C16—C15—H15 | 120.7397 |
| O1—C8—H8c | 109.4713 | C11—C16—C15 | 120.9 (2) |
| H8a—C8—H8b | 109.4713 | C11—C16—H16 | 119.5302 |
| H8a—C8—H8c | 109.4709 | C15—C16—H16 | 119.5303 |
| H8b—C8—H8c | 109.4714 | ||
| C8—O1—C3—C2 | 103.6 (2) | C11—N1—C1—C2 | −176.48 (16) |
| C8—O1—C3—C4 | −77.6 (2) | C1—N1—C11—C12 | −140.37 (18) |
| C9—O2—C4—C3 | −88.7 (2) | C1—N1—C11—C16 | 43.7 (3) |
| C9—O2—C4—C5 | 94.54 (19) | N1—C1—C2—C3 | −159.30 (17) |
| C10—O3—C5—C4 | 174.08 (16) | N1—C1—C2—C7 | 20.0 (3) |
| C10—O3—C5—C6 | −7.2 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| ?—?···? | ? | ? | ? | ? |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: FK2021).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Brandenburg, K. & Putz, H. (2005). DIAMOND Crystal Impact GbR, Bonn, Germany.
- Burla, M. C., Camalli, M., Carrozzini, B., Cascarano, G. L., Giacovazzo, C., Polidori, G. & Spagna, R. (2003). J. Appl. Cryst.36, 1103.
- Hadjoudis, E., Rontoyianni, A., Ambroziak, K., Aziembowska, T. & Mavridis, I. M. (2004). J. Photochem. Photobiol. A, 162, 521–530.
- Hijji, Y. M., Barare, B., Kennedy, A. P. & Butcher, R. (2009). Sens. Actuators B, 136, 297–302.
- Karakas, A., Univer, H. & Elmali, A. (2008). J. Mol. Struct.877, 152–157.
- Khalaji, A. D., Fejfarová, K. & Dušek, M. (2010). Acta Chim. Slov.57, 257–261. [PubMed]
- Khalaji, A. D. & Harrison, W. T. A. (2008). Anal. Sci.24, x3–x4.
- Khalaji, A. D. & Simpson, J. (2009). Acta Cryst. E65, o553. [DOI] [PMC free article] [PubMed]
- Khalaji, A. D., Slawin, A. M. Z. & Woollins, J. D. (2007). Acta Cryst. E63, o4257.
- Khalaji, A. D., Weil, M., Gotoh, K. & Ishida, H. (2009). Acta Cryst. E65, o436. [DOI] [PMC free article] [PubMed]
- Khalaji, A. D., Welter, R., Amirnasr, M. & Barry, A. H. (2008). Anal. Sci.24, x138–x139.
- Oxford Diffraction (2009). CrysAlis PRO Oxford Diffraction Ltd, Yarnton, England.
- Petříček, V., Dušek, M. & Palatinus, L. (2006). JANA2006 Institute of Physics, Prague, Czech Republic.
- Yildiz, M., Unver, H., Dulger, B., Erdener, D., Ocak, N., Erdonmez, A. & Durlu, T. N. (2008). J. Mol. Struct.738, 253–260.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810028163/fk2021sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810028163/fk2021Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report