Abstract
γ-Tubulin localizes to microtubule-organizing centers in animal and fungal cells where it is important for microtubule nucleation. Plant cells do not have morphologically defined microtubule organizing centers, however, and γ-tubulin is distributed in small, discrete structures along microtubules. The great difference in distribution has prompted speculation that plant γ-tubulins function differently from animal and fungal γ-tubulins. We tested this possibility by expressing Arabidopsis γ-tubulin in the fission yeast Schizosaccharomyces pombe. At high temperatures, the plant γ-tubulin was able to bind to microtubule-organizing centers, nucleate microtubule assembly, and support the growth and replication of S. pombe cells lacking endogenous γ-tubulin. However, the distribution of microtubules was abnormal as was cell morphology, and at low temperatures, cells were arrested in mitosis. These results reveal that Arabidopsis γ-tubulin can carry out essential functions in S. pombe and is, thus, functionally conserved. The morphological abnormalities reveal that it cannot carry out some nonessential functions, however, and they underscore the importance of γ-tubulin in morphogenesis of fission yeast cells and in maintaining normal interphase microtubule arrays.
Nucleation of microtubule assembly is a key step in the cellular organization of microtubules (for review, see Brinkley, 1985; Oakley, 1992; Joshi, 1994; Gislene and Schiebel, 1997; Oakley and Akkari, 1999), and γ-tubulin is an indispensable component of microtubule nucleation complexes in a variety of organisms including fungi and animal cells (Oakley et al., 1990; Horio et al., 1991; Stearns et al., 1991; Zheng et al., 1991; Joshi et al., 1992; Felix et al., 1994). Previously, we have reported that human γ-tubulin can replace the endogenous γ-tubulin of Schizosaccharomyces pombe (Horio and Oakley, 1994). This finding demonstrated that γ-tubulin is functionally conserved in phylogenetically distant organisms with microtubule-organizing centers (MTOCs) that differ vastly in size and appearance. However, the spindles of these species do share the common feature that microtubules extend from discrete MTOCs, spindle pole bodies (SPBs) in fungi, and centrosomes in higher animal cells.
In contrast, in plant cells, spindle microtubules are less focused than in typical animal cells and no discrete MTOCs equivalent to the centrosomes in animal cells are present (for review, see Vaughn and Harper, 1998; Canaday et al., 2000; Wasteneys, 2002). The localization of γ-tubulin in plant cells has been examined extensively (Liu et al., 1993, 1994, 1995; Binarova et al., 2000; Kumagai et al., 2003). In most microtubule arrays of plant cells, γ-tubulin is distributed along the microtubules, often in very small, punctate structures. Although γ-tubulin was sometimes enriched in regions containing the minus ends of the microtubules, it did not localize to, or help to define, MTOCs (Liu et al., 1993, 1994; for review, see Joshi and Palevitz, 1996; Vaughn and Harper, 1998; Canaday et al., 2000). These observations gave rise to the hypothesis that γ-tubulin in plants has functions along the microtubule array distinct from the nucleation of microtubules at the MTOCs (Liu et al., 1993; Vaughn and Harper, 1998; Canaday et al., 2000).
To determine to what extent plant γ-tubulin is functionally different from its animal or fungal counterparts, we expressed a flowering plant, Arabidopsis, γ-tubulin (Liu et al., 1994) in the fission yeast S. pombe and determined whether it is able to function in place of the endogenous γ-tubulin. We found that the Arabidopsis γ-tubulin was able to support the growth of S. pombe at high temperatures. Examination of the cytological phenotype of the Arabidopsis γ-tubulin-expressing cells at high temperatures revealed that mitotic spindles and cytoplasmic microtubules were present and that Arabidopsis γ-tubulin localized to MTOCs. However, cells exhibited morphological abnormalities, and the distribution of microtubules was abnormal. At lower temperatures, growth was inhibited and cells were blocked in mitosis. These data reveal that Arabidopsis γ-tubulin, like fungal and animal γ-tubulins, is able to bind to MTOCs and nucleate microtubule assembly. However, not surprisingly, it does not carry out all functions as well as S. pombe γ-tubulin, and the abnormalities in morphology and microtubule distribution we have observed illustrate the importance of γ-tubulin in maintaining normal interphase arrays of microtubules and in morphogenesis of yeast cells.
RESULTS
Expression of Arabidopsis γ-Tubulin in the Fission Yeast S. pombe
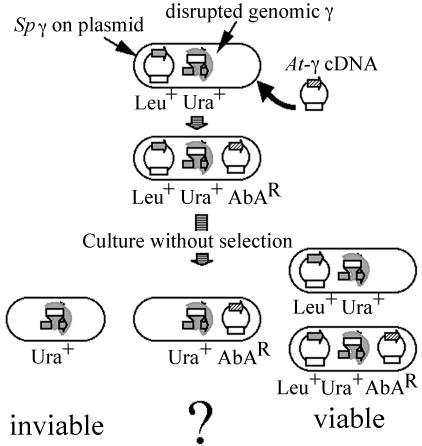
The facts that γ-tubulin is distributed along microtubules in plant cells and shows, at most, a partial enrichment at the minus ends of microtubules have led to the suggestion that plant γ-tubulins may function differently from those of fungi or animal cells. To test this hypothesis, we expressed Arabidopsis γ-tubulin in the fission yeast S. pombe. We constructed a plasmid (pTH1197) containing an expressible Arabidopsis γ-2 γ-tubulin cDNA and transformed it into AH001, a γ-tubulin gene disruptant strain carrying the wild-type S. pombe γ-tubulin gene, gtb1+, on a plasmid (Fig. 1). pTH1197 transformed AH001 at a frequency comparable with that of the control plasmid pAUR224 and the transformants grew as well as the control. This result indicates that expression of Arabidopsis γ-tubulin in S. pombe is not dominantly toxic (see Fig. 2). Next, we cultured the pTH1197 transformants in a nonselective medium and looked for colonies that lost the plasmid carrying S. pombe γ-tubulin (and its Leu+ marker). Because γ-tubulin is indispensable for growth (Horio et al., 1991), these cells can lose the plasmid only if the Arabidopsis γ-tubulin can function in place of endogenous γ-tubulin. For our first attempts, a culture temperature of 32°C (the optimal growth temperature for S. pombe) was used and no leu- colonies were isolated from the screening. We repeated the same screen using 36°C or 20°C for the culture temperature. Two different colony types that were clearly distinguished by the difference in their sizes were formed when the culture was incubated at 36°C and almost all of the small colonies tested were leu- while retaining the AbAR phenotype. Such colonies should carry the Arabidopsis γ-tubulin plasmid and should not carry the S. pombe γ-tubulin plasmid. One of these strains (AH120) was selected and used for further analysis. We tested the growth of AH120 at different temperatures. As shown in Figure 2, AH120 grew reasonably well, although slower than the control, at 36°C. However, it grew very poorly at 32°C, and did not grow at all at 20°C. This result explains our failure to isolate leu- colonies at 32°C and 20°C.
Figure 1.
Schematic diagram of the plasmid-shuffling procedure.
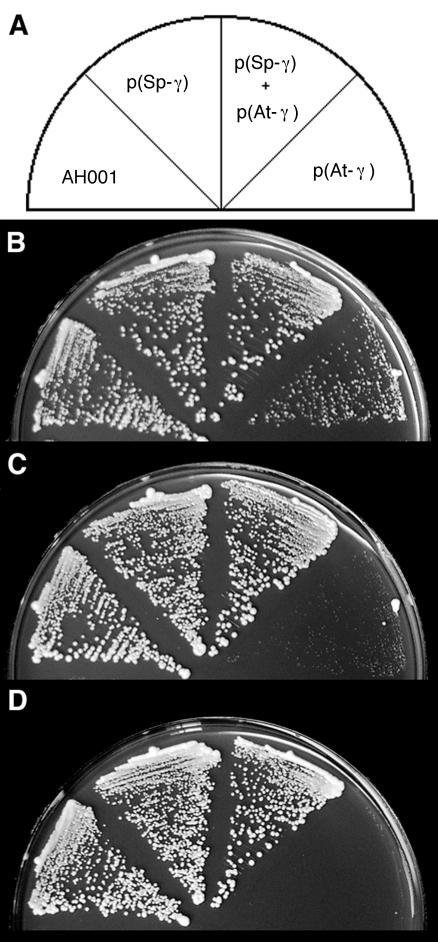
Figure 2.
Growth of Arabidopsis γ-tubulin-expressing strains. The strains indicated in A were streaked onto 0.5% [w/v] yeast extract and 3% [w/v] Glc (YE) medium and incubated at 36°C (B), 32°C (C), and 20°C (D). All strains carry a disruption of the chromosomal γ-tubulin gene. AH001 carries pCT134 (the wild-type S. pombe γ-tubulin gene on a multicopy vector). Strain AH125 [p(Sp-γ)], carries a plasmid (pTH1203) with the S. pombe γ-tubulin cDNA under control of the cytomegalovirus (CMV) promoter. Strain AH119 [p(Sp-γ), p(At-γ)], carries pCT134 and a plasmid (pTH1197) with the Arabidopsis γ-tubulin cDNA under control of the CMV promoter. Strain AH120 [p(At-γ)] carries pTH1197 only [p(At-γ)].
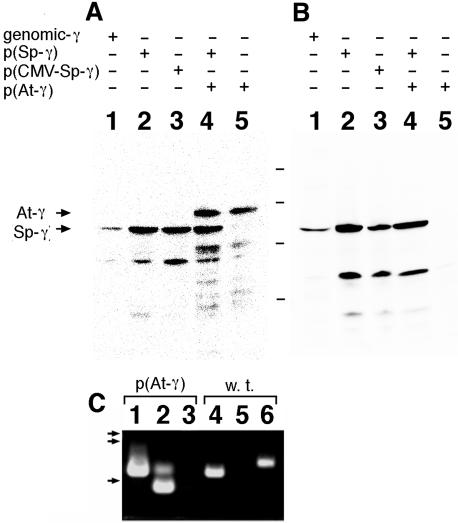
The expression of the Arabidopsis γ-tubulin and the absence of endogenous γ-tubulin in AH120 cells was confirmed by western blotting using monoclonal anti-γ-tubulin antibodies and reverse transcriptase (RT)-PCR (Fig. 3). With one of the antibodies, G9, which reacts to the γ-tubulins of a broad range of species (Jung et al., 2001; Horio et al., 2002; Kumagai et al., 2003), including S. pombe and Arabidopsis, a band slightly larger than the endogenous γ-tubulin was detected in the extracts prepared from the strains carrying pTH1197 (Fig. 3A). Arabidopsis γ-tubulin is slightly larger than S. pombe γ-tubulin and this result indicates that Arabidopsis γ-tubulin is expressed in strains carrying pTH1197. In another blot, the G58 antibody, which recognizes an epitope mapped to the C-terminal variable region of S. pombe γ-tubulin, did not give any detectable band in protein extracts from AH120. This result confirmed the absence of the endogenous S. pombe γ-tubulin in AH120 cells. The expression of Arabidopsis γ-tubulin and the absence of the S. pombe γ-tubulin in AH120 cells were further confirmed by RT-PCR. Although amplification using primers specific for Arabidopsis γ-tubulin gave a band of the expected size, another set of primers specific for S. pombe γ-tubulin failed to give a detectable band when cDNA from AH120 cell was used as the template (Fig. 3C).
Figure 3.
Expression of Arabidopsis γ-tubulin detected by western blotting and RT-PCR. Crude extracts of S. pombe strains, wild type 972 (lane 1), AH001 (lane 2), AH125 (lane 3), AH119 (lane 4), and AH120 (lane 5) were run on a SDS gel and probed with the anti-γ-tubulin antibody G9 (A) or anti-S. pombe γ-tubulin antibody G58 (B). Types of γ-tubulin genes carried by each strain are indicated on top of each lane. The positions of Arabidopsis γ-tubulin (At-γ) and S. pombe γ-tubulin (Sp-γ) are indicated by arrows. The lower bands detected by each antibody are presumably products of proteolytic degradation due to the overproduction of the γ-tubulin. Bars in the middle indicate the positions of Mr markers (94, 67, 43, and 20 kD from top to bottom). C, cDNA fragments amplified from mRNA of AH120 (lanes 1-3) or wild-type HM123 (lanes 4-6). Oligonucleotide primers corresponding to the coding sequence of S. pombe actin gene (lanes 1 and 4), the Arabidopsis γ-tubulin gene (lanes 2 and 5), or the S. pombe γ-tubulin gene (lanes 3 and 6) were used for amplification. Arrows on the left indicate migrated positions of size markers (2.3, 2.0, and 0.56 kb from top to bottom).
AH120 Exhibits Abnormalities in Morphology and Cytoplasmic Microtubule Organization
AH120 grew more slowly than control strains even at 36°C, the permissive temperature. This suggested that, even under permissive conditions, one or more cellular processes required for cell proliferation is delayed or partly defective in AH120 cells. We consequently examined the cytological phenotype of AH120 incubated at 36°C to identify the defect(s).
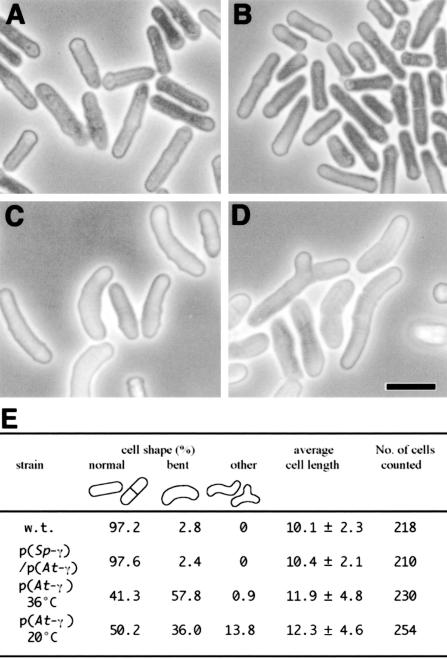
We examined the cellular morphology of AH120 by phase contrast microscopy (Fig. 4, A-D). At 36°C, more than one-half (57.8%, n = 230, see Fig. 4E) of the AH120 cells were curved, and the average cell length was longer than the control strain (Fig. 4E). We also found cells with other morphological abnormalities such as branched or kinked cells, but the frequency of cells of these types was much lower than that of curved cells. We examined five independent Arabidopsis γ-tubulin-expressing transformants. All of these strains were severely cold sensitive as the case of AH120 and exhibited the same curved cell phenotype (data not shown), indicating that the alternation of cell shape does not result from an additional mutation that has accidentally occurred in AH120, but from the expression of Arabidopsis γ-tubulin. The cell shape of the strain expressing the S. pombe γ-tubulin and the Arabidopsis γ-tubulin was essentially indistinguishable from that of the wild type (Fig. 4, B and E). This result indicated that the aberrant morphology caused by the Arabidopsis γ-tubulin is recessive and can be suppressed by the expression of S. pombe γ-tubulin.
Figure 4.
Aberrant cellular morphology observed in the Arabidopsis γ-tubulin-expressing cells. Phase contrast micrographs of the wild-type strain 972 (A), a strain expressing S. pombe γ-tubulin and Arabidopsis γ-tubulin AH119 [p(Sp-γ), p(At-γ)] (B), and strain AH120 (which expresses only Arabidopsis γ-tubulin) incubated at 36°C [p(At-γ) 36°C] (C) or 20°C [p(At-γ) 20°C] (D). Bar = 10 μm. The length and shape of the cells in each culture was determined (E).
We also observed morphological abnormalities in cells grown at 36°C and then shifted to 20°C for 12 h. Although 36.0% of the cells (n = 254) still exhibited a curved morphology, 13.8% of the cells exhibited other aberrant morphologies, including abnormally swollen, branched, and twisted cell shapes (Fig. 4, D and E).
It is known that strains with defects in microtubule organization exhibit aberrant cell morphologies. We consequently examined microtubule organization using the α-tubulin antibody TAT1. In mitotic cells, the mitotic spindles were visible as intensely stained rod-like structures. In the majority of the mitotic cells, mitotic spindles and properly segregating chromosomes were observed. However, in some mitotic cells, condensed chromosomes failed to move to the poles in spite of the presence of elongated spindles (Fig. 5G, indicated by arrow). In wild-type cells, dividing chromatids are tightly packed together and are observed as a single DAPI staining body (see Fig. 6D). In AH120 cells, abnormally stretched or spread chromatids were observed in one or both dividing nuclei in 13.5% of cells (17 cases in 126 mitotic cells). These results indicate that Arabidopsis γ-tubulin does not support completely normal spindle function in S. pombe, but spindles function well enough to support growth.
Figure 5.
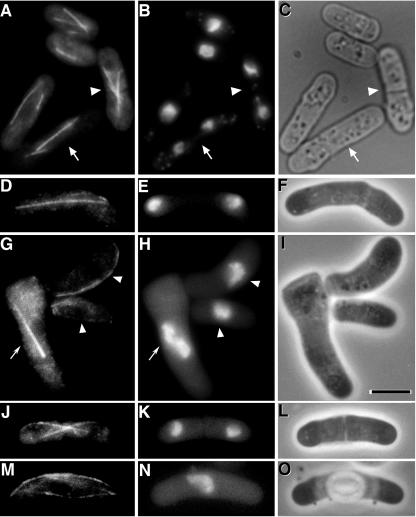
Abnormal microtubule arrays observed in the Arabidopsis γ-tubulin-expressing cells at a permissive temperature. Microtubules of S. pombe wild type (A-C) and AH120 (D--L) were stained using the anti-α-tubulin antibody TAT1. Immunofluorescent staining of α-tubulin (A, D, G, J, and M), corresponding 4′6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI) staining (B, E, H, K, and N), and phase contrast images (C, F, I, L, and O) are shown. In A through C, wild-type cells in mitosis (arrows) and postmitotic stage (arrowheads) are indicated. Cells with curved microtubules and a mitotic cell in which condensed chromosomes are left behind on the stretched spindle are indicated by arrowheads and an arrow in G and H, respectively. In M through O, an interphase cell with extensively stained microtubules along the outside edge of the cell and a few microtubules in other locations of cytoplasm is shown. Bar = 5 μm.
Figure 6.
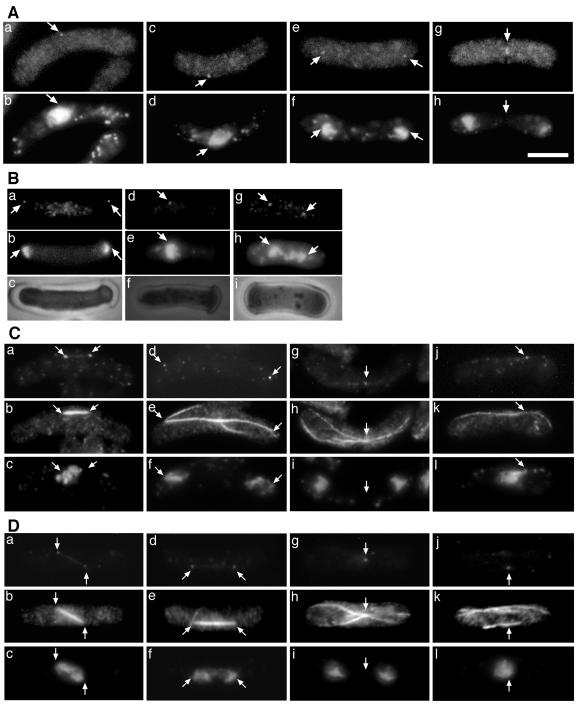
Arabidopsis γ-tubulin localizes to MTOCs. Immunofluorescent staining of Arabidopsis γ-tubulin-expressing strains unextracted (A and C) and partially extracted (B) cells and wild-type green fluorescent protein (GFP)-α-tubulin expressing cells (D). Localization of the γ-tubulin was detected by using G9 antibody (A, a, c, e, and g; B, a, d, and g; C, a, d, g, and j; and D, d, g, and j). GFP-α-tubulin was detected by anti-GFP antibodies (C, b, e, h, and k, and D, b, e, h, and k). Corresponding DAPI staining (A, b, d, f, and h; B, b, e, and h; C,c, f, i, and l; and D, c, f, i, and l) and phase contrast (B, c, f, and i,) images are shown. Positions of G9-stained bodies and corresponding locations in DAPI and microtubule panels are indicated by arrows. G9-stained bodies are SPBs except in Ag, Cg, and Dg, where they are cytoplasmic MTOCs. Bar = 5 μm.
In S. pombe, cytoplasmic microtubules extend from cytoplasmic MTOCs immediately after mitosis (Fig. 5, A-C, indicated by arrowheads, also see Fig. 6D). However, these cytoplasmic MTOCs are not detectable in later stages of interphase. We found that microtubule arrays in immediately postmitotic cells were relatively normal (Fig. 5, J-L). Microtubules extended from cytoplasmic MTOCs as in wild-type cells. However, in later stages of interphase, cytoplasmic microtubule arrays were often abnormal. In many of the curved cells, cytoplasmic microtubules were present only along the outside (longer side) edge of the cells (Fig. 5G, indicated by arrowheads) or as a brightly stained bundle of microtubules along the outside edge of the cells accompanied by faintly stained microtubules in other areas of the cytoplasm (Fig. 5M). The extensive cytoplasmic microtubule abnormalities in AH120 indicate that γ-tubulin is essential for normal cytoplasmic microtubule arrays in S. pombe and that Arabidopsis γ-tubulin does not function in S. pombe to establish normal arrays.
Arabidopsis γ-Tubulin Localizes to the SPBs
Because Arabidopsis γ-tubulin does not localize to morphologically defined MTOCs in Arabidopsis, we were very interested in determining if Arabidopsis γ-tubulin bound to SPBs, the major S. pombe MTOCs. We consequently stained AH120 cells using the G9 anti-γ-tubulin antibody. In unextracted cells, G9 gave bright cytoplasmic staining with brighter spots at the periphery of the nuclei and at the ends of dividing nuclei (Fig. 6A), the positions of SPBs. Double staining of the γ-tubulin and the GFP-tagged microtubules revealed that the Arabidopsis γ-tubulin localizes to the both ends of mitotic spindles (Fig. 6C, a-f). Cytoplasmic MTOCs, which appear specifically during the postnuclear division stage, were also detected by G9 (Fig. 6, A, e and f, and C, g-i). These results indicated that a considerable amount of the Arabidopsis γ-tubulin is located at the SPBs and cytoplasmic MTOCs, as is the case with the endogenous γ-tubulin (Fig. 6D, also see Horio et al., 1991). Intense cytoplasmic background staining of the γ-tubulin is one of the typical phenotypes of γ-tubulin-overexpressing strains in S. pombe (Horio and Oakley, 1994; Horio et al., 1999). We consequently prepared cells in which the cytoplasm was partially extracted before fixation. As shown in Figure 6B, brightly stained spots were seen at the periphery of the nuclei and at the ends of mitotic spindles with G9. In unextracted and partially extracted cells, we did not find any indication of the accumulation of γ-tubulin along the position of the spindle microtubules or the cytoplasmic microtubules. It also should be noted that in interphase cells, as shown by G9 staining, SPBs were almost always located near the outside edge of the curved cells. The position of the SPBs coincided with the major population of cytoplasmic microtubules (Fig. 6C, j-l). This suggests that SPBs may interact with cytoplasmic microtubules.
DISCUSSION
Our results clearly indicate that a plant γ-tubulin functions well enough in S. pombe to support growth. This is, perhaps, surprising given that plants lack morphologically defined MTOCs and that the distribution of γ-tubulin in plant cells is quite different from the distribution in S. pombe. Our results do not rule out the possibility that Arabidopsis γ-tubulin may have plant-specific functions, but they demonstrate clearly that Arabidopsis γ-tubulin, like animal and fungal γ-tubulins, can bind to MTOCs and nucleate microtubule assembly. These findings have important implications. First, they indicate that Arabidopsis γ-tubulin has functional microtubule nucleation domains. It is highly unlikely that microtubule nucleation domains would be preserved in plant γ-tubulins if they did not play an important role in microtubule nucleation in plants. Second, the binding of γ-tubulin to the SPB is probably not direct but due to a binding of γ-tubulin to two conserved proteins (Knop et al., 1997; Knop and Schiebel, 1998; Oegema et al., 1999). These proteins are homologs of the Saccharomyces cerevisiae proteins Spc97p and Spc98p and have a variety of different designations in different species. Our results imply that the domains of γ-tubulin responsible for binding to these proteins are preserved in Arabidopsis and thus that the Arabidopsis homologs of these proteins probably bind to Arabidopsis γ-tubulin.
We did not find any obvious accumulation of γ-tubulin along spindle microtubules or cytoplasmic microtubules. In fungi, γ-tubulin is located mainly at MTOCs, with no obvious association with cytoplasmic microtubules (Oakley et al., 1990; Horio et al., 1991; Sobel and Snyder, 1995; Marschall et al., 1996; Spang et al., 1996). Overexpression of S. pombe γ-tubulin at moderate levels results in excess γ-tubulin being distributed throughout the cytoplasm without any particular preferred location (Horio et al., 1999). In plant cells, the localization of γ-tubulin overlaps significantly with the distribution of microtubules (Liu et al., 1993, 1994). This overlap has been observed in every type of microtubule array of plant cells, including mitotic spindles, cortical microtubules, phragmoplasts, and preprophase bands. The possibility that γ-tubulin functions along microtubules, or even incorporates into the lattice of the microtubule, has been proposed based on these observations (Liu et al., 1993; Vaughn and Harper, 1998; Canaday et al., 2000). However, our results indicate that Arabidopsis γ-tubulin has no tendency to stick to or to be incorporated into the microtubules of S. pombe. This is not simply a function of abundance because our results, western blotting (Fig. 3) and immunofluorescence staining (Fig. 6), show that Arabidopsis γ-tubulin is somewhat overexpressed in AH120 relative to S. pombe γ-tubulin in wild-type cells. Our data demonstrate that the localization of γ-tubulin along microtubules in plant cells (Liu et al., 1993, 1994; Joshi and Palevitz, 1996; Vaughn and Harper, 1998) is not solely a property of plant γ-tubulins. It might reflect a specific interaction of plant γ-tubulins with plant α- or β-tubulins or it might reflect the fact that cofactors are present in plants that are required for localization of γ-tubulin along microtubules and these cofactors are absent from S. pombe. Wasteneys (2002) pointed out the possibility that katanin proteins sever the microtubules near the minus end, with kinesin-related proteins delivering the γ-tubulin complex along the length of the microtubules to next microtubule nucleation site. These molecules are certainly candidates for plant-specific cofactors that are responsible for the dispersed distribution of the γ-tubulin in plant cells. The significance of the lateral association of γ-tubulin with microtubules in plants remains open to debate. Our data argue that the primary function of the γ-tubulin is to nucleate microtubules as is the case of animal and fungal counterparts, but allow the possibility that association of the γ-tubulin to the microtubule lattice is important for other functions.
Although Arabidopsis γ-tubulin functions well enough in S. pombe at 36°C to support growth, it does not support growth at normal rates. The reduced growth rate appears to be due to some spindles not segregating chromosomes properly (see Fig. 5, G and H) and to cytoplasmic microtubule abnormalities that result in abnormal cellular morphologies. We can rule out the possibility that these abnormalities are due to overexpression of Arabidopsis γ-tubulin because in strains expressing Arabidopsis and S. pombe γ-tubulins, growth was normal (see Fig. 4). If expression of Arabidopsis γ-tubulin at the levels that occur in our cells were of itself harmful, one would expect that the harmful effects would occur regardless of the presence or absence of S. pombe γ-tubulin. Rather, Arabidopsis γ-tubulin behaves as a recessive allele in S. pombe. This implies that the abnormalities we see are due to absent or reduced function rather than alteration or disruption of function due to overexpression. Absence or reduced function of Arabidopsis γ-tubulin in S. pombe is not surprising, given the phylogenetic distance between the two organisms. There may be subtle functions of S. pombe γ-tubulin that are simply not carried out by Arabidopsis γ-tubulin. Alternatively, some absent or reduced functions may reflect absence or weak interactions of Arabidopsis γ-tubulin with S. pombe proteins. For example, the interaction between Arabidopsis γ-tubulin and the S. pombe α-/β-tubulin dimer could be very weak at 20°C and this would cause failure of spindle microtubule assembly at this temperature.
Finally, our results indicate that Arabidopsis γ-tubulin can be used as a conditionally lethal γ-tubulin allele that should be very useful for studying not only the microtubule organization system, but also the role of MTOCs in cellular morphogenesis.
MATERIALS AND METHODS
Strains and Media
Experiments were carried out with Schizosaccharomyces pombe strain AH001 (h-, leu1-32, ura4-D18, ade6-210, gtb1::ura4+, pCT134; Horio and Oakley, 1994), which carries a disruption of the chromosomal γ-tubulin gene as well as a plasmid (pCT134) with the wild-type γ-tubulin gene (gtb1+). Wild-type strains 972 (h-) and HM123 (h-, leu1-32) were used as control strains. Strains expressing GFP-labeled α-tubulin, YY105 (h90, leu1-32, ura4-D18, lys1+::nmt1p-GFP-atb2 [Ding et al., 1998] kindly provided by Dr. Da-Qiao Ding) and HR577 (h+, leu1-32, his2, ura4-D18, ade6-210, lys1+::nda3p-GFP-atb2+; kindly provided by Dr. Hirohisa Masuda) were used. Medium (YE) and YE supplemented with 70 μg mL-1 adenine sulfate were used as complete media. Edinburgh minimal medium (EMM; Moreno et al., 1991) was used as a minimal medium with appropriate supplements. Aureobasidine A (AbA; Takara Syuzo, Kyoto; 0.4 μg mL-1) was added to the medium when needed.
Construction of Plasmids
An Arabidopsis γ-2 γ-tubulin full-length cDNA (Liu et al., 1994; a kind gift from Dr. Pete Snustad, University of Minnesota, St. Paul) was ligated into the XhoI- and SacII-digested vector pAUR224 (Takara Syuzo) and the resulting plasmid was named pTH1197. In this plasmid, expression of the γ-tubulin cDNA is under the control of the CMV promoter, which is known to function in S. pombe as a constitutive promoter. pAUR224 carries the aur1R gene that confers AbA resistance. An S. pombe full-length γ-tubulin cDNA was ligated into PstI- and BamHI-digested pAUR224 to create plasmid pTH1203. The orientation of the γ-tubulin cDNA in pTH1203 also places it under the control of the CMV promoter.
Construction of Strains
AH001 was transformed with pAUR224, pTH1197, or pTH1203. Transformants were selected by incubating on YE supplemented with AbA. Selected transformants were spread onto YE solid medium and incubated at 32°C until visible colonies formed. Colonies were inoculated to YE liquid medium and incubated overnight at 32°C or 37°C or for 2 d at 20°C. Each culture was diluted and spread onto YE medium. Colonies were tested to determine if they could grow after loss of either plasmid (the resident plasmid pCT134, which carries gtb1+, or the transforming plasmid) by transferring the colonies to EMM supplemented with adenine and YE supplemented with AbA. A strain expressing Arabidopsis γ-tubulin and GFP-α-tubulin, AH133, was obtained by crossing AH120 and HR577.
Preparation of Antibodies and Western Blotting
Several distinct mouse monoclonal antibodies were raised against S. pombe γ-tubulin expressed in bacteria (Horio et al., 1999). The reactive epitope of each monoclonal antibody was mapped using bacterially expressed γ-tubulins of various species and truncated constructs of S. pombe γ-tubulin. The reactivity of one of these monoclonal antibodies, G9, against Arabidopsis γ-tubulin was verified by western blotting using in vitro-translated Arabidopsis γ-tubulin. For western blots, whole cell extracts of S. pombe strains were prepared by crushing the cells with glass beads. An extract of each strain equivalent to 2.5 × 106 cells was separated by SDS-PAGE and electroblotted following standard procedures. Signals were detected by chemiluminescence using an enhanced chemiluminescence western blotting detection kit (Amersham Pharmacia Biotech, Piscataway, NJ). Signals were recorded with a Luminescent Image Analyzer (LAS-1000; Fuji Photo Film, Tokyo).
RT-PCR
Cultures of the strains AH120 and HM123 in exponential growth phase were harvested and total RNA of each strain was isolated by disrupting the cells using glass beads in the presence of phenol:chloroform. Harvested cells were suspended in RNA extraction buffer (50 mm Tris-HCl, 100 mm EDTA, and 100 mm NaCl, pH 8.0), and an equal volume of phenol:chloroform was added. This cell suspension was mixed with glass beads and the cells were crushed by vigorous vortexing. The aqueous phase was recovered, extracted with phenol:chloroform again, and then precipitated by adding ammonium acetate and ethanol. Isolated RNA was stored at -30°C until use. To synthesize first strand DNA corresponding to the population of mRNAs in the cell, reverse transcription was carried out using the isolated RNA, M-MLV reverse transcriptase (Promega, Madison, WI), and the oligo-dT primer supplied by the manufacturer. PCR amplification was performed by using Arabidopsis γ-tubulin-specific primers (#127; 5′-AATCAGATGGAAACGAGT-3′, #128; 5′-GTACTTTGCCTGACTAGC-3′), S. pombe γ-tubulin-specific primers (#1; 5′-TCTCTAGAGGGTTTCTCGCTTTTACA-3′, #12; 5′-CGGATCCTTAAAGAGATAAATAATTGGGATC-3′), or primers corresponding to the S. pombe actin gene (act1+) sequence (#160; 5′-CGTCACCATGGTATTATG-3′, #161; 5′-CAATACCGGGATACATAG-3′). The sizes of DNA fragments expected to be amplified by these primer sets are 436, 951, and 814 bp, respectively.
Measurement of Cell Length and Shape
Phase-contrast micrographs of wild-type and Arabidopsis γ-tubulin-expressing strains were recorded using a CCD camera (ATTO Corporation, Tokyo). The length of each cell was measured on printed pictures. The radii of the curvature of curved cells were determined in the following way. The innermost points of each end of each curved cell were connected by a straight line. The farthest point of the inside surface of the cell from the line was measured. Using this value, b, and the length of the cell, l, the radius of the curvature, r, was calculated as:
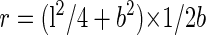 |
We scored each cell as curved if r was less than 80 μm.
Fluorescence and Immunofluorescence Microscopy
Cells cultured in YE supplemented with 70 μg mL-1 adenine sulfate at permissive or restrictive temperatures were fixed and processed for DAPI staining or immunofluorescence staining. For DAPI staining, cells were fixed by heating at 70°C for 5 min. A drop of staining solution (1 μg mL-1 DAPI, 1 mg mL-1 p-phenylenediamine, and 50% [v/v] glycerol) was placed on the sample, which was then sealed with a coverslip and subjected to microscopic observation.
Immunofluorescent staining of unextracted cells was carried out following the procedure described by Horio and Oakley (1994). For double staining, the fixation procedure was modified as follows. Cells growing in liquid culture were harvested by centrifugation and suspended in fixative solution (3.7% [w/v] formaldehyde, 10% [v/v] dimethyl sulfoxide, 100 mm PIPES, 2 mm EGTA, and 1 mm MgSO4) and were incubated for 1 h at culture temperature. Fixed cells were processed following the previously described procedure. Anti-α-tubulin monoclonal antibody TAT1 (Woods et al., 1989; kindly provided by Dr. Keith Gull), anti-γ-tubulin monoclonal antibody G9, and anti-GFP polyclonal antibodies (BD Biosciences, Franklin Lakes, NJ) were used for microtubule staining and γ-tubulin staining. The anti-GFP antibodies were preadsorbed with an S. pombe acetone powder (Harlow and Lane, 1988) before use. Cy-3-conjugated goat anti-mouse immunoglobulin G antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) and Alexa 488-conjugated anti-rabbit immunoglobulin G antibodies (Molecular Probes, Eugene, OR) were preadsorbed with an S. pombe acetone powder and used as secondary antibodies. Antibody-stained cells were stained with 0.1 μg mL-1 DAPI in phosphate-buffered saline and were mounted on a coverslip. Images were recorded on film (T-Max 400; Eastman Kodak, Rochester, NY) or were captured with a digital camera (DMX1200; Nikon, Tokyo).
Permeabilized cells were prepared using a method described previously (Horio and Oakley, 1994). Briefly, AH120 cells cultured in YE supplemented with adenine and AbA were harvested and the cell wall was digested in EMM containing 1 m sorbitol supplemented with Leu and adenine. Cells were harvested and washed twice in 100 mm MES, pH 6.5, 5 mm EDTA, 1 mm trolox, 10% [v/v] dimethyl sulfoxide, and 0.5 mm phenylmethylsulfonyl fluoride (PBL buffer) containing 1 m sorbitol. For cells cultured at 36°C, cell wall digestion was carried out at 36°C. The cells were then incubated in PBL containing 1% (v/v) Triton X-100 for 12 min, washed in PBL twice, and fixed.
Acknowledgments
We thank Dr. Keith Gull for the TAT1 antibody and Dr. Pete Snustad for Arabidopsis γ2-tubulin cDNA. We also thank Dr. Masayuki Yamato, Chie Mori, Miyuki Shimizu, and Akiko Taniwaki for preparation of the antibodies and technical assistance. The authors are grateful to Drs. Da-Qiao Ding, Hirohisa Masuda, and Yasushi Hiraoka for strains, and to Drs. Mikiko Ito and Yutaka Taketani for helpful discussions.
This work was supported by the National Science Foundation (grant no. MCB-9808480) and by The National Institutes of Health (grant no. GM31837) to B.R.O.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.027367.
References
- Binarova P, Cenklova V, Hause B, Kubatova E, Lysak M, Dolezel J, Bogre L, Draber P (2000) Nuclear γ-tubulin during acentriolar plant mitosis. Plant Cell 12: 433-442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley BR (1985) Microtubule organizing centers. Annu Rev Cell Biol 1: 145-172 [DOI] [PubMed] [Google Scholar]
- Canaday J, Stoppin-Mellet V, Mutterer J, Lambert AM, Schmit AC (2000) Higher plant cells: γ-tubulin and microtubule nucleation in the absence of centrosomes. Microsci Res Technol 49: 487-495 [DOI] [PubMed] [Google Scholar]
- Ding D-Q, Chikashige Y, Haraguchi T, Hiraoka Y (1998) Oscillatory nuclear movement in fission yeast meiotic prophase is driven by astral microtubules, as revealed by continuous observation of chromosomes and microtubules in living cells. J Cell Sci 111: 701-712 [DOI] [PubMed] [Google Scholar]
- Felix M-A, Antony C, Wright M, Maro B (1994) Centrosome assembly in vitro: role of γ-tubulin recruitment in Xenopus sperm aster formation. J Cell Biol 124: 19-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gislene P, Schiebel E (1997) Centrosome-microtubule nucleation. J Cell Sci 110: 295-300 [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D (1988) Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Horio T, Basaki A, Takeoka A, Yamato M (1999) Lethal level overexpression of γ-tubulin in fission yeast causes mitotic arrest. Cell Motil Cytoskel 44: 284-295 [DOI] [PubMed] [Google Scholar]
- Horio T, Kimura N, Basaki A, Tanaka Y, Noguchi T, Akashi T, Tanaka K (2002) Molecular and structural characterization of the spindle pole bodies in the fission yeast Schizosaccharomyces japonicus var. japonicus. Yeast 19: 1335-1350 [DOI] [PubMed] [Google Scholar]
- Horio T, Oakley BR (1994) Human γ-tubulin functions in fission yeast. J Cell Biol 126: 1465-1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T, Uzawa S, Jung MK, Oakley BR, Tanaka K, Yanagida M (1991) The fission yeast γ-tubulin is essential for mitosis and is localized at microtubule organizing centers. J Cell Sci 99: 693-700 [DOI] [PubMed] [Google Scholar]
- Joshi HC (1994) Microtubule organizing centers and γ-tubulin. Curr Opin Cell Biol 6: 55-62 [DOI] [PubMed] [Google Scholar]
- Joshi HC, Palacios MJ, McNamara L, Cleveland DW (1992) γ-Tubulin is a centrosomal protein required for cell cycle-dependent microtubule nucleation. Nature 356: 80-83 [DOI] [PubMed] [Google Scholar]
- Joshi HC, Palevitz BA (1996) γ-Tubulin and microtubule organization in plants. Trends Cell Biol 6: 41-44 [DOI] [PubMed] [Google Scholar]
- Jung MK, Prigozhina N, Oakley CE, Nogales E, Oakley BR (2001) Alanine-scanning mutagenesis of Aspergillus γ-tubulin yields diverse and novel phenotypes. Mol Biol Cell 12: 2119-2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop M, Pereira G. Geissler S, Grein K, Schiebel E (1997) The spindle pole body component Spc97p interacts with the γ-tubulin of Saccharomyces cerevisiae and functions in microtubule organization and spindle pole body duplication. EMBO J 16: 1550-1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop M, Schiebel E (1998) Receptors determine the cellular localization of a γ-tubulin complexes and thereby the site of microtubule formation. EMBO J 17: 3952-3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai F, Nagata T, Yahara N, Moriyama Y, Horio T, Naoi K, Hashimoto T, Hasezawa S (2003) γ-Tubulin distribution during cortical microtubule reorganization at the M/G1 interface in tobacco BY-2 cells. Eur J Cell Biol 82: 43-51 [DOI] [PubMed] [Google Scholar]
- Liu B, Joshi HC, Palevitz BA (1995) Experimental manipulation of γ-tubulin distribution in Arabidopsis using anti-microtubule drugs. Cell Motil Cytoskel 31: 113-129 [DOI] [PubMed] [Google Scholar]
- Liu B, Joshi HC, Wilson TJ, Silflow CD, Palevitz BA, Snustad DP (1994) γ-Tubulin in Arabidopsis: gene sequence, immunoblot, and immunofluorescence studies. Plant Cell 6: 303-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Marc J, Joshi HC, Palevitz BA (1993) A γ-tubulin-related protein associated with the microtubule arrays of higher plants in a cell cycle-dependent manner. J Cell Sci 104: 1217-1228 [DOI] [PubMed] [Google Scholar]
- Marschall LG, Jeng RL, Mullholland J, Stearns T (1996) Analysis of Tub4p, a yeast tubulin-like protein: implication for microtubule-organizing center function. J Cell Biol 134: 443-454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194: 795-823 [DOI] [PubMed] [Google Scholar]
- Oakley BR (1992) γ-Tubulin: The microtubule organizer? Trends Cell Biol 2: 1-5 [DOI] [PubMed] [Google Scholar]
- Oakley BR, Akkari YN (1999) γ-Tubulin at ten: progress and prospects. Cell Struct Funct 24: 365-372 [DOI] [PubMed] [Google Scholar]
- Oakley BR, Oakley CE, Yoon Y, Jung MK (1990) γ-Tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergillus nidulans. Cell 61: 1289-1301 [DOI] [PubMed] [Google Scholar]
- Oegema K, Wiese C, Martin OC, Milligan RA, Iwamatsu A, Mitchison TJ, Zheng Y (1999) Characterization of two related Drosophila γ-tubulin complexes that differ in their ability to nucleate microtubules. J Cell Biol 144: 721-733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel SG, Snyder M (1995) A highly divergent γ-tubulin gene is essential for cell growth and proper microtubule organization in Saccharomyces cerevisiae. J Cell Biol 131: 1775-1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A, Geissler S, Grein K, Schiebel E (1996) γ-Tubulin-like Tub4p of Saccharomyces cerevisiae is associated with the spindle pole body substructures that organize microtubule and is required for mitotic spindle formation. J Cell Biol 134: 429-441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T, Evans L, Kirschner M (1991) γ-Tubulin is a highly conserved component of the centrosome. Cell 65: 825-836 [DOI] [PubMed] [Google Scholar]
- Vaughn KC, Harper DI (1998) Microtubule-organizing centers and nucleating sites in land plants. Int Rev Cytol 181: 75-149 [DOI] [PubMed] [Google Scholar]
- Wasteneys GO (2002) Microtubule organization in the green kingdom: chaos or self-order? J Cell Sci 115: 1345-1354 [DOI] [PubMed] [Google Scholar]
- Woods A, Sherwin T, Sasse R, MacRae TH, Baines AJ, Gull K (1989) Definition of individual components within the cytoskeleton of Trypanosoma burucei by a library of monoclonal antibodies. J Cell Sci 93: 491-500 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Jung MK, Oakley BR (1991) γ-Tubulin is present in Drosophila melanogaster and Homo sapiens and is associated with the centrosome. Cell 65: 817-823 [DOI] [PubMed] [Google Scholar]