Abstract
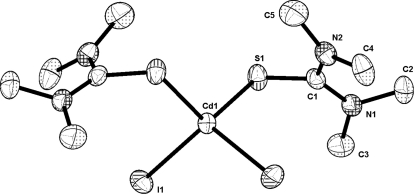
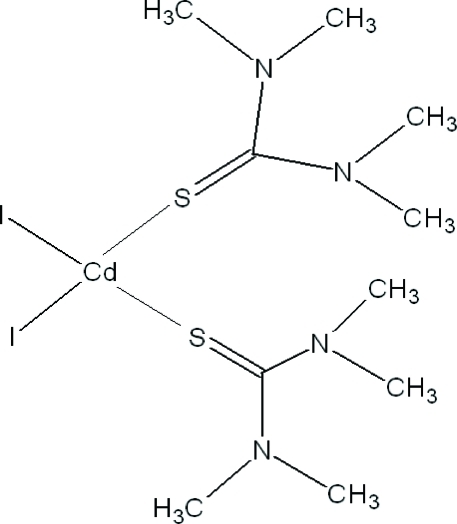
In the title compound, [CdI2(C5H12N2S)2], the CdII ion is located on a twofold rotation axis and is coordinated in a distorted tetrahedral mode by two iodide ions and by two tetramethylthiourea (tmtu) ligands through their S atoms. The crystal structure is stabilized by C—H⋯N and C—H⋯S hydrogen bonds.
Related literature
For background to thiourea complexes of group 12 elements, see: Ahmad et al. (2009 ▶); Bell et al. (2001 ▶, 2004 ▶); Lobana et al. (2008 ▶); Marcos et al. (1998 ▶); Matsunaga et al. (2005 ▶); Moloto et al. (2003 ▶); Wazeer et al. (2007 ▶). The structure of the title compound is isotypic with [Cd(tmtu)2Br2] (Nawaz et al., 2010a
▶) and [Hg(tmtu)2Cl2] (Nawaz et al., 2010b
▶).
Experimental
Crystal data
[CdI2(C5H12N2S)2]
M r = 630.65
Monoclinic,
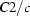
a = 18.985 (5) Å
b = 10.395 (3) Å
c = 13.719 (4) Å
β = 130.740 (4)°
V = 2051.4 (9) Å3
Z = 4
Mo Kα radiation
μ = 4.27 mm−1
T = 294 K
0.33 × 0.22 × 0.20 mm
Data collection
Bruker SMART APEX area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.333, T max = 0.482
13642 measured reflections
2557 independent reflections
2235 reflections with I > 2σ(I)
R int = 0.022
Refinement
R[F 2 > 2σ(F 2)] = 0.023
wR(F 2) = 0.054
S = 1.04
2557 reflections
91 parameters
H-atom parameters constrained
Δρmax = 0.67 e Å−3
Δρmin = −0.58 e Å−3
Data collection: SMART (Bruker, 2008 ▶); cell refinement: SAINT (Bruker, 2008 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810028114/wm2373sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810028114/wm2373Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C2—H2A⋯N2 | 0.96 | 2.51 | 2.859 (6) | 101 |
| C4—H4A⋯N1 | 0.96 | 2.52 | 2.853 (5) | 100 |
| C5—H5A⋯S1 | 0.96 | 2.66 | 3.026 (5) | 103 |
Acknowledgments
We gratefully acknowledge King Fahd University of Petroleum and Minerals, Dhahran, Saudi Arabia, for providing the X-ray facility.
supplementary crystallographic information
Comment
A considerable amount of work has been done in recent years on the synthesis and characterization of cadmium(II) and mercury(II) complexes of thiourea type ligands due to their variable binding modes and because of the their importance in biological systems (Ahmad et al., 2009; Bell et al., 2001, 2004; Lobana et al., 2008; Marcos et al., 1998; Matsunaga et al., 2005; Moloto et al., 2003; Wazeer et al., 2007). Cadmium(II) complexes with thiones possess a variety of structures ranging from four- to six-coordinate species with tetrahedral and octahedral environments for the CdII atom, respectively. In some cases, these units further aggregate to form polymeric structures (Bell et al., 2001, 2004; Lobana et al., 2008; Matsunaga et al., 2005; Moloto, et al., 2003; Wazeer et al., 2007). We report here the crystal structure of a cadmium(II) iodide complex with tetramethylthiourea (tmtu).
In the title complex, the cadmium atom is bonded to two I- ions and to two tetramethylthiourea ligands through the sulfur atoms in a distorted tetrahedral mode (Fig. 1). The compound is isotypic with [Cd(tmtu)2Br2] (Nawaz et al., 2010a) and [Hg(tmtu)2Cl2] (Nawaz et al., 2010b).
For a more detailed description of the structure, see: Nawaz et al. (2010a).
Experimental
To 0.37 g (1.0 mmol) cadmium(II) iodide in 10 ml water was added to two equivalents of tetramethylthiourea in 15 ml methanol. A clear solution was obtained that was stirred for 30 minutes. The colorless solution was filtered and the filtrate was kept at room temperature for crystallization. As a result, a white crystalline product was obtained, that was washed with methanol and dried.
Refinement
H atoms were placed in calculated positions with a C—H distance of 0.96 Å and Uiso(H) = 1.5 Ueq(C).
Figures
Fig. 1.
The molecular structure of title compound with atomic numbering scheme. Displacement ellipsoids drawn at the 30% probability level. H-atoms were omitted for clarity.
Crystal data
| [CdI2(C5H12N2S)2] | F(000) = 1192 |
| Mr = 630.65 | Dx = 2.042 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -C 2yc | Cell parameters from 13642 reflections |
| a = 18.985 (5) Å | θ = 2.4–28.3° |
| b = 10.395 (3) Å | µ = 4.27 mm−1 |
| c = 13.719 (4) Å | T = 294 K |
| β = 130.740 (4)° | Block, colorless |
| V = 2051.4 (9) Å3 | 0.33 × 0.22 × 0.20 mm |
| Z = 4 |
Data collection
| Bruker SMART APEX area-detector diffractometer | 2557 independent reflections |
| Radiation source: normal-focus sealed tube | 2235 reflections with I > 2σ(I) |
| graphite | Rint = 0.022 |
| ω scans | θmax = 28.3°, θmin = 2.4° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −25→25 |
| Tmin = 0.333, Tmax = 0.482 | k = −13→13 |
| 13642 measured reflections | l = −18→18 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.023 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.054 | H-atom parameters constrained |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0229P)2 + 2.9795P] where P = (Fo2 + 2Fc2)/3 |
| 2557 reflections | (Δ/σ)max = 0.002 |
| 91 parameters | Δρmax = 0.67 e Å−3 |
| 0 restraints | Δρmin = −0.58 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cd1 | 1.0000 | 0.71325 (3) | 0.2500 | 0.04562 (8) | |
| I1 | 1.155686 (14) | 0.56794 (2) | 0.35345 (2) | 0.06074 (8) | |
| S1 | 1.03756 (5) | 0.84000 (9) | 0.43960 (7) | 0.05829 (19) | |
| N1 | 0.92464 (19) | 0.7668 (2) | 0.4801 (3) | 0.0568 (6) | |
| N2 | 0.85712 (18) | 0.8932 (3) | 0.3011 (2) | 0.0569 (6) | |
| C1 | 0.93150 (19) | 0.8328 (3) | 0.4031 (3) | 0.0462 (6) | |
| C2 | 0.8634 (3) | 0.8067 (4) | 0.5045 (4) | 0.0777 (10) | |
| H2A | 0.8343 | 0.8869 | 0.4619 | 0.116* | |
| H2B | 0.8992 | 0.8168 | 0.5954 | 0.116* | |
| H2C | 0.8165 | 0.7423 | 0.4722 | 0.116* | |
| C3 | 0.9920 (3) | 0.6671 (4) | 0.5676 (4) | 0.0870 (12) | |
| H3A | 1.0154 | 0.6268 | 0.5308 | 0.130* | |
| H3B | 0.9622 | 0.6037 | 0.5808 | 0.130* | |
| H3C | 1.0425 | 0.7054 | 0.6486 | 0.130* | |
| C4 | 0.7619 (2) | 0.8470 (4) | 0.2310 (4) | 0.0808 (11) | |
| H4A | 0.7637 | 0.7632 | 0.2621 | 0.121* | |
| H4B | 0.7299 | 0.8422 | 0.1408 | 0.121* | |
| H4C | 0.7297 | 0.9054 | 0.2444 | 0.121* | |
| C5 | 0.8660 (3) | 0.9963 (4) | 0.2369 (4) | 0.0840 (11) | |
| H5A | 0.9259 | 1.0360 | 0.2972 | 0.126* | |
| H5B | 0.8184 | 1.0594 | 0.2048 | 0.126* | |
| H5C | 0.8594 | 0.9610 | 0.1666 | 0.126* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cd1 | 0.04989 (15) | 0.04876 (16) | 0.04920 (15) | 0.000 | 0.03717 (13) | 0.000 |
| I1 | 0.05619 (12) | 0.06315 (14) | 0.06036 (13) | 0.01437 (9) | 0.03692 (11) | 0.00562 (9) |
| S1 | 0.0509 (4) | 0.0769 (5) | 0.0529 (4) | −0.0122 (4) | 0.0365 (3) | −0.0185 (4) |
| N1 | 0.0677 (15) | 0.0552 (14) | 0.0648 (15) | 0.0083 (12) | 0.0508 (14) | 0.0065 (12) |
| N2 | 0.0576 (14) | 0.0651 (15) | 0.0490 (13) | 0.0072 (12) | 0.0352 (12) | −0.0014 (11) |
| C1 | 0.0517 (14) | 0.0473 (14) | 0.0470 (13) | −0.0003 (11) | 0.0355 (12) | −0.0087 (11) |
| C2 | 0.092 (3) | 0.090 (3) | 0.091 (3) | 0.005 (2) | 0.077 (2) | 0.003 (2) |
| C3 | 0.109 (3) | 0.067 (2) | 0.103 (3) | 0.025 (2) | 0.077 (3) | 0.030 (2) |
| C4 | 0.0502 (17) | 0.118 (3) | 0.068 (2) | 0.0085 (19) | 0.0360 (17) | −0.014 (2) |
| C5 | 0.102 (3) | 0.081 (3) | 0.064 (2) | 0.019 (2) | 0.052 (2) | 0.0211 (19) |
Geometric parameters (Å, °)
| Cd1—S1 | 2.5670 (9) | C2—H2B | 0.9600 |
| Cd1—S1i | 2.5670 (10) | C2—H2C | 0.9600 |
| Cd1—I1i | 2.7489 (7) | C3—H3A | 0.9600 |
| Cd1—I1 | 2.7489 (7) | C3—H3B | 0.9600 |
| S1—C1 | 1.731 (3) | C3—H3C | 0.9600 |
| N1—C1 | 1.335 (4) | C4—H4A | 0.9600 |
| N1—C2 | 1.465 (4) | C4—H4B | 0.9600 |
| N1—C3 | 1.466 (4) | C4—H4C | 0.9600 |
| N2—C1 | 1.330 (4) | C5—H5A | 0.9600 |
| N2—C5 | 1.464 (5) | C5—H5B | 0.9600 |
| N2—C4 | 1.468 (4) | C5—H5C | 0.9600 |
| C2—H2A | 0.9600 | ||
| S1—Cd1—S1i | 118.23 (5) | H2A—C2—H2C | 109.5 |
| S1—Cd1—I1i | 107.41 (2) | H2B—C2—H2C | 109.5 |
| S1i—Cd1—I1i | 105.36 (2) | N1—C3—H3A | 109.5 |
| S1—Cd1—I1 | 105.36 (2) | N1—C3—H3B | 109.5 |
| S1i—Cd1—I1 | 107.41 (2) | H3A—C3—H3B | 109.5 |
| I1i—Cd1—I1 | 113.34 (3) | N1—C3—H3C | 109.5 |
| C1—S1—Cd1 | 100.59 (9) | H3A—C3—H3C | 109.5 |
| C1—N1—C2 | 122.5 (3) | H3B—C3—H3C | 109.5 |
| C1—N1—C3 | 121.9 (3) | N2—C4—H4A | 109.5 |
| C2—N1—C3 | 114.3 (3) | N2—C4—H4B | 109.5 |
| C1—N2—C5 | 121.3 (3) | H4A—C4—H4B | 109.5 |
| C1—N2—C4 | 122.9 (3) | N2—C4—H4C | 109.5 |
| C5—N2—C4 | 114.9 (3) | H4A—C4—H4C | 109.5 |
| N2—C1—N1 | 119.4 (3) | H4B—C4—H4C | 109.5 |
| N2—C1—S1 | 121.3 (2) | N2—C5—H5A | 109.5 |
| N1—C1—S1 | 119.3 (2) | N2—C5—H5B | 109.5 |
| N1—C2—H2A | 109.5 | H5A—C5—H5B | 109.5 |
| N1—C2—H2B | 109.5 | N2—C5—H5C | 109.5 |
| H2A—C2—H2B | 109.5 | H5A—C5—H5C | 109.5 |
| N1—C2—H2C | 109.5 | H5B—C5—H5C | 109.5 |
Symmetry codes: (i) −x+2, y, −z+1/2.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C2—H2A···N2 | 0.96 | 2.51 | 2.859 (6) | 101 |
| C4—H4A···N1 | 0.96 | 2.52 | 2.853 (5) | 100 |
| C5—H5A···S1 | 0.96 | 2.66 | 3.026 (5) | 103 |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: WM2373).
References
- Ahmad, S., Sadaf, H., Akkurt, M., Sharif, S. & Khan, I. U. (2009). Acta Cryst. E65, m1191–m1192. [DOI] [PMC free article] [PubMed]
- Bell, N. A., Branston, T. N., Clegg, W., Parker, L., Raper, E. S., Sammon, C. & Constable, C. P. (2001). Inorg. Chim. Acta, 319, 130–136.
- Bell, N. A., Clegg, W., Coles, S. J., Constable, C. P., Harrington, R. W., Hursthouse, M. B., Light, M. E., Raper, E. S., Sammon, C. & Walker, M. R. (2004). Inorg. Chim. Acta, 357, 2091–2099.
- Bruker (2008). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Lobana, T. S., Sharma, R., Sharma, R., Sultana, R. & Butcher, R. J. (2008). Z. Anorg. Allg. Chem.634, 718–723.
- Marcos, C., Alía, J. M., Adovasio, V., Prieto, M. & García-Granda, S. (1998). Acta Cryst. C54, 1225–1229.
- Matsunaga, Y., Fujisawa, K., Amir, N., Miyashita, Y. & Okamoto, K.-I. (2005). J. Coord. Chem.58, 1047–1061.
- Moloto, M. J., Malik, M. A., O’Brien, P., Motevalli, M. & Kolawole, G. A. (2003). Polyhedron, 22, 595–603.
- Nawaz, S., Sadaf, S., Fettouhi, M., Fazal, A. & Ahmad, S. (2010a). Acta Cryst. E66, m950. [DOI] [PMC free article] [PubMed]
- Nawaz, S., Sadaf, H., Fettouhi, M., Fazal, A. & Ahmad, S. (2010b). Acta Cryst. E66, m952. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Wazeer, M. I. M., Isab, A. A. & Fettouhi, M. (2007). Polyhedron, 26, 1725–1730.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810028114/wm2373sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810028114/wm2373Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report