Abstract
A functional explanation for the regulation of grain nitrogen (N) accumulation in cereal by environmental and genetic factors remains elusive. Here, new mechanistic hypotheses of grain N accumulation are proposed and tested for wheat (Triticum aestivum). First, we tested experimentally the hypothesis that grain N accumulation is mostly source regulated. Four contrasting cultivars, in terms of their grain N concentrations and yield potentials, were grown with non-limiting N supply. Grain number per ear was reduced by removing the top part of the ear at anthesis. Reduction in grain number gave a significant increase in N content per grain for all cultivars, showing that grain N accumulation was source regulated. However, on a per ear basis, cultivars with a high grain number fully compensated their N accumulation for reduced grain number at anthesis. Cultivars with a lower grain number did not compensate completely, and grain N per ear was decreased by 16%. Second, new mechanistic hypotheses of the origins of grain N source regulation and its response to environment were tested by simulation. The hypotheses were: (a) The regulation by N sources of grain N accumulation applies only for the storage proteins (i.e. gliadin and glutenin fractions); (b) accumulation of structural and metabolic proteins (i.e. albumin-globulin and amphiphilic fractions) is sink-regulated; and (c) N partitioning between gliadins and glutenins is constant during grain development and unmodified by growing conditions. Comparison of experimental and simulation results of the accumulation of grain protein fractions under wide ranges of N fertilization, temperatures, and irrigation supported these hypotheses.
One challenge for global nutrition in the next decade is to increase food yield per unit ground area in a sustainable manner while maintaining its end use value (Cassman, 1999; Tilman, 1999; Tilman et al., 2002). Grain protein concentration and composition are major determinants of grain nutritional value (Feil, 1997). The concentration of Lys in grain, the most limiting amino acid in cereals for human and monogastric animals, increases with increasing grain protein concentration (Feil, 1997) despite the decrease of its concentration in total protein (Mossé et al., 1985). Grain protein concentration and composition are also the major determinants of flour functional properties (Weegels et al., 1996; Shewry and Halford, 2002). However, the inverse relationship between grain yield and protein concentration, reported for several species, may prevent breeders from improving these two traits simultaneously (Stewart and Dwyer, 1990; Delzer et al., 1995; Feil, 1997; Brancourt-Hulmel et al., 2003). To break this inverse relationship, genetic increments in grain protein yield must keep pace with those in grain yield. Therefore, efforts to overcome the inverse relationship between grain yield and protein concentration must concentrate on improving grain protein accumulation per square meter and per grain (Feil, 1997; Triboï and Triboï-Blondel, 2002).
An increase in grain protein content may come from either improved capacity of the grain to accumulate nitrogen (N) or through greater N supply to the grains (Triboï and Triboï-Blondel, 2002). Several studies have shown some degree of control over grain N by intrinsic grain characteristics for wheat (Triticum aestivum; Borghi et al., 1986), barley (Hordeum vulgare; Mattsson et al., 1993), and maize (Zea mays; Wyss et al., 1991). Although others have shown control of grain N accumulation by the level of N supply for wheat (Barlow et al., 1983; Barneix and Guitman, 1993; Ma et al., 1995, 1996), barley (Dreccer et al., 1997; Voltas and Araus, 1997), maize (Wyss et al., 1991), pea (Pisum sativum; Lhuillier-Soundele et al., 1999a, 1999b), and soybean (Glycine max; Saravitz and Raper, 1995; Nakasathien et al., 2000). Comparison of the capacity of in vitro-cultured grains or seeds from low- and high-protein genotypes of wheat (Donovan et al., 1977), maize (Wyss et al., 1991), and soybean (Hayati et al., 1996) to accumulate N has led to the conclusion that genetic differences in grain or seed N content and concentration are caused, at least in part, by differences in protein synthetic capacity. The opposite conclusion was reached for barley when comparing a high-protein accession of wild barley (Hordeum spontaneum Koch), with low-protein barley cv Ruth, which were able to accumulate 300 and 350 g proteins kg-1 dry mass, respectively (Corke and Atsmon, 1988). Hence, it is still not clear if environmental and genetic differences in grain protein accumulation are regulated by process within the grains or by the N supply from the vegetative organs or are colimited by both (Feil, 1997; Triboï and Triboï-Blondel, 2002). Nevertheless, most crop and plant simulation models assume grain N accumulation to be sink regulated (e.g. Porter, 1993; for full reference, see Jamieson and Semenov, 2000). Most studies of the regulation of grain N accumulation for cereals have not considered the partitioning of N in the grain. We believe that to make progress in our understanding of the regulation of grain N accumulation, we should consider the physiological function of N in the grain.
Grain proteins can be divided into structural/metabolic (Nstru) and storage (Nsto) proteins (Shewry and Halford, 2002). Structural/metabolic proteins consist of albumin, globulin, and amphiphilic proteins. In wheat, storage proteins are divided into two broad fractions. These are gliadins (Ngli), which are present as monomers, and glutenins (Ngln), which form polymers. Structural/metabolic protein fractions accumulate mainly during the early phase of grain growth, when most endosperm cells are still dividing, whereas the accumulation of storage proteins fractions occurs later when cell division as stopped and grain growth is only due to cell expansion (Stone and Nicolas, 1996; Triboï et al., 2003). Although grain protein composition depends primarily on genotype, it is significantly affected by environmental factors and their interactions (Graybosch et al., 1996; Huebner et al., 1997; Triboï et al., 2000; Zhu and Khan, 2001). However, the mechanism by which genotype and environmental factors modified the accumulation of the protein fractions are unknown, and, to date, no attempt has been made to model the partitioning of grain or seed N to different protein fractions.
In this study, we manipulated the sink to source ratio of four contrasted wheat cultivars to show that, overall, grain N is regulated by the supply of N to the grain. This was further confirmed by a simulation study using the wheat simulation model Sirius (Jamieson and Semenov, 2000), in which grain N accumulation is driven by N availability in the sources. We were able to explain a wide variation in observed grain N concentration at the canopy level, induced by N fertilization and postanthesis high and low air temperatures and water deficit. In this paper, we extended the concepts of grain N dynamic in Sirius to include a functional explanation for the regulation of the source regulation of grain N accumulation in a series of new mechanistic hypotheses formalized as a simulation model of the accumulation of grain protein fractions. The main hypotheses were: (a) The apparent overall source regulation of grain N accumulation is due to the synthesis of storage proteins, (b) the synthesis of structural and metabolic proteins is sink regulated, and (c) the allocation of N between the storage protein fractions gliadin and glutenin is constant during grain filling and is not modified by growing conditions. Comparison of experimental and simulated results for a wide range of environmental conditions provided a strong support to these functional hypotheses.
RESULTS
Grain N Accumulation Is Source Regulated for Both High- and Low-Yielding Cultivars
First, we analyzed the level of supply limitation of grain N accumulation in four cultivars with different potential grain numbers per square meter, an increase in which has been one of the major factors contributing to grain yield increases over the last 40 years (Reynolds et al., 1999; Brancourt-Hulmel et al., 2003). Sink to source ratio was modified by removing the top part of the ear on the main stems at anthesis or 250 degree-days (°Cd) later. The experiment was done in the field under non-limiting soil N supply.
Grain yield, yield components, and N content and concentration for the four cultivars and the different treatments show that, under normal conditions, grain number per ear was highest for the cultivars Arche and Récital, intermediate for Renan, and lowest for Tamaro (Table I). Grain yield was not significantly different for the cultivars Arche, Récital, and Renan but was 52% to 60% lower for Tamaro compared with the three other cultivars. The four cultivars analyzed could be separated as low (Arche and Récital) and high (Renan and Tamaro) protein cultivars (Table I).
Table I.
Grain yield components, N content, and protein concentration for wheat crops grown in the field with non-limiting N supply
The top parts of the ears were removed either at anthesis (treatment Abl.) or 250 °Cd later (Abl.250). Data are means ± se (n = 3 repetitions each of 10 plants). Within columns, different letters indicate significant differences for a variety due to ear halving treatment using a one-way ANOVA (α = 0.05) followed by an lsd test (P < 0.05). Significance of cultivar and treatment effects and their interactions were measured using a multifactor ANOVA (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
| Cultivars
|
Treatments
|
Grain No.
|
Grain Dry Mass
|
Grain N
|
|||
|---|---|---|---|---|---|---|---|
| grain ear−1 | grain m−2 | g ear−1 | g m−2 | mg N grain−1 | mg N g−1dry mass | ||
| Arche | Control | 36.3 ± 0.4c | 22,452 ± 616c | 1.26 ± 0.01c | 778 ± 15b | 0.80 ± 0.02a | 22.9 ± 0.6a |
| Abl. | 26.8 ± 0.8b | 16,549 ± 515b | 1.14 ± 0.04b | 706 ± 22b | 1.09 ± 0.02b | 25.4 ± 0.2b | |
| Abl.250 | 22.0 ± 0.6a | 13,603 ± 367a | 0.90 ± 0.02a | 554 ± 10a | 1.03 ± 0.02b | 25.2 ± 0.3b | |
| Récital | Control | 38.9 ± 0.6c | 18,370 ± 634c | 1.31 ± 0.05b | 685 ± 58b | 0.75 ± 0.02a | 22.3 ± 0.4a |
| Abl. | 28.3 ± 0.3b | 14,758 ± 153b | 1.22 ± 0.01b | 636 ± 7b | 1.07 ± 0.01c | 24.8 ± 0.2b | |
| Abl.250 | 23.4 ± 1.0a | 12,216 ± 523a | 0.89 ± 0.03a | 466 ± 17a | 0.94 ± 0.02b | 24.5 ± 0.4b | |
| Renan | Control | 26.0 ± 0.8b | 16,160 ± 514b | 1.09 ± 0.01b | 678 ± 30b | 1.11 ± 0.02a | 26.4 ± 0.3a |
| Abl. | 17.6 ± 1.5a | 10,942 ± 964a | 0.79 ± 0.08a | 492 ± 50a | 1.38 ± 0.04c | 30.7 ± 0.4b | |
| Abl.250 | 15.7 ± 0.4a | 9,774 ± 245a | 0.66 ± 0.02a | 414 ± 10a | 1.23 ± 0.03b | 29.1 ± 0.2b | |
| Tamaro | Control | 23.4 ± 1.2b | 10,760 ± 584b | 0.89 ± 0.05b | 407 ± 23b | 1.02 ± 0.01a | 27.0 ± 0.6a |
| Abl. | 17.1 ± 0.6a | 7,845 ± 277a | 0.64 ± 0.01a | 293 ± 6a | 1.16 ± 0.03b | 31.1 ± 0.6b | |
| Abl.250 | 14.6 ± 1.2a | 6,728 ± 532a | 0.54 ± 0.05a | 249 ± 24a | 1.11 ± 0.01ab | 30.1 ± 0.7b | |
| Main Effects | |||||||
| Treatment | *** | *** | *** | *** | *** | *** | |
| Cultivar | *** | *** | *** | *** | *** | *** | |
| Interaction | |||||||
| Treatment × cultivar | * | n.s.a | * | n.s. | ** | n.s. | |
n.s., Not significant.
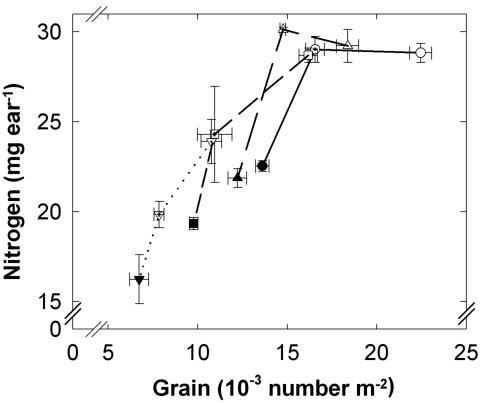
The ear halving treatment at anthesis reduced the number of grains per ear (i.e. per square meter) by 26% to 32%. This treatment leaded to an increase in N content per grain of 37%, 43%, 25%, and 14% for Arche, Récital, Renan, and Tamaro, respectively (Table I). However, not all cultivars fully compensated for the reduced grain number. Grain N per ear decreased by 16% for the two cultivars with the lower grain number per square meter (i.e. Renan and Tamaro; Fig. 1). In contrast, grain N per ear was not modified by the reduction in grain number per ear at anthesis for the two cultivars with the higher grain number per square meter (i.e. Arche and Récital).
Figure 1.
Final quantity of total grain N per ear versus the grain number per square meter of intact (control; white symbols) and halved (either at anthesis, crossed symbols; or at 250 °Cd after anthesis, black symbols) ears for four varieties of wheat grown in the field under non-limiting soil N fertilization (Arche, circles; Récital, face-up triangles; Renan, squares; and Tamaro, face-down triangles). Data are means ± 1 se for n = 3 replicates each of 30 ears.
Ear halving at 250 °Cd after anthesis reduced the sink size by 37% to 40%, leading to an increase of N content per grain of 29%, 24%, 12%, and 9% for Arche, Récital, Renan, and Tamaro, respectively (Table I), whereas grain N per ear decreased by 22% to 33% for all four cultivars (Fig. 1).
Variations of Grain N Can Be Predicted Based on the Level of N Supply from the Plant
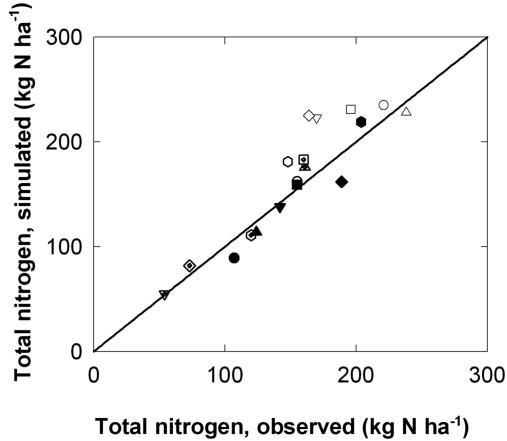
Regulation of grain N accumulation was further analyzed by simulating N uptake and redistribution for wheat crops grown in the field with a combination of rates and timings of N fertilization and in controlled environments, where different postanthesis temperatures and watering regimes were applied postanthesis. The wheat simulation model Sirius was used to simulate dry matter and N accumulation in the different organs of the crops for these experiments. Simulated and observed kinetics of grain N accumulation for the different experimental treatments agreed well (data not shown), and simulated and observed final grain N were well correlated (r2 = 0.83, 16 degrees of freedom [d.f.]; Fig. 2). The square root of the mean square error of prediction was 2.5 g N m-2 over a range of 5.4 to 23.8 g N m-2.
Figure 2.
Relationship between observed and simulated total N for mature grains obtained from crops of wheat. Simulations were performed using the Sirius V99 simulation model. Crops were grown either in the controlled environment closed-top chambers with different postanthesis temperatures (○, treatment -5; ▵, 0; □,+5; ▿,+5/+10; and ⋄,+10/+5) or with different postanthesis temperatures and watering regimes ([hexagon], -5W; ○ with cross, 5D; ▵ with cross, +5W; and □ with cross, +5D), or in the field with different rates and timings of N fertilization (▿ with cross, L0; ⋄ with cross, L3; [hexagon] with cross, L15; •, M0; ▴, M3; ▪, M15; ▾, H0; ♦, H3; and [hexagonblack], H15). Treatments are denoted as outlined in “Materials and Methods.” The solid line is y = x.
Sink/Source Regulation of Grain N Accumulation Revisited
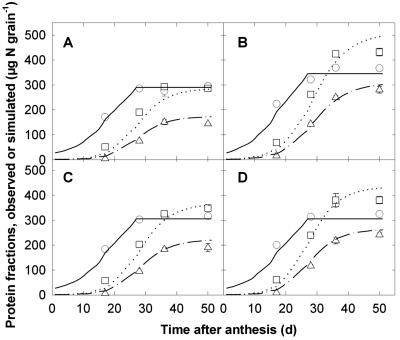
Consideration of the protein fractions in the grain gives a new perspective to the supply regulation of grain N accumulation. An example of the kinetics of accumulation of Nstru, Ngli, and Ngln obtained for crops grown in the field with a combination of two rates and timings of N fertilization is shown in Figure 3. Pre-anthesis N fertilization increased only slightly (7%) the final quantity of Nstru but increased the final quantities of Ngli and Ngln by 33% and 22%, respectively (Fig. 3, A and C). Under conditions of preanthesis N shortage, N fertilization at anthesis increased the final quantity of Nstru by 25% but that of Ngli and Ngln by 95% and 49%, respectively (Fig. 3, A and B). Under conditions of normal pre-anthesis N fertilization, postanthesis N fertilization increased the final quantity of Nstru, Ngli, and Ngln by 3%, 26%, and 9%, respectively (Fig. 3, C and D). Thus, the accumulation of Ngli and Ngln are significantly enhanced by N fertilization, whereas Nstru is little affected.
Figure 3.
Observed (symbols) and simulated (lines) quantities of structural/metabolic (○, ——), gliadin (▵, —·—), and glutenin (□,...) proteins versus the number of days after anthesis for grains obtained from wheat crops grown in the field. Crops received either 0 g N m-2 (treatment L) or 10 g N m-2 (H) at the beginning of stem elongation, followed by either 3 (L3 or H3) or 15 (L15 or H15) g N m-2 at anthesis. A, Treatment L3; B, L15; C, H3; D, H15. Observed data are means ± 1 se for n = 3 replicates.
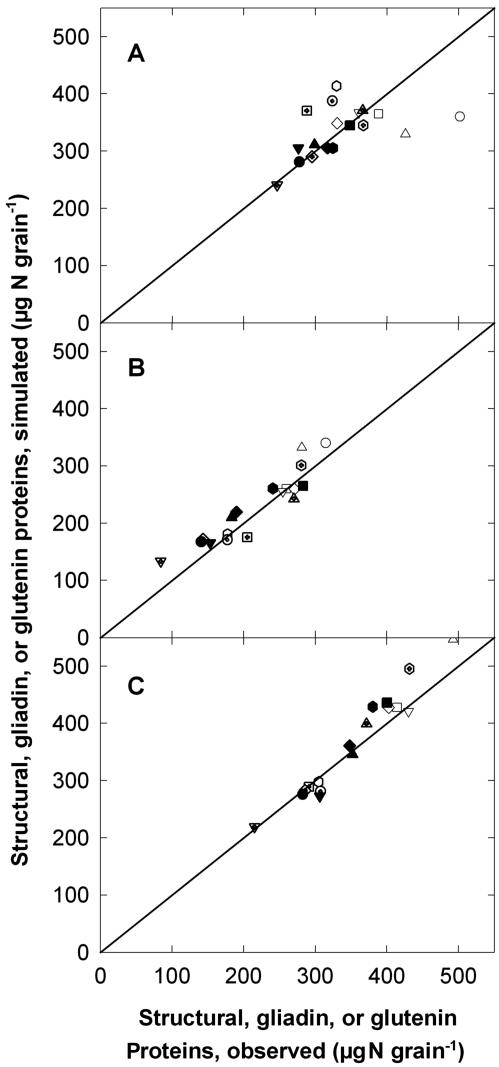
The model of accumulation of grain protein fractions described here gave accurate simulations of the accumulation of Nstru, Ngli, and Ngln, even for conditions of non-limiting soil N supply, such as the treatment H15 (Fig. 3). Similar agreement was observed for the 14 other treatments of Figure 2 (data not shown). Simulated and observed Ngli (r2 = 0.86, 16 d.f.) and Ngln (r2 = 0.96, 16 d.f.) at harvest ripeness were well correlated (Fig. 4). The square root of the mean square error of prediction was 26 μg N grain-1 over a range of 84 to 315 μg N grain-1 for Ngli and 31 μg N grain-1 over a range of 215 to 508 μg N grain-1 for Ngln.
Figure 4.
Relationship between observed and simulated quantity of protein fractions for mature grain obtained from crops of wheat. A, Structural/metabolic; B, gliadin; C, glutenin proteins. Simulations were performed using the model of grain N partitioning described in “Materials and Methods.” Crops were grown either in the controlled environment closed-top chamber with different postanthesis temperatures (○, treatment -5; ▵, 0; □, +5; ▿, +5/+10; and ⋄, +10/+5) or with different postanthesis temperatures and watering regimes ([hexagon], -5W; ○ with cross, -5D; ▵ with cross, +5W; and □ with cross, +5D), or in the field with different rates and timings of N fertilization (▿ with cross, L0; ⋄ with cross, L3; [hexagon] with cross, L15; •, M0; ▴, M3; ▪, M15; ▾, H0; ♦, H3; and [hexagonblack], H15). Treatments are denoted as outlined in “Materials and Methods.” The solid lines are y = x.
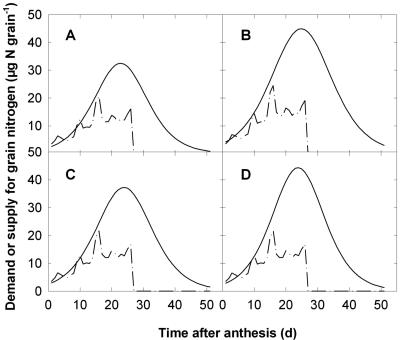
The supply limitation of grain N uptake may apply predominantly at a particular stage of the development of the grain. Hence, we used our model of grain N partitioning to analyze the joint evolution of the demand for Nstru (Nstrudemand) and the supply of total N (Ntotsupply; Fig. 5). During the first 10 to 15 d after anthesis, Ntotsupply balanced Nstrudemand, indicating that grain N accumulation during that period was sink regulated. Nevertheless, during the period of accumulation of storage protein, i.e. from approximately 15 d after anthesis to grain maturity, Ntotsupply was 2 to 3 times higher than Nstrudemand, indicating that the accumulation of grain N was then limited by the supply of N.
Figure 5.
Time course of simulated demand for structural N (—·—) and supply of total N (——) during the development of wheat grains grown in the field with different rates and timing of N fertilization. Crops received either 0 (treatment L) or 10 (H) g N m-2 at the beginning of stem elongation, followed by either 3 (L3 or H3) or 15 (L15 or H15) g N m-2 at anthesis. A, Treatment L3; B, L15; C, H3; D, H15.
DISCUSSION
The experiments and simulations reported here were designed to analyze the source/sink regulation of grain N accumulation and to assess its genetic variability. Several lines of evidence from studies on detached ears cultured in vitro (Barlow et al., 1983; Corke and Atsmon, 1988) and isolated plants cultivated in pots under controlled environments (Barneix and Guitman, 1993; Dreccer et al., 1997) have suggested that grain N accumulation for many cereal species is source regulated, but apparently this never has been investigated at the canopy level under field or controlled environment conditions, and most studies have been limited to one genotype. Moreover, no functional hypothesis has been proposed to account for the source regulation of grain N accumulation. We modified the sink to source ratio of four contrasted genotypes of wheat grown in the field. The results show that the level of source regulation of grain N accumulation depends on the genotypes, but none of the genotypes were sink limited. The hypotheses framed above have been formulated in a simulation model that predicted the dynamic changes of grain protein composition, an important nutritional and economic trait for cereals. The simulation results presented here support these hypotheses over a broad range of environmental conditions.
Ear halving increases the availability of N to the remaining grains either at anthesis or 250 °Cd later, when cell division has ended and grain growth is solely due to cell expansion (Gleadow et al., 1982; Singh and Jenner, 1982). Ear halving has been shown to increase the final number of cells per grain for wheat (Brocklehurst, 1977). For both ear halving treatments, N content per grain increased for all four cultivars, indicating that the storage capacities of the grains were not reached for the control treatments; thus, the capacity of the sink to synthesize proteins did not regulate grain N accumulation for the four cultivars. If the N sources were the major regulators, one would expect grain N per ear to be constant, i.e. independent of grain number. In the experiments presented here, the two cultivars with the lower grain number per square meter and per ear (Renan and Tamaro) were unable to compensate completely for the reduced grain number per ear, so grain N accumulation for these cultivars became sink regulated. In contrast, the two cultivars with the higher grain number per square meter and per ear (Arche and Récital) were able to fully compensate for the reduced grain number per ear. Thus, grain N accumulation for these cultivars was still supply regulated. The only way we could introduce a sink limitation of grain N accumulation in these two cultivars was to artificially reduce the total sink number too late for compensation to occur. This latter result suggests that the compensation observed for Arche and Récital when ablated at anthesis was due to an increased cell number per endosperm.
We were able accurately to predict total grain N accumulation over a large range of grain N for Thésée, a high-yield potential and grain number cultivar, by assuming grain N accumulation to be determined by the size of the source of N, defined as the total nonstructural crop N at anthesis. This gives further support to the previous conclusion that, overall, the accumulation of grain N, at least for high-yielding cultivars with high grain number, is regulated by the source of N and not by the activity of the grain.
Using the model of accumulation of protein fractions described here, the comparison of the simulated demand and supply of grain N suggested that grain N accumulation was sink limited or colimited by both source and sink for the first 10 to 15 d after anthesis. This emphasizes the importance of the early stage of grain development, characterized by active cell division in the endosperm, in setting the potential grain size and Nstru. In contrast with the early phase of grain development, grain N accumulation was always source limited during the grain filling period, even when soil N was non-limiting. Moreover, simulations and observations of the accumulation of grain protein fractions for developing grains obtained from plants grown in the field with different rates and timings of N fertilization and in the controlled environment chambers with different postanthesis temperatures and watering regimes agreed well, verifying the hypothesis that the supply limitation of grain N accumulation results from the accumulation of storage proteins and not from that of structural proteins. Thus, the sink/source limitation of grain protein accumulation is related to differences in the timing of deposition between structural/metabolic proteins versus storage proteins, as postulated earlier for barley (Dreccer et al., 1997).
The source regulation of the accumulation of storage proteins gives a mechanistic explanation of the effect of overexpressing glutenin genes on protein composition and concentration where the transformation of wheat with high-Mr glutenin subunit genes results in increased quantities and proportions of the high-Mr glutenin subunits (Altpeter et al., 1996; Blechl and Anderson, 1996; Barro et al., 1997; Alvarez et al., 2000) but with no difference in total protein quantity and concentration (Rooke et al., 1999). The source regulation of the accumulation of storage proteins is also in good agreement with the presence of two regulatory elements in the promoter region of the genes of several grain storage proteins: the “endosperm motif,” which act as a positive element under high-N conditions; and the “GNC4-like motif,” which act as a negative element under low-N conditions for grains of barley (Hammond-Kosack et al., 1993; Müller and Knudsen, 1993).
The hypothesis introduced in our simulation model of grain protein accumulation that the partitioning coefficient for Ngli and Ngln is constant during grain development and is not modified by the growth conditions was verified. This implies that any modification of the gliadins to glutenins ratio is only the result of modification of total N content per grain and that the processes leading to the synthesis of storage proteins in the grain are not affected by the concentration of N. We observed similar result for the albumin-globulin and the amphiphilic proteins, the constituent of Nstru (data not shown). Preliminary data indicate that this is true for the other cultivars studied here, i.e. Arche, Récital, Renan, and Tamaro (V. Samoil, P. Martre, and E. Triboï, unpublished data), and, importantly, the same partitioning parameters applied. These results imply that the protein fractions and amino acids composition of wheat grains from widely different cultivars can be deduced directly from the total quantity of N per grain. Furthermore, we suggest that the genotype-environment interactions for the composition of protein fractions reported earlier (Graybosch et al., 1996; Triboï et al., 2000) act only via variations of total grain N and, thus, N availability and not via the allocation of N between the different protein fractions.
Functional genomics and proteomics studies aiming at understanding the regulation of grain protein level and composition for cereals, especially wheat (Clarke et al., 2001; Lagudah et al., 2001; Shewry et al., 2001) and rice (Oryza sativa; Tyagi and Mohanty, 2000), have focused on the “protein warehouse” (i.e. the grain). These studies are valuable to better understand the development of the grain and to genetically modify grain protein composition and increase the sink demand. However, the supply limitation of grain N accumulation, as shown here, means that to increase grain yield while maintaining high nutritional and processing values requires understanding of the functioning of the “protein factory” (i.e. the vegetative organs) and its interaction with the grain.
MATERIALS AND METHODS
All experiments were at Clermont-Ferrand, France (45°47′ N, 3°10′ E, 329-m elevation) with winter wheat (Triticum aestivum) cv Thésée, Arche, Récital, Renan, and Tamaro.
Ear Halving Experiments
Source/sink regulation of grain N accumulation was studied in the field for four cultivars (Arche, Récital, Renan, and Tamaro) with contrasting potential grain number, grain yield, and grain protein concentration. One main plot of 202 m2 was sown for each cultivar on November 7, 2001 at a density of 300 grains m-2. The crops were rain fed. Accumulated rainfall from sowing to anthesis and from anthesis to grain maturity was 124 to 174 and 115 to 135 mm depending on the cultivar, respectively. Average air temperature from sowing to anthesis and from anthesis to grain maturity was 6.8°C to 7.3°C and 17.8°C to 18.7°C depending on the cultivar, respectively. The crops received 10 g N m-2 on March 3, 2002 and 15 g N m-2 on April 30, 2002. Anthesis was recorded on May 24, 15, 21, and 28, 2002 for Arche, Récital, Renan, and Tamaro, respectively. The source to sink ratio was artificially manipulated by removing the top one-half or so of the ears from the main stems on three 0.5-m-2 subplots per cultivar. At the same time, three 0.5-m-2 control subplots were identified for each cultivar. Ear halving was performed either at anthesis or 249, 277, 254, and 244 °Cd later for Arche, Récital, Renan, and Tamaro, respectively. Samples of 0.5 m2 were taken in each subplot at the time when ears were halved and at grain maturity. Three replicates were used per N treatment.
Temperature and Drought Experiments
To study the effects of postanthesis temperature and drought at the canopy level, crops of wheat cv Thésée were grown in 2-m2 containers in controlled environment closed-top chambers under natural light (Triboï et al., 2003). From 5 d after anthesis to grain maturity, five air temperatures relative to ambient air temperature were applied in the chambers: -5°C (treatment termed -5, average temperature of 14.9°C); 0°C (0, average temperature of 19.5°C); +5°C (+5, average temperature of 22.3°C); +5°C until 300 °Cd, base 0°C after anthesis, then +10°C until harvest maturity (+5/+10, average temperature of 24.7°C); and +10°C until 300 °Cd after anthesis then +5°C until harvest maturity (+10/+5, average temperature of 23.7°C).
Interactions between postanthesis temperature and drought were studied in a 2nd year of experiments where two air temperatures (-5°C and +5°C, average temperature of 12.6°C and 19.9°C, respectively) were applied from 5 d after anthesis to grain maturity. The crops were rain fed from sowing to anthesis and received 226 mm of rainfall during that period. One container for each temperature treatment received 25 to 50 mm of water every 4 to 7 d until harvest maturity to replace measured crop evapotranspiration (treatments -5W and +5W), whereas the other container received 5% to 15% of the measured crop evapotranspiration from anthesis to harvest maturity (treatments -5D and +5D). Crop evapotranspiration and, thus, crop water requirements under the controlled environment chambers were computed from measurements of the volume of water condensed on the cold exchanger of the chambers and the difference of air vapor pressure between the outlet and inlet of the chambers.
One controlled environment chamber/container was used per treatment. To study the dynamic accumulation of total N and protein fractions, three replicates each of 20 plants (approximately 0.25 m2) were collected every 50 to 130 °Cd from anthesis to grain maturity. Plants were sampled from the northern side of the containers through their southern side. To minimize the border effects, for each sampling date, the northernmost raw was discarded, and after each sampling, a net of the high of the crop was placed in place of the last raw removed.
N Experiments
The effect of N availability at anthesis in relation to the level of N nutrition before anthesis was studied in a field experiment for crops of wheat cv Thésée sown at a density of 300 seeds m-2 (Triboï et al., 2003). Crops were sown in plots that had not received N fertilizer since 1948. Three rates of N were supplied at the beginning of stem elongation: 0, 5, and 10 g N m-2 (treatments L, M, and H), respectively. The H treatments were on plots where leaves of sugar beet (Beta vulgaris) from a previous cultivation and a cut of alfalfa (Medicago sativa) had been buried. At anthesis, each plot was split into three subplots to which 0 (treatments L0, M0, and H0), 3 (L3, M3, and H3), or 15 (L15, M15, and H15) g N m-2 were applied. Crops were rain fed. Accumulated rainfall from sowing to anthesis and from anthesis to grain maturity was 344 and 61 mm, respectively. Average air temperature from sowing to anthesis and from anthesis to grain maturity was 7.8°C and 19.6°C, respectively. Samples of 0.2 m2 were taken in each subplot at anthesis and 290, 505, 712, and 900 °Cd later. Three replicates were used per N treatment.
Plant Sampling, Protein Extraction, and Total N Content Determination
Grains were separated, and their dry mass was determined on sub-samples after oven drying at 70°C to constant mass. The remaining grains were frozen in liquid N, freeze dried, and stored at 4°C before analysis.
The protein fractions albumin-globulin, amphiphilic, gliadin, and glutenin were sequentially extracted from whole meal flour (Triboï et al., 2003). The residue fraction, which represents 1.5% to 9% of the total N content, was pooled with the glutenin fraction. Total N content for the different protein fractions were determined by the Kjeldhal method using a Kjeltec 2300 analyzer (Foss Tecator AB, Hoeganaes, Sweden). One sequential extraction and N content analysis were performed for each of the three independent replicates. As a control, total N content of the whole meal flour was systematically determined by the Kjeldhal method on a different subsample, and analyses were redone if the difference between whole meal flour N content and the sum of N content for the different protein fractions was greater than 5%.
The Sirius Wheat Simulation Model
We used the wheat simulation model Sirius V99 (Jamieson and Semenov, 2000) to analyze the regulation of grain N accumulation of the crop from the experimental treatments described above. Detailed description of Sirius is given elsewhere (Jamieson et al., 1998; Jamieson and Semenov, 2000). In short, in Sirius from emergence to anthesis, N demand is set in proportion to the increment of green area index and structure each day, whereas extra nonstructural N can be stored in proportion to stem biomass. The major assumptions are that specific leaf N concentration is constant at 1.5 g m-2 of leaf, structural N is 0.5% of biomass accumulated until anthesis, and the crop can store N equivalent to 1% of the stem biomass. At anthesis, all nonstructural shoot N (i.e. both stored nonstructural N and N in green tissue) is considered to be available for transfer to the grain. Grain N accumulates at a constant rate, in thermal time, from 100 °Cd after anthesis until either the total senescence of the canopy or the unconstrained end of grain filling, whichever occurs first. The unconstrained duration of grain filling is assumed to be under genetic control and constant in thermal time. The flux rate of N to the grain in the Sirius model is set at anthesis such as all the nonstructural N would be transferred by the end of unconstraint grain filling. Grain N is supplied from three different sources, accessed in sequence. The first is excess stem N and N released by natural leaf senescence. If these are insufficient, soil N is taken. Should these combined sources be insufficient, then the required N is obtained by accelerating leaf senescence.
The initial quantity of soil organic N at sowing was adjusted in Sirius to match the observed crop N content at anthesis using the treatments 0 and L0 for the controlled environment closed-top chamber and field experiments, respectively. Phenological development was not part of this study. Thus, the phyllochron in Sirius was adjusted so that the simulated and observed anthesis dates matched. A phyllochron value of 93 °Cd was used for the controlled environment chamber experiments, and 112 °Cd was used for the field experiments. Where appropriate, others genetic parameters in Sirius were set as for wheat cv Claire.
Modeling the Accumulation of Grain Protein Fractions
Although grain yield and protein content are regulated at the square meter scale (Jamieson and Semenov, 2000), grain N partitioning appeared to be regulated at the grain scale (Stone and Nicolas, 1996; Triboï et al., 2003). Thus, accumulation of grain protein fractions were modeled at the grain scale. The total grain N (Ntot) was divided into structural (Nstru) and storage (Nsto) N:
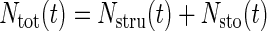 |
1 |
where Nstru is composed of the albumin-globulin and amphiphilic protein fractions, and Nsto is composed of the gliadin (Ngli) and glutenin (Ngln) protein fractions. From the analysis of treatment 0 of the experiment in the controlled environment chambers described above, we assumed a constant partitioning of Nsto between Ngli and Ngln: 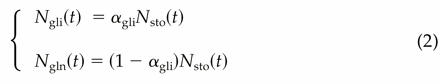 where αgli is the partitioning coefficient for Ngli.
where αgli is the partitioning coefficient for Ngli.
The daily flux of Nstru was expressed as the minimum of the daily demand for Nstru (Nstrudemand) and the daily supply of total N (Ntotsupply). Based on previous work (Stone and Nicolas, 1996), we made several hypotheses to model Nstrudemand. During the initial cell division phase, accumulation of Nstru is exponential. During the cell expansion phase, the flux of Nstru is determined by the size of the pool of Nstru at the end of the cell division phase. We also assumed that the end of accumulation of Nstru coincides with the end of the DNA endoreduplication phase: 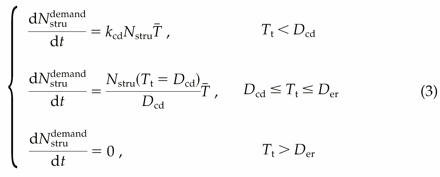 where kcd is the initial relative rate of accumulation of Nstru, T̄ is the average daily temperature, Tt is the thermal time after anthesis, base 0°C, and Dcd and Der are the durations in thermal time of the cell division and DNA endoreduplication phases, respectively.
where kcd is the initial relative rate of accumulation of Nstru, T̄ is the average daily temperature, Tt is the thermal time after anthesis, base 0°C, and Dcd and Der are the durations in thermal time of the cell division and DNA endoreduplication phases, respectively.
Based on previous work for grain of maize (Zea mays; Tsai et al., 1980), we assumed that the accumulation of storage proteins was source regulated. The daily flux of Nsto was expressed as the difference between the daily flux of Nstru and Ntotsupply, whereas Ntotsupply was defined as the first time derivative of a logistic function fitted to total grain N.
Using treatment 0 of the experiment in the controlled environment chambers, kcd and αgli were estimated using regression analysis as 8.44 × 10-3 (°Cd)-1 and 0.38 (dimensionless), respectively, and Nstru (Tt = 0) and Nsto (Tt = 250) as 27.27 and 3.50 μg N grain-1, respectively. For Dcd, we used the values of 250 °Cd found for wheat (Gleadow et al., 1982; Singh and Jenner, 1982) and maize grains (Engelen-Eigles et al., 2000), and for Der, we used the values of 450 °Cd found for maize grains (Engelen-Eigles et al., 2000).
Acknowledgments
The authors thank Drs. Stefan M. Henton and Jean-Francois Soussana and Mr. Robert F. Zyskowski for helpful discussions, and Drs. Gérard Branlard, Maarten J. Chrispeels, Tony Fischer, Peter R. Shewry, and Thomas R. Sinclair for comments on an earlier version of this manuscript. Miss Sandrire Revaillot, Mrs. Joëlle Messaoud, Mr. Bernard Bonnemoy, Mr. Michel Martignac, and Mr. Robert Falcimagne are thanked for their skillful technical assistance.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.030585.
References
- Altpeter F, Vasil V, Srivastava IK, Vasil IK (1996) Integration and expression of the high-molecular-weight glutenin subunit 1Ax1 gene into wheat. Nat Biotechnol 14: 1155-1159 [DOI] [PubMed] [Google Scholar]
- Alvarez ML, Guelman S, Halford NG, Lustig S, Reggiardo MI, Ryabushkina N, Shewry P, Stein J, Vallejos RH (2000) Silencing of HMW glutenins in transgenic wheat expressing extra HMW subunits. Theor Appl Genet 100: 319-327 [Google Scholar]
- Barlow EWR, Donovan GR, Lee JW (1983) Water relationships and composition of wheat ears grown in liquid culture: effect of carbon and nitrogen. Aust J Plant Physiol 10: 99-108 [Google Scholar]
- Barneix AJ, Guitman MR (1993) Leaf regulation of the nitrogen concentration in the grain of wheat plants. J Exp Bot 44: 1607-1612 [Google Scholar]
- Barro F, Rooke L, Békés F, Gras P, Tatham AS, Fido R, Lazzeri P, Shewry PR, Barcelo P (1997) Transformation of wheat with high molecular weight subunit genes results in improved functional properties. Nat Biotechnol 15: 1295-1299 [DOI] [PubMed] [Google Scholar]
- Blechl AE, Anderson OD (1996) Expression of a novel high-molecular-weight glutenin subunit gene in transgenic wheat. Nat Biotechnol 14: 875-879 [DOI] [PubMed] [Google Scholar]
- Borghi B, Corbellini M, Cattaneo M, Fornasari M, Zucchelli L (1986) Modification of the sink/source relationships in bread wheat and its influence on grain protein content. J Agric Crop Sci 157: 245-254 [Google Scholar]
- Brancourt-Hulmel M, Doussinault G, Lecomte C, Bérard P, Le Buanec B, Trottet M (2003) Genetic improvement of agronomic traits of winter wheat cultivars released in France from 1946 to 1992. Crop Sci 43: 37-45 [Google Scholar]
- Brocklehurst PA (1977) Factors controlling grain weight in wheat. Nature 266: 348-349 [Google Scholar]
- Cassman KG (1999) Ecological intensification of cereal production systems: yield potential, soil quality, and precision agriculture. Proc Natl Acad Sci USA 96: 5952-5959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke BC, Larroque O, Békés F, Somers DE, Appels R (2001) The frequent classes of expressed genes in wheat endosperm tissue as possible sources of genetic markers. Aust J Agric Res 52: 1181-1193 [Google Scholar]
- Corke H, Atsmon D (1988) Effect of nitrogen nutrition on endosperm protein synthesis in wild and cultivated barley grown in spike culture. Plant Physiol 87: 523-528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delzer BW, Bush RH, Hareland GA (1995) Recurrent selection for grain protein in hard red spring wheat. Crop Sci 35: 730-735 [Google Scholar]
- Donovan GR, Lee JW, Hill RD (1977) Compositional changes in the developing grain of high- and low-protein wheats: II. Starch and protein synthetic capacity. Cereal Chem 54: 646-656 [Google Scholar]
- Dreccer MF, Grashoff C, Rabbinge R (1997) source-sink ratio in barley (Hordeum vulgare L.) during grain filling: effects on senescence and grain protein concentration. Field Crops Res 49: 269-277 [Google Scholar]
- Engelen-Eigles G, Jones RJ, Phillips RL (2000) DNA Endoreduplication in maize endosperm cells: the effect of exposure to short-term high temperature. Plant Cell Environ 23: 657-663 [Google Scholar]
- Feil B (1997) The inverse yield-protein relationship in cereals: possibilities and limitations for genetically improving the grain protein yield. Trends Agron 1: 103-119 [Google Scholar]
- Gleadow RM, Dalling MJ, Halloran GM (1982) Variation in endosperm characteristics and nitrogen content in six wheat lines. Aust J Plant Physiol 9: 539-551 [Google Scholar]
- Graybosch RA, Peterson CJ, Shelton DR, Baenziger PS (1996) Genotypic and environmental modification of wheat flour protein composition in relation to end-use quality. Crop Sci 36: 296-300 [Google Scholar]
- Hammond-Kosack MCU, Holdsworth M, Bevan MW (1993) In vivo foot-printing of a low molecular weight glutenin gene (LMWG-1D1) in wheat endosperm. EMBO J 12: 545-554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayati R, Egli DB, Crafts-Brandner SJ (1996) Independence of nitrogen supply and seed growth in soybean: studies using an in vitro culture system. J Exp Bot 47: 33-40 [Google Scholar]
- Huebner FR, Nelsen TC, Chung OK, Bietz JA (1997) Protein distributions among hard red winter wheat varieties as related to environment and baking quality. Cereal Chem 74: 123-128 [Google Scholar]
- Jamieson PD, Semenov MA (2000) Modelling nitrogen uptake and redistribution in wheat. Field Crops Res 68: 21-29 [Google Scholar]
- Jamieson PD, Semenov MA, Brooking IR, Francis GS (1998) Sirius: a mechanistic model of wheat response to environmental variation. Eur J Agron 8: 161-179 [Google Scholar]
- Lagudah ES, Dubcovsky J, Powell W (2001)Wheat genomics. Plant Physiol Biochem 39: 335-344 [Google Scholar]
- Lhuillier-Soundele A, Munier-Jolain N, Ney B (1999a) Dependence of seed nitrogen concentration on plant nitrogen availability during the seed filling in pea. Eur J Agron 11: 157-166 [Google Scholar]
- Lhuillier-Soundele A, Munier-Jolain N, Ney B (1999b) Influence of nitrogen availability on seed nitrogen accumulation in pea. Crop Sci 39: 1741-1748 [Google Scholar]
- Ma YZ, Mackown CT, Van Sanford DA (1995) Kernel mass and assimilate accumulation of wheat: cultivar responses to 50% spikelet removal at anthesis. Field Crop Res 42: 93-99 [Google Scholar]
- Ma YZ, Mackown CT, Van Sanford DA (1996) Differential effects of partial spikelet removal and defoliation on kernel growth and assimilate partitioning among wheat cultivars. Field Crop Res 47: 201-209 [Google Scholar]
- Mattsson M, Lundborg T, Larsson CM (1993) Nitrogen utilization in N-limited barley during vegetative growth: IV. Translocation and remobilization of nitrogen. J Exp Bot 44: 537-546 [Google Scholar]
- Mossé J, Huet JC, Baudet J (1985) The amino acid composition of wheat grain as a function of nitrogen content. J Cereal Sci 3: 115-130 [Google Scholar]
- Müller M, Knudsen S (1993) The nitrogen response of a barley C-hordein promoter is controlled by positive and negative regulation of the GCN4 and endosperm box. Plant J 4: 343-355 [DOI] [PubMed] [Google Scholar]
- Nakasathien S, Israel DW, Wilson RF, Kwanyuen P (2000) Regulation of seed protein concentration in soybean by supra-optimal nitrogen supply. Crop Sci 40: 1277-1284 [Google Scholar]
- Porter JR (1993) AFRCWHEAT2: a model of the growth and development of wheat incorporating responses to water and nitrogen. Eur J Agron 2: 69-82 [Google Scholar]
- Reynolds MP, Rajaram S, Sayre KD (1999) Physiological and genetic changes of irrigated wheat in the post-green revolution period and approaches for meeting projected global demand. Crop Sci 39: 1611-1621 [Google Scholar]
- Rooke L, Békés F, Fido R, Barro F, Gras P, Tatham AS, Barcelo P, Lazzeri P, Shewry PR (1999) Overexpression of a gluten protein in transgenic wheat results in greatly increased dough strength. J Cereal Sci 30: 115-120 [Google Scholar]
- Saravitz CH, Raper CD (1995) Responses to sucrose and glutamine by soybean embryos grown in vitro. Physiol Plant 93: 799-805 [Google Scholar]
- Shewry PR, Halford NG (2002) Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot 53: 947-958 [DOI] [PubMed] [Google Scholar]
- Shewry PR, Tatham AS, Halford NG (2001) Nutritional control of storage protein synthesis in developing grain of wheat and barley. Plant Growth Regul 34: 105-111 [Google Scholar]
- Singh BK, Jenner CF (1982) A modified method for the determination of cell number in wheat endosperm. Plant Sci Lett 26: 273-278 [Google Scholar]
- Stewart DW, Dwyer LW (1990) Yields and protein trends of spring wheat (Triticum aestivum L.) on the Canadian prairies, 1961-1982. Can J Plant Sci 70: 33-44 [Google Scholar]
- Stone PJ, Nicolas ME (1996) Varietal differences in mature protein composition of wheat resulted from different rates of polymer accumulation during grain filling. Aust J Plant Physiol 23: 727-737 [Google Scholar]
- Tilman D (1999) Global environmental impacts of agricultural expansion: the need for sustainable and efficient practices. Proc Natl Acad Sci USA 96: 5995-6000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418: 671-677 [DOI] [PubMed] [Google Scholar]
- Triboï E, Abad A, Michelena A, Lloveras J, Ollier JL, Daniel C (2000) Environmental effects on the quality of two wheat genotypes: I. quantitative and qualitative variation of storage proteins. Eur J Agron 13: 47-64 [Google Scholar]
- Triboï E, Martre P, Triboï-Blondel AM (2003) Environmentally-induced changes of protein composition for developing grains of wheat are related to changes in total protein content. J Exp Bot 54: 1731-1742 [DOI] [PubMed] [Google Scholar]
- Triboï E, Triboï-Blondel AM (2002) Productivity and grain or seed composition: a new approach to an old problem: invited paper. Eur J Agron 16: 163-186 [Google Scholar]
- Tsai CY, Huber DM, Warren HL (1980) A proposed role of zein and glutelin as N sinks in maize. Plant Physiol 66: 330-333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi AK, Mohanty A (2000) Rice transformation for crop improvement and functional genomics. Plant Sci 158: 1-18 [DOI] [PubMed] [Google Scholar]
- Voltas JIR, Araus JL (1997) Grain size and nitrogen accumulation in sink-reduced barley under Mediterranean conditions. Field Crop Res 52: 117-126 [Google Scholar]
- Weegels PL, Hamer RJ, Schofield JD (1996) Critical review: functional properties of wheat glutenin. J Cereal Sci 23: 1-18 [Google Scholar]
- Wyss CS, Czyzewicz JR, Below FE (1991) Source-sink control of grain composition in maize strains divergently selected for protein concentration. Crop Sci 31: 761-766 [Google Scholar]
- Zhu J, Khan K (2001) Effects of genotype and environment on glutenin polymers and breadmaking quality. Cereal Chem 78: 125-130 [Google Scholar]