Abstract
We investigated whether and how mitochondria from durum wheat (Triticum durum Desf.) and potato (Solanum tuberosum), isolated from etiolated shoots and a cell suspension culture, respectively, oxidize externally added NADH via the mitochondrial shuttles; in particular, we compared the shuttles and the external NADH dehydrogenase (NADH DHExt) with respect to their capacity to oxidize external NADH. We found that external NADH and NADPH can be oxidized via two separate DHExt, whereas under conditions in which the activities of NAD(P)H DHExt are largely prevented, NADH (but not NADPH) is oxidized in the presence of external malate (MAL) and MAL dehydrogenase, in a manner sensitive to several non-penetrant compounds according to the occurrence of the MAL/oxaloacetate (OAA) shuttle. In durum wheat mitochondria and potato cell mitochondria, the rate of NADH oxidation was limited by the rate of a novel carrier, the MAL/OAA antiporter, which is different from other carriers thought to transport OAA across the mitochondrial membrane. No NAD(P)H oxidation occurred arising from the MAL/Aspartate and the α-glycerophosphate/dihydroxyacetonphosphate shuttles. We determined the kinetic parameters of the enzymes and the antiporter involved in NADH oxidation, and, on the basis of a kinetic analysis, we showed that, at low physiological NADH concentrations, oxidation via the MAL/OAA shuttle occurred with a higher efficiency than that due to the NADH DHExt (about 100- and 10-fold at 1 μm NADH in durum wheat mitochondria and in potato cell mitochondria, respectively). The NADH DHExt contribution to NADH oxidation increased with increasing NADH concentration.
One of the most outstanding problems in plant biochemistry concerns the interaction between mitochondria, cytosol, and other organelles, which involves the transfer of NAD(P)H reducing equivalent across the inner mitochondrial membrane (Raghavendra et al., 1994; Krömer, 1995).
In particular, in addition to NADH produced by glycolysis, NAD(P)H concentration can increase in plant cell cytosol under certain conditions, e.g. when increased reduction of chloroplast electron carriers occurs (Raghavendra et al., 1994; Krömer, 1995). However, how cytosolic NAD(P)H is oxidized by mitochondria is still a matter of debate. Plant mitochondria contain two external rotenone-insensitive NAD(P)H dehydrogenases [NAD(P)H DHExt] coupled to the electron transport chain, one of which is specific for NADH and the other for NADPH (Roberts et al., 1995; Møller, 2001). However, the occurrence of malate (MAL) dehydrogenase (MDH) in both the cytosol and in the mitochondria and the mitochondrial permeability to both MAL (Douce, 1985, and refs. therein; Rustin and Valat, 1986; Pastore et al., 2001) and oxaloacetate (OAA; Douce, 1985, and refs. therein; Hanning et al., 1999) could allow the operation of the MAL/OAA shuttle, thus raising the question as to whether and with which efficiency reducing equivalents can be transferred from the cytosol to the mitochondria via this mitochondrial shuttle.
In this regard, the OAA concentration on both sides of the mitochondrial membrane was proposed to govern the efflux and influx of OAA in plant mitochondria (Neuburger and Douce, 1980), thus regulating traffic of reducing power. On the other hand, in light of the redox gradient between the NADH/NAD+ systems in the mitochondria and cytosol, the MAL/OAA shuttle was suggested to occur only for the export of redox equivalents outside mitochondria (Zoglowek et al., 1988; Krömer and Heldt, 1991; Hanning and Heldt, 1993; Raghavendra et al., 1998), thus permitting peroxisomal conversion of hydroxypyruvate to glycerate during photorespiration (Krömer, 1995; Raghavendra et al., 1998) and cytosolic nitrate reduction in non-photosynthesizing cells (Krömer, 1995).
These controversial findings, together with the availability of spectroscopic methods adequate to ascertain the occurrence of the MAL/OAA shuttle in transferring reducing equivalents into mitochondria (Passarella et al., 1984, 2003), prompted us to investigate whether plant mitochondria can oxidize externally added NAD(P)H via the MAL/OAA shuttle and/or via the other shuttles that may provide cytosolic NADH oxidation, namely the MAL/Aspartate (Asp) shuttle and the α-glycerophosphate/dihydroxyacetonphosphate (α-GP/DHAP) shuttle. Moreover, we compared the MAL/OAA shuttle and the external NADH dehydrogenase (NADH DHExt) with respect to their capacity to oxidize external NADH.
We show that in mitochondria from both the monocotyledonous durum wheat (Triticum durum Desf.) and from the dicotyledonous potato (Solanum tuberosum), external NADH oxidation occurs mostly via the MAL/OAA shuttle with a rate limited by the novel MAL/OAA antiporter.
RESULTS
External NAD(P)H Oxidation via DHExt
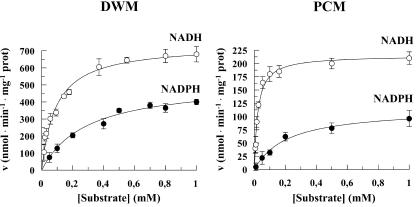
To investigate how external NAD(P)H oxidation can occur, use was made of coupled durum wheat mitochondria (DWM) and potato cell mitochondria (PCM), both of which showed high membrane intactness (see “Materials and Methods”). Both DWM and PCM were checked with respect to their capability to oxidize either NADH or NADPH via the NAD(P)H DHExt. Added Ca2+ (in the range 1-100 μm) had no effect on NADH oxidation, which requires very low Ca2+ concentration (see also Pastore et al., 1999b), whereas the rate of NADPH oxidation was maximal at 30 μm Ca2+. The dependence of the rate of either NADH or NADPH oxidation as a function of the substrate concentration was studied (Fig. 1) and, as expected, in both cases was hyperbolic. In three different experiments, Vmax and Km values for NADH oxidation were 740 ± 48 nmol min-1 mg-1 protein and 0.115 ± 0.025 mm in DWM and 215 ± 13 nmol min-1 mg-1 protein and 0.020 ± 0.004 mm in PCM. For NADPH oxidation, Vmax and Km values were 520 ± 30 nmol min-1 mg-1 protein and 0.29 ± 0.059 mm in DWM and 116 ± 8 nmol min-1 mg-1 protein and 0.21 ± 0.04 mm in PCM. In both DWM and PCM, the Vmax values for the NADH and NADPH oxidation were statistically different (Student's t test, P < 0.001).
Figure 1.
Dependence of the rate of NAD(P)H oxidation by NAD(P)H DHExt on increasing NAD(P)H concentrations in DWM and PCM. For details, see “Materials and Methods.” ○, NADH oxidation; •, NADPH oxidation. The data are reported as mean of three experiments ± se.
Appearance of OAA outside DWM and PCM Because of Externally Added MAL and Lack of α-GP/DHAP and MAL/Asp Shuttles
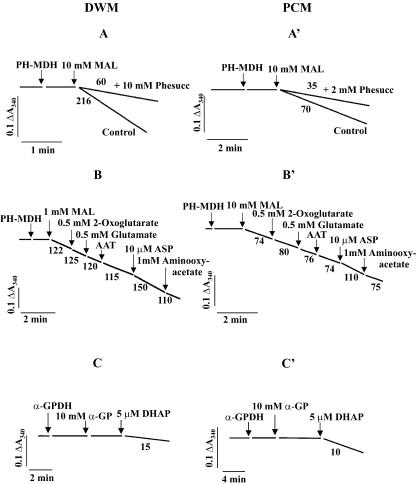
To ascertain whether the mitochondrial shuttles, namely the MAL/OAA and the MAL/Asp and α-GP/DHAP shuttles already described in mammalian and plant cells (for refs., see Laloi, 1999; Passarella et al., 2003; Shen et al., 2003) have a role in NADH oxidation by DWM and PCM, 0.2 mm NADH was added to mitochondria and was found to be oxidized rapidly as shown by the absorbance decrease at 340 nm. Further addition of 10 mm EGTA plus 10 mm EDTA resulted in a progressive inhibition of the rate of absorbance decrease observed, as expected because this treatment removes calcium ions strictly required for NAD(P)H DHExt function (Møller, 1997, 2001, and refs. therein): In 1 min, NADH oxidation was about 95% inhibited, after which the residual rate of NADH oxidation was instrumentally zeroed as reported in “Materials and Methods” (not shown). The OAA concentration in the extramitochondrial phase is negligible. In fact, addition of porcine heart (PH)-MDH (0.5 enzymic units [EU]) resulted in no NADH oxidation (Fig. 2, A and A'). Externally added 10 mm MAL caused NADH oxidation (216 and 70 nmol min-1 mg-1 protein for DWM and PCM, respectively), thus indicating the OAA appearance outside the mitochondria. This reaction was strongly impaired by phenylsuccinate (Phesucc, 10 mm in DWM and 2 mm in PCM), which inhibits a number of transport processes in mitochondria from different sources (Passarella et al., 1984; Douce, 1985; Fratianni et al., 2001) and by other compounds including butylmalonate (see below), which cannot inhibit OAA uptake in pea (Pisum sativum) leaf mitochondria via the OAA carrier (Oliver and Walker, 1984). When cytosolic extracts from durum wheat shoots and potato cells, both having high MDH activity, were used instead of PH-MDH, NADH oxidation was found to occur as above (not shown). On the other hand, no decrease in absorbance was found when NADPH was used in the presence of either PH-MDH or the cytosolic extract. It should be noted that the standard medium contained phosphate and ADP to allow a high oxidation rate in the course of the experiment; when phosphate and ADP were absent, the rate of NADH oxidation was reduced by about 50% (not shown). In agreement with Passarella et al. (1984), a possible explanation of these findings is the following (see also Scheme 1):
Figure 2.
The existence of a MAL/OAA shuttle and the lack of both MAL/Asp and α-GP/DHAP shuttles in DWM (A-C) and PCM (A'-C'). A and A', Reconstruction of the MAL/OAA shuttle. DWM and PCM (0.1 mg of protein) were incubated at 25°C in 2 mL of the standard medium containing 0.3 m mannitol, 5 mm MgCl2, 10 mm KCl, 1 mm ADP, 10 mm K-phosphate (pH 7.20), and 0.1% (w/v) defatted bovine serum albumin (BSA); addition was also made of 0.2 mm NADH and 10 mm EDTA plus 10 mm EGTA, which inhibit the NADH DHExt; the residual activity of NADH DHExt was zeroed as reported in “Materials and Methods.” The reaction was started by adding 0.5 EU PH-MDH and 10 mm MAL, and the A340 was continuously monitored. Where indicated, Phesucc, 10 mm in DWM and 2 mm in PCM, was also present in the medium. B and B', Reconstruction of the MAL/Asp shuttle. This was carried out as above, but 0.5 mm 2-oxoglutarate, 0.5 mm Glu, and 0.5 EU Asp aminotransferase (AAT) were also added to mitochondria. Then, 10 μm Asp and 1 mm aminooxyacetate were added to test the revealing system and to inhibit the transaminase, respectively. C and C', Reconstruction of the α-GP/DHAP shuttle. DWM and PCM were treated as in A and A', but the reaction was started by the additions of 0.5 EU α-glycerophosphate dehydrogenase (α-GPDH) and 10 mm α-GP. DHAP (5 μm) was also added to test the revealing system. The numbers alongside the traces refer to the nanomoles of NADH oxidized per minute per milligram of protein.
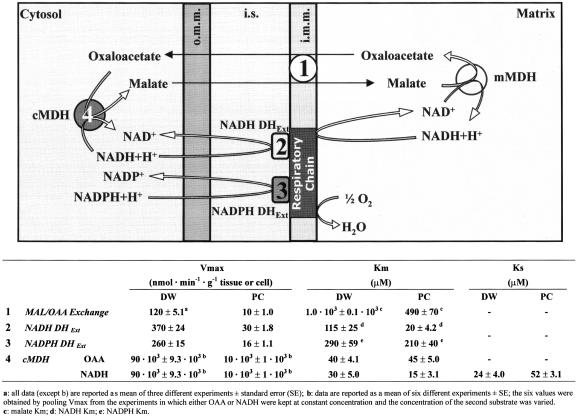
Scheme 1.
: Oxidation of cytosolic NAD(P)H by DWM and PCM and kinetic parameters of the enzymes involved. Cytosolic NADH is oxidized by the MAL/OAA shuttle: MAL enters mitochondria, then it is converted via mitochondrial MDH (mMDH) in OAA, which is released in the extramitochondrial phase in exchange with entering MAL; once in the extramitochondrial phase, OAA is converted in MAL via cytosolic MDH (cMDH) with NADH consumption. On the basis of the kinetic parameters summarized in Scheme (see later for explanation), at physiological concentration of NADH (less than 1 μm), the oxidation by the NADH DHExt is expected to be negligible (see also Fig. 6). NADPH oxidation occurs as due to NADPH DHExt, whereas cMDH shows no specificity toward NADPH. i.m.m., inner mitochondrial membrane; i.s., intermembrane space; o.m.m., outer mitochondrial membrane.
MAL can enter mitochondria in exchange with endogenous OAA (or phosphate or dicarboxylates); once inside the matrix, MAL is oxidized via the very active mitochondrial MDH (mMDH) to OAA (Douce, 1985; Pastore et al., 1999b, 2001), which, in turn, can exit in a Phesucc-sensitive manner outside the mitochondria, where it is reduced, thus oxidizing NADH in the reconstructed MAL/OAA shuttle.
In a parallel experiment, the substrates 2-oxoglutarate and glutamate (Glu; 0.5 mm each), and 0.5 EU AAT were added to MAL-oxidizing mitochondria, thus providing the components of the MAL/Asp shuttle (Fig. 2, B and B'). A small decrease in the rate of absorbance decrease was found that was, however, also observed in control experiments carried out without mitochondria (data not shown). This result was possibly due to the transamination of the newly effluxed OAA, thus showing the failure of DWM and PCM to export Asp from the matrix and to provide further OAA outside mitochondria via transamination. As a control, further addition of 10 μm Asp, increased the rate of NADH oxidation in a manner inhibited by both cycloserine (not shown) and aminooxyacetate, transaminase inhibitors that cannot enter or can penetrate mitochondria, respectively (Pastore et al., 2001).
In another experiment carried out as above (Fig. 2, C and C'), no NADH oxidation was found when adding 0.5 EU α-GPDH and 10 mm α-GP to either DWM or PCM. Control was also made that 5 μm DHAP caused NADH oxidation outside mitochondria. Similarly, no evidence for the existence of MAL/Asp or of α-GP/DHAP shuttles was observed when NADH was replaced with NADPH plus cytosolic extract (not shown).
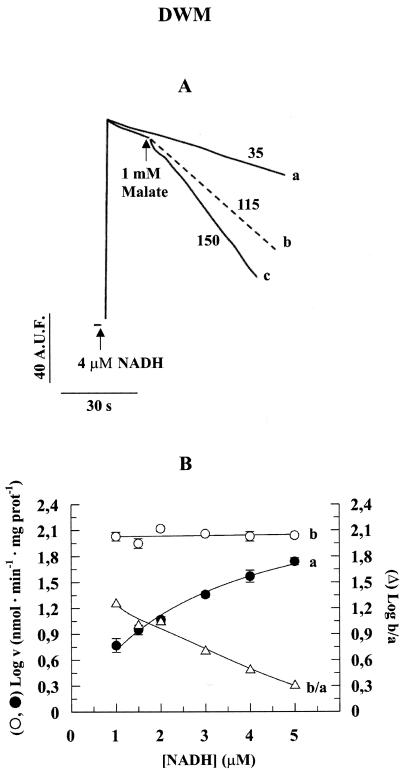
In light of the differences occurring between the in vitro and in vivo conditions and on the assumption that the physiological cytosolic NADH concentration is very low (<1 μm; Heineke et al., 1991), we investigated fluorimetrically the rate of NADH oxidation by DWM in EDTA/EGTA-free medium containing PH-MDH and 1 mm MAL as a function of its concentration in the range 1 to 5 μm. In a typical experiment (Fig. 3A), the rate of NADH (4 μm) oxidation due to the DHExt was measured (trace a). Addition of 1 mm MAL increased the rate due to the simultaneous operation of the DHExt and of MAL/OAA shuttle (trace c). The rate of the NADH oxidation via the shuttle was then calculated as the difference between the rates in traces c and a (trace b). In Figure 3B, the logarithm of the rate of NADH DHExt (a), of the MAL/OAA shuttle (b), and of the ratio b to a are reported for a range of 1 to 5 μm NADH. Notice that the y axis shows the logarithm of the rate to better plot the very different values of the b to b/a curves. NADH was found to be oxidized by the shuttle at a constant rate in the entire concentration range, and the rate was always higher than that due to the NADH DHExt (compare a with b). In particular, the rate of the shuttle-dependent oxidation of 1 μm NADH was found to be about 20 times higher with respect to that due to the NADH DHExt (curve b/a).
Figure 3.
Fluorimetric measurement of NADH oxidation rate at low NADH concentrations by the external dehydrogenase and by MAL/OAA shuttle in DWM. A, Mitochondria (0.05 mg of protein) were incubated in 2 mL of the standard medium containing 10 EU PH-MDH; then, 4 μm NADH was added, and the fluorescence (λex 340 nm; λem 456 nm) was continuously monitored (trace a). In the case of trace c, MAL was added at the time indicated by the arrow. Trace b was calculated as: c - a. The numbers alongside the traces refer to the nanomoles of NADH oxidized per minute per milligram of protein. A.U.F., Arbitrary units of fluorescence. In this experiment, PH-MDH activity was used giving a ratio of external-MDH activity to MAL-OAA antiporter activity roughly similar to the one due to cytosolic MDH (cMDH) under physiological condition (see Scheme 1). B, The same experiment was repeated using 1 to 5 μm NADH, and the logarithm of the rates (v) of NADH oxidation was reported as a function of NADH concentrations: a, NADH oxidation rate due to NADH DHExt; and b, NADH oxidation rate due to MAL/OAA exchange. The data are reported as mean of three experiments ± se.
Inhibition of the MAL-Induced OAA Efflux from DWM and PCM
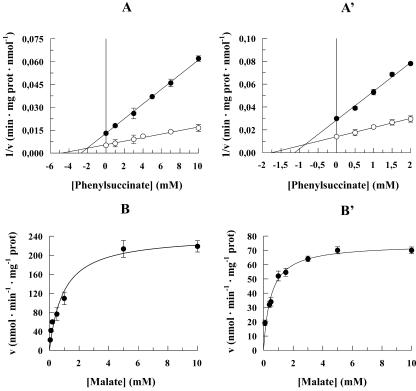
To determine whether the rate of OAA appearance outside mitochondria reflects either the rate of the OAA transport across the mitochondrial membrane or the activity of the mMDH, the control strength criterion was applied (Pastore et al., 2002; Passarella et al., 2003, and refs. therein) using Phesucc, which inhibits OAA transport (Fig. 2, A and A') but cannot enter plant mitochondria (Fratianni et al., 2001). Thus, the rate of OAA appearance outside DWM and PCM was investigated at two MAL concentrations (0.5 and 10 mm) in the absence and presence of increasing Phesucc concentrations, and data were then plotted using the Dixon plot. The y axis intercepts of the lines fitting the experimental points determined in the presence of Phesucc proved to coincide with the experimental points obtained at zero inhibitor concentration, demonstrating that the rate of OAA appearance outside mitochondria mirrors the rate of the inhibited process, i.e. the rate of the OAA transport. Consistently, mMDH activity in DWM was found to be very high (50 ± 5.2 EU mg-1 protein; Pastore et al., 2001). Interestingly, Figure 4, A and A', also show: (a) that OAA efflux cannot occur in a manner insensitive to externally added inhibitors, i.e. via diffusion (Douce, 1985), and (b) that Phesucc inhibits the MAL-induced appearance of OAA outside mitochondria in a competitive manner: Ki was 2 mm in DWM (Fig. 4A) and 0.8 mm in PCM (Fig. 4A').
Figure 4.
Phesucc sensitivity and saturation kinetics of MAL-induced OAA efflux in DWM (A and B) and PCM (A' and B'). The rate (v) of OAA efflux, measured as reported in Figure 1A and A', is reported as a function of Phesucc concentration using a Dixon plot, using 10 mm (○) and 0.5 mm (•) MAL (A and A') and as a function of MAL concentration using a Michaelis-Menten plot (B and B'). The data are reported as mean of three experiments ± se.
In DWM, competitive inhibition with respect to MAL was also found with other inhibitors, including the dicarboxylate analogs phthalonate (Ki = 10 μm) and butylmalonate (Ki = 1 mm) and the thiol reagent p-chloromercuribenzene sulfonate (Ki = 1 mm). These inhibitors were added together with MAL, thus preventing, mainly in the case of the thiol reagent, protein conformation changes. The competitive inhibition by p-chloromercuribenzene sulfonate suggests the existence of -SH group/s at or near the substrate-binding site. Moreover, the OAA efflux due to addition of 1 mm MAL to mitochondria was found to be 95% inhibited by 0.1 mm bathophenanthroline and 85% inhibited by 0.2 mm mersalyl. On the contrary, methylfumarate, which can inhibit the fumarate/MAL and fumarate/phosphate antiporters in rat liver mitochondria (Atlante et al., 1985), and N-ethylmaleimide, which can inhibit the exchange between intramitochondrial phosphate and extramitochondrial anions in purified bean (Vigna sinensis) mitochondria (De Santis et al., 1976), did not affect MAL/OAA exchange up to 20 and 1 mm, respectively. Moreover, the MAL/OAA exchange was insensitive to benzene-1,2,3-tricarboxylate, which is known to inhibit citrate transport in a variety of mitochondria (Klingenberg, 1972; Genchi et al., 1999) and to citrate, both up to 10 mm.
Dependence on the MAL Concentration of the MAL-Induced OAA Efflux from DWM and PCM
The rate of MAL-induced OAA efflux was studied in both DWM and PCM as a function of increasing MAL concentrations (Fig. 4, B and B'). In both cases, in agreement with the occurrence of carrier-mediated transport, saturation kinetics were found with Vmax and Km values equal to 240 ± 10 nmol min-1 mg-1 protein and 1.0 ± 0.1 mm in DWM and 70 ± 7 nmol min-1 mg-1 protein and 0.49 ± 0.07 mm in PCM (n = 3).
NAD(P)H DHExt and MAL/OAA Shuttle Activities
Because in vivo the rate of NADH oxidation via the MAL/OAA shuttle could depend on the activity of the cMDH, this enzyme was assayed in cytosolic extracts free of any organelle contamination. The initial rate of MDH reaction conformed to an ordered bi-bi mechanism (Dixon and Webb, 1979):
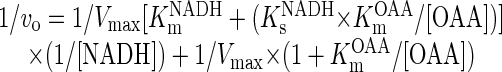 |
1 |
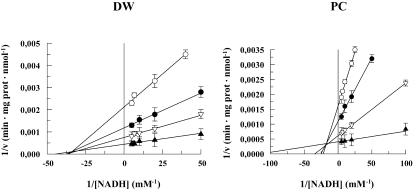
The KsNADH, Vmax, KmNADH, and KmOAA were determined (see “Materials and Methods”) to calculate the rate of the reaction occurring in a particular substrate concentration range. In particular, KsNADH was obtained as reported in Figure 5. MDH Vmax measured in the cytosolic fraction was equal to 5.60 × 103 ± 0.58 × 103 nmol min-1 mg-1 protein and 2.94 × 103 ± 0.29 × 103 nmol min-1 mg-1 protein in DWM and PCM, respectively. When NADPH was used instead of NADH, negligible cMDH activity was observed.
Figure 5.
Determination of KsNADH for cMDH in durum wheat (DW) and potato cell (PC). Measurements were carried out as reported in “Materials and Methods.” The initial reaction rate (v) was plotted as 1/v versus 1/[NADH]. Both in DW and PC, KsNADH was calculated on the basis of the intercept of the lines obtained using 10 (○), 50 (•), 200 (▿), and 600 (▴) μm OAA
All the parameters relative to the MAL/OAA exchange, NAD(P)H DHExt and cMDH, are reported in Scheme 1, where the mechanisms of NAD(P)H-oxidizing processes is also reported. To compare cytosolic and mitochondrial reactions, the rates were expressed as nanomoles of NADH oxidized per gram of either tissue or cell. The calculation was carried out as reported in “Materials and Methods” by assuming that, in the course of the isolation procedure of mitochondria and cytosol, the recoveries of the MAL/OAA exchange and NAD(P)H DH and of the cMDH resemble the recoveries of the cytochrome c oxidase (COX) and the lactate dehydrogenase (LDH), marker enzymes of the inner mitochondrial membrane, and the cytosolic fraction, respectively. The results of a typical experiment are reported in Table I; they were obtained by adopting a gentle homogenization procedure giving intact and fully functional mitochondria (Pastore et al., 1999b; Fratianni et al., 2001).
Table I.
Recovery of proteins in the homogenate and activities of COX and LDH in the homogenate and in the mitochondrial or cytosolic fraction, respectively
| Proteins and Enzyme Activities | DWM | PCM |
|---|---|---|
| Milligrams of protein in the homogenate per gram of fresh tissue or cells | 16.8 | 3.58 |
| Enzyme-specific activity | ||
| COX in the homogenate (natom oxygen min−1 mg−1 protein) | 33.3 | 27.0 |
| COX in the mitochondrial fraction (natom oxygen min−1 mg−1 protein) | 1120 | 690 |
| LDH in the homogenate (nmol min−1 mg−1 protein) | 9.1 | 7.9 |
| LDH in the cytosolic fraction (nmol min−1 mg−1 protein) | 9.5 | 8.3 |
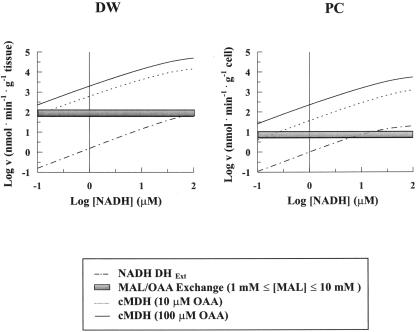
The reaction rates (v) of NADH DHExt and cMDH as a function of NADH concentration were calculated by inserting the kinetic parameters in the Michaelis-Menten equation and in Equation 1, respectively. The resulting graphs are reported in logarithmic form in Figure 6 for durum wheat and potato. The rate of the MAL/OAA exchange was also reported for comparison. cMDH reaction rate was calculated for physiological OAA concentrations ranging from 10 to 100 μm (Douce, 1985; Heineke et al., 1991; Krömer, 1995), whereas the MAL/OAA exchange was calculated in a 1 to 10 mm MAL range (Martinoia and Rentsch, 1994). The vertical line indicates a NADH concentration of 1 μm, close to the physiological one (Heineke et al., 1991).
Figure 6.
Dependence of the rate of NADH oxidation by NADH DHExt and cMDH on increasing NADH concentrations. On the basis of data from Scheme 1, calculation was made of the reaction rate of the NADH DHExt and of cMDH at different NADH concentrations (see text). The cMDH reaction rate was calculated for both 10 and 100 μm OAA concentration. Data were plotted using a logarithmic scale to plot very different concentrations and rates. The vertical line indicates 1 μm NADH. The rates of the MAL/OAA exchange at 1 and 10 mm MAL (horizontal lines) are also reported on the same graph for comparison.
Interestingly, up to about 100 and 10 μm NADH in durum wheat and potato, respectively, NADH oxidation occurs mostly via the MAL/OAA shuttle rather than by the NADH DHExt. Assuming that cytosol NADH concentration is about 1 μm, shuttle-dependent oxidation rate is about 100- and 10-fold higher than that due to the external dehydrogenase in durum wheat and potato, respectively. A very high cMDH reaction rate was found both in durum wheat and potato, which does not limit the maximal rates of MAL/OAA exchange even in the presence of physiological concentration of the product pair (1 mm MAL and 0.5 mm NAD+, not shown).
DISCUSSION
In the last decade, the role of mitochondria in plant cell biochemistry has been the subject of intense investigation: Oxidative phosphorylation has been suggested to be the main process supplying ATP for the cytosol both in light and in the dark (Gardeström and Lernmark, 1995; Krömer, 1995; Gardeström, 1996). On the other hand, mitochondria can regulate both the redox and the energy state of the cell by virtue of the existence of a number of metabolite carriers (Laloi, 1999), of a respiratory chain which is rather different from the animal counterpart (Møller, 2001, and refs. therein), and by energy-dissipating systems, including the alternative oxidase (Vanlerberghe and McIntosh, 1997), the plant-uncoupling mitochondrial protein (Vercesi et al., 1995), and the potassium channel (Pastore et al., 1999a).
In this paper, we show that the oxidation of external NADH via shuttles by mitochondria isolated from both durum wheat and potato, a mono- and a dicotyledonous plants, respectively, occurs mostly via the MAL/OAA shuttle, whereas the activity of the other shuttles is negligible. It should be noted that although the proposal that MAL oxidation can take place through the mediation of the MAL/OAA shuttle has been already reported (see above), the transport mechanism by which this occurred was not clearly indicated. Here, we confirm the occurrence of a MAL/OAA shuttle, but, in addition, we show the regulation of the shuttle activity by a novel MAL/OAA antiporter. Plant cells generally are thought to be more aligned energetically to export NADH from mitochondria than to import it (see above). Nonetheless, several findings, including inhibition of mitochondrial ATP synthesis, which can enhance photoinhibition (Saradadevi and Raghavendra, 1992) and decrease reduction potential in both chloroplast and cytosol (Krömer and Heldt, 1991; Krömer et al., 1993), and the cooperation of noncyclic photosynthetic and mitochondrial electron transport (Krömer and Heldt, 1991, and refs. therein), are consistent with redox-equivalent shift from photosynthetic electron transport for mitochondrial oxidation (see also Raghavendra et al., 1994). This could take place mostly when a decrease of cytosolic and chloroplast reduction potential occurs, as under environmental stress conditions, when mitochondrial oxidations can eliminate excessive redox equivalents, thus preventing an over-reduction of the carriers of photosynthetic electron transport (Krömer, 1995, and refs. therein). Under stress conditions, NADH oxidation via the mitochondrial external dehydrogenase, which we show can oxidize external (cytosolic NADH) poorly at physiological NADH concentration, is expected to increase. These points will be discussed separately.
NAD(P)H Oxidation via External Dehydrogenases
Here, we have demonstrated that NADH and NADPH oxidation due to DHExt differ from each other as shown by the different Vmax and Km values. This confirms that, as with other plant mitochondria (Møller, 2001), DWM and PCM contain two separate external dehydrogenases. In light of data from Figure 1 and Scheme 1 and assuming that the in vivo cytosolic concentration of NADH and NADPH are less than 1 and 150 μm, respectively (Heineke et al., 1991), NADPH should be oxidized under physiological conditions at a rate at least 28- and 4-fold higher than NADH in DWM and PCM, respectively (see also Krömer, 1995, and refs. therein). On the other hand, because in in vitro experiments NADPH DHExt requires Ca2+ concentrations higher than that measured in the cytosol (0.1-1 μm, Kauss, 1987; or 0.1-0.2 μm, Knight et al., 1996), the above hypothesis should be investigated in more detail. It should be noted that NADPH was oxidized by the NADPH DHExt but not by the shuttles.
MAL/OAA Antiport and Shuttle
The occurrence of the MAL/OAA carrier was shown by using spectroscopic techniques previously used (Passarella et al., 1984), in which coupled mitochondria with mostly active metabolism were studied. The main criteria we used to establish carrier-mediated transport included the occurrence of saturation kinetics and inhibition due to non-penetrant compounds. Consistently, the application of the control strength criterion using Phesucc allows us to rule out the possibilities that OAA can be formed in the extra mitochondrial compartment at a significant rate and that OAA can efflux by free diffusion. Moreover, in light of the sensitivity to butylmalonate and Phesucc, the MAL/OAA exchange appears to be due to a MAL/OAA antiporter rather than to the combined activities of possible electrogenic uniporters of MAL and OAA linked to each other for the charge compensation as reported by Zoglowek et al. (1988).
The Vmax value for MAL/OAA antiport in the presence of ADP and Pi can account for the rate of state 3 MAL oxidation by DWM (Pastore et al., 2001). The MAL/OAA shuttle is more active in durum wheat than in potato; notice that DWM converted MAL to OAA but not to pyruvate (Pastore et al., 2001) due to the very high activity of mMDH and to the negligible activity of the mitochondrial malic enzyme (Pastore et al., 1999b, 2001). In this regard, DWM resemble animal mitochondria rather than potato and other plant mitochondria. In contrast to mammalian mitochondria, the permeability of plant mitochondria is far from being exhaustively investigated and, as a consequence, at present we cannot easily distinguish the MAL/OAA carrier from others already reported in studies with plant mitochondria. However, by assuming that mitochondria from different plants share similar transport features, in light of the failure of the benzene-1,2,3-tricarboxylate to inhibit the OAA efflux, we suggest that this carrier is different: (a) from the putative tricarboxylate carrier (Day and Wiskich, 1981 and refs. therein), (b) from the protein recently proposed as a novel dicarboxylate-tricarboxylate carrier (Picault et al., 2002), and (c) from the MAL transporter reported by Taniguchi and Sugiyama (1996). On the other hand, the inhibition by butylmalonate rules out the possibility that the carrier that transports OAA outside mitochondria is the putative OAA translocator of pea leaf mitochondria (Oliver and Walker, 1984). In reconstituted systems of membrane proteins from potato tubers and pea leaf mitochondria, OAA was found to be transported in an obligatory counter-exchange with MAL, 2-oxoglutarate, succinate, citrate, or Asp (Hanning et al., 1999); because we show here that the MAL/OAA exchange is insensitive to citrate, we conclude that in DWM, the metabolite traffic due to MAL/OAA carrier is different from that reported by Hanning et al. (1999). Such a discrepancy could arise from differences in the mitochondrial functions in different plants and organs and to special, non-physiological transport processes occurring in the artificial systems used (proteoliposomes; see Passarella et al., 2003). Even though OAA transport in plant mitochondria is not a total “mystery” (Day and Wiskich, 1981), we believe that the elucidation of the mechanism by which OAA traffic occurs across the mitochondrial membrane in plants requires further investigation.
We also show that in durum wheat and potato cells, the MAL/OAA shuttle can work to transport reducing equivalent from the extramitochondrial phase to the matrix. In this regard, notice that the shuttle cannot oxidize NADPH due to the specificity of the cMDH for NADH. Furthermore, we show that that the antiporter regulates the rate of the overall process of NADH oxidation via the MAL/OAA shuttle. Apart from the ADP/ATP antiporter (Pastore et al., 1999b, and refs. therein), this is, to the best of our knowledge, the only case in which the rate of a process involving both cytosol and mitochondria depends on the rate of mitochondrial transport.
Finally, we observed no operation of the MAL/Asp shuttle in oxidizing external NADH in either of our plant systems. In this regard, DWM and PCM are rather different from other plant mitochondria: The MAL/Asp shuttle was shown to account for 12% to 20% of the activity of the MAL/OAA shuttle in the export of reducing power from mitochondria in pea and spinach (Spinacia oleracea; Krömer, 1995 and refs. therein), whereas Dry et al. (1987) reported a 50% contribution in pea. DWM and PCM cannot oxidize NAD(P)H via the α-GPDH/DHAP shuttle, whereas such a shuttle was shown in Arabidopsis thaliana (Shen et al., 2003).
In conclusion, the picture emerging from this paper is the following (Scheme 1): At cytosolic NADH concentrations, NADH oxidation by mitochondria occurs essentially due to the MAL/OAA shuttle with the rate of NADH oxidation regulated by the MAL/OAA antiporter. On the other hand, the contribution of the NADH DHExt to NADH oxidation can increase (Fig. 6) under conditions in which a decrease in the cytosolic reduction potential occurs; for example, as observed under environmental stress, when plants suffer over-reduction of the carriers of the photosynthetic electron transport (Raghavendra et al., 1994; Krömer, 1995).
MATERIALS AND METHODS
Materials and Isolation of DWM and PCM
All reagents and enzymes were purchased from Sigma (St. Louis). Mitochondrial substrates were of the purest grade available. Certified seeds of durum wheat (Triticum durum Desf. cv Ofanto) were kindly supplied by Dr. Natale Di Fonzo (Italian Cereal Crop Institute, Foggia). Cell suspension from leaf callus of potato (Solanum tuberosum), dihaploid clone SVP11, was kindly provided by Prof. Donato Chiatante (University of Insubria, Italy). DWM were purified as described by Pastore et al. (1999b) from 72-h-old etiolated shoots of durum wheat seedlings and suspended in a medium containing 0.3 m sucrose, 1 mm EDTA, 10 mm Tris-HCl (pH 7.40), and 0.1% (w/v) defatted BSA. Washed PCM were obtained as described by Fratianni et al. (2001) from 3-d-old cell suspension cultures in the early logarithmic phase and suspended in a medium containing 0.3 m sucrose, 10 mm K-phosphate (pH 7.10), and 0.2% (w/v) defatted BSA. Mitochondrial protein content was determined by the Lowry method modified as reported by Harris (1987), using BSA as a standard. In the case of PCM experiments, the protein content was divided by 2.2 to account for the non-mitochondrial proteins; this correction was based on preliminary experiments in which we calculated a ratio, “COX activity in purified PCM/COX activity in washed PCM,” equal to 2.2. The intactness of the outer and inner membranes was checked by measuring the latency of both COX and MDH (Neuburger et al., 1982; Douce et al., 1987); DWM and PCM showed 95% and 90% intactness of the inner membrane and 90% and 87% of the outer membrane, respectively (Pastore et al., 1999b, 2000; Fratianni et al., 2001). Using succinate as a substrate, both DWM and PCM proved to be coupled, showing good respiratory control (2.3 ± 0.2 DWM, 2.2 ± 0.4 PCM, n = 10) and ADP/O ratios (1.7 ± 0.1 DWM, n = 10; 1.7 ± 0.2 PCM, n = 10), as measured at 25°C in a medium consisting of 0.3 m mannitol, 5 mm MgCl2, 10 mm KCl, 10 mm K-phosphate buffer (pH 7.20), and 0.1% (w/v) defatted BSA by means of a GILSON oxygraph (model 5/6-servo channel, pH 5, Gilson, Middletown, WI).
Photometric and Fluorimetric Assays
Reconstruction of the MAL/OAA Shuttle
OAA appearance outside mitochondria was monitored by using the OAA detecting system consisting of 0.2 mm NAD(P)H plus 0.5 EU MDH (l-MAL: NAD+ oxidoreductase, EC 1.1.1.37) from PH; the NAD(P)H oxidation caused by externally added MAL was photometrically followed at 340 nm (ε340 = 6.22 mm-1 cm-1). In a typical experiment, either DWM or PCM (0.25 mg of protein) were added in 5 mL of a medium consisting of 0.3 m mannitol, 5 mm MgCl2, 10 mm KCl, 1 mm ADP, 10 mm K-phosphate (pH 7.20), and 0.1% (w/v) defatted BSA (standard medium) at 25°C; then, two aliquots of the resulting suspension (2 mL each) were put in two cuvettes held in the double-beam Jasco V 560 UV/Vis spectrophotometer holder (Jasco Corporation, Tokyo). The OAA detecting system was added to both of them. Then, at the same time, MAL (20 μL) and medium (20 μL) were added in the sample and reference cuvette, respectively, with recording made of the NADH oxidation due to OAA efflux.
The NADH oxidation due to the NADH DHExt was largely prevented by adding 10 mm EGTA plus 10 mm EDTA. Control was also made that the residual rate of NADH oxidation due to incomplete inhibition by EDTA/EGTA of the NADH DHExt proved to remain constant for more than 10 min.
Fluorimetric determinations were carried out by using an LS-50B luminescence spectrometer (Perkin-Elmer Applied Biosystems, Foster City, CA) with excitation and emission wavelengths set at 340 and 456 nm, respectively, but in EDTA/EGTA-free medium.
The rate of both absorbance and fluorescence change were obtained as tangents at the initial part of the progress curve and expressed as nanomoles of NADH oxidized per minute per milligram of protein.
Reconstruction of the MAL/Asp Shuttle
Asp appearance outside mitochondria was checked essentially as above with the addition to both the cuvettes of 0.5 mm 2-oxoglutarate, 0.5 mm Glu and of 0.5 EU AAT (l-Asp: 2-oxoglutarate aminotransferase, EC 2.6.1.1) from PH to the sample cuvette only.
Reconstruction of the α-GP/DHAP Shuttle
DHAP appearance outside mitochondria was monitored by using the DHAP-detecting system consisting of 0.2 mm NAD(P)H and 0.5 EU α-GPDH (sn-glycerol-3-phosphate: NAD+ 2-oxidoreductase, EC 1.1.1.8) from rabbit muscle. Control experiments were carried out to confirm that all of the compounds used in this study did not affect the enzymes used to reveal metabolite appearance outside mitochondria and that the revealing enzymes cannot limit the whole reaction rate under the adopted experimental conditions. For further details, see the figure legends.
Enzymatic Assays
MDH Assay
The kinetic parameters (Vmax, Km, and Ks values) of cMDH were evaluated by using 0.05 mg of protein of the supernatant obtained by centrifugation at 20,000g for 16 min of the homogenate from durum wheat shoots and at 12,000g for 20 min of the homogenate from potato cells. Control was made that contamination by non-cytosolic proteins was negligible. MDH reaction was checked at 25°C in 2 mL of the standard medium containing either 0.2 mm NAD(P)H and increasing OAA concentrations or 0.6 mm OAA and increasing NAD(P)H concentrations. In both cases, the reaction was started by rapidly adding the variable substrate. The reaction was monitored by following photometrically the absorbance decrease at 340 nm due to NAD(P)H oxidation.
NAD(P)H DHExt Assay
NAD(P)H oxidation by NAD(P)H DHExt was studied by incubating either DWM or PCM (0.1 mg of protein) at 25°C in 2 mL of the standard medium added with 30 μm CaCl2. Then, the reaction was started by adding either NADH or NADPH at different concentrations. The rate was calculated as tangent of the initial part of either the progress curve of A340 [up to 0.2 mm NAD(P)H] or oxygen uptake [NAD(P)H concentration > 0.2 mm]. Control was made that the two methods used gave results in a fairly good accordance. The rates were expressed as nanomoles of NAD(P)H oxidized per minute per milligram of protein.
Calculations and Computing
The activity of NADH DHExt, NADPH DHExt and MAL/OAA antiporter, measured as described above, were expressed on a gram of whole (W) fresh tissue or cells basis according to the following equation:
 |
where ACTIVITYW is activity of NADH DHExt, NADPH DHExt, or MAL/OAA antiporter in the whole tissue or cell expressed as nanomoles per minute per gram of tissue or cells; ACTIVITYM is activity of NADH DHExt, NADPH DHExt, or MAL/OAA antiporter in the mitochondrial fraction expressed as nanomoles per minute per milligram of mitochondrial protein; COXH is activity of COX in the homogenate expressed as natom (oxygen) per minute per milligram of homogenate protein; and COXM is activity of COX in the mitochondrial fraction expressed as natom (oxygen) per minute per milligram of mitochondrial protein.
Similarly, cMDH activity was expressed on a gram of whole tissue or cells basis according to the following equation:
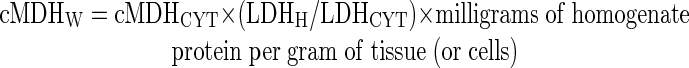 |
where cMDHW is activity of cMDH in the whole tissue or cell expressed as nanomoles per minute per gram of tissue or cells; cMDHCYT is activity of MDH in the cytosolic fraction expressed as nanomoles per minute per milligram of cytosolic protein; LDHH is activity of LDH in the homogenate expressed as nanomoles per minute per milligram of homogenate protein; and LDHCYT is activity of LDH in the cytosolic fraction expressed as nanomoles per minute per milligram of cytosolic protein.
COX and LDH, used as marker enzymes of mitochondria and cytosol, respectively, were assayed as reported by Pastore et al. (1999b).
Acknowledgments
The skillful cooperation of Dr. Gianluca Paventi and Dr. Maura Nicoletta Laus and the critical reading of Dr. Steve Reshkin are gratefully acknowledged.
This work was supported in part by the MURST project (Progetto di Rilevante Interesse Nazionale: “Bioenergetica: aspetti genetici, biochimici e fisiopatologici”), by the Italian Council of Research (project “Cell organelles and metabolism regulation”), and by the University of Molise (“Fondi per la ricerca di Ateneo” to D.P.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.028548.
References
- Atlante A, Passarella S, Giannattasio S, Quagliariello E (1985) Fumarate permeation in rat liver mitochondria: fumarate/malate and fumarate/phosphate translocators. Biochem Biophys Res Commun 132: 8-18 [DOI] [PubMed] [Google Scholar]
- Day DA, Wiskich JT (1981) Effect of phthalonic acid on respiration and metabolite transport in higher plant mitochondria. Arch Biochem Biophys 211: 100-107 [DOI] [PubMed] [Google Scholar]
- De Santis A, Arrigoni O, Palmieri F (1976) Carrier-mediated transport of metabolites in purified bean mitochondria. Plant Cell Physiol 17: 1221-1233 [Google Scholar]
- Dixon M, Webb EC (1979) Enzymes, Ed 3. Academic Press, New York
- Douce R (1985) Mitochondria in higher plants. Structure, Function and Biogenesis. Academic Press, Orlando, FL
- Douce R, Bourguignon J, Brouquisse R, Neuburger M (1987) Isolation of plant mitochondria: general principles and criteria of integrity. Methods Enzymol 148: 403-415 [Google Scholar]
- Dry IB, Dimitriadis E, Ward AD, Wiskich JT (1987) The photorespiratory hydrogen shuttle. Synthesis of phthalonic acid and its use in the characterization of the malate/aspartate shuttle in pea (Pisum sativum) leaf mitochondria. Biochem J 245: 669-675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratianni A, Pastore D, Pallotta ML, Chiatante D, Passarella S (2001) Increase of membrane permeability of mitochondria isolated from water stress adapted potato cells. Biosci Rep 21: 81-91 [DOI] [PubMed] [Google Scholar]
- Gardeström P (1996) Interactions between mitochondria and chloroplasts. Biochim Biophys Acta 1275: 38-40 [Google Scholar]
- Gardeström P, Lernmark U (1995) The contribution of mitochondria to energetic metabolism in photosynthetic cells. J Bioenerg Biomembr 27: 415-421 [DOI] [PubMed] [Google Scholar]
- Genchi G, Spagnoletta A, De Santis A, Stefanizzi L, Palmieri F (1999) Purification and characterization of the reconstitutively active citrate carrier from maize mitochondria. Plant Physiol 120: 841-847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanning I, Baumgarten K, Schott K, Heldt HW (1999) Oxaloacetate transport into plant mitochondria. Plant Physiol 119: 1025-1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanning I, Heldt HW (1993) On the function of mitochondrial metabolism during photosynthesis in spinach (Spinacia oleracea L.) leaves. Plant Physiol 103: 1147-1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DA (1987) Spectrophotometric assays. In CL Bashford, DA Harris, eds, Spectrophotometry and Spectrofluorimetry: a Practical Approach. IRL Press, Oxford, pp 59-61
- Heineke D, Riens B, Grosse H, Hoferichter P, Peter U, Flügge UI, Heldt HW (1991) Redox transfer across the inner chloroplast envelope membrane. Plant Physiol 95: 1131-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H (1987) Some aspects of calcium-dependent regulation in plant metabolism. Annu Rev Plant Physiol Plant Mol Biol 38: 47-72 [Google Scholar]
- Klingenberg M (1972) Kinetic study of tricarboxylate carrier in rat liver mitochondria. Eur J Biochem 26: 587-594 [DOI] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR (1996) Cold calcium signalling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8: 489-503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krömer S (1995) Respiration during photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 46: 45-70 [Google Scholar]
- Krömer S, Heldt HW (1991) Respiration of pea leaf mitochondria and redox transfer between the mitochondrial and extramitochondrial compartment. Biochim Biophys Acta 1057: 42-51 [Google Scholar]
- Krömer S, Malmberg G, Gardeström P (1993) Mitochondrial contribution to photosynthetic metabolism (a study with barley (Hordeum vulgare L.) leaf protoplasts at different light intensities and CO2 concentration. Plant Physiol 102: 947-955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloi M (1999) Plant mitochondrial carriers: an overview. Cell Mol Life Sci 56: 918-944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E, Rentsch D (1994) Malate compartmentation-responses to a complex metabolism. Annu Rev Plant Physiol Plant Mol Biol 45: 447-467 [Google Scholar]
- Møller IM (1997) The oxidation of cytosolic NAD(P)H by external NAD(P)H dehydrogenases in the respiratory chain of plant mitochondria. Physiol Plant 100: 85-90 [Google Scholar]
- Møller IM (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 52: 561-591 [DOI] [PubMed] [Google Scholar]
- Neuburger M, Douce R (1980) Effect of bicarbonate and oxaloacetate on malate oxidation by spinach leaf mitochondria. Biochim Biophys Acta 589: 176-189 [DOI] [PubMed] [Google Scholar]
- Neuburger M, Journet EP, Bligny R, Card JP, Douce R (1982) Purification of plant mitochondria by isopycnic centrifugation in density gradients of percoll. Arch Biochem Biophys 217: 312-323 [DOI] [PubMed] [Google Scholar]
- Oliver DJ, Walker GH (1984) Characterization of the transport of oxaloacetate by pea leaf mitochondria. Plant Physiol 76: 409-413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarella S, Atlante A, Valenti D, de Bari L (2003) The role of mitochondrial transport in energy metabolism. Mitochondrion 2: 319-343 [DOI] [PubMed] [Google Scholar]
- Passarella S, Barile M, Atlante A, Quagliariello E (1984) Oxaloacetate uptake into rat brain mitochondria and reconstruction of the malate/oxaloacetate shuttle. Biochem Biophys Res Commun 119: 1039-1046 [DOI] [PubMed] [Google Scholar]
- Pastore D, Fratianni A, Di Pede S, Passarella S (2000) Effects of fatty acids, nucleotides and reactive oxygen species on durum wheat mitochondria. FEBS Lett 470: 88-92 [DOI] [PubMed] [Google Scholar]
- Pastore D, Laus MN, Di Fonzo N, Passarella S (2002) Reactive oxygen species inhibit the succinate oxidation-supported generation of membrane potential in wheat mitochondria. FEBS Lett 516: 15-19 [DOI] [PubMed] [Google Scholar]
- Pastore D, Stoppelli MC, Di Fonzo N, Passarella S (1999a) The existence of the K+ channel in plant mitochondria. J Biol Chem 274: 26683-26690 [DOI] [PubMed] [Google Scholar]
- Pastore D, Trono D, Laus MN, Di Fonzo N, Passarella S (2001) Alternative oxidase in durum wheat mitochondria: activation by pyruvate, hydroxypyruvate and glyoxylate and physiological role. Plant Cell Physiol 42: 1373-1382 [DOI] [PubMed] [Google Scholar]
- Pastore D, Trono D, Passarella S (1999b) Substrate oxidation and ADP/ATP exchange in coupled durum wheat (Triticum durum Desf.) mitochondria. Plant Biosystems 133: 219-228 [Google Scholar]
- Picault N, Palmieri L, Pisano I, Hodges M, Palmieri F (2002) Identification of a novel transporter for dicarboxylates and tricarboxylates in plant mitochondria: bacterial expression, reconstitution, functional characterization and tissue distribution. J Biol Chem 277: 24204-24211 [DOI] [PubMed] [Google Scholar]
- Raghavendra AS, Padmasree K, Saradadevi K (1994) Interdependence of photosynthesis and respiration in plant cells: interactions between chloroplast and mitochondria. Plant Sci 97: 1-14 [Google Scholar]
- Raghavendra AS, Reumann S, Heldt HW (1998) Participation of mitochondrial metabolism in photorespiration. Reconstituted system of peroxisomes and mitochondria from spinach leaves. Plant Physiol 116: 1333-1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TH, Fredlund KM, Møller IM (1995) Direct evidence for the presence of two external NAD(P)H dehydrogenases coupled to the electron transport chain in plant mitochondria. FEBS Lett 373: 307-309 [DOI] [PubMed] [Google Scholar]
- Rustin P, Valat M (1986) The control of malate dehydrogenase activity by adenine nucleotides in purified potato tuber (Solanum tuberosum L.) mitochondria. Arch Biochem Biophys 247: 62-67 [DOI] [PubMed] [Google Scholar]
- Saradadevi K, Raghavendra AS (1992) Dark respiration protects photosynthesis against photoinhibition in mesophyll protoplasts of pea (Pisum sativum). Plant Physiol 99: 1232-1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Wie Y, Dauk M, Zheng Z, Zou J (2003) Identification of a mitochondrial glycerol-3-phosphate dehydrogenase from Arabidopsis thaliana: evidence for a mitochondrial glycerol-3-phosphate shuttle in plants. FEBS Lett 536: 92-96 [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Sugiyama T (1996) Isolation, characterization and expression of cDNA clones encoding a mitochondrial malate translocator from Panicum miliaceum L. Plant Mol Biol 30: 51-64 [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L (1997) Alternative oxidase: from gene to function. Annu Rev Plant Physiol Plant Mol Biol 48: 703-734 [DOI] [PubMed] [Google Scholar]
- Vercesi AE, Martins IS, Silva MAP, Leite HMF, Cuccovia IM, Chaimovich H (1995) PUMPing plants. Nature 375: 24 [Google Scholar]
- Zoglowek C, Krömer S, Heldt HW (1988) Oxaloacetate and malate transport by plant mitochondria. Plant Physiol 87: 109-115 [DOI] [PMC free article] [PubMed] [Google Scholar]