Abstract
In the asymmetric unit of the title compound, C19H27ClN4O·0.5C2H6O·1.5H2O, there are two molecules of the Schiff base, which has a rigid adamantyl cage at one end of the C(= O)NH–N=CH– chain and an almost planar [torsion angles = 1.3 (1) and 7.9 (2)° imidazolyl ring at the other end, three molecules of water and one molecule of ethanol. In both independent molecules of the Schiff base, this chain displays an extended zigzag configuration. All their amino groups function as hydrogen-bond donors to water molecules; these are linked to other acceptor atoms, generating a layer structure. O—H⋯O and O—H⋯N interactions involving the water molecules also occur.
Related literature
For the cyclization of this class of Schiff bases to pharmaceutically useful chemicals, see: Kadi et al. (2007 ▶).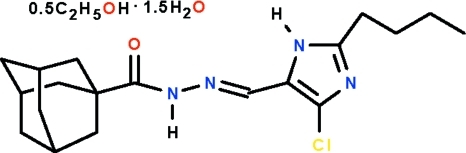
Experimental
Crystal data
C19H27ClN4O·0.5C2H6O·1.5H2O
M r = 412.95
Triclinic,
a = 7.9867 (6) Å
b = 16.8478 (13) Å
c = 16.9656 (13) Å
α = 97.341 (1)°
β = 100.376 (1)°
γ = 97.505 (1)°
V = 2199.3 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.20 mm−1
T = 100 K
0.40 × 0.10 × 0.10 mm
Data collection
Bruker SMART APEX diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.924, T max = 0.980
21291 measured reflections
10050 independent reflections
7547 reflections with I > 2σ(I)
R int = 0.036
Refinement
R[F 2 > 2σ(F 2)] = 0.043
wR(F 2) = 0.113
S = 1.02
10050 reflections
552 parameters
11 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.37 e Å−3
Δρmin = −0.43 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810029260/bt5304sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810029260/bt5304Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯O3i | 0.86 (1) | 2.00 (1) | 2.841 (2) | 166 (2) |
| N3—H3⋯O1wii | 0.86 (1) | 1.95 (1) | 2.806 (2) | 170 (2) |
| N6—H6⋯O2w | 0.88 (1) | 1.95 (1) | 2.829 (2) | 174 (2) |
| N8—H8⋯O3wiii | 0.86 (1) | 1.94 (1) | 2.778 (2) | 164 (2) |
| O3—H3o⋯O1w | 0.84 (1) | 1.84 (1) | 2.673 (2) | 177 (2) |
| O1w—H11⋯O1ii | 0.84 (1) | 2.00 (1) | 2.821 (2) | 166 (2) |
| O1w—H12⋯N5 | 0.84 (1) | 1.91 (1) | 2.751 (2) | 175 (3) |
| O2w—H21⋯O2 | 0.85 (1) | 2.07 (1) | 2.905 (2) | 172 (2) |
| O2w—H22⋯O2iv | 0.84 (1) | 1.93 (1) | 2.764 (2) | 176 (2) |
| O3w—H31⋯N4 | 0.84 (1) | 1.94 (1) | 2.773 (2) | 169 (2) |
| O3w—H32⋯O2w | 0.85 (1) | 1.92 (1) | 2.766 (2) | 174 (2) |
Symmetry codes: (i) 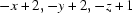 ; (ii)
; (ii) 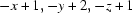 ; (iii)
; (iii) 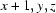 ; (iv)
; (iv) 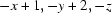 .
.
Acknowledgments
We thank King Saud University and the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
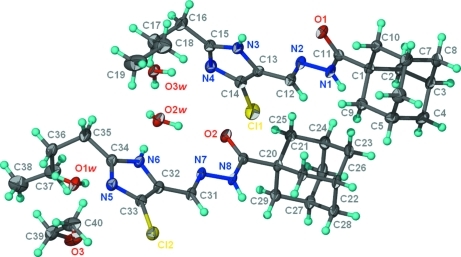
Adamantane-1-carbohydrazide is a commercially available compound that condenses with primary amines to form Schiff bases; some of these Schiff bases can be cyclized to yield pharmaceutically useful compounds (Kadi et al., 2007). However, unlike other aryolhydrazides, there have been no reports of the crystal structures of these Schiff bases. The condensation product with 2-n-butyl-5-chloro-1H-imidazole-4-carboxaldehyde crystallizes from aqueous ethanol as a sesquihydrate hemiethanol solvate (Scheme 1). There are two independent Schiff base molecules in the asymmetric unit (Fig. 1). The molecule of C20H27ClN4O has a rigid adamantyl cage at one end of the C(═ O)NH–N═CH– chain and a planar imidazolyl ring at the other end; the chain displays an extended zigzag configuration.
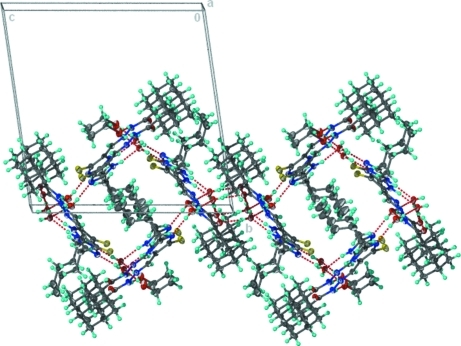
The amino groups all function as hydrogen-bond donors to water molecules; these are linked to other acceptor atoms to generate a layer structure (Fig. 2).
Experimental
Adamantane-1-carbohydrazide (1.94 g, 1 mmol) and 2-n-butyl-5-chloro-1H-imidazole-4-carboxaldehyde (1.87 g, 1 mmol) were heated in ethanol (50 ml) for 1 h. The solvent was removed and the product recrystallized from aqueous ethanol to afford colorless primatic crystals in 90% yield.
Refinement
Carbon-bound H-atoms were placed in calculated positions (C—H 0.95 to 0.98 Å) and were included in the refinement in the riding model approximation, with U(H) set to 1.2 to 1.5U(C).
The amino and water H-atoms were located in a difference Fourier map, and were refined isotropically with distance restraints of N–H 0.86±0.01 Å and O–H 0.84±0.01 Å.
Figures
Fig. 1.
Anisotropic ellipsoid plot (Barbour, 2001) of C19H27ClN4O.0.5C2H5OH.1.5H2O at the 70% probability level; hydrogen atoms are drawn as spheres of arbitrary radius.
Fig. 2.
Hydrogen-bonded layer structure.
Crystal data
| C19H27ClN4O·0.5C2H6O·1.5H2O | Z = 4 |
| Mr = 412.95 | F(000) = 888 |
| Triclinic, P1 | Dx = 1.247 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 7.9867 (6) Å | Cell parameters from 4692 reflections |
| b = 16.8478 (13) Å | θ = 2.5–28.2° |
| c = 16.9656 (13) Å | µ = 0.20 mm−1 |
| α = 97.341 (1)° | T = 100 K |
| β = 100.376 (1)° | Prism, colorless |
| γ = 97.505 (1)° | 0.40 × 0.10 × 0.10 mm |
| V = 2199.3 (3) Å3 |
Data collection
| Bruker SMART APEX diffractometer | 10050 independent reflections |
| Radiation source: fine-focus sealed tube | 7547 reflections with I > 2σ(I) |
| graphite | Rint = 0.036 |
| ω scans | θmax = 27.5°, θmin = 1.6° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −10→10 |
| Tmin = 0.924, Tmax = 0.980 | k = −22→21 |
| 21291 measured reflections | l = −21→21 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.043 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.113 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.0579P)2] where P = (Fo2 + 2Fc2)/3 |
| 10050 reflections | (Δ/σ)max = 0.001 |
| 552 parameters | Δρmax = 0.37 e Å−3 |
| 11 restraints | Δρmin = −0.43 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 0.76837 (6) | 1.16482 (3) | 0.27186 (3) | 0.02855 (12) | |
| Cl2 | 0.96970 (5) | 0.80656 (2) | 0.35728 (3) | 0.02268 (10) | |
| O1 | 0.75877 (14) | 1.40939 (7) | 0.65640 (7) | 0.0201 (3) | |
| O2 | 0.73265 (14) | 1.07788 (7) | 0.06758 (7) | 0.0188 (2) | |
| O3 | 0.78300 (16) | 0.63333 (8) | 0.50491 (8) | 0.0284 (3) | |
| O1W | 0.54116 (15) | 0.70102 (7) | 0.41892 (7) | 0.0196 (3) | |
| O2W | 0.46191 (15) | 0.95403 (7) | 0.08834 (7) | 0.0201 (3) | |
| O3W | 0.27823 (15) | 1.00424 (7) | 0.20395 (7) | 0.0216 (3) | |
| N1 | 0.92004 (18) | 1.38006 (8) | 0.56315 (8) | 0.0180 (3) | |
| N2 | 0.78186 (17) | 1.32710 (8) | 0.51751 (8) | 0.0178 (3) | |
| N3 | 0.50609 (18) | 1.21097 (8) | 0.43693 (8) | 0.0173 (3) | |
| N4 | 0.46659 (18) | 1.13115 (8) | 0.31913 (8) | 0.0204 (3) | |
| N5 | 0.62851 (18) | 0.77835 (8) | 0.29533 (8) | 0.0195 (3) | |
| N6 | 0.60000 (18) | 0.86304 (8) | 0.20631 (8) | 0.0176 (3) | |
| N7 | 0.83450 (17) | 0.98033 (8) | 0.16998 (8) | 0.0158 (3) | |
| N8 | 0.95038 (17) | 1.04002 (8) | 0.15190 (8) | 0.0151 (3) | |
| C1 | 1.0617 (2) | 1.46542 (9) | 0.69156 (9) | 0.0157 (3) | |
| C2 | 1.1519 (2) | 1.52998 (9) | 0.64919 (10) | 0.0182 (3) | |
| H2A | 1.1853 | 1.5032 | 0.6002 | 0.022* | |
| H2B | 1.0716 | 1.5675 | 0.6321 | 0.022* | |
| C3 | 1.3131 (2) | 1.57772 (10) | 0.70820 (10) | 0.0214 (4) | |
| H3A | 1.3706 | 1.6198 | 0.6811 | 0.026* | |
| C4 | 1.4376 (2) | 1.51865 (11) | 0.73189 (11) | 0.0238 (4) | |
| H4A | 1.4708 | 1.4921 | 0.6828 | 0.029* | |
| H4B | 1.5435 | 1.5487 | 0.7688 | 0.029* | |
| C5 | 1.3512 (2) | 1.45452 (10) | 0.77393 (10) | 0.0218 (4) | |
| H5 | 1.4330 | 1.4164 | 0.7895 | 0.026* | |
| C6 | 1.3012 (2) | 1.49608 (11) | 0.85039 (10) | 0.0250 (4) | |
| H6A | 1.2465 | 1.4548 | 0.8783 | 0.030* | |
| H6B | 1.4057 | 1.5264 | 0.8881 | 0.030* | |
| C7 | 1.1755 (2) | 1.55423 (10) | 0.82642 (10) | 0.0218 (4) | |
| H7 | 1.1428 | 1.5813 | 0.8762 | 0.026* | |
| C8 | 1.2614 (2) | 1.61865 (10) | 0.78384 (11) | 0.0249 (4) | |
| H8A | 1.1803 | 1.6563 | 0.7684 | 0.030* | |
| H8B | 1.3648 | 1.6504 | 0.8213 | 0.030* | |
| C9 | 1.1886 (2) | 1.40708 (10) | 0.71633 (10) | 0.0184 (3) | |
| H9A | 1.1329 | 1.3654 | 0.7435 | 0.022* | |
| H9B | 1.2203 | 1.3793 | 0.6674 | 0.022* | |
| C10 | 1.0134 (2) | 1.50723 (10) | 0.76836 (10) | 0.0183 (3) | |
| H10A | 0.9319 | 1.5449 | 0.7534 | 0.022* | |
| H10B | 0.9559 | 1.4661 | 0.7956 | 0.022* | |
| C11 | 0.8996 (2) | 1.41698 (9) | 0.63612 (9) | 0.0153 (3) | |
| C12 | 0.8028 (2) | 1.28451 (9) | 0.45310 (9) | 0.0173 (3) | |
| H12A | 0.9093 | 1.2909 | 0.4354 | 0.021* | |
| C13 | 0.6573 (2) | 1.22641 (9) | 0.40851 (10) | 0.0165 (3) | |
| C14 | 0.6272 (2) | 1.17570 (10) | 0.33610 (10) | 0.0185 (3) | |
| C15 | 0.3969 (2) | 1.15408 (10) | 0.38198 (10) | 0.0198 (4) | |
| C16 | 0.2199 (2) | 1.11990 (11) | 0.39062 (11) | 0.0251 (4) | |
| H16A | 0.1425 | 1.1094 | 0.3366 | 0.030* | |
| H16B | 0.1759 | 1.1605 | 0.4256 | 0.030* | |
| C17 | 0.2146 (2) | 1.04091 (11) | 0.42715 (12) | 0.0308 (4) | |
| H17A | 0.0940 | 1.0127 | 0.4158 | 0.037* | |
| H17B | 0.2833 | 1.0051 | 0.4003 | 0.037* | |
| C18 | 0.2832 (3) | 1.05454 (13) | 0.51753 (13) | 0.0377 (5) | |
| H18A | 0.2149 | 1.0907 | 0.5443 | 0.045* | |
| H18B | 0.4039 | 1.0825 | 0.5287 | 0.045* | |
| C19 | 0.2776 (3) | 0.97727 (16) | 0.55442 (17) | 0.0588 (8) | |
| H19A | 0.3189 | 0.9906 | 0.6133 | 0.088* | |
| H19B | 0.3515 | 0.9426 | 0.5311 | 0.088* | |
| H19C | 0.1589 | 0.9486 | 0.5428 | 0.088* | |
| C20 | 1.0200 (2) | 1.15241 (9) | 0.07997 (9) | 0.0143 (3) | |
| C21 | 1.1515 (2) | 1.19510 (10) | 0.15687 (9) | 0.0173 (3) | |
| H21A | 1.0903 | 1.2215 | 0.1962 | 0.021* | |
| H21B | 1.2121 | 1.1546 | 0.1827 | 0.021* | |
| C22 | 1.2828 (2) | 1.25884 (10) | 0.13452 (10) | 0.0191 (4) | |
| H22A | 1.3672 | 1.2860 | 0.1846 | 0.023* | |
| C23 | 1.1891 (2) | 1.32202 (10) | 0.09564 (11) | 0.0239 (4) | |
| H23A | 1.1275 | 1.3491 | 0.1344 | 0.029* | |
| H23B | 1.2736 | 1.3637 | 0.0818 | 0.029* | |
| C24 | 1.0604 (2) | 1.28048 (10) | 0.01893 (11) | 0.0233 (4) | |
| H24 | 0.9992 | 1.3218 | −0.0064 | 0.028* | |
| C25 | 0.9281 (2) | 1.21643 (10) | 0.04083 (11) | 0.0201 (4) | |
| H25A | 0.8645 | 1.2429 | 0.0790 | 0.024* | |
| H25B | 0.8439 | 1.1900 | −0.0088 | 0.024* | |
| C26 | 1.1560 (2) | 1.23945 (11) | −0.04119 (11) | 0.0259 (4) | |
| H26A | 1.0730 | 1.2130 | −0.0911 | 0.031* | |
| H26B | 1.2402 | 1.2805 | −0.0562 | 0.031* | |
| C27 | 1.2497 (2) | 1.17624 (10) | −0.00257 (10) | 0.0206 (4) | |
| H27 | 1.3125 | 1.1496 | −0.0419 | 0.025* | |
| C28 | 1.3788 (2) | 1.21725 (10) | 0.07450 (10) | 0.0216 (4) | |
| H28A | 1.4657 | 1.2579 | 0.0607 | 0.026* | |
| H28B | 1.4395 | 1.1763 | 0.0996 | 0.026* | |
| C29 | 1.1182 (2) | 1.11208 (9) | 0.01915 (10) | 0.0181 (3) | |
| H29A | 1.1784 | 1.0706 | 0.0435 | 0.022* | |
| H29B | 1.0355 | 1.0849 | −0.0306 | 0.022* | |
| C30 | 0.8874 (2) | 1.08749 (9) | 0.09866 (9) | 0.0147 (3) | |
| C31 | 0.8957 (2) | 0.93466 (9) | 0.21920 (9) | 0.0170 (3) | |
| H31A | 1.0157 | 0.9405 | 0.2410 | 0.020* | |
| C32 | 0.7734 (2) | 0.87396 (9) | 0.23988 (9) | 0.0168 (3) | |
| C33 | 0.7857 (2) | 0.82064 (10) | 0.29406 (10) | 0.0182 (3) | |
| C34 | 0.5179 (2) | 0.80624 (10) | 0.24081 (10) | 0.0188 (3) | |
| C35 | 0.3269 (2) | 0.77994 (10) | 0.21929 (11) | 0.0235 (4) | |
| H35A | 0.2761 | 0.8135 | 0.1804 | 0.028* | |
| H35B | 0.2786 | 0.7898 | 0.2688 | 0.028* | |
| C36 | 0.2740 (2) | 0.69046 (10) | 0.18198 (10) | 0.0223 (4) | |
| H36A | 0.3137 | 0.6569 | 0.2233 | 0.027* | |
| H36B | 0.1466 | 0.6782 | 0.1680 | 0.027* | |
| C37 | 0.3442 (2) | 0.66585 (11) | 0.10660 (11) | 0.0273 (4) | |
| H37A | 0.4717 | 0.6740 | 0.1209 | 0.033* | |
| H37B | 0.3106 | 0.7011 | 0.0660 | 0.033* | |
| C38 | 0.2775 (3) | 0.57763 (13) | 0.06950 (12) | 0.0390 (5) | |
| H38A | 0.3292 | 0.5636 | 0.0224 | 0.058* | |
| H38B | 0.1518 | 0.5700 | 0.0523 | 0.058* | |
| H38C | 0.3085 | 0.5426 | 0.1099 | 0.058* | |
| C39 | 0.7041 (2) | 0.59637 (11) | 0.56315 (11) | 0.0292 (4) | |
| H39A | 0.7416 | 0.5431 | 0.5676 | 0.035* | |
| H39B | 0.5773 | 0.5871 | 0.5446 | 0.035* | |
| C40 | 0.7512 (3) | 0.64869 (12) | 0.64503 (11) | 0.0317 (4) | |
| H40A | 0.7063 | 0.6193 | 0.6850 | 0.048* | |
| H40B | 0.7011 | 0.6986 | 0.6423 | 0.048* | |
| H40C | 0.8769 | 0.6623 | 0.6611 | 0.048* | |
| H3o | 0.709 (2) | 0.6547 (12) | 0.4769 (11) | 0.040 (6)* | |
| H11 | 0.4462 (17) | 0.6699 (11) | 0.4039 (12) | 0.039 (6)* | |
| H12 | 0.561 (3) | 0.7241 (15) | 0.3798 (11) | 0.068 (9)* | |
| H21 | 0.547 (2) | 0.9885 (11) | 0.0863 (14) | 0.047 (7)* | |
| H22 | 0.402 (2) | 0.9418 (11) | 0.0416 (7) | 0.028 (5)* | |
| H31 | 0.348 (2) | 1.0401 (11) | 0.2379 (11) | 0.050 (7)* | |
| H32 | 0.328 (3) | 0.9889 (13) | 0.1658 (10) | 0.047 (7)* | |
| H1 | 1.0165 (16) | 1.3843 (11) | 0.5468 (11) | 0.022 (5)* | |
| H3 | 0.491 (2) | 1.2324 (11) | 0.4835 (7) | 0.030 (5)* | |
| H6 | 0.553 (2) | 0.8878 (11) | 0.1671 (9) | 0.032 (6)* | |
| H8 | 1.0584 (13) | 1.0385 (11) | 0.1682 (11) | 0.029 (5)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0305 (3) | 0.0324 (2) | 0.0219 (2) | 0.00006 (19) | 0.01191 (19) | −0.00352 (18) |
| Cl2 | 0.0199 (2) | 0.0231 (2) | 0.0234 (2) | 0.00125 (16) | −0.00039 (17) | 0.00668 (16) |
| O1 | 0.0146 (6) | 0.0253 (6) | 0.0187 (6) | −0.0019 (5) | 0.0044 (5) | 0.0003 (5) |
| O2 | 0.0139 (6) | 0.0229 (6) | 0.0171 (6) | −0.0021 (5) | −0.0006 (5) | 0.0038 (5) |
| O3 | 0.0208 (7) | 0.0456 (8) | 0.0228 (7) | 0.0111 (6) | 0.0065 (6) | 0.0107 (6) |
| O1W | 0.0161 (6) | 0.0242 (6) | 0.0177 (6) | −0.0031 (5) | 0.0048 (5) | 0.0044 (5) |
| O2W | 0.0151 (6) | 0.0263 (7) | 0.0163 (6) | −0.0022 (5) | −0.0003 (5) | 0.0045 (5) |
| O3W | 0.0147 (6) | 0.0240 (6) | 0.0226 (7) | −0.0009 (5) | 0.0018 (5) | −0.0030 (5) |
| N1 | 0.0125 (7) | 0.0218 (7) | 0.0166 (7) | −0.0038 (6) | 0.0030 (6) | −0.0012 (6) |
| N2 | 0.0152 (7) | 0.0191 (7) | 0.0162 (7) | −0.0024 (5) | 0.0006 (6) | 0.0007 (5) |
| N3 | 0.0159 (7) | 0.0194 (7) | 0.0155 (7) | 0.0012 (6) | 0.0029 (6) | 0.0009 (6) |
| N4 | 0.0193 (8) | 0.0203 (7) | 0.0191 (7) | 0.0003 (6) | 0.0003 (6) | 0.0012 (6) |
| N5 | 0.0180 (7) | 0.0199 (7) | 0.0211 (7) | 0.0002 (6) | 0.0058 (6) | 0.0052 (6) |
| N6 | 0.0143 (7) | 0.0188 (7) | 0.0197 (7) | −0.0002 (6) | 0.0036 (6) | 0.0053 (6) |
| N7 | 0.0151 (7) | 0.0164 (7) | 0.0146 (7) | −0.0035 (5) | 0.0047 (5) | 0.0010 (5) |
| N8 | 0.0111 (7) | 0.0167 (7) | 0.0159 (7) | −0.0026 (5) | 0.0021 (5) | 0.0029 (5) |
| C1 | 0.0137 (8) | 0.0160 (8) | 0.0161 (8) | 0.0000 (6) | 0.0015 (6) | 0.0015 (6) |
| C2 | 0.0155 (8) | 0.0178 (8) | 0.0210 (8) | 0.0000 (6) | 0.0035 (7) | 0.0047 (7) |
| C3 | 0.0157 (8) | 0.0199 (8) | 0.0259 (9) | −0.0040 (7) | 0.0034 (7) | 0.0018 (7) |
| C4 | 0.0138 (8) | 0.0291 (9) | 0.0248 (9) | 0.0008 (7) | 0.0008 (7) | −0.0030 (7) |
| C5 | 0.0185 (9) | 0.0260 (9) | 0.0191 (9) | 0.0073 (7) | −0.0019 (7) | 0.0011 (7) |
| C6 | 0.0248 (10) | 0.0287 (9) | 0.0175 (9) | 0.0023 (8) | −0.0011 (7) | −0.0017 (7) |
| C7 | 0.0182 (9) | 0.0241 (9) | 0.0202 (9) | 0.0012 (7) | 0.0033 (7) | −0.0049 (7) |
| C8 | 0.0185 (9) | 0.0193 (8) | 0.0313 (10) | −0.0011 (7) | −0.0010 (8) | −0.0042 (7) |
| C9 | 0.0188 (9) | 0.0167 (8) | 0.0182 (8) | 0.0019 (6) | 0.0012 (7) | 0.0010 (6) |
| C10 | 0.0169 (8) | 0.0191 (8) | 0.0175 (8) | 0.0008 (7) | 0.0037 (7) | −0.0011 (6) |
| C11 | 0.0165 (8) | 0.0144 (7) | 0.0156 (8) | 0.0017 (6) | 0.0038 (6) | 0.0042 (6) |
| C12 | 0.0154 (8) | 0.0193 (8) | 0.0169 (8) | 0.0000 (6) | 0.0045 (7) | 0.0034 (6) |
| C13 | 0.0147 (8) | 0.0184 (8) | 0.0171 (8) | 0.0027 (6) | 0.0038 (6) | 0.0040 (6) |
| C14 | 0.0198 (9) | 0.0208 (8) | 0.0150 (8) | 0.0022 (7) | 0.0039 (7) | 0.0032 (6) |
| C15 | 0.0170 (9) | 0.0197 (8) | 0.0207 (9) | 0.0015 (7) | −0.0001 (7) | 0.0030 (7) |
| C16 | 0.0147 (9) | 0.0299 (10) | 0.0275 (10) | −0.0006 (7) | 0.0004 (7) | 0.0021 (8) |
| C17 | 0.0235 (10) | 0.0261 (10) | 0.0420 (12) | −0.0028 (8) | 0.0130 (9) | 0.0003 (8) |
| C18 | 0.0370 (12) | 0.0422 (12) | 0.0415 (12) | 0.0121 (10) | 0.0177 (10) | 0.0152 (10) |
| C19 | 0.0459 (15) | 0.0676 (17) | 0.088 (2) | 0.0273 (13) | 0.0378 (14) | 0.0514 (16) |
| C20 | 0.0138 (8) | 0.0145 (7) | 0.0128 (7) | −0.0010 (6) | 0.0009 (6) | 0.0010 (6) |
| C21 | 0.0155 (8) | 0.0184 (8) | 0.0151 (8) | −0.0037 (6) | 0.0021 (6) | −0.0004 (6) |
| C22 | 0.0159 (8) | 0.0181 (8) | 0.0192 (8) | −0.0048 (6) | 0.0016 (7) | −0.0015 (6) |
| C23 | 0.0231 (9) | 0.0150 (8) | 0.0320 (10) | −0.0036 (7) | 0.0092 (8) | −0.0007 (7) |
| C24 | 0.0201 (9) | 0.0187 (8) | 0.0302 (10) | −0.0001 (7) | 0.0007 (8) | 0.0098 (7) |
| C25 | 0.0159 (8) | 0.0188 (8) | 0.0255 (9) | 0.0022 (7) | 0.0027 (7) | 0.0057 (7) |
| C26 | 0.0273 (10) | 0.0272 (9) | 0.0201 (9) | −0.0100 (8) | 0.0039 (8) | 0.0077 (7) |
| C27 | 0.0206 (9) | 0.0203 (8) | 0.0203 (9) | −0.0026 (7) | 0.0104 (7) | −0.0014 (7) |
| C28 | 0.0162 (8) | 0.0215 (8) | 0.0267 (9) | −0.0025 (7) | 0.0074 (7) | 0.0031 (7) |
| C29 | 0.0191 (9) | 0.0168 (8) | 0.0168 (8) | 0.0000 (6) | 0.0048 (7) | −0.0020 (6) |
| C30 | 0.0155 (8) | 0.0148 (7) | 0.0120 (7) | 0.0001 (6) | 0.0025 (6) | −0.0013 (6) |
| C31 | 0.0157 (8) | 0.0186 (8) | 0.0150 (8) | −0.0015 (6) | 0.0028 (6) | 0.0008 (6) |
| C32 | 0.0162 (8) | 0.0182 (8) | 0.0142 (8) | −0.0006 (6) | 0.0017 (6) | 0.0007 (6) |
| C33 | 0.0177 (8) | 0.0190 (8) | 0.0175 (8) | 0.0016 (7) | 0.0032 (7) | 0.0026 (6) |
| C34 | 0.0188 (9) | 0.0177 (8) | 0.0207 (8) | 0.0005 (7) | 0.0074 (7) | 0.0035 (7) |
| C35 | 0.0183 (9) | 0.0240 (9) | 0.0292 (10) | −0.0001 (7) | 0.0077 (7) | 0.0080 (7) |
| C36 | 0.0183 (9) | 0.0238 (9) | 0.0248 (9) | −0.0013 (7) | 0.0040 (7) | 0.0085 (7) |
| C37 | 0.0262 (10) | 0.0362 (10) | 0.0208 (9) | 0.0086 (8) | 0.0015 (8) | 0.0095 (8) |
| C38 | 0.0404 (13) | 0.0435 (12) | 0.0278 (11) | 0.0141 (10) | −0.0064 (9) | −0.0027 (9) |
| C39 | 0.0210 (10) | 0.0311 (10) | 0.0358 (11) | 0.0006 (8) | 0.0022 (8) | 0.0149 (8) |
| C40 | 0.0294 (11) | 0.0440 (12) | 0.0294 (10) | 0.0146 (9) | 0.0119 (8) | 0.0166 (9) |
Geometric parameters (Å, °)
| Cl1—C14 | 1.7142 (17) | C16—C17 | 1.537 (3) |
| Cl2—C33 | 1.7186 (17) | C16—H16A | 0.9900 |
| O1—C11 | 1.2300 (19) | C16—H16B | 0.9900 |
| O2—C30 | 1.2344 (19) | C17—C18 | 1.510 (3) |
| O3—C39 | 1.428 (2) | C17—H17A | 0.9900 |
| O3—H3o | 0.84 (1) | C17—H17B | 0.9900 |
| O1W—H11 | 0.84 (1) | C18—C19 | 1.514 (3) |
| O1W—H12 | 0.84 (1) | C18—H18A | 0.9900 |
| O2W—H21 | 0.85 (1) | C18—H18B | 0.9900 |
| O2W—H22 | 0.84 (1) | C19—H19A | 0.9800 |
| O3W—H31 | 0.84 (1) | C19—H19B | 0.9800 |
| O3W—H32 | 0.85 (1) | C19—H19C | 0.9800 |
| N1—C11 | 1.360 (2) | C20—C30 | 1.525 (2) |
| N1—N2 | 1.3682 (18) | C20—C25 | 1.535 (2) |
| N1—H1 | 0.86 (1) | C20—C21 | 1.547 (2) |
| N2—C12 | 1.278 (2) | C20—C29 | 1.547 (2) |
| N3—C15 | 1.345 (2) | C21—C22 | 1.533 (2) |
| N3—C13 | 1.385 (2) | C21—H21A | 0.9900 |
| N3—H3 | 0.86 (1) | C21—H21B | 0.9900 |
| N4—C15 | 1.327 (2) | C22—C23 | 1.529 (2) |
| N4—C14 | 1.361 (2) | C22—C28 | 1.535 (2) |
| N5—C34 | 1.335 (2) | C22—H22A | 1.0000 |
| N5—C33 | 1.366 (2) | C23—C24 | 1.529 (2) |
| N6—C34 | 1.344 (2) | C23—H23A | 0.9900 |
| N6—C32 | 1.378 (2) | C23—H23B | 0.9900 |
| N6—H6 | 0.88 (1) | C24—C26 | 1.529 (3) |
| N7—C31 | 1.282 (2) | C24—C25 | 1.538 (2) |
| N7—N8 | 1.3784 (18) | C24—H24 | 1.0000 |
| N8—C30 | 1.354 (2) | C25—H25A | 0.9900 |
| N8—H8 | 0.86 (1) | C25—H25B | 0.9900 |
| C1—C11 | 1.522 (2) | C26—C27 | 1.527 (2) |
| C1—C10 | 1.536 (2) | C26—H26A | 0.9900 |
| C1—C9 | 1.544 (2) | C26—H26B | 0.9900 |
| C1—C2 | 1.549 (2) | C27—C28 | 1.532 (2) |
| C2—C3 | 1.538 (2) | C27—C29 | 1.533 (2) |
| C2—H2A | 0.9900 | C27—H27 | 1.0000 |
| C2—H2B | 0.9900 | C28—H28A | 0.9900 |
| C3—C8 | 1.527 (2) | C28—H28B | 0.9900 |
| C3—C4 | 1.536 (2) | C29—H29A | 0.9900 |
| C3—H3A | 1.0000 | C29—H29B | 0.9900 |
| C4—C5 | 1.527 (2) | C31—C32 | 1.441 (2) |
| C4—H4A | 0.9900 | C31—H31A | 0.9500 |
| C4—H4B | 0.9900 | C32—C33 | 1.365 (2) |
| C5—C9 | 1.534 (2) | C34—C35 | 1.496 (2) |
| C5—C6 | 1.536 (2) | C35—C36 | 1.531 (2) |
| C5—H5 | 1.0000 | C35—H35A | 0.9900 |
| C6—C7 | 1.531 (2) | C35—H35B | 0.9900 |
| C6—H6A | 0.9900 | C36—C37 | 1.519 (2) |
| C6—H6B | 0.9900 | C36—H36A | 0.9900 |
| C7—C10 | 1.532 (2) | C36—H36B | 0.9900 |
| C7—C8 | 1.535 (2) | C37—C38 | 1.524 (3) |
| C7—H7 | 1.0000 | C37—H37A | 0.9900 |
| C8—H8A | 0.9900 | C37—H37B | 0.9900 |
| C8—H8B | 0.9900 | C38—H38A | 0.9800 |
| C9—H9A | 0.9900 | C38—H38B | 0.9800 |
| C9—H9B | 0.9900 | C38—H38C | 0.9800 |
| C10—H10A | 0.9900 | C39—C40 | 1.503 (3) |
| C10—H10B | 0.9900 | C39—H39A | 0.9900 |
| C12—C13 | 1.441 (2) | C39—H39B | 0.9900 |
| C12—H12A | 0.9500 | C40—H40A | 0.9800 |
| C13—C14 | 1.367 (2) | C40—H40B | 0.9800 |
| C15—C16 | 1.494 (2) | C40—H40C | 0.9800 |
| C39—O3—H3o | 108.2 (15) | H19A—C19—H19B | 109.5 |
| H11—O1W—H12 | 108 (2) | C18—C19—H19C | 109.5 |
| H21—O2W—H22 | 107 (2) | H19A—C19—H19C | 109.5 |
| H31—O3W—H32 | 109 (2) | H19B—C19—H19C | 109.5 |
| C11—N1—N2 | 116.73 (13) | C30—C20—C25 | 109.75 (13) |
| C11—N1—H1 | 124.0 (13) | C30—C20—C21 | 112.26 (12) |
| N2—N1—H1 | 119.0 (12) | C25—C20—C21 | 108.69 (13) |
| C12—N2—N1 | 118.29 (14) | C30—C20—C29 | 108.65 (12) |
| C15—N3—C13 | 107.94 (14) | C25—C20—C29 | 108.58 (13) |
| C15—N3—H3 | 127.7 (13) | C21—C20—C29 | 108.83 (13) |
| C13—N3—H3 | 124.2 (13) | C22—C21—C20 | 110.09 (13) |
| C15—N4—C14 | 104.80 (13) | C22—C21—H21A | 109.6 |
| C34—N5—C33 | 104.56 (13) | C20—C21—H21A | 109.6 |
| C34—N6—C32 | 108.41 (14) | C22—C21—H21B | 109.6 |
| C34—N6—H6 | 126.6 (13) | C20—C21—H21B | 109.6 |
| C32—N6—H6 | 125.0 (13) | H21A—C21—H21B | 108.2 |
| C31—N7—N8 | 116.86 (14) | C23—C22—C28 | 109.68 (14) |
| C30—N8—N7 | 117.28 (13) | C23—C22—C21 | 109.51 (14) |
| C30—N8—H8 | 124.6 (13) | C28—C22—C21 | 109.28 (13) |
| N7—N8—H8 | 117.2 (13) | C23—C22—H22A | 109.5 |
| C11—C1—C10 | 109.09 (13) | C28—C22—H22A | 109.5 |
| C11—C1—C9 | 109.09 (12) | C21—C22—H22A | 109.5 |
| C10—C1—C9 | 108.73 (13) | C24—C23—C22 | 109.42 (13) |
| C11—C1—C2 | 111.73 (13) | C24—C23—H23A | 109.8 |
| C10—C1—C2 | 109.51 (13) | C22—C23—H23A | 109.8 |
| C9—C1—C2 | 108.64 (13) | C24—C23—H23B | 109.8 |
| C3—C2—C1 | 109.50 (13) | C22—C23—H23B | 109.8 |
| C3—C2—H2A | 109.8 | H23A—C23—H23B | 108.2 |
| C1—C2—H2A | 109.8 | C23—C24—C26 | 109.71 (14) |
| C3—C2—H2B | 109.8 | C23—C24—C25 | 109.66 (14) |
| C1—C2—H2B | 109.8 | C26—C24—C25 | 109.38 (14) |
| H2A—C2—H2B | 108.2 | C23—C24—H24 | 109.4 |
| C8—C3—C4 | 109.85 (14) | C26—C24—H24 | 109.4 |
| C8—C3—C2 | 109.70 (14) | C25—C24—H24 | 109.4 |
| C4—C3—C2 | 108.74 (13) | C20—C25—C24 | 110.06 (13) |
| C8—C3—H3A | 109.5 | C20—C25—H25A | 109.6 |
| C4—C3—H3A | 109.5 | C24—C25—H25A | 109.6 |
| C2—C3—H3A | 109.5 | C20—C25—H25B | 109.6 |
| C5—C4—C3 | 109.80 (14) | C24—C25—H25B | 109.6 |
| C5—C4—H4A | 109.7 | H25A—C25—H25B | 108.2 |
| C3—C4—H4A | 109.7 | C27—C26—C24 | 109.48 (14) |
| C5—C4—H4B | 109.7 | C27—C26—H26A | 109.8 |
| C3—C4—H4B | 109.7 | C24—C26—H26A | 109.8 |
| H4A—C4—H4B | 108.2 | C27—C26—H26B | 109.8 |
| C4—C5—C9 | 109.74 (13) | C24—C26—H26B | 109.8 |
| C4—C5—C6 | 109.39 (14) | H26A—C26—H26B | 108.2 |
| C9—C5—C6 | 109.32 (14) | C26—C27—C28 | 109.75 (14) |
| C4—C5—H5 | 109.5 | C26—C27—C29 | 109.49 (14) |
| C9—C5—H5 | 109.5 | C28—C27—C29 | 109.40 (13) |
| C6—C5—H5 | 109.5 | C26—C27—H27 | 109.4 |
| C7—C6—C5 | 109.40 (14) | C28—C27—H27 | 109.4 |
| C7—C6—H6A | 109.8 | C29—C27—H27 | 109.4 |
| C5—C6—H6A | 109.8 | C27—C28—C22 | 109.47 (13) |
| C7—C6—H6B | 109.8 | C27—C28—H28A | 109.8 |
| C5—C6—H6B | 109.8 | C22—C28—H28A | 109.8 |
| H6A—C6—H6B | 108.2 | C27—C28—H28B | 109.8 |
| C6—C7—C10 | 109.82 (14) | C22—C28—H28B | 109.8 |
| C6—C7—C8 | 109.62 (14) | H28A—C28—H28B | 108.2 |
| C10—C7—C8 | 108.98 (14) | C27—C29—C20 | 109.95 (12) |
| C6—C7—H7 | 109.5 | C27—C29—H29A | 109.7 |
| C10—C7—H7 | 109.5 | C20—C29—H29A | 109.7 |
| C8—C7—H7 | 109.5 | C27—C29—H29B | 109.7 |
| C3—C8—C7 | 109.75 (13) | C20—C29—H29B | 109.7 |
| C3—C8—H8A | 109.7 | H29A—C29—H29B | 108.2 |
| C7—C8—H8A | 109.7 | O2—C30—N8 | 121.49 (14) |
| C3—C8—H8B | 109.7 | O2—C30—C20 | 122.90 (14) |
| C7—C8—H8B | 109.7 | N8—C30—C20 | 115.61 (13) |
| H8A—C8—H8B | 108.2 | N7—C31—C32 | 116.67 (15) |
| C5—C9—C1 | 109.88 (13) | N7—C31—H31A | 121.7 |
| C5—C9—H9A | 109.7 | C32—C31—H31A | 121.7 |
| C1—C9—H9A | 109.7 | C33—C32—N6 | 104.13 (14) |
| C5—C9—H9B | 109.7 | C33—C32—C31 | 133.72 (16) |
| C1—C9—H9B | 109.7 | N6—C32—C31 | 122.04 (15) |
| H9A—C9—H9B | 108.2 | C32—C33—N5 | 111.78 (15) |
| C7—C10—C1 | 110.06 (13) | C32—C33—Cl2 | 126.87 (13) |
| C7—C10—H10A | 109.6 | N5—C33—Cl2 | 121.32 (12) |
| C1—C10—H10A | 109.6 | N5—C34—N6 | 111.12 (15) |
| C7—C10—H10B | 109.6 | N5—C34—C35 | 126.11 (15) |
| C1—C10—H10B | 109.6 | N6—C34—C35 | 122.77 (15) |
| H10A—C10—H10B | 108.2 | C34—C35—C36 | 113.43 (14) |
| O1—C11—N1 | 121.44 (15) | C34—C35—H35A | 108.9 |
| O1—C11—C1 | 122.50 (14) | C36—C35—H35A | 108.9 |
| N1—C11—C1 | 116.03 (14) | C34—C35—H35B | 108.9 |
| N2—C12—C13 | 116.98 (15) | C36—C35—H35B | 108.9 |
| N2—C12—H12A | 121.5 | H35A—C35—H35B | 107.7 |
| C13—C12—H12A | 121.5 | C37—C36—C35 | 114.70 (14) |
| C14—C13—N3 | 103.96 (14) | C37—C36—H36A | 108.6 |
| C14—C13—C12 | 133.60 (15) | C35—C36—H36A | 108.6 |
| N3—C13—C12 | 122.43 (14) | C37—C36—H36B | 108.6 |
| N4—C14—C13 | 111.82 (14) | C35—C36—H36B | 108.6 |
| N4—C14—Cl1 | 121.52 (12) | H36A—C36—H36B | 107.6 |
| C13—C14—Cl1 | 126.65 (13) | C36—C37—C38 | 111.99 (16) |
| N4—C15—N3 | 111.47 (14) | C36—C37—H37A | 109.2 |
| N4—C15—C16 | 123.95 (15) | C38—C37—H37A | 109.2 |
| N3—C15—C16 | 124.58 (15) | C36—C37—H37B | 109.2 |
| C15—C16—C17 | 113.03 (15) | C38—C37—H37B | 109.2 |
| C15—C16—H16A | 109.0 | H37A—C37—H37B | 107.9 |
| C17—C16—H16A | 109.0 | C37—C38—H38A | 109.5 |
| C15—C16—H16B | 109.0 | C37—C38—H38B | 109.5 |
| C17—C16—H16B | 109.0 | H38A—C38—H38B | 109.5 |
| H16A—C16—H16B | 107.8 | C37—C38—H38C | 109.5 |
| C18—C17—C16 | 113.12 (16) | H38A—C38—H38C | 109.5 |
| C18—C17—H17A | 109.0 | H38B—C38—H38C | 109.5 |
| C16—C17—H17A | 109.0 | O3—C39—C40 | 111.29 (15) |
| C18—C17—H17B | 109.0 | O3—C39—H39A | 109.4 |
| C16—C17—H17B | 109.0 | C40—C39—H39A | 109.4 |
| H17A—C17—H17B | 107.8 | O3—C39—H39B | 109.4 |
| C17—C18—C19 | 113.7 (2) | C40—C39—H39B | 109.4 |
| C17—C18—H18A | 108.8 | H39A—C39—H39B | 108.0 |
| C19—C18—H18A | 108.8 | C39—C40—H40A | 109.5 |
| C17—C18—H18B | 108.8 | C39—C40—H40B | 109.5 |
| C19—C18—H18B | 108.8 | H40A—C40—H40B | 109.5 |
| H18A—C18—H18B | 107.7 | C39—C40—H40C | 109.5 |
| C18—C19—H19A | 109.5 | H40A—C40—H40C | 109.5 |
| C18—C19—H19B | 109.5 | H40B—C40—H40C | 109.5 |
| C11—N1—N2—C12 | −172.14 (15) | C16—C17—C18—C19 | 179.65 (17) |
| C31—N7—N8—C30 | 178.67 (14) | C30—C20—C21—C22 | 179.26 (13) |
| C11—C1—C2—C3 | 179.27 (13) | C25—C20—C21—C22 | −59.15 (17) |
| C10—C1—C2—C3 | 58.29 (17) | C29—C20—C21—C22 | 58.94 (17) |
| C9—C1—C2—C3 | −60.33 (16) | C20—C21—C22—C23 | 60.07 (17) |
| C1—C2—C3—C8 | −59.13 (17) | C20—C21—C22—C28 | −60.09 (17) |
| C1—C2—C3—C4 | 61.03 (17) | C28—C22—C23—C24 | 59.73 (17) |
| C8—C3—C4—C5 | 59.27 (17) | C21—C22—C23—C24 | −60.19 (17) |
| C2—C3—C4—C5 | −60.79 (17) | C22—C23—C24—C26 | −60.06 (17) |
| C3—C4—C5—C9 | 60.18 (17) | C22—C23—C24—C25 | 60.10 (18) |
| C3—C4—C5—C6 | −59.74 (17) | C30—C20—C25—C24 | −177.94 (13) |
| C4—C5—C6—C7 | 60.22 (18) | C21—C20—C25—C24 | 58.94 (17) |
| C9—C5—C6—C7 | −59.97 (18) | C29—C20—C25—C24 | −59.30 (17) |
| C5—C6—C7—C10 | 59.72 (18) | C23—C24—C25—C20 | −60.05 (18) |
| C5—C6—C7—C8 | −59.99 (18) | C26—C24—C25—C20 | 60.30 (18) |
| C4—C3—C8—C7 | −58.87 (18) | C23—C24—C26—C27 | 60.04 (17) |
| C2—C3—C8—C7 | 60.61 (18) | C25—C24—C26—C27 | −60.28 (18) |
| C6—C7—C8—C3 | 59.44 (17) | C24—C26—C27—C28 | −59.71 (17) |
| C10—C7—C8—C3 | −60.78 (17) | C24—C26—C27—C29 | 60.39 (17) |
| C4—C5—C9—C1 | −59.58 (17) | C26—C27—C28—C22 | 59.40 (17) |
| C6—C5—C9—C1 | 60.39 (17) | C29—C27—C28—C22 | −60.76 (17) |
| C11—C1—C9—C5 | −178.64 (13) | C23—C22—C28—C27 | −59.40 (17) |
| C10—C1—C9—C5 | −59.78 (17) | C21—C22—C28—C27 | 60.66 (17) |
| C2—C1—C9—C5 | 59.34 (16) | C26—C27—C29—C20 | −60.15 (17) |
| C6—C7—C10—C1 | −59.86 (18) | C28—C27—C29—C20 | 60.16 (17) |
| C8—C7—C10—C1 | 60.24 (17) | C30—C20—C29—C27 | 178.60 (13) |
| C11—C1—C10—C7 | 178.20 (13) | C25—C20—C29—C27 | 59.28 (17) |
| C9—C1—C10—C7 | 59.33 (17) | C21—C20—C29—C27 | −58.88 (17) |
| C2—C1—C10—C7 | −59.24 (17) | N7—N8—C30—O2 | 0.1 (2) |
| N2—N1—C11—O1 | −7.1 (2) | N7—N8—C30—C20 | −179.67 (12) |
| N2—N1—C11—C1 | 170.95 (13) | C25—C20—C30—O2 | 17.2 (2) |
| C10—C1—C11—O1 | −5.6 (2) | C21—C20—C30—O2 | 138.20 (15) |
| C9—C1—C11—O1 | 113.00 (17) | C29—C20—C30—O2 | −101.38 (17) |
| C2—C1—C11—O1 | −126.86 (16) | C25—C20—C30—N8 | −163.04 (13) |
| C10—C1—C11—N1 | 176.36 (14) | C21—C20—C30—N8 | −42.05 (18) |
| C9—C1—C11—N1 | −65.00 (17) | C29—C20—C30—N8 | 78.37 (16) |
| C2—C1—C11—N1 | 55.14 (18) | N8—N7—C31—C32 | 178.15 (13) |
| N1—N2—C12—C13 | 176.98 (14) | C34—N6—C32—C33 | 0.49 (17) |
| C15—N3—C13—C14 | −0.20 (18) | C34—N6—C32—C31 | −176.12 (14) |
| C15—N3—C13—C12 | −179.78 (15) | N7—C31—C32—C33 | −173.00 (17) |
| N2—C12—C13—C14 | 174.59 (17) | N7—C31—C32—N6 | 2.4 (2) |
| N2—C12—C13—N3 | −6.0 (2) | N6—C32—C33—N5 | −0.36 (18) |
| C15—N4—C14—C13 | −0.44 (19) | C31—C32—C33—N5 | 175.66 (16) |
| C15—N4—C14—Cl1 | 178.68 (12) | N6—C32—C33—Cl2 | −178.57 (12) |
| N3—C13—C14—N4 | 0.40 (18) | C31—C32—C33—Cl2 | −2.6 (3) |
| C12—C13—C14—N4 | 179.91 (17) | C34—N5—C33—C32 | 0.08 (18) |
| N3—C13—C14—Cl1 | −178.66 (12) | C34—N5—C33—Cl2 | 178.41 (12) |
| C12—C13—C14—Cl1 | 0.8 (3) | C33—N5—C34—N6 | 0.24 (17) |
| C14—N4—C15—N3 | 0.30 (19) | C33—N5—C34—C35 | −179.45 (15) |
| C14—N4—C15—C16 | −178.97 (16) | C32—N6—C34—N5 | −0.47 (18) |
| C13—N3—C15—N4 | −0.06 (19) | C32—N6—C34—C35 | 179.23 (15) |
| C13—N3—C15—C16 | 179.20 (15) | N5—C34—C35—C36 | −63.0 (2) |
| N4—C15—C16—C17 | 84.5 (2) | N6—C34—C35—C36 | 117.35 (17) |
| N3—C15—C16—C17 | −94.6 (2) | C34—C35—C36—C37 | −56.7 (2) |
| C15—C16—C17—C18 | 76.1 (2) | C35—C36—C37—C38 | −176.31 (15) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···O3i | 0.86 (1) | 2.00 (1) | 2.841 (2) | 166 (2) |
| N3—H3···O1wii | 0.86 (1) | 1.95 (1) | 2.806 (2) | 170 (2) |
| N6—H6···O2w | 0.88 (1) | 1.95 (1) | 2.829 (2) | 174 (2) |
| N8—H8···O3wiii | 0.86 (1) | 1.94 (1) | 2.778 (2) | 164 (2) |
| O3—H3o···O1w | 0.84 (1) | 1.84 (1) | 2.673 (2) | 177 (2) |
| O1w—H11···O1ii | 0.84 (1) | 2.00 (1) | 2.821 (2) | 166 (2) |
| O1w—H12···N5 | 0.84 (1) | 1.91 (1) | 2.751 (2) | 175 (3) |
| O2w—H21···O2 | 0.85 (1) | 2.07 (1) | 2.905 (2) | 172 (2) |
| O2w—H22···O2iv | 0.84 (1) | 1.93 (1) | 2.764 (2) | 176 (2) |
| O3w—H31···N4 | 0.84 (1) | 1.94 (1) | 2.773 (2) | 169 (2) |
| O3w—H32···O2w | 0.85 (1) | 1.92 (1) | 2.766 (2) | 174 (2) |
Symmetry codes: (i) −x+2, −y+2, −z+1; (ii) −x+1, −y+2, −z+1; (iii) x+1, y, z; (iv) −x+1, −y+2, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BT5304).
References
- Barbour, L. J. (2001). J. Supramol. Chem.1, 189–191.
- Bruker (2009). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Kadi, A. A., El-Brollosy, N. R., Al-Deeb, O. A., Habib, E. E., Ibrahim, T. M. & El-Emam, A. A. (2007). Eur. J. Med. Chem.42, 235–242. [DOI] [PubMed]
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst.43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810029260/bt5304sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810029260/bt5304Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report