Abstract
The cyanobacterial CO2-concentrating mechanism (CCM) allows photosynthesis to proceed in CO2-limited aquatic environments, and its activity is modulated in response to inorganic carbon (Ci) availability. Real-time reverse transcriptase-PCR analysis was used to examine the transcriptional regulation of more than 30 CCM-related genes in Synechococcus sp. strain PCC7942 with an emphasis on genes encoding high-affinity Ci transporters and carboxysome-associated proteins. This approach was also used to test hypotheses about sensing of Ci limitation in cyanobacteria. The transcriptional response of Synechococcus sp. to severe Ci limitation occurs rapidly, being maximal within 30 to 60 min, and three distinct temporal responses were detected: (a) a rapid, transient induction for genes encoding carboxysome-associated proteins (ccmKLMNO, rbcLS, and icfA) and the transcriptional regulator, cmpR; (b) a slow sustained induction of psbAII; and (c) a rapid sustained induction of genes encoding the inducible Ci transporters cmpABCD, sbtA, and ndhF3-D3-chpY. The Ci-responsive transcripts investigated had half-lives of 15 min or less and were equally stable at high and low Ci. Through the use of a range of physiological conditions (light and Ci levels) and inhibitors such as 3-(3,4-dichlorophenyl)-1,1dimethylurea, glycolaldehyde, dithiothreitol, and ethoxyzolamide, we found that no strict correlation exists between expression of genes known to be induced under redox stress, such as psbAII, and the expression of the Ci-responsive CCM genes. We argue that redox stress, such as that which occurs under high-light stress, is unlikely to be a primary signal for sensing of Ci limitation in cyanobacteria. We discuss the data in relation to current theories of CO2 sensing in cyanobacteria.
In response to restrictions on the availability of CO2 in aquatic environments, cyanobacteria have evolved a unique ability to actively acquire inorganic carbon (Ci) and elevate CO2 up to 1,000-fold around the active site of Rubisco. In the unicellular, freshwater strains Synechococcus sp. PCC7942 and Synechocystis sp. PCC6803, this CO2-concentrating mechanism (CCM) consists of at least four Ci separate transport activities for the active uptake of both CO2 and HCO3 (for review, see Price et al., 2002; Shibata et al., 2002; Badger and Price, 2003). Ci is accumulated as HCO3- inside the cell but is converted to CO2 by a specifically localized carbonic anhydrase (CA) inside a microcompartment known as the carboxysome, which houses cellular Rubisco. The activity of the CCM is modulated according to the external availability of Ci and can occupy two extreme states—a low-affinity, constitutive state and a high-affinity, induced state—in addition to intermediate states (for review, see Kaplan and Reinhold, 1999; Price et al., 2002; Badger and Price, 2003).
Many of the genes encoding components of the cyanobacterial CCM have been identified in Synechococcus sp. PCC7942 and Synechocystis sp. PCC6803 (referred to as Synechococcus and Synechocystis hereafter), and a full genome sequence is available for Synechocystis. Two high-affinity, inducible HCO3- transport activities have been identified in these strains—an ABC-transporter, BCT1, encoded by the cmp operon (Omata et al., 1999), and a Na+-dependent HCO3- uptake activity encoded by the sbtA gene (Shibata et al., 2001). The active transport of CO2 is believed to occur through the activity of specialized NDH-1 dehydrogenase complexes that convert CO2 to HCO3- within the cell. The ndhF4/ndhD4/chpX (cupB) genes are thought to encode components for a low-affinity constitutive CO2 transporter termed NDH-14, whereas an inducible, high-affinity CO2-transporter, NDH-13, requires expression of the ndhD3/ndhF3/chpY (cupA) genes (Ohkawa et al., 2000a, 2000b; Shibata et al., 2001; Maeda et al., 2002; hereafter, this work conforms to the terminology of Maeda et al. [2002] to describe genes encoding CO2 transport complexes). Carboxysome-associated genes are encoded by the ccmKLMNO genes, the rbcLS genes, and icfA (ccaA), which encodes carboxysomal-CA (Fukuzawa et al., 1992; Yu et al., 1992). A family of three Lys-R-type transcriptional regulators, related to the CbbR regulators of Calvin cycle gene expression in proteobacteria, have been identified in Synechocystis (Figge et al., 2001). Of these, ndhR and cmpR encode transcriptional regulators of the ndhF3/D3/chpY (cupA) and cmp operons, respectively (Figge et al., 2001; Omata et al., 2001), whereas RbcR is thought to be involved in control of the rbc operon (Mori et al., 2002). Despite the rapid progress in defining the genetic basis of the cyanobacterial CCM, the first integrated study of the transcriptional response of CCM-related genes to Ci limitation was published only recently, from work in Synechocystis (McGinn et al., 2003). One of the key findings of this study was that the expression of inducible, CCM-related genes is modulated in response to changes in external Ci in as little as 15 min.
The signaling pathways that lead to up-regulation of the CCM in response to Ci limitation remain to be defined. It is unclear what sort of signal is actually perceived by cells subject to this stress. A number of competing theories have been proposed (for review, see Kaplan and Reinhold, 1999). These include direct sensing of external Ci or the internal Ci pool, the detection of changes in the concentration of photorespiratory or Calvin cycle intermediates, or changes in the redox potential of the photosynthetic electron transport chain. In support of the latter theory, a link between light stress and the low-Ci response of the CCM has recently been suggested with reports that some CCM-related genes are light responsive in Synechocystis (Hihara et al., 2001; Huang et al., 2002). However, this finding was not replicated in another recent study (McGinn et al., 2003).
The nature of the low-Ci signal remains elusive, and an integrated picture of the transcriptional regulation and physiological expression of the CCM is not yet available in Synechococcus, which has been favored for physiological and genetic studies of the CCM (Price et al., 2002). Accordingly, this study had two goals. The first goal was to describe aspects of the transcriptional response of Synechococcus cells to severe and intermediate Ci limitation using highly sensitive real-time PCR assays and to place these observations in a physiological context. The second goal of this study was to examine some competing theories about the nature of the low-Ci signal that elicits induction of a high-affinity CCM. We detected distinct temporal responses to Ci limitation at the transcriptional level, and our results suggest it is unlikely that Synechococcus cells sense Ci limitation through a redox-sensing mechanism such as that which underpins the high-light response. Instead, we propose that depletion of the internal Ci pool or changes in phororespiratory activity may be the primary signal that is sensed by a Ci-limited cell.
RESULTS
Early Transcriptional Response of Synechococcus to Severe Ci Limitation
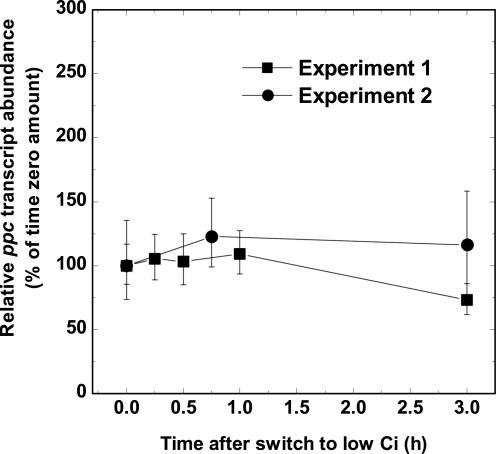
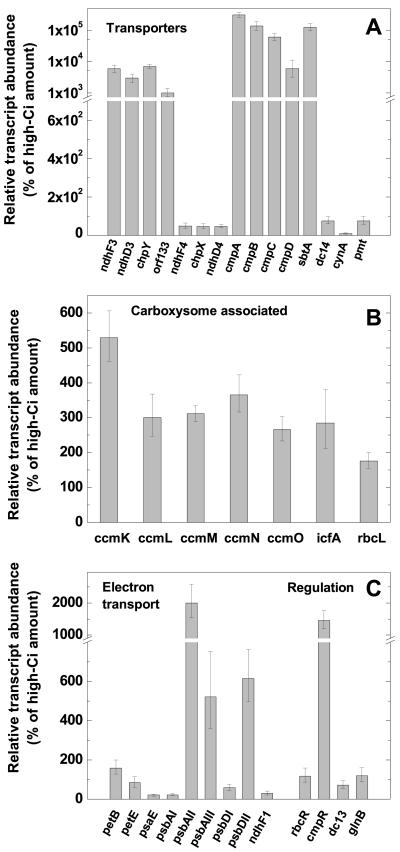
The responsiveness of several potential housekeeping genes to inorganic Ci limitation, including gap1 and ppc, encoding glyceraldehyde-3-phosphate dehydrogenase and phosphoenolpyruvate carboxylase, respectively, was determined. Exponentially growing Synechococcus high-Ci cells (bubbled with 1.7% CO2 in air) were harvested and immediately transferred to CO2-free air equilibrated buffer and aerated with CO2-free air for 3 h. First-strand cDNA was generated from normalized total RNA extracts from treated cells and quantitative real-time reverse transcriptase (RT)-PCR assays, using SYBR Green I to monitor product formation, were performed using ppc-specific primers (Table I). The gap1 transcript was found to vary considerably (results not shown), but the abundance of ppc was found to be relatively unresponsive to Ci limitation, varying by less than 35% in two independent experiments (Fig. 1.) Accordingly, ppc was used to normalize for small variations in starting template concentration and the efficiency of cDNA synthesis in all further experiments. To provide a “snapshot” of the early transcriptional response in Synechococcus, cells were subjected to a severe Ci limitation (as above) for 30 min. As a control, a culture was harvested, resuspended in high-Ci equilibrated medium, and returned to aeration with 1.7% CO2. The abundance of 35 transcripts encoding membrane transporters, carboxysome-associated genes, electron transport components, and regulatory factors in low-Ci-induced cells relative to high-Ci-grown cells was determined by real-time PCR (Fig. 2) using the gene-specific primers listed in Table I.
Table I.
Sequences of the gene-specific primers used in real-time PCR assays of gene expression in Synechococcus sp. PCC7942
| Gene | GenBank Accession No. | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| ccmK | M96929 | TTTTTAAAGGACGTGGACTC | GCAGAGACAGAAGCTTGAAC |
| ccmL | M96929 | TTCTTGGTTGTTCAGTTCTTG | TATCGATGATGGCAATGAC |
| ccmM | M96929 | AGTCGCGATTACTCACAAAG | AGGAATCTCTACGTCTTGGAC |
| ccmN | M30808 | AGAGTATTGTCGGTCGTCAG | ACTGTTCCTTGCCGTAGAC |
| ccmO | M30808 | TAGACGACTTGATGGACAGC | GTCGCTCTTCTGCTAAAAGTC |
| chpX (cupB) | AY029339 | TGCCTACTCTCGTAATCTCTG | CATCGAGAAAGGTATCTAATCC |
| chpY (cupA) | AY029338 | GCTTTACCTGGCTCTATGAAG | AGCAAGTAGGGTGGTAAGAAG |
| cmpA | M32999 | TCGCAACTTCTTTAACGTG | TTGTAGGGATAGGAGACACTG |
| cmpB | D26358 | TCGAAAAGCAAGTACCTAATG | AACAGCACCGATATAAATCAC |
| cmpC | D26358 | AGGATTGATCATGAGTTTATTTG | CAACTCTAGACCAGCAATCAG |
| cmpD | D26358 | CAGAGGACTTGTGTCATGC | AAGATTGACTCCATCCAAAAC |
| cmpR | AB047379 | ATTACTGTGCAACCATTTCTC | CGTTCAAATTGAGGTTATGAG |
| cynA | AF001333 | CAATTTACCTGACGACTGAAG | CGTATTTCTGCTTTTGAGATG |
| dc13 | U62616 | CTCAGAAATTCCAAGTGGTC | AGATTGGTCAGTTCACGTTC |
| dc14 (ictB) | U62616 | CAGCTTATGTCTGATCCTCAC | AAGTTGTTGCTGCTGTCTTC |
| glnB | AF079137 | GAGTTCCCTTGAAGAAGATTG | AAAACTCAACCGTGTATTCC |
| icfA (ccaA) | M77095 | CTGTTCATTACCTGCTCAGAC | CAACATGCTCAATGTTCAAAG |
| ndhD3 | AY029338 | AGTTTGTTGATGGGCTTTG | CCAGAAAAATCAGGCTGTAG |
| ndhD4 | AY029339 | GCACTCTTCTTCCAGTTCG | AATGATCAGGAAGGTTAGGAG |
| ndhF1 | AY017305 | TACATGTTCCGCATCTACTTC | CCAGTAGACCGATCAGAGC |
| ndhF3 | AY029338 | CTACACGCCAATCCTTTATC | GCATCAGGAAGAAGACACTG |
| ndhF4 | AY029339 | CAATGGCAGCTGTTAGTCAC | CGATCGATGTAGAAGTCGTAG |
| ORF133 | AY029338 | CAACTAGCTCGAATTCTCAAG | GTTATCACAGGCAATATCAGC |
| PEP carboxylase (ppc) | M11198 | GCCTCAAGCTCTCCTATATTC | GAATCAGCTTGAGATCTTCG |
| petB | U33285 | TTGTTTTCTCATTCAGTTTGC | GAAACCACCAGTCAGATAAAC |
| petE | U20147 | TCGGTAGCTTCTTCCTCTC | GAAGGTAGCTTCAAAGGTTTC |
| pmta | AF428100 | AAAGACCTTACCAAGATGAGC | ACCGAAGCTACTGATCAAGAC |
| psaE | M99432 | GAGGAAGCATCAGTTATGG | AGCCGTTGTAGTTCACTTTC |
| psbAI | X04616 | CCTTTACAACCTCAAGATCG | AATGAACGCAACGATGAAGC |
| psbAII | X04616 | TAGTTCAACTAAGGACTCATC | GATGAACGCAACGATGAAGC |
| psbAIII | X04616 | ATTCACTGGACTCAAAACATC | GATGAACGCAACGATGAAGC |
| psbDI | M20814 | AGTACGGCAAGAGGTTTTAG | CTAAATACGCACAGGGAAAC |
| psbDII | M20815 | CAAATATCTGGAGATTGCTAAG | CTAAATACGCACAGGGAAAC |
| rbcL | J01536 | CTATTACACCCCCGATTACAC | GTAGCACTTGCCTTTGTACC |
| rbcR | AY017305 | AAGGTGATTGACCAAGTGC | GGTTGTAGATTACCGATAGGG |
| sbtA | unpublished datab | AATATGCTCAGCAAGAGTCTG | CTTTACGCAGCTCACTAATTC |
a Putative membrane transporter. b See “Materials and Methods.”
Figure 1.
Phosphoenolpyruvate carboxylase (ppc) transcript abundance in Ci-limited Synechococcus cells. Exponentially growing high-Ci cells were swapped to buffer equilibrated with CO2-free air and bubbled with CO2-free air for 3 h. The abundance of ppc transcript relative to time zero was determined by real-time PCR using cDNA pools generated from normalized, total RNA extracts from cells collected at various time points. Symbols represent the extent of induction or repression after the shift to low Ci at each time point as a percentage of the high-Ci amount (set at 100%) ± se for three separate measurements. The results of two independent experiments are shown.
Figure 2.
Early transcriptional response of Synechococcus cells subject to a severe Ci limitation. Exponentially growing high-Ci cells were swapped to CO2-free air equilibrated buffer and bubbled with CO2-free air for 30 min. The relative abundance of specific transcripts encoding known or putative Ci transporters (A), carboxysome associated proteins (B), or electron transport or regulatory proteins (C), was determined by real-time PCR. Bars represent the extent of induction or repression after the shift to low Ci as a percentage of the high-Ci amount (set at 100%) ± se for three separate measurements. Similar data were obtained from a replicate experiment (data not shown). Note the break in the y axis in A and C. The scale is exponential after the break in A.
As has been observed in Synechocystis, we found that expression of transcripts encoding the known inducible Ci transport activities was induced by Ci limitation in Synechococcus. Previously, only expression of the cmp operon had been confirmed as being low-Ci responsive in this strain (Omata et al., 1999, 2001). Genes contributing to the high-affinity CO2 transporter NDH-I3, ndhF3-ndhD3-chpY (cupA) are cotranscribed in Synechocystis, and expression of members of this operon has been reported to be induced by 100% to 200% upon Ci limitation (Ohkawa et al., 1998; McGinn et al., 2003). By contrast, we found the degree of induction of these transcripts to be to an order of magnitude higher in Synechococcus. The low-Ci induction of the sbtA and cmpA-D transcripts, encoding the two inducible, high-affinity HCO3- transporters was also orders of magnitude higher in Synechococcus than has been reported for Synechocystis (McGinn et al., 2003). This difference may reflect the fact that real-time PCR assays have a much greater dynamic range than other methods of gene expression analysis such as semiquantitative RT-PCR and microarrays, which tend to underestimate large changes (Schmittgen et al., 2000). Like Synechocystis, the genes encoding the low-affinity, constitutive CO2-transport activity, ndhF4/D4 and chpX, were found to be constitutively expressed. We also assayed the Ci-responsive expression of three other putative membrane transporters. It has previously been proposed that dc14 might encode a HCO3- transport activity in Synechococcus (Bonfil et al., 1998), whereas cynA, the substrate binding protein from an ABC-transporter of the mono-anion group (Maeda et al., 2000), although ascribed a function in CN- transport, could potentially bind HCO3-. Also, expression of a gene encoding a putative membrane transporter (termed pmt in this study) has been suggested to be low-Ci responsive in Synechococcus (O.A. Koksharova, submission notes for GenBank accession no. AF428100). Our results suggest that the expression of all of these three genes is unresponsive to Ci limitation.
Carboxysomes are known to increase in number in Synechococcus cells in response to Ci limitation (McKay et al., 1993), and corresponding increases in Rubisco and carboxysomal-CA activity (Price et al., 1992) and rbcL/S and ccmKLMNO transcript abundance have been reported (Omata et al., 2001). Compared with transcripts encoding the inducible transporters, we detected relatively small increases (approximately 200%-500%) in the abundance of rbcL and ccmKLMNO in response to Ci limitation and detected for the first time, to our knowledge, induction of the icfA transcript, which encodes carboxysomal CA. We could not detect expression of the open reading frame ccmJ (result not shown) under either Ci regime. Some studies have found no induction of carboxysome-associated genes (McGinn et al., 2003) after 60 min in Synechocystis, whereas one study detected a small increase (60%-70%) in rbcLXS transcript abundance in cells subject to 90-min Ci limitation (Omata et al., 2001). These results may reflect a genuine difference between the strains given a recent finding that Ci limitation is not accompanied by an increase in carboxysome number in Synechocystis cells (So et al., 2002).
Severe Ci limitation is somewhat analogous to a high-light stress because depression of CO2 fixation would cause over-reduction of the plastoquinone pool. A preliminary report of the low-Ci inducibility of psbAII has emerged from another group (T. Omata, personal communication). We also found low-Ci-responsive expression of transcripts encoding other components of the photosynthetic electron transport chain. The psbAI/II/III and psbDI/II gene families, encoding alternative D1 and D2 proteins, responded to Ci limitation as they do to high-light stress (Schaefer and Golden, 1989; Bustos and Golden, 1991). That is, we found that psbAI expression was down-regulated in response to Ci limitation, whereas psbAII/III and psbDII expression was up-regulated. Other genes such as glnB, encoding the PII-signaling protein, and dc13, encoding a putative methyltransferase, have been proposed to have a potential regulatory role in low-Ci sensing (Ruppert et al., 2002; Amoroso et al., 2003), however, neither was transcriptionally regulated by a 30-min Ci limitation.
As with other Lys-R-type transcriptional regulators, expression of ndhR, a controller of the ndhD3/F3/chpY operon, and the cmpA-D operon regulator, cmpR, is stress responsive, and transcript abundance for these genes is induced in response to Ci limitation (Figge et al., 2001; McGinn et al., 2003). Although cmpR has been identified in Synechococcus (Omata et al., 2001), an ndhR homolog has not yet been identified in this strain. We also found that cmpR transcript is more abundant in Ci-limited cells in this strain. Additionally, the third cbbR homolog, rbcR, which is believed to encode a regulator of the rbcLS operon by virtue of the function of its proteobacterial homolog (Mori et al., 2002), was not transcriptionally induced by Ci limitation in this study.
Temporal Response of CCM-Related Gene Expression in Synechococcus Cells Subject to Severe Ci Limitation
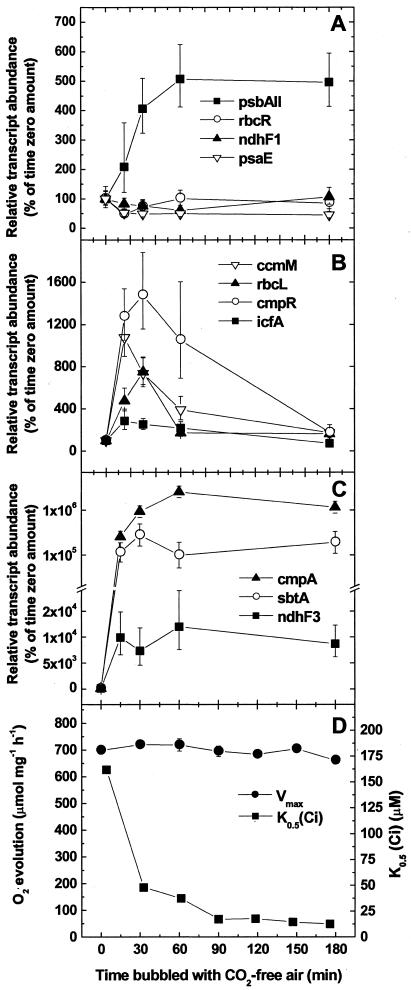
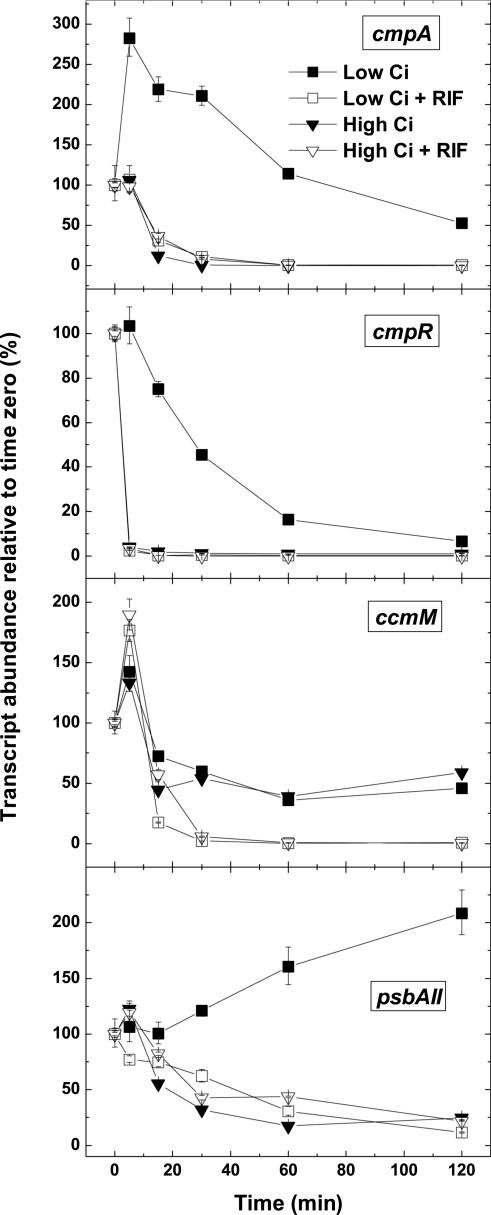
To date, there has been no report of the kinetics of low-Ci-inducible gene expression using quantitative gene expression assays. High-Ci-grown Synechococcus cells were transferred to low Ci as described above, and cultures were sampled at 15, 30, 60, and 180 min after the switch. Changes in the abundance of a representative subset of transcripts from the full set previously assayed, compared with time zero, were quantified as before using real-time PCR assays. The low-Ci-inducible transcripts exhibited kinetic patterns of three types. First, the abundance of one transcript, psbAII, increased steadily over the first 60 min and was sustained at this level for 3 h (Fig. 3A). A second group of transcripts, encoding carboxysome-associated proteins and cmpR, was rapidly induced but, in the case of ccmM, rbcL, and icfA, returned to uninduced levels approximately 60 min after induction and in the case of cmpR, by 3 h (Fig. 3B). It is possible that carboxysome gene expression may also be strongly but transiently Ci responsive in Synechocystis and that there is no significant difference in the regulation of these genes between the two strains. A third group of transcripts, encoding the inducible Ci transport activities, was rapidly induced to maximum amounts by 30 to 60 min (Fig. 3C) and was maintained at relatively high amounts over the 3-h time course. A slight transient reduction in the amount of rbcR transcript was detected at 15 min, whereas the amount of transcript for psaE slowly declined over the 3 h (Fig. 3A).
Figure 3.
Time course of CCM-related transcript expression in severely Ci-limited Synechococcus cells and induction of a high-affinity CCM. A through C, Relative abundance of a subset of CCM-related transcripts as determined by real-time PCR. Symbols represent the extent of induction or repression after the shift to low Ci at each time point as a percentage of the high-Ci amount (set at 100%) ± se for three separate measurements. D, Maximum photosynthetic rate (Vmax) and K0.5(Ci) for cells collected at each time point. Similar data were obtained from a replicate experiment (data not shown). Note the break in the y axis in part C. The scale is exponential after the break in C.
The physiological response of Synechococcus cells to Ci limitation was also monitored for 3 h after the transition from bubbling with 1.7% CO2 to bubbling with CO2-free air. The maximum photosynthetic rate of cells during this period was largely constant, indicating that cells were not photo-inhibited (Fig. 3D). The relative affinity for Ci, as determined by net K0.5 (Ci), started to increase soon after transfer to Ci limitation (Fig. 3D). The maximal increase in affinity was achieved between 1 and 1.5 h (Fig. 3D), similar to what has been reported recently in Synechocystis (McGinn et al., 2003) under comparable conditions. This response is faster than previously observed for Synechococcus (Yu et al., 1994) and is largely due to a more rapid transfer between Ci conditions. The initial increase in affinity for Ci at 30 and 60 min was abolished by treating cells with 200 μg mL-1 protein synthesis inhibitor chloramphenicol (results not shown). The initial increase in affinity, therefore, was due to a genuine increase in de novo protein synthesis and is unrelated to the fast induction response in Synechococcus that appears to result from allosteric activation of an existing HCO3- transporter (Sültemeyer et al., 1998).
Relaxation of the High-Affinity CCM in Synechococcus Cells
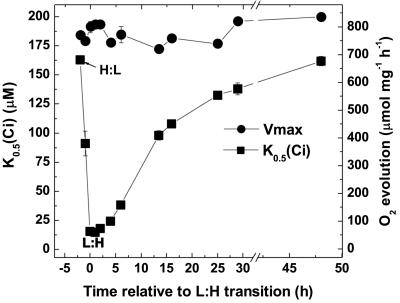
Relatively little data exists about the relaxation of high-affinity CCMs and consequently the rate of turnover of CCM components. To address this question, exponentially growing high-Ci Synechococcus cells were transferred to CO2-free equilibrated growth medium and bubbled for 2 h with CO2-free air. Cells were then swapped to bubbling with 1.7% CO2 (designated time zero) and supplemented with NaHCO3 to a final concentration of 5 mm. This amount of supplementary Ci equals the total concentration of Ci found in actively growing cultures bubbled with 1.7% CO2 under steady-state conditions (data not shown). Cultures were sampled over the next 48 h to determine the relative affinity for Ci, as K0.5 (Ci) (Fig. 4). The K0.5 (Ci) decreased from 163 to 15 μm during the initial CO2-free air induction. For 25 h after the return to high-CO2 conditions, the K0.5 (Ci) rose steadily to 133 μm, about 80% of the initial high-CO2 value, but cells required a further 24 h growth at high CO2 to return to the initial low-affinity state. Previous work on the relaxation of the high-affinity CCM in Synechocystis found that it took considerably longer (over 40 h) for cells to approach a low-affinity state (Benschop et al., 2003), suggesting that a difference exists in regulation of this response between the two strains.
Figure 4.
Relaxation of the high-affinity CCM in Synechococcus cells. Exponentially growing high-Ci cells were swapped to CO2-free air equilibrated buffer and bubbled with CO2-free air for 2 h (termed a H:L transition) and then returned to aeration with 1.7% CO2 for 48 h (termed a L:H transition). Symbols represent the relative affinity for Ci, K0.5 (Ci) at each time point or the maximum photosynthetic rate (Vmax). Values are averages of at least two independent measurements ± se.
To examine the turnover and stability of CCM-related transcripts in cells subject to either Ci limitation or Ci sufficiency, an exponentially growing high-Ci Synechococcus cell culture was transferred to CO2-free equilibrated growth medium and bubbled for 30 min with CO2-free air. This period is sufficient to strongly induce Ci-responsive CCM transcripts but brief enough to capture the transient increases observed for transcripts such as ccmM and cmpR (Fig. 3, A-C). Subsequently, the culture was split four ways. Two cultures were supplemented with 5 mm NaHCO3 (designated time zero) and swapped to bubbling with 1.7% CO2 in the presence or absence of 200 μg mL-1 of the transcriptional inhibitor rifampicin for a further 2 h. The remaining two cultures were supplemented with 5 mm NaCl, to balance sodium, and bubbled with CO2-free air with or without rifampicin as above. The four cultures were sampled for transcript analysis at various times after the treatments. Changes in the abundance of cmpA, sbtA, chpY, cmpR, ccmM, and psbAII compared with time zero, were quantified as before using real-time PCR assays (Fig. 5). The cmpA transcript had a half-life of approximately 15 min at both high and low Ci. However, it was even more rapidly degraded in high-Ci cells in the absence of rifampicin, being two-thirds less abundant at 15 min than in high-Ci cells treated with rifampicin. This result suggests that the synthesis of a factor is required for rapid degradation of the cmpA transcript. The sbtA and chpY transcripts responded similarly to cmpA (results not shown), however, both sbtA and chpY exhibited a transient insensitivity to rifampicin treatment followed by rapid degradation. This type of response has been observed in Escherichia coli and is thought possibly to result from different sigma factors influencing the interaction of rifampicin with the RNA polymerase holoenzyme (Selinger et al., 2003). The cmpR transcript had a half-time of less than 5 min at high and low Ci and was equally rapidly degraded at high Ci in the absence of rifampicin. The ccmM transcript was initially insensitive to any of the treatments, but by 15 min, approximately 50% and 75% of the initial transcript pool had decayed at high and low Ci, respectively. The trajectory apparent for ccmM abundance in the absence of rifampicin indicates that active transcription still occurred after 2 h at high Ci. The psbAII transcript had a half-life of approximately 30 min under both Ci conditions, which is similar to previously reported values for this transcript in cells grown with Ci sufficiency at low or high light (Kulkarni et al., 1992). At high Ci, psbAII was slightly more rapidly degraded in the absence of rifampicin. In summary, the CCM-related transcripts investigated had relatively short half-lives (5-15 min), were in general equally stable at high and low Ci, and with the exception of ccmM, were not actively transcribed at high Ci. This indicates that changes in transcript abundance in response to variations in external Ci availability result from transcriptional control of these genes rather than changes in mRNA stability.
Figure 5.
Turnover of Ci-responsive CCM-related transcripts in Synechococcus cells. Exponentially growing high-Ci cells were swapped to CO2-free air equilibrated buffer, bubbled with CO2-free air for 30 min, and then swapped again to either CO2-free air or 1.7% CO2 in the presence or absence of 200 μg mL-1 rifampicin (RIF). The relative abundance of cmpA, cmpR, ccmM, and psbAII at various time points was determined by real-time PCR. Symbols represent the abundance of transcripts at each time point as a percentage of the initial 30-min, low-Ci-induced amount (set at 100%) ± se for three separate measurements.
Effect of Light on CCM-Related Gene Expression
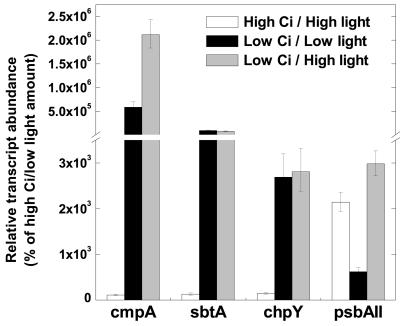
To assess whether high-light stress is sufficient to induce CCM-related gene expression, as has been reported in Synechocystis (Hihara et al., 2001; Huang et al., 2002), a Synechococcus culture bubbled with 1.7% CO2 and illuminated with 85 μmol photons m-2 s-1 was grown to exponential phase and harvested. An equal number of cells were resuspended in media equilibrated with 1.7% CO2 or CO2-free air and then bubbled with 1.7% CO2 or CO2-free air, respectively. Duplicate cultures were illuminated with 85 or 500 μmol photons m-2 s-1 (termed low or high light) for 30 min. At high Ci, high-light treatment was not found to be sufficient to induce increased expression for any of the CCM-related genes tested, whereas psbAII transcript abundance increased around 2 × 103% (Fig. 6). At low Ci, a high-light treatment increased the degree of induction around 4-fold for the cmpA transcript and by about 50% for psbAII compared with that observed in cells subject to a low-Ci/low-light treatment (Fig. 6). The abundance of sbtA and chpY transcripts was unresponsive to light under either Ci regime.
Figure 6.
Effect of light on CCM-related transcript abundance in Synechococcus cells. Exponentially growing high-Ci cells illuminated with 85 μmol photons m-2 s-1 (low light) were transferred to bubbling with CO2-free air (low Ci) and illuminated with either 85 or 500 μmol photons m-2 s-1 (high light) for 30 min. A parallel culture was illuminated with 85 μmol photons m-2 s-1 and bubbled with 1.7% CO2 as a control. The relative abundance of cmpA, sbtA, chpY, and psbAII transcripts was determined by real-time PCR. Bars represent the extent of induction or repression after the shift to low Ci as a percentage of the high-Ci/low-light amount (set at 100%) ± se for three separate measurements. Note the break in the y axis.
Effect of Redox Modifiers and Inhibitors of CO2 Fixation or CO2 Transport on CCM-Related Gene Expression
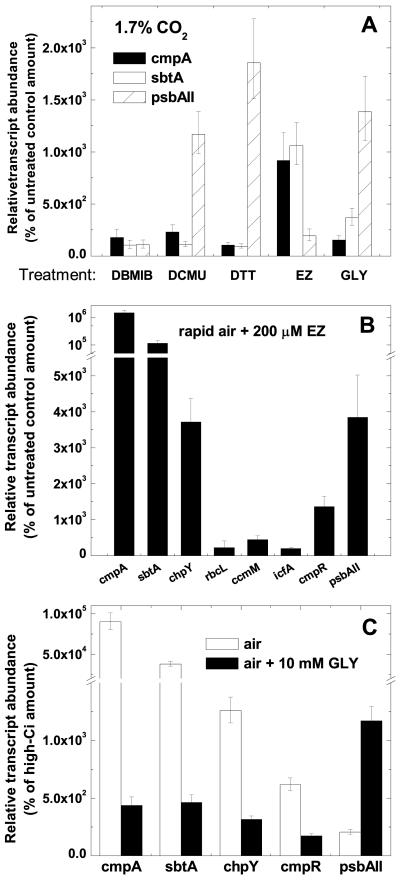
To examine some competing theories about the nature of the low-Ci signal that is sensed by Synechococcus cells, high-Ci-grown cells were treated for 30 min with 20 μm 3-(3,4-dichlorophenyl)-1,1dimethylurea (DCMU) or 20 μm 2,5-dibromo-3-methyl-6-isopropyl-benzoquinone (DBMIB)—agents that increase the reduction state of the electron carriers between photosystems II and I. Cells were also treated with 5 mm dithiothreitol (DTT)red, 200 μm CA of the inhibitor ethoxyzolamide (EZ), and 10 mm of the carbon fixation inhibitor glycolaldehyde (GLY). The concentration of GLY used has only a minor effect on CO2 transport (Salon et al., 1998). Changes in cmpA, sbtA, and psbAII transcript abundance relative to untreated control cells were determined using real-time PCR assays (Fig. 7A). As previously reported in Synechocystis, psbAII transcript abundance increased substantially in cells treated with DTTred and DCMU (Sippola and Aro, 1999; Li and Sherman, 2000). GLY treatment, which would be expected to emulate a high-light stress due to over-reduction of the photosynthetic electron transport chain, also resulted in an increase in psbAII abundance. These treatments did not alter cmpA or sbtA transcript abundance, with the exception of the GLY treatment, which elicited a small increase in sbtA transcript abundance. However, this increase was 3 orders of magnitude less than that typically observed in low-Ci cells (Fig. 2A). The EZ treatment resulted in nearly a 10-fold increase in the abundance of cmpA and sbtA transcripts, however, this is less than 1% of the response typically observed in low-Ci cells. It has previously been shown that the effect of EZ treatment can be overcome by increasing the availability of Ci (Price and Badger, 1989b). Accordingly, to increase the ratio of EZ to CO2 within the culture medium, Synechoccocus cells were instead grown with air delivered through a fritted sparge (approximately 0.4 L min-1). This rapid bubbling technique does not induce a high-affinity CCM (Yu et al., 1994), and compared with high-Ci cells, transcripts such as cmpA were induced to a degree that was less than 1% of that typically observed in severely Ci-limited cells (data not shown). By contrast, with the exception of the rbcL transcript, inducible CCM-related transcripts and psbAII increased significantly in abundance in rapidly air-bubbled cells treated with EZ (Fig. 7B), to a degree comparable with that typically seen in cells subject to a severe Ci limitation (Fig. 2).
Figure 7.
Effect of redox modifiers and inhibitors of CO2 fixation or CO2 transport on CCM-related transcript abundance in Synechococcus cells. As determined by real-time PCR, bars represent the extent of induction or repression after the treatment as a percentage of the untreated control amount (set at 100%) ± se for three separate measurements. A, Relative abundance of cmpA, sbtA, and psbAII in high-Ci cells treated for 30 min with 20 μm 2,5-dibromo-3-methyl-6-isopropyl-benzoquinone, 20 μm DCMU, 5 mm DTTred, 200 μm EZ, or 10 mm GLY. B, Relative abundance of a subset of CCM-related transcripts in cells bubbled with air through a fritted sparge (rapid air; approximately 0.4 L min-1) and treated for 30 min with 200 μm EZ. y axis scale is exponential after the break. C, Relative abundance of a subset of CCM-related transcripts in high cells swapped to medium air sparging (approximately 0.15 L min-1) for 30 min in the presence or absence of 10 mm GLY. Note the break in the y axis in B and C.
We also tested the effect of GLY on low-Ci induction of CCM-related gene expression. Equal volumes of cells from an exponentially growing high-Ci Syn- echococcus culture were harvested and resuspended in medium containing approximately 100 μm total Ci. Cells were then bubbled slowly with air, using a standard pipette (approximately 0.15 L min-1) for 30 min to create a moderate level of Ci limitation. At the swap over, one of the cultures was treated with 10 mm GLY, and cells were incubated in the dark for 5 min. As a control, an equivalent number of high-Ci cells were resuspended in high-Ci-equilibrated medium and returned to bubbling with 1.7% CO2. Treatment of cells with GLY reduced the low-Ci induction of chpY and cmpR by more than 80% and that of cmpA and sbtA by more than 99% (Fig. 7C). By contrast, GLY treatment resulted in a 10-fold greater induction in psbAII expression than that observed in the untreated air-bubbled culture.
DISCUSSION
We have profiled changes in the expression of genes involved in the CCM of Synechococcus when cells are transferred from Ci sufficiency to conditions of Ci limitation. We have also tested a number of ideas about the sensory mechanisms leading to induction of a high-affinity CCM. As has been observed in Synechocystis, the transcriptional response of this strain to severe Ci limitation occurs rapidly, reaching a maximum within 30 to 60 min (Fig. 3). One of our key findings includes the characterization of three distinct temporal responses of low-Ci-inducible transcripts. The temporal responses of these transcripts to Ci limitation align with functional groupings of the encoded proteins: (a) a slow, sustained induction for psbAII, (b) a rapid, transient induction for genes encoding the carboxysome-associated proteins (ccmKLMNO and rbcL) and cmpR, and (c) a rapid, sustained induction for genes encoding the inducible Ci uptake systems (sbtA, cmpABCD, and ndhF3-D3-chpY). In all cases, the transcriptional response of low-Ci-inducible CCM-related genes preceded the full physiological response by 30 to 60 min, however, the three distinct temporal responses are suggestive of the existence of alternative controls on expression of subsets of these genes. Furthermore, the pattern of decay of CCM-related transcripts at high- and low-Ci suggests that the abundance of these transcripts is primarily controlled at the level of transcription rather than at the level of mRNA stability (Fig. 5).
Previous work has shown that light is a prerequisite condition for induction of CCM-related gene expression (McGinn et al., 2003), and under low-Ci conditions, high light tends to accentuate induction of some CCM-related genes (P. McGinn, unpublished data). However, the nature of the primary signal that elicits induction of a high-affinity CCM is unknown. Direct sensing of external or internal Ci availability, changes in the redox potential of the photosynthetic electron transport chain or in the amounts of intermediates in the Calvin cycle, and photorespiratory pathways have all been proposed (for review, see Kaplan and Reinhold, 1999). It is well established that light-regulated expression of the psbA family occurs through a redox-sensing mechanism in cyanobacterial cells (Sippola and Aro, 1999; Alfonso et al., 2000; Li and Sherman, 2000; van Waasbergen et al., 2002). Given the finding that the expression of members of the psbA and pbsD families is responsive to Ci availability and reports that some CCM-related gene expression is light-responsive (Hihara et al., 2001; Huang et al., 2002), a redox-sensing mechanism modulating CCM-related gene expression seems to be an attractive theory. However, the reported culturing conditions of Huang et al. (2002) involved air-mixing of Synechocystis cells at 100 rpm, possibly causing a Ci limitation that would be exacerbated by increasing the light intensity. Hihara et al. (2001) found that a low- to high-light transition for Synechocystis bubbled with 1% CO2 lead to induction of ndhF3-D3 transcripts, but this result has not been reproduced in another study (McGinn et al., 2003) or in the present study involving Synechococcus (Fig. 6).
We detected multiple conditions under which the regulation of a classic light-inducible gene, psbAII, and CCM-related genes is uncoupled in Synechococcus cells. First, a high-light treatment at high-Ci levels, although sufficient to induce psbAII expression, failed to significantly induce any of the CCM genes tested at high Ci (Fig. 6). The only effect of high light was found at low Ci, where cmpA transcript abundance was 4-fold higher in cells exposed to the higher irradiance. Our results indicate that any high-light responsiveness of CCM-related gene expression in Synechococcus is confined to conditions under which Ci is limiting, and this has also been confirmed in Synechocystis (McGinn et al., 2003; P. McGinn, unpublished data). Second, our inhibitor studies in Synechococcus also show that the regulation of psbAII and CCM-related transcripts can be uncoupled (Fig. 7). At high-Ci, psbAII expression was strongly induced under conditions known to produce redox stress, namely by treatment with DCMU, DTTred, and GLY, but the expression of cmpA and sbtA was unaffected. Also, at low Ci, GLY treatment largely abolished the low-Ci induction of cmpA, sbtA, and chpY but enhanced psbAII induction. Collectively, our results suggest that it is unlikely that Synechococcus cells detect low-Ci availability primarily through a redox-sensing mechanism shared with light stress-signaling pathways. In addition, our data indicate that an increased concentration of reactive oxygen species, which is known to potentiate signaling in cyanobacteria (He and Harder, 2002), is unlikely to signal Ci limitation. This is because light stress in isolation failed to induce CCM genes, and GLY treatment, which would be predicted to increase ROS, actually prevented induction (Fig. 7). The results of our inhibitor studies are also inconsistent with the direct perception by cells of reduced external Ci concentration, because GLY and EZ treatment uncoupled the relationship between external Ci concentration and the degree of CCM-transcript induction.
The preceding arguments point to just two of the proposed sensory mechanisms, namely that changes within the internal Ci pool are perceived directly and dictate the degree of induction of the CCM in Synechococcus or that cells sense altered photorespiratory activity. In support of the latter theory, it is already established that Anabaena variabilils cells grown at low O2 are slow to adapt to Ci limitation (Marcus et al., 1983). The CA inhibitor EZ is known to severely reduce the internal Ci pool size in Ci-limited Synechococcus cells due to inhibition of active CO2-uptake systems (Price and Badger, 1989a). It would also be predicted to increase photorespiratory activity because of the decreased ratio of CO2 to O2 inside the cell. In this study, the EZ treatment of Synechococcus cells grown with rapid air sparging led to increases in the expression of all inducible CCM-related genes tested, to a degree comparable with that observed in cells subjected to severe Ci limitation (Fig. 7). A relatively small effect of EZ on cmpA and sbtA abundance was also observed in cells grown at 1.7% CO2, even though it would be predicted that CO2 diffusion should be sufficient under these conditions to maintain pool sizes that saturate Rubisco (Price and Badger, 1989c; Reinhold et al., 1991). It is possible that reduced flux through the NDH-14 complex, in the presence of EZ, might elicit this minor effect. In GLY-treated cells at low Ci, we found that normally low-Ci-inducible transcripts encoding CCM components were only weakly responsive to Ci limitation. When CO2-fixation is blocked by inhibitors such as GLY, a larger intracellular Ci pool develops (Salon et al., 1996). However, GLY acts to inhibit ribulose 1,5-bisphosphate regeneration (Sicher, 1984), inhibiting photorespiration as well as Ci pool depletion. We are currently exploring Ci pool- and photorespiratory-dependent sensory mechanisms further using available mutants with altered internal Ci pools and wild-type cells in which photorespiration has been manipulated.
MATERIALS AND METHODS
Cyanobacterial Strains and Culture Conditions
Cells of the cyanobacterium Synechococcus sp. strain PCC7942 were cultured in modified BG-11 medium (Price and Badger, 1989a) containing 20 mm HEPES-KOH, pH 8.0, at 28°C with a light intensity of approximately 85 μmol photons m-2 s-1. Aeration was delivered through a pipette with a 1-mm annulus at a flow rate of approximately 0.15 L min-1 unless otherwise indicated. To rapidly induce a high-affinity CCM, exponentially growing cells (OD730 of 0.3-0.6) that had been bubbled with an air-CO2 mixture containing 1.7% CO2 were harvested by centrifugation at 4,800g for 6 min and resuspended in an equivalent volume of CO2-free medium. The CO2-free medium was generated by bubbling growth medium with air passed through CO2-absorbing soda lime (path-length approximately 1 m) for at least 24 h. Cultures were returned to the original conditions but aerated with CO2-free air.
Isolation of Total RNA and First-Strand cDNA Synthesis
Isolation of total RNA from harvested cell pellets stored at -80°C was essentially as previously described (McGinn et al., 2003). First-strand cDNA synthesis from 3 to 5 μg of normalized total RNA using 0.1 μm each of the 3′ gene-specific primers described in Table I was performed using the Superscript First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.
Real-Time RT-PCR Assays
Primers were designed using Jellyfish software (LABVELOCITY, v1.5) and, with the exception of the psbA primers, forward and reverse primers in every pair had melting temperatures that varied by less than 3°C and GC contents not greater than 55%. For specificity, it was necessary to anchor the psbAI/II/III forward primers in the 5′-untranslated region and to relax design constraints slightly. Primers for sbtA were designed after sequencing of a fragment of sbtA obtained with degenerate primers (GenBank accession no. AY365060). All other sequences were obtained from GenBank. The relative amount of specific cDNA templates between different samples was quantified using real-time PCR. HotstarTaq PCR kit components (Qiagen, Hilden, Germany) were used in 20-μL reactions containing 3 mm MgCl2, 200 μm each of dATP, dTTP, dCTP, and dGTP, 0.5 μm each of forward and reverse primer, 0.5 to 1 unit Taq, and cDNA template equivalent to 25 ng of total RNA. For every reaction, an RNA sample without reverse transcriptase was included to control for genomic DNA contamination. Product formation was monitored by the inclusion of SYBR Green I at a final dilution of 1:40,000 (Fisher Biotech, Springfield, NJ). Thermocycling was conducted in a Rotorgene 2000 Thermal Cycler (Corbett Research, Sydney, Australia) for 35 cycles consisting of denaturation for 30 s at 95°C, annealing at 54°C for 30 s, extension at 72°C for 30 s, and fluorescence acquisition at 84°C for 15 s. Cycling was preceded by a 15-min 95°C activation step. Specificity of amplification was confirmed through a melt curve analysis of final PCR products by ramping the rotor temperature from 55°C to 99°C at 0.2°C s-1 with fluorescence acquired after every 1°C increase.
Calculation of Changes in Transcript Abundance
The amplification efficiencies of primer pairs used in real-time PCR assays (Table I) varied by less than 10% compared with that of the normalizer ppc, which was 1.9. These values were determined by the slope of the curve generated by amplification of serially diluted cDNA over at least 3 orders of magnitude (r ≥ 0.965). Accordingly, all primer pairs were nominated as having an efficiency of 2 and -fold changes in transcript abundance were calculated using the 2-ΔΔCt method (Livak and Schmittgen, 2001), where ΔCt expresses the difference in Ct between a target sample and the Ct of the sample from the basal condition. The value ΔΔCt expresses the difference between the ΔCt of the normalizer (ppc) and the target. -Fold changes were converted to percent change whereby a value of 100% equals no change. All reactions were carried out in triplicate, and the error was propagated using standard methods. The final error for any normalized -fold change incorporates the error for both the basal condition and the normalizer and is asymmetrical (for discussion, see Livak and Schmittgen, 2001).
Mass Spectrometric Measurements
Cells were prepared and analyzed in the mass spectrometer as previously described (Sültemeyer et al., 1995; McGinn et al., 2003). Cells were assayed at a chlorophyll density of 2 μg mL-1 in BG11 medium buffered with 50 mm BisTrisPropane-HCl (pH 7.9) and where NaNO3 had been replaced with 20 mm NaCl. Assays were performed in a thermostatted (30°C) mass spectrometer cuvette allowing membrane inlet analysis of O2 (mass 32) and CO2 (mass 44). In the presence of 25 μg mL-1 bovine CA, the CO2 signal was used as a surrogate measure [Ci] in real time. The maximum rate of net O2 evolution (Vmax) was measured in the presence of 3 mm NaHCO3, and the photosynthetic affinity for Ci was determined as K0.5 (Ci), that is, the Ci concentration required to reach half the maximum rate of net O2 evolution. Measurements at low levels of Ci were initiated at around 25 μm O2 and allowed to progressively increase throughout the Ci range. The light intensity used was 950 μmol photons m-2 s-1. The concentration of total Ci in culture was determined as previously described (Badger et al., 1994).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.029728.
References
- Alfonso M, Perewoska I, Kirilovsky D (2000) Redox control of psbA gene expression in the cyanobacterium Synechocystis PCC 6803: involvement of the cytochrome b(6)/f complex. Plant Physiol 122: 505-515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoroso G, Seimetz N, Sültemeyer D (2003) The dc13 gene upstream of ictB is involved in rapid induction of the high affinity Na+-dependent HCO3- transporter in cyanobacteria. Photosynth Res 77: 127-138 [DOI] [PubMed] [Google Scholar]
- Badger MR, Palmqvist K, Yu JW (1994) Measurement of CO2 and HCO3- fluxes in cyanobacteria and microalgae during steady-state photosynthesis. Physiol Plant 90: 529-536 [Google Scholar]
- Badger MR, Price GD (2003) CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. J Exp Bot 54: 609-622 [DOI] [PubMed] [Google Scholar]
- Benschop JJ, Badger MR, Price GD (2003) Characterisation of CO2 and HCO3- uptake in the cyanobacterium Synechocystis sp. PCC6803. Photosynth Res 77: 117-126 [DOI] [PubMed] [Google Scholar]
- Bonfil DJ, Ronentarazi M, Sultemeyer D, Liemanhurwitz J, Schatz D, Kaplan A (1998) A putative HCO3- transporter in the cyanobacterium Synechococcus sp. strain PCC7942. FEBS Lett 430: 236-240 [DOI] [PubMed] [Google Scholar]
- Bustos SA, Golden SS (1991) Expression of the psbDII gene in Synechococcus sp. strain PCC 7942 requires sequences downstream of the transcription start site. J Bacteriol 173: 7525-7533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figge RM, Cassier-Chauvat C, Chauvat F, Cerff R (2001) Characterization and analysis of an NAD(P)H dehydrogenase transcriptional regulator critical for the survival of cyanobacteria facing inorganic carbon starvation and osmotic stress. Mol Microbiol 39: 455-468 [DOI] [PubMed] [Google Scholar]
- Fukuzawa H, Suzuki E, Komukai Y, Miyachi S (1992) A gene homologous to chloroplast carbonic anhydrase (icfA) is essential to photosynthetic carbon dioxide fixation by Synechococcus PCC7942. Proc Natl Acad Sci USA 89: 4437-4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YY, Harder D (2002) Reactive oxygen species and UV-B: effect on cyanobacteria. Photochem Photobiol Sci 1: 729-736 [DOI] [PubMed] [Google Scholar]
- Hihara Y, Kamei A, Kanehisa M, Kaplan A, Ikeuchi M (2001) DNA microarray analysis of cyanobacterial gene expression during acclimation to high light. Plant Cell 13: 793-806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, McCluskey MP, Ni H, LaRossa RA (2002) Global gene expression profiles of the cyanobacterium Synechocystis sp. strain PCC 6803 in response to irradiation with UV-B and white light. J Bacteriol 184: 6845-6858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A, Reinhold L (1999) CO2 concentrating mechanisms in photosynthetic microorganisms [review]. Annu Rev Plant Physiol Plant Mol Biol 50: 539-570 [DOI] [PubMed] [Google Scholar]
- Kulkarni RD, Schaefer MR, Golden SS (1992) Transcriptional and post-transcriptional components of psbA response to high light intensity in Synechococcus sp. strain PCC 7942. J Bacteriol 174: 3775-3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Sherman LA (2000) A redox-responsive regulator of photosynthesis gene expression in the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol 182: 4268-4277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔCt) method. Methods 25: 402-408 [DOI] [PubMed] [Google Scholar]
- Maeda S, Badger MR, Price GD (2002) Novel gene products associated with NdhD3/D4-containing NDH-1 complexes are involved in photosynthetic CO2 hydration in the cyanobacterium, Synechococcus sp. PCC7942. Mol Microbiol 43: 425-435 [DOI] [PubMed] [Google Scholar]
- Maeda S, Price GD, Badger MR, Enomoto C, Omata T (2000) Bicarbonate binding activity of the CmpA protein of the cyanobacterium Synechococcus sp strain PCC 7942 involved in active transport of bicarbonate. J Biol Chem 275: 20551-20555 [DOI] [PubMed] [Google Scholar]
- Marcus Y, Harel E, Kaplan A (1983) Adaptation of the cyanobacterium Anabaena variabilis to low CO2 concentration in their environment. Plant Physiol 71: 208-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinn PJ, Price GD, Maleszka R, Badger MR (2003) Inorganic carbon limitation and light control the expression of transcripts related to the CO2-concentrating mechanism in the cyanobacterium Synechocystis sp. strain PCC6803. Plant Physiol 132: 218-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay RML, Gibbs SP, Espie GS (1993) Effect of dissolved inorganic carbon on the expression of carboxysomes, localization of Rubisco and the mode of inorganic carbon transport in cells of the cyanobacterium Synechococcus UTEX 625. Arch Microbiol 159: 21-29 [Google Scholar]
- Mori S, Castoreno A, Lammers PJ (2002) Transcript levels of rbcR1, ntcA, and rbcL/S genes in cyanobacterium Anabaena sp PCC 7120 are down-regulated in response to cold and osmotic stress. FEMS Microbiol Lett 213: 167-173 [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Pakrasi HB, Ogawa T (2000a) Two types of functionally distinct NAD(P)H dehydrogenases in Synechocystis sp. strain PCC6803. J Biol Chem 275: 31630-31634 [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Price GD, Badger MR, Ogawa T (2000b) Mutation of ndh genes leads to inhibition of CO2 uptake rather than HCO3- uptake in Synechocystis sp strain PCC 6803. J Bacteriol 182: 2591-2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Sonoda M, Katoh H, Ogawa T (1998) The use of mutants in the analysis of the CO2-concentrating mechanism in cyanobacteria. Can J Bot 76: 1035-1042 [Google Scholar]
- Omata T, Gohta S, Takahashi Y, Harano Y, Maeda S (2001) Involvement of a CbbR homolog in low CO2-induced activation of the bicarbonate transporter operon in cyanobacteria. J Bacteriol 183: 1891-1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T, Price GD, Badger MR, Okamura M, Gohta S, Ogawa T (1999) Identification of an ATP-binding cassette transporter involved in bicarbonate uptake in the cyanobacterium Synechococcus sp. strain PCC 7942. Proc Natl Acad Sci USA 96: 13571-13576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GD, Badger MR (1989a) Ethoxyzolamide inhibition of CO2 uptake in the cyanobacterium Synechococcus PCC7942 without apparent inhibition of internal carbonic anhydrase activity. Plant Physiol 89: 37-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GD, Badger MR (1989b) Ethoxyzolamide inhibition of CO2-dependent photosynthesis in the cyanobacterium Synechococcus PCC7942. Plant Physiol 89: 44-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GD, Badger MR (1989c) Isolation and characterization of high CO2-requiring-mutants of the cyanobacterium Synechococcus PCC7942: two phenotypes that accumulate inorganic carbon but are apparently unable to generate CO2 within the carboxysome. Plant Physiol 91: 514-525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GD, Coleman JR, Badger MR (1992) Association of carbonic anhydrase activity with carboxysomes isolated from the cyanobacterium Synechococcus PCC7942. Plant Physiol 100: 784-793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GD, Maeda S, Omata T, Badger MR (2002) Modes of active inorganic carbon uptake in the cyanobacterium Synechococcus sp. PCC7942. Funct Plant Biol 29: 131-149 [DOI] [PubMed] [Google Scholar]
- Reinhold L, Kosloff R, Kaplan A (1991) A model for inorganic carbon fluxes and photosynthesis in cyanobacterial carboxysomes. Can J Bot 69: 984-988 [Google Scholar]
- Ruppert U, Irmler A, Kloft N, Forchhammer K (2002) The novel protein phosphatase PphA from Synechocystis PCC6803 controls dephosphorylation of the signalling protein P-II. Mol Microbiol 44: 855-864 [DOI] [PubMed] [Google Scholar]
- Salon C, Li QL, Canvin DT (1998) Glycolaldehyde inhibition of CO2 transport in the cyanobacterium Synechococcus UTEX625. Can J Bot 76: 1-11 [Google Scholar]
- Salon C, Mir NA, Canvin DT (1996) HCO3- and CO2 leakage from Synechococcus UTEX 625. Plant Cell Environ 19: 260-274 [Google Scholar]
- Schaefer MR, Golden SS (1989) Differential expression of members of a cyanobacterial psbA gene family in response to light. J Bacteriol 171: 3973-3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW (2000) Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem 285: 194-204 [DOI] [PubMed] [Google Scholar]
- Selinger DW, Saxena RM, Cheung KJ, Church GM, Rosenow C (2003) Global RNA half-life analysis in Escherichia coli reveals positional patterns of transcript degradation. Genome Res 13: 216-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Ohkawa H, Kaneko T, Fukuzawa H, Tabata S, Kaplan A, Ogawa T (2001) Distinct constitutive and low-CO2-induced CO2 uptake systems in cyanobacteria: genes involved and their phylogenetic relationship with homologous genes in other organisms. Proc Natl Acad Sci USA 98: 11789-11794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Ohkawa H, Katoh H, Shimoyama M, Ogawa T (2002) Two CO2 uptake systems in cyanobacteria: four systems for inorganic carbon acquisition in Synechocystis sp. strain PCC6803. Funct Plant Biol 29: 123-129 [DOI] [PubMed] [Google Scholar]
- Sicher RC (1984) Glycolaldehyde inhibition of photosynthetic carbon assimilation by isolated chloroplasts and protoplasts. In C Sybesma, eds, Advances in Photosynthetic Research, Vol 3. Martinus Nijhoff/Dr. W. Junk, The Hague, The Netherlands, pp 413-416 [Google Scholar]
- Sippola K, Aro EM (1999) Thiol redox state regulates expression of psbA genes in Synechococcus sp. PCC 7942. Plant Mol Biol 41: 425-433 [DOI] [PubMed] [Google Scholar]
- So AKC, John-McKay M, Espie GS (2002) Characterization of a mutant lacking carboxysomal carbonic anhydrase from the cyanobacterium Synechocystis PCC6803. Planta 214: 456-467 [DOI] [PubMed] [Google Scholar]
- Sültemeyer D, Amoroso G, Fock H (1995) Induction of intracellular carbonic anhydrases during the adaptation to low inorganic carbon concentrations in wild-type and ca-1 mutant cells of Chlamydomonas reinhardtii. Planta 196: 217-224 [Google Scholar]
- Sültemeyer D, Klughammer B, Badger MR, Price GD (1998) Fast induction of high-affinity HCO3- transport in cyanobacteria. Plant Physiol 116: 183-192 [Google Scholar]
- van Waasbergen LG, Dolganov N, Grossman AR (2002) nblS, a gene involved in controlling photosynthesis-related gene expression during high light and nutrient stress in Synechococcus elongatus PCC 7942. J Bacteriol 184: 2481-2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JW, Price GD, Badger MR (1994) Characterisation of CO2 and HCO3- uptake during steady-state photosynthesis in the cyanobacterium Synechococcus PCC 7942. Aust J Plant Physiol 21: 185-195 [Google Scholar]
- Yu JW, Price GD, Song L, Badger MR (1992) Isolation of a putative carboxysomal carbonic anhydrase gene from the cyanobacterium Synechococcus PCC7942. Plant Physiol 100: 794-800 [DOI] [PMC free article] [PubMed] [Google Scholar]