Abstract
Glyceraldehyde-3-phosphate dehydrogenases catalyze key steps in energy and reducing power partitioning in cells of higher plants. Phosphorylated non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase (GAPN) present in heterotrophic cells of wheat (Triticum aestivum) was activated up to 3-fold by MgCl2. The effect was not observed with the non-phosphorylated enzyme found in leaves. The divalent cation also affected the response of the enzyme from endosperm and shoots to adenine nucleotides and inorganic pyrophosphate. Gel filtration chromatography, co-immunoprecipitation followed by immunostaining, and the use of a phosphopeptide containing a canonical binding motif showed that MgCl2 actually disrupted the interaction between GAPN and a 14-3-3 regulatory protein. After interaction with 14-3-3, phosphorylated GAPN exhibits a 3-fold lower Vmax and higher sensitivity to inhibition by ATP and pyrophosphate. Results suggest that GAPN is a target for regulation by phosphorylation, levels of divalent cations, and 14-3-3 proteins. The regulatory mechanism could be critical to maintain levels of energy and reductants in the cytoplasm of heterotrophic plant cells.
Three different enzymes capable of catalyzing the oxidation of glyceraldehyde-3-phosphate (Ga3P) have been isolated from plants, and their distinctive properties have been documented (Pupillo and Faggiani, 1979; Habenicht, 1997; Gomez Casati et al., 2000). Two of them catalyze a inorganic orthophosphate (Pi)-dependent, reversible oxidation of d-Ga3P to 1,3-bisphosphoglycerate; one (EC 1.2.1.12; glyceraldehyde-3-phosphate dehydrogenase C [GAPC]) is NAD+ specific and found in the cytoplasm of both photosynthetic and non-photosynthetic tissues, whereas the other (EC 1.2.1.13; glyceraldehyde-3-phosphate dehydrogenase AB) uses either NAD+ or NADP+ and is confined to the chloroplast (Cerff and Chambers, 1979; Pupillo and Faggiani, 1979; Mateos and Serrano, 1992; Plaxton, 1996). The third enzyme (EC 1.2.1.9; glyceraldehyde-3-phosphate dehydrogenase [GAPN]), the non-phosphorylating Ga3P dehydrogenase, irreversibly converts Ga3P to 3-phosphoglycerate with the coupled reduction of NADP+ to NADPH. Analysis of amino acid sequence alignment has shown that GAPN shares 20% to 30% identity with the superfamily of aldehyde dehydrogenases (Habenicht, 1997). GAPN is not related to glyceraldehyde-3-phosphate dehydrogenase AB/GAPC, which differs in both molecular mass and structure (Iglesias et al., 1987; Iglesias and Losada, 1988; Habenicht, 1997).
GAPN has been described and characterized in photosynthetic tissues of a number of higher plants (Habenicht, 1997) and in the green alga Chlamydomonas reinhardtii (Iglesias et al., 1987). It has been proposed that GAPN could participate, associated with chloroplastic GAPAB and the triose-phosphate/Pi translocator of the chloroplast envelope, in the indirect shuttling of photosynthetically generated NADPH to the cytosol (Kelly and Gibbs, 1973). This agrees with the occurrence of GAPN being a specific feature of those organisms with chloroplasts or cyanelles (Mateos and Serrano, 1992). However, the presence of this enzyme in non-photosynthetic tissues of plants, protists, and heterotrophic bacteria suggests that GAPN might also perform another role, not related to photosynthetic carbon metabolism. It was proposed that the plant enzyme could take part of an alternative cytosolic glycolysis, in a pathway also involving inorganic pyrophosphate (PPi)-dependent phosphofructokinase and phosphoenolpyruvate phosphatase (Plaxton, 1996; Givan, 1999).
Alternative roles for GAPN could be relevant for carbon metabolism in non-photosynthetic tissues of higher plants because they depend upon the supply of Suc from the leaf and, thus, perform metabolic pathways totally opposite from those occurring in photosynthetic cells. For example, the amyloplast imports metabolites from the cytoplasm as precursors of carbon skeletons and energy, which is an opposite route to that taking place in leaf cells, where the chloroplast is the source of assimilates (Emes and Neuhaus, 1997). Despite the importance that GAPN could play in heterotrophic cells, no experimental evidence is currently available to support its alternative functions, and the enzyme from non-photosynthetic tissues of higher plants has been poorly studied.
In agreement with the model that proposes a role for GAPN in heterotrophic plant tissues, different from that played by the enzyme in photosynthetic cells, we recently found that in wheat (Triticum aestivum) endosperm and shoots (but not in leaves), GAPN is posttranslationally modified by phosphorylation (Bustos and Iglesias, 2002). This distinctive modification of the enzyme from heterotrophic plant cells supposes the existence of specific mechanisms for regulating its activity. In the present work, we characterize the regulatory properties of GAPN from wheat endosperm and shoots, and we report that the enzyme from these tissues is not only phosphorylated but also interacting with proteins of the type 14-3-3. Results are discussed in the context of the physiological relevance of regulating the flux of carbon to produce energetic or reducing power in the cytoplasm of heterotrophic plant cells.
RESULTS
Regulation of Partially Purified GAPN by Adenine Nucleotides and PPi
Recently, we reported that GAPN is extracted in a phosphorylated state from heterotrophic tissues (endosperm and shoot) of wheat, which is a distinctive feature with respect to what is found in leaf (Bustos and Iglesias, 2002). In vitro phosphorylation/dephosphorylation experiments showed that partially purified endosperm enzyme exhibits 3-fold lower Vmax when it is phosphorylated with no changes observed in the affinity for substrates (Bustos and Iglesias, 2002).
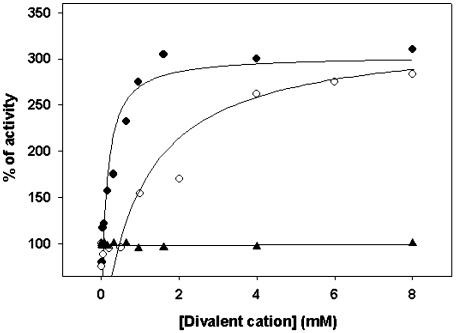
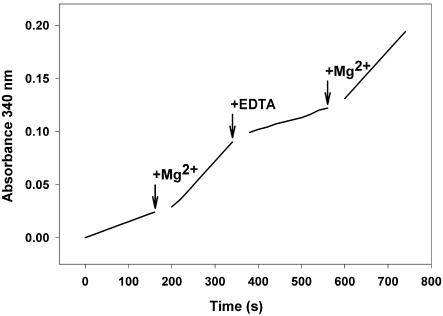
To better understand differences in kinetic and regulatory properties of the modified enzyme, we performed a comparative study on the effect of several compounds on GAPN from wheat leaf, endosperm, and shoot. These studies were performed using enzymes partially purified (about 25-fold) after ammonium sulfate (35%-60% saturation) fractionation and anion-exchange chromatography on DEAE-Sepharose. A main difference between GAPNs partially purified from leaf and endosperm was the response to the presence of Mg2+ in the assay medium. Figure 1 shows that the leaf enzyme was insensitive to the divalent cation, whereas the endosperm enzyme was activated up to 3-fold by Mg2+, with an apparent effector concentration giving 50% of maximal activation (A0.5) of 0.4 mm (Hill coefficient of 0.5). As shown in Figure 1, Ca2+ was also effective to activate the endosperm enzyme by 3-fold, although with a markedly lower affinity (A0.5 of 4 mm; Hill coefficient of 0.7). Monovalent cations were much less effective to activate GAPN because KCl activated by near 2-fold (A0.5 of 19 mm), and NaCl exhibited no effect (data not shown). Figure 2 shows that the activation of phosphorylated GAPN by Mg2+ is a reversible process. Thus, the activatory effect exerted by the divalent cation was reversed by addition of EDTA to the enzyme assay medium. Concurrently, further addition of Mg2+ overcame the reversion of the chelating compound, activating again the enzyme activity (Fig. 2).
Figure 1.
Effect of MgCl2 and CaCl2 on GAPN activity. The enzyme partially purified from wheat endosperm was assayed as described with the addition of the stated concentrations of Mg2+ (black circles) or Ca2+ (white circles). The enzyme from leaf was assayed under identical conditions in the presence of specified levels of Mg2+ (triangles).
Figure 2.
Reversibility of the effect of MgCl2 on GAPN activity. The enzyme partially purified from wheat endosperm was assayed spectrophotometrically, and at the stated times (arrows), additions of 1 mm MgCl2, 1 mm EDTA, and 5 mm MgCl2 were consecutively performed.
The distinctive effect of Mg2+ on GAPN from wheat endosperm was not only exerted by the cation itself but also by modifying the response of the enzyme to adenine nucleotides and PPi. Table I shows that the presence of 5 mm Mg2+ in the assay medium distinctively affected the response of the endosperm enzyme to ATP, ADP, AMP, or PPi. On the other hand, the leaf enzyme showed a different affinity to the latter effectors and was insensitive to Mg2+. Thus, ATP showed no effect on GAPN from endosperm in the presence of 5 mm Mg2+, but it inhibited the enzyme in the absence of the cation (Table I). Contrarily, the endosperm enzyme was inhibited (to near 40% inhibition) by 3 mm ADP or AMP only in the presence of Mg2+ (Table I). This behavior was different from that exhibited by wheat leaf GAPN, which was inhibited by ATP, ADP, or AMP independently of the presence of the divalent cation (Table I). The effect of PPi was also distinctive for GAPN from endosperm because it inhibited this enzyme when assayed in the absence of Mg2+ but showed no effect on the enzyme from leaf (Table I).
Table I.
Regulation of GAPN activity by MgCl2, adenine nucleotides, and PPi
The state compounds were included in the assay medium at 3 mm concentration, without or with further addition of 5 mm MgCl2. Values are means of four independent experiments made by triplicate.
| Compound
|
GAPN Activity
|
|||
|---|---|---|---|---|
| Endosperm
|
Leaf
|
|||
| −Mg2+ | +Mg2+ | −Mg2+ | +Mg2+ | |
| milliunits mg−1 | ||||
| None | 120 ± 6.0 | 330 ± 16.1 | 450 ± 20.1 | 400 ± 25.9 |
| AMP | 110 ± 5.5 | 200 ± 10.2 | 380 ± 19.5 | 360 ± 22.2 |
| ADP | 110 ± 5.0 | 210 ± 10.1 | 320 ± 20.8 | 330 ± 17.6 |
| ATP | 81 ± 3.2 | 330 ± 18.3 | 350 ± 21.4 | 300 ± 17.5 |
| PPi | 84 ± 3.7 | 340 ± 15.0 | 430 ± 25.0 | 410 ± 23.3 |
As a whole, results in Table I show that GAPN from wheat leaf is poorly regulated by adenine nucleotides and that this is not dependent on divalent cations. These results are in agreement with previous reports on the characterization of the enzyme from different photosynthetic organisms (Iglesias et al., 1987; Iglesias and Losada, 1988; Trost and Pupillo, 1993; Habenicht, 1997; Gomez Casati et al., 2000). Interestingly, ATP, ADP, AMP, or PPi (together with Mg2+) affected the endosperm enzyme activity to a higher degree when compared with the effect on the leaf enzyme. Although the inhibition exerted by adenine nucleotides and PPi on endosperm GAPN was relatively low, the combined effect of them with Mg2+ can produce changes of up to near 6-fold in the enzyme activity (Table I).
To explore if the above distinctive regulatory properties exhibited by GAPN from wheat endosperm are related with heterotrophic properties of the plant cell, the same studies were performed with the enzyme partially purified from wheat shoots. Regulatory properties of the enzyme from the latter source were identical to those above described for the protein from endosperm. Because it has been reported that in these non-photosynthetic tissues GAPN is found posttranslationally modified (Bustos and Iglesias, 2002), results shown in the present work suggest that when phosphorylated, GAPN exhibits a distinctive, more sensitive response to Mg2+, adenylates, and PPi.
Phosphorylated GAPN Interacts with 14-3-3 Proteins
To further comparatively analyze properties of GAPN from wheat endosperm and leaf, we attempted purification of the enzyme from both sources to a higher degree. After the procedure employed to purify GAPN from celery (Apium graveolens; Gomez Casati et al., 2000), we performed an affinity chromatography on Reactive Red 120 agarose column as a next purification step. The endosperm enzyme eluted at 0.9 m NaCl from the affinity column, exhibiting a near 10-fold increase in specific activity, reaching a value of 1.12 units mg-1. Unexpectedly, after the affinity chromatography step, GAPN from endosperm was no longer activated by Mg2+, and it lost the distinctive behavior respect to PPi (data not shown). These results suggested that activation by the divalent cation could require some protein component to be lost during the purification step. Conversely, the enzyme extracted and purified from leaves was not affected by the divalent cation, and it exhibited similar kinetic and regulatory properties along the different purification steps.
Because GAPN from wheat endosperm is a phosphorylated protein (which is different from the leaf enzyme; see Bustos and Iglesias, 2002), the above results were related with reports showing that this posttranslational regulation is usually associated with proteins (i.e. 14-3-3 proteins) that interact with the target enzyme exerting changes in its regulatory properties (for review, see Comparot et al., 2003). Interestingly, the mechanism operating in plants for the latter specified protein-protein interaction requires divalent cations (Lu et al., 1994; Athawal et al., 1998), and the dissociation between proteins after affinity chromatography was observed with another plant enzyme that interacts with regulatory proteins (Fullone et al., 1998; Provan et al., 2000).
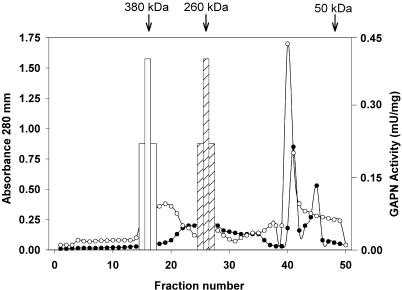
To explore this possibility, GAPNs partially purified from leaf and endosperm (or shoots) were chromatographed on Superose 12 in the absence or presence of Mg2+. The cation showed no effect on the elution profile of the 250- ± 20-kD leaf enzyme (data not shown). Conversely, Figure 3 shows that elution of endosperm GAPN activity from the size exclusion column was different for each condition. The endosperm enzyme eluted as a 380- ± 35- or 260- ± 20-kD protein depending on the absence or presence, respectively, of 5 mm MgCl2 in the running buffer (Fig. 3). The elution profile of GAPN exhibiting the lower molecular mass also shows a new protein peak of about 60 to 70 kD (Fig. 3), suggesting that Mg2+ disrupted the interaction between both proteins.
Figure 3.
Dissociation of GAPN from a regulatory protein. Gel filtration chromatography of GAPN partially purified from wheat endosperm was performed in the absence (white symbols) or in the presence (black symbols) of 5 mm MgCl2. A280 (circles) and GAPN activity (assayed in the presence of 1 mm EDTA to assure similar conditions for samples derived from chromatography with or without divalent cation; bars) were determined in the different fractions.
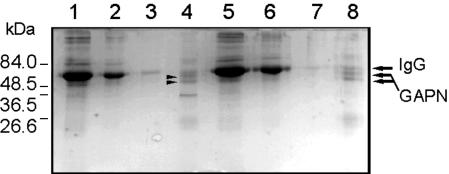
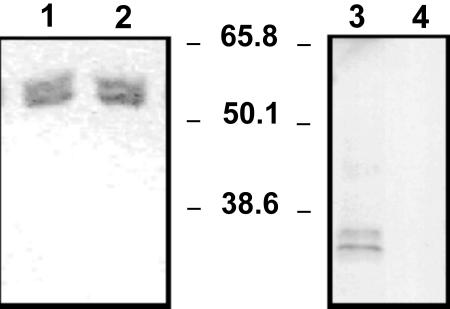
Co-immunoprecipitation studies also showed the interaction of wheat endosperm (or shoots) GAPN with another proteins only in the absence of a divalent cation. As illustrated in Figure 4, in extracts from endosperm containing no Mg2+ serum, anti-GAPN coprecipitated the enzyme together with a protein of molecular mass about 35 kD, whereas when the extract contained 5 mm MgCl2 only, GAPN precipitated with the antibody (Fig. 4). The latter was the pattern observed for extracts from leaves either containing or not containing MgCl2 (data not shown) and, thus, in agreement with other results reported above. The identity of the proteins co-immunoprecipitated in endosperm extracts was established by western blots revealed with antisera raised against celery GAPN and against spinach (Spinacia oleracea) 14-3-3 (Fig. 5). As shown in Figure 5, the coprecipitate obtained from wheat endosperm extracts containing no Mg2+ (Fig. 4, lane 4) gave positive immunostaining to GAPN and to 14-3-3 (Fig. 5, lanes 1 and 3, respectively), whereas in extracts obtained with Mg2+ (Fig. 4, lane 8), only GAPN was immunodetected (Fig. 5, lanes 2 and 4). Similar results that those shown in Figures 4 and 5 were obtained when co-immunoprecipitation was performed using anti-14-3-3 serum (data not shown). In addition, the anti-14-3-3 serum was utilized to identify the 60- to 70-kD peak eluted from the Superose 12 column run in the presence of 5 mm MgCl2 (Fig. 3). Thus, western-blot analysis revealed that this peak was recognized by the antibody raised against spinach 14-3-3 protein (data not shown).
Figure 4.
Co-immunoprecipitation of GAPN in wheat endosperm extracts. Extracts in media without (lanes 1-4) or with (lanes 5-8) 5 mm MgCl2 were precipitated using serum anti-GAPN from celery leaf. Supernatants after washings (lanes 1-3 and 5-7) and after elution (lanes 4 and 8) were run in 10% (w/v) SDS-PAGE. Proteins in the gel were revealed by Coomassie Blue staining.
Figure 5.
Western-blot analysis of co-immunoprecipitate samples from wheat endosperm. Eluted samples from precipitate obtained using serum anti-GAPN from celery leaf from wheat extracts without (lanes 1 and 3) or with (lanes 2 and 4) 5 mm MgCl2 were subjected to SDS-PAGE followed by electroblotting. Nitrocellulose membranes were revealed with rabbit anti-celery GAPN serum (lanes 1 and 2) or goat anti-spinach 14-3-3 serum (lanes 3 and 4) and phosphatase-conjugated anti-rabbit or anti-goat, respectively, IgG.
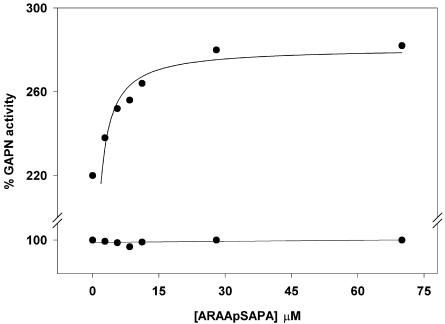
To further characterize the identity of the protein interacting with phosphorylated GAPN from endosperm and shoot, studies were performed to determine the competence exerted by a phosphopeptide carrying the canonical 14-3-3-binding motif A-R-A-A-pS-A-P-A (pS being phospho-Ser; Moorhead et al., 1999; Milne et al., 2002). Figure 6 shows that GAPN was activated up to near 3-fold by the presence of the phosphopeptide in the assay medium. Only phosphorylated GAPN (not the leaf enzyme; data not shown) was activated by the phosphopeptide, and this was strictly dependent on the presence of Mg2+ (Fig. 6). This is in agreement with previous reports demonstrating the capacity of the same phosphopeptide to bind 14-3-3 in a competitive manner with respect to other enzymes of plant metabolism (Moorhead et al., 1999). Because the phosphopeptide exhibits a high specificity to bind 14-3-3 proteins in the presence of Mg2+ (Moorhead et al., 1999; Milne et al., 2002), the above results strongly suggest that the interaction of a protein of the type 14-3-3 with GAPN is what produces the decrease in GAPN activity. In addition, it is assumed that such an interaction between phosphorylated GAPN and a 14-3-3 protein in released by divalent cations (Mg2+ and less effectively Ca2+).
Figure 6.
Activation of GAPN by a phosphopeptide carrying the canonical 14-3-3 binding motif. The enzyme partially purified from wheat endosperm was assayed with the stated additions of the phosphopeptide in the absence (white circles) or presence (black circles) of 0.1 mm MgCl2.
GAPN Interacting with 14-3-3 Localizes in the Cytoplasm
One recognized function of 14-3-3s in animals and plants is to mediate organellar trafficking after binding to target proteins (Hachiya et al., 1993; Roberts, 2000). For example, it has been demonstrated that 14-3-3 proteins form a guidance complex with chloroplast precursor proteins in plants (May and Soll, 2000). To know the intracellular localization of GAPN in heterotrophic tissues of plants is critical to understand the role of the enzyme in the metabolism of non-photosynthetic cells. Because of this after determining that the enzyme is phosphorylated and interacts with 14-3-3 proteins, we performed studies to establish the intracellular compartment where GAPN is found in wheat endosperm and shoot. Table II shows that after plastidial subcellular fractionation was performed with both heterotrophic plant tissues (endosperm and shoot), the activity of GAPN was almost entirely recovered outside the plastid.
Table II.
Subcellular localization of GAPN activity in endosperm and shoot cells of wheat
| Enzyme
|
Activity
|
||
|---|---|---|---|
| Total | in Supernatant | in Pellet | |
| units g fresh wt−1a | % | ||
| Endosperm | |||
| GAPC | 75 | 95 | 2.0 |
| Soluble starch synthase | 4.7 | 3.2 | 98 |
| ADPGlc pyrophosphorylase | 5.2 | 5.1 | 92 |
| GAPN | 3.0 | 1.5 | 88 |
| Shoot | |||
| GAPC | 130.0 | 98 | n.d.b |
| Glc 6-P dehydrogenase | 43 | 10 | 88 |
| Aldolase | 17.8 | 92 | 1.2 |
| GAPN | 6.0 | 95 | n.d. |
a Units of activity per gram of fresh wt. b n.d., Not detected.
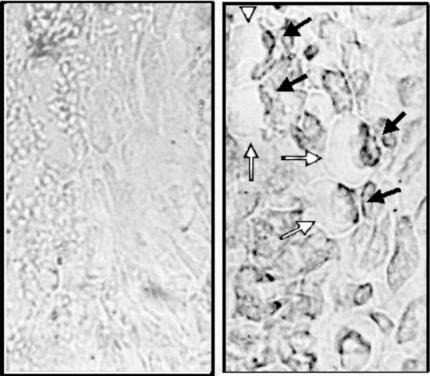
In agreement with the latter results were data obtained on the immunolocalization of GAPN in wheat endosperm. Figure 7 shows that rabbit serum anti-GAPN from spinach leaf revealed that the enzyme is not localized in the major plastidic region containing starch but in the periphery of the endosperm cell, corresponding to the cytosol. Localization of the enzyme in the cytoplasm of leaf cell had been reported (Habenicht, 1997) and was also found in our experiments performed in wheat leaves (data not shown). Thus, results suggest that the interaction of phosphorylated GAPN with 14-3-3 does not modify the intracellular localization of the enzyme.
Figure 7.
Immunolocalization of GAPN. Thin sections of wheat endosperm were prepared as described under “Materials and Methods” and then stained using rabbit pre-immune (A) or anti-GAPN (B) sera. White and black head arrows show cell borders and immunostained GAPN, respectively.
DISCUSSION
The presence of GAPN has been documented in leaves and roots of higher plants (Mateos and Serrano, 1992). In photosynthetic cells, the enzyme is involved in a transport shuttle mechanism for the export of photosynthesized NADPH from the chloroplast to the cytosol (Kelly and Gibbs, 1973; Habenicht, 1997). A less clear-cut role is the physiological function of the enzyme in non-photosynthetic tissues. The recent finding that in endosperm and shoot of wheat GAPN undergoes posttranslational phosphorylation (Bustos and Iglesias, 2002) strongly suggesting a distinctive regulation of the enzyme activity (with respect to leaf) with a different metabolic scenario. In the present work, we show that the interaction of the phosphorylated enzyme with 14-3-3 proteins is a mechanism exerting main changes in the regulation of its activity in heterotrophic plant cells.
A number of differences were established between leaf and endosperm (or shoot) GAPNs with respect to regulatory properties. In agreement with previous reports (Iglesias et al., 1987; Iglesias and Losada, 1988; Trost and Pupillo, 1993; Habenicht, 1997; Gomez Casati et al., 2000), the activity of the enzyme from photosynthetic cells was found insensitive to Mg2+ and to PPi, whereas it exhibited a relatively low sensitivity to adenine nucleotides. Conversely, GAPN partially purified from wheat endosperm (and shoot) was activated by about 3-fold by the divalent cations Mg2+ and Ca2+, the former exhibiting a near 10-fold higher affinity. In addition, Mg2+ modified the response of the endosperm enzyme to adenine nucleotides and PPi.
Because the above distinctive properties exhibited by GAPN partially purified from endosperm were lost after a further purification step by affinity chromatography, we searched for a possible interaction of the phosphorylated enzyme with other protein. In addition, the latter was related with previous reports showing other enzymes modified by phosphorylation that exhibit a divalent cation-mediated interaction with regulatory proteins (Lu et al., 1994; Athawal et al., 1998). Data derived from size exclusion chromatography and co-immunoprecipitation showed that Mg2+ exerts the dissociation of a protein from native GAPN. Results suggest that the activatory effect exhibited by the divalent cation is consequence of the disruption of a protein-protein interaction between GAPN and a homodimer of 70 kD.
Different enzymes of the carbon and nitrogen metabolism in plants undergo posttranslational phosphorylation followed by interaction with regulatory proteins named 14-3-3 (Moorhead et al., 1999; Roberts, 2000; DeLille et al., 2001; Rosenquist et al., 2001; Huber et al., 2002; Comparot et al., 2003). These regulatory proteins have a molecular mass of about 30 to 35 kD and usually form a dimeric native structure that interact with the target enzyme (DeLille et al., 2001; Huber et al., 2002). Phosphorylated GAPN found in heterotrophic tissues of wheat seems to interact with a 14-3-3-like regulatory polypeptide forming a complex stable in the absence of divalent cation. The identity of the protein interacting with GAPN was established by immunodetection with serum anti-spinach 14-3-3, and it was further determined by studies with a phosphopeptide carrying a canonical 14-3-3-binding motif. As exemplified with other plant enzymes (Moorhead et al., 1999; Huber et al., 2002), the phosphopeptide was able to increase the enzyme activity nearly 3-fold after its competitive binding to the 14-3-3 protein interacting with GAPN. The difference of 140 kD observed for samples analyzed by size exclusion chromatography in the absence or the presence of Mg2+ suggests a quaternary structure of the complex of 1 mol tetrameric GAPN to 2 mol dimeric 14-3-3 protein. A distinctive property of this interaction is that it is disrupted by Mg2+, which was found as relevant for the association of 14-3-3 proteins with many plant enzymes (DeLille et al., 2001; Huber et al., 2002). To our knowledge, the case of GAPN is the first example showing an opposite effect of divalent cations as dissociating 14-3-3 from a target protein.
Recently, we reported that phosphorylation of GAPN in cells from wheat endosperm and shoot produces a main effect on Vmax, reducing it nearly 3-fold without exerting changes in the affinity of the enzyme for substrates (Bustos and Iglesias, 2002). In the present work, we further characterized posttranslational modification of GAPN in heterotrophic plant tissues, showing that it is not phosphorylation itself but the interaction of the modified enzyme with 14-3-3 proteins that actually affects its activity. In addition to a change in Vmax, phosphorylated GAPN interacting with 14-3-3 also exhibited a distinctive response to regulation by adenine nucleotides and PPi. The main effect of phosphorylation and interaction with regulatory proteins, thus, is to produce a modified form of GAPN that is more sensitive to regulation, with no change in its intracellular localization as occurs with other 14-3-3 target enzymes in plants (Moorhead et al., 1999; Roberts, 2000; Huber et al., 2002). From the analysis of the sequence of wheat endosperm GAPN (GeneBank accession no. AAM77679) and assuming phosphorylation of the Ser residue, one possible 14-3-3-binding consensus motif can be proposed: 401RINpSVEE. This motif is similar to what was reported for the binding of 14-3-3 to other proteins (Campbell et al., 1997; Huber et al., 2002; Pozuelo Rubio et al., 2003).
Phosphorylation alone renders a form of GAPN that is insensitive to ATP or PPi and that is inhibited by ADP and AMP. In this state, the enzymes from endosperm and shoot are quite similar to the leaf non-phosphorylated enzyme (Bustos and Iglesias, 2002) except that the latter is slightly inhibited by ATP (Iglesias et al., 1987; Iglesias and Losada, 1988; Trost and Pupillo, 1993; Habenicht, 1997; Gomez Casati et al., 2000). In addition to having a near 3-fold lower Vmax, the complex between phosphorylated GAPN and 14-3-3 exhibits different regulatory properties because it is inhibited by ATP and PPi but neither affected by ADP nor by AMP. The whole picture suggest that phosphorylation, together with 14-3-3 proteins, establish a divalent cation-dependent regulatory mechanism to inhibit GAPN activity at high energy content.
The cytosol of higher plant cells contains little or no soluble pyrophosphatase, and a significant pool of PPi may accumulate as a result of the reversible reaction of PPi-dependent phosphofructokinase (mainly found in heterotrophic plant cells; Plaxton, 1996; Stitt, 1998). Thus, not only ATP but also PPi levels are related with energy content in plant tissues (Stitt, 1998). Increases in ATP and/or PPi constitute a signal for high energy content in the cytosol of non-photosynthetic cells that, in combination with low levels of divalent cations, would produce a fine regulation of GAPN bound to 14-3-3, leading to a marked inhibition of its activity. Considering the presence of GAPN and GAPC, alternative pathways are established at the level of Ga3P in the glycolytic pathway occurring in the cytosol of plant cells (Iglesias, 1989; Plaxton, 1996). One pathway is the classical route through GAPC and P-glycerate kinase, rendering 1 mol of ATP per mol of Ga3P oxidized to 3-phosphoglycerate. The other pathway involving only GAPN produces no ATP but NADPH (Iglesias, 1989). In this scenario, a mechanism to modulate GAPN activity, involving posttranslational modification and interaction with regulatory proteins, may be relevant for plant metabolism. It is tempting to speculate that regulation of the above bifurcation in a central metabolic pathway needs to be finely exerted to effectively modulate levels of energy and reductants in the cytoplasm of the heterotrophic plant cell.
MATERIALS AND METHODS
Materials
Wheat (Triticum aestivum) leaves were obtained from plants grown in the greenhouse at 18°C ± 2°C under a 14-h photoperiod. Shoots were developed for 2 d in the dark in 5 mm Pi (pH 7.0)-buffered medium. Endosperm (20 DPA) was obtained from plants grown in the field. DEAE-Sepharose fast flow, Mono Q HR5/5, and Superose 6 were from Pharmacia (Uppsala). All other reagents were of the highest purity.
Enzyme Assays and Protein Measurements
Total protein was determined according to Bradford (1976), using bovine serum albumin (BSA) as the standard. All enzyme assays were performed at 30°C. GAPN was assayed spectrophotometrically by monitoring NADPH generation at 340 nm, essentially as previously described (Gomez Casati et al., 2000). The standard assay medium (final volume of 0.75 mL) contained 50 mm Tricine (pH 8.5), 0.4 mm NADP+, 1 mm d-Ga3P (eventually generated by 2 mm Fru 1,6-bisphosphate and 1 unit of aldolase), and 1 mm 2-mercaptoethanol. GAPC activity was determined under the same conditions, except that NAD+ was utilized instead of NADP+, and 5 mm Naarsenate was included in the medium. Aldolase (EC 4.1.2.13) was assayed by coupling the production of Ga3P from Fru 1,6-bisP to the reaction of GAPC. Glc 6-P dehydrogenase (EC 1.1.1.49) activity was measured by monitoring NADPH production as described (Bergmeyer, 1974). ADP-Glc pyrophosphorylase (EC 2.7.7.27) and soluble starch synthase (EC 2.4.1.21) were assayed radiochemically by measuring synthesis of [14C]ADP-Glc from radioactive Glc 1-P or incorporation of 14C into glucan from [14C]ADP-Glc, respectively, as described elsewhere (Smith, 1991; Gomez Casati et al., 2001). In all cases, 1 unit of activity is defined as the amount of enzyme catalyzing synthesis of 1 μmol of product min-1.
Purification of GAPN
The phosphorylated form of GAPN was partially purified from wheat endosperm as described previously (Bustos and Iglesias, 2002). The purification protocol was similar to that utilized to purify the enzyme from leaves (Gomez Casati et al., 2000), and it renders a 25-fold purified endosperm GAPN exhibiting a specific activity of 0.12 units mg-1 after ammonium sulfate fractionation and DEAE-Sepharose fast-flow chromatography (Bustos and Iglesias, 2002). Eventually, GAPN was further purified by adsorption onto a Reactive Red-120 agarose column (2.3 × 5 cm) equilibrated with buffer A (100 mm Tris-HCl [pH 7.0], 1 mm EDTA, and 20 mm 2-mercaptoethanol). After washing with 40 mL of loading buffer, the enzyme was eluted with a linear gradient of 0 to 1 m NaCl in buffer A.
Gel Filtration Chromatography
The molecular mass of native GAPN was determined by using a Superose 12 HR10/30 column (FPLC, Pharmacia, Uppsala, Sweden) calibrated with standard protein markers, as specified previously (Gomez Casati et al., 2000). Running was performed in 50 mm MOPS-NaOH (pH 7.5), 1 mm dithiothreitol, and 50 NaCl, with or without the addition of 5 mm MgCl2.
Protein Electrophoresis
Electrophoresis under denaturating conditions (SDS-PAGE) was perfomed in 10% (w/v) running gels as previously described (Laemmli, 1970). After electrophoresis, gels were stained for protein using Coomasie Brilliant Blue.
Co-Immunoprecipitation
Co-immunoprecipitation experiments were performed using partially purified endosperm GAPN in buffer A, plus or minus the addition of 5 mm MgCl2. The enzyme was incubated under gentle stirring at 4°C for 40 min with 2 μL of rabbit anti-celery (Apium graveolens) leaf GAPN antiserum (affinity purified), followed by 1 h of incubation with 0.2 mL of streptavidin-coated paramagnetic beads (Streptavidin Magnesphere, Promega, Madison, WI) and biotin-labeled anti-rabbit IgG (Life Technologies/Gibco-BRL, Cleveland). Beds were washed twice with 1 mL of buffer containing Tris-HCl (pH 7.5) and 120 mm NaCl. Anti-GAPN and coprecipitated proteins were released from the beads with 100 mm Gly (pH 2.2). Protein in the resulting samples (washing steps and protein released from the beads) were precipitated with 10% (w/v) trichloroacetic acid, boiled in Laemmli buffer for 5 min, and subjected to SDS-PAGE. Gels were stained for protein or electroblotted. Nitrocellulose membranes were treated with rabbit anti-celery leaf GAPN or goat anti-spinach (Spinacia oleracea) leaf 14-3-3 antisera, and the antigen-antibody complexes were visualized with phosphatase-conjugated anti-rabbit or anti-goat, respectively, IgG followed by staining with 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium (Bollag and Edelstein, 1991). Co-immunoprecipitation experiments using goat anti-spinach 14-3-3β were performed basically under the same conditions, except that protein G agarose (from Amersham Bioscience, Uppsala, Sweden) was utilized instead of biotin labeled anti-rabbit IgG. The efficiency of immunoprecipitation was higher than 90%.
Immunocytochemistry
Transverse sections of developing grains of wheat were fixed for 1 h in 4% (v/v) paraformaldehyde buffered at pH 7.0 with 20 mm PIPES. The fixed samples were dehydrated, embedded in paraffin, and sectioned with a microtome. Thin sections were incubated for 45 min with a drop of phosphate-buffered saline (PBS; 0.15 m NaCl in 10 mm K-phosphate [pH 7.2]) containing 1% (w/v) BSA, followed by 45 min of incubation with anti-GAPN serum diluted in PBS plus BSA. Nonspecifically bound antibodies were removed by washing several times with PBS. The sections were then floated for 45 min on a drop of secondary antibody (phosphatase-conjugated anti-rabbit IgG). Samples were washed with PBS and then stained with 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium (Bollag and Edelstein, 1991).
Subcellular Fractionation
Amyloplasts were isolated by homogenizing wheat endosperm in a buffer containing 50 mm HEPES-KOH (pH 7.5), 0.8 m sorbitol, 1 mm EDTA, 1 mm KCl, 2 mm MgCl2, and 1 mg mL-1 BSA, followed by isopycnic centrifugation in Percoll as described by Tetlow et al., 1993). Shoot plastids were isolated from 72-h-old etiolated seedlings of wheat essentially as described previously (Gruissem et al., 1988). The grinding medium contained 30 mm Tris-HCl (pH 7.50), 0.5 m Suc, 1 mm EDTA, 4 mm Cys, and 0.1% (w/v) BSA. The washing medium was similar, except that 10 mm Tris-HCl (pH 7.40) was used as the buffer. The final plastid suspension, containing intact plastids, was diluted to reach a 0.3 m Suc concentration.
Acknowledgments
The authors wish to thank Dr. Carol MacKintosh (MRC Protein Phosphorylation Unit, School of Life Sciences, University of Dundee, UK) and Dr. Greg Moorhead (Department of Biological Sciences, University of Calgary, Canada) who kindly provided the phosphopeptide (CMK) and the anti-spinach 14-3-3 antiserum (CMK and GM) utilized in this study.
This work was supported in part by Agencia Nacional de Promoción Científica y Tecnológica (Argentina; grant no. PICT'99 1-6074).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.030981.
References
- Athawal GS, Huber JL, Huber SC (1998) Biological significance of divalent metal ion binding to 14-3-3 proteins in relationship to nitrate reductase inactivation. Plant Cell Physiol 39: 1065-1072 [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU (1974) Methods of enzymatic analysis. Verlag Chemie, Weinheim, Germany
- Bollag DM, Edelstein SJ (1991) Protein Methods. Wiley, New York
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254 [DOI] [PubMed] [Google Scholar]
- Bustos DM, Iglesias AA (2002) Non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase is post-translationally phosphorylated in heterotrophic cells of wheat (Triticum Aestivum). FEBS Lett 530: 169-173 [DOI] [PubMed] [Google Scholar]
- Campbell JK, Gurung R, Romero S, Speed CJ, Andrews RK, Berndt MC, Mitchell CA (1997) Activation of the 43-Kda inositol polyphosphate 5-phosphatase by 14-3-3. Biochemistry 36: 15363-15370 [DOI] [PubMed] [Google Scholar]
- Cerff R, Chambers SE (1979) Subunit structure of higher plant glyceraldehyde-3-phosphate dehydrogenases (EC 1.2.1.12 and EC 1.2.1.13). J Biol Chem 254: 6094-6098 [PubMed] [Google Scholar]
- Comparot S, Lingiah G, Martin T (2003) Function and specificity of 14-3-3 proteins in the regulation of carbohydrate and nitrogen metabolism. J Exp Bot 54: 595-604 [DOI] [PubMed] [Google Scholar]
- DeLille JM, Sehnke PC, Ferl RJ (2001) The Arabidopsis 14-3-3 family of signaling regulators. Plant Physiol 126: 35-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emes M, Neuhaus H (1997) Metabolism and transport in non-photosynthetic plastids. J Exp Bot 48: 1995-2005 [Google Scholar]
- Fullone MR, Visconti S, Marra M, Fogliano V, Aducci P (1998) Fusicoccin effect on the in vitro interaction between plant 14-3-3 proteins and plasma membrane H+-ATPase. J Biol Chem 273: 7698-7702 [DOI] [PubMed] [Google Scholar]
- Givan CV (1999) Evolving concepts in plant glycolysis: two centuries of progress. Biol Rev 74: 277-309 [Google Scholar]
- Gomez Casati DF, Aon MA, Cortassa S, Iglesias AA (2001) Measurement of the glycogen synthetic pathway in permeabilized cells of cyanobacteria. FEMS Microbiol Lett 194: 7-11 [DOI] [PubMed] [Google Scholar]
- Gomez Casati DF, Sesma JI, Iglesias AA (2000) Structural and kinetic characterization of NADP-dependent, non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase from celery leaves. Plant Sci 154: 107-115 [DOI] [PubMed] [Google Scholar]
- Gruissem W, Barkan A, Deng XW, Stern D (1988) Transcriptional and post-transcriptional control of plastid mRNA levels in higher plants. Trends Genet 4: 258-263 [DOI] [PubMed] [Google Scholar]
- Habenicht A (1997) The non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase: biochemistry, structure, occurrence and evolution. Biol Chem 378: 1413-1419 [PubMed] [Google Scholar]
- Hachiya N, Alam R, Sakasegawa Y, Sakaguchi M, Milhara K, Omura T (1993) A mitochondrial import factor purified from rat liver cytosol is an ATP-dependent conformational modulator for precursor proteins. EMBO J 12: 1579-1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SC, MacKintosh C, Kaiser WM (2002) Metabolic enzymes as targets for 14-3-3 proteins. Plant Mol Biol 50: 1053-1063 [DOI] [PubMed] [Google Scholar]
- Iglesias AA (1989) On the metabolism of triose-phosphates in photosynthetic cells: their involvement on the traffic of ATP and NADPH. Biochem Educ 18: 2-5 [Google Scholar]
- Iglesias AA, Losada M (1988) Purification and kinetic and structural properties of spinach leaf NADP-dependent nonphosphorylating glyceraldehyde-3-phosphate dehydrogenase. Arch Biochem Biophys 260: 830-840 [DOI] [PubMed] [Google Scholar]
- Iglesias AA, Serrano A, Guerrero MG, Losada M (1987) Purification and properties of NADP-dependent non-phosphorylating glyceraldehyde-3-phosphate dehydrogenase from the green alga Chlamydomonas reinhardtii. Biochim Biophys Acta 925: 1-10 [Google Scholar]
- Kelly GJ, Gibbs M (1973) A mechanism of the indirect transfer of photosynthetically reduced nicotinamide adenine dinucleotide phosphate from the chloroplast to the cytoplasm. Plant Physiol 52: 1023-1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680-685 [DOI] [PubMed] [Google Scholar]
- Lu G, Sehnke PC, Feiler HS (1994) Phosphorylation and calcium binding properties of an Arabidopsis GF14 brain protein homolog. Plant Cell 6: 501-510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos MI, Serrano A (1992) Occurrence of phosphorylating and nonphosphorylating NADP-dependent glyceraldehyde-3-phosphate dehydrogenases in photosynthetic organisms. Plant Sci 84: 163-170 [Google Scholar]
- May T, Soll J (2000) 14-3-3 Proteins form a guidance complex with chloroplast precursor proteins in plants. Plant Cell 12: 53-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne FC, Moorhead G, Pozuelo Rubio M, Wong B, Kulma A, Harthill JE, Villadsen D, Cotelle V, MacKintosh C (2002) Affinity purification of diverse plant and human 14-3-3-binding partners. Biochem Soc Trans 30: 379-381 [DOI] [PubMed] [Google Scholar]
- Moorhead G, Douglas P, Cotelle V, Harthill J, Morrice N, Meek S, Deiting U, Stitt M, Scarabel M, Aitken A et al. (1999) Phosphorylation-dependent interactions between enzymes of plant metabolism and 14-3-3 proteins. Plant J 18: 1-12 [DOI] [PubMed] [Google Scholar]
- Plaxton WC (1996) The organization and regulation of plant glycolysis. Annu Rev Plant Physiol Plant Mol Biol 47: 185-214 [DOI] [PubMed] [Google Scholar]
- Pozuelo Rubio M, Peggie M, Wong B, Morrice N, MacKintosh C (2003) 14-3-3s regulate fructose-2,6-bisphosphate levels by binding to PKB-phosphorylated cardiac fructose-2,6-bisphosphate kinase/phosphatase. EMBO J 22: 3514-3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provan F, Aksland LM, Meyer C, Lillo C (2000) Deletion of the nitrate reductase N-terminal domain still allows binding of 14-3-3 proteins but affects their inhibitory properties. Plant Physiol 123: 757-764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupillo P, Faggiani R (1979) Subunit structure of three glyceraldehyde 3-phosphate dehydrogenases of some flowering plants. Arch Biochem Biophys 194: 581-592 [DOI] [PubMed] [Google Scholar]
- Roberts MR (2000) Regulatory 14-3-3 protein-protein interactions in plant cells. Curr Opin Plant Biol 3: 400-405 [DOI] [PubMed] [Google Scholar]
- Rosenquist M, Alsterfjord M, Larsson C, Sommarin M (2001) Data mining the Arabidopsis genome reveals fifteen 14-3-3 genes: expression is demonstrated for two out of five novel genes. Plant Physiol 127: 142-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM (1991) Enzymes of starch synthesis. In PM Dey, JB Harborna, eds, Methods in Plant Biochemistry. Academic Press, London, pp 93-102
- Stitt M (1998) Pyrophosphate as an energy donor in the cytosol of plant cells: an enigmatic alternative to ATP. Bot Acta 111: 167-175 [Google Scholar]
- Tetlow IJ, Blissett K, Emes M (1993) A rapid method for the isolation of purified amyloplasts from wheat endosperm. Planta 189: 597-600 [Google Scholar]
- Trost P, Pupillo P (1993) Inhibition of spinach d-glyceraldehyde 3-phosphate: NADP+ oxidoreductase (nonphosphorylating) by adenylate compounds: the effect of dead-end inhibitors on a steady state random reaction mechanism. Arch Biochem Biophys 306: 76-82 [DOI] [PubMed] [Google Scholar]