Abstract
Histone H3 Lysine 4 (H3K4) tri-methylation (H3K4me3) at the promoter region of genes has been linked to transcriptional activation. In the present study, we found that hypoxia (1 % oxygen) increased H3K4me3 in both normal human bronchial epithelial Beas-2B cells and human lung carcinoma A549 cells. The increase of H3K4me3 by hypoxia was likely caused by the inhibition of H3K4 demethylating activity, as hypoxia still increased H3K4me3 in methionine-deficient medium. Furthermore, an in vitro histone demethylation assay demonstrated that 1% oxygen decreased the activity of H3K4 demethylases in Beas-2B nuclear extract since ambient oxygen tensions were required for the demethylation reaction to proceed. Hypoxia only minimally increased H3K4me3 in the BEAS-2B cells with knockdown of JARID1A, which is the major histone H3K4 demethylase in this cell line. However, the mRNA and protein levels of JARID1A were not affected by hypoxia. GeneChip and pathway analysis in JARID1A knockdown Beas-2B cells revealed that JARID1A regulates the expression of hundreds of genes involved in different cellular functions, including tumorigenesis. Knocking down of JARID1A increased H3K4me3 at the promoters of HMOX1 and DAF genes. Thus, these results indicate that hypoxia may target JARID1A activity which in turn increases H3K4me3 at both the global and gene specific levels, leading to the altered programs of gene expression and tumor progression.
Keywords: Hypoxia, Histone H3 lysine 4 methylation, JARID1A, epigenetics, lung cancer
Introduction
Cancer cells experience severe hypoxia, resulting from reduced oxygen supply from blood vessels because of the rapid cell proliferation characteristic of solid tumors. Activation of the major transcription factor hypoxia-inducible factor 1 (HIF-1), as well as other important transcription factors such as NF-κB, AP-1 (activator protein 1), p53 and c-Myc, drives much of hypoxic gene expression or repression (1). This will result in transcriptional activation of genes that increase angiogenesis, glycolysis, metastasis, and oppose apoptosis. Consequently, this shift in gene expression allows the cancer cell to survive proliferation and metastasis in a hypoxic environment. However, the mechanisms by which cancer cells can activate or repress gene expression remains unclear.
The fundamental unit of chromatin is the nucleosome, which consists of 146 bp of DNA wrapped twice around an octomer formed from two copies of each histone H2A, H2B, H3 and H4. Histone modifications at N-terminal tails which protrude from the nucleosomes are now recognized as critical epigenetic marks that modulate gene expression and genomic function. At least eight types of histone modifications have been identified. Among them, acetylation, methylation and phosphorylation have been most studied. These modifications act in combination to modulate chromatin structure and regulate gene expression (2). Generally, H3K9, H3K27 and H4K20 methylation are found in the promoter of gene that are transcriptionally silent, whereas H3K4, H3K36, and H3K79 methylation of gene promoters are associated with active transcription (3).
Active promoters are marked by H3K4me3 and this mark has been linked to transcriptional activation in a variety of eukaryotic species (4) (5) (6). H3K4me3 is catalyzed by a group of methyltransferases that contain a SET domain. Demethylation of this site can be reversed by four JARID1 family histone demethylases that are capable of removing the methyl groups from methylated H3K4. The JARID1 family in humans consists of 4 enzymes: KDM5A/RBP2/JARID1A, KDM5B/PLU-1/JARID1B, and 2 highly homologous proteins encoded by sex-chromosome-specific genes, KDM5C/SMCX/JARID1C, found on the X chromosome, and KDM5D/SMCY/JARID1D, found on the Y chromosome (7–11). These demethylases are members of dioxygenase superfamily and they require oxygen, iron and ascorbic acid as essential cofactors to oxidatively demethylate tri-methylated H3K4. Thus they are less active under hypoxic condition. Since they remove a gene-activating mark, they have been associated with transcriptional repression (11) (12). Experimental evidence indicates that individual members of the JARID1 family of H3K4 demethylases have unique functional properties and divergent expression profiles. JARID1A was originally described as a binding partner for the tumor suppressor protein Retinoblastoma (RB) (13). JARID1A is distinct from other histone-modifying enzymes in that it has a DNA binding motif within its AT-rich interaction domain (ARID), and this is essential for its transcriptional regulation and demethylation of H3K4me3 marks (14). JARID1A functions in the regulation of cell differentiation (15). The genome-wide location analysis revealed that JARID1A has a high correlation with H3K4me3 at gene promoters and it regulates two functionally distinct classes of genes: differentiation-independent and differentiation-dependent JARID1A target genes (15, 16). JARID1B has been described as a cancer antigen that is over-expressed in 90% of breast carcinomas (17). Very little is known about the molecular function of JARID1C, with the exception that it escapes X inactivation and is mutated in X-linked mental retardation (18). JARID1D has not yet been associated with any form of human disease.
In this study, we have demonstrated that hypoxia increased H3K4me3 at the global level in A549 and Beas-2B cell lines, and this effect is attributed to the inhibition of the demethylation process, particularly the H3K4 demethylase JARID1A. GeneChip and functional analysis suggested that JARID1A regulates the expression of genes involved in several distinct cellular categories. Knocking down of JARID1A increased H3K4me3 at the promoters of HMOX1 and DAF genes.
Materials and Methods
Cell culture
Cells were grown at 37°C in an incubator with a humidified atmosphere containing 5% CO2. A549 cells were cultured in F-12K medium (Mediatech, Inc., Herndon, VA) and Beas-2B cells were grown in DMEM medium. Both A549 and Beas-2B cell lines were purchased from American Type Culture Collection (ATCC) (Manassas, VA).
All medium was supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cells were exposed to hypoxic conditions in a chamber with a continuous flow of a hypoxic gas mixture with 1% oxygen at 37°C. The levels of oxygen in chambers were verified using a gas monitor (SKC, Inc., Eighty Four, PA).
Preparation of histones, whole cell lysate and measurement of HIF-1α
The cells were 80–90% confluent before collection. Histoneswere extracted from the cells as described previously (19, 20). Whole cell lysates were extracted by incubating with ice-cold radioimmunoprecipitation assay (RIPA) buffer for 20 min on ice, followed by centrifugation at 14000 × g for 15 min. The supernatant was collected. The cell extracts for HIF-1α measurement were prepared as described previously(21). The immunoblottings were performed with HIF-1α antibodies (Novus Biologicals, Littleton, CO) at 1:500 dilution.
Western blotting
The protein concentration was determined using the Bio-Rad DC protein assay (Bio-Rad, Hercules, CA), and 5 μg histones were separated by 15% SDS-PAGE gel and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad). Immunoblotting was performed using tri-methyl H3K4 (1:5000; Abcam) primary antibodies, and HRP-conjugated anti-rabbit secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). The detection was accomplished by chemical fluorescence following an ECL Western blotting protocol (Amersham, Piscataway, NJ). After transfer to PVDF membranes, the gels were stained with Bio-safe Coomassie stain (Bio-Rad) to assess the loading of histones. The immunoblots were scanned and analyzed using ImageJ software, and values were normalized to that obtained in the control sample(s).
Transient transfection of RNAi
Transient transfection of RNAi was done in Beas-2B cells using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol. 72 h after transfection, the cell extracts were prepared either for Western blotting or semi-quantitative RT-PCR. JARID1A RNAi was purchased from Invitrogen (Carlsbad, CA).
Histone H3K4 demethylation assay
Nuclear extract were prepared using a CelLytic NuCLEAR extraction kit (Sigma). 130 ug of freshly prepared nuclear extract from Beas-2B cells were incubated with 5 ug histones (upstate) in histone demethylation buffer (50 mM HEPES, PH 8.0, 2 ug/ml bovine serum albumin, 0.1 mM DL-dithiothreitol, 100 uM FeSO4, 2 mM ascorbate, 1 mM a-ketoglutarate and protease inhibitors) in a final volume of 50 ul at 37°C. Before mixing and incubating in hypoxia, nuclear extract, histones, histone demethylation buffer and water were all pre-equilibrated at 1% oxygen atmosphere for 1 h. The reaction in hypoxia was carried out in a glove box (Biospherix) with 1 % oxygen which was verified using a gas monitor (SKC, Inc., Eighty Four, PA). Following an overnight incubation, the demethylation reaction was terminated by the addition of EDTA to a final concentration of 1 mM. The reaction mixture was analyzed by Western blotting using H3K4me3 antibody. The experiments were carried out in duplicate.
Semi-quantitative RT-PCR and Real-time RT-PCR
Total RNA was extracted from cells immediately after exposure using Trizol reagent (Invitrogen), and following the manufacturer’s protocol. RNA concentration was determined by absorbance at 260 nm. First strand cDNA was synthesized using SuperScript III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen). Semi-quantitative PCR was performed using Taq DNA polymerase (Roche) and the specific primers indicated below: JARID1A: 5′-GGAGCCTCTGAGTGATCTGG-3′ (forward) and 5′-TCCAATAAGTAGCGAAGCAG-3′ (reverse); COL1A2: 5′-TTGACCCTAACCAAGGATGC-3′ (forward) and 5′-ATGCAATGCTGTTCTTGCAG-3′ (reverse); HMOX1: 5′-ACATCTATGTGGCCCTGGAG-3′ (forward), and 5′-TGTTGGGGAAGGTGAAGAAG-3′ (reverse); β-actin: 5′-TCACCCACACTGTGCCCATCTACGA-3′ (forward) and 5′-CAGCGGAACCGCTCATTGCCAATGG-3′ (reverse). Finally, PCR products were visualized by ethidium bromide on 1% agarose gel. Real-tim RT-PCR was performed using the 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA) with Fast SYBR Green Master Mix reagent (Applied Biosystems). Genes were amplified using the following primers: JARID1A: 5′-GCTTGGCAATGGGAACAAAA-3′ (forward) and 5′-CCGTTGTCTCATTTGCATGTTAA-3′ (reverse); JARID 1 B: 5′-AGTGCAGTGGCGCGATCT-3′ (forward) and 5′-GGCAGAAGAATTGCTGGAATCTAG-3′ (reverse); JARID1C: 5′-GCAAAAATATTGGCTCCTTGCT-3′ (forward) and 5′-ACGTGTGTTACACTGCACAAGGTT-3′ (reverse); JARID1D: 5′-GCCTAGCTGGGCTGAATTCC-3′ (forward) and 5′-GATGCCAGACTTCTCTGCTATGG-3′ (reverse) and β-actin: 5′-ATCGTCCACCGCAAATGCTTCTA-3′ (forward) and 5′-AGCCATGCCAATCTCATCTTGTT-3′ (reverse). All quantifications were normalized to β-actin. cDNA amplified by the same conditions.
Chromatin immunoprecipitation assays
Beas-2B cells were cross-linked using 37% formaldehyde to a final concentration of 1%. The ChIP assay was performed using EZ ChIP Kit (Millipore) according to the manufacturer’s protocol. Antibodies against tri-methyl H3K4 (Abcam) and normal IgG were used for immunoprecipitation. Semi-quantitative PCR was performed using the specific primers indicated below: HMOX1: 5′-GAGCCTGCAGCTTCTCAGAT-3′ (forward) and 5′-AACAGCTGATGCCCACTTTC-3′ (reverse); DAF: 5′-TAAGCTCCCCACGTGATTCT-3′ (forward) and 5′-ATTCACCAGTGTGCGTGTGT-3′ (reverse); DUSP2: 5′-AAAAACGGAGGGGTGCTAGT -3′ (forward) and 5′-ACCATACAAGGGCAGAGCAG-3′ (reverse). PCR products were separated on 2% agarose gel and visualized by ethidium bromide staining.
Results
Hypoxia increases global levels of H3K4me3
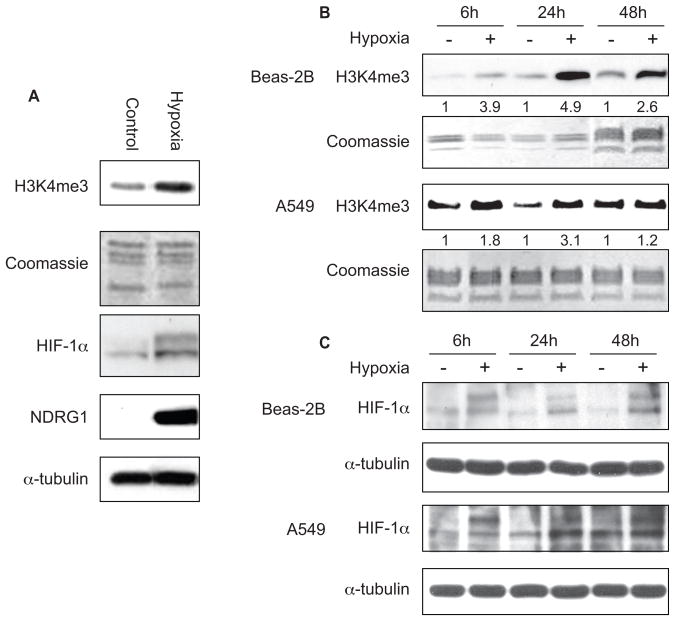
To experimentally measure if exposure to hypoxia results in changes in H3K4me3, Beas-2B cells were exposed to hypoxia (1 % oxygen). Exposure of BEAS-2B cells to hypoxia for 24 h increased the global level of H3K4me3. In parallel samples, intracellular HIF-1α protein level was found to be increased by hypoxia (Fig. 1A). It was noticed that both isoforms of HIF-1α that result from alternative splicing were present in Beas-2B cells (Fig. 1A) as were previously found in Hela and A549 cells(22, 23). N-myc downstream-regulated gene 1 (NDRG1), which is strongly up-regulated by HIF-1α.under hypoxia (24, 25), was also increased (Fig. 1A).
Figure 1.
A, Beas-2B cells were exposed to hypoxia (1% oxygen) for 24 h, and histones were then extracted. H3K4me3 was detected using Western blotting as described in Materials and Methods. In parallel samples, the intracellular HIF-1α and NDRG1 protein levels were measured using Western blotting. The same membranes were stripped and re-probed with α-tubulin antibody as loading control. B, The effect of hypoxia on H3K4me3 in A549 and Beas-2B cells after 6 h, 24 h and 48 h hypoxia exposure. The numbers below the figure represent the relative intensity of the bands. C, The intracellular HIF-1α protein levels were measured using Western blotting with anti- HIF-1α antibody after 6 h, 24 h and 48 h hypoxia exposure. After HIF-1α immunoblotting, the same membrane was stripped and re-blotted with α-tubulin to assess the protein loading.
The hypoxia-induced H3K4me3 was further studied at different time intervals in Beas-2B cells and in A549 cells. Exposure of BEAS-2B cells to hypoxia increased the global level of H3K4me3 at 6 h and 48 h in addition to 24 h, while exposure of A549 cells to hypoxia only increased the global level of H3K4me3 at 6 h and 24 h, but failed to increase H3K4me3 at 48 h compared to normoxia controls (Fig. 1A and B). It should be noted that both cell monolayers were sub-confluent and comparable in cell density. We next assessed the level of HIF-1α to determine if a hypoxic response was initiated in the cancerous A549 cells compared to the immortalized normal BEAS-2B cells at the 48 hr time interval. HIF-1α was induced compared to normoxia after 6 h, 24 h and 48 h hypoxia exposure in Beas-2B cells (Fig. 1C). In our previous study, we have shown that hypoxia can induce HIF-1α in A549 cells in a time-dependent manner (23). Here we demonstrated that hypoxia induced HIF-1α compared to normoxia only at 6 h and 24 h in A549 cells (Fig. 1C). The HIF-1α at 48 h was induced to the same level by both hypoxia and normoxia in A549 cells (Fig. 1C). This suggested that A549 cells maintained at normal oxygen level became hypoxic at the later time interval (48 h) which increased H3K4me3 in normoxic A549 cells to the level of H3K4me3 induced by 48 h of hypoxia (Fig. 1B).
Hypoxia increases H3K4me3 by inhibiting the H3K4 demethylating activity
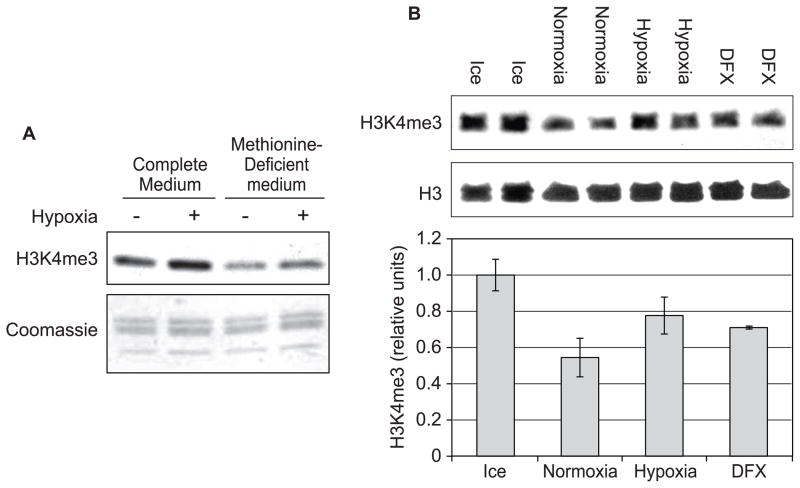
Since methionine is essential for S-adenosyl methionine (SAM) synthesis and SAM has a short half-life in cells, the withdrawal of methionine in the culture medium leads to a lowered intracellular SAM pool and a generalized inhibition of methyl transfer reactions. Beas-2B cells were pre-incubated with complete DMEM or methionine-deficient DMEM for 4 h, and cells were exposed to hypoxia for 24 h. Histones were extracted and subjected to Western blotting analysis with antibody directed against tri-methylated H3K4. In cells maintained in methionine-deficient medium, the basal level of H3K4me3 was decreased but hypoxia still elevated H3K4me3 compared to untreated cells (Fig. 2A). This result suggested that the removal of histone H3K4 methylation was inhibited by hypoxia.
Figure 2.
A, Beas-2B cells were seeded with DMEM complete medium. On the second day, cells cells were pre-incubated with complete DMEM or methionine-deficient DMEM for 4 h, and cells were exposed to hypoxia for 24 h. Histones were extracted and immunoblotted with anti-H3K4me3 antibody. The gel was stained with coomassie blue as a loading control. The results were repeated in another independent experiments; one representative blot is shown here. B, Hypoxia inhibited the activity of histone H3K4 demethylase in vitro. The histone H3K4 demethylation assay was performed as described in Materials and Methods. The reaction mixture was incubated on ice, or incubated at 37°C in nomoxia, hypoxia (1% oxygen) and in the presence of 1 mM DFX overnight. The same membrane was stripped and re-blotted with H3 antibody to verify the loading. Each condition was used in duplicate. The intensity of the bands was quantified, and values were normalized to the samples that were incubated on ice and were plotted in the graph. Error bars represent standard deviation.
To further study if hypoxia inhibits the H3K4 demethylase enzyme activity, an in vitro histone H3K4 demethylation assay was performed. Histones that contain H3K4me3 were incubated with nuclear extracts from Beas-2B cells at 37°C overnight with or without hypoxia, and were subjected to Western blotting using tri-methyl H3K4 antibody. As shown in Fig. 2B, incubation of histones with nuclear extract overnight at normoxia led to a decrease in tri-methyl H3K4 level at 37°C, compared to the reaction mixture incubated on ice which abolished the demethylase activity. However, the reaction was attenuated by hypoxia, as well as by addition of iron chelator deferoxamine (DFX). The result indicated that hypoxia inhibited H3K4 demethylase activity which likelycaused the increase of H3K4me3 in the living cells.
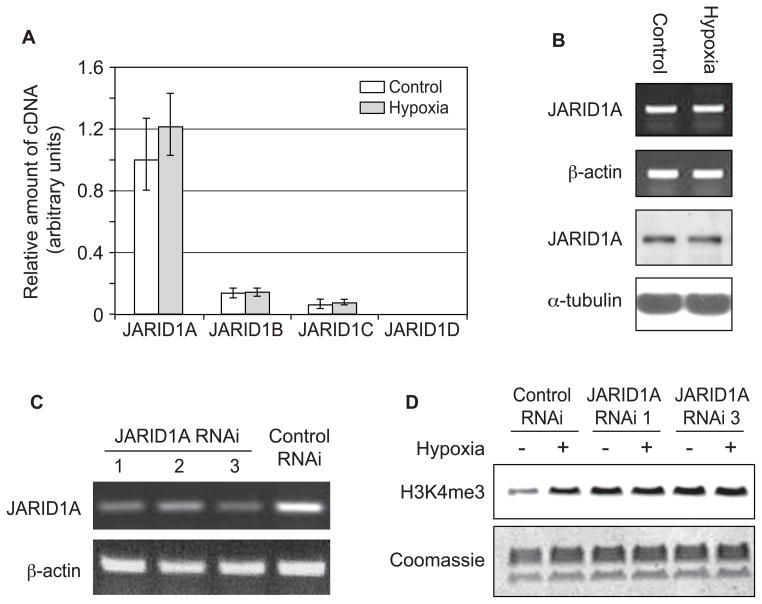
By examining the microarray data and the real-time RT-PCR results in wild-type Beas-2B cells, we identified that JARID1A is highly expressed in Beas-2B cells (Table 1 and Fig. 3A) (See Supplementary Table 1 and Supplementary Fig. 1 for A549 cells). To examine the role of JARID1A in hypoxia induced H3K4me3, JARID1A mRNA levels were measured in Beas-2B cells following hypoxia exposure. It was found that hypoxia did not cause any measurable changes of JARID1A mRNA level at 24 h (Fig. 3A and Fig. 3B upper panel). We also examined the effect of hypoxia on the level of JARID1A protein. Bease-2B cells were exposed to hypoxia for 24 h, and whole cell lysates were isolated and subjected to Western blotting analysis with antibody directed against JARID1A. As shown in Fig. 3B lower panel, hypoxia had no effect on JARID1A protein level at 24 h.
Table 1.
List of the expression levels (raw data) of JARID1A, JARID1B, JARID1C and JARID1D in the GeneChip in Beas-2B cells.
| Beas-2B | Probe | Raw |
|---|---|---|
| JARID1A | 202040_s_at | 236.6 |
| JARID1B | 201548_s_at | 203.8 |
| JARID1C | 202383_at | 122.3 |
| JARID1D | No hits | |
Figure 3.
A, Real-time RT-PCR analysis of the relative mRNA expression level of JARID1A, JARID1B, JARID1C and JARID1D in control (white) and 24 h hypoxia (grey) treated Beas-2B cells. The experiments were done in triplicate. Error bars represent standard deviation. B, Beas-2B cells were exposed to hypoxia. After 24 h, mRNA was extracted and whole cell lysates were collected. Semi-quantitative RT-PCR was then performed using JARID1A primers. The cDNA product was visualized in the 1% agarose gels using ethidium bromide staining. β-actin was used as a loading control. JARID1A protein level was analyzed by Western blotting with antibody against JARIDA. The same membrane was stripped and re-blotted with α-tubulin to assess the protein loading. The results were repeated in another independent experiment; one representative blot is shown here. C, JARID1A was knocked down in Beas-2B cells. 72h after JARID1A RNAi transfection, cells were collected and total mRNA was extracted. Semi-quantitative RT-PCR was then performed using JARID1A primers. The cDNA product was visualized in the 1% agarose gels using ethidium bromide staining. β-actin was used as a loading control. D, H3K4me3 was not increased by 24 h hypoxia exposure in JARID1A knockout cells. 48 h after JARID1A RNAi transfection, cells were treated hypoxia for 24 h. histones were extracted and immunoblotted with anti-H3K4me3 antibody. The gel was stained with coomassie blue as a loading control. The results were repeated in another independent experiments; one representative blot is shown here.
We further knocked down JARID1A in Beas-2B cells to see if H3K4me3 could still be increased by hypoxia to the same degree after knocking down of JARID1A. The knockdown efficiencies of different JARID1A RNAis were determined prior to this experiment. As is shown in Fig. 3C, JARID1A RNAi 1 and 3 have better knockdown efficiency efficiencies than 2. To avoid any possible off target effect, we used both JARID1A RNAi 1 and 3. Knocking down JARID1A using both RNAi oligos increased global H3K4me3, and hypoxia did not further increase H3K4me3 in these cells (Fig. 3D). These results indicated that hypoxia increased H3K4me3 by inhibiting the demethylating process, in particular, the JARID1A H3K4 demethylase.
Modulation of gene expression by JARID1A in Beas-2B cells
Since JARID1A is very important in hypoxia-induced H3K4me3, we next used the GeneChip microarray to study which genes were regulated by JARID1 A. Gene expression arrays were performed using Affymetrix GeneChip in wild type and JARID1A knockout out cell lines. GeneSpring 7.0 program (Silicon Genetics, Redwood City, CA) was used to filter gene expression level. Results were visualized using a Venndiagram, and known genes whose expression levels exhibit changes of at least 2-fold (P < 0.05) upon JARID1A knockdown were listed in Supplementary Table 2 and Supplementary Table 3. There were 91 genes up-regulated (Supplementary Table 2) and 291 genes down-regulated (Supplementary Table 3) genes when JARID1A was knocked down.
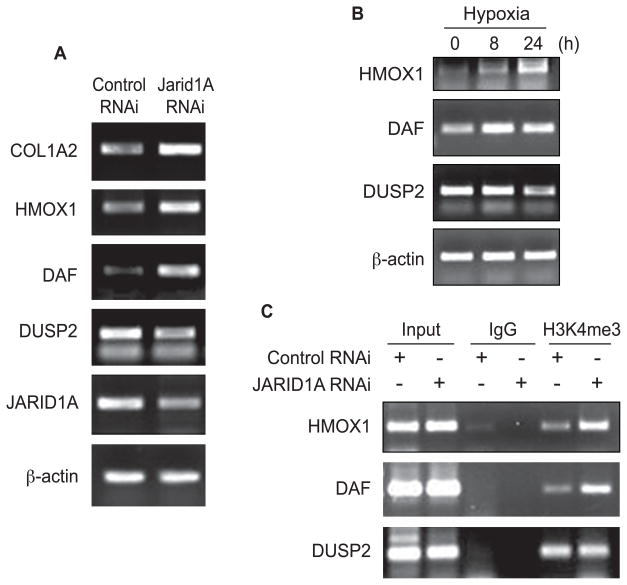
To validate the GeneChip data, the expression levels of selected differentially expressed genes were assessed by semi-quantitative RT-PCR (Fig. 4A). The genes include 3 up-regulated genes (COL1A2, HMOX1 and DAF) and 1 down-regulated gene (DUSP2). The results were consistent with the GeneChip findings.
Figure 4.
A, Semi-quantitative RT-PCR analysis of 4 differentially expressed genes in Beas-2B cells. After 72 h of JARID1A RNAi transfection, cells were collected and total mRNA was extracted. Semi-quantitative RT-PCR was then performed. The PCR amplified cDNA were separated by 1% agarose gel electrophoresis containing ethidium bromide. β-actin was used as a loading control. B, Semi-quantitative RT-PCR analyses of HMOX1, DAF and DUSP2 genes in Beas-2B cells. After 8 h or 24 h of hypoxia exposure, cells were collected and total mRNA was extracted. Semi-quantitative RT-PCR was then performed. The PCR amplified cDNA were separated by 1% agarose gel electrophoresis containing ethidium bromide. β-actin was used as a loading control. C, Knocking down of JARID1A induced enrichment of H3K4me3 in HMOX1 and DAF promoter in Beas-2B. After 72 h of JARID1A RNAi transfection, the ChIP assays were performed using tri-methyl H3K4 antibody. Normal rabbit IgG was used as negative control. The specific primers were used for the PCR amplification as indicated in the methods.
Pathway analysis of differential regulated genes in JARID1A knockdown Beas-2B cells
To gain further information into the biological functions of the JARID1A regulated genes, we classified the genes by utilizing three different pathway databases: KEGG, BIOCATRA and Func anot. The significant (p<0.05) KEGG pathway involved by the 91 commonly up-regulated genes is insulin signaling pathway, and among the 291 down-regulated genes, the significant (p<0.05) KEGG pathway involved are regulation of actin cytoskeleton, focal adhesion, p53 signaling pathway, melanoma and ECM-receptor interaction. In the BIOCARTA categories, signal transduction through IL1R, Fc Epsilon Receptor I signaling in mast cells, links between Pyk2 and Map Kinases, oxidative stress induced gene expression via Nrf2, angiotensin II mediated activation of JNK pathway via Pyk2 dependent signaling, role of MAL in Rho-mediated activation of SRF and keratinocyte differentiation are in 91 up-regulated genes, while only CXCR4 signaling pathway was found in down-regulated genes (p<0.05). The complete list and functional categories of the differentially expressed genes by JARID1A knockdown in Beas-2B cell line are in Supplementary Table 4.
Knocking down of JARID1A induces H3K4me3 at the HMOX1 and DAF promoters in Beas-2B cells
We next knocked down JARID1A to study whether the level of H3K4me3 at the promoters of specific genes was increased using chromatin immunoprecipitation (ChIP) assay. We analyzed two up-regulated genes, Heme oxygenase-1(HMOX1) and decay accelerating factor (DAF, or CD55); and one down-regulated gene, dual specificity phosphatase 2 (DUSP2) from the JARID1A knockdown GeneChip results. HMOX1 catalyzes the oxidative catabolism of heme to form biliverdin and CO, and it is proposed to protect cells or tissues from oxidative injury (26–29). DAF exists in most of the malignant tumors, and it functions as an inhibitor of the complement system (for review, see (30)). DUSP2 dephosphorylates ERK and p38, which positively regulates the inflammatory response(for review, see (31)). It should be noted that the mRNA of HMOX1 and DAF were increased by hypoxia, and the mRNA of DUSP2 was decreased by hypoxia (Fig. 4B). The lower bands are probably due to primer self-extension. As shown in Fig. 4C, knocking down of JARID1A increased the amount of H3K4me3 transcription activating mark at the HMOX1 and DAF promoters, but not H3K4me3 at the DUSP2 promoter.
Discussion
Several studies have indicated that hypoxia was able to alter epigenetic homeostasis and these changes may play a role in promoting tumorigenesis. It has been shown that hypoxia increased localized histone acetylation surrounding activated genes (32–34) Studies from our lab has demonstrated that di-methylated H3K9 was elevated following hypoxia exposure, and this effect was mediated by increase of G9a methyltransferase protein and enzyme activity, as well as by inhibiting histone H3K9 demethylases (23). In addition, di-methylated H3K9 at the promoter of the hypoxia repressed genes MLH1 and DHFR were found to be increased by hypoxia. A more recent study has investigated changes in histone methylation on HIF target genes when cells are challenged with hypoxia (32). In that study, the promoters of the hypoxia-induced genes VEGF and EGR1 and repressed genes AFP and ALB were investigated. When cells were exposed to hypoxia, there was an observed increase of H3K4me3 and decrease of H3K27 tri-methylation in all the promoters of genes, regardless of whether they were activated or repressed. However, the mechanisms and identity of the methylases and demethylases involved in this modification remains unclear. In the present study, we also found that exposure of Beas-2Bcells to hypoxia increased H3K4me3 at 6 h, 24 h and 48 h and exposure of A549 cells to hypoxia increased H3K4me3 at 6 h and 24 h. At 48 h, however, the H3K4me3 in the normoxic A549 cells was higher and hypoxia failed to increase H3K4me3 compared with control. We attributed this to the oxygen deprivation in the medium of untreated A549 cells at later time interval (48 h) which made the cells hypoxic and increased H3K4me3 due to the demethylase inhibition. In 1973, Goldblatt and collaborators (35) had observed that rat embryo cells grown for a prolonged period in vitro developed some degree of hypoxia due to insufficient diffusion of ambient oxygen into the stationary layer of medium and this hypoxia caused malignant transformation. A549, as a cancerous cell line, forms clusters at higher density while Beas-2B cells do not have this growth pattern (Supplementary Fig. 2). Therefore, A549 cells at later time interval likely developed pericellular hypoxia because of their growth pattern.
To study the mechanisms by which hypoxia increase H3K4me3, a transcriptional activating mark in chromatin, Beas-2B cells were pre-incubated in the methionine-deficient medium prior to exposure to hypoxia. Our data suggested that hypoxia increased H3K4me3 by inhibiting the demethylating activity rather than activating the methylating enzymes, as was evident from the finding that hypoxia still increased H3K4me3 when theintracellular methylation process was suppressed by the absenceof methionine. Further studies confirmed that hypoxia inhibited the enzymatic activity of H3K4 demethylases that demethylate tri-methyl H3K4 by in vitro histone H3K4 demethylation assay, therefore preventing the removal of the methyl group from H3K4me3 and increasing the tri-methylation levels of H3K4. As a major tri-methylated H3K4 demethylase, the role of JARID1A in hypoxia-induced H3K4me3 was investigated. Our results suggested that hypoxia did not affect JARID1AmRNA and protein level. These results are contradictory to the findings by Xia and co-workers (36). In their study, JARID1A mRNA was found to be up-regulated in HepG2 cells by hypoxia and in glioblastoma multiforme (36). The conflicting observations were likely due to the different hypoxic conditions and cell lines (or tumor tissue). In their study, HepG2 cells were exposed to more severe hypoxia (0.5%) for 4, 8, and 12 h and mRNA expression levels were determined by Affymetrix GeneChip. The mRNA levels were analyzed in glioblastoma following 0.5% hypoxia challenge. Our study was done by semi-quantitative RT-PCR in Beas-2B cells exposed to hypoxia (1%) for 24 h. Xia’s GeneChip results were not confirmed by RT-PCR. In addition to JARID1A, JARID1B and JARID1C are also expressed in Beas-2B cells. However, they may not function and bind to the promoters of the genes in Beas-2B under our experimental condition, although they could be inhibited by hypoxia. This is supported by our result that hypoxia did not further increase H3K4me3 in the Beas-2B cells after knock down of JARID1A. This might suggest that JARID1A is the predominant demethylase regulating global H3K4me3 level in the cell lines used in the present study. It’s likely that the ‘open’ chromatin structure created by increase of H3K4me3 at the promoters facilitates the recruitment of HIF to their target genes under hypoxic condition.
To identify the genes regulated by JARID1A, we used GeneChip microarray technique following the knocking down of JARID1A in Beas-2B cells. It was expected that knocking down of JARID1A should increase the expression of genes, since knocking down JARID1A inhibits the removal of methyl group from the transcription activating mark H3K4me3, resulting in a higher level of gene expression. However, more genes were down-regulated than up-regulated by JARID1A knockdown in our study. This may due to the indirect effect of JARID1A in regulating gene expression, as is confirmed by the ChIP assay that H3K4me3 at the promoter of DUSP2, the most down-regulated gene (7.5 fold) in the GeneChip assay, were not altered by when JARID1A was knocked down.(Fig. 4C).
JARID1A preferentially binds to DNA through CCGCCC motif (14). We identified several CCGCCC sequences in the promters of HMOX1 and DAF. Therefore, it’s likely that JARID1A binds directly to the promters of HMOX1 and DAF. However, we indentified several JARID1A binding sites at the promoter region of DUSP2 as well, but JARID1A knockdown had no effect on the H3K4me3 level at this promoter site. The reason could be that the JARID1A binding motif only has six bases and its short length may limit its specificity.
In conclusion, this study suggests that hypoxia exposure is able to induce global as well as gene specific H3K4me3, which plays an important role in altering gene expression during hypoxia. We propose that the increase of H3K4me3 induced by hypoxia may due to inhibition of only one of the H3K4 demethylases, JARID1A. Knocking down of JARID1A, which mimic the inactivation of JARID1A by hypoxia, induced H3K4me3 at the promoter of HMOX1 and DAF genes. Further studies will be directed to investigate which gene promoters are associated with altered H3K4me3 in the genome and the extent of change in the amount of this modification that is induced by hypoxia using ChIP-seq, and perform ChIP-seq with JARID1A antibody following similar treatment with hypoxia to correlate the changes in H3K4me3 with the location of the JARID1A.
Supplementary Material
Acknowledgments
This work was supported by grant numbers, ES014454, ES005512, ES000260 from the National Institutes of Environmental Health Sciences, and grant number CA16087 from the National Cancer Institute.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Kenneth NS, Rocha S. Regulation of gene expression by hypoxia. Biochem J. 2008;414:19–29. doi: 10.1042/BJ20081055. [DOI] [PubMed] [Google Scholar]
- 2.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–5. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 3.Peterson CL, Laniel MA. Histones and histone modifications. Curr Biol. 2004;14:R546–51. doi: 10.1016/j.cub.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol. 2005;6:838–49. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- 5.Mikkelsen TS, Ku M, Jaffe DB, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–60. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heintzman ND, Stuart RK, Hon G, et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet. 2007;39:311–8. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 7.Christensen J, Agger K, Cloos PA, et al. RBP2 belongs to a family of demethylases, specific for tri-and dimethylated lysine 4 on histone 3. Cell. 2007;128:1063–76. doi: 10.1016/j.cell.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Iwase S, Lan F, Bayliss P, et al. The X-linked mental retardation gene SMCX/JARID1C defines a family of histone H3 lysine 4 demethylases. Cell. 2007;128:1077–88. doi: 10.1016/j.cell.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Klose RJ, Yan Q, Tothova Z, et al. The retinoblastoma binding protein RBP2 is an H3K4 demethylase. Cell. 2007;128:889–900. doi: 10.1016/j.cell.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Seward DJ, Cubberley G, Kim S, et al. Demethylation of trimethylated histone H3 Lys4 in vivo by JARID1 JmjC proteins. Nat Struct Mol Biol. 2007;14:240–2. doi: 10.1038/nsmb1200. [DOI] [PubMed] [Google Scholar]
- 11.Kim TD, Shin S, Janknecht R. Repression of Smad3 activity by histone demethylase SMCX/JARID1C. Biochem Biophys Res Commun. 2008;366:563–7. doi: 10.1016/j.bbrc.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Pasini D, Hansen KH, Christensen J, Agger K, Cloos PA, Helin K. Coordinated regulation of transcriptional repression by the RBP2 H3K4 demethylase and Polycomb-Repressive Complex 2. Genes Dev. 2008;22:1345–55. doi: 10.1101/gad.470008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Defeo-Jones D, Huang PS, Jones RE, et al. Cloning of cDNAs for cellular proteins that bind to the retinoblastoma gene product. Nature. 1991;352:251–4. doi: 10.1038/352251a0. [DOI] [PubMed] [Google Scholar]
- 14.Tu S, Teng YC, Yuan C, et al. The ARID domain of the H3K4 demethylase RBP2 binds to a DNA CCGCCC motif. Nat Struct Mol Biol. 2008;15:419–21. doi: 10.1038/nsmb.1400. [DOI] [PubMed] [Google Scholar]
- 15.Benevolenskaya EV, Murray HL, Branton P, Young RA, Kaelin WG., Jr Binding of pRB to the PHD protein RBP2 promotes cellular differentiation. Mol Cell. 2005;18:623–35. doi: 10.1016/j.molcel.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Bigas N, Kisiel TA, Dewaal DC, et al. Genome-wide analysis of the H3K4 histone demethylase RBP2 reveals a transcriptional program controlling differentiation. Mol Cell. 2008;31:520–30. doi: 10.1016/j.molcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett A, Madsen B, Copier J, et al. PLU-1 nuclear protein, which is upregulated in breast cancer, shows restricted expression in normal human adult tissues: a new cancer/testis antigen? Int J Cancer. 2002;101:581–8. doi: 10.1002/ijc.10644. [DOI] [PubMed] [Google Scholar]
- 18.Jensen LR, Amende M, Gurok U, et al. Mutations in the JARID1C gene, which is involved in transcriptional regulation and chromatin remodeling, cause X-linked mental retardation. Am J Hum Genet. 2005;76:227–36. doi: 10.1086/427563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Ke Q, Kluz T, Yan Y, Costa M. Nickel ions increase histone H3 lysine 9 dimethylation and induce transgene silencing. Mol Cell Biol. 2006;26:3728–37. doi: 10.1128/MCB.26.10.3728-3737.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Sun H, Ellen TP, Chen H, Costa M. Arsenite alters global histone H3 methylation. Carcinogenesis. 2008;29:1831–6. doi: 10.1093/carcin/bgn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidson TL, Chen H, Di Toro DM, D’Angelo G, Costa M. Soluble nickel inhibits HIF-prolyl-hydroxylases creating persistent hypoxic signaling in A549 cells. Mol Carcinog. 2006;45:479–89. doi: 10.1002/mc.20176. [DOI] [PubMed] [Google Scholar]
- 22.Gothie E, Richard DE, Berra E, Pages G, Pouyssegur J. Identification of alternative spliced variants of human hypoxia-inducible factor-1alpha. J Biol Chem. 2000;275:6922–7. doi: 10.1074/jbc.275.10.6922. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Yan Y, Davidson TL, Shinkai Y, Costa M. Hypoxic stress induces dimethylated histone H3 lysine 9 through histone methyltransferase G9a in mammalian cells. Cancer Res. 2006;66:9009–16. doi: 10.1158/0008-5472.CAN-06-0101. [DOI] [PubMed] [Google Scholar]
- 24.Cangul H. Hypoxia upregulates the expression of the NDRG1 gene leading to its overexpression in various human cancers. BMC Genet. 2004;5:27. doi: 10.1186/1471-2156-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellen TP, Ke Q, Zhang P, Costa M. NDRG1, a growth and cancer related gene: regulation of gene expression and function in normal and disease states. Carcinogenesis. 2008;29:2–8. doi: 10.1093/carcin/bgm200. [DOI] [PubMed] [Google Scholar]
- 26.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61:748–55. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AM. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J Clin Invest. 1999;103:1047–54. doi: 10.1172/JCI5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A. 1997;94:10925–30. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vile GF, Basu-Modak S, Waltner C, Tyrrell RM. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci U S A. 1994;91:2607–10. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mikesch JH, Buerger H, Simon R, Brandt B. Decay-accelerating factor (CD55): a versatile acting molecule in human malignancies. Biochim Biophys Acta. 2006;1766:42–52. doi: 10.1016/j.bbcan.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008;27:253–61. doi: 10.1007/s10555-008-9123-1. [DOI] [PubMed] [Google Scholar]
- 32.Johnson AB, Denko N, Barton MC. Hypoxia induces a novel signature of chromatin modifications and global repression of transcription. Mutat Res. 2008;640:174–9. doi: 10.1016/j.mrfmmm.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang F, Zhang R, Beischlag TV, Muchardt C, Yaniv M, Hankinson O. Roles of Brahma and Brahma/SWI2-related gene 1 in hypoxic induction of the erythropoietin gene. J Biol Chem. 2004;279:46733–41. doi: 10.1074/jbc.M409002200. [DOI] [PubMed] [Google Scholar]
- 34.Jung JE, Lee HG, Cho IH, et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 2005;19:1296–8. doi: 10.1096/fj.04-3099fje. [DOI] [PubMed] [Google Scholar]
- 35.Goldblatt H, Friedman L, Cechner RL. On the malignant transformation of cells during prolonged culture under hypoxic conditions in vitro. Biochem Med. 1973;7:241–52. doi: 10.1016/0006-2944(73)90079-3. [DOI] [PubMed] [Google Scholar]
- 36.Xia X, Lemieux ME, Li W, et al. Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Proc Natl Acad Sci U S A. 2009;106:4260–5. doi: 10.1073/pnas.0810067106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.