Abstract
Objective
To examine changes in utilization and expenditures for infliximab in RA patients associated with the two changes implemented by the Medicare Modernization Act (MMA) of 2003, namely (1) reductions in physician reimbursement for Part B drugs between 2003 and 2005 and (2) availability of alternative RA biologicals in 2006.
Methods
Using 2002 to 2006 5% Medicare files, nationally representative estimates of infliximab use and expenditures were estimated in annual cross-sectional samples of RA beneficiaries. Infliximab initiation and continuation rates were estimated in two-year longitudinal cohorts (2005–2006 vs. 2002–2003, 2003–2004, and 2004–2005).
Results
Total payments (in 2006 dollars) for infliximab increased from $357 million in 2002 to $492 million in 2006. The largest annual increase in infliximab payments occurred in the pre-MMA period from 2002 to 2003, wherein payments per RA patient increased by 31%. From 2003 to 2004, despite the reduction in payments brought by MMA, there was a 4% increase in total expenditures for infliximab per RA patient driven by an increase in utilization factors. Total payments for infliximab per RA patient actually decreased from 2004 to 2005, when reimbursement was further reduced. Continuation and initiation rates for infliximab use remained unchanged in 2006 as compared to previous years.
Conclusion
Infliximab expenditures increased from the 2002 to 2006, yet the passage of the MMA was associated with a remarkable slow down in the rate of increase in expenditures. There was no evidence of significant substitution of infliximab with other biologics made available in 2006.
Keywords: Rheumatoid arthritis, infliximab, Medicare, Medicare Modernization Act
The Medicare Prescription Drug Improvement and Modernization Act (MMA) of 2003 not only established an outpatient prescription benefit or Part D program but significantly changed the reimbursement of physician dispensed outpatient drugs already covered under Part B. Before 2004, Medicare reimbursed physicians for Part B drugs at the lesser of the billed charge or 95 percent of the average wholesale price (AWP) of the drug. However, several government investigations revealed that physicians purchased these drugs at prices significantly lower than the AWP.1–4
In order to address this overpayment issue, the MMA established a new methodology for Medicare Part B reimbursement of most covered drugs.5 Starting in January 1, 2004, reimbursement for most Part B drugs was reduced from 95% of their AWP to 85% of their AWP (Table 1). Beginning in January 1, 2005, reimbursement was further reduced to 106% of the average sale price (ASP) or wholesale acquisition cost (WAC), whichever is lower. To partially offset these reductions, the MMA increased physician reimbursement for Part B drug administration service.6 Yet, the mark-up on Part B drugs that physicians could earn prior to 2004 has significantly been reduced over time.
Table 1.
Changes Introduced by the Medicare Modernization Act (MMA) of 2003
| Date | Period | Medicare Part B drug payment system |
Medicare Part D |
RA Biological covered by Medicare (Part B or D) |
|---|---|---|---|---|
| January 1, 2002 | Pre-MMA | 95% of AWP | No | Infliximab (Part B) |
| January 1, 2003 | Pre-MMA | 95% of AWP | No | Infliximab (Part B) |
| January 1, 2004 | Post-MMA | 85% of AWP | No | Infliximab (Part B) |
| January 1, 2005 | Post-MMA | 106% of ASP/WAC | No | Infliximab (Part B) |
| January 1, 2006 | Post-MMA | 106% of ASP/WAC or physicians procure drugs from contractor* |
Yes | Infliximab, rituximab, and abatacept (Part B) Etanercept, adalimumab, and anakinra (Part D) |
Note: AWP – Average Wholsesale Price, ASP – Average Sales Price, WAC – Wholesale Acquisition Cost
Starting January 1, 2006, physicians had the option to continue their own drug purchasing, with the 106% ASP/WAC reimbursement in place for 2005, or obtain Part B drugs from a contractor in their area selected under a new competitive acquisition program (CAP). If physicians chose the latter option, they will no longer seek reimbursement for Part B drugs from CMS. These functions were now assumed by the contractors, along with any associated profit or loss. Physician participation in the CAP program was extremely low and this program was recently discontinued by CMS.
These new reimbursement methods directly reduced physician payments for infliximab, the only Medicare covered biological agent for the management of rheumatoid arthritis (RA) available prior to the MMA. This tumor necrosis factor alpha biologic agent covered under Medicare Part B soon after its introduction on the market in late 1999 was the predominant RA biological used in the Medicare population and ranked 8th in terms of percent share of Medicare Part B drug expenditures by 2002.7
In addition to directly reducing infliximab spending, it is likely that lower Medicare reimbursement rates might have made some rheumatologists reluctant to see Medicare patients or administer infliximab less often in their clinic, thus further reducing total infliximab payments. On the other hand, previous literature on other types of services suggests that as Medicare payment rates decline the treatment rates increase (i.e. there may be physician induced demand to compensate for the physician’s loss of income due to the reduction in reimbursement rates).8–18 Hence, it is difficult to predict how these Part B drug reimbursement changes affected infliximab use and spending.
Furthermore, the new Medicare Part D program implemented by the MMA on January 1, 2006 started covering self-administered RA biologic agents, which were not covered under Medicare Part B since they do not require administration by or under supervision of physicians (Table 1). In addition, two newly FDA approved physician-administered infusible biologics, abatacept and rituximab, became competing alternatives for infliximab under Part B in 2006. It is unknown whether availability of alternative choices of otherwise expensive biologics further influenced infliximab use and spending.
The objective of this study was to examine the changes in utilization and expenditures for infliximab in RA patients associated with (a) the reductions in physician reimbursement for Part B drugs between 2003 and 2005 and (b) the availability of alternative RA biologicals in 2006.
MATERIALS AND METHODS
Data sources
The study used data from the 2002 to 2006 5% Medicare standard analytical files (SAF) which include Medicare claims and enrollment data for a 5% random sample of the Medicare population. For the purpose of this study we used the 5% Medicare denominator files, inpatient, outpatient, and carrier SAFs.19 The denominator files contain beneficiary information such as age, gender, race, date of death, and monthly entitlement (Part A/B/both) and participation in Medicare managed care. The inpatient files contain claims submitted by inpatient hospital providers and the outpatient files contain claims submitted by institutional outpatient providers (e.g., hospital outpatient departments). The carrier files contain claims data submitted by non-institutional providers for services provided under Medicare Part B, and are used to identify physician-administered Part B biologicals.
Study Sample
The study sampling frame consisted of all Medicare beneficiaries who had full year fee-for-service Part A and B coverage and were alive during the entire year. Our primary sample included annual cross-sections of beneficiaries from 2002 to 2006 with ≥ 1 inpatient claim or ≥ 2 outpatient or carrier claims with an RA diagnosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] 714.xx) in the year.
We also created two-year longitudinal cohorts of RA patients to further address the aim of examining changes in infliximab use associated with the availability of alternative RA biologicals in 2006. Four sets of two-year cohorts of patients identified with RA (using the same diagnostic criteria outlined above) in the base year but having at least one full calendar year of follow-up data were created (i.e. 2002–03, 2003–04, 2004–05, 2005–06). Each two-year cohort was further divided into two subsets; the first included RA patients who were infliximab users in the base year (to examine continuation of infliximab in the follow-up year) and the second included non-users of infliximab in the base year (to examine initiation of infliximab in the follow-up year).
Infliximab Use and Expenditures
Infliximab use was identified using a Health Care Procedure Classification Code (HCPCS) of J1745 in the carrier claim. Infliximab is billed in terms of number of units of infliximab administered where one unit represents infliximab 10 mg. Infliximab administration services were identified by using the Current Procedural Terminology (CPT) codes (90780 and 90781 in 2002–2004, G0359 and G0360 in 2005, 96413 and 96415 in 2006).corresponding to intravenous infusions within infliximab-related claims in each year
Our main outcome variable was infliximab expenditures measured as the total Medicare allowed payment due to the provider. It was comprised of the sum of the payment made by Medicare, the payment responsibility of the beneficiary or other secondary payer (20% coinsurance plus Part B deductible amount, if any), and the payment made by the primary payer, if any. We created two estimates of total infliximab expenditures, one including only the infliximab drug payments and the second including drug payments for infliximab and its associated administration fees. The expenditures were adjusted for inflation using the prescription drugs component of the Consumer Price Index (CPI). In order to understand the relative contribution of factors influencing the changes in infliximab expenditures, we separated out the total infliximab payments in each year into two categories: price and utilization (i.e. volume) factors.
The price factors examined were allowed payment per unit of infliximab or alternatively the allowed payment per unit plus the administration fees per unit of infliximab in each year. The price factors were also converted into 2006 dollars using the same adjustment used for total expenditures.
The utilization factors included the number of infliximab users (i.e. number of RA patients times the percentage of infliximab users), the number of infliximab claims per user, and the number of units per infliximab claim in each year. The multiplication of all utilization factors results in the total infliximab utilization i.e. the total number of units of infliximab administered in each year to RA patients. The total infliximab utilization when multiplied by the allowed payment per unit of infliximab results in the total infliximab expenditures. Since the number of RA patients increased across years we also computed total infliximab utilization and expenditures per RA patient.
Alternative RA Biologics and Potential Impact on Infliximab Use
Since Medicare Part D data were not yet available for research we were unable to directly examine the utilization of the three self-administered RA biologics available through Part D and their impact on infliximab use in 2006. However, we indirectly tested the impact of the availability of these alternative RA biologics via a longitudinal analysis of two-year cohorts. The outcomes examined in the subset of RA patients who were users of infliximab in the base year included infliximab continuation in the follow-up year and the number of infliximab claims among continuers. The outcomes examined in the subset of RA patients who were non-users of infliximab in the base year included infliximab initiation in the follow-up year and the number of infliximab claims among initiators. We hypothesized that if coverage of the subcutaneous RA biologics under Part D resulted in switching of previous infliximab users to these agents or initiation of new biologic users directly on the Part D covered biologics rather than infliximab, then the rates of continuation and initiation on infliximab would be lower in 2006 compared to previous years. Furthermore, if infliximab continuers or initiators in 2006 switched to other alternatives sometime during the year 2006 then the number of infliximab claims per patient would be lower in 2006 compared to previous years.
The two newly approved physician-administered infusible biologics (abatacept and rituximab) were directly examined using 2006 carrier claims (Appendix 2). The percentage of infliximab users switching to these competing Part B alternatives was calculated using the 2005–06 longitudinal cohort.
Analysis
Nationally representative estimates of infliximab expenditures in each year from 2002 to 2006 were obtained by multiplying the estimates from our 5% files by a factor of 20. We tracked absolute and relative changes in total infliximab expenditures and each price and utilization factor across two annual cross-sections corresponding to each policy change, namely (1) 2003–2004 (i.e. 95% AWP to 85% AWP), (2) 2004–2005 (i.e. 85% AWP to 106% ASP), and (3) 2005–2006 (i.e. 106% ASP to 106% ASP + Part D). The changes across the 2002–2003 period served as a reference trend prior to the introduction of any MMA changes. Multivariate regressions were used to examine the adjusted changes in the mean infliximab expenditures and the utilization factors across each policy change. In all our models, the main independent variables of interest were binary indicators of the annual year of observation. Model covariates included patient sociodemographic characteristics and clinical severity measures (listed in Table 2). Using the two-year longitudinal cohorts, we compared the infliximab continuation and initiation rates and number of infliximab claims among continuers and initiators, respectively in pre-Part D years with similar estimates from 2006.
Table 2.
Characteristics of Medicare Beneficiaries with Rheumatoid Arthritis from 2002 to 2006
| Year | |||||
|---|---|---|---|---|---|
| Characteristics | 2002 | 2003 | 2004 | 2005 | 2006 |
| N (unweighted) | 29,033 | 30,829 | 31,884 | 32,699 | 32,294 |
| Age (years) | |||||
| ≤ 64(%) | 17.0 | 17.6 | 18.6* | 19.5* | 19.3* |
| 65–74 (%) | 38.0 | 37.7 | 38.0* | 37.7* | 37.4* |
| 75– 84 (%) | 35.4 | 34.7 | 33.6* | 33.2* | 33.0* |
| ≥ 85 (%) | 9.6 | 9.9 | 9.8* | 9.6* | 10.3* |
| Male (%) | 26.3 | 26.4 | 26.3 | 26.2 | 25.9 |
| Race | |||||
| White (%) | 84.7 | 84.2* | 83.8 | 83.3* | 83.7 |
| Black (%) | 10.0 | 10.0* | 10.4 | 10.4* | 10.1 |
| Hispanic (%) | 2.5 | 2.6* | 2.6 | 3.1* | 3.1 |
| Other race (%) | 2.8 | 3.2* | 3.1 | 3.2* | 3.1 |
| Census region | |||||
| Northeast (%) | 20.1 | 19.5 | 18.8* | 19.1 | 20.1* |
| Midwest (%) | 23.4 | 23.3 | 22.9* | 22.6 | 23.0* |
| South (%) | 40.3 | 40.5 | 41.0* | 40.7 | 40.7 |
| West (%) | 16.1 | 16.7 | 17.2* | 17.6 | 16.3* |
| RxHCC Risk Score | 1.4 | 1.5* | 1.5* | 1.5* | 1.5* |
|
Any inpatient claim with RA diagnosis (%) |
19.8 | 19.8 | 19.8 | 18.9* | 18.7 |
|
Number of evaluation and management visits with RA as principal diagnosis |
2.6 | 2.5 | 2.4* | 2.3* | 2.3 |
Note: RA=rheumatoid arthritis, RxHCC= Prescription drug hierarchical condition category
Significantly different from estimate in previous column (year) at p<0.05.
Results
The number of beneficiaries with RA ranged from 0.58 million in 2002 to 0.65 million in 2006, representing 1.9% to 2.2% of the total number of fee-for-service Medicare beneficiaries with full year Part A and B coverage, respectively. Beneficiaries with RA were predominantly female, white, and aged 65–74 years old. Most beneficiary characteristics were quite similar across the five year period, although small differences across consecutive years are statistically significant given the large sample sizes (Table 2).
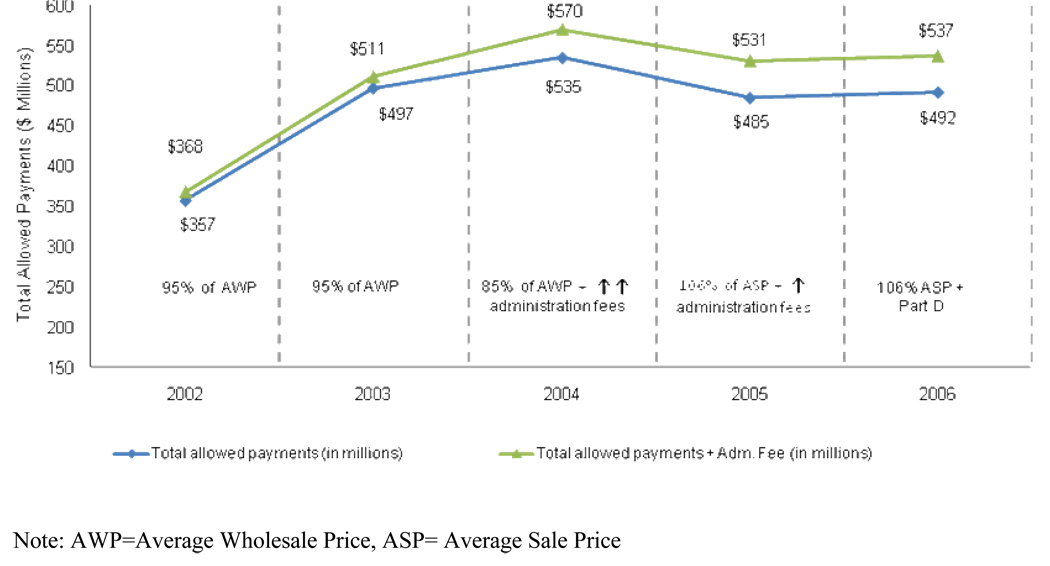
Figure 1 displays the total infliximab expenditures (in 2006 dollars) with and without administration fees from 2002 to 2006. The total payments for only infliximab increased from $357 million in 2002 to $492 million in 2006. Medicare payments for infliximab administration fees also increased considerably from $11 million in 2002 to $45 million in 2006. The largest annual increase in infliximab payments occurred in the pre-MMA period from 2002 to 2003, wherein payments increased by 39%. From 2003 to 2004, despite the MMA reduction in Part B drug reimbursements from 95% AWP to 85% AWP, there was an increase in total expenditures for infliximab; however, the rate of increase was significantly lower relative to the pre-MMA period. Total payments for infliximab actually decreased from 2004 to 2005, when reimbursement was further reduced with the transition from the AWP to ASP payment system. Infliximab expenditures increased slightly from 2005 to 2006.
Figure 1.
Total Payments (in 2006 dollars) for Infliximab with and without Administration Fees Included in Medicare Beneficiaries with Rheumatoid Arthritis
Table 3 tracks the annual-level individual utilization and price factors influencing the changes in total infliximab expenditures observed in Figure 1. Quarterly-level utilization and price factors are presented in Appendix 2. As seen from the annual estimates in the first panel of the table all utilization factors generally increased or remained stable between 2002 and 2006. The second panel of the table highlights the relative changes in the price and utilization factors during each policy change introduced by the MMA. In the period prior to the introduction of the MMA changes (i.e. 2002 to 2003), all utilization factors increased substantially whereas the price factors decreased marginally. While there was a relative increase of 6.2% in the number of RA patients, the largest increases occurred in the percentage of infliximab users (16.7%) and the mean number of units per infliximab claim (9.5%). As a result the total number of units of infliximab administered per RA patient increased by 36% from 2002 to 2003 and largely explained the 31% increase in total infliximab payments per RA patient in the pre-MMA period.
Table 3.
Utilization and Price Factors Influencing Changes in Total Infliximab Expenditures
| Individual Utilization Factors | Price Factors (in 2006 dollars)* |
Total Payments (in 2006 dollars)* |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of RA Patients |
Percent infliximab users |
Number of infliximab claims per user |
Number of units per infliximab claim |
Total number of units |
Number of units per RA patient |
Allowed payment per unit |
Allowed payment per unit + Adm. fee per unit |
Total allowed payments |
Total allowed payments + Adm. Fee |
Total allowed payments per RA patient |
Total allowed payments + Adm. fee per RA patient |
|
| Annual Estimates |
||||||||||||
| 2002 | 0.58 M | 4.8% | 5.1 | 33.8 | 4,773,680 | 8.2 | $74.86 | $77.03 | $357 M | $368 M | $615 | $633 |
| 2003 | 0.62 M | 5.6% | 5.4 | 37.0 | 6,897,400 | 11.2 | $72.09 | $74.10 | $497 M | $511 M | $806 | $829 |
| 2004 | 0.64 M | 6.1% | 5.7 | 38.3 | 8,505,160 | 13.3 | $62.89 | $67.05 | $535 M | $570 M | $839 | $894 |
| 2005 | 0.65 M | 6.0% | 5.7 | 39.3 | 8,758,940 | 13.4 | $55.39 | $60.57 | $485 M | $531 M | $742 | $811 |
| 2006 | 0.65 M | 6.1% | 5.8 | 40.1 | 9,193,140 | 14.2 | $53.53 | $58.36 | $492 M | $537 M | $762 | $831 |
| Relative changes |
||||||||||||
| 2002 to 2003 | 6.20% | 16.7% | 4.9% | 9.5% | 44.5% | 36.1% | −3.7% | −3.8% | 39.2% | 38.9% | 31.0% | 30.9% |
| 2003 to 2004 | 3.40% | 8.9% | 6.2% | 3.6% | 23.3% | 19.2% | −12.8% | −9.5% | 7.6% | 11.5% | 4.0% | 7.9% |
| 2004 to 2005 | 2.60% | −1.6% | 0.1% | 2.7% | 3.0% | 0.4% | −11.9% | −9.7% | −9.3% | −6.8% | −11.6% | −9.3% |
| 2005 to 2006 | −1.20% | 1.7% | 1.3% | 2.1% | 5.0% | 6.3% | −3.4% | −3.6% | 1.4% | 1.1% | 2.7% | 2.4% |
As expected the first Part B drug reimbursement reduction (95% to 85% of AWP), was directly associated with an observed reduction of 12.8% in the mean allowed payment per unit of infliximab from 2003 to 2004. Due to concurrent increases in administration fees, the net reduction after including administration fees was 9.5%. However, this reduction in price factors was accompanied by a 19% increase in total infliximab utilization per RA patient. As a result, the total allowed payments for infliximab per RA patient increased by 4% (or 7.9% after including administration fees) between 2003 and 2004.
When Part B drug reimbursement transitioned from the AWP to ASP payment system in 2005, the mean allowed payment per unit of infliximab further decreased by 11.9% (or 9.7% after accounting for administration fee increases). This additional decrease in price factors was accompanied by a negligible increase in total infliximab utilization per RA patient and hence, the total allowed payments for infliximab per RA patient dropped by 11.6% (or 9.3% after including administration fees) between 2004 and 2005.
In 2006, the second year after the change to the ASP-based reimbursement system for Part B drugs, the price factors for infliximab declined marginally by about 3%. On the other hand, total infliximab utilization per RA patient increased slightly (6%). As a result total allowed payments for infliximab per RA patient increased only marginally by 2.7% (or 2.4% after including administration fees) between 2005 and 2006.
Multivariate analysis confirmed that all utilization factors significantly increased in 2004 relative to 2003 and only the number of units per infliximab claim significantly increased in 2005 and 2006 relative to 2004 and 2005, respectively.
Table 4 presents results from our longitudinal analysis of two year cohorts to indirectly test the impact of the introduction of Part D in 2006. We find that 82.3% of infliximab users in 2005 continued use of infliximab in 2006 and had on average 6.2 infliximab claims in the year. In addition, 1.1% of non-users of infliximab in 2005 initiated its use in 2006 and had on average 4.5 infliximab claims in the year. Similar continuation and initiation rates and levels of infliximab use were observed in the years prior to the introduction of Part D. Multivariate analyses confirmed that there were no significant differences in the rates of use or the number of claims per user across the four longitudinal cohorts.
Table 4.
Continuation and Initiation Rates of Infliximab Use in 2006 Relative to Prior Years
| Year | Infliximab users in base year (N) |
Continuation rate in follow-up year |
Claims per user among continuers in follow-up year |
Non-users of infliximab in base year (N) |
Initiation rate in follow- up year |
Claims per user among new user in follow-up year |
|---|---|---|---|---|---|---|
| 2002 to 2003 | 26,260 | 79.8% | 6.0 | 510,920 | 1.8% | 4.4 |
| 2003 to 2004 | 33,040 | 79.6% | 6.1 | 536,680 | 1.4% | 4.8 |
| 2004 to 2005 | 36,460 | 79.7% | 6.1 | 542,320 | 1.1% | 4.4 |
| 2005 to 2006 | 35,840 | 82.3% | 6.2 | 544,080 | 1.1% | 4.5 |
Note: At 5% level, there were no significant differences in the rates of use or the number of claims per user across the four longitudinal cohorts. Analysis of the 2005–06 longitudinal cohort indicated that only 3% and 2% of infliximab users in 2005 switched to abatacept and rituximab in 2006, respectively (Appendix 1).
Discussion
This is the first study to present national estimates on infliximab use and expenditures in Medicare patients with RA before and after the enactment of the MMA in December 2003. Total infliximab payments to physicians increased 1.4 times in the pre-MMA period between 2002 to 2003. Overall the changes introduced by the MMA were associated with a remarkable slow down in the rate of increase in infliximab expenditures, which reached approximately half a billion dollars in 2006.
Given that the MMA introduced policy changes at the national level for the entire Medicare program, we lack a contemporaneous control group of Medicare beneficiaries that had not been subject to these policy changes. Hence, it is plausible that our observed results are influenced by other external unmeasured factors and concurrent trends. For instance, part of the observed changes in infliximab utilization may be explained by the usual trajectory of uptake after a new drug has been brought to market. To test the robustness of our results we aggregated our analysis on a quarterly level (Appendix 2). We found that major changes in total spending, price, and utilization coincided with the first quarter of the calendar year. The finding that the timing of the changes in infliximab expenditure patterns is closely associated with the timing of the changes introduced by the MMA strengthens the validity of our results. Another concern may be that our findings on changes in infliximab spending are an artifact of the underlying changes in the study sample. However, we found that the composition of our annual cross-sections of RA beneficiaries and infliximab users was stable between 2002 and 2006. We were unable to control for direct measures of severity of RA symptoms as these data are unavailable in claims. Nevertheless, it is unlikely that sudden changes in RA severity could have occurred over our one-year periods of annual level policy changes. Finally, there were no major changes in the clinical guidelines for the treatment of RA during this time period that may explain the observed changes in utilization that we observed.
The observed changes in infliximab use and expenditures associated with each of the annual changes introduced by the MMA were varied in their direction and magnitude. First, despite the initial reduction in Part B drug payment rates total infliximab payments per RA patient relatively increased by 4% to 8% between 2003 and 2004. This inverse relationship was largely driven by an accompanying significant increase in utilization factors. There may be several explanations for this finding. One potential explanation may be the physician induced demand or the target income hypothesis reported in the literature8–18 particularly given that payments for Part B drugs, mainly infliximab, consisted of 50 percent of the total payments for all Medicare services provided by rheumatologists in 2003.12 This result may also partly be explained by the concomitant large increases in administration fees for infliximab introduced by the MMA that partially offset the Part B drug payment reductions and hence may have encouraged rheumatologists, particularly those with practice-based infusion centers to continue or increase administration of infliximab. Alternatively, one might interpret these results to suggest that the Part B reimbursement reductions considerably slowed down the growth in infliximab utilization which otherwise could have potentially been much higher in the absence of the MMA (relative increase of 19% between 2003 and 2004 vs. 36% between 2002 and 2003).
Second, an additional reduction in physician reimbursement with the transition from the 85% AWP in 2004 to 106% ASP system in 2005 was associated with a more intuitive 9% to 12% reduction in total infliximab payments per RA patient. It is possible that a cumulative decline of approximately 23% in Part B payments per unit of infliximab (18% after accounting for the increases in administration fees) over two consecutive years had a larger negative impact on physician incentives to administer infliximab in 2005. Total number of infliximab units administered did not increase over this period. In general, these findings are consistent with a recent MedPAC report to the Congress on the effects of Part B drug payment changes under the MMA.12
Third, the implementation of Part D in 2006 was accompanied by no change in continuation and initiation rates for infliximab in this year as compared to previous years. In contrast to our expectation, substitution of infliximab with Part B alternatives (abatacept and rituximab) was low (Appendix 1) and with Part D alternatives (etarnecept, adalimumab, and anakinra) “appeared” low. This apparent lack of substitution has important implications given that infliximab has been shown unlikely to be cost-effective for the management of RA in the Medicare population compared with either etanercept or adalimumab.20 On the other hand, comparative effectiveness reviews have reported similar effectiveness for infliximab, etanercept, and adalimumab.21, 22
The reasons for the apparently “low” levels of substitution of infliximab with Part D alternatives may be multifactorial. Physicians may have patient’s financial interest in mind given that many patients were likely to enter the coverage gap (i.e. “doughnut hole”) under Part D upon using the self-injectable biologics. The infusible biologics under Part B have no such gaps in coverage and only require a 20 percent coinsurance after the initial Part B deductible is met. Nevertheless, once a patient reaches the catastrophic limit under Part D its coverage is more comprehensive than Part B since patients are only responsible for a 5 percent coinsurance for the rest of the calendar year. In addition to out-of-pocket costs, patient treatment preference may be influenced by issues such as route and frequency of administrations. For instance, patients may tend to prefer infusion biologics like infliximab given the clinical assistance received in terms of administration by a physician or nurse as opposed to the subcutaneous biologics covered under Part D that need to be self-injected and more so often than infliximab.23, 24 It is also likely that while profit margins have been reduced on infliximab due to the reimbursement changes under the MMA, physicians still have a personal financial interest to prescribe such infusion drugs as opposed to the self-injectable RA biologics wherein they receive no reimbursements at all.25
Finally, it is possible that it might be too early to assess the full impact of the implementation of Part D. Hence, it will be imperative for additional studies to examine changes in utilization and expenditures of infliximab and other RA biologicals under Part B in future years.
Our study has several limitations that need acknowledgement. As we lack a contemporaneous control group we cannot attribute a causal relationship between the policy changes introduced by the MMA and the observed changes in infliximab utilization and spending. Also currently there is no literature on the appropriateness of current infliximab usage levels and prescribing patterns. Hence, we can only comment on reductions in the growth of use of infliximab that occurred over this time period but not whether this is desirable or not from a health and quality of life perspective. Another limitation of the study is its reliance on diagnosis codes from claims data to identify RA patients. The use of a single ICD-9-CM code of 714.xx has been reported to have high sensitivity (100%) in identifying RA patients but low specificity (55%) in veterans administration databases.26 To avoid false positives our study required a more stringent definition of ≥ 1 inpatient claim or ≥ 2 outpatient or carrier claims with ICD-9-CM 714.xx. Finally, given the unavailability of Part D claims data for research at the time of this study we were unable to conduct a detailed assessment of the impact of the Part D implementation on use and spending for infliximab.
In summary, we find that total infliximab payments to physicians increased from the 2002 to 2006, yet the passage of the MMA in 2003 was associated with a remarkable slow down in the rate of increase in spending. Evidence suggests that there was no significant substitution of infliximab with self-injectable biologics made available under Part D. As data becomes available in the future, ongoing research is needed to monitor the effects of these major policy changes under Medicare on the use and spending of biologic therapies for the management of rheumatoid arthritis in the Medicare population.
Supplementary Material
Acknowledgments
Funding Source: This study was funded by the Leonard Davis Institute of Health Economics and the National Institute of Health NIH/NIA (1-R01-AG024451-01)
Appendix 1
Alternative Part B Covered RA Biologics and Impact on Infliximab Use
We also captured utilization and expenditures for abatacept and rituximab which became competing alternatives for infliximab under Part B in 2006. Abatacept did not have a HCPCS code assigned in 2006, its first year on market, and was grouped by CMS under a miscellaneous code of J3590 for unclassified biologics. Hence, we report a range of estimates for abatacept use and expenditures based on three different definitions applied to claims with a HCPCS code of J3590 identified in our sample of RA patients in 2006: (1) all such claims; (2) subset of claims with an ICD-C-9-CM code for RA; and (3) subset of claims that were filed by rheumatologists. While rituximab had an assigned HCPCS code of J9310 due to its availability on the market prior to 2006 for the indication of Non-Hodgkins lymphoma, we still used the same three definitions above in order to capture rituximab used only for the indication of RA. We examined the percent use and spending on these two new Part B biologics introduced in 2006. To examine the impact of availability of these alternative Part B biologics on infliximab use, we used the two-year longitudinal cohort samples to examine switching from infliximab to abatacept or rituximab.
Table A1.
Abatacept and Rituximab Use and Expenditures in Medicare Beneficiaries with Rheumatoid Arthritis in 2006
| Abatacept | Rituximab | |||
|---|---|---|---|---|
| Identification algorithm | Percent users |
Total allowed payments |
Percent users |
Total allowed payments |
| (1) All claims with relevant HCPCS code* | 0.61% | $22.6 M | 0.46% | $38.5 M |
| (2) Subset of claims in (1) with associated RA diagnosis | 0.49% | $19.8 M | 0.28% | $16.3 M |
| (3) Subset of claims in (1) filed by rheumatologists | 0.43% | $16.8 M | 0.16% | $9.8 M |
HCPCS code J3590 for abatacept and J9310 for rituximab
Table A1 presents the use and expenditures associated with the two new Part B biologic alternatives to infliximab that became available in 2006. Regardless of which identification algorithm was applied, the rate of use of abatacept was quite low (0.43% to 0.61%) in our annual cross-section of RA patients in 2006. Rituximab use was even lower (0.16% to 0.46%), albeit total expenditures were relatively high since it is more expensive than abatacept [$9.8 M to $38.5M for rituximab and $16.8 M to $22.6 M for abatacept]. Moreover, a longitudinal analysis of the 2005–2006 cohort of infliximab users indicated a low rate of switching from infliximab to these new Part B biologicals. Using the least conservative identification algorithm, only 3% and 2% of infliximab users in 2005 switched to abatacept and rituximab in 2006, respectively.
Appendix 2
Results by Quarter from 2002 to 2006
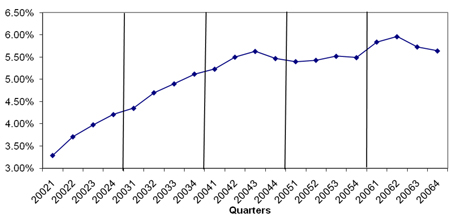 Figure A1 Percentage of Infliximab Users by Quarter from 2002 to 2006
Figure A1 Percentage of Infliximab Users by Quarter from 2002 to 2006
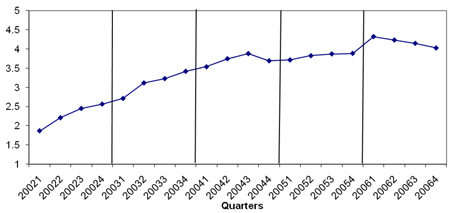 Figure A2 Total Number of Infliximab Units per RA patient by Quarter from 2002 to 2006
Figure A2 Total Number of Infliximab Units per RA patient by Quarter from 2002 to 2006
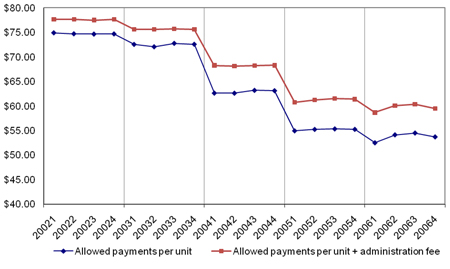 Figure A3 Allowed payments per unit of Infliximab (with and without administration fee) by Quarter from 2002 to 2006
Figure A3 Allowed payments per unit of Infliximab (with and without administration fee) by Quarter from 2002 to 2006
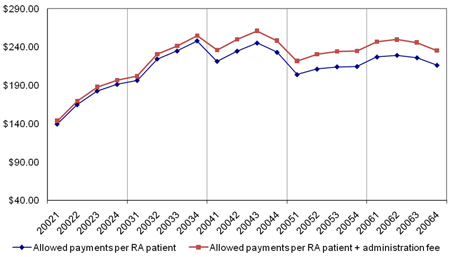 Figure A4 Allowed payments per RA patient (with and without administration fee) by Quarter from 2002 to 2006
Figure A4 Allowed payments per RA patient (with and without administration fee) by Quarter from 2002 to 2006
REFERENCES
- 1.Office of Inspector General, Department of Health and Human Services. Washington, DC: OIG; 1996. May, Appropriateness of medicare prescription drug allowances. OEI-03-96-00420. [Google Scholar]
- 2.Office of Inspector General, Department of Health and Human Services. Washington, DC: OIG; 1997. May, Appropriateness of medicare prescription drug allowances. OEI-03-96-00420. [Google Scholar]
- 3.Office of Inspector General, Department of Health and Human Services. Washington, DC: OIG; 2001. Jan, Medicare reimbursement of prescription drugs. OEI-03097-00290. [Google Scholar]
- 4.General Accounting Office. Washington, DC: 2001. Sep, Medicare: Payments for covered outpatient drugs exceed providers' cost. GAO-011118. [Google Scholar]
- 5.Danzon PM, Wilensky GR, Means KE. Alternative strategies for medicare payment of outpatient prescription drugs--part B and beyond. Am J Manag Care. 2005;11(3):173–180. [PubMed] [Google Scholar]
- 6.Federal register/ rules and regulations. 2004 November 15 Monday;Vol. 69(No. 219) [Google Scholar]
- 7.Data book: Section 9 drugs. 2003. Medicare Payment Advisory Commission. [Google Scholar]
- 8.Gabel JR, Rice TH. Reducing public expenditures for physician services: The price of paying less. J Health Polit Policy Law. 1985;9(4):595. doi: 10.1215/03616878-9-4-595. [DOI] [PubMed] [Google Scholar]
- 9.McGuire TG, Pauly MV. Physician response to fee changes with multiple payers. Journal of health economics. 1991;10(4):385–410. doi: 10.1016/0167-6296(91)90022-f. [DOI] [PubMed] [Google Scholar]
- 10.Yip WC. Physician response to medicare fee reductions: Changes in the volume of coronary artery bypass graft (CABG) surgeries in the medicare and private sectors. J Health Econ. 1998;17(6):675–699. doi: 10.1016/s0167-6296(98)00024-1. [DOI] [PubMed] [Google Scholar]
- 11.Medicare Payment Advisory Commission. Effect of medicare payment changes on oncology services. Washington, DC: 2006 Available from: http://www.medpac.gov/documents/Jan07_PartB_mandated_report.pdf.
- 12.Medicare Payment Advisory Commission. Report to congress: Impact of changes in medicare payments for part B drugs. Washington, DC: 2007
- 13.Christensen S. Volume response to exogenouse changes in medicare's payment policies. Health Serv Res. 1992;27(1):65. [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Medicare & Medicaid Services (CMS), HHS. Baltimore, MD: CMS; 1998. Physician volume and intensity response. [Google Scholar]
- 15.Rice TH. The impact of changing medicare reimbursement rates on physician-induced demand. Med Care. 1983;21(8):803–815. doi: 10.1097/00005650-198308000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Rice T. Physician-induced demand for medical care: New evidence from the medicare program. Adv Health Econ Health Serv Res. 1984;5:129–160. [PubMed] [Google Scholar]
- 17.Rice TH, Labelle RJ. Do physicians induce demand for medical services? J Health Polit Policy Law. 1989;14(3):587. doi: 10.1215/03616878-14-3-587. [DOI] [PubMed] [Google Scholar]
- 18.Wedig G, Mitchell JB, Cromwell J. Can price controls induce optimal physician behavior? J Health Polit Policy Law. 1989;14(3):601. doi: 10.1215/03616878-14-3-601. [DOI] [PubMed] [Google Scholar]
- 19.Medicare data file descriptions. [Accessed July 23, 2009];2009 Available at: http://www.resdac.umn.edu/Medicare/data_file_descriptions.asp#rif.
- 20.Wailoo AJ, Bansback N, Brennan A, Michaud K, Nixon RM, Wolfe F. Biologic drugs for rheumatoid arthritis in the medicare program. ARTHRITIS & RHEUMATISM. 2008;58(4):939–946. doi: 10.1002/art.23374. [DOI] [PubMed] [Google Scholar]
- 21.Donahue KE, Gartlehner G, Jonas DE, et al. Systematic review: Comparative effectiveness and harms of disease-modifying medications for rheumatoid arthritis. Ann Intern Med. 2008;148(2):124. doi: 10.7326/0003-4819-148-2-200801150-00192. [DOI] [PubMed] [Google Scholar]
- 22.Donahue KE, Gartlehner G, Jonas DE, et al. Comparative effectiveness of drug therapy for rheumatoid arthritis and psoriatic arthritis in adults. [Accessed September 22, 2008.];Agency for Healthcare Research and Quality. 2007 Available from: www.effectivehealthcare.ahrq.gov/reports/final.cfm. [PubMed]
- 23.Fraenkel L, Bogardus S, Concato J, Felson D, Wittink D. Patient preferences for treatment of rheumatoid arthritis. Br Med J. 2004;63(11):1372–1378. doi: 10.1136/ard.2003.019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarry JL, Coambs RB, De Maio FG, Rattray AH, Balaram R, Russell A. Projected patterns of compliance associated with remicade (infliximab) and enbrel (etarcept) [Google Scholar]
- 25.DeWitt EM, Glick HA, Albert DA, Joffe MM, Wolfe F. Medicare coverage of tumor necrosis factor {alpha} inhibitors as an influence on physicians' prescribing behavior. Arch Intern Med. 2006;166(1):57–63. doi: 10.1001/archinte.166.1.57. [DOI] [PubMed] [Google Scholar]
- 26.Singh JA, Holmgren AR, Noorbaloochi S. Accuracy of veterans administration databases for a diagnosis of rheumatoid arthritis. Arthritis & Rheumatism. 2004;51(6) doi: 10.1002/art.20827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.