Abstract
Liver failure due to ischemia and reperfusion (IR) and subsequent acute kidney injury are significant clinical problems. We showed previously that liver IR selectively reduced plasma sphinganine-1-phosphate levels without affecting sphingosine 1-phosphate (S1P) levels. Furthermore, exogenous sphinganine 1-phosphate protected against both liver and kidney injury induced by liver IR. In this study, we elucidated the signaling mechanisms of sphinganine 1-phosphate-mediated renal and hepatic protection. A selective S1P1 receptor antagonist blocked the hepatic and renal protective effects of sphinganine 1-phosphate whereas a selective S1P2 or S1P3 receptor antagonist was without effect. Moreover, a selective S1P1 receptor agonist, SEW-2871, provided similar degree of liver and kidney protection compared with sphinganine-1-phosphate. Furthermore, in vivo gene knock-down of S1P1 receptors with small interfering RNA abolished the hepatic and renal protective effects of sphinganine 1-phosphate. In contrast to sphinganine 1-phosphate, S1P’s hepatic protection was enhanced with an S1P3 receptor antagonist. Inhibition of extracellular signal-regulated kinase, Akt or pertussis toxin-sensitive G-proteins blocked sphinganine-1-phosphate-mediated liver and kidney protection in vivo. Taken together, our results show that sphinganine 1-phosphate provided renal and hepatic protection after liver IR injury in mice via selective activation of S1P1 receptors and pertussis toxin-sensitive G-proteins with subsequent activation of ERK and Akt.
Keywords: Akt, dihydrosphingosine 1-phosphate, endothelial cell, extracellular signal-regulated kinase, necrosis, sphingolipid, sphingosine 1-phosphate
Introduction
Hepatic ischemia and reperfusion (IR) is a major clinical problem complicating liver transplantation and major hepatic resection (1,2). Hepatic IR frequently leads to remote organ injury including the kidney, lung and heart (3). In particular, acute kidney injury (AKI) after major liver IR is extremely common (40–85% incidence) and the development of AKI after liver injury greatly increases patient mortality and morbidity during the perioperative period (3). We recently characterized a mouse model of AKI induced by liver IR with prominent early renal endothelial cell apoptosis and dysfunction with subsequent proximal tubule inflammation and necrosis (4). We also unexpectedly discovered rapid and profound depletion of a physiologically uncharacterized sphingolipid molecule sphinganine 1-phosphate (also called dihydrosphingosine 1-phosphate) in mouse plasma after hepatic IR (5). Moreover, we showed that exogenous repletion of sphinganine 1-phosphate provided a powerful protection against liver and kidney injury after liver IR in mice (5). We were able to demonstrate that mice treated with exogenous sphinganine 1-phosphate showed dramatically improved endothelial cell integrity and vascular dysfunction.
Unlike the better characterized cytoprotective effects of S1P, the cellular mechanism(s) of sphinganine 1-phosphate-mediated liver and kidney protection after liver IR has not been elucidated. For example, in our previous study, we implicated a sphingosine 1-phosphate (S1P) receptor utilizing an antagonist for S1P1/3 receptors (VPC23019); however the specific subtype of S1P receptor involved is still unclear (5). Activation of S1P1 receptors in vascular endothelial cells initiates several cytoprotective kinase signaling cascades including ERK mitogen activated protein kinase (MAPK) and Akt via a pertussis toxin-sensitive G-protein (Gi/o) dependent pathway (6–8). Since ERK MAPK and Akt signaling pathways are known to protect against endothelial cell apoptosis (9,10) and since hepatic IR induced AKI directly causes renal endothelial cell apoptosis with subsequent vascular dysfunction and neutrophil infiltration (4), we hypothesized that sphinganine 1-phosphate via S1P1 receptor-mediated activation of ERK MAPK and Akt signaling pathways protect against renal endothelial cell apoptosis and reduce AKI after liver IR. In addition, we have shown previously that enhanced phosphorylation as well as increased synthesis of heat shock protein 27 (HSP27) protected against endothelial cell apoptosis and vascular compromise after hepatic IR. Therefore, we postulated that sphinganine 1-phosphate may also increase HSP27 phosphorylation and upregulation. Finally, since endothelial nitric oxide synthase (iNOS) upregulation with subsequently enhanced release of NO protects against vascular endothelial cell injury, and since S1P receptor activation is known to activate eNOS to increase NO levels in the vasculature (11), we postulated that sphinganine 1-phosphate activation of S1P1 receptors may protect against liver and kidney injury via stimulating the eNOS pathway.
In this study, we tested the hypothesis that sphinganine 1-phosphate protects against liver IR induced hepatic and renal dysfunction via S1P1 receptor activation coupled to pertussis toxin-sensitive G-proteins (Gi/o) with subsequent activation of cytoprotective kinases including ERK MAPK and Akt and induction of HSP27 and eNOS in the kidney and liver. We also determined in this study the S1P receptor subtype(s) involved in S1P-mediated hepatic and renal protection utilizing both pharmacologic as well as gene knock-down approaches.
Materials and Methods
Reagents
Sphinganine 1-phosphate and (R)-3-Amino-(3-hexylphenylamino)-4-oxobutylphosphonic acid (W146, a selective S1P1 receptor antagonist) were purchased from Avanti Polar Lipids, Inc (Alabaster, AL). 5-[4-Phenyl-5-(trifluoromethyl)thiophen-2-yl]-3-[3-(trifluoromethyl)phenyl] 1,2,4-oxadiazole (SEW-2871, a selective S1P1 receptor agonist) and 1-[1,3-Dimethyl-4-(2-methylethyl)-1H-pyrazolo[3,4-b]pyridin-6-yl]-4-(3,5-dichloro-4-pyridinyl)-semicarbazide (JTE-013, a selective S1P2 receptor antagonist) were purchased from Tocris Bioscience (Ellisville, MO). 2-undecyl-thiazolidine-4-carboxylic acid (BML-241, a selective S1P3 receptor antagonist) was purchased from Cayman Chemical (Ann Arbor, MI). Wortmannin (a selective PI3K inhibitor) and L-N5-(1-Iminoethyl)ornithine (L-NIO, a selective eNOS inhibitor) were purchased from EMD Chemicals, Inc (Gibbstown, NJ). Unless otherwise specified, all other reagents including PD98059 (a selective MEK1 inhibitor) were purchased from Sigma (St. Louis, MO).
Murine model of hepatic IR
All protocols were approved by the Institutional Animal Care and Use Committee of Columbia University. Male C57BL/6 mice (20–25 g, Harlan, Indianapolis, IN) were subjected to liver IR injury as described previously (4). This method of partial hepatic ischemia for 60 min. results in a segmental (~70%) hepatic ischemia but spares the right lobe of the liver and prevents mesenteric venous congestion by allowing portal decompression through the right and caudate lobes of the liver. Sham operated mice were subjected to laparotomy and identical liver manipulations without the vascular occlusion. Plasma as well as liver and kidney tissues were collected 24 hrs after liver IR injury.
Sphinganine 1-phosphate administration
We have demonstrated previously that sphinganine 1-phosphate (0.01–0.1, mg/kg i.v. prior to reperfusion and 0.02–0.2 mg/kg s.c. 2 hrs after reperfusion) produced dose-dependent protection against liver and kidney injury after liver IR with the peak protection observed with the dose of 0.1 mg/kg i.v. before reperfusion and 0.2 mg/kg s.c. 2 hrs after reperfusion (5). In this study, sphinganine 1-phosphate was dissolved in warm (50°C) methanol and the aliquots were stored at −20°C. The solution was evaporated under nitrogen immediately before use, and the powder redissolved in 4 mg/mL fatty acid-free bovine serum albumin solution as a carrier as described by Van Brocklyn et al. (12). The sphinganine-1-phosphate dose that produced the maximal liver and kidney protection was given to mice in this study (0.1 mg/kg i.v. immediately before reperfusion and 0.2 mg/kg s.c. 2 hrs after reperfusion). Vehicle-treated mice received injections of 0.4% fatty acid free BSA. We also tested whether a single injection of sphinganine-1-phosphate also could provide liver and kidney protection after liver IR injury. In separate cohorts of mice, a single dose of sphinganine-1-phosphate was given immediately before (0.1 mg/kg, i.v.) or 2 hrs after (0.2 mg/kg, s.c.) reperfusion of the liver.
In another cohort of mice, we also gave a dose of S1P (0.1 mg/kg i.v. immediately prior to reperfusion and 0.2 mg/kg s.c. 2 hrs after reperfusion dissolved in 4 mg/mL fatty acid-free bovine serum albumin solution) to test whether S1P also provided liver and kidney protection. Our preliminary data showed that sphinganine 1-phosphate, S1P or vehicle injection alone in sham-operated mice had no effect on any of the injury parameters tested in the liver or in the kidney.
Plasma ALT activity and creatinine level
The plasma ALT activities were measured using the Infinity™ ALT assay kit according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA). Plasma creatinine was measured by an enzymatic creatinine reagent kit according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA). This method of creatinine measurement largely eliminates the interferences from mouse plasma chromagens well known to the Jaffe method (13).
Determining S1P receptor subtype(s) involved in sphinganine 1-phosphate- and S1P-mediated renal and hepatic protection after liver IR
To determine the S1P receptor subtype(s) involved in sphinganine 1-phosphate- and S1P-mediated renal and hepatic protection after liver IR, mice were treated with a selective S1P1 (W146, 0.05, 0.1 or 0.2 mg/kg i.p.), S1P2 (JTE013: 0.05, 0.1 or 0.2 mg/kg i.p.) or S1P3 (BML-241, 0.05 or 0.1 mg/kg i.p.) receptor antagonist 20 min. before sphinganine 1-phosphate or S1P treatment. In separate cohorts of mice, we also treated mice with the selective S1P1 receptor agonist SEW-2871 (1 mg/kg, i.p.) in lieu of sphinganine 1-phosphate 30 min. prior to liver ischemia. The doses of S1P1 receptor antagonists and SEW-2871 were obtained from previous in vivo studies (14–17).
siRNA preparation and delivery to mice in vivo
A chemically synthesized 21 nucleotide siSTABLE™ (Stability enhanced siRNA) sequences specific for S1P1 receptors were custom made and purchased from Dharmacon Research (Lafayette, CO) in 2′-hydroxyl, annealed, desalted and dialyzed duplex form for in vivo use. The siSTABLE™ is a modified siRNA with improved resistance against nuclease degradation and enhanced silencing duration in vivo. The double stranded sequence for S1P1 receptor siRNA was 5′-CCTGTGACATCCTGTACAA-3′. Mice were injected with 50 μg of siRNA (100 μl) i.v. 48 hrs prior to liver ischemia.
Potential signaling intermediates of sphinganine 1-phosphate-mediated renal and hepatic protection after liver IR
To test the hypothesis that ERK MAPK, Akt and/or eNOS activation participate in sphinganine 1-phosphate-mediated protection against liver IR induced AKI and liver injury, we pretreated the mice with PD98059 (an inhibitor of MEK1 to inhibit ERK phosphorylation, 1 mg/kg, i.p.), wortmannin (an inhibitor of PI3K to inhibit Akt phosphorylation, 1 mg/kg, i.p.) or L-NIO (an inhibitor of eNOS, 10 mg/kg i.p.) 20 min. before sphinganine 1-phosphate treatment. The doses of PD98059 and wortmannin were selected based on previous in vivo studies (6,18). In addition, we performed preliminary experiments to demonstrate that the dosage and method of administration of PD98059 and wortmannin we used effectively blocked the phosphorylation of ERK and Akt in vivo, respectively (6). The dose of L-NIO has been demonstrated previously to selectively block the eNOS activation in vivo (19). For determination of the role of pertussis-toxin sensitive G-protein (Gi/o) in sphinganine 1-phosphate-mediated renal and hepatic protection, mice were pretreated with pertussis toxin (25 μg/kg i.p.) 48 hrs before sphinganine 1-phosphate injection as described previously (7,20).
Histological evaluations of hepatic and renal injury
For histological preparations, liver or kidney tissues were fixed in 10% formalin solution overnight. After automated dehydration through a graded alcohol series, transverse liver or kidney slices were embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin-eosin (H&E). To quantify the degree of hepatic necrosis, H&E stains were digitally photographed and the percent of necrotic area was quantified with NIH IMAGE (Image-J, 1.37v) software by a person (SWC) who was blinded to the treatment each animal had received. Twenty random sections were investigated per slide to determine the percentage of necrotic area. Liver H&E sections were also graded for IR injury by a pathologist (VDD) blinded to the samples using the system devised by Suzuki et al. (21). In this classification, 3 liver injury indices are graded: sinusoidal congestion (0–4), hepatocyte necrosis (0–4), and ballooning degeneration (0–4) are graded for a total score of 0–12. No necrosis, congestion, or centrilobular ballooning is given a score of 0 whereas severe congestion/ballooning and >60% lobular necrosis is given a value of 4. Renal H&E sections were evaluated for the severity (score: 0–3) of renal cortical vacuolization, peritubular/proximal tubule leukocyte infiltration, proximal tubule simplification and proximal tubule hypereosinophilia by an experienced pathologist (VDD) who was blinded to the treatment each animal had received.
Cell culture
Human renal glomerular endothelial cells (ScienCell Research Laboratories, Carlsbad, CA) were grown in endothelial cell medium (ECM consisting of 5% fetal bovine serum, 1% endothelial cell growth supplement plus 1% of penicillin/streptomycin solution, ScienCell Research Laboratories, Carlsbad, CA) at 37°C in a 100% humidified atmosphere of 5% CO2–95% air. These cells are not immortalized so they were plated and used when confluent. Human renal proximal tubule (HK-2, immortalized human proximal tubular cell line, American Type Culture Collection, Manassas, VA) cells were grown and passaged in culture medium (50:50 mixture of DMEM low glucose and F12 plus 5% serum) and antibiotics (100 U/ml of penicillin G, 100 μg/ml of streptomycin, and 0.25 μg/ml of amphotericin B) at 37°C in a 100% humidified atmosphere of 5% CO2–95% air. Human renal endothelial cells or HK-2 cells were treated with 1 μM sphinganine 1-phosphate for 5 min. to 16 hrs. We also pretreated some cells with 1 μM W146 (a selective S1P1 receptor antagonist) 30 min. prior to sphinganine 1-phosphate treatment.
Kidney and liver tissue preparation and immunoblotting analyses
For determination of the signaling pathways after sphinganine-1-phosphate injection, livers and kidneys were isolated 15 min after 0.1 mg/kg sphinganine-1-phosphate injection. Liver tissues or mouse kidney cortical tissues (including corticomedullary junction) were dissected on ice and immediately placed in ice-cold RIPA buffer (150 mM NaCl, 50 mM Tris-HCl, 1 mM EDTA, and 1% Triton-X [pH 7.4]) and homogenized for 10 s on ice. The samples were centrifuged for 30 min at 50,000 xg. The supernatant was collected and used for immunoblotting as described previously (6,18). We measured the phosphorylation of ERK MAPK, Akt and HSP27 and the same blots were stripped and reprobed for total ERK MAPK, Akt and HSP27.
Immunoblot analyses of human renal endothelial cells
Immunoblotting analyses of human renal endothelial cell and proximal tubule (HK-2) cell lysates were performed as described previously (8,22) after treating the cells with either sphinganine-1-phosphate or with vehicle (0.4% BSA) for 5 min. to 16 hrs. The primary antibodies for phospho-ERK1/2 and total ERK were from Santa Cruz Biotechnologies (Santa Cruz, CA). The primary antibody for phospho-Akt and total Akt1 were from Cell Signaling Technologies (Danvers, MA). The primary antibodies for pHSP27 and HSP27 were obtained from Millipore (Billerica, MA). All of the phospho-ERK, phospho-Akt and phospho-HSP27 blots were stripped and reprobed for total ERK, Akt and HSP27, respectively. The secondary antibody (goat anti-rabbit or anti-mouse IgG conjugated to horseradish peroxidase at 1:5000 dilution) was detected with enhanced chemiluminescence immunoblotting detection reagents (Amersham), with subsequent exposure to a CCD camera coupled to a UVP Bio-imaging System (Upland, CA) and a personal computer. The band intensities of the immunoblots were within the linear range of exposure for all experiments.
Reverse transcription polymerase chain reaction (RT-PCR) analyses
We also performed a semi-quantitative RT-PCR assay for mouse HSP27 from total RNA extracted from renal cortices of mice injected either vehicle or with sphinganine-1-phosphate 5 hrs prior as described previously (Table 1) (23). We also extracted total RNA from human renal endothelial cells or renal proximal tubule (HK-2) cells treated with either vehicle (4 hrs) or with sphinganine 1-phosphate (2 or 4 hrs) and performed RT-PCR for human HSP27 as described (Table 1) (23). To determine the specificity as well as the degree of reduction in S1P1 receptors after siRNA treatment in mice in vivo, we also performed semi-quantitative RT-PCR assay for mouse S1P1–5 receptor subtypes in the kidney and liver tissues extracted 48 hrs after siRNA injection i.v. For each experiment, we also performed semiquantitative RT-PCR under conditions that yielded linear results for glyceraldehyde-3-phosphate dehydrogenase (Table 1) to confirm equal RNA input. RT-PCR products were analyzed on a 6% acrylamide gel stained with SYBR green (Invitrogen, Carlsbad, CA) for analysis with a UVP Bio-imaging System (Upland, CA). Semi-quantitative analysis of mRNA expression gene was accomplished by obtaining the ratio of the band density of the mRNA’s of interest to that of GAPDH (a housekeeping gene) from the same sample.
Table 1.
RT-PCR primers used in this study. GAPDH (glyceraldehyde 3-phosphate dehydrogenase), HSP27 (heat shock protein 27), S1P1–5R (sphingosine 1-phosphate receptor subtypes 1–5).
| Primers | Species | Size (bp) | Sequence (Sense/Antisense) | Annealing °C/Cycle# |
|---|---|---|---|---|
| GAPDH | Mouse | 450 | 5′-ACCACAGTCCATGCCATCAC-3′ 5′-CACCACCCTGTTGCTGTAGCC-3 |
65/15 |
| HSP27 | Mouse | 373 | 5′-CCTAAGGTCTGGCATGGTA-3′ 5′-AGGAAGCTCGTTGTTGAAGC-3′ |
66/25 |
| HSP27 | Human | 286 | 5′-CACGAGGAGCGGCAGGACGAG-3′ 5′-CAGTGGCGGCAGCAGGGGTGG-3′ |
65/13 |
| S1P1R | Mouse | 393 | 5′-CGGTGTAGACCCAGAGTCCT-3′ 5′-AGCAGCAGATGAGAATGAAC-3′ |
64/20 |
| S1P2R | Mouse | 317 | 5′-AAAACCAACCACTGGCTGTC-3′ 5′-GAGTGGAACTTGCTGTT-3′ |
60/25 |
| S1P3R | Mouse | 452 | 5′-AAGCCTAGCGGGAGAGAAAC-3′ 5′-GGCAATCAAAACCATCAGGT-3′ |
64/22 |
| S1P4R | Mouse | 403 | 5′-GCAGAAGTCTCCACGTCCTC-3′ 5′-GCTGAGTGACCGAGAAGTCC-3′ |
62/23 |
| S1P5R | Mouse | 335 | 5′-ACACCAAATGCCCAGCTTAC-3′ 5′-ACCAAGAGCACAGCCAAGTTC-3′ |
62/32 |
Statistical analysis
All data are reported as mean ± standard error. The overall significance of the results was examined using one-way analysis of variance and the significant differences between the groups were considered at a P<0.05 with the appropriate Tukey’s post hoc test made for multiple comparisons. The ordinal values of the liver and kidney injury scores were analyzed by the Mann-Whitney nonparametric test.
Results
Sphinganine 1-phosphate protects against hepatic and renal injury after liver IR
The plasma level of ALT and creatinine (Cr) in the vehicle-treated sham-operated mice was 72±9 U/L (N=6) and 0.43±0.03 mg/dL (N=6), respectively. The plasma level of ALT and Cr in the sphinganine 1-phosphate-treated sham-operated mice was 80±6 U/L (N=6) and 0.46±0.05 mg/dL (N=6), respectively. The plasma level of ALT increased significantly 24 hrs after 60 min. liver ischemia and reperfusion in mice treated with vehicle (15076±1174 U/L, N=6, Fig. 1). The mice subjected to liver IR after vehicle treatment also developed AKI with rises in plasma Cr (1.08±0.07 mg/dL, N=6, P<0.01 vs. sham-operated mice) 24 hrs after reperfusion. In contrast, mice treated with sphinganine 1-phosphate (0.1 mg/kg i.v. before reperfusion and 0.2 mg/kg s.c. 2 hrs after reperfusion), the increases in ALT (7474±557 U/L, N=6, P<0.001, Figure 1) and Cr (0.55±0.05 mg/dL, N=6, P<0.001, Figure 1) were significantly suppressed at 24 hrs after reperfusion. In this study, we also tested whether a single dose of sphinganine 1-phosphate would provide hepatic and renal protection when given immediately before reperfusion (0.1 mg/kg, i.v.) or 2 hr after reperfusion (0.2 mg/kg, s.c.). We show that sphinganine 1-phosphate given before reperfusion was protective (ALT=7197±753 U/L, N=6 and Cr=0.58±0.06 mg/dL, N=6) whereas the dose given 2 hrs after reperfusion was not protective (ALT=14762±1732 U/L, N=6 and Cr=0.98±0.06 mg/dL, N=6).
Figure 1.
Sphinganine 1-phosphate administered 0.1 mg/kg i.v. immediately prior to reperfusion and 0.2 mg/kg s.c. 2 hrs after reperfusion (Sphinganine 1-P IR (iv+sc), N=6) or 0.1 mg/kg i.v. immediately prior to reperfusion (Sphinganine 1-P IR (iv), N=6) protects against hepatic (A, ALT) and renal (B, creatinine) injury in C57BL/6 mice subjected to 60 min. liver ischemia and 24 hrs reperfusion. Sphinganine 1-phoshate when only given 2 hrs after reperfusion s.c. (Sphinganine 1-P IR (sc), N=6) did not produce liver or kidney protection. Data are presented as mean ± SEM. *P<0.05 vs. vehicle-treated IR (Vehicle IR) group.
We also tested whether exogenous S1P protected against liver IR induced hepatic and renal dysfunction. S1P (0.1 mg/kg i.v. prior to reperfusion and 0.2 mg/kg s.c. 2 hrs after reperfusion) also produced significant (but to a lesser degree than Sg1P) hepatic (ALT=9178±1822, N=9) and renal protection (Cr=0.72±0.13, N=9) 24 hrs after liver IR.
Sphinganine 1-phosphate provides protection against hepatic and renal injury after liver IR via S1P1 receptor activation
We also determined the S1P receptor subtype involved in sphinganine 1-phosphate-mediated hepatic and renal protection by pretreating mice with a highly selective pharmacological antagonist for S1P1 (W146), S1P2 (JTE-013) or S1P3 (BML-241) receptors. We found that blockade of S1P1 receptors but not S1P2 or S1P3 receptors blocked the sphinganine 1-phosphate-mediated liver and kidney protection after liver IR. W146 (0.05–0.2 mg/kg) caused complete inhibition of sphinganine 1-phosphate’s protective effects against liver and kidney injury. For example, W146 at 0.05 mg/kg i.p. 10 min. prior to liver ischemia completely abolished the sphinganine 1-phosphate induced hepatic and renal protection (Figure 2) 24 hrs after liver IR. SEW-2871, a selective S1P1 receptor agonist (1 mg/kg i.p. 30 min. prior to liver ischemia and 5 min prior to reperfusion) also provided equivalent degree of liver (ALT=6502±552 U/L, N=6) and renal (Cr=0.63±0.08 mg/dL, N=6) protection when given in lieu of sphinganine 1-phosphate. Neither S1P2 nor S1P3 receptor antagonist prevented the sphinganine 1-phosphate-mediated hepatic and renal protection against injury after liver IR (Figure 2).
Figure 2.
Selective S1P1 receptor antagonist W146 prevents hepatic (A, ALT) and renal (B, creatinine) protection by sphinganine 1-phosphate and S1P in C57BL/6 mice subjected to 60 min. liver ischemia and 24 hrs reperfusion. Mice were pretreated with vehicle (0.4% fatty acid free BSA, Veh, N=6), W146 (a selective S1P1 receptor antagonist, 0.05 mg/kg i.p., N=6), JTE-013 (JTE, a selective S1P2 receptor antagonist, 0.1 mg/kg i.p., N=6) or BML-241 (BML, a selective S1P3 receptor antagonist, 0.1 mg/kg i.p., N=6) 20 min. prior to sphinganine 1-phosphate or S1P treatment. Sphinganine 1-phosphate or S1P was administered 0.1 mg/kg i.v. immediately prior to reperfusion and 0.2 mg/kg s.c. 2 hrs after reperfusion. Data are presented as means ± SEM. *P<0.05 vs. Vehicle-treated hepatic IR group. #P<0.05 vs. Vehicle-treated sphinganine 1-phosphate hepatic IR group. $P<0.05 vs. Vehicle-treated S1P hepatic IR group.
Similar to sphinganine 1-phopshate, S1P-mediated hepatic and renal protection was inhibited by W146 (a selective S1P1 receptor antagonist, Figure 2). Surprisingly, the S1P-mediated hepatic protection was significantly enhanced by an S1P3 receptor antagonist (BML-241, Figure 2). S1P2 receptor selective antagonist (JTE-013) has no effect on S1P-mediated hepatic and renal protection (Figure 2).
In vivo siRNA targeting of S1P1 receptor blocked sphinganine 1-phosphate-induced hepatic and renal protection after liver IR
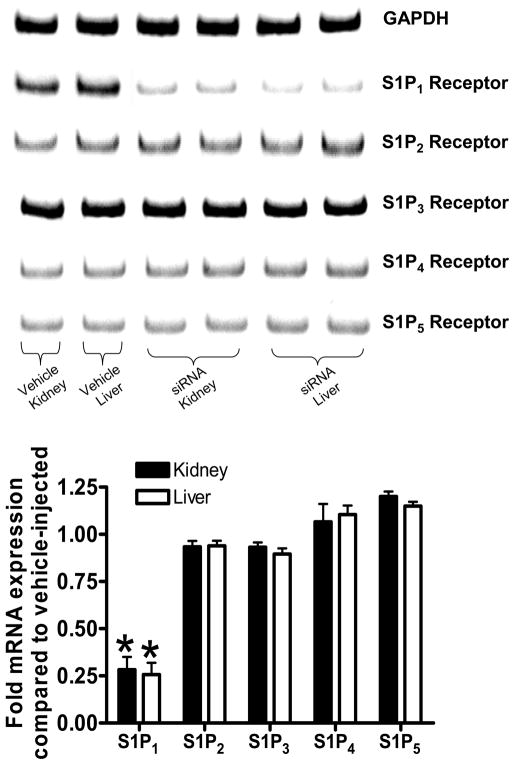
Mice were injected with siSTABLE™ siRNA sequences specific for murine S1P1 receptors 48 hrs before liver ischemia. We first demonstrate that siRNA injection selectively and significantly reduced S1P1 receptor mRNA expression in the liver and kidney (Figure 3). We also show that selective knock-down of S1P1 receptors with siRNA completely abolished the hepatic and renal protective effects of sphinganine 1-phosphate (ALT=15882±610 U/L, N=7 and Cr=1.36±0.08 mg/dL, N=7, p<0.001 vs. sphinganine 1-phopshate injected mice subjected to liver IR). siSTABLE™ S1P1 siRNA injection had no effect on hepatic and renal function in vehicle injected mice subjected to liver IR (ALT=16680±560 U/L, N=7 and Cr=1.29±0.06 mg/dL, N=7).
Figure 3.
Mice injected with siSTABLE™ targeting S1P1 receptors show selective reduction in S1P1 receptor mRNA without affecting other S1P receptor subtypes (S1P2–5). Densitometric quantification of relative mRNA band intensities normalized to GAPDH from RT-PCR reactions are shown in the bottom panel (N=7). Data are presented as means ± SEM. *P<0.05 vs. Vehicle-injected mice.
Signaling pathways of sphinganine 1-phosphate-mediated renal protection: critical role for the pertussis toxin sensitive G-proteins (Gi/o), ERK and Akt
We probed the renal and hepatic protective signaling pathways activated by sphinganine 1-phosphate treatment in mice subjected to liver IR. To determine whether Gi/o, ERK MAPK, Akt and/or eNOS signaling mediate the sphinganine 1-phosphate-mediated renal and hepatic protection after hepatic IR, mice were pretreated with pertussis toxin (an inhibitor of Gi/o signaling), PD98059 (a selective MEK1 inhibitor), wortmannin (a selective PI3K inhibitor) or L-NIO (a selective eNOS inhibitor) prior to sphinganine 1-phosphate treatment. We have demonstrated previously that the doses of pertussis toxin, PD98059 and wortmannin used effectively blocked phosphorylation of ERK and Akt, respectively, in mice in vivo (6,18). We found that the inhibition of Gi/o, MEK1 or PI3K prevented the renal and hepatic protection with sphinganine 1-phosphate treatment after hepatic IR (Figure 4). A selective eNOS inhibitor (L-NIO) had no effects on sphinganine 1-phosphate-mediated hepatic and renal protection after liver IR (Figure 4). Inhibitors alone had no effect on renal function after IR injury (Figure 4).
Figure 4.
Inhibition of pertussis toxin-sensitive G-proteins, ERK MAPK or Akt but not eNOS prevents hepatic (A, ALT) and renal (B, creatinine) protection by sphinganine 1-phosphate in C57BL/6 mice subjected to 60 min. liver ischemia and 24 hrs reperfusion. Mice were pretreated with PD98059 (PD, an inhibitor of MEK1 to inhibit ERK phosphorylation, 1 mg/kg, i.p., N=6), with wortmannin (Wort, an inhibitor of PI3K to inhibit Akt phosphorylation, 1 mg/kg, i.p., N=6) or with N-iminoethyl-L-ornithine (L-NIO, a selective inhibitor of eNOS, 10 mg/kg i.p. N=6) 20 min. before vehicle or sphinganine 1-phosphate treatment. Some mice were pretreated with pertussis toxin (PTX, 25 μg/kg, i.p.) 48 hrs prior to sphinganine 1-phosphate treatment. Sphinganine 1-phosphate was administered 0.1 mg/kg i.v. immediately prior to reperfusion and 0.2 mg/kg s.c. 2 hrs after reperfusion. Data are presented as means ± SEM. *P<0.05 vs. Vehicle-treated IR group. #P<0.05 vs. Vehicle-treated sphinganine 1-phosphate-treated hepatic IR group.
Sphinganine 1-phosphate-mediated reduction in hepatic necrosis and renal injury are blocked by a selective S1P1 receptor antagonist and inhibitors of ERK MAPK, Akt and Gi/o
Representative histological slides (magnification, 40X) from liver tissues from vehicle-treated or sphinganine 1-phosphate-treated mice subjected to 60 min ischemia and 24 hrs reperfusion or to sham-operation are shown in Figure 5. Sixty min of partial hepatic IR in vehicle-treated mice produced large necrotic areas of livers after reperfusion (Figure 5). Correlating with significantly improved function, reduced necrosis was observed in mice treated with sphinganine 1-phosphate and subjected to hepatic IR (Figure 5). The average percent necrotic areas for vehicle-treated mice were 92±2% (N=6) and sphinganine 1-phosphate-treatment reduced this percent necrosis to 44±8% (N=7, P<0.05). We failed to detect necrosis in liver sections from sham-operated mice. Livers were also analyzed for the degree of hepatocellular damage using the Suzuki’s criteria (Figure 5B) (24). The ischemic lobes in the control group showed severe hepatocyte vacuolization, necrosis and sinusoidal congestion (Suzuki score=8.7±0.3, N=5). Mice treated with sphinganine 1-phosphate revealed significantly less necrosis/sinusoidal congestion and better preservation of lobular architecture (Suzuki score=5.2±0.8, N=5, P<0.01, Figure 5B). Pre-treating mice with W146 (a selective S1P1 receptor antagonist), PD98059 (a selective MEK1 inhibitor), wortmannin (a selective PI3K inhibitor) or pertussis toxin (an inhibitor of Gi/o signaling) prior to sphinganine 1-phosphate treatment reduced the protective effects of sphinganine 1-phosphate on liver histology. Necrotic areas in the liver after IR also increased significantly in mice treated with W146, PD98059, wortmannin or pertussis toxin (data not shown).
Figure 5.

A). Representative photomicrographs of hematoxylin and eosin staining of the liver sections (40X). C57BL/6 mice were subjected liver ischemia reperfusion after vehicle- or sphinganine 1-phosphate treatment (0.1 mg/kg i.v. immediately prior to reperfusion and 0.2 mg/kg s.c. 2 hrs after reperfusion). Necrotic hepatic tissue appears as light pink (*) with inflammation/vascular congestion near the portal triad (arrow). Some mice were pretreated with PD98059 (PD, an inhibitor of MEK1 to inhibit ERK phosphorylation, 1 mg/kg, i.p., N=6) or with wortmannin (Wort, an inhibitor of PI3K to inhibit Akt phosphorylation, 1 mg/kg, i.p., N=6) 20 min. before vehicle or sphinganine 1-phosphate treatment. Some mice were pretreated with pertussis toxin (PTX, 25 μg/kg, i.p.) 48 hrs prior to sphinganine 1-phosphate treatment. Photographs are representative of 6 independent experiments. B). Summary of Suzuki liver injury scores (scale 0–12) from mice subjected to liver IR after vehicle or sphinganine 1-phosphate treatment. Mice pretreated with PD98059, wortmannin or pertussis toxin showed increased indices of hepatic injury. *P<0.05 vs. Vehicle-treated IR group. #P<0.05 vs. sphinganine 1-phosphate-treated hepatic IR group.
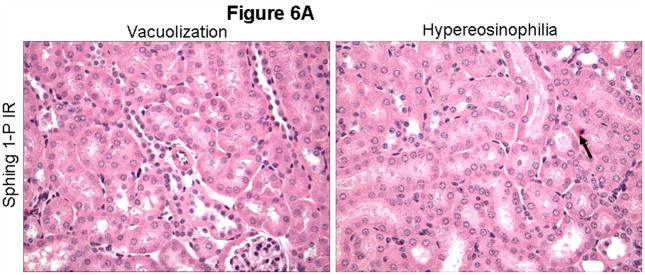
Representative kidney H&E slides from vehicle-treated and sphinganine 1-phosphate-treated mice subjected to 60 min ischemia and 24 hrs reperfusion are shown in Figure 6A (magnification, 400X). When we examined the kidneys from the mice injected with vehicle and subjected to liver IR, we observed multifocal acute tubular injury including S3 segment proximal tubule necrosis, cortical tubular simplification, cytoplasmic vacuolization and dilated lumina as well as focal granular bile/heme casts (Figure 6A). Correlating with significantly improved renal function, mice treated with sphinganine 1-phosphate showed less renal cortical vacuolization, peritubular/proximal tubule leukocyte infiltration, proximal tubule simplification and proximal tubule hypereosinophilia (Figure 6A). The summary of renal injury scores for percent renal tubular hypereosinophilia, percent peritubular leukocyte margination and percent cortical vacuolization are shown in Figure 6B. Blockade of S1P1 receptors, MEK1, PI3K or Gi/o by pre-treating mice with W146, PD98059, wortmannin or pertussis toxin, respectively, prior to sphinganine 1-phosphate treatment reduced the protective effects of sphinganine 1-phosphate on renal histology (Figure 6B).
Figure 6.

A). Representative photomicrographs of 6 experiments (hematoxylin and eosin staining, magnification 400X) demonstrating vacuolization (*) and hypereosinophillia (arrows) in kidneys from C57BL/6 mice 24 hrs after being subjected sham-operation or to liver ischemia reperfusion after vehicle- or sphinganine 1-phosphate treatment (0.1 mg/kg i.v. immediately prior to reperfusion and 0.2 mg/kg s.c. 2 hrs after reperfusion). Photographs are representative of 6 independent experiments. B). Summary of renal injury scores (scale 0–3) for renal cortical vacuolization, peritubular leukocyte margination, proximal tubule simplification and renal tubular hypereosinophilia for kidneys from mice subjected to liver IR after vehicle or sphinganine 1-phosphate treatment. Some mice were pretreated with PD98059 (PD, an inhibitor of MEK1 to inhibit ERK phosphorylation, 1 mg/kg, i.p., N=6) or with wortmannin (Wort, an inhibitor of PI3K to inhibit Akt phosphorylation, 1 mg/kg, i.p., N=6) 20 min. before vehicle or sphinganine 1-phosphate treatment. Some mice were pretreated with pertussis toxin (PTX, 25 μg/kg, i.p.) 48 hrs prior to sphinganine 1-phosphate treatment. Mice pretreated with PD98059, wortmannin or pertussis toxin showed increased indices of renal injury. *P<0.05 vs. Vehicle-treated IR group. #P<0.05 vs. sphinganine 1-phosphate-treated hepatic IR group.
Sphinganine-1-phosphate treatment phosphorylates ERK MAPK, Akt and HSP27 and induces HSP27 mRNA and protein in mouse kidney and liver
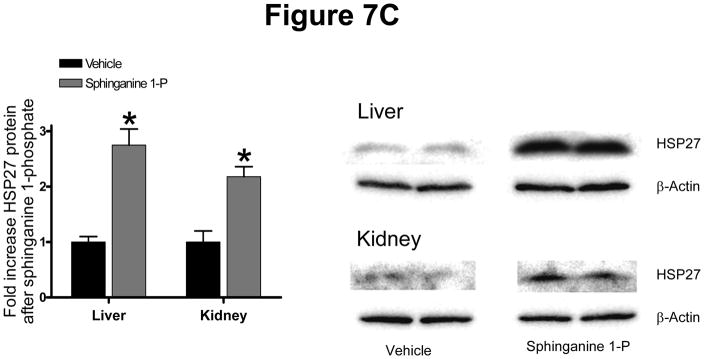
Mice were injected with sphinganine 1-phophate i.v. and their kidney and liver tissues were extracted at 15 min. (for immunoblotting of phosphorylated proteins), at 5 hrs (for RTPCR) and at 24 hrs (for immunoblotting of total HSP27) after injection. Sphinganine 1-phosphate induced HSP27 mRNA of the liver and kidney in mice (Figure 7A). Sphinganine 1-phosphate treatment also resulted in phosphorylation of ERK MAPK and Akt as well as phosphorylation of renal and hepatic HSP27 in mice (Figure 7B). Finally, we show that sphinganine 1-phosphate treatment increased total HSP27 protein in the liver and kidney in mice (Figure 7C).
Figure 7.
(A) Representative gel images of RT-PCR results GAPDH and mouse HSP27 mRNAs extracted after treatment with vehicle (0.4% fatty acid free BSA) or 0.1 mg/kg sphinganine 1-phosphate i.v. 5 hrs prior. Densitometric quantification of relative mRNA band intensities normalized to GAPDH from RT-PCR reactions are shown in the bottom panel (N=6). Data are presented as means ± SEM. *P<0.05 vs. Vehicle group. (B) Representative immunoblots for phospho (p)- and total-ERK, phospho (p)-and total-Akt, phospho (p)- and total-HSP27 from renal cortices and liver of mice (right panel) after injection with vehicle or 0.1 mg/kg sphinganine 1-phosphate i.v. 15 min. prior. C) Representative immunoblots for total-HSP27 and actin (showing equal lane loading) from renal cortices and liver of mice after injection with vehicle or 0.1 mg/kg sphinganine 1-phosphate i.v. 24 hrs prior. Densitometric quantifications of relative band intensities are also shown (left panel, N=4). *P<0.05 vs vehicle. Error bars, 1 SEM.
Sphinganine-1-phosphate phosphorylates ERK MAPK, Akt and HSP27 and induces HSP27 in human renal endothelial cells
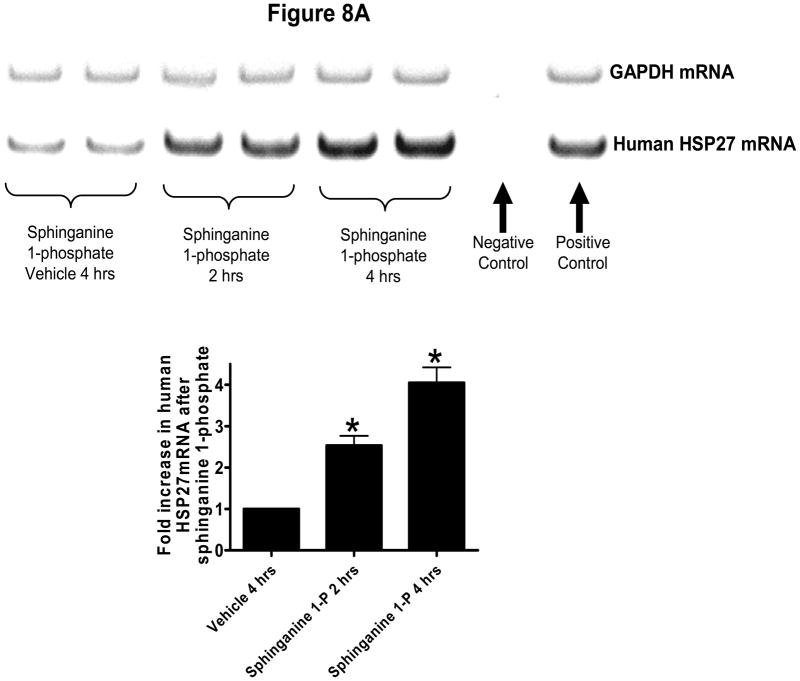
The next series of experiments were performed in cultured human renal vascular endothelial cells to further elucidate the mechanistic aspect of sphinganine-1-phosphate mediated renal endothelial protection. Human renal endothelial cells were treated with sphinganine 1-phosphate and their mRNA and protein were extracted for analyses. Figure 8A shows that sphinganine-1-phosphate induces HSP27 mRNA in cultured human renal endothelial cells. Figure 8B shows that sphinganine-1-phosphate phosphorylates 2 well known anti-apoptotic kinases (ERK MAPK and AKT) in human renal endothelial cells in a time-dependent manner. Moreover, we also demonstrate that sphinganine-1-phosphate phosphorylates and induces HSP27 (Figure 8). Blockade of S1P1 receptors with W146 completely abolished the effects of sphinganine 1-phosphate in human renal endothelial cells (data not shown). In contrast to the effects on human endothelial cells, sphinganine 1-phosphate failed to phosphorylate ERK MAPK, Akt and HSP27 and induce HSP27 in HK-2 (human renal proximal tubule epithelial) cells (data not shown).
Figure 8.
(A) Representative gel images of RT-PCR results GAPDH and human HSP27 mRNAs extracted from human renal endothelial cells in culture. Cells were treated with vehicle (0.4% fatty acid free BSA for 4 hrs) or with sphinganine 1-phosphate (Sphinganine 1-P, 1 μM for 2 or 4 hrs). Densitometric quantification of relative mRNA band intensities normalized to GAPDH from RT-PCR reactions are shown in the bottom panel. Data are presented as means ± SEM. *P<0.05 vs. Vehicle 4 hrs group. (B) Human renal endothelial cells were treated with 1 μm sphinganine-1-phosphate for indicated periods and probed for phosphorylation of anti-apoptotic kinases ERK and Akt and phospho- and total HSP27 (representative of 5 experiments).
Discussion
The major findings of this study are that sphinganine 1-phosphate protects against liver IR induced hepatic and renal injury via activation of the S1P1 receptors with subsequent signaling through Gi/o, ERK and Akt-mediated mechanisms (Figure 9). Both pharmacological (W146) as well as gene deletion approaches (with siSTANLE™ siRNA) demonstrated essential roles for S1P1 receptors in sphinganine 1-phosphate-mediated hepatic and renal protection after liver IR. Sphinganine 1-phosphate phosphorylated cytoprotective kinase ERK MAPK, Akt and HSP27 in human glomerular renal endothelial cells in vitro as well as in mouse kidney and liver in vivo. However, sphinganine 1-phosphate failed to activate the cytoprotective kinase phosphorylation and HSP27 induction in human proximal tubule cells in culture. We also determined sphinganine 1-phosphate-mediated liver and kidney protection is independent of the eNOS pathway in vivo. In contrast, the mechanisms of S1P-mediated hepatic protection are more complex as a selective S1P1 receptor antagonist blocked whereas a selective S1P3 receptor antagonist potentiated S1P’s hepatic protective effects.
Figure 9.
Proposed cellular mechanisms of sphinganine 1-phosphate-mediated renal and hepatic protection after liver IR. Based on the data generated from our studies, we propose that sphinganine 1-phosphate activates the S1P1 receptor subtype in endothelial cells which couples to pertussis toxin-sensitive G-proteins, resulting in the phosphorylation of ERK MAPK, Akt as well as HSP27. We also propose induction of HSP27 mRNA as well as protein after sphinganine 1-phosphate treatment.
Development of AKI associated with liver injury is a devastating clinical complication with an extremely high mortality (3). Neither effective prevention nor therapy exists for hepatic IR induced liver and kidney injury and the current management remains largely supportive (2). We used a murine model of liver IR that not only produces severe liver dysfunction but also rapidly and reproducibly develops AKI with the degree of hepatic dysfunction directly correlating with the degree of AKI (4). Hepatic IR induced AKI in mice mimicked the histological (renal tubular injury and juxtaglomerular apparatus hyperplasia) as well as biochemical (plasma creatinine, inflammatory markers) changes observed with human AKI associated with liver failure (4). Importantly, we noted that AKI after liver IR in our model was associated with a rapid development of renal endothelial cell apoptosis with subsequent vascular impairment, neutrophil infiltration and renal proximal tubule cell necrosis (4). Therefore, we hypothesized and explored ways to improve endothelial integrity that will subsequently reduce renal and hepatic dysfunction after liver IR.
Sphingolipids including sphingosine and sphinganine (dihydrosphingosine) are ubiquitous but essential structural and functional components of the cell. In addition, sphingolipid metabolites including S1P have important biological roles in various physiological as well as pathophysiological events (25). Sphinganine 1-phosphate as well as S1P is produced by the ATP-dependent phosphorylation of sphinganine by sphingosine kinases (26,27). Sphingosine kinase (SK) is a conserved lipid kinase with two mammalian isoforms (SK1 and SK2) (26,27). The biological role of S1P has been extensively characterized including cell growth and survival and inflammation (28). Furthermore, S1P produces powerful anti-apoptotic and pro-survival signaling in endothelial cells (29). In contrast to the well characterized biological and physiological roles of S1P (29,30), sphinganine 1-phosphate has not been widely studied and little is known about its function.
We unexpectedly discovered recently that plasma levels of sphinganine 1-phosphate (but not S1P) fell significantly after liver IR in mice (5). Moreover, in our present and previous studies, we demonstrated that exogenous sphinganine 1-phosphate treatment immediately before reperfusion significantly attenuated the elevation of plasma ALT and creatinine levels after hepatic IR. We propose that sphinganine 1-phosphate is biologically potent, is depleted after massive liver IR injury and may have important cytoprotective functions to defend against endothelial cell dysfunction after liver IR. Although sphinganine 1-phosphate is structurally similar to S1P, it differs from S1P by being cell impermeable (31) and lacks the trans double bond at the 4 position (32). Liver IR results in depletion of systemic as well as hepatic ATP (a necessary cofactor of the SK enzyme) levels which may decrease the activities and/or efficiencies of SK. However, it is unclear as to why a selective depletion of plasma sphinganine 1-phosphate and not S1P occurs after liver IR as both sphinganine 1-phosphate and S1P synthesis depend on the same enzyme, SK. Preferential synthesis of sphinganine 1-phosphate over S1P has been demonstrated with SK1 overexpression (33). Berdyshev et al. have demonstrated that SK1 overexpression in several primary cells and cultured cell lines resulted in a predominant upregulation of sphinganine 1-phosphate synthesis relative to S1P (33). In their study, SK1 overexpression preferentially directed the metabolic flow of newly formed sphingoid bases from de novo ceramide formation toward the synthesis of sphinganine 1-phosphate. These studies suggest that SK1 preferentially synthesizes sphinganine 1-phoshate from simple de novo sphingolipids produced whereas formation of S1P is via separate and complex catabolic pathways.
Although S1P → S1P receptor signaling has been extensively studied, sphinganine 1-phosphate-mediated cell signaling has not been studied in detail. Since the structures of sphinganine 1-phosphate and S1P are similar, we postulated that sphinganine 1-phosphate acting on the cell surface S1P receptors may mediate hepatic and renal protection after liver IR. Protective effects of S1P receptor signaling to protect against liver and kidney injury have been demonstrated previously in vivo. For example, FTY720 (2-amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diol) protected against liver IR in rats presumably via activation of S1P receptor modulation (34,35). Moreover, several S1P receptor agonists, including S1P, FTY-720 and SEW-2871 (a selective ligand for S1P1 receptor), protected against renal IR injury in vivo via reducing renal proximal tubule influx of T-lymphocytes with subsequent reduction in necrosis and inflammation (17,36,37).
We show in this study that sphinganine 1-phosphate-mediated liver and kidney protection after liver IR is S1P1 receptor-mediated as a selective S1P1 receptor antagonist (W146) blocked the protective effects of sphinganine 1-phosphate. Selective S1P2 (JTE-013) and S1P3 (BML-241) antagonists had no effect on sphinganine 1-phosphate-mediated liver and kidney protection after liver IR. All of these antagonists for S1P receptors provide extreme selectivity for their respective receptor subtypes (14–16). To further evaluate the role of S1P1 receptors in sphinganine 1-phosphate-mediated liver and kidney protection, we utilized siRNA targeting S1P1 receptors in mice in vivo to complement the data obtained with pharmacological inhibitor (W146) studies. We were able to selectively downregulate S1P1 receptors in adult mice with siSTABLE™ constructs in vivo (Figure 3) which resulted in complete loss of sphinganine 1-phosphate-mediated hepatic and renal protection after liver IR.
We also show in this study that sphinganine 1-phosphate via S1P1 receptor activation leads to phosphorylation of ERK MAPK, Akt and HSP27 as well as induction of HSP27 in mouse kidney and liver (Figure 7) as well as cultured human renal endothelial cells (Figure 8). Endothelial selectivity is suggested as sphinganine 1-phosphate failed to phosphorylate ERK MAPK, Akt and HSP27 in human kidney proximal tubule epithelial cell (HK-2) line. The differential molecular mechanisms for these signaling differences between endothelial cells and proximal tubules cells remain to be elucidated. Activation of ERK MAPK is strongly associated with enhanced protection against several forms of injury including necrosis and apoptosis (9,38). The serine/threonine kinase Akt is an important component of cell survival pathways in many cell types (39,40). In particular, Akt has diverse functions to counteract apoptosis including inhibition of mitochondrial cytochrome c and phosphorylation of several pro-apoptotic factors (e.g., bad, caspase 9, glycogen synthase kinase 3) (10,41).
HSP27 is a member of family of chaperone proteins that are up-regulated in response to a wide range of cellular stresses including hypoxia, ischemia and exposure to toxic drugs (42–45). Increased expression of HSP27 serves to defend a cell against injury or death by acting as chaperones facilitating proper polypeptide folding and aberrant protein removal (46–48). Furthermore, HSP27 is a potent anti-apoptotic protein and is a key stabilizer of the actin cytoskeleton; both of these cellular effects lead to increased resistance against cell death (49–51). Both phosphorylated and non-phosphorylated forms of HSP27 can reduce cellular injury against diverse forms of stress including renal injury. It remains to be determined whether a direct link exists between HSP27 phosphorylation/induction and sphinganine 1-phosphate-mediated liver and kidney protection.
In this study, we were surprised to discover that the hepatic protection with S1P was not only attenuated by an S1P1 receptor antagonist but was also improved by an S1P3 selective antagonist. These findings suggest that exogenous S1P activation of S1P1 receptor provides protective signaling cascade in the liver, however S1P can also initiate potentially detrimental effects via S1P3 receptor activation as well. S1P3 receptor activation in pulmonary epithelial cells leads to disruption of tight junctions, possibly by activating Rho resulting in increased lung vascular permeability (52). Moreover, the S1P3 but not the S1P1 receptor subtype has been implicated in non-selective S1P receptor agonist induced bradycardia (53). Indeed, FTY-720 (an immunomodulator analog of S1P) has been shown to not only produce expected lymphomenia but also produced undesirable dose-dependent bradycardia in clinical trials (53). Therefore, in contrast to the protective effects of S1P1 receptor activation, S1P3 receptor activation may trigger detrimental effects against organ injury. We propose that S1P produces activation of multiple S1P receptor subtypes (including S1P1 and S1P3) resulting in conflicting physiological effects. This is in contrast to the lack of S1P3 receptor-mediated effects observed with sphinganine 1-phosphate-mediated hepatic protection (Figure 2).
A limitation of the study is that S1P4 and S1P5 receptor selective antagonists currently are not available, therefore, we cannot rule of the roles for these receptor subtypes in sphinganine 1-phosphate mediated liver and kidney protection. However, although S1P receptors are ubiquitously expressed in almost every cell type, in the vascular endothelial system S1P1, S1P2 and S1P3 receptor subtypes predominate in expression and function (54). Another limitation is that, although we implicate endothelial cells as the target of sphinganine 1-phosphate-mediated protection as this drug shows selective phosphorylation of renal endothelial but not renal epithelial cell line, with in vivo studies it is impossible to delineate for certain the target cell type(s) involved in sphinganine 1-phosphate-mediated protection. Future in vitro studies to complement our current in vivo studies are required to determine whether other parenchymal cell types of interest (e.g., hepatocyte) are also involved.
In conclusion, we determined the mechanisms of sphinganine 1-phosphate (a relatively unknown sphingolipid molecule)-mediated protection against liver IR induced renal and hepatic injury in mice. Our study demonstrates that activation of the S1P1 receptor via sphinganine 1-phosphate protects against liver IR induced AKI and hepatic injury via, Gi/o, ERK and Akt-mediated mechanisms and the protection is independent of the eNOS pathway. In contrast, activation of S1P3 receptors attenuated the hepatic protective effects of exogenous S1P after liver IR. We propose that sphinganine 1-phosphate via selective S1P1 receptor activation without affecting the S1P3 receptors is superior to S1P in attenuating hepatic IR injury and may be a promising pharmacological agent for protecting both liver and kidney function after hepatic IR.
Acknowledgments
This work was supported by National Institute of Health Grant RO1 DK-58547 and RO1 GM-067081.
Footnotes
Disclosure
None of the authors had financial interests or ties to commercial companies.
References
- 1.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160–166. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 2.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86–93. doi: 10.1016/s0014-4800(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 3.Davis CL, Gonwa TA, Wilkinson AH. Pathophysiology of renal disease associated with liver disorders: implications for liver transplantation. Part I. Liver Transpl. 2002;8:91–109. doi: 10.1053/jlts.2002.31516. [DOI] [PubMed] [Google Scholar]
- 4.Lee HT, Park SW, Kim M, D’Agati VD. Acute kidney injury after hepatic ischemia and reperfusion injury in mice. Lab Invest. 2009;89:196–208. doi: 10.1038/labinvest.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SW, Kim M, Chen SWC, D’Agati VD, Lee HT. Sphinganine-1-phosphate attenuates both hepatic and renal injury induced by hepatic ischemia and reperfusion in mice. Shock. 2009 doi: 10.1097/SHK.0b013e3181c02c1f. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joo JD, Kim M, Horst P, Kim J, D’Agati VD, Emala CW, Sr, Lee HT. Acute and delayed renal protection against renal ischemia and reperfusion injury with A1 adenosine receptors. Am J Physiol Renal Physiol. 2007;293:F1847–F1857. doi: 10.1152/ajprenal.00336.2007. [DOI] [PubMed] [Google Scholar]
- 7.Lee HT, Emala CW. Protein kinase C and G(i/o) proteins are involved in adenosine- and ischemic preconditioning-mediated renal protection. J Am Soc Nephrol. 2001;12:233–240. doi: 10.1681/ASN.V122233. [DOI] [PubMed] [Google Scholar]
- 8.Lee HT, Emala CW. Characterization of adenosine receptors in human kidney proximal tubule (HK-2) cells. Exp Nephrol. 2002;10:383–392. doi: 10.1159/000065306. [DOI] [PubMed] [Google Scholar]
- 9.Buckley S, Driscoll B, Barsky L, Weinberg K, Anderson K, Warburton D. ERK activation protects against DNA damage and apoptosis in hyperoxic rat AEC2. Am J Physiol. 1999;277:L159–L166. doi: 10.1152/ajplung.1999.277.1.L159. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy SG, Kandel ES, Cross TK, Hay N. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol. 1999;19:5800–5810. doi: 10.1128/mcb.19.8.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igarashi J, Michel T. Sphingosine-1-phosphate and modulation of vascular tone. Cardiovasc Res. 2009;82:212–220. doi: 10.1093/cvr/cvp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Brocklyn JR, Tu Z, Edsall LC, Schmidt RR, Spiegel S. Sphingosine 1-phosphate-induced cell rounding and neurite retraction are mediated by the G protein-coupled receptor H218. J Biol Chem. 1999;274:4626–4632. doi: 10.1074/jbc.274.8.4626. [DOI] [PubMed] [Google Scholar]
- 13.SLOT C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17:381–387. doi: 10.3109/00365516509077065. [DOI] [PubMed] [Google Scholar]
- 14.Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, Cahalan MD, Wong CH, Rosen H. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol. 2006;2:434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- 15.Koide Y, Hasegawa T, Takahashi A, Endo A, Mochizuki N, Nakagawa M, Nishida A. Development of novel EDG3 antagonists using a 3D database search and their structure-activity relationships. J Med Chem. 2002;45:4629–4638. doi: 10.1021/jm020080c. [DOI] [PubMed] [Google Scholar]
- 16.Inoki I, Takuwa N, Sugimoto N, Yoshioka K, Takata S, Kaneko S, Takuwa Y. Negative regulation of endothelial morphogenesis and angiogenesis by S1P2 receptor. Biochem Biophys Res Commun. 2006;346:293–300. doi: 10.1016/j.bbrc.2006.05.119. [DOI] [PubMed] [Google Scholar]
- 17.Awad AS, Ye H, Huang L, Li L, Foss FW, Jr, Macdonald TL, Lynch KR, Okusa MD. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290:F1516–F1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- 18.Joo JD, Kim M, D’Agati VD, Lee HT. Ischemic preconditioning provides both acute and delayed protection against renal ischemia and reperfusion injury in mice. J Am Soc Nephrol. 2006;17:3115–3123. doi: 10.1681/ASN.2006050424. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Ohgami Y, Dai Y, Quock RM. Antagonism of nitrous oxide-induced anxiolytic-like behavior in the mouse light/dark exploration procedure by pharmacologic disruption of endogenous nitric oxide function. Psychopharmacology (Berl) 2003;166:366–372. doi: 10.1007/s00213-002-1363-0. [DOI] [PubMed] [Google Scholar]
- 20.Lee HT, Gallos G, Nasr SH, Emala CW. A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2004;15:102–111. doi: 10.1097/01.asn.0000102474.68613.ae. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Lee HT, Emala CW. Adenosine attenuates oxidant injury in human proximal tubular cells via A(1) and A(2a) adenosine receptors. Am J Physiol Renal Physiol. 2002;282:F844–F852. doi: 10.1152/ajprenal.00195.2001. [DOI] [PubMed] [Google Scholar]
- 23.Kim M, Chen SW, Park SW, Kim M, D’Agati VD, Yang J, Lee HT. Kidney-specific reconstitution of the A1 adenosine receptor in A1 adenosine receptor knockout mice reduces renal ischemia-reperfusion injury. Kidney Int. 2009;75:809–823. doi: 10.1038/ki.2008.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Jo SK, Bajwa A, Awad AS, Lynch KR, Okusa MD. Sphingosine-1-phosphate receptors: biology and therapeutic potential in kidney disease. Kidney Int. 2008;73:1220–1230. doi: 10.1038/ki.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Stunff H, Milstien S, Spiegel S. Generation and metabolism of bioactive sphingosine-1-phosphate. J Cell Biochem. 2004;92:882–899. doi: 10.1002/jcb.20097. [DOI] [PubMed] [Google Scholar]
- 27.Liu H, Chakravarty D, Maceyka M, Milstien S, Spiegel S. Sphingosine kinases: a novel family of lipid kinases. Prog Nucleic Acid Res Mol Biol. 2002;71:493–511. doi: 10.1016/s0079-6603(02)71049-0. [DOI] [PubMed] [Google Scholar]
- 28.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee YM, Wu M, Parikh NS, Khan F, Proia RL, Hla T. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J. 2006;397:461–471. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chalfant CE, Spiegel S. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J Cell Sci. 2005;118:4605–4612. doi: 10.1242/jcs.02637. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Diez M, Rodriguez C, Badimon L, Martinez-Gonzalez J. Prostacyclin induction by high-density lipoprotein (HDL) in vascular smooth muscle cells depends on sphingosine 1-phosphate receptors: effect of simvastatin. Thromb Haemost. 2008;100:119–126. doi: 10.1160/TH07-11-0675. [DOI] [PubMed] [Google Scholar]
- 32.Fossetta J, Deno G, Gonsiorek W, Fan X, Lavey B, Das P, Lunn C, Zavodny PJ, Lundell D, Hipkin RW. Pharmacological characterization of human S1P4 using a novel radioligand, [4,5-3H]-dihydrosphingosine-1-phosphate. Br J Pharmacol. 2004;142:851–860. doi: 10.1038/sj.bjp.0705856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berdyshev EV, Gorshkova IA, Usatyuk P, Zhao Y, Saatian B, Hubbard W, Natarajan V. De novo biosynthesis of dihydrosphingosine-1-phosphate by sphingosine kinase 1 in mammalian cells. Cell Signal. 2006;18:1779–1792. doi: 10.1016/j.cellsig.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Anselmo DM, Amersi FF, Shen XD, Gao F, Katori M, Lassman C, Ke B, Coito AJ, Ma J, Brinkmann V, Busuttil RW, Kupiec-Weglinski JW, Farmer DG. FTY720 pretreatment reduces warm hepatic ischemia reperfusion injury through inhibition of T-lymphocyte infiltration. Am J Transplant. 2002;2:843–849. doi: 10.1034/j.1600-6143.2002.20906.x. [DOI] [PubMed] [Google Scholar]
- 35.Man K, Ng KT, Lee TK, Lo CM, Sun CK, Li XL, Zhao Y, Ho JW, Fan ST. FTY720 attenuates hepatic ischemia-reperfusion injury in normal and cirrhotic livers. Am J Transplant. 2005;5:40–49. doi: 10.1111/j.1600-6143.2004.00642.x. [DOI] [PubMed] [Google Scholar]
- 36.Lai LW, Yong KC, Igarashi S, Lien YH. A sphingosine-1-phosphate type 1 receptor agonist inhibits the early T-cell transient following renal ischemia-reperfusion injury. Kidney Int. 2007 doi: 10.1038/sj.ki.5002203. [DOI] [PubMed] [Google Scholar]
- 37.Lien YH, Yong KC, Cho C, Igarashi S, Lai LW. S1P(1)-selective agonist, SEW2871, ameliorates ischemic acute renal failure. Kidney Int. 2006;69:1601–1608. doi: 10.1038/sj.ki.5000360. [DOI] [PubMed] [Google Scholar]
- 38.Fryer RM, Hsu AK, Gross GJ. ERK and p38 MAP kinase activation are components of opioid-induced delayed cardioprotection. Basic Res Cardiol. 2001;96:136–142. doi: 10.1007/s003950170063. [DOI] [PubMed] [Google Scholar]
- 39.Hausenloy D, Mocanu M, Yellon D. Cross-talk between the survival kinases during reperfusion in ischaemic preconditioning. Cardiovasc J S Afr. 2004;15:S11. doi: 10.1016/j.cardiores.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–H976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 41.Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM. Serine/threonine protein kinases and apoptosis. Exp Cell Res. 2000;256:34–41. doi: 10.1006/excr.2000.4836. [DOI] [PubMed] [Google Scholar]
- 42.Garrido C, Bruey JM, Fromentin A, Hammann A, Arrigo AP, Solary E. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J. 1999;13:2061–2070. doi: 10.1096/fasebj.13.14.2061. [DOI] [PubMed] [Google Scholar]
- 43.Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- 44.Arrigo AP, Firdaus WJ, Mellier G, Moulin M, Paul C, Diaz-Latoud C, Kretz-Remy C. Cytotoxic effects induced by oxidative stress in cultured mammalian cells and protection provided by Hsp27 expression. Methods. 2005;35:126–138. doi: 10.1016/j.ymeth.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 45.Arrigo AP. Hsp27: novel regulator of intracellular redox state. IUBMB Life. 2001;52:303–307. doi: 10.1080/152165401317291156. [DOI] [PubMed] [Google Scholar]
- 46.Wyttenbach A, Sauvageot O, Carmichael J, Diaz-Latoud C, Arrigo AP, Rubinsztein DC. Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum Mol Genet. 2002;11:1137–1151. doi: 10.1093/hmg/11.9.1137. [DOI] [PubMed] [Google Scholar]
- 47.Samali A, Robertson JD, Peterson E, Manero F, van Zeijl L, Paul C, Cotgreave IA, Arrigo AP, Orrenius S. Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones. 2001;6:49–58. doi: 10.1379/1466-1268(2001)006<0049:hpmotc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP. Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol. 2002;22:816–834. doi: 10.1128/MCB.22.3.816-834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weber NC, Toma O, Wolter JI, Wirthle NM, Schlack W, Preckel B. Mechanisms of xenon- and isoflurane-induced preconditioning - a potential link to the cytoskeleton via the MAPKAPK-2/HSP27 pathway. Br J Pharmacol. 2005;146:445–455. doi: 10.1038/sj.bjp.0706324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landry J, Huot J. Regulation of actin dynamics by stress-activated protein kinase 2 (SAPK2)-dependent phosphorylation of heat-shock protein of 27 kDa (Hsp27) Biochem Soc Symp. 1999;64:79–89. [PubMed] [Google Scholar]
- 51.Huot J, Houle F, Spitz DR, Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996;56:273–279. [PubMed] [Google Scholar]
- 52.Shikata Y, Birukov KG, Garcia JG. S1P induces FA remodeling in human pulmonary endothelial cells: role of Rac, GIT1, FAK, and paxillin. J Appl Physiol. 2003;94:1193–1203. doi: 10.1152/japplphysiol.00690.2002. [DOI] [PubMed] [Google Scholar]
- 53.Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, Rosen H. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 54.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, Yamashita T, Proia RL. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]