Abstract
Bilateral interference, referring to the tendency of movements of one arm to disrupt the intended movements made simultaneously with the other arm, is often observed in a task that involves differential planning of each arm movement during sensorimotor adaptation. In the present study, we examined two questions: 1) how does the compatibility between visuomotor adaptation tasks performed with both arms affect bilateral interference during bimanual performance? and 2) how do variations in bilateral interference affect transfer of visuomotor adaptation between bilateral and unilateral conditions? To examine these questions, we manipulated visuomotor compatibility using two kinematic variables (direction of required hand motion, direction of an imposed visual rotation). Experiment 1 consisted of two conditions in which the direction of visual rotations for both arms was either in the same or opposing directions, whereas the target direction for both arms was always the same. In experiment 2, we examined the pattern of generalization between the bilateral and unilateral conditions when both the target and rotation directions were opposing between the arms. In both experiments, subjects first adapted to a 30° visual rotation with one arm (preunilateral), then with both arms (bilateral), and finally with the arm that was not used in the first session (postunilateral). Our results show that bilateral interference was smallest when both variables were the same between the arms. Our data also show extensive transfer of visuomotor adaptation between bilateral and unilateral conditions, regardless of degree of bilateral interference.
INTRODUCTION
Many daily activities, such as opening a jar, require simultaneous movements of both arms. Previous research has demonstrated a tendency or preference of individuals to perform highly synchronized bilateral movements, which led to the hypothesis that the nervous system establishes “coordinative structures” (Kelso et al. 1979) that maintain tight synchrony between the movements of the arms (see Swinnen 2002; Wiesendanger and Serrien 2004). It is well established that bilateral movements recruit unique neural resources (e.g., Brinkman 1981, 1984; Sadato et al. 1997), suggesting that motor learning under bilateral and unilateral conditions may show limited transfer. If true, this has substantial implications for neurorehabilitation, where training is often focused on a single arm at one time (Wolf et al. 2008). The limited transfer hypothesis is supported by a recent study by Nozaki and colleagues (2006), which indicated limitations in transfer of learning between bilateral and unilateral movement conditions when individuals adapted to novel force fields imposed by a robotic manipulandum. The authors concluded that the neural circuits underlying bilateral and unilateral adaptation are only partially shared, which results in limited transfer of learning between these conditions.
More recently, we examined transfer of learning visuomotor rotations between unilateral and bilateral conditions. Surprisingly, our findings indicated extensive transfer of motor learning from bilateral to unilateral conditions (Wang and Sainburg 2009). In that study, our subjects first adapted to rotated visual feedback during targeted reaching with one arm (preunilateral), then with both arms simultaneously (bilateral), and finally with the other arm that was not used during the initial adaptation session (postunilateral). Under bilateral movement conditions, however, our subjects showed substantial interference between the arms. This interference in bilateral performance appeared to limit the transfer of visuomotor adaptation from the unilateral to the bilateral conditions, but not from bilateral to unilateral conditions. This study suggested that apparent limitations in transfer of learning from unilateral to bilateral conditions might be due to bilateral interference during performance, but not due to limitations in transfer of learning. If this hypothesis is true, then manipulating the degree of bilateral interference between the arms should not affect the extent of transfer of learning between bilateral and unilateral conditions.
A great deal of work has been done on bilateral interference involving motor performance per se (e.g., Franz et al. 1991; Heuer 1996; Swinnen and Walter 1988; Swinnen et al. 2001), indicating that bilateral interference occurs most often when joint movements are not mirror-imaged between the arms. However, research on bilateral interference during sensorimotor adaptation is virtually nonexistent. During visuomotor rotation adaptation tasks, we expect two variables to manipulate the extent of bilateral interference: 1) compatibility of instructed movement directions and 2) compatibility of visuomotor rotation directions (clockwise [CW] vs. counterclockwise [CCW]). In the present study, we separately manipulated these two sources of interference to determine the conditions that will yield the highest and the lowest degrees of interference during bilateral adaptation to novel visuomotor conditions. This, then, allowed us to determine the effects of varying bilateral interference on generalization of visuomotor learning between bilateral and unilateral conditions.
We conducted two separate experiments, to achieve the intended variations in these factors: in experiment 1, we compared between two experimental conditions in which the direction of visual rotations was either the same or opposing between the arms in extrinsic space, whereas the target directions were always the same (i.e., movement directions in parallel). In experiment 2, we used a condition in which both the target and the rotation directions were opposing between the arms (i.e., mirror-imaged movements in the extrinsic space). Its counterpart condition (i.e., the same rotation directions and opposing target directions) has been explored in our previous study (Wang and Sainburg 2009).
METHODS
Subjects
Subjects were 36 neurologically intact right-handed adults (18 female, 18 male; ages from 18 to 28 yr). They were recruited from the university community and were paid for their participation. Informed consent approved by the Internal Review Board of the Pennsylvania State University was obtained prior to participation.
Apparatus
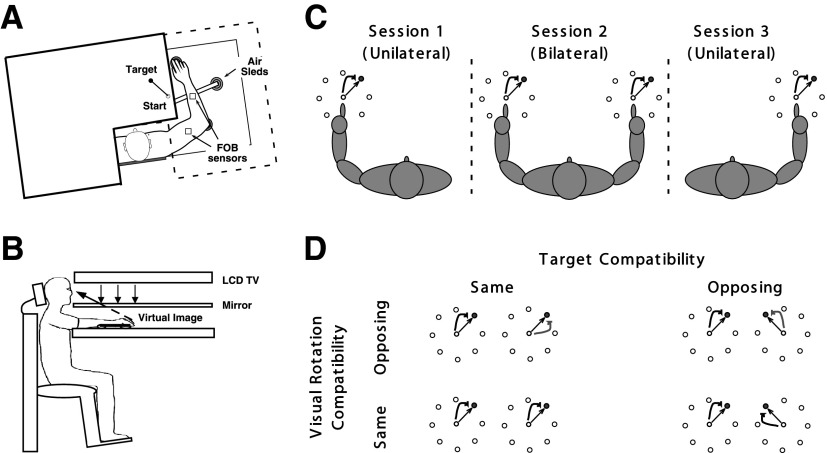
Our subjects sat in front of a table with both arms supported over the tabletop that was positioned just below shoulder height, by a frictionless air jet system (Fig. 1A). A start circle, target, and cursor representing the index finger position were projected onto a horizontal 52-in. liquid crystal display TV positioned above the arm (Fig. 1B), which were reflected through a mirror that was positioned below this TV. The setup was arranged in such a way to give the illusion that the display was in the same horizontal plane as the hand. The Flock of Birds (Ascension Technology, Milton, VT) magnetic 6 degrees of freedom movement recording system was used to sample the index finger-tip position at 103 Hz for both arms simultaneously. For detailed information, see Sainburg and Wang (2002).
Fig. 1.
A, top view: the positions of the Flock of Birds sensors are shown. B, top view: subjects were seated in a chair with the arm supported by an air jet system that removed the effects of friction on arm movement. Targets and the cursor representing hand position were projected on a TV screen placed above the arm. A mirror placed below this screen reflected the image, such that the projection was perceived in the plane of the arm. C: target schemes in the same visual rotation conditions (experiment 1): subjects first adapted to 30° counterclockwise (CCW) rotation by making movements with the left arm (preunilateral). During the bilateral session, they made movements in 2 target directions that were the same for 2 arms, and so were visual rotation directions. Following this, subjects made reaching movement with the right arm, which was not used during the preunilateral session. Straight arrows indicate expected hand paths without visual rotations, and curved arrows with visual rotations (30° CCW). D: target and visual rotation schemes in different consistency conditions: schemes illustrated in the left column represent 2 conditions in experiment 1. Schemes illustrated in the right column, top, represent the condition in experiment 2. Those in the right column, bottom, represent the condition reported in Wang and Sainburg (2009). Arrows curved in the same direction between the arms indicate 30° CCW rotations; those in the opposite directions indicate 30° CCW rotation for the left and 30° clockwise (CW) rotation for the right arm.
Experimental design
In experiment 1, visual rotation directions were either the same or opposing between the arms in the extrinsic space, whereas target directions were always the same (see Fig. 1, C and D). In experiment 2, visual rotation directions and target directions both were opposing (i.e., mirror-imaged movements). Its counterpart condition (i.e., the same rotation directions and opposing target directions) has been tested in our previous study (Wang and Sainburg 2009). Data from that study were not included in the data analyses of the current study, although a summarized description of the data is provided at the end of the results section.
Both experiments consisted of four sessions: baseline (no visual rotation), preunilateral adaptation, bilateral adaptation, and postunilateral adaptation sessions. The baseline session was provided so that subjects could get familiar with our reaching task in general, using both arms simultaneously. During the adaptation sessions, the position of the cursor was rotated 30° either CW or CCW about the start circle. For each trial, one of eight targets (Fig. 1C, 2 cm in diameter), presented in a pseudorandom sequence, was displayed prior to movement. Each session consisted of 192 trials, which were organized into 24 cycles, with each cycle containing eight consecutive trials (i.e., a set of movements made toward all eight target directions). Subjects were instructed to move directly from the starting circle to the target using a single, rapid motion in response to an auditory “go” signal. During the movement, visual feedback was provided as a screen cursor. At the end of each trial, knowledge of results (KR) was provided in the form of a hand path between the starting circle and the target and by points awarded for spatial accuracy (two-dimensional distance between the target and the final hand position): 1 point for accuracy <4 cm, 3 points for accuracy <2 cm, and 10 points for accuracy <1 cm. No points were given for movements that took >400 ms.
There were four subject groups in experiment 1 (six subjects per group): a half of all subjects received opposing visual rotations for the two arms, whereas the other half received the same rotations (Fig. 1D). Prior to the experiment, each of these subject groups was further divided into two groups: a half of them performed the adaptation task with the right arm first and the other half with the left arm first. Experiment 2 consisted of only two subject groups (six subjects per group; no subject participated in both experiments): one group performed the adaptation task with the right arm first and the other half with the left arm first.
Each subject received the same direction of visual rotation in the same arm performances across all adaptation sessions in both experiments. Thus for example, if a subject adapted to a CCW rotation with the left arm in the preunilateral condition, her left arm experienced the same CCW rotation during the bilateral session as well and her right arm experienced a CW (when the rotation directions were opposing) or CCW (when the rotation directions were the same) rotation during both the bilateral and postunilateral sessions. Table 1 summarizes these conditions.
Table 1.
Experimental design
| Adaptation (30° Rotation) Session |
||||||
|---|---|---|---|---|---|---|
| Experiment | Target Direction Between Hands | Rotation Direction Between Hands | Preunilateral | Bilateral | Postunilateral | |
| 1 | Same | Opposing | R/CW | L/CCW | R/CW | L/CCW |
| L/CCW | L/CCW | R/CW | R/CW | |||
| Same | R/CCW | L/CCW | R/CCW | L/CCW | ||
| L/CCW | L/CCW | R/CCW | R/CCW | |||
| 2 | Opposing | Opposing | R/CW | L/CCW | R/CW | L/CCW |
| L/CCW | L/CCW | R/CW | R/CW | |||
R, right; L, left; CW, clockwise; CCW, counterclockwise.
Data analysis
We calculated hand-path direction error at peak velocity (Vmax), which was calculated as the angular difference between the vectors defined by the target and by the hand-path position at movement start and at Vmax. The absolute values of these errors were calculated for each trial and collapsed across trials when subjected to statistical analyses. We have previously shown that this performance measure can be used effectively to characterize visuomotor adaptation processes (Wang and Sainburg 2009).
An epoch is the mean of two consecutive cycles. In experiment 1, a repeated-measures ANOVA was conducted to examine the main effects of, and the interaction effects among, the four following variables: rotation direction (same, opposing), order (left arm first, right arm first), session (preunilateral, bilateral, postunilateral), and epoch (1–12), with the last two variables as within-subject factors; 24 cycles in each session, with each cycle as the mean of eight consecutive trials (i.e., eight different targets), reorganized into 12 epochs for the purpose of data reduction in statistical analyses. In experiment 2, a repeated-measures ANOVA was conducted to examine the main effects of, and the interaction effects among, order, session, and epoch, with the last two variables as within-subject factors.
Because the main purposes of this study were not only to investigate changes in the pattern of bilateral interference during bilateral adaptation across the conditions with different combinations of target and rotation directions, but also to examine the effect of bilateral interference on generalization of visuomotor adaptation between bilateral and unilateral conditions, we were most interested in post hoc pairwise comparisons within each subject group. Following the ANOVAs, thus post hoc pairwise comparisons were conducted using paired t-test, with an alpha level of 0.05. These pairwise comparisons were made among the three adaptation sessions at the first epoch, defined as the mean of cycles 1 and 2, and among those at the last epoch, defined as the mean of cycles 23 and 24. The comparisons between the preunilateral and bilateral sessions at the first epoch were made to examine the degree of bilateral interference observed during the bilateral session compared with unilateral performance with the same arm; and the comparisons between the bilateral and postunilateral sessions at the first epoch were made to assess the amount of immediate transfer from bilateral training to subsequent unilateral performance. Those at the last epoch were made to compare the final level of adaptation across sessions. Additional pairwise comparisons were made between the preunilateral and bilateral session at the last epoch, also to determine an interference effect. The direction of visual rotation was always the same between any two adaptation sessions examined in the pairwise comparisons (e.g., left arm performances compared between the bilateral and postunilateral sessions both received a CW rotation).
RESULTS
Experiment 1: same target directions
In this experiment, we examined the effects of visuomotor rotation directions on bilateral performance, when the target direction was always the same.
Opposing rotations between the arms.
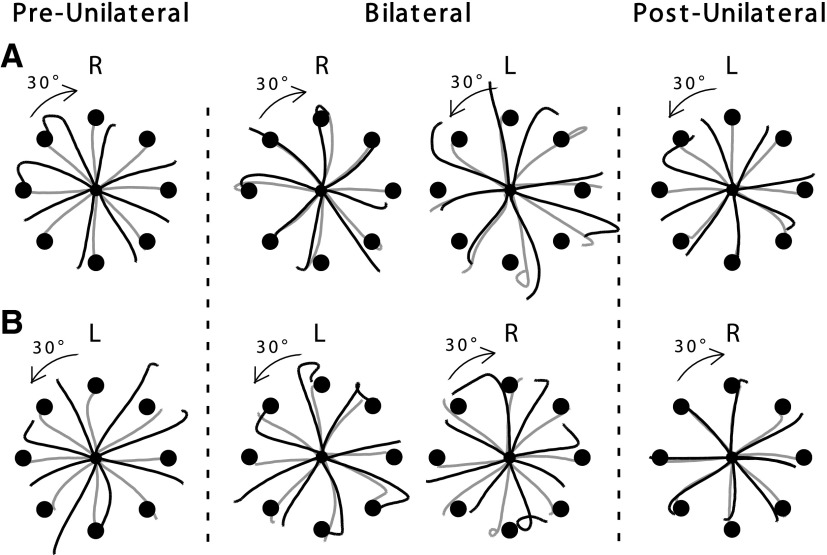
Figure 2 shows typical hand paths from representative subjects during the preunilateral, bilateral, and postunilateral adaptation sessions. These examples are from our first group of subjects who adapted to opposing rotations (CW for the right, CCW for the left arm). During the preunilateral session, hand paths (black lines) are initially directed either CW (right) or CCW (left) to the target, with a large “hook” at the end of motion that reflects an error correction. Following adaptation to the visual rotation, these hand paths (gray lines) become relatively straight and substantially more accurate. During the bilateral session, the left and right arm performances appeared substantially different: following initial adaptation with the unilateral right arm (row 1), right arm performance during bilateral adaptation was accurate in the beginning of the bilateral session (column 2, black), whereas left arm performance remained inaccurate even at the end of the adaptation session (column 3, gray). Following initial adaptation with the left arm (row 2), left arm performance during bilateral adaptation was poor at the beginning and end (column 2, black and gray, respectively), whereas right arm performance was similar to its performance during unilateral conditions (column 3).
Fig. 2.
Hand paths from representative subjects during the 3 adaptation sessions, when the arms experienced the same target directions and opposing rotation directions. Black lines represent the first 8 consecutive trials in each session and gray lines the last 8 consecutive trials. Black circles around the start position represent actual target locations viewed by subjects. Arrows below L or R indicate the direction of visual rotation. A and B: hand paths from the subject groups who used the right arm first, and the left arm first, during the preunilateral session, respectively. L above the hand paths indicates left arm, R right arm.
The consistency of these effects across subjects and throughout adaptation is demonstrated in Fig. 3, which shows the average direction error at peak velocity (mean ± SE across subjects) for each epoch (i.e., an average across 16 consecutive trials) of each adaptation session. Repeated-measures ANOVA revealed a significant four-way interaction effect (rotation direction × order × session × epoch; P < 0.05), indicating that the changes in performance across epochs were different among the three adaptation sessions, which also varied depending on the rotation direction and the arm that was first used. Thus we performed pairwise comparisons among the three adaptation sessions. Pairwise comparisons among the adaptation sessions at the first epoch and at the last epoch, when the rotation directions were opposing between the arms, are illustrated in the bar graphs to the far right of Fig. 3. The horizontal lines shown above or below the bar graphs indicate significant pairwise comparisons (P < 0.05) between two given first or last epochs, respectively.
Fig. 3.
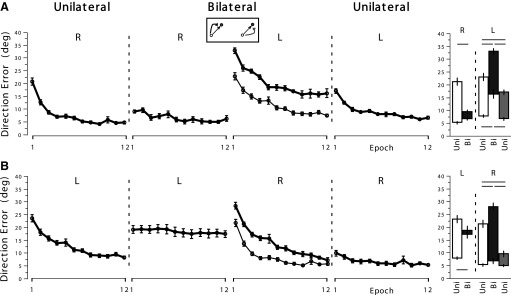
Mean performance measures of direction error at peak velocity (Vmax) in experiment 1, with the same target directions and opposing rotation directions. Here and in the following figures: columns 1–4: every data point in each epoch represents the mean of 16 consecutive trials averaged across all subjects (mean ± SE). In column 3, a thin line on each row is borrowed from the other subject group for comparison purposes (e.g., a thin line shown in column 3, row 2 is the same as the line shown in column 1, row 1). Column 5: this graph provides summary of data shown in columns 1–4, for statistical comparisons. White bar in the left panel represents performance shown in column 1, black bar performance in column 2. Black bar in the right panel represents performance in column 3, gray bar performance in column 4. White bar in the right panel is borrowed from the other subject group (e.g., white bar in the right panel of column 5 on row 1 is the same as the white bar in the left panel of column 5 on row 2). Top of each bar represents mean (±SE) at epoch 1, bottom of each bar mean (±SE) at epoch 12. Horizontal lines above or below these bars indicate that the comparisons between 2 given first or last epochs, respectively, are significant at P < 0.05. A and B: direction errors from the subject groups who used the right arm first, and the left arm first, during the preunilateral session, respectively.
During the preunilateral session, both subject groups adapted substantially to the novel rotation condition with the given arm. Their bilateral performances, however, were quite different depending on which arm was used first. A pairwise comparison between the preunilateral and bilateral performances with the right arm (Fig. 3, row 1) shows a significant difference (at P < 0.001) at the first epoch (column 5, left panel, indicated by a horizontal line on top). This indicates substantial generalization of visuomotor adaptation from the unilateral to the bilateral session performed by the right arm. However, a small, but significant, detriment in performance from the end of unilateral performance to the beginning of bilateral performance (P < 0.05) was also observed, indicating the presence of some bilateral interference effect. In contrast, a pairwise comparison between the preunilateral and bilateral performances with the left arm (Fig. 3, row 2) failed to show a significant difference at the first epoch (column 5, left panel, indicated by the absence of a horizontal line on top). This indicates the lack of generalization from the unilateral to the bilateral sessions performed by the left arm. This was also accompanied by a significant detriment in performance from the end of unilateral performance to the beginning of bilateral performance (P < 0.001), indicating the presence of a large bilateral interference effect.
With regard to the performance of the opposite arm (i.e., one that was not used in the preceding session) during the bilateral session, both arms experienced some bilateral interference effect in both subject groups, as indicated by their poorer performances (Fig. 3, thick lines in column 3), compared with their unilateral performances observed in the opposite subject groups (thin lines in column 3; see figure legends for descriptions). Pairwise comparisons confirmed a substantial detriment in performance from the preunilateral to the bilateral session, indicated by a significant difference between the two sessions at the first epoch for both arms. (White bars shown in the right panels of column 5 correspond to thin lines shown in column 3.)
Despite the observed bilateral interference, generalization of visuomotor adaptation did occur from the bilateral to postunilateral sessions. As illustrated in Fig. 3, the initial performances of both arms during the postunilateral session (column 4) were substantially better, compared with the initial performances of the same arms during the bilateral session (column 3, thick lines) or during the preunilateral session by the opposite subject groups (column 3, thin lines). Pairwise comparisons confirmed that the differences between the bilateral and postunilateral sessions at the first epoch were significant for both arms; those between the last epoch of the bilateral session and the first epoch of the postunilateral session were not for either arm.
Same rotations between the arms.
In general, changes in the pattern of visuomotor adaptation from the unilateral to bilateral, and back to unilateral sessions, when the rotation directions were the same between the arms, are similar to those observed when the rotation directions were opposing, although the bilateral interference effects were somewhat smaller. In fact, no bilateral interference was observed for the right arm (Fig. 4, column 2, row 1), as indicated by its superb performance at the first epoch, which was significantly different from its unilateral performance at the first epoch (Fig. 4, column 5, row 1, left panel) and not statistically different from its unilateral performance at the last epoch. Again, the left arm performance during the bilateral session (Fig. 4, column 2, row 2) demonstrated a high degree of bilateral interference, indicated by the lack of significant difference between the preunilateral and bilateral performances at the first epoch (Fig. 4, column 5, row 2, left panel).
Fig. 4.
Mean performance measures of direction error at Vmax in experiment 1, with the same target directions and the same rotation directions.
With regard to the performance of the opposite arm during the bilateral session, neither arm experienced any bilateral interference effect, indicated by their decent performances (Fig. 4, thick lines in column 3), compared with their unilateral performances observed in the opposite subject groups (Fig. 4, thin lines in column 3). Pairwise comparisons indicated no difference between the two sessions at either the first epoch or the last epoch, for either arm.
Bilateral adaptation in this condition also generalized substantially to the unilateral session. As illustrated in Fig. 4, both arm performances during the postunilateral session at the first epoch (column 4) were not significantly different from those observed during the bilateral session at the last epoch (column 3), indicating nearly complete transfer from the bilateral to unilateral condition.
Experiment 2: opposing target directions
Opposing rotations between the arms.
The results are similar to those observed in experiment 1. Repeated-measures ANOVA revealed a significant three-way interaction effect (order × session × epoch; P < 0.05), indicating that the difference among the three adaptation sessions in terms of performance changes across epochs also varied, depending on the two groups of subjects who used either the left or right arm in the preunilateral session. Thus we performed pairwise comparisons for each subject group separately.
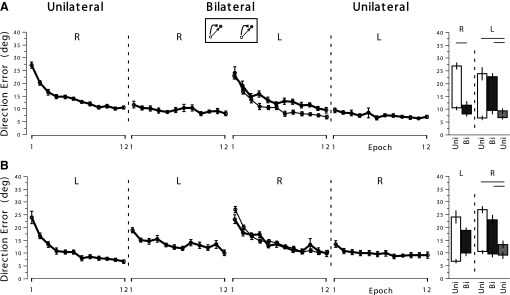
The patterns of data in this condition are comparable between the two subject groups (Fig. 5). They both adapted substantially to the visual rotation with the given arm during the preunilateral session and substantial generalization from the preunilateral to the bilateral performance with the same arm occurred for both arms, reflected by their decent performances at the first epoch of the bilateral session (Fig. 5, column 2), which were significantly different from their unilateral performances at the same epoch (Fig. 5, column 5, left panels). However, the data also indicate the presence of bilateral interference for both arms, in that their performances at the first epoch during the bilateral session were significantly worse than those at the last epoch during the preunilateral session (P < 0.01).
Fig. 5.
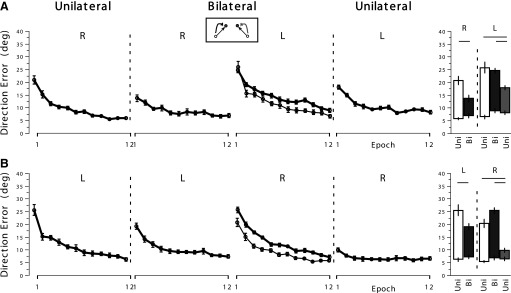
Mean performance measures of direction error at Vmax in experiment 2, with opposing target directions and opposing rotation directions.
Regarding the performance of the opposite arm during the bilateral session, the right arm appears to have experienced some bilateral interference effect, as illustrated in Fig. 5 (thick line vs. thin line in column 3, row 2), although the pairwise comparisons at the first and last epochs did not show significant differences (Fig. 5, column 5, row 2, right panel). The left arm did not experience a significant bilateral interference effect either (Fig. 5, column 5, row 1, right panel).
Substantial generalization of visuomotor adaptation from the bilateral to unilateral session also occurred in this condition. The postunilateral performance at the first epoch was significantly better than the same-arm bilateral performance at the first epoch for both arms (Fig. 5, column 5, right panels). The amount of generalization, however, was greater for the right arm, in that the postunilateral performance at the first epoch was not significantly different from the bilateral performance at the last epoch for the right arm, whereas it was for the left arm (P < 0.01), indicating limited transfer.
Same rotations between the arms.
This condition in which the rotations were the same and the target directions opposing between the arms was tested in our previous study (Wang and Sainburg 2009; experiment 1, Fig. 3). The results from that study were almost identical to those observed in the condition in which the rotations were opposing and the target directions were the same (see Fig. 3), except the following: First, the right arm performance during the bilateral session by the subject group who started with the left arm was not statistically different from the unilateral right arm performance by the other subject group (analogous to the comparison between the thick and the thin lines in column 3, row 2; reported in Fig. 3, column 3, row 1 in our previous study) and, second, the amount of generalization from the bilateral to the unilateral performance with the left arm was greater (analogous to the comparison between the last epoch of the bilateral performance and the first epoch of the postunilateral performance shown in columns 3 and 4, row 1; reported in Fig. 3, columns 3 and 4, row 2 in our previous study).
DISCUSSION
In this study, we manipulated two sources of interference to determine the conditions that would yield the highest and the lowest degrees of interference during bilateral adaptation to novel visuomotor conditions: 1) compatibility between the arms in terms of target direction and 2) compatibility between the arms in terms of visuomotor rotation direction. These manipulations varied the extent of bilateral interference across our four experimental conditions, which in turn influenced the pattern of generalization between the bilateral and unilateral conditions differently across the conditions. However, we also showed an effect of arm, such that the left nondominant arm showed substantial interference effects from right arm adaptation under all our conditions, whereas interference for the right dominant arm was minimal under compatible visuomotor conditions. For both arms, bilateral interference was lowest in the condition in which both rotation and target directions were compatible between the arms (see Fig. 6). In this condition, right arm performance showed no detriment in performance from the preunilateral to the bilateral sessions, whereas it showed a statistically significant detriment in all other conditions. Although the left nondominant arm showed substantial interference under all conditions, the variation in interference with compatibility was generally consistent with that of the dominant arm. We were also able to assess interference using a group comparison between same arm performance during naïve unilateral performance in the first experimental block and same arm performance during the bilateral block. This comparison revealed a significant detriment in performance with the left arm during the bilateral session in the conditions in which the target directions were the same and the visual rotations were opposing, or vice versa (Wang and Sainburg 2009), whereas a significant detriment with the right arm was observed only when the target directions were the same and the rotations opposing.
Fig. 6.
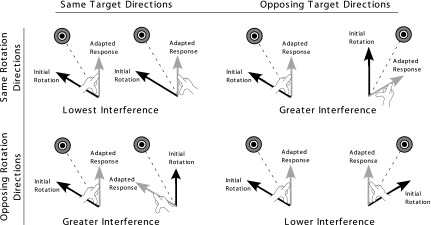
Target and visual rotation schemes in different consistency conditions: schemes illustrated in the left column represent 2 conditions in experiment 1. Schemes illustrated in the right column (top) represent the condition in experiment 2. Those in the right column (bottom) represent the condition reported in Wang and Sainburg (2009). Initial rotation arrows indicate direction of hand movement on initial exposure to visual rotation (30° CCW for left, 30° CW for right arm). Adapted response arrows indicate direction of hand movement following full adaptation to visual rotation.
Our findings indicate that opposing rotations with matched target directions resulted in the greatest bilateral interference, reflected by significant detriments in performance observed for both arms. It is likely that this condition of opposing visual rotations and the same target directions required differential processing of visual feedback and differential specifications of movement kinematics for the two arms, respectively, which resulted in greatest interference. Our results indicating the least bilateral interference when both target directions and visual rotations were the same in extrinsic coordinates may seem to contradict previous findings that bilateral coordination is less stable for movements requiring different joint motions (e.g., Franz et al. 1991; Heuer 1996; Swinnen and Walter 1988). However, we expect that the requirement to adapt to visuomotor rotations places the stress of this task on visual feedback conditions, which are best reflected by extrinsic rather than joint-based coordinates (Sainburg and Wang 2002; Wang and Sainburg 2003). This idea is consistent with previous studies that have examined the effects of manipulating visual feedback on coordination. Although bilateral coordination is normally unstable when subjects are required to produce movements that are matched in Cartesian space, but require opposing rotations in joint space, Bogaerts et al. (2003) recently showed that virtual feedback that displays the parallel rotations of the hand as opposing rotations enables stable performance. These findings indicate that “mirror-imaged” joint configurations are not always a requirement for optimal bilateral coordination, which supports the notion that a bias toward movement symmetry in intrinsic coordinates can be modified or even reversed under altered feedback conditions (Howard et al. 2009).
Previous demonstrations that optimal coordination between the arms occurs when movements are mirror-imaged (e.g., Kelso et al. 1979; Semjen et al. 1995; Swinnen et al. 1998) caused some to posit that identical neural commands could be sent to both arms when a given task required intrinsically consistent movements, thereby facilitating symmetrical activations of homologous muscles. One of our conditions in which both target and rotation directions were opposing between the arms (i.e., Fig. 5) would be analogous to the above-mentioned condition and, indeed, bilateral interference observed in that condition was relatively small. However, in the condition in which the direction of both variables was the same between the arms (i.e., Fig. 4), we showed the lowest bilateral interference. This indicates that a bilateral task involving the same arm movements in the extrinsic coordinate frame might simplify coordination, probably because the computational demands to process visual information are reduced when the two arms move in parallel, relative to extrinsic coordinate space. This is consistent with previous work indicating that spatial coupling of bimanual movements occurs within a visually based extrinsic space (Weigelt and Cardoso de Oliveira 2003) and provides further support to the idea that visual information processing can play a major role in bilateral coordination (Bogaerts et al. 2003; Mechsner et al. 2000). Our current findings are thus consistent with the idea that bilateral coordination is facilitated when movements are consistent between the arms in the coordinate frame of either the visual or proprioceptive system, depending on which system has greater computational demands (e.g., Kelso et al. 1979; Semjen et al. 1995; Swinnen et al. 1998). That is, when the role of visual feedback is minimized during cyclic bilateral movements, for example, proprioceptive processing demands are higher than those of vision. In that case, coordination is best when movements are matched in joint coordinates. However, when visual information processing demands are high, as in the current study and previous studies using virtual feedback (Bogaerts et al. 2003), bilateral coordination can be most stable when movements are matched in extrinsic coordinates.
Our data also indicate that, under each condition, bilateral interference was larger for the left nondominant arm. We propose two explanations, which are not mutually exclusive, to account for this finding. First, it is plausible that our subjects may have focused their attention on the performance of the dominant arm. In fact, previous studies have emphasized an important role of visual attention in adapting to novel sensorimotor transformations (Ingram et al. 2000; Redding et al. 1992; Taylor and Thoroughman 2007). Although our experiment did not control for attentional selectivity between right and left arm movements, there was no bias in the experimental design for either right or left arm attention. Nevertheless, it may be natural to focus attention on the dominant arm under such novel adaptation conditions. Second, we have previously documented that the dominant arm is more proficient at coordinating task dynamics for accurate control of movement trajectories (e.g., Sainburg 2002, 2005; Sainburg and Kalakanis 2000). Consistent with this, Dounskaia et al. (2010) showed that during bilateral tasks, the relative deficits of the nondominant arm in coordinating intersegmental dynamics becomes amplified, resulting in substantial errors in continuous circle drawing, compared with movements of the dominant arm. It is plausible that this coordination difference resulted in the relatively poorer nondominant arm performance during bilateral movements in the current study, which in turn resulted in less transfer from the unilateral to bilateral movement conditions for the nondominant arm.
In fact, our current model of motor lateralization proposes that both hemispheres contribute specialized functions to control of unilateral movement of each arm: the hemisphere contralateral to the dominant arm contributes processes that control task dynamics to each arm and the hemisphere contralateral to the nondominant arm contributes processes that control limb impedance to the each arm (Sainburg 2005; Wang and Sainburg 2007). This model has recently been supported by a series of studies in the ipsilesional arm of stroke patients (Haaland et al. 2009; Schaefer et al. 2007, 2009a,b). Damage to the left hemisphere in right-handed stroke patients produces deficits in intersegmental coordination in the ipsilesional arm, that is, the arm on the same side as the stroke that is often thought of as intact with respect to motor control. In contrast, damage to the right hemisphere produces deficits in control of stable limb position, or limb impedance (Haaland et al. 2009; Schaefer et al. 2007). A recent study showed that these differences are amplified when patients adapt to novel visuomotor rotations. Most relevant to this study, left hemisphere damage produced substantial deficits in trajectory adaptation in the left arm of patients (Schaefer et al. 2009b). These studies in stroke thereby support our bihemispheric model of motor lateralization. It is plausible that bilateral movements challenge the demands on this system because both arms require resources from each hemisphere. The fact that the left arm experienced the most profound interference in trajectory adaptation is thus consistent with this model of motor lateralization.
Remarkably, our findings indicate unambiguous generalization of visuomotor adaptation, not only from the unilateral to bilateral, but also from the bilateral to unilateral movement conditions, despite the extent of bilateral interference. Initial unilateral adaptation with the right arm transferred to facilitate the subsequent same-arm performance during the bilateral session in all conditions (completely in one condition). Left arm adaptation also transferred in the two conditions with the intrinsic target directions (experiment 2 in the present study; experiment 1 in our previous study), although the amount of transfer was rather limited. It must be stressed that an accurate measure of “transfer” from unilateral to bilateral conditions was precluded by the effects of bilateral interference discussed earlier.
However, bilateral adaptation transferred almost completely to subsequent unilateral performance with the right arm in every condition, and also to the left arm in most conditions. It is noteworthy here that our previous study (Wang and Sainburg 2009) demonstrated that bilateral interference can suppress improvement in motor performance, but not necessarily motor learning per se. In the condition used in that study (i.e., extrinsic rotations, intrinsic target directions), the degree of interference observed for the left arm during the bilateral session was large and did not improve much during the course of the bilateral session. Surprisingly, however, left arm performance at the beginning of the subsequent unilateral session was substantially improved over the performance at the end of the previous bilateral session. This indicated that visuomotor adaptation occurred, despite the presence of bilateral interference in performance. That study also showed that the adaptation obtained during the preunilateral session was fully retained in the postunilateral performance with the same arm, despite large interference effects observed during the bilateral session (see experiment 3 in our previous study). Our current findings confirm our previous finding that substantial generalization can occur between bilateral and unilateral movement conditions, regardless of the presence of bilateral interference in performance.
GRANTS
This research was supported by National Institute of Child Health and Human Development Grants K01-HD-050245 and R01-HD-039311.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- Bogaerts et al., 2003. Bogaerts H, Buekers MJ, Zaal FT, Swinnen SP. When visuo-motor incongruence aids motor performance: the effect of perceiving motion structures during transformed visual feedback on bimanual coordination. Behav Brain Res 138: 45–57, 2003 [DOI] [PubMed] [Google Scholar]
- Dounskaia et al., 2010. Dounskaia NV, Nogueira KG, Swinnen SP, Drummond E. Limitations on coupling of bimanual movements caused by arm dominance: when the muscle homology principle fails. J Neurophysiol 103: 2027–2038, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz et al., 1991. Franz EA, Zelaznik HN, McCabe G. Evidence of common timing processes in the control of manual, orofacial, and speech movements. J Mot Behav 24: 281–287, 1991 [DOI] [PubMed] [Google Scholar]
- Haaland et al., 2009. Haaland KY, Schaefer SY, Knight RT, Adair J, Magalhaes A, Sadek J, Sainburg RL. Ipsilesional trajectory control is related to contralesional arm paralysis after left hemisphere damage. Exp Brain Res 196: 195–204, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer, 1996. Heuer H. Coordination. In: Handbook of Perception and Action: Motor Skills, edited by Heuer H, Keele SW. San Diego, CA: Academic Press, 1996, vol. 2, p. 121–180 [Google Scholar]
- Howard et al., 2009. Howard IS, Ingram JN, Körding KP, Wolpert DM. Statistics of natural movements are reflected in motor errors. J Neurophysiol 102: 1902–1910, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram et al., 2000. Ingram HA, van Donkelaar P, Cole J, Vercher JL, Gauthier GM, Miall RC. The role of proprioception and attention in a visuomotor adaptation task. Exp Brain Res 132: 114–126, 2000 [DOI] [PubMed] [Google Scholar]
- Kelso et al., 1979. Kelso JA, Southard DL, Goodman D. On the nature of human interlimb coordination. Science 203: 1029–1031, 1979 [DOI] [PubMed] [Google Scholar]
- Mechsner et al., 2001. Mechsner F, Kerzel D, Knoblich G, Prinz W. Perceptual basis of bimanual coordination. Nature 414: 69–73, 2001 [DOI] [PubMed] [Google Scholar]
- Nozaki et al., 2006. Nozaki D, Kurtzer I, Scott SH. Limited transfer of learning between unimanual and bimanual skills within the same limb. Nat Neurosci 9: 1364–1366, 2006 [DOI] [PubMed] [Google Scholar]
- Redding et al., 1992. Redding GM, Rader SD, Lucas DR. Cognitive load and prism adaptation. J Mot Behav 24: 238–246, 1992 [DOI] [PubMed] [Google Scholar]
- Sainburg, 2002. Sainburg RL. Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res 142: 241–258, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg, 2005. Sainburg RL. Handedness: differential specializations for control of trajectory and position. Exerc Sport Sci Rev 33: 206–213, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg and Kalakanis, 2000. Sainburg RL, Kalakanis D. Differences in control of limb dynamics during dominant and nondominant arm reaching. J Neurophysiol 83: 2661–2675, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg and Wang, 2002. Sainburg RL, Wang J. Interlimb transfer of visuomotor rotations: independence of direction and final position information. Exp Brain Res 145: 437–447, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer et al., 2007. Schaefer SY, Haaland KY, Sainburg RL. Ipsilesional motor deficits following stroke reflect hemispheric specializations for movement control. Brain 130: 2146–2158, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer et al., 2009a. Schaefer SY, Haaland KY, Sainburg RL. Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy [Erratum in: Neuropsychologia 48;1178–1180;2010]. Neuropsychologia 47: 2953–2966, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer et al., 2009b. Schaefer SY, Haaland KY, Sainburg RL. Dissociation of initial trajectory and final position errors during visuomotor adaptation following unilateral stroke [Erratum in: Brain Res 1321;180–181;2010]. Brain Res 1298: 78–91, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semjen et al., 1995. Semjen A, Summers JJ, Cattaert D. Hand coordination in bimanual circle drawing. J Exp Psych Hum Percept Perform 21: 1139–1157, 1995 [Google Scholar]
- Swinnen, 2002. Swinnen SP. Intermanual coordination: from behavioural principles to neural–network interactions. Nat Rev Neurosci 3: 348–359, 2002 [DOI] [PubMed] [Google Scholar]
- Swinnen et al., 2001. Swinnen SP, Dounskaia N, Levin O, Duysens J. Constraints during bimanual coordination: the role of direction in relation to amplitude and force requirements. Behav Brain Res 123: 201–218, 2001 [DOI] [PubMed] [Google Scholar]
- Swinnen and Walter, 1988. Swinnen SP, Walter CB. Constraints in coordinating limb movements. In: Cognition and Action in Skilled Behaviour, edited by Colley AM, Beech JR. Amsterdam: North-Holland, 1988, p. 127–143 [Google Scholar]
- Taylor and Thoroughman, 2007. Taylor JA, Thoroughman KA. Divided attention impairs human motor adaptation but not feedback control. J Neurophysiol 98: 317–326, 2007 [DOI] [PubMed] [Google Scholar]
- Wang and Sainburg, 2003. Wang J, Sainburg RL. Mechanisms underlying interlimb transfer of visuomotor rotations. Exp Brain Res 149: 520–526, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang and Sainburg, 2005. Wang J, Sainburg RL. Adaptation to visuomotor rotations remaps movement vectors, not final positions. J Neurosci 25: 4024–4030, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang and Sainburg, 2007. Wang J, Sainburg RL. The dominant and nondominant arms are specialized for stabilizing different features of task performance. Exp Brain Res 178: 565–570, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang and Sainburg, 2009. Wang J, Sainburg RL. Generalization of visuomotor learning between bilateral and unilateral conditions. J Neurophysiol 102: 2790–2799, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt and Cardoso de Oliveira, 2003. Weigelt C, Cardoso de Oliveira S. Visuomotor transformations affect bimanual coupling. Exp Brain Res 148: 439–450, 2003 [DOI] [PubMed] [Google Scholar]
- Wiesendanger and Serrien, 2004. Wiesendanger M, Serrien DJ. The quest to understand bimanual coordination. Prog Brain Res 143: 491–505, 2004 [DOI] [PubMed] [Google Scholar]
- Wolf et al., 2008. Wolf SL, Winstein CJ, Miller JP, Thompson PA, Taub E, Uswatte G, Morris D, Blanton S, Nichols-Larsen D, Clark PC. Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: the EXCITE randomised trial. Lancet Neurol 7: 33–40, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]