Abstract
The electrophysiological properties of substantia nigra pars compacta (SNC) dopamine neurons can influence their susceptibility to degeneration in toxin-based models of Parkinson's disease (PD), suggesting that excitotoxic and/or hypoactive mechanisms may be engaged during the early stages of the disease. It is unclear, however, whether the electrophysiological properties of SNC dopamine neurons are affected by genetic susceptibility to PD. Here we show that deletion of PD-associated genes, PINK1 or HtrA2/Omi, leads to a functional reduction in the activity of small-conductance Ca2+-activated potassium channels. This reduction causes SNC dopamine neurons to fire action potentials in an irregular pattern and enhances burst firing in brain slices and in vivo. In contrast, PINK1 deletion does not affect firing regularity in ventral tegmental area dopamine neurons or substantia nigra pars reticulata GABAergic neurons. These findings suggest that changes in SNC dopamine neuron excitability may play a role in their selective vulnerability in PD.
INTRODUCTION
Mitochondrial dysfunction has been widely implicated in the pathogenesis of Parkinson's disease (PD) (Abou-Sleiman et al. 2006; Henchcliffe and Beal 2008; Schapira 2008), although how this dysfunction triggers the selective loss of substantia nigra pars compacta (SNC) dopamine neurons remains unclear. Studies on familial forms of PD have identified mutations in several genes that encode mitochondrial proteins, including PINK1, DJ-1, parkin, and HtrA2/Omi (Abou-Sleiman et al. 2006). SNC dopamine neurons, however, do not preferentially express these genes, and therefore some other factors must convey their selective vulnerability (Sulzer 2007). Of the many suggestions, one influential idea is that the unusual electrophysiological properties of dopamine neurons play a central role. For example, their unusual reliance on voltage-dependent L-type Ca2+ channels confers a susceptibility to mitochondrial toxins used to create animal models of PD (Chan et al. 2007). Moreover, mitochondrial toxins induce hypoactivity of SNC dopamine neurons through the activation of ATP-sensitive potassium (KATP) channels, suggesting that a loss of electrical activity can also contribute to the degeneration of these neurons (Liss et al. 2005). Indeed inactivation of either L-type Ca2+ channels or KATP channels protects SNC dopamine neurons in toxin-based mouse models of PD (Chan et al. 2007; Liss et al. 2005). These studies suggest that excitotoxic and/or hypoactive mechanisms may be engaged during the early stages of PD and provide evidence of selective changes that can influence dopamine neuron survival. Consequently, we hypothesized that genetic susceptibility to PD, in particular the loss of mitochondria-associated genes, may alter the excitability of SNC dopamine neurons.
Among the familial PD genes known, PTEN-induced kinase 1 (PINK1) provides the most direct link to mitochondria. PINK1 is a serine/threonine kinase that has been shown to be localized to mitochondria both in vitro and in vivo (Gandhi et al. 2006; Muqit et al. 2006; Silvestri et al. 2005). Although the targets for PINK1 remain unknown, several PD-associated genes have been found to interact with PINK1 including the E3 ubiquitin ligase Parkin (Clark et al. 2006; Exner et al. 2007; Park et al. 2006) and the stress-protective protease HtrA2/Omi (Plun-Favreau et al. 2007). In Drosophila, loss of PINK1 causes severe mitochondrial pathology and leads to both muscle and neuronal degeneration (Park et al. 2006; Yang et al. 2006). In mammals, studies on PINK1-deficient mice have reported mitochondrial dysfunction in the absence of dopamine neuron loss (Gautier et al. 2008; Gispert et al. 2009; Kitada et al. 2007). Despite this, PINK1-deficient mice display deficiencies in striatal dopamine release (Gispert et al. 2009; Kitada et al. 2007) and altered synaptic plasticity (Kitada et al. 2007) that can lead to age-dependent motor impairments (Gispert et al. 2009). We, therefore, took advantage of this mouse model to directly address whether the loss of a mitochondrial-associated PD gene can affect SNC dopamine neuron excitability. To do this, we conducted electrophysiological recordings from individual SNC dopamine neurons in ex vivo brain slice and in vivo.
METHODS
Animals
PINK1-deficient mice were generated by Lexicon Genetics (The Woodlands, TX), and HtrA2/Omi-deficient mice were generated by Martins et al. (2004). Generation procedures for PINK1- and HtrA2/Omi-deficient mice can be found here (Martins et al. 2004; Wood-Kaczmar et al. 2008). Mice were initially backcrossed on a C57BL/6 background and then interbred to generate study populations of animals containing all three possible genotypes [wild type, heterozygous, and homozygous (PINK1-deficient)]. Experimental wild type and PINK1-deficient mice were subsequently identified using PCR-based genotyping. Animal husbandry and experimental procedures were performed in full compliance with the United Kingdom Animal (Scientific Procedures) Act of 1986.
Slice preparation
Electrophysiological recordings were performed on adult PINK1 mice aged ∼3–4 mo and HtrA2/Omi mice aged ∼1–2 mo. To prepare SNC slices, animals were anesthetized with isoflurane and decapitated. Thin 220 μm horizontal midbrain slices were cut with a Vibratome (Leica VT1000S) while being bathed in an ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM) 120 NaCl, 25 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 2 CaCl, 1 MgCl2, and 10 glucose, bubbled with a mixture of 95% O2-5% CO2. After sectioning, slices were allowed to recover for ≥45 min before being placed in the recording chamber and superfused with oxygenated ACSF at 31–32°C at a rate of 2–4 ml/min. Midbrain slices containing a clearly defined SNC at the level of the medial terminal nucleus (MT, medial terminal nucleus of the accessory optic tract) were used for the experiments.
In vitro electrophysiology
Cells were visualized using infrared differential interference contrast video microscopy. Conventional tight-seal (>3GΩ) whole cell patch clamp and cell-attached recordings were made using an NPI SEC-10LX amplifier (npi) and WinWCP software (Courtesy of John Dempster, University of Strathclyde) or Spike2 v5 (CED). For cell-attached recordings, electrodes (2–5 MΩ) made from borosilicate glass (Harvard Apparatus) were filled with 120 mM NaCl. For whole cell recordings, electrodes were filled with internal solution containing (in mM) 140 K-gluconate, 5 NaCl, 1 MgCl2, 10 HEPES, 1 EGTA, 2 Mg-ATP, and 0.5 Li-GTP. Neurobiotin (0.10%) was also added to the intracellular solution for labeling of recorded neurons. Records were filtered at 1 kHz and digitized at 3–5 kHz. Dopamine neurons were identified by having a spontaneous pacemaker activity of 1–4 Hz and the presence of a large Ih-dependent voltage sag following injection of hyperpolarizing current. Neurochemical identity was subsequently confirmed using co-immunohistochemical-labeling for neurobiotin and tyrosine hydroxylase (see following text). Spontaneous firing activity was observed in current-clamp mode immediately after forming a whole cell configuration. Ih current was assayed for in voltage-clamp by using a holding voltage of −50 mV and applying a series of hyperpolarizing voltage steps for 1 s in −10 mV increments to −120 mV. Initial currents following the capacitive spike were used as a measure of input resistance. Linear fits of the data near resting membrane potential were then used to determine an estimate of Rinput (Supplementary Fig. S1).1 During SK pharmacology experiments, the following concentrations of drugs were used: apamin (300 nM), 1-ethyl-2-benzimidazolinone (1-EBIO, 200 μM). During experiments to isolate the intrinsic excitability of SNC dopamine neurons the following drugs were used: picrotoxin (100 μM) to block GABAA receptors, CGP35348 (30 μM) to block GABAB receptors, 2,3-dioxo-6-nitro-1,2,3,4- tetrahydrobenzo[f]quinoxaline-7-sulfonamide (NBQX; 5 μM) and d(−)-2-amino-5-phosphonopentanoic acid (d-APV; 50 μM) to block AMPA and N-methyl-d-aspartate (NMDA) receptors, respectively, A-methyl-4-carboxyphenylglycine (MCPG) (1 mM) to block metabotropic glutamate receptors (mGluRs), and haloperidol (10 μM) to block dopamine D2 receptors. For experiments examining the effects of intracellular Ca2+ release, the following drugs were used: cyclopiazonic acid (CPA, 10 μM), which depletes endoplasmic reticulum (ER) Ca2+ stores, and CGP-37157 (10 μM), which inhibits mitochondrial Na+/Ca2+ exchanger-mediated Ca2+ release. For examining neuronal excitability, NMDA (20 μM) was used. Drugs were dissolved in ACSF and applied by bath perfusion for ≥10 min before responses were measured. Apamin, 1-EBIO, CPA, and CGP-37157 were obtained from Tocris. All other drugs were obtained from Sigma Aldrich UK.
Action potential waveforms were analyzed using WinWCP (University of Strathclyde) and Spike2 v5 (CED). The average basal instantaneous firing frequency and interspike interval (ISI) were determined across 60 successive action potentials in cell-attached mode or immediately after forming whole cell configuration with the neuron. The coefficient of variation of the ISI (CV-ISI) was calculated as the ratio of the SD ISI to the mean ISI. Average action potential waveforms for each neuron were obtained using Spike2 software to obtain values for the action potential peak, threshold, peak of the afterhyperpolarization (AHP), and time-to-peak of the AHP. Any bursts were defined as beginning when two action potentials occur within 80 ms of each other and ending when an action potential fails to occur for 160 ms.
During experiments to isolate SK channel activity, neurons were voltage-clamped at −50 mV (near their resting membrane potential) and a 2 ms depolarizing step was applied to +20 mV to trigger an unclamped action potential. This produced an outward tail current that could be inhibited by apamin (300 nM). On application of apamin, a fast transient outward current insensitive to apamin could be observed to decay within 20 ms. The apamin-sensitive current, representing SK channel conductance, peaked at ∼20 ms after the test pulse, and decayed within 300 ms. Consistent with this, the tail current could be fitted with two exponentials containing a fast deactivating (<20 ms) component and a second component which decayed slower over 300 ms. Therefore we routinely calculated the integral of the outward current from 20 to 300 ms after the test pulse to assess the charge transfer representing SK channel activity. SK channel conductance was evoked in an all-or-none manner and could be abolished by TTX (1 μM). Further increasing the test pulse amplitude or prolonging the test pulse duration added a TTX-insensitive component (Supplementary Fig. S2). Reversal potentials of isolated SK currents were determined by using a depolarizing pulse to +20 mV from a holding potential of −50 mV, followed by test potentials ranging from −100 to −50 mV in 10 mV increments. Averaged amplitudes of SK currents were then plotted as a function of holding potential and reversal potential estimated.
For examination of whole cell Ca2+ currents, potassium gluconate was substituted with cesium methanesulphonate (140 mM) in the intracellular solution, and the extracellular ACSF was supplemented with tetrodotoxin (TTX, 1 μM) and tetraethylammonium (TEA, 20 mM) to block voltage-gated Na+ and K+ channels respectively. Neurons were voltage-clamped at −70 mV and a 250 ms ramp to +30 mV was applied to measure voltage-dependent Ca2+ currents. To selectively isolate low-voltage-activated T-type Ca2+ currents, nifedipine (10 μM) was added to the ACSF to block high-voltage-activated L-type Ca2+ channels, and neurons were depolarized to −40 mV from a holding potential of −100 mV. After recording baseline currents, T-type Ca2+ currents were then inhibited in some neurons with mibefradil (10 μM) by bath perfusion (10 min). Linear leakage current was not subtracted. Cells the access resistance and capacitance of which increased significantly during the course of recording (>20%) were discarded.
Statistical analysis of in vitro data
All averaged values are expressed as means ± SD. The Kolmogorov-Smirnov test and the D'Agostino-Pearson omnibus test were used to confirm normality of data sets. Data were then analyzed using either Student t-test or two-way ANOVAs with a probability level of P < 0.05 qualifying as statistically significant. When differences were found using ANOVA, Bonferroni's post hoc test was used for multiple pairwise comparisons (Prism, Graphpad).
In vivo electrophysiology
Wild type and PINK1-deficient mice were anesthetized with urethan (2.0 g/kg ip; Sigma); supplemental doses of ketamine (20 mg/kg ip; Ketaset, Willows Francis, UK) and xylazine (2 mg/kg ip; Rompun, Bayer, Germany) were given if required. Body temperature was maintained using a homeothermic heating device (Harvard Apparatus). The depth of anesthesia was assessed by testing reflexes to a hindpaw pinch. Corneal dehydration was prevented with application of Lacri-lube eye ointment (Allergan Pharmaceuticals). A craniotomy on either side of the sagittal suture was performed centered above the SNC on either side of the sagittal suture. Dura mater overlying the exposed cortex following craniotomy was removed gently by using a very fine-toothed forceps. Saline solution (0.9% wt/vol NaCl) was applied to the exposed cortex to prevent dehydration during recording.
Glass microelectrodes were lowered into the SNC using a micromanipulator (LSS-8000 Inchworm Microdrive System, Burleigh) to a depth of 3.5–4.4 mm from the dural surface and using the following stereotaxic coordinates: anterior, −3.08 to −3.4 mm; lateral, −0.8 to −1.3 mm. Extracellular neuronal activity was monitored using the glass microelectrode in 0.5 M NaCl or physiological saline, which was broken back to give a final tip diameter of 1–2 μm and a resistance of 6–15 MΩ (in situ). Extracellular recordings were AC coupled, amplified (1,000 times), band-pass filtered between 0.3 and 5.0 kHz (NeuroLog System, Digitimer) and acquired with Spike2 software (version 5.08, Cambridge Electronic Design) on a PC. Electrical interference from analog signals was minimized by using HumBug (Quest Scientific, Canada). The signals were then displayed on a digital oscilloscope (Tektronics) and captured using a 1401plus A-D converter (Cambridge Electronic Design). Data were collected from neurons exhibiting broad triphasic action potentials and a spontaneous firing rate <10 Hz. SNC neurons with these properties in vivo are uniformly dopaminergic (Brown et al. 2009; Grace and Bunney 1983). Spike2 software was used to analyze data off-line. Neuronal activity was typically measured for 2–3 min each, providing the baseline firing profile of individual neurons. At the end of experiments, some mice were given a lethal overdose of anesthetic, and brain slices were examined for histological verification of the recording sites.
The baseline firing rate of each neuron and CV-ISI was quantified over a 2 min period (with band-pass filter settings of 0.3–5 kHz). Bursts were measured using the baseline recordings and were defined as beginning when two action potentials occur within 80 ms of each other and ending when an action potential fails to occur for 160 ms.
Statistical analysis of in vivo data
All averaged values are expressed as means ± SD. Statistical comparison of firing rates, CV-ISI and percentage of spikes in bursts between wild type and PINK1-deficient neurons were made by using Student t-test with a probability level of P < 0.05 qualifying as statistically significant.
Immunohistochemistry
Following in vitro recordings, brain slices were fixed with 4% paraformaldehyde in PBS, pH 7.4, for 30 min at room temperature. The fixative was removed with four washes of PBS solution. Slices were treated for 30 min with a blocking solution containing 10% normal donkey serum (Jackson Laboratories), 0.2% BSA, and 0.5% Triton-X (Sigma) for permeabilization in PBS. Primary antibody, chicken anti-tyrosine hydroxylase (1:1,000; Abcam), was applied overnight in a carrier solution consisting of 1% donkey serum, 0.2% BSA, and 0.5% Triton-X in PBS. Afterward, slices were washed four times in PBS for 10 min and then incubated with the following secondary antibodies: AlexaFluor488 goat anti-chicken IgG (1:1,000; Molecular Probes) and streptavidin-AlexaFluor555 (1:1,000; Molecular probes for 90 min at room temperature in 0.5% Triton X in PBS). Subsequently, slices were washed six times in PBS for 5 min and mounted in Vectorshield Mounting Medium (Vector Laboratories). Confocal laser scanning microscopy was performed using a Leica SP confocal microscope through a ×40 or ×63 Plan-Apochromat 1.32 numerical aperture oil immersion objective. AlexaFluor 488 was excited by a 488 nm line of an Argon laser and AlexaFluor 555 by a 561 nm line of a steady state laser. To reduce spectral bleed-through, the emission filter bands for AlexaFluor 488 and AlexaFluor 555 were restricted to 498–550 and 580–640 nm, respectively. Images were taken at a resolution of 1,024 × 1,024 and processed using Leica Confocal Software (Leica Microsystems) and Adobe Photoshop CS3 (Adobe Systems).
Following in vivo experiments, animals were given a lethal dose of anesthetic then transcardially perfused with 200 ml of 0.1 M phosphate-buffered saline (PBS) solution at pH 7.4 followed by 400 ml of 4% wt/vol paraformaldehyde (PFA) solution. The brain was subsequently removed and postfixed in 4% PFA. Following perfusion and fixation, the whole mouse brain was cryoprotected in 30% sucrose in PBS, embedded in optimal cutting temperature (OCT) medium, frozen in isopentane at −50°C and sectioned at 15–30 μm on a cryostat (Leica CM1800, Leica Microsystems). The floating sections were rinsed in PBS and then processed as described for in vitro experiments. Anatomical localization of recorded neurons was assessed by examining electrode tracts in combination with immunolabeling for tyrosine hydroxylase to identify SNC dopamine neurons.
RESULTS
PINK1-deficient SNC dopamine neurons display irregular firing patterns
We compared the firing activity of individual dopamine neurons within the SNC of adult (age ∼3–4 mo) wild type and PINK1-deficient mice. In brain slices, SNC dopamine neurons typically display highly regular “pacemaker” firing activity at a frequency of ∼1 – 4 Hz (Grace and Onn 1989). Using whole cell recordings, we found that PINK1-deficient dopamine neurons had the same action potential firing rates as wild type neurons [Fig. 1, A–C; PINK1: 2.13 ± 0.54 (SD) Hz, n = 54; wild type (WT): 1.98 ± 0.33 Hz, n = 33; NS]. Unexpectedly, however, PINK1-deficient dopamine neurons fired action potentials in a more irregular pattern as shown by the variability in ISIs between action potentials (Fig. 1D; CV-ISI, PINK: 1 0.29 ± 0. 19, n = 54; WT: 0.16 ± 0.10, n = 33; P < 0.05). No differences were observed in other basic properties including cell capacitance (PINK1: 29.94 ± 6.91 pF, n = 54; WT 31.24 ± 5.23 pF, n = 33; NS), input resistance (Supplementary Fig. S1; PINK1: 176 ± 65 MΩ, n = 54; WT: 161 ± 56 MΩ, n = 33; NS), and hyperpolarization-activated cation (Ih) currents (Supplementary Fig. S1). To confirm that the firing irregularity was not an artifact of the whole cell configuration, we conducted cell-attached recordings which also revealed a difference in firing regularity between PINK1-deficient and wild type dopamine neurons (Fig. 1D; CV-ISI, PINK1: 0.27 ± 0.19, n = 14; WT: 0.15 ± 0.08, n = 17, P < 0.05) without any changes in firing rate (PINK1: 1.98 ± 0.46 Hz, n = 14; WT: 2.13 ± 0.84 Hz,, n = 17; ns).
Fig. 1.
PINK1-deficient substantia nigra pars compacta (SNC) dopamine neurons display irregular firing patterns. Representative examples of an individual wild type (A) and PINK1-deficient (B) SNC dopamine neuron showing spontaneous activity (scale bars: cell-attached 25 pA, 1 s; whole cell 10 mV, 1 s), interspike interval plots, and immunohistochemical identification (scale bar: 10 μm) C: PINK1-deficient neurons have a similar rate of firing activity to wild type neurons. However, raw data of CV-ISI for 50 wild type and 68 PINK1-deficient neurons shows that PINK1-deficient mice have a greater proportion of irregularly firing neurons D: comparison of action potential waveforms between wild type and PINK1-deficient neurons reveals a significant reduction in the time-to-peak of the afterhyperpolarization (TTP-AHP; E), indicating a disruption of SK channel-mediated conductances. NS, not significant; * = P < 0.05.
PINK1-deficient SNC dopamine neurons have reduced SK channel function
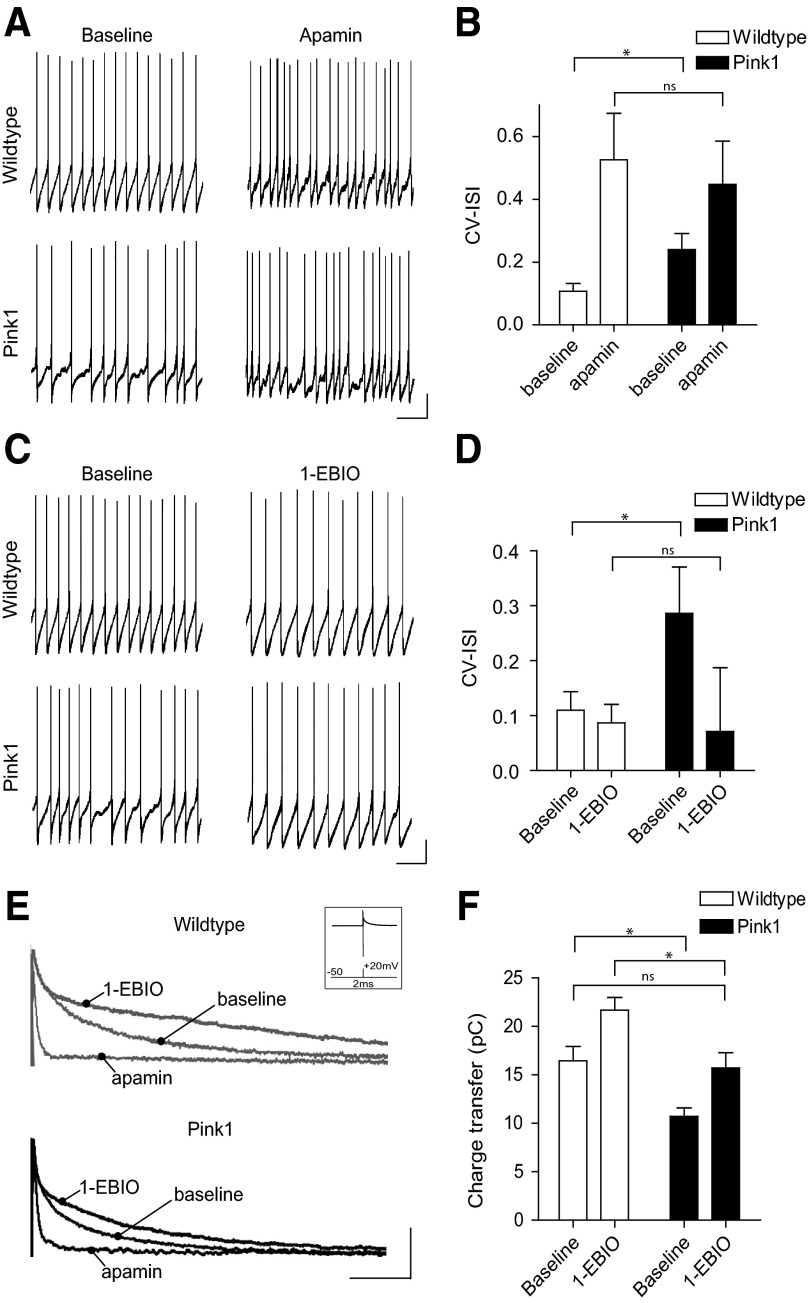
Firing regularity in SNC dopamine neurons is strongly controlled by small conductance Ca2+-activated potassium channels (SK) (Wolfart and Roeper 2002; Wolfart et al. 2001). SK channels are activated by the transient elevation of intracellular Ca2+ during an action potential, and their activity contributes to a prolonged AHP during which the return to baseline reflects the decay of intracellular Ca2+ levels (Stocker 2004). We therefore tested the possibility that a reduction in SK channel function was causing firing irregularity in PINK1-deficient neurons. Consistent with this, comparison of action potential waveforms between wild type and PINK1-deficient neurons showed that the time to peak of the AHP (TTP-AHP) was selectively reduced in PINK1-deficient neurons (Fig. 1E). Moreover, pharmacological blockade of SK channels, with apamin (300 nM), normalized the differences in firing irregularity between PINK1-deficient and wild type neurons (Fig. 2, A and B; PINK1 CV-ISI, baseline: 0.24 ± 0.05, apamin: 0.45 ± 0.13, n = 8; WT, baseline: 0.11 ± 0.02, apamin: 0.52 ± 0.15, n = 7; P < 0.05). Conversely, pharmacological facilitation of SK channel function, with 1-EBIO (200 μM), restored the firing regularity of PINK1-deficient neurons to that observed in wild type neurons (Fig. 2, C and D; CV-ISI, PINK1 baseline: 0.29 ± 0.08, n = 8; WT baseline: 0.11 ± 0.03, n = 6; P < 0.05; PINK1 1-EBIO: 0.07 ± 0.12, n = 8; WT 1-EBIO: 0.09 ± 0.03, >n = 6; NS).
Fig. 2.
PINK1-deficient SNC dopamine neurons have reduced SK channel function. A and B: the SK channel blocker, apamin, normalizes the differences in firing patterns between PINK1-deficient and wild type neurons, as shown by their coefficient of variation of the interspike interval (CV-ISI). C and D: conversely, pharmacological facilitation of SK channel function, with 1-ethyl-2-benzimidazolinone (1-EBIO), restores the firing regularity of PINK1-deficient neurons to wildtype levels. E and F: analysis of SK channel-mediated currents using voltage-clamp, shown inset, confirms a reduction of SK channel activity in PINK1-deficient dopamine neurons. Moreover, this reduction can be restored to wildtype baseline levels using 1-EBIO. Scale bars: current-clamp 10 mV, 1 s; voltage-clamp 100 pA, 50 ms. NS = not significant, * = P < 0.05.
These pharmacological experiments are consistent with the hypothesis that there is a functional reduction in SK channel activation in PINK1-deficient neurons. To directly examine this, we isolated SK channel-mediated currents. Neurons were voltage-clamped at –50 mV (near their resting membrane potential) and a 2 ms depolarizing step to +20 mV was used to evoke a single unclamped action potential (Supplementary Fig. S2). This protocol produced an outward tail current lasting ≤300 ms. The fast component of this current was insensitive to apamin and decayed within 20 ms. A slower apamin-sensitive SK channel-mediated component peaked at 10–20 ms (Fig. 2E). No significant differences were observed in apamin-insensitive currents between wild type and PINK1-deficient neurons (PINK1: 1.28 ± 0.47 pC, n = 33; WT: 1.37 ± 0.34 pC, n = 24; ns). Consequently, we calculated the integral of the outward tail current from 20 to 300 ms after the test pulse to assess the charge-transfer representing SK channel activity. This analysis revealed a reduction in SK channel-mediated currents in PINK1-deficient neurons compared with wild type (Fig. 2E; PINK1: 10.98 ± 2.61 pC, n = 33; WT: 16.44 ± 3.63 pC, n = 24; P < 0.05). Furthermore, increasing the open probability of SK channels using 1-EBIO restored tail current charge transfer in PINK1-deficient neurons to wild type baseline levels (Fig. 2E; charge transfer, PINK1: 15.72 ± 1.92 pC, n = 6; WT: 21.68 ± 1.48 pC, n = 5; P < 0.05). We did not observe any differences in SK channel reversal potential (WT: −87.10 ± 6.35 mV, n = 7; PINK1: −87.52 ± 9.55 mV, n = 6; P > 0.05). Moreover, when we administered longer test pulses to prolong Ca2+ entry into the neuron and saturate SK channel activity, we no longer observed a significant difference between PINK1-deficient and wild type neurons (Supplementary Fig. S3, A–D). This suggests that wild type levels of functional SK channels are present in PINK1-deficient neurons but are suboptimally activated during pacemaker firing. Consistent with this, using double immunolabeling for tyrosine hydroxylase (the rate-limiting enzyme in dopamine synthesis) and SK3 protein (the most common SK channel-subtype in the SNC) (Wolfart et al. 2001), we found no overt differences in channel expression between PINK1-deficient and wild type mice (Supplementary Fig. S3E).
Although our data are indicative of an intrinsic reduction of SK channel activity in PINK1-deficient neurons, we wanted to confirm this by isolating the intrinsic excitability of SNC dopamine neurons. To do this, we carried out current- and voltage-clamp recordings in ACSF containing synaptic blockers (antagonists of GABAA, GABAB, AMPA, NMDA, mGluR, and D2 receptors; see methods). Even in the presence of this pharmacological blockade, we still observed increased firing irregularity in PINK1-deficient neurons compared with wild type (CV-ISI, PINK1: 0.20 ± 0.06, n = 16; WT: 0.12 ± 0.05 Hz, n = 13; P < 0.05). Similar to our previous data, we also found that PINK1-deficient and wild type neurons had identical firing rates (Hz, PINK1: 2.51 ± 0.81 Hz, n = 16; WT: 2.67 ± 0.72 Hz, n = 13; NS). Moreover, voltage-clamp recordings confirmed a functional reduction in SK channel activation was still present in PINK1-deficient neurons under these conditions (charge transfer, PINK1: 11.24 ± 2.84 pC, n = 16; WT: 15.23 ± 3.36 pC, n = 13; P < 0.05). It should also be noted that in the presence of TTX (which will eliminate action potential-dependent synaptic activity), we also observed a reduced depolarization-induced SK current in PINK1-deficient mice compared with controls using brief test pulses (Supplementary Fig. S3, C and D).
Impaired intracellular Ca2+ signaling underlies SK channel dysfunction
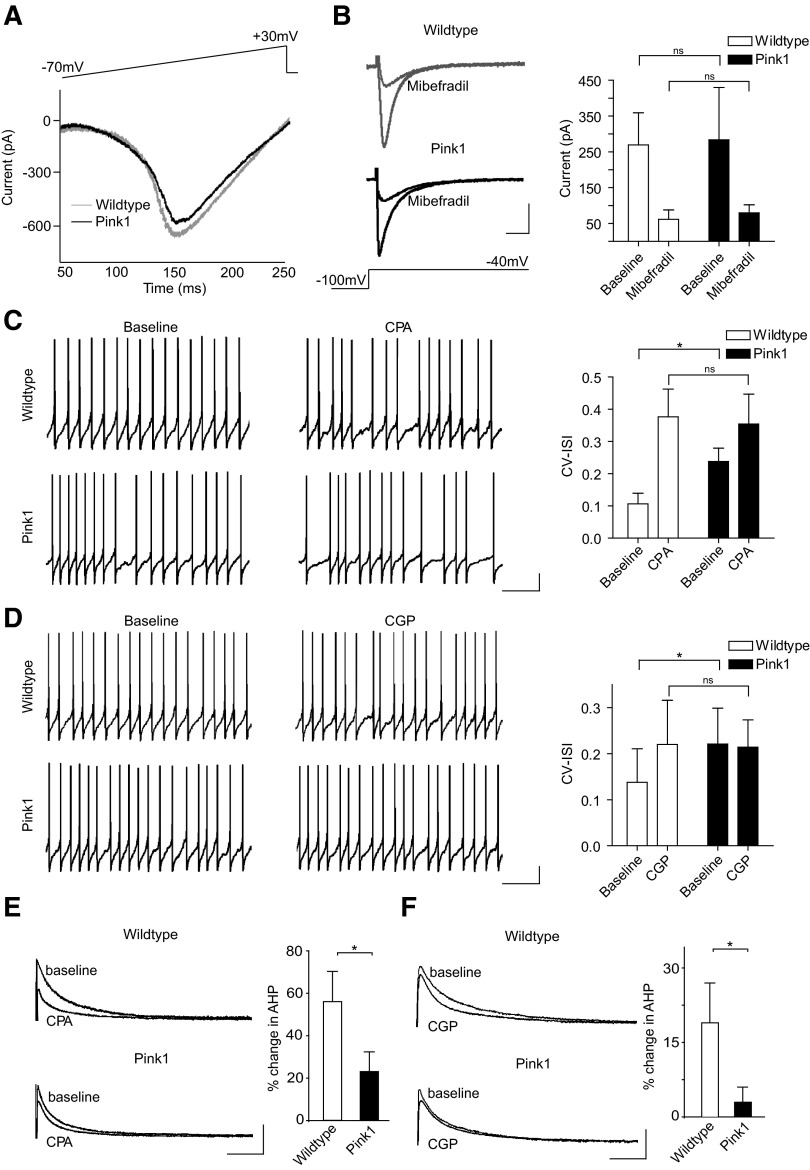
A deficit in SK channel function could be the result of either reduced Ca2+ influx through voltage-dependent T-type Ca2+ channels (Wolfart and Roeper 2002) or impaired Ca2+-induced Ca2+ release from ER stores; this is known to provide amplification of local Ca2+ microdomains that facilitate SK channel activity (Bond et al. 2005; Wolfart and Roeper 2002). To examine these possibilities, we measured whole cell Ca2+ currents induced by voltage ramp from −70 to +30 mV. We found no significant difference in the amplitude of total peak Ca2+ currents between PINK1-deficient and wild type neurons (Fig. 3A; PINK1: −593.93 ± 21.16 pA, n = 11; WT: −597.74 ± 36.88 pA, n = 9; NS). Although we would expect any change in T-type Ca2+ current activity to be evident in this overall Ca2+ current, we wanted to confirm this by selectively isolating low-voltage-activated Ca2+ channels. To do this, nifedipine (10 μM) was added to the ACSF to block high-voltage-activated L-type Ca2+ channels, and neurons were depolarized to −40 mV from a holding potential −100 mV. After recording baseline currents, the presence of T-type Ca2+ currents was confirmed in some neurons by inhibiting their activity with mibefradil (10 μM). We observed no difference in the activity of low-voltage-activated T-type Ca2+ channels between PINK1-deficient and wild type neurons (Fig. 3B; PINK1: 283.54 ± 146.54 pA, n = 11; WT: 269.42 ± 89.82 pA, n = 12; NS; mifefradil, PINK1: 79.67 ± 22.47 pA, n = 3; WT: 61.66 ± 26.31 pA, n = 3; NS). Together these results show that rapid Ca2+ signaling through voltage-sensitive Ca2+ channels is normal in PINK1-deficient neurons. Next we examined whether blockade of ER Ca2+ release could differentially affect SK channel activity in PINK1-deficient and wild type neurons. To do this, we bath applied CPA (10 μM) to neurons, which depletes ER Ca2+ stores (Seidler et al. 1989), and examined its effect on firing activity and SK channel-mediated currents. We found CPA had a smaller effect on the firing regularity of PINK1-deficient neurons compared with wild type (Fig. 3C; CV-ISI: PINK1, baseline: 0.24 ± 0.04, CPA: 0.39 ± 0.05, n = 5; WT, baseline: 0.11 ± 0.03, CPA: 0.38 ± 0.09, n = 6; P < 0.05). Moreover, AHP currents recorded in voltage-clamp showed a significantly smaller reduction in PINK1-deficient neurons following CPA treatment compared with wild type (Fig. 3E; percentage change in charge transfer in CPA, PINK1: 23 ± 9%, n = 5; WT: 56 ± 14%, n = 6; P < 0.05). Together, these results suggest that ER Ca2+ release-dependent regulation of SK channel activity is impaired in PINK1-deficient neurons.
Fig. 3.
PINK1-deficient neurons have impaired endoplasmic reticulum (ER) and mitochondrial Ca2+ release that regulate SK channel function. Comparison of whole cell Ca2+ currents (A) and low-voltage-activated T-type Ca2+ currents (B) between PINK1-deficient and wild type SNC dopamine neurons reveals that the loss of SK channel activity in PINK1-deficient neurons does not coincide with a reduction in Ca2+ influx through voltage-dependent Ca2+ channels. Whole cell Ca2+ current traces presented in A were selected for their similar amplitudes. (Scale bars: 100 pA, 50 ms). C: blockade of ER Ca2+ release with cyclopiazonic acid (CPA) normalizes the firing regularity of PINK1-deficient and wild type neurons. D: inhibition of mitochondrial Na+/Ca2+ exchanger-mediated Ca2+ release using CGP-37157 significantly increases the irregularity of firing patterns in a proportion of wild type neurons but has no significant effect on PINK1-deficient neurons. Consistent with current-clamp recordings, voltage-clamp recordings reveal a smaller reduction in SK channel-mediated currents in PINK1-deficient neurons following CPA (E) and CGP treatment. (scale bars: current-clamp, 10 mV, 1 s; voltage-clamp, 100 pA, 50 ms). *, P < 0.05.
Because PINK1 is predominantly localized and functions in the mitochondria (Deas et al. 2009), one crucial question is whether PINK1 deficiency and mitochondria play a role in regulating SK channel function. Mitochondria can directly influence the Ca2+ concentration in the cytosol of the cell by importing Ca2+ via the mitochondrial Ca2+ uniporter or transporting Ca2+ from the interior of the organelle into the cytosol by means of Na+/Ca2+ or H+/Ca2+ exchangers (Brini 2003). Uptake and release of Ca2+ from mitochondria can also directly regulate ER Ca2+ release. Moreover, mitochondria can sequester Ca2+ released from the ER as well as provide Ca2+ for replenishing ER stores (Brini 2003; Rizzuto et al. 1998). Importantly, impaired Na+/Ca2+ exchanger-mediated Ca2+ release occurs following loss of PINK1 function in dopamine neurons (Gandhi et al. 2009). We therefore examined whether inhibition of the Na+/Ca2+ exchanger with the specific inhibitor CGP-37157 could affect SK channel function in PINK1-deficient and wild type neurons. Current-clamp recordings showed that CGP-37157 (10 μM) had no significant effect on the regularity of PINK1-deficient neurons. In contrast, wild type neurons increased firing irregularity following CGP-37157 [Fig. 3D; (CV-ISI): PINK1, baseline: 0.22 ± 0.07, CGP: 0.24 ± 0.09, n = 12; WT, baseline: 0.14 ± 0.07, CGP: 0.20 ± 0.10, n = 14; P < 0.05]. Consistent with our current-clamp recordings, we observed a reduction in SK channel-mediated currents in wild type neurons following CGP-37157. Strikingly, this effect was largely absent in PINK1-deficient neurons (Fig. 3F; percentage change in charge transfer in CGP-37157, PINK1: 3 ± 3%, n = 6; WT: 19 ± 8%, n = 6; P < 0.05). These results show that mitochondrial Na+/Ca2+ exchanger-mediated Ca2+ release regulates SK channel activity and is impaired in PINK1-deficient neurons.
Selective effects of PINK1 deletion on SNC dopamine neurons
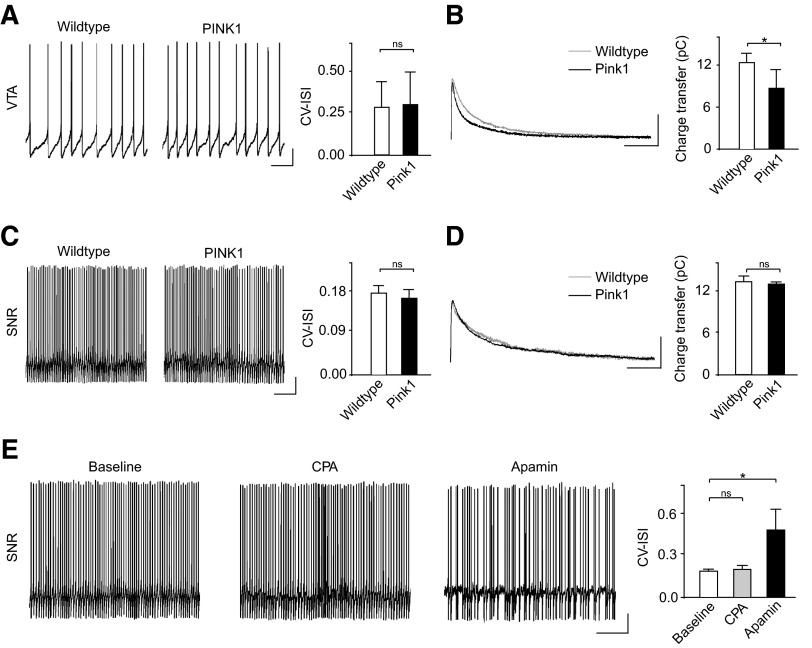
Selective vulnerability of SNC dopamine neurons is a central feature of PD (Damier et al. 1999). Therefore one key question is whether a functional reduction in SK channel activation is found in other neurons following PINK1 deletion. To directly address this, we examined firing activity and SK channel activity in ventral tegmental area (VTA) dopamine neurons and GABA neurons of the SNR. Both of these neuronal subgroups have been shown to express functional SK channels (Wolfart et al. 2001; Yanovsky et al. 2005).
In PINK1-deficient and wild type VTA dopamine neurons, we observed identical firing patterns, as shown by the CV-ISI (Fig. 4A; CV-ISI, PINK1: 0.29 ± 0.19, n = 10; WT: 0.28 ± 0.15, n = 13; P > 0.05). In general, we found that wild type VTA dopamine neurons showed more irregular firing patterns than the SNC. This is consistent with previous findings and is thought to be the consequence of a lower expression and dependence on SK channels in the VTA (Wolfart et al. 2001). Strikingly, however, a significant reduction in SK channel activity could still be observed in PINK1-deficient VTA dopamine neurons under voltage-clamp (Fig. 4B; charge transfer, PINK1: 8.66 ± 2.50 pC, n = 6; WT: 12.24 ± 1.22 pC, n = 8; P < 0.05).
Fig. 4.
Differential effect of PINK1-deficiency on VTA dopamine neurons and SNR GABA neurons. A: representative examples of firing patterns from VTA dopamine neurons in wild type and PINK1-deficient mice. No differences were observed in the CV-ISI between the groups. However, voltage-clamp recordings showed a small but significant reduction in SK channel-mediated currents in PINK1-deficient neurons compared with wild type (B). Firing patterns and SK channel-mediated currents in SNR GABA neurons were identical in wild type and PINK1-deficient mice (C and D). Interestingly, however, firing regularity of SNR GABA neurons are insensitive to CPA but show a significant increase in irregularity in apamin (E). This reveals cell-type specific differences in how SK channels are regulated by intracellular Ca2+ signaling (Scale bars: current-clamp, 10 mV, 1 s; voltage-clamp, 100 pA, 50 ms). *, P < 0.05.
Next we recorded the activity of SNR GABA neurons in PINK1-deficient and wild type mice. SNR GABA neurons show a distinct electrophysiological phenotype compared with dopamine neurons: fast spontaneous firing rates at ∼20–30 Hz, short-duration spikes (<1 ms), and little or no Ih current (Nakanishi et al. 1987; Yanovsky et al. 2005). Unlike VTA dopamine neurons, PINK1 deficiency had no effect on firing patterns (Fig. 4C; CV-ISI, PINK1: 0.16 ± 0.02, n = 3; WT: 0.17 ± 0.01, n = 3; P > 0.05) or SK channel-mediated currents (Fig. 4D; charge transfer, PINK1, 12.72 ± 0.18 pC, n = 3; WT: 13.07 ± 0.71, n = 3; P > 0.05) in these neurons. This result is striking because SK channels are known to play a role in maintaining firing regularity of SNR GABA neurons (Atherton and Bevan 2005; Yanovsky et al. 2005). However, SNR GABA neurons express the SK2 subtype of SK channels compared with SK3 found in dopamine neurons (Yanovsky et al. 2005). Importantly, the AHP of action potentials recorded from SNR GABA neurons have also been shown to be insensitive to CPA (Yanovsky et al. 2005), indicating that SK channel function is not regulated by ER Ca2+ release in these neurons. This further suggests that CPA will not affect firing regularity. To test this hypothesis, we examined whether the activity of wild type SNR GABA neurons could be disrupted using CPA. Importantly, we found that SNR GABA neurons were insensitive to CPA (10 μM; Fig. 4E; CV-ISI, baseline: 0.18 ± 0.01, CPA: 0.19 ± 0.02, n = 3; P > 0.05) despite having a strong dependency on SK channels for firing regularity as shown by their sensitivity to apamin (300 nM; Fig. 4E; CV-ISI, baseline: 0.18 ± 0.01, apamin: 0.47 ± 0.14, n = 3; P < 0.05). Taken together, our results suggest that cell-type specific differences in intracellular Ca2+ signaling and SK channel-subtype expression may underlie the differential effects of PINK1 deletion on SNC dopamine neurons compared with VTA dopamine neurons and SNR GABA neurons.
PINK1-deficient SNC dopamine neurons display hyperexcitability in vivo
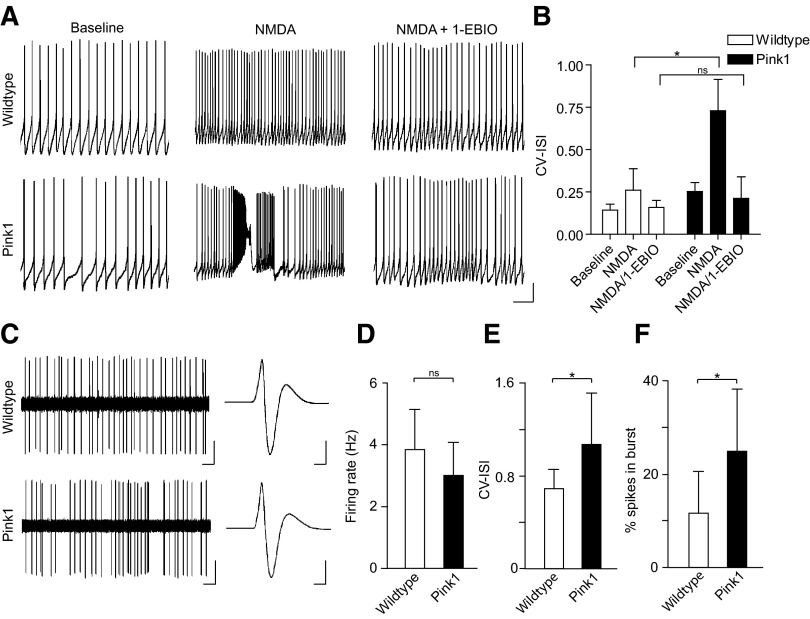
Dopamine neurons in vivo fire action potentials as single spikes or in bursts (typically doublets or triplets) (Grace and Bunney 1984a,b). Bursts are thought to have important consequences for dopamine signaling (Overton and Clark 1997; Redgrave et al. 2008; Schultz 2002), but they may increase the cytosolic Ca2+ burden and subsequent intracellular stress (Chan et al. 2009; Kuznetsov et al. 2006; Sulzer 2007). Burst firing is controlled through an interplay of synaptic inputs and SK channels (Blythe et al. 2007; Ji and Shepard 2006; Ji et al. 2009; Komendantov et al. 2004; Ping and Shepard 1999; Wolfart et al. 2001). We therefore hypothesized that PINK1-deficient dopamine neurons would be more likely to burst fire compared with wild type neurons. Indeed, when we applied NMDA (a glutamate receptor agonist), we found that PINK1-deficient neurons were more likely to fire in a “bursting” manner compared with wild type neurons (Fig. 5, A and B; CV-ISI, PINK1: baseline, 0.25 ± 0.05; NMDA, 0.73 ± 0.19, n = 12; WT: baseline, 0.14 ± 0.04, NMDA, 0.26 ± 0.13; n = 14; P < 0.05). More importantly, the hyperexcitability observed in PINK1-deficient neurons could be blocked by application of the SK channel opener, 1-EBIO, confirming that SK channel facilitation can dampen excitability in these neurons and restore normal activity (Fig. 5, A and B; CV-ISI: PINK1, NMDA/1-EBIO: 0.21 ± 0.13, n = 6; NMDA/1-EBIO: 0.16 ± 0.04, n = 6; P > 0.05).
Fig. 5.
PINK1-deficient SNC dopamine neurons display hyperexcitability in ex vivo brain slice and in vivo. A and B: PINK1-deficient neurons show a greater propensity to fire in a “bursting” manner when treated with N-methyl-d-aspartate (NMDA) ex vivo compared with wild type neurons. Moreover, treatment with the SK channel facilitator, 1-EBIO, can dampen excitability in PINK1-deficient neurons and prevent onset of bursts. (Scale bars: 10 mV, 2 s) C: comparison of extracellular recordings in vivo shows that SNC dopamine neurons from PINK1-deficient mice have similar firing rates to wild type neurons (D) but are often more irregular (E) and show a greater percentage of spikes in bursts (F). (Scale bars: firing pattern, 0.5 mV, 1 s; action potential waveform, 0.5 mV, 1 ms). *, P < 0.05.
We next wanted to confirm that PINK1-deficient dopamine neurons exhibited increased endogenous burst firing in vivo. Consequently we conducted in vivo extracellular recordings from putative SNC dopamine neurons in anesthetized mice (Fig. 5, C–F). Consistent with our ex vivo findings, SNC dopamine neurons in PINK1-deficient mice and wild type mice had similar firing rates (Fig. 5D; PINK1: 3.01 ± 1.04 Hz, n = 30; WT: 3.84 ± 1.48, n = 28; P > 0.05). However, PINK1-deficient neurons had more irregular firing patterns (Fig. 5E; CV-ISI, PINK1: 1.07 ± 0.44, n = 30; WT: 0.69 ± 0.16, n = 28; P < 0.05) and exhibited increased burst firing compared with wild type neurons (Fig. 5F; percentage spikes in bursts, PINK1: 24.85 ± 13.15, n = 30; WT: 11.69 ± 8.68, n = 28; P < 0.05). Taken together these experiments show that PINK1-deficiency leads to hyperexcitability and increased burst firing in SNC dopamine neurons.
HtrA2/omi-deficient SNC dopamine neurons display hyperexcitability ex vivo
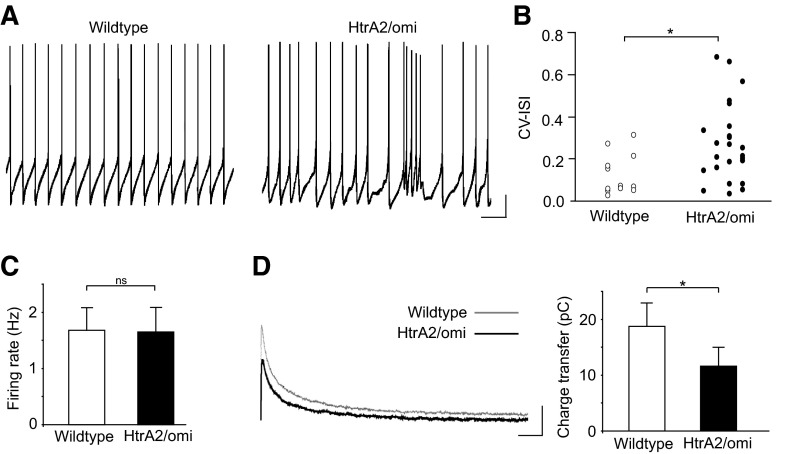
To broaden the scope of our findings, we examined firing activity and SK channel currents in HtrA2/Omi-deficient mice (Martins et al. 2004; Strauss et al. 2005). Mutations in HtrA2/Omi (Strauss et al. 2005) and altered expression of the protein (Bogaerts et al. 2008) may be susceptibility factors for PD (but see Kruger et al. 2009). In particular, we chose to examine these mice because HtrA2/Omi and PINK1 have been shown to interact in vitro and are thought to be involved in a common stress-protective pathway (Plun-Favreau et al. 2007). Interestingly, similar to the effects of PINK1 deletion, loss of HtrA2/Omi protease activity has also been shown to impair mitochondrial Ca2+ handling in mouse embryonic fibroblasts (Jones et al. 2003). We therefore hypothesized that SNC dopamine neurons in HtrA2/Omi-deficient mice would also exhibit a functional reduction in SK channel activation. Indeed consistent with our PINK1 whole cell recordings in brain slices, we found that HtrA2/Omi-deficient neurons fired more irregularly compared with wild type neurons (Fig. 6, A and B; CV-ISI, HtrA2/Omi: 0.35 ± 0.37, n = 25; WT: 0.13 ± 0.09, n = 12; P < 0.05) but with no difference in firing rate (Fig. 6C; firing rate, HtrA2/Omi: 1.65 ± 0.42 Hz, n = 25; WT: 1.68 ± 0.39 Hz, n = 12; P > 0.05). Moreover, voltage-clamp recordings confirmed a functional reduction of SK channel activation in HtrA2/Omi-deficient SNC dopamine neurons (Fig. 6D; charge transfer, HtrA2/Omi: 11.59 ± 3.28 pC, n = 14; WT: 18.74 ± 4.05 pC, n = 6; P < 0.05). Strikingly, 20% of HtrA2/Omi-deficient neurons displayed spontaneous burst firing (in the absence of NMDA) comparable to that seen in PINK1-deficient neurons after application of NMDA (Fig. 6A; HtrA2/Omi: 20%, n = 25; WT: 0%, n = 12). These results suggest that functional reduction of SK channel activation may be a common consequence of Parkinson's disease-associated mitochondrial dysfunction.
Fig. 6.
HtrA2/omi-deficient SNC dopamine neurons display hyperexcitability ex vivo. A: representative examples of spontaneous firing activity from wild type and HtrA2/omi-deficient neurons. Some HtrA2/omi-deficient neurons have irregular firing and bursts under control conditions. (Scale bars: 10 mV, 1 s) B: raw data of CV-ISI analysis shows HtrA2/omi-deficient mice have a greater number of irregularly firing neurons, without a change in firing rate (C). D: voltage-clamp analysis (using 2 ms depolarizing pulses from −50 to +20 mV) reveals a reduction in SK channel-mediated currents in HtrA2/omi-deficient SNC dopamine neurons compared with wildtype (Scale bars: 50 pA, 50 ms). *, P < 0.05.
DISCUSSION
We have found that deletion of PD-associated genes leads to a form of hyperexcitability in SNC dopamine neurons. In particular, we show that deletion of PINK1 or HtrA2/Omi causes dopamine neurons to fire action potentials in an irregular manner and makes them more likely to fire bursts of action potentials. We have observed this deficit in vitro using both whole cell and cell-attached recording methods and in vivo using extracellular recordings, which indicate that it is not an artifact of recording approach or experimental preparation. This irregularity occurs as a consequence of impaired intracellular Ca2+ signaling by ER and mitochondria, leading to a functional reduction of SK channel activation that regulates firing activity. Furthermore, we show that this deficit can be pharmacologically rescued by an SK channel facilitator, suggesting a potentially neuroprotective therapeutic target in the early stages of PD. Consistent with this possibility, SK channel facilitation with 1-EBIO has recently been shown to be neuroprotective in a 6-hydroxy-dopamine lesion mouse model of PD (Aumann et al. 2008).
It has been suggested that the selective vulnerability of dopamine neurons in PD is the consequence of multiple factors (Sulzer 2007). Recently particular emphasis has been placed on the role high Ca2+ plays in exacerbating cellular stress (Chan et al. 2009; Mosharov et al. 2009). Because SNC dopamine neuron activity entails relatively high Ca2+ fluxes, particularly through the involvement of L-type Ca2+ channels, it has been proposed that homeostatic Ca2+ stress is a key determinant in their selective vulnerability (Chan et al. 2007; Foehring et al. 2009). Indeed Ca2+ needs to be maintained within a tight physiological range in neurons or it can increase reactive oxygen species or, in more severe cases, trigger excitotoxicity leading to cell death (Beal 1998; Kress and Reynolds 2005; Schulz 2007). Our results suggest that genetic susceptibility may further increase the Ca2+ burden in SNC dopamine neurons by increasing their propensity to burst fire in vivo, a mechanism that is associated with additional Ca2+ loading through L-type Ca2+ channels and other voltage-gated channels (Deister et al. 2009; Johnson and Wu 2004; Kuznetsov et al. 2006). Consequently, a feed-forward mechanism may be established whereby Ca2+ influx from burst firing leads to additional mitochondrial dysfunction and cellular stress.
The proper functioning of mitochondria is critical for the normal physiology of neurons the specialized structure and function of which mean that a large amount of ATP supply is devoted to the maintenance of ion homeostasis. PINK1 deficiency has been shown to severely disrupt mitochondrial function in vitro and in Drosophila (Deas et al. 2009). In PINK1-deficient mice, however, mitochondrial functional defects have been shown to be relatively subtle and fail to create a major energy crisis (Gautier et al. 2008). Therefore reduced ATP levels are unlikely to be the cause of the electrophysiological deficits found in our study. A recent report has demonstrated that a loss of PINK1 affects the capacity of the mitochondrial Na+/Ca2+ exchanger to release Ca2+, leading to mitochondrial Ca2+ overload. More importantly, this deficit appears to precede respiratory chain dysfunction, increased production of ROS, and, ultimately, cell death (Gandhi et al. 2009). Consistent with this, our results suggest that there is a functional reduction in SK channel activation, in part, because of a failure of mitochondrial Ca2+ release via the Na+/Ca2+ exchanger. The milder phenotype found in PINK1-deficient mice may therefore reflect the early stages of PINK1 deficiency observed in cell models. Although it is still possible that impaired ER and mitochondrial Ca2+ release is a result of impaired respiration and ATP production, compromised ATP levels would be expected to activate KATP channels in SNC dopamine neurons and cause hypoactivity, as has been observed following mitochondrial inhibition in toxin-based PD mouse models (Liss et al. 2005). Thus the hyperexcitability observed in our study may represent a distinct pathophysiological mechanism that can be triggered through mitochondrial dysfunction and provides an insight into how genetic factors may modulate susceptibility in PD.
Recent studies have sparked renewed appreciation for the remarkably dynamic nature of mitochondria, particularly in the context of PINK1 and PD (Deng et al. 2008; Poole et al. 2008). Mitochondria constantly fuse and divide and are actively transported to specific subcellular locations (Karbowski and Youle 2003; Yaffe 1999). The dynamic nature of these organelles will undoubtedly be important in their role as regulators of Ca2+ signaling. Although mitochondria in PINK1-deficient mice appear to be structurally intact and preserved in total number, there is a selective increase in larger mitochondria, which would be consistent with a role of PINK1 in the promotion of mitochondrial fission (Gautier et al. 2008). Therefore it is possible that a deficit in mitochondrial Ca2+ flux in our study is a result of impaired mitochondrial dynamics. It will be interesting to examine whether mitochondrial trafficking and cellular distribution are also impaired in PINK1-deficient mice because mitochondrial motility is regulated by intracellular Ca2+ (Wang and Schwarz 2009). In addition, the kinase activity of PINK1 could directly or indirectly modulate the Na+/Ca2+ exchanger and/or other mitochondrial ion channels.
It is not yet clear how mitochondrial Ca2+ signaling can affect SK channel function. However, mitochondria can form close contacts with the ER (de Brito and Scorrano 2008; Rizzuto et al. 1998, 2009) and the plasma membrane (Malli et al. 2003) with the ER forming a continuous intracellular network in SNC dopamine neurons that can extend throughout the somatodendritic tree (Choi et al. 2006; Schwyn and Fox 1974). The ability of mitochondria to uptake and release Ca2+ allows them to operate as a dynamic and reversible Ca2+ storage compartment that can modulate ER Ca2+ stores and influence the spatiotemporal pattern of intracellular Ca2+ signals (Brini 2003; Castaldo et al. 2009). Given the high buffering capacity of mitochondria, it is possible that PINK1 deficiency triggers mitochondrial Ca2+ overload similar to that observed in other studies when the Na+/Ca2+ exchanger is impaired (Gandhi et al. 2009). In turn, this may suppress local ER Ca2+ signaling or impair reloading of ER Ca2+ stores, diminishing the Ca2+ microdomain that facilitates SK channel activation. Thus voltage-gated Ca2+ channels, along with ER and mitochondrial Ca2+ stores, may collectively regulate the Ca2+ microdomain that shapes SK channel activity in dopamine neurons.
Although the precise Ca2+ dynamics that regulate SK channel function are beyond the scope of this study, the intrinsic pacemaker activity of SNC dopamine neurons may be particularly prone to disruption during mitochondrial impairment. This is suggested by the lack of SK channel dysfunction in SNR GABA neurons, where SK channels play a similar role in maintaining firing regularity but have no reliance on intracellular Ca2+ release to facilitate their activity (Atherton and Bevan 2005; Yanovsky et al. 2005). In addition, using prolonged depolarizing steps to elevate intracellular Ca2+ levels (beyond that achieved during single action potentials) can restore SK channel activity in PINK1-deficient SNC dopamine neurons, suggesting that the deficit is specific to the temporal and spatial dynamics of tonic firing activity. VTA dopamine neurons, which are also spontaneously active, have a similar functional reduction in SK channel activation to SNC neurons. However, these neurons have less reliance on SK channels for maintaining firing regularity (Wolfart et al. 2001), which could explain the differential effects of PINK1 deficiency on the firing activity of SNC and VTA dopamine neurons.
The selective effect of PINK1 deficiency on SNC dopamine neurons has important implications toward understanding their vulnerability in PD. Although no neurodegeneration occurs in PINK1-deficient mice, it is likely that genetic mouse models require secondary “hits” to trigger neurodegeneration. This is suggested by the fact that PINK1-deficient mice show enhanced sensitivity to oxidative stressors such as H202 and mild heat shock (Gautier et al. 2008). Similarly, Parkin-deficient mice have increased vulnerability to inflammatory stimuli, suggesting that an environmental trigger is sufficient to induce neurodegeneration (Frank-Cannon et al. 2008). Therefore in future studies, it will be important to examine whether SNC dopamine neurons from PINK1-deficient mice show a greater sensitivity to Ca2+-induced cell death or other cellular stressors.
Here, we propose that PINK1 and HtrA2/omi deficiency leads to a feed-forward interaction between mitochondrial function and electrophysiological activity that contributes to the vulnerability of SNC dopamine neurons in PD. Because mitochondrial dysfunction is also implicated in sporadic forms of PD (Abou-Sleiman et al. 2006), changes in dopamine neuron excitability may be a common pathophysiological mechanism preceding neurodegeneration.
GRANTS
This work was supported by U.K. Medical Research Council grants U120085816 to M. A. Ungless and U120081321 to R. Festenstein, a University Research Fellowship from The Royal Society to M. A. Ungless, an MRC Senior Clinical Research Fellowship to R. Festenstein, and MRC Programme Grant G0400000 to N. W. Wood.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
The experiments were conceived and designed by M. W. Bishop., A. Dougalis, N. W. Wood, R. Festenstein, and M. A.Ungless. The electrophysiological data were collected and analyzed by M. W. Bishop, S. Chakraborty, G.A.C. Matthews, and A. Dougalis. The manuscript was prepared by M. Bishop, R. Festenstein, and M. A. Ungless and commented on by all authors.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Abou-Sleiman et al., 2006. Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nat Rev Neurosci 7: 207–219, 2006 [DOI] [PubMed] [Google Scholar]
- Atherton and Bevan, 2005. Atherton JF, Bevan MD. Ionic mechanisms underlying autonomous action potential generation in the somata and dendrites of GABAergic substantia nigra pars reticulata neurons in vitro. J Neurosci 25: 8272–8281, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumann et al., 2008. Aumann TD, Gantois I, Egan K, Vais A, Tomas D, Drago J, Horne MK. SK channel function regulates the dopamine phenotype of neurons in the substantia nigra pars compacta. Exp Neurol 213: 419–430, 2008 [DOI] [PubMed] [Google Scholar]
- Beal, 1998. Beal MF. Excitotoxicity and nitric oxide in Parkinson's disease pathogenesis. Ann Neurol 44: S110–114, 1998 [DOI] [PubMed] [Google Scholar]
- Blythe et al., 2007. Blythe SN, Atherton JF, Bevan MD. Synaptic activation of dendritic AMPA and NMDA receptors generates transient high-frequency firing in substantia nigra dopamine neurons in vitro. J Neurophysiol 97: 2837–2850, 2007 [DOI] [PubMed] [Google Scholar]
- Bogaerts et al., 2008. Bogaerts V, Nuytemans K, Reumers J, Pals P, Engelborghs S, Pickut B, Corsmit E, Peeters K, Schymkowitz J, De Deyn PP, Cras P, Rousseau F, Theuns J, Van Broeckhoven C. Genetic variability in the mitochondrial serine protease HTRA2 contributes to risk for Parkinson disease. Hum Mutat 29: 832–840, 2008 [DOI] [PubMed] [Google Scholar]
- Bond et al., 2005. Bond CT, Maylie J, Adelman JP. SK channels in excitability, pacemaking and synaptic integration. Curr Opin Neurobiol 15: 305–311, 2005 [DOI] [PubMed] [Google Scholar]
- Brini, 2003. Brini M. Ca(2+) signalling in mitochondria: mechanism and role in physiology and pathology. Cell Calcium 34: 399–405, 2003 [DOI] [PubMed] [Google Scholar]
- Brown et al., 2009. Brown MT, Henny P, Bolam JP, Magill PJ. Activity of neurochemically heterogeneous dopaminergic neurons in the substantia nigra during spontaneous and driven changes in brain state. J Neurosci 29: 2915–2925, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaldo et al., 2009. Castaldo P, Cataldi M, Magi S, Lariccia V, Arcangeli S, Amoroso S. Role of the mitochondrial sodium/calcium exchanger in neuronal physiology and in the pathogenesis of neurological diseases. Prog Neurobiol 87: 58–79, 2009 [DOI] [PubMed] [Google Scholar]
- Chan et al., 2009. Chan CS, Gertler TS, Surmeier DJ. Calcium homeostasis, selective vulnerability and Parkinson's disease. Trends Neurosci 32: 249–256, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan et al., 2007. Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. “Rejuvenation” protects neurons in mouse models of Parkinson's disease. Nature 447: 1081–1086, 2007 [DOI] [PubMed] [Google Scholar]
- Choi et al., 2006. Choi YM, Kim SH, Chung S, Uhm DY, Park MK. Regional interaction of endoplasmic reticulum Ca2+ signals between soma and dendrites through rapid luminal Ca2+ diffusion. J Neurosci 26: 12127–12136, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark et al., 2006. Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441: 1162–1166, 2006 [DOI] [PubMed] [Google Scholar]
- Damier et al., 1999. Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain 122: 1437–1448, 1999 [DOI] [PubMed] [Google Scholar]
- Deas et al., 2009. Deas E, Plun-Favreau H, Wood NW. PINK1 function in health and disease. EMBO Mol Med 1: 152–165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito and Scorrano, 2008. de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605, 2008 [DOI] [PubMed] [Google Scholar]
- Deister et al., 2009. Deister CA, Teagarden MA, Wilson CJ, Paladini CA. An intrinsic neuronal oscillator underlies dopaminergic neuron bursting. J Neurosci 29: 15888–15897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng et al., 2008. Deng H, Dodson MW, Huang H, Guo M. The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci USA 105: 14503–14508, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner et al., 2007. Exner N, Treske B, Paquet D, Holmstrom K, Schiesling C, Gispert S, Carballo-Carbajal I, Berg D, Hoepken HH, Gasser T, Kruger R, Winklhofer KF, Vogel F, Reichert AS, Auburger G, Kahle PJ, Schmid B, Haass C. Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci 27: 12413–12418, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foehring et al., 2009. Foehring RC, Zhang XF, Lee JC, Callaway JC. Endogenous calcium buffering capacity of substantia nigral dopamine neurons. J Neurophysiol 102: 2326–2333, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Cannon et al., 2008. Frank-Cannon TC, Tran T, Ruhn KA, Martinez TN, Hong J, Marvin M, Hartley M, Trevino I, O'Brien DE, Casey B, Goldberg MS, Tansey MG. Parkin deficiency increases vulnerability to inflammation-related nigral degeneration. J Neurosci 28: 10825–10834, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi et al., 2006. Gandhi S, Muqit MM, Stanyer L, Healy DG, Abou-Sleiman PM, Hargreaves I, Heales S, Ganguly M, Parsons L, Lees AJ, Latchman DS, Holton JL, Wood NW, Revesz T. PINK1 protein in normal human brain and Parkinson's disease. Brain 129: 1720–1731, 2006 [DOI] [PubMed] [Google Scholar]
- Gandhi et al., 2009. Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, Duchen MR, Abramov AY. PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell 33: 627–638, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier et al., 2008. Gautier CA, Kitada T, Shen J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci USA 105: 11364–11369, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispert et al., 2009. Gispert S, Ricciardi F, Kurz A, Azizov M, Hoepken HH, Becker D, Voos W, Leuner K, Muller WE, Kudin AP, Kunz WS, Zimmermann A, Roeper J, Wenzel D, Jendrach M, Garcia-Arencibia M, Fernandez-Ruiz J, Huber L, Rohrer H, Barrera M, Reichert AS, Rub U, Chen A, Nussbaum RL, Auburger G. Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PLoS One 4: e5777, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace and Bunney, 1983. Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons. I. Identification and characterization. Neuroscience 10: 301–315, 1983 [DOI] [PubMed] [Google Scholar]
- Grace and Bunney, 1984a. Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 4: 2877–2890, 1984a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace and Bunney, 1984b. Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci 4: 2866–2876, 1984b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace and Onn, 1989. Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci 9: 3463–3481, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henchcliffe and Beal, 2008. Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 4: 600–609, 2008 [DOI] [PubMed] [Google Scholar]
- Ji et al., 2009. Ji H, Hougaard C, Herrik KF, Strobaek D, Christophersen P, Shepard PD. Tuning the excitability of midbrain dopamine neurons by modulating the Ca2+ sensitivity of SK channels. Eur J Neurosci 29: 1883–1895, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji and Shepard, 2006. Ji H, Shepard PD. SK Ca2+-activated K+ channel ligands alter the firing pattern of dopamine-containing neurons in vivo. Neuroscience 140: 623–633, 2006 [DOI] [PubMed] [Google Scholar]
- Johnson and Wu, 2004. Johnson SW, Wu YN. Multiple mechanisms underlie burst firing in rat midbrain dopamine neurons in vitro. Brain Res 1019: 293–296, 2004 [DOI] [PubMed] [Google Scholar]
- Jones et al., 2003. Jones JM, Datta P, Srinivasula SM, Ji W, Gupta S, Zhang Z, Davies E, Hajnoczky G, Saunders TL, Van Keuren ML, Fernandes-Alnemri T, Meisler MH, Alnemri ES. Loss of Omi mitochondrial protease activity causes the neuromuscular disorder of mnd2 mutant mice. Nature 425: 721–727, 2003 [DOI] [PubMed] [Google Scholar]
- Karbowski and Youle, 2003. Karbowski M, Youle RJ. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ 10: 870–880, 2003 [DOI] [PubMed] [Google Scholar]
- Kitada et al., 2007. Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci USA 104: 11441–11446, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komendantov et al., 2004. Komendantov AO, Komendantova OG, Johnson SW, Canavier CC. A modeling study suggests complementary roles for GABAA and NMDA receptors and the SK channel in regulating the firing pattern in midbrain dopamine neurons. J Neurophysiol 91: 346–357, 2004 [DOI] [PubMed] [Google Scholar]
- Krüger et al. Krüger R, Sharma M, Riess O, Gasser T, Van Broeckhoven C, Theuns J, Aasly J, Annesi G, Bentivoglio AR, Brice A, Djarmati A, Elbaz A, Farrer M, Ferrarese C, Gibson JM, Hadjigeorgiou GM, Hattori N, Ioannidis JP, Jasinska-Myga B, Klein C, Lambert JC, Lesage S, Lin JJ, Lynch T, Mellick GD, de Nigris F, Opala G, Prigione A, Quattrone A, Ross OA, Satake W, Silburn PA, Tan EK, Toda T, Tomiyama H, Wirdefeldt K, Wszolek Z, Xiromerisiou G, Maraganore DM; for the Genetic Epidemiology of Parkinson's disease consortium A large-scale genetic association study to evaluate the contribution of Omi/HtrA2 (PARK13) to Parkinson's disease. Neurobiol Aging In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress and Reynolds, 2005. Kress GJ, Reynolds IJ. Dopaminergic neurotoxins require excitotoxic stimulation in organotypic cultures. Neurobiol Dis 20: 639–645, 2005 [DOI] [PubMed] [Google Scholar]
- Kuznetsov et al., 2006. Kuznetsov AS, Kopell NJ, Wilson CJ. Transient high-frequency firing in a coupled-oscillator model of the mesencephalic dopaminergic neuron. J Neurophysiol 95: 932–947, 2006 [DOI] [PubMed] [Google Scholar]
- Liss et al., 2005. Liss B, Haeckel O, Wildmann J, Miki T, Seino S, Roeper J. K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nat Neurosci 8: 1742–1751, 2005 [DOI] [PubMed] [Google Scholar]
- Malli et al., 2003. Malli R, Frieden M, Osibow K, Zoratti C, Mayer M, Demaurex N, Graier WF. Sustained Ca2+ transfer across mitochondria is essential for mitochondrial Ca2+ buffering, sore-operated Ca2+ entry, and Ca2+ store refilling. J Biol Chem 278: 44769, 2003 [DOI] [PubMed] [Google Scholar]
- Martins et al., 2004. Martins LM, Morrison A, Klupsch K, Fedele V, Moisoi N, Teismann P, Abuin A, Grau E, Geppert M, Livi GP, Creasy CL, Martin A, Hargreaves I, Heales SJ, Okada H, Brandner S, Schulz JB, Mak T, Downward J. Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol Cell Biol 24: 9848–9862, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosharov et al., 2009. Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y, Krantz DE, Kobayashi K, Edwards RH, Sulzer D. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron 62: 218–229, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muqit et al., 2006. Muqit MM, Abou-Sleiman PM, Saurin AT, Harvey K, Gandhi S, Deas E, Eaton S, Payne Smith MD, Venner K, Matilla A, Healy DG, Gilks WP, Lees AJ, Holton J, Revesz T, Parker PJ, Harvey RJ, Wood NW, Latchman DS. Altered cleavage and localization of PINK1 to aggresomes in the presence of proteasomal stress. J Neurochem 98: 156–169, 2006 [DOI] [PubMed] [Google Scholar]
- Nakanishi et al., 1987. Nakanishi H, Kita H, Kitai ST. Intracellular study of rat substantia nigra pars reticulata neurons in an in vitro slice preparation: electrical membrane properties and response characteristics to subthalamic stimulation. Brain Res 437: 45–55, 1987 [DOI] [PubMed] [Google Scholar]
- Overton and Clark, 1997. Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev 25: 312–334, 1997 [DOI] [PubMed] [Google Scholar]
- Park et al., 2006. Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 441: 1157–1161, 2006 [DOI] [PubMed] [Google Scholar]
- Ping and Shepard, 1999. Ping HX, Shepard PD. Blockade of SK-type Ca2+-activated K+ channels uncovers a Ca2+-dependent slow afterdepolarization in nigral dopamine neurons. J Neurophysiol 81: 977–984, 1999 [DOI] [PubMed] [Google Scholar]
- Plun-Favreau et al., 2007. Plun-Favreau H, Klupsch K, Moisoi N, Gandhi S, Kjaer S, Frith D, Harvey K, Deas E, Harvey RJ, McDonald N, Wood NW, Martins LM, Downward J. The mitochondrial protease HtrA2 is regulated by Parkinson's disease-associated kinase PINK1. Nat Cell Biol 9: 1243–1252, 2007 [DOI] [PubMed] [Google Scholar]
- Poole et al., 2008. Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA 105: 1638–1643, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave et al., 2008. Redgrave P, Gurney K, Reynolds J. What is reinforced by phasic dopamine signals? Brain Res Rev 58: 322–339, 2008 [DOI] [PubMed] [Google Scholar]
- Rizzuto et al., 2009. Rizzuto R, Marchi S, Bonora M, Aguiari P, Bononi A, De Stefani D, Giorgi C, Leo S, Rimessi A, Siviero R, Zecchini E, Pinton P. Ca(2+) transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta 1787: 1342–1351, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto et al., 1998. Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280: 1763–1766, 1998 [DOI] [PubMed] [Google Scholar]
- Schapira, 2008. Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet Neurol 7: 97–109, 2008 [DOI] [PubMed] [Google Scholar]
- Schultz, 2002. Schultz W. Getting formal with dopamine and reward. Neuron 36: 241–263, 2002 [DOI] [PubMed] [Google Scholar]
- Schulz, 2007. Schulz JB. Mechanisms of neurodegeneration in idiopathic Parkinson's disease. Parkinsonism Relat Disord 13, Suppl 3: S306–308, 2007 [DOI] [PubMed] [Google Scholar]
- Schwyn and Fox, 1974. Schwyn RC, Fox CA. The primate substantia nigra: a Golgi and electron microscopic study. J Hirnforsch 15: 95–126, 1974 [PubMed] [Google Scholar]
- Seidler et al., 1989. Seidler NW, Jona I, Vegh M, Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca 21-ATPase of sarcoplasmic reticulum. J Biol Chem 264: 17816–17823, 1989 [PubMed] [Google Scholar]
- Silvestri et al., 2005. Silvestri L, Caputo V, Bellacchio E, Atorino L, Dallapiccola B, Valente EM, Casari G. Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum Mol Genet 14: 3477–3492, 2005 [DOI] [PubMed] [Google Scholar]
- Stocker, 2004. Stocker M. Ca(2+)-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci 5: 758–770, 2004 [DOI] [PubMed] [Google Scholar]
- Strauss et al., 2005. Strauss KM, Martins LM, Plun-Favreau H, Marx FP, Kautzmann S, Berg D, Gasser T, Wszolek Z, Muller T, Bornemann A, Wolburg H, Downward J, Riess O, Schulz JB, Kruger R. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson's disease. Hum Mol Genet 14: 2099–2111, 2005 [DOI] [PubMed] [Google Scholar]
- Sulzer, 2007. Sulzer D. Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci 30: 244–250, 2007 [DOI] [PubMed] [Google Scholar]
- Wang and Schwarz, 2009. Wang X, Schwarz TL. The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell 136: 163–174, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfart et al., 2001. Wolfart J, Neuhoff H, Franz O, Roeper J. Differential expression of the small-conductance, calcium-activated potassium channel SK3 is critical for pacemaker control in dopaminergic midbrain neurons. J Neurosci 21: 3443–3456, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfart and Roeper, 2002. Wolfart J, Roeper J. Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. J Neurosci 22: 3404–3413, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood-Kaczmar et al., 2008. Wood-Kaczmar A, Gandhi S, Yao Z, Abramov AY, Miljan EA, Keen G, Stanyer L, Hargreaves I, Klupsch K, Deas E, Downward J, Mansfield L, Jat P, Taylor J, Heales S, Duchen MR, Latchman D, Tabrizi SJ, Wood NW. PINK1 is necessary for long term survival and mitochondrial function in human dopaminergic neurons. PLoS One 3: e2455, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe, 1999. Yaffe MP. Dynamic mitochondria. Nat Cell Biol 1: E149–150, 1999 [DOI] [PubMed] [Google Scholar]
- Yang et al., 2006. Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW, Yang L, Beal MF, Vogel H, Lu B. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci USA 103: 10793–10798, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky et al., 2005. Yanovsky Y, Zhang W, Misgeld U. Two pathways for the activation of small-conductance potassium channels in neurons of substantia nigra pars reticulata. Neuroscience 136: 1027–1036, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.