Abstract
Synchronization of local and distributed neuronal assemblies is thought to underlie fundamental brain processes such as perception, learning, and cognition. In neurological disease, neuronal synchrony can be altered and in epilepsy may play an important role in the generation of seizures. Linear cross-correlation and mean phase coherence of local field potentials (LFPs) are commonly used measures of neuronal synchrony and have been studied extensively in epileptic brain. Multiple studies have reported that epileptic brain is characterized by increased neuronal synchrony except possibly prior to seizure onset when synchrony may decrease. Previous studies using intracranial electroencephalography (EEG), however, have been limited to patients with epilepsy. Here we investigate neuronal synchrony in epileptic and control brain using intracranial EEG recordings from patients with medically resistant partial epilepsy and control subjects with intractable facial pain. For both epilepsy and control patients, average LFP synchrony decreases with increasing interelectrode distance. Results in epilepsy patients show lower LFP synchrony between seizure-generating brain and other brain regions. This relative isolation of seizure-generating brain underlies the paradoxical finding that control patients without epilepsy have greater average LFP synchrony than patients with epilepsy. In conclusion, we show that in patients with focal epilepsy, the region of epileptic brain generating seizures is functionally isolated from surrounding brain regions. We further speculate that this functional isolation may contribute to spontaneous seizure generation and may represent a clinically useful electrophysiological signature for mapping epileptic brain.
INTRODUCTION
The coordinated activity and interaction of local and distributed neuronal assemblies underlies the brain's ability to perform sensorimotor and cognitive tasks. The concept of functional connectivity is widely used to describe the interaction strength between neuronal populations involved in these critical brain functions (Bullmore and Sporns 2009; David et al. 2004; Horwitz 2003; Stephan et al. 2008). Functional connectivity does not have a specific definition but rather is typically inferred from the statistical dependencies among signals measured from different neuronal assemblies. Synchrony of signals obtained from functional MRI, magnetoencephalography (MEG), and electroencephalography (EEG) have been widely used to characterize the functional connectivity between different brain regions. Higher levels of signal synchrony are interpreted as evidence of stronger functional connectivity between putative neural assemblies. In this paper, we use intracranial EEG (iEEG) synchrony to characterize the functional connectivity in epileptic and control brain.
The unsurpassed temporal resolution of EEG has led to its widespread use by clinicians and scientists investigating normal brain function and disease. In patients with medically resistant partial epilepsy undergoing evaluation for epilepsy surgery, scalp-recorded interictal (between seizures) and ictal (during seizure) EEG often fails to definitively localize the region of epileptic brain generating seizure (Engel et al. 2005). Invasive EEG recordings using intracranial electrodes to record local field potential (LFP) activity directly from the cortex are often required prior to resective surgery (Alper et al. 2008). Localization of the seizure-generating region of epileptic brain using iEEG typically emphasizes the analysis of seizure onsets and interictal epileptiform activity (Bartolomei et al. 2001; Blumenfeld et al. 2004; Faingold 2004; McCormick and Contreras 2001; Pan et al. 2005; Spencer 2002). More recently, investigators have begun to explore measures of neuronal synchrony to map epileptic brain (Schevon et al. 2007) as well as identify periods of increased seizure probability (Mormann et al. 2006).
There are multiple measures of neural synchrony (Doesburg and Ward 2009; Perez Velazquez et al. 2009), but perhaps the most common ways of measuring synchrony are linear cross-correlation (Aarabi et al. 2007; Amor et al. 2005; Kramer et al. 2008; Schindler et al. 2007, 2008) and mean phase correlation (Mormann et al. 2000; Schevon et al. 2007). The fact that correlation is directly dependent on LFP amplitude is a potential confound given that LFP amplitude is affected by impedance of the electrode-brain interface and orientation of the LFP generators. Alternatively, the mean phase coherence has the limitation that it only has clear physical meaning within a discrete band of frequencies where a clear rhythm can be identified. Last, the impact of the reference electrode signal is a well known confound requiring careful interpretation (Guevara et al. 2005; Schiff 2005; Zaveri et al. 2000). Nonetheless, neuronal synchronization in iEEG is widely regarded as an important neurophysiological measure (Aarabi et al. 2007; Elger and Lehnertz 1998; Iasemidis et al. 1990; Le Van Quyen et al. 1998–2001; Lopes da Silva et al. 2003; Martinerie et al. 1998; Rulkov et al. 1995; Zeng et al. 2007).
Studies investigating measures of LFP synchrony recorded using iEEG report increased mean phase coherence (Mormann et al. 2000; Schevon et al. 2007), magnitude squared coherence (Zaveri et al. 2009), and nonlinear correlation (Bettus et al. 2008) in interictal epileptic brain recordings. A fundamental limitation, however, of these iEEG studies has been the absence of control data (Stead et al. 2010). Because of the invasiveness of iEEG (Van Gompel et al. 2008), it is not ethically possible to obtain iEEG from healthy human control subjects. There are, however, emerging applications of iEEG for patients without epilepsy (Lima and Fregni 2008). Here we investigate the spatial organization of local synchrony from brain regions generating spontaneous seizures, seizure onset zone (SOZ), and brain regions not generating seizures (non-SOZ) in patients with epilepsy and from “normal” control brain of patients without epilepsy undergoing subdural electrode implantation for experimental treatment of intractable facial pain (Lima and Fregni 2008). By analyzing the iEEG of control brain from patients without epilepsy and patients with epilepsy, we are able to directly compare the LFP synchrony in the SOZ and non-SOZ in epileptic brain with control brain from patients without epilepsy.
METHODS
The data analyzed here are from a Mayo Clinic Institutional Review Board approved study. All patients provided informed consent. The patients with epilepsy underwent intracranial electrode implantation because noninvasive studies (video scalp-EEG, MRI, and functional imaging) did not adequately localize the SOZ. Patients were presented at a multidisciplinary surgery conference attended by neurosurgeons, neurologists, radiologists, and psychologists. The consensus clinical opinion was for implantation with intracranial electrodes for SOZ localization in patients with epilepsy and for an experimental trial of therapeutic motor cortex stimulation in the patients with intractable facial pain.
Six consecutive patients, two control patients (patients A and B) with facial pain and four patients with well localized neocortical partial epilepsy (Wetjen et al. 2009; Worrell et al. 2004) (patients 1–4), were investigated (see Table 1). The control patients were implanted with a subdural electrode grid for cortical stimulation to treat facial pain. The patients with epilepsy underwent cortical grid implantation and evaluation for epilepsy surgery (Brinkmann et al. 2009; Van Gompel et al. 2008). For patients A and 1–3, iEEG was recorded from a 24 contact subdural grid consisting of a silastic sheet embedded with 2.3 mm diam platinum-iridium alloy electrodes spaced every 10 mm in a 4 × 6 array. For patient B, the grid consisted of a similar 4 × 4 array, and for patient 4, the grid analyzed consisted of a 6 × 6 array.
Table 1.
Patients analyzed
| Patient | Diagnosis (pathology) | Channels analyzed | Duration, min | SOZ (# electrodes) seizure onset pattern |
|---|---|---|---|---|
| A | Facial pain (N/A) | 22 | 60 | N/A |
| B | Facial pain (N/A) | 13 | 60 | N/A |
| 1 | Partial epilepsy (cortical dysplasia) | 22 | 60 | Left frontal (3 electrodes) |
| Focal beta frequency onset | ||||
| 2 | Partial epilepsy (cortical dysplasia) | 21 | 60 | Left frontal (6 electrodes) |
| Focal gamma frequency onset | ||||
| 3 | Partial epilepsy (N/A*) | 23 | 45 | Left temporal (5 electrodes) |
| Focal gamma frequency onset | ||||
| 4 | Partial epilepsy (ependymoma) | 31 | 30 | Right frontal (7 electrodes) |
| Focal beta frequency onset |
Patients with facial pain and Patient 3 did not have surgical resection and therefore underlying tissue pathology is not known. Patient 3 was not a candidate for resective epilepsy surgery because the SOZ was localized to eloquent cortex. Not applicable (N/A). Seizure onset zone (SOZ).
All iEEG was acquired with a common reference, a stainless steel scalp suture placed in the vertex region of the scalp, midline between the Cz and Fz electrode positions (international 10–20). The scalp suture electrode was relatively isolated from the intracranial electrodes by the intervening layers of cerebrospinal fluid, bone, muscle, and scalp. These layers serve to distribute signal in such a way that ∼7 cm2 of coordinated cortical activity is required to produce a clear deflection detectable on the scalp (Tao et al. 2005). In practice the amplitude of the scalp reference signal is small compared with the direct cortical recordings obtained from the subdural electrodes (Hu et al. 2010).
The data were acquired with a DC-capable Neuralynx electrophysiology system, and digitized at 32,556 Hz. For analysis in this paper, the data were decimated off-line to a frequency of 5,000 Hz using FIR band-pass filters from 0.5 to 500 Hz each with a Bartlett-Hanning window. A notch filter from 45 to 75 Hz was applied to eliminate line noise.
Seizure onset zone (SOZ) electrodes and time of seizure were determined from the clinical report and verified independently. The clinical SOZ is defined by the subdural electrodes with the earliest intracranial electroencephalographic seizure discharge. Seizure onset times and location were determined by visual identification of a clear electrographic seizure discharge, followed by looking back in the record for the earliest electroencephalographic change contiguously associated with the seizure. We have previously used this approach for identification of neocortical SOZ (Wetjen et al. 2009; Worrell et al. 2004) In this study, we studied only patients with well localized focal seizure onsets.
Prior to quantitative analysis, continuous iEEG from each patient was reviewed using a custom Matlab viewer (Brinkmann et al. 2009). We removed all channels with excessive artifacts or line noise. All analyzed data from patients with epilepsy were ≥2 h removed from the time of seizure onsets. Using a custom annotation tool, channels and time segments containing significant artifact were labeled and not included in subsequent analysis. This process was completed with the reader blinded to all clinical information. To determine the effect of interictal epileptiform activity on LFP synchrony, we created duplicate data sets with the interictal epileptiform spikes, sharp waves, and after-coming slow waves removed. The average signal over all the LFP signals was computed, and the SD was computed over all time and all signals. Channel signals that deviated >5 SD from the average signal were marked as spikes. LFP amplitude distributions in 500 ms windows were calculated and activity with amplitude falling outside 5 SD was identified and the surrounding ±500 ms removed. If an interictal spike is detected on any channel, data of all channels for that time interval are removed. This process was validated by visual review of the data to ensure that selected events were epileptiform discharges. We have previously used a similar approach to detect and isolate interictal high-frequency epileptiform oscillations (Worrell et al. 2008).
For synchrony analysis, balancing the issues of signal nonstationarity and adequate sample size, we selected 1 s as the sliding window size (n = 5000 data points window) and calculated the cross-correlation of lag n within these nonoverlapping windows, for two electrode signals, and sj(t) and sk(t), as
where μi and σi are the means ± SD of signal si(t).
For mean phase coherence (Mormann et al. 2000), an analytic signal z(t) = s(t) + is̃(t) was generated using the Hilbert transform of the signal s(t)
where P.V. is the Cauchy principal value. The instantaneous phase of the signal is then
The mean phase coherence Rjk of the phase of two signals sj(t) and sk(t), ϕj and ϕk, is measured over time
Note that the issue of optimal time delay is already incorporated in the assignment of phase in the Hilbert transform. Because the mean phase coherence (MPC) measures the coherence of the phase difference between two signals within a window, as long as the time delay is constant, it has no effect on the MPC. A snapshot of signal pairs and the measured linear correlation and mean phase coherence in Fig. 1 show they can be qualitatively similar.
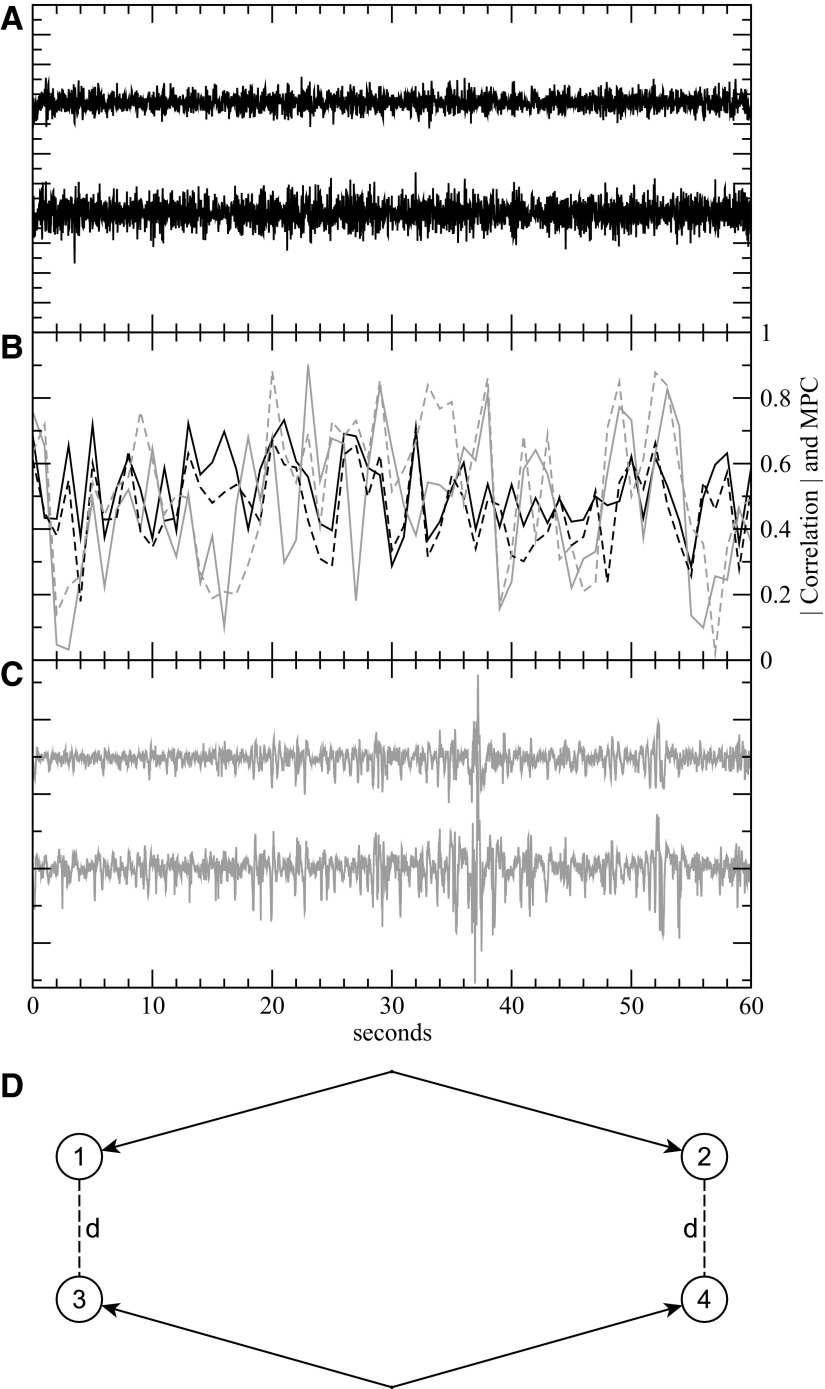
Fig. 1.
Data from patient with intractable facial pain (black) and patient with epilepsy (gray). Data from patients with intractable facial pain and no history of seizures serve as control recordings for quantitative comparison with epileptic cortex. A: sample signals from 2 electrodes of the control brain recording. B: the correlation magnitudes (solid lines) and mean phase coherence (dashed lines) of the signal pairs in A and C. Both the correlation and mean phase coherence (MPC) show significant temporal variability over the course of 60 s with values primarily ranging from 0.2 to 0.7. C: sample signals from 2 electrodes in epileptic cortex inside the seizure onset zone. D: a sample layout of the bipolar reference pair measurement. The distance d between 1 corresponding pair of electrodes 1 and 3 is equal to the distance between the other pair, electrodes 2 and 3, and this is the distance referenced in our bipolar measurements (Fig. 3).
Figure 1 shows sample iEEG signals and correlations from control and epilepsy patients. Zero-lag (n = 0) cross-correlation was analyzed first. Later, to explore the effect of distance and time lag, we computed cross-correlation using fast Fourier transforms (FFTs) to find the lag with the maximum correlation.
As mentioned in the preceding text, common references can add sizable spurious correlations/synchrony to EEG measurements (Guevara et al. 2005; Hu et al. 2010) because changes in the common reference potential can be misinterpreted as synchrony. To address this issue, we analyzed the correlations between the bipolar pair differences of voltage signals, such as s12 = s1 − s2 and s34 = s2 − s4, as shown in Fig. 1D. Bipolar electrode pairs were chosen so that the distances between corresponding electrodes in different bipolar pairs were identical, i.e., d13 = d24 = d in the figure, the distance referenced in Fig. 3. In addition, to isolate the effect of distance on corresponding electrode pairs while minimizing the effects of the other electrodes, corresponding distances d were chosen to be significantly smaller than other distances–d12, d14, d23, and d34. For corresponding electrode distances d ≥ 2 cm, distances between both opposite electrodes in a bipolar pair (d12 and d34) and noncorresponding electrodes (d23 and d14) were greater than or equal to 2d. For corresponding electrode distances of d = 3 cm, bipolar pair and noncorresponding distances were ≥5 cm. To investigate the effect of interictal epileptiform activity on local field potential synchrony, two independent reviewers identified and removed time intervals with interictal epileptiform activity. The analysis of local field synchrony was performed separately with the raw signals and also with all interictal epileptiform activity eliminated (Fig. 5).
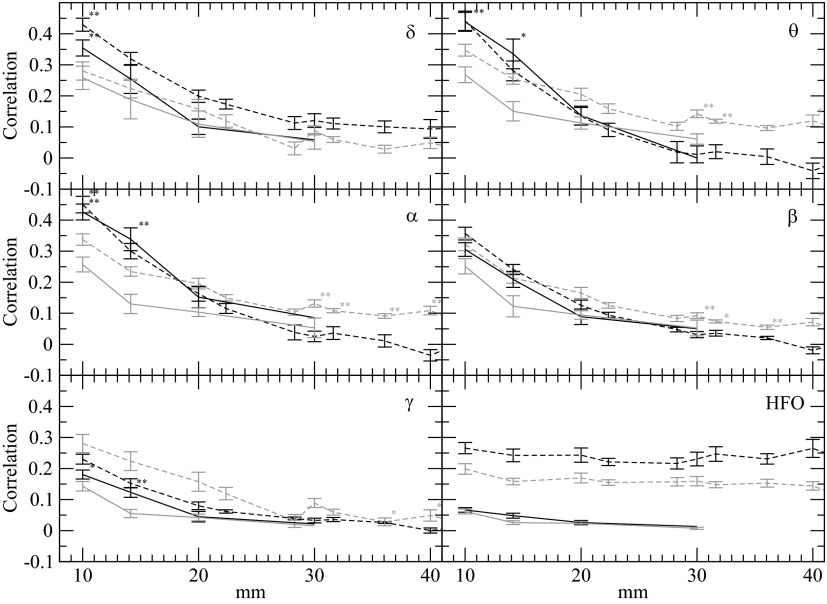
Fig. 3.
Average correlation as a function of distance between electrode pairs for 6 frequency bands: δ (0.5–4 Hz, A), θ (4–8 Hz, B), α (8–12 Hz, C), β (12–25 Hz, D), γ (25–45 Hz, E), and HFO (75–500 Hz, F). Average correlation as a function of distance from all electrode pairs in control brain (black) and epileptic brain (gray). Dashed lines, correlations between electrodes with a common reference; solid lines, bipolar pairs as a function of interelectrode distance. SEs are given except for the points lacking sufficient data. The correlation shows a significant decrease with electrode distance up to ∼30 mm for δ, θ, α, β, and for γ, the correlations fall more rapidly and are not significant beyond 14 mm. Correlations <0.07 are not significant as determined by the Bartlett estimator (Bartlett 1946). For high-frequency (75–500 Hz), the common reference has a significant impact and artificially increases correlations, as is demonstrated by the bipolar analysis. Unexpectedly, for δ, θ, α, the correlation at 10 mm is higher in the control brain compared with epileptic brain. Results with asterisk indicate significantly differing medians (P > 0.05) with a Mann-Whitney rank sum test, and results with double asterisk indicate highly significantly differing medians (P > 0.01).
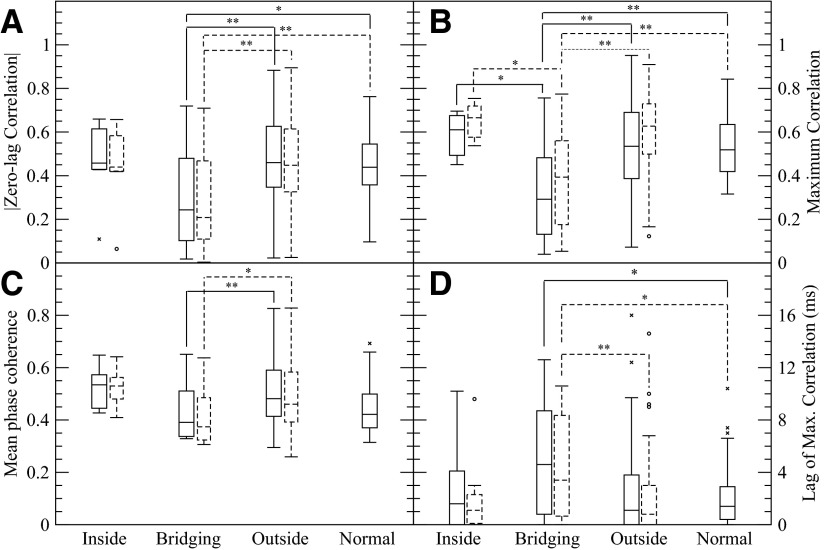
Fig. 5.
Comparison of different synchrony measures using zero-lag correlation, maximum correlation, and mean phase coherence (0.5–25 Hz band). A: the magnitude of zero-lag correlation, B: maximum correlation, C: mean phase coherence, and D: lag of max correlation. Both the entire data set (solid) and the data set with interictal spike time intervals removed (dashed) show similar results. The zero-lag correlation and maximum correlation for electrode pairs that bridge between SOZ and regions outside the SOZ is reduced compared with other electrode pairs in epileptic brain and electrode pairs in control brain. The MPC shows significantly lower synchrony for bridging electrode pairs in comparison to outside pairs. The time lag producing maximum correlation D is increased for bridging electrode pairs compared regions outside the SOZ in epileptic brain. Statistical comparisons of the data without epileptiform spikes to the control patient data were not made. To emphasize the body of the distribution, several outliers in the bridging pair distribution (lag = 140, 159, 208, 268, and 303 ms), the outside pair distribution (lag = 85, 131, 172, and 182 ms), and normal pair distribution (lag = 40 ms) are not shown. For the data with the interictal spikes removed, several outliers in the bridging pair distribution (lag = 146, 158, 162, 223, and 498 ms) and the outside pair distribution (lag = 79, 135, 179, and 291 ms) are similarly not shown. Brackets with * and ** indicate significantly different mean values at (P > 0.05) and (P > 0.01) with a Mann-Whitney rank sum test.
Statistical comparisons of signals were performed using the nonparametric Mann-Whitney rank sum test except for bipolar data analysis. A number of the pairs of electrode pairs in the bipolar analysis shared a common electrode pair, and so they were not truly independent samples. This precluded the use of a Mann-Whitney rank sum test, so a modified Student's t-test was performed.
RESULTS
The clinical diagnosis, pathological tissue diagnosis, location and number of implanted electrodes, duration of data analyzed, and seizure onset description, is shown in Table 1. The patients with epilepsy had well localized focal neocortical onset seizures. Three of the four patients with epilepsy underwent focal cortical resection of the SOZ and are now seizure free. One of the patients with epilepsy (patient 3, Table 1) was not a candidate for resective epilepsy surgery because the SOZ co-localized with eloquent cortex (language function). Tissue diagnosis from the three patients that had focal cortical resection showed cortical dysplasia for two patients and an ependymoma in one patient. The two patients with medically resistant facial pain went on to have chronic subdural electrical stimulation and significant improvement in their facial pain.
Amplitude of LFP
LFP recorded from a clinical subdural electrode primarily represents the synchronous synaptic input to the dendrites of pyramidal cells (Mitzdorf 1985). Thus the amplitude of the LFP is not only a measure of overall level of activity but also of local synchrony. In Fig. 2, A–C, histograms of the average LFP amplitude within 0.5–25 Hz for the different brain regions (SOZ, non-SOZ, control) are shown. Within the SOZ, as shown by the long tail in the histogram, there is a greater likelihood of higher iEEG amplitude compared with non-SOZ and control brain. Because of the significant contribution of the scalp common reference, the results at high-frequency (>25 Hz) are unreliable and not included here (see Fig. 3). While the artifact from muscle can be removed by analyzing bipolar data, the signals are no longer localized to a single subdural electrode.
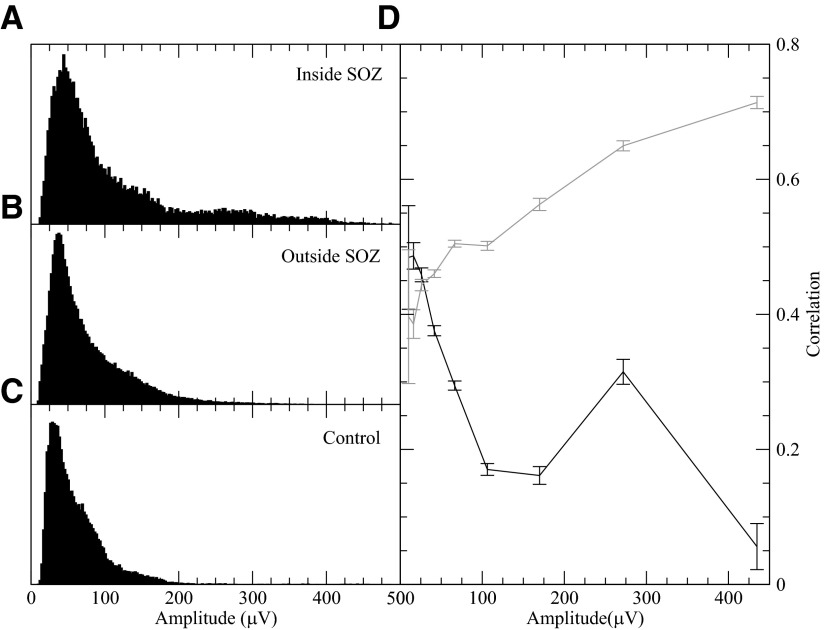
Fig. 2.
Distributions of the amplitude (root mean square) of the demeaned electroencephalographic (EEG) signals inside the seizure onset zone (SOZ) of epileptic brain (A), outside the SOZ (B), and in control patient brain (C). The median of the SOZ signal amplitude is significantly higher than control brain signal amplitude (P > 0.05). D: comparison of a SOZ electrode's average correlation with a neighbor inside the SOZ (gray) and a neighbor outside the SOZ (black) as a function of the SOZ electrode's amplitude. The correlations shown are averaged over all possible neighboring triplets (SOZ–SOZ–non-SOZ). Using a Mann-Whitney test, for all but the smallest amplitude bin, median differences were highly significant (P > 0.01). Confidence intervals are shown. For all these figures, the correlations and amplitudes of 1,200 windows (20 min) from each of the 4 patients with epilepsy were used. The higher value around amp = 275 μV for bridging pairs in D is largely due to patient 2, who had generally higher amplitude activity and a drop off in correlation at a higher amplitude.
Correlation versus distance
The iEEG signals were filtered into the six traditional frequency bands: δ (0.5–4 Hz),θ (4–8 Hz), α (8–12 Hz), β (12–25 Hz), γ (25–45 Hz), and a high-frequency band (75–500 Hz). Figure 3 shows that, not surprisingly, zero-lag correlation falls with interelectrode distance for patients with epilepsy (patient 1) and the control patient (patient A). Comparing the two patients, the results (the dashed lines in Fig. 3) show for distances <2 cm, significantly higher synchrony in control brain signals than the LFP from patients with epilepsy in (δ, θ, α, γ) but not the β and high-frequency bands.
The results for distances >2 cm may appear less clear. Most strikingly, for 75–500 Hz, correlations were measured to be roughly between 0.2 and 0.3 for common reference measurements and independent of distance. However, using bipolar signals, whereby the contribution of common scalp reference is eliminated (solid lines in Fig. 3), the synchrony in the high-frequency bands and distances >2 cm falls rapidly, supporting the idea that at high frequencies muscle artifact from common reference contributes significantly to the correlation (Guevara et al. 2005; Schiff 2005). The predominance of muscle artifact in the high frequency osciliation (HFO) band was verified by the fact that the maximum correlation recorded in this band always occurred at zero lag irrespective of distance as would be expected from common reference artifact.
We also explored how distance was defined on the convoluted brain. In particular, for neighboring electrodes, the existence of a sulcus between the electrodes could weaken and delay signal spread and as a result cause an effect on correlation value change. To account for the effective cortical surface distance, we used the patients' MRI and co-registered electrodes (Ken et al. 2007) to estimate the cortical surface distance between the two electrodes. The distance between the electrodes determined anatomically, accounting for brain invaginations (sulcii), did not significantly change the correlation and mean phase coherence results.
Seizure onset zone
With data from all the patients, we compared the nearest neighbor correlations inside the SOZ (SOZ–SOZ), bridges between inside and outside of the onset zone (SOZ–non-SOZ), outside the seizure onset zone (non-SOZ—non-SOZ), and in control brain of patients without epilepsy. Figure 4 shows that in a spectrum of different frequency bands the zero-lag correlations of connections bridging the SOZ and the non-SOZ were significantly less than those of the other electrode pairs—electrode pairs inside the SOZ, pairs outside the SOZ and pairs in the normal (control) brain.
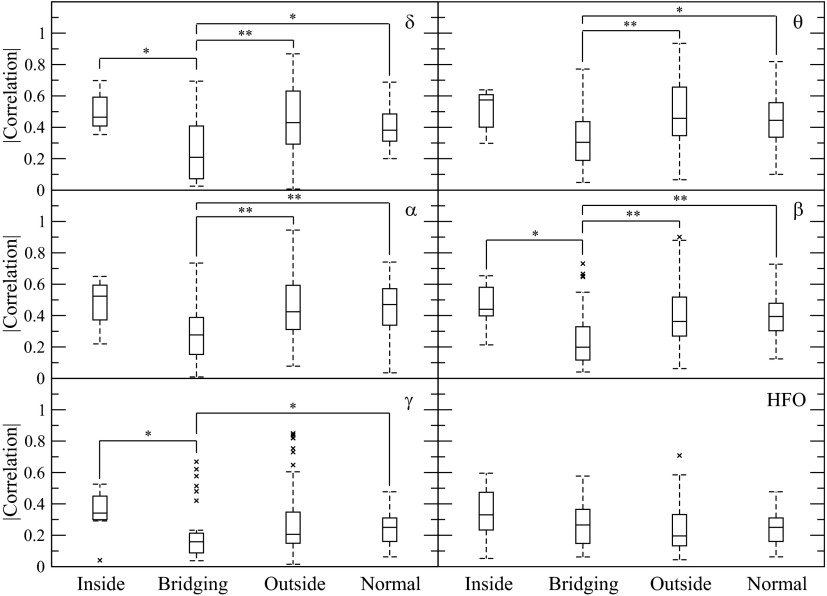
Fig. 4.
Average nearest neighbor correlations for electrode pairs inside the SOZ of patients with epilepsy, bridging between the SOZ and outside, outside the SOZ, and in the control patients. The frequency bands are those in Fig. 3. Bridging connections have significantly lower correlations over a range of frequency bands (δ, β, and γ) compared with those inside the SOZ, outside the SOZ in the δ, θ, α, β frequency bands, and in control brain for δ, θ, α, β, and γ frequency bands. Although there was a trend for increased correlations inside the SOZ compared with outside and for control brain, this did not reach significance. Brackets with * and ** indicate significantly different mean values at (P > 0.05) and (P > 0.01) with a Mann-Whitney rank sum test.
Correlation versus amplitude
To test whether decreased correlations between neighboring SOZ and non-SOZ electrodes were due to decreased connectivity between the regions or to input from different field potential generators, the correlations of bridging pairs were compared with the SOZ's correlation with another neighboring SOZ electrode. As Fig. 2D shows, the correlation between the bridging neighbors dropped as the amplitude of the SOZ electrode increased, in contrast to the other neighboring SOZ electrode. This agrees with the hypothesis that the bridging SOZ–non-SOZ pairs correlations reflected different generators and were functionally rather than anatomically disconnected from each other.
Lag cross-correlation
To test the hypothesis that the lower correlations observed for neighbor electrodes bridging the boundary between epileptic brain and surrounding brain (SOZ—non-SOZ) were a reflection of a persistent phase lag in LFP oscillations between near neighbor electrodes, the maximum cross-correlation over the range of lags was calculated.
As shown in Fig. 5, A and B, the maximum correlation showed results similar to zero-lag correlation. In calculating maximum correlation, two different weighting procedures were followed. In the first procedure, the correlations of every window were weighted identically in the average. In the second procedure, to assure that correlations reflected the bulk of signal activity rather than noisy low-level activity, each correlation was weighted according to the product of the SDs of the two signals in that window, effectively the amplitudes of the signals. The results of this second procedure are shown in Fig. 5, B and D. Both procedures gave qualitatively identical results: the maximum correlation of bridging pairs is lower than outside pairs and normal pairs with high significance (P > 0.01), the maximum correlation of bridging pairs is significantly lower than inside pairs (P > 0.05), and the lag for bridging connections is significantly higher (P > 0.05) than outside pairs. An example of significant phase lags between SOZ and non-SOZ pairs is shown in Fig. 6. The considerable amount of effort that went into finding suitable epilepsy patient data for this figure, specifically signals similar enough to discern similar phase relationships, suggests that lower correlation for bridging connections is a stronger effect.
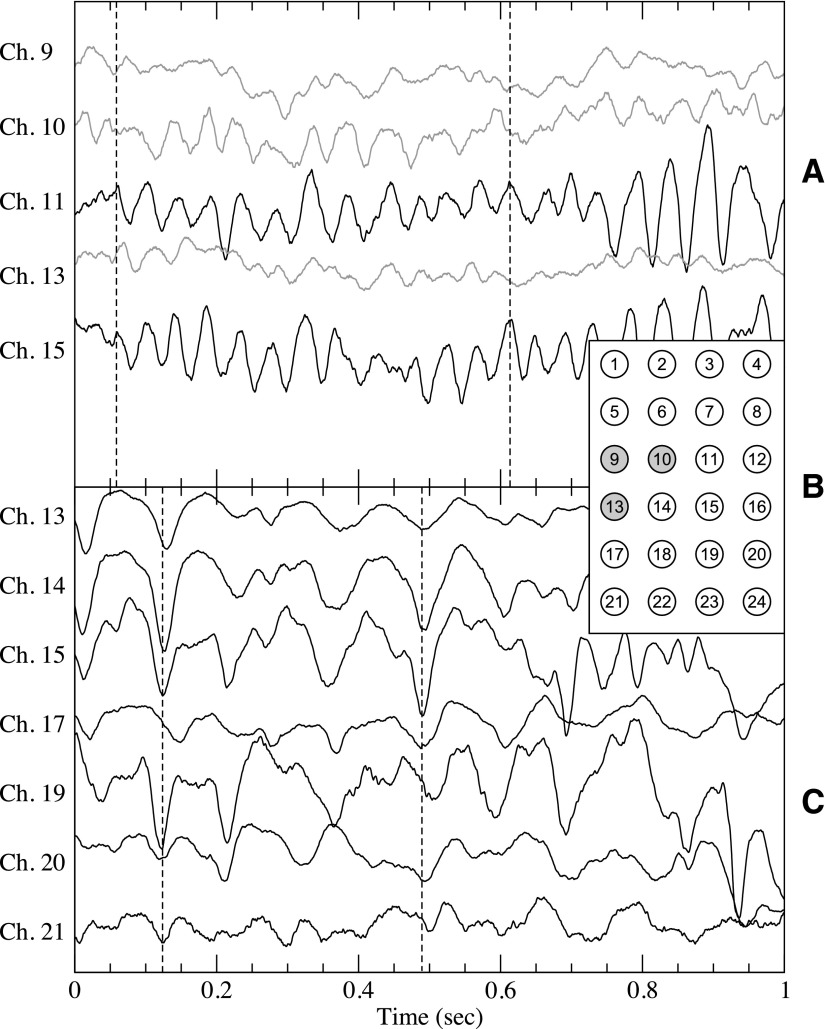
Fig. 6.
A: sample interictal intracranial electroencephalographic signals from patient 1 with epilepsy from both inside the SOZ, shown in gray, and near signals outside the SOZ (black). Dashed line, significant phase lag between inside and outside the SOZ. B: spatial layout of the intracranial electrodes for patients 1 and A with the SOZ electrodes (9, 10 and 13) of patient 1 shown in gray. C: sample signals from the control patient A. The spatial numbering is as shown in B. For clarification, signals are offset vertically.
Although the mean phase coherence of the signals (Fig. 5C) followed the qualitative trends of correlation, there is significant difference only between bridging connections and connections outside the SOZ. For both correlation and MPC, the synchrony (correlation or MPC) of pairs inside the SOZ trends high, similar to a previous study (Mormann et al. 2000), but does not reach significance. To analyze patient-specific variation, we first calculated the average zero-lag correlation differences between each patient's inside-the-SOZ pairs and its outside-the-SOZ pairs and then used a rank sum test, but again it did not yield a significant difference (P = 0.07) between the inside pairs and outside pairs. This lack of significance is likely to be due to the limited size of SOZs that limits the number of electrodes. Fewer connections within the SOZ means more limited statistical power. In contrast, the most populous group, the outside pairs, have the most significant differences with the bridging pairs. When Ansari-Asl et al. (2006) measured mean phase coherence on their coupled neuronal population, they found that it was less sensitive to increased coupling than linear correlation measures. In the presence of noise and a broad spectrum signal, mean phase coherence lacks intuitive interpretation (Sun and Small 2009). As the dashed results show in Fig. 5, the synchrony results on the data remaining after the epileptiform activity removal were similar to those of the entire data set.
The question arises: which is more important or relevant, the intertemporal variation of an electrode pair or the variation among pairs. Given that we measured ≥1,200 windows for each electrode pair, the SE in each of these pair averages is quite small. We used the more stringent measure of treating each electrode pair's average correlation over time as a data point in all figures shown, the zero-lag correlations within the SOZ have a trend of higher synchrony compared with outside connections (SOZ—non-SOZ), but that difference is not significant. If, however, the correlation of each window of each pair is a data point, then highly significant differences would be found between all groups.
DISCUSSION
The idea that hypersynchrony of pathological neuronal assemblies is the generator of epileptiform activity has long been a central tenet of the electrophysiology of epileptic brain (Jasper and Penfield 1954). Jasper and Penfield speculated that the local high-amplitude interictal epileptiform activity recorded directly from human cortex was generated by a burst of hypersynchronous neuronal activity. In studies of LFPs recorded directly from cortex with macroscopic clinical electrodes (∼1–10 mm2), like Jasper and Penfield's original work, the LFP is largely generated by synchronous postsynaptic currents associated with the coordinated input into the dendritic arbor of pyramidal cells. In effect, the LFP amplitude may be a measure of the synchronous network input, suggesting the large amplitude epileptiform transients represent more synchronous synaptic input. Alternatively, it could be increased activity. Interestingly, based on this line of reasoning they also reported seizures that began with an apparent “asynchronous state” low-amplitude LFP activity that evolved into a hypersynchronous state with high-amplitude rhythmic activity (Jasper and Penfield 1954). These findings were descriptive, but the terminology has remained as one of the central themes of the electrophysiology of epileptic brain.
Here we investigated the degree of LFP synchrony using iEEG recorded from clinical subdural grid electrodes. By the nature of the clinical recording, our data are limited to macroscopic LFP recorded from ∼10 mm2 surface area electrodes spaced 10 mm apart. Our results address the large-scale spatial structure of neuronal synchronization. Recent studies using a range of computational techniques have investigated the interictal and ictal synchrony of LFP obtained from iEEG. Multiple studies have reported increased local synchrony, i.e., hypersynchrony, within epileptic brain using a range of quantitative measures of synchrony, including spectral coherence (Towle et al. 1998, 1999), magnitude squared coherence (Zaveri et al. 2009), and mean phase coherence (Schevon et al. 2007). In general, the regions of interictal local hypersynchrony correlate with brain regions generating interictal epileptiform activity but not necessarily with the seizure onset zone (Schevon et al. 2007). In addition, investigations of LFP synchrony during spontaneous human seizures have consistently demonstrated a decrease in local LFP synchrony at seizure onset compared with baseline using mean phase coherence (Mormann et al. 2000), correlation and coherence (Wendling et al. 2003), and eigenvalue spectrum of zero-lag correlations (Schindler et al. 2008).
A major challenge with these studies has been the absence of control data. For this reason, it has been unclear whether the local regions of increased interictal synchrony are specific to epileptic brain. Here we analyzed long records of interictal iEEG from patients with medically resistant epilepsy and control subjects with intractable facial pain. We found that for normal (control) and epileptic brain the synchronization of LFP fell rapidly with the distance between electrodes and more rapidly at higher frequency. This finding is consistent with the hypothesis the generators of high-frequency oscillations are more spatially localized (Logothetis et al. 2007).
We find that control patients actually have greater LFP synchrony when averaged across all electrodes than interictal data from patients with epilepsy. The difference in average synchrony between control and epileptic brain is shown to be from isolated regions of markedly reduced synchrony. In particular, we show that these regions of reduced synchrony are the bridging connections between the SOZ and surrounding brain, and furthermore, we show that the synchrony in these bridging regions is reduced with greater activity in the SOZ. Within the context of functional connectivity (Bullmore and Sporns 2009; David et al. 2004; Horwitz 2003), which can be used to characterize the strength of interaction between different neuronal assemblies, the results show there is reduced functional connectivity between the epileptic neuronal assembly generating focal seizures and surrounding brain regions. In effect, SOZ is functionally disconnected from surrounding brain regions.
The loss of synchrony simply indicates a loss of the statistical interdependency of LFP measured at the recording sites separated by 10 mm. The loss of synchrony between specific electrode pairs could be the result of a range of possible mechanisms. Our recordings using clinical subdural electrodes provided a macroscale view of LFP synchrony and do not directly address possible mechanisms for the reduced synchrony between electrodes in the SOZ and surrounding brain. However, it is interesting that multiple studies have demonstrated significant decrease in the LFP synchrony in the SOZ at seizure onset (Cymerblit-Sabba and Schiller 2010; Netoff and Schiff 2002; Schindler et al. 2008). We speculate that the decreased neuronal synchrony may be fundamental property of epileptic brain, whereby the region of epileptic brain generating seizures is functionally disconnected from surrounding brain regions.
While we cannot directly determine whether the functional disconnection of the SOZ is a cause or an effect of epilepsy, it is consistent with recent experimental and theoretical models. An inhibitory surround of the epileptic foci (i.e., barrages of inhibitory postsynaptic potentials in the tissue surrounding the foci generating interictal epileptiform discharges) has been reported from intra- and extracellular recordings in a penicillin model of focal epilepsy in cats (Prince and Wilder 1967). Also in a tetanus toxin model, upregulation of brain-derived neurotrophic factor in the epileptic focus and downregulation in the surrounding tissue suggest changes in plasticity that could functionally isolate the SOZ (Liang et al. 1998). It has also been demonstrated that permanently isolated neocortex develops bursting epileptiform discharges within weeks of the injury (Avramescu and Timofeev 2008; Avramescu et al. 2009; Houweling et al. 2005). Within this theory the homeostatic plasticity that is responsible for a balance of inhibitory and excitatory input leads to the spontaneous bursts of epileptiform activity induced by low neuronal activity after deafferentation. While the mechanism underlying the development of epileptiform activity was not addressed in this model, recent studies of the cellular correlates of seizures have demonstrated that while there is often an increase in the firing rate of neurons (Bower and Buckmaster 2008; Cymerblit-Sabba and Schiller 2010), there is consistently a desynchronization of LFP and single unit activity at (Netoff and Schiff 2002) or even prior to seizure onset (Cymerblit-Sabba and Schiller 2010). It is speculated, and there is theoretical support for (Golomb 1998; Gutkin et al. 2001), the concept that the desynchronization of local neuronal assemblies allows higher firing rates to be sustained.
In summary, we show evidence that epileptic neocortex in human partial epilepsy is functionally disconnected from surrounding brain regions. We speculate that functional disconnection of the SOZ plays a role in the spontaneous generation of focal seizures and may be a clinically useful electrophysiological signature for spatial mapping of the SOZ for epilepsy surgery.
GRANTS
This research was supported by the National Institute of Neurological Disorders and Stroke Grant R01-NS-063039 to G. A. Worrell, Mayo Clinic Discovery Translation Grant, Minnesota Partnership for Biotechnology and Medical Genomics, and National Science Foundation of China Grant No. 61070127 to S. Hu.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We appreciate the technical support provided by C. Nelson and K. Crockett.
REFERENCES
- Aarabi et al., 2007. Aarabi A, Wallois F, Grebe R. Does spatiotemporal synchronization of EEG change prior to absence seizures? Brain Res 1188: 207–211, 2007 [DOI] [PubMed] [Google Scholar]
- Alper et al., 2008. Alper K, Raghavan M, Isenhart R, Howard B, Doyle W, John R, Prichep L. Localizing epileptogenic regions in partial epilepsy using three-dimensional statistical parametric maps of background EEG source spectra. Neuroimage 39: 1257–1265, 2008 [DOI] [PubMed] [Google Scholar]
- Amor et al., 2005. Amor F, Rudrauf D, Navarro V, N′diaye K, Garnero L, Martinerie J, Le Van Quyen M. Imaging brain synchrony at high spatio-temporal resolution: application to MEG signals during absence seizures. Signal Processing 85: 2101–2111, 2005 [Google Scholar]
- Ansari-Asl et al., 2006. Ansari-Asl K, Senhadji L, Bellanger JJ, Wendling F. Quantitative evaluation of linear and nonlinear methods characterizing interdependencies between brain signals. Phys Rev E Stat Nonlin Soft Matter Phys 74: 031916, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramescu et al., 2009. Avramescu S, Nita DA, Timofeev I. Neocortical post-traumatic epileptogenesis is associated with loss of GABAergic neurons. J Neurotrauma 26: 799–812, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramescu and Timofeev, 2008. Avramescu S, Timofeev I. Synaptic strength modulation after cortical trauma: a role in epileptogenesis. J Neurosci 28: 6760–6772, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett, 1946. Bartlett MS. On the theoretical specification and sampling properties of autocorrelated time-series. J R Stati Soc B 8: 27–41, 1946 [Google Scholar]
- Bartolomei et al., 2001. Bartolomei F, Wendling F, Bellanger JJ, Régis J, Chauvel P. Neural networks involving the medial temporal structures in temporal lobe epilepsy. Clin Neurophysiol 112: 1746–1760, 2001 [DOI] [PubMed] [Google Scholar]
- Bettus et al., 2008. Bettus G, Wendling F, Guye M, Valton L, Régis J, Chauvel P, Bartolomei F. Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res 81: 58–68, 2008 [DOI] [PubMed] [Google Scholar]
- Blumenfeld et al., 2004. Blumenfeld H, McNally KA, Vanderhill SD, Paige AL, Chung R, Davis K, Norden AD, Stokking R, Studholme C, Novotny EJ, Zubal IG, Spencer SS. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex 14: 892–902, 2004 [DOI] [PubMed] [Google Scholar]
- Bower and Buckmaster, 2008. Bower MR, Buckmaster PS. Changes in granule cell firing rates precede locally recorded spontaneous seizures by minutes in an animal model of temporal lobe epilepsy. J Neurophysiol 99: 2431–2442, 2008 [DOI] [PubMed] [Google Scholar]
- Brinkmann et al., 2009. Brinkmann BH, Bower MR, Stengel KA, Worrell GA, Stead M. Large-scale electrophysiology: acquisition, compression, encryption, and storage of big data. J Neurosci Methods 180: 185–192, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore and Sporns, 2009. Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10: 186–198, 2009 [DOI] [PubMed] [Google Scholar]
- Cymerblit-Sabba and Schiller, 2010. Cymerblit-Sabba A, Schiller Y. Network dynamics during development of pharmacologically induced epileptic seizures in rats in vivo. J Neurosci 30: 1619–1630, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David et al., 2004. David O, Cosmelli D, Friston KJ. Evaluation of different measures of functional connectivity using a neural mass model. Neuroimage 21: 659–673, 2004 [DOI] [PubMed] [Google Scholar]
- Doesburg and Ward, 2009. Doesburg SM, Ward LM. Synchronization between sources: emerging methods for understanding large-scale Functional networks in the human brain. In: Coordinated Activity in the Brain: Measurements and Relevance to Brain Function and Behavior. New York: Springer, 2009. p. 25–42 [Google Scholar]
- Elger and Lehnertz, 1998. Elger CE, Lehnertz K. Seizure prediction by non-linear time series analysis of brain electrical activity. Eur J Neurosci 10: 786–789, 1998 [DOI] [PubMed] [Google Scholar]
- Engel et al., 2005. Engel AK, Moll CK, Fried I, Ojemann GA. Invasive recordings from the human brain: clinical insights and beyond. Nat Rev Neurosci 6: 35–47, 2005 [DOI] [PubMed] [Google Scholar]
- Faingold, 2004. Faingold CL. Emergent properties of CNS neuronal networks as targets for pharmacology: application to anticonvulsant drug action. Prog Neurobiol 72: 55–85, 2004 [DOI] [PubMed] [Google Scholar]
- Golomb, 1998. Golomb D. Models of neuronal transient synchrony during propagation of activity through neocortical circuitry. J Neurophysiol 79: 1–12, 1998 [DOI] [PubMed] [Google Scholar]
- Guevara et al., 2005. Guevara R, Velazquez JLP, Nenadovic V, Wennberg R, Senjanovic G, Dominguez LG. Phase synchronization measurements using electroencephalographic recordings. Neuroinformatics 3: 301–313, 2005 [DOI] [PubMed] [Google Scholar]
- Gutkin et al., 2001. Gutkin BS, Laing CR, Colby CL, Chow CC, Ermentrout GB. Turning on and off with excitation: the role of spike-timing asynchrony and synchrony in sustained neural activity. J Comput Neurosci 11: 121–134, 2001 [DOI] [PubMed] [Google Scholar]
- Horwitz, 2003. Horwitz B. The elusive concept of brain connectivity. Neuroimage 19: 466–470, 2003 [DOI] [PubMed] [Google Scholar]
- Houweling et al., 2005. Houweling AR, Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Homeostatic synaptic plasticity can explain post-traumatic epileptogenesis in chronically isolated neocortex. Cereb Cortex 15: 834–845, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al., 2010. Hu S, Stead M, Dai Q, Worrell GA. On the recording reference contribution to EEG correlation, phase synchrony, and coherence. IEEE Trans Syst Man Cybern B Cybern, 40: 1294–1304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iasemidis et al., 1990. Iasemidis LD, Sackellares JC, Zaveri HP, Williams WJ. Phase space topography and the Lyapunov exponent of electrocorticograms in partial seizures. Brain Topogr 2: 187–201, 1990 [DOI] [PubMed] [Google Scholar]
- Jasper and Penfield, 1954. Jasper H, Penfield WG. Epilepsy and the Functional Anatomy of the Human Brain. Boston, MA: Little, Brown, 1954 [Google Scholar]
- Ken et al., 2007. Ken S, Di Gennaro G, Giulietti G, Sebastiano F, De Carli D, Garreffa G, Colonnese C, Passariello R, Lotterie JA, Maraviglia B. Quantitative evaluation for brain CT/MRI coregistration based on maximization of mutual information in patients with focal epilepsy investigated with subdural electrodes. Magn Reson Imaging 25: 883–888, 2007 [DOI] [PubMed] [Google Scholar]
- Kramer et al., 2008. Kramer MA, Kolaczyk ED, Kirsch HE. Emergent network topology at seizure onset in humans. Epilepsy Res 79: 173–186, 2008 [DOI] [PubMed] [Google Scholar]
- Le Van Quyen et al., 1998. Le Van Quyen M, Adam C, Baulac M, Martinerie J, Varela FJ. Nonlinear interdependencies of EEG signals in human intracranially recorded temporal lobe seizures. Brain Res 792: 24–40, 1998 [DOI] [PubMed] [Google Scholar]
- Le Van Quyen et al., 2000. Le Van Quyen M, Adam C, Martinerie J, Baulac M, Clémenceau S, Varela F. Spatio-temporal characterizations of non-linear changes in intracranial activities prior to human temporal lobe seizures. Eur J Neurosci 12: 2124–2134, 2000 [DOI] [PubMed] [Google Scholar]
- Le Van Quyen et al., 1999. Le Van Quyen M, Martinerie J, Baulac M, Varela F. Anticipating epileptic seizures in real time by a non-linear analysis of similarity between EEG recordings. Neuroreport 10: 2149–2155, 1999 [DOI] [PubMed] [Google Scholar]
- Le Van Quyen et al., 2001. Le Van Quyen M, Martinerie J, Navarro V, Boon P, D'Havé M, Adam C, Renault B, Varela F, Baulac M. Anticipation of epileptic seizures from standard EEG recordings. Lancet 357: 183–188, 2001 [DOI] [PubMed] [Google Scholar]
- Liang et al., 1998. Liang F, Le LD, Jones EG. Reciprocal up- and down-regulation of BDNF mRNA in tetanus toxin-induced epileptic focus and inhibitory surround in cerebral cortex. Cereb Cortex 8: 481–491, 1998 [DOI] [PubMed] [Google Scholar]
- Lima and Fregni, 2008. Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology 70: 2329–2337, 2008 [DOI] [PubMed] [Google Scholar]
- Logothetis et al., 2007. Logothetis NK, Kayser C, Oeltermann A. In vivo measurement of cortical impedance spectrum in monkeys: implications for signal propagation. Neuron 55: 809–823, 2007 [DOI] [PubMed] [Google Scholar]
- Lopes da Silva et al., 2003. Lopes da Silva FH, Blanes W, Kalitzin SN, Parra J, Suffczynski P, Velis DN. Dynamical diseases of brain systems: different routes to epileptic seizures. IEEE Trans Biomed Eng 50: 540–548, 2003 [DOI] [PubMed] [Google Scholar]
- Martinerie et al., 1998. Martinerie J, Adam C, Le Van Quyen M, Baulac M, Clemenceau S, Renault B, Varela FJ. Epileptic seizures can be anticipated by non-linear analysis. Nat Med 4: 1173–1176, 1998 [DOI] [PubMed] [Google Scholar]
- McCormick and Contreras, 2001. McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu Rev Physiol 63: 815–846, 2001 [DOI] [PubMed] [Google Scholar]
- Mitzdorf, 1985. Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev 65: 37–100, 1985 [DOI] [PubMed] [Google Scholar]
- Mormann et al., 2006. Mormann F, Elger CE, Lehnertz K. Seizure anticipation: from algorithms to clinical practice. Curr Opin Neurol 19: 187–193, 2006 [DOI] [PubMed] [Google Scholar]
- Mormann et al., 2000. Mormann F, Lehnertz K, David P, Elger CE. Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Phys D: Nonlinear Phenomena 144: 358–369, 2000 [Google Scholar]
- Netoff and Schiff, 2002. Netoff TI, Schiff SJ. Decreased neuronal synchronization during experimental seizures. J Neurosci 22: 7297–7307, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan et al., 2005. Pan JW, Kim JH, Cohen-Gadol A, Pan C, Spencer DD, Hetherington HP. Regional energetic dysfunction in hippocampal epilepsy. Acta Neurol Scand 111: 218–224, 2005 [DOI] [PubMed] [Google Scholar]
- Perez Velazquez et al., 2009. Perez Velazquez JL, Guevara Erra R, Wennberg R, Dominquez LG. Correlations of cellular activities in the nervous system: physiological and methodological considerations, In: Coordinated Activity in the Brain: Measurements and Relevance to Brain Function and Behavior. New York: Springer, 2009. p. 1–24 [Google Scholar]
- Prince and Wilder, 1967. Prince DA, Wilder BJ. Control mechanisms in cortical epileptogenic foci. “Surround” inhibition. Arch Neurol 16: 194–202, 1967 [DOI] [PubMed] [Google Scholar]
- Rulkov et al., 1995. Rulkov NF, Sushchik MM, Tsimring LS, Abarbanel HD. Generalized synchronization of chaos in directionally coupled chaotic systems. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics 51: 980–994, 1995 [DOI] [PubMed] [Google Scholar]
- Schevon et al., 2007. Schevon CA, Cappell J, Emerson R, Isler J, Grieve P, Goodman R, McKhann G, Weiner H, Doyle W, Kuzniecky R, Devinsky O, Gilliam F. Cortical abnormalities in epilepsy revealed by local EEG synchrony. Neuroimage 35: 140–148, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff, 2005. Schiff SJ. Dangerous phase. Neuroinformatics 3: 315–318, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler et al., 2007. Schindler K, Elger CE, Lehnertz K. Increasing synchronization may promote seizure termination: evidence from status epilepticus. Clin Neurophysiol 118: 1955–1968, 2007 [DOI] [PubMed] [Google Scholar]
- Schindler et al., 2008. Schindler KA, Bialonski S, Horstmann MT, Elger CE, Lehnertz K. Evolving functional network properties and synchronizability during human epileptic seizures. Chaos 18: 033119, 2008 [DOI] [PubMed] [Google Scholar]
- Spencer, 2002. Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia 43: 219–227, 2002 [DOI] [PubMed] [Google Scholar]
- Stead et al., 2010. Stead M, Bower MR, Brinkmann BH, Lee K, Marsh WR, Meyer FB, Litt B, Van Gompel J, Worrell GA. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain 133: 2789–2797, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan et al., 2008. Stephan KE, Riera JJ, Deco G, Horwitz B. The Brain Connectivity Workshops: moving the frontiers of computational systems neuroscience. Neuroimage 42: 1–9, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun and Small, 2009. Sun J, Small M. Unified framework for detecting phase synchronization in coupled time series. Phys Rev E Stat Nonlin Soft Matter Phys 80: 046219, 2009 [DOI] [PubMed] [Google Scholar]
- Tao et al., 2005. Tao JX, Ray A, Hawes-Ebersole S, Ebersole JS. Intracranial EEG substrates of scalp EEG interictal spikes. Epilepsia 46: 669–676, 2005 [DOI] [PubMed] [Google Scholar]
- Towle et al., 1999. Towle VL, Carder RK, Khorasani L, Lindberg D. Electrocorticographic coherence patterns. J Clin Neurophysiol 16: 528–547, 1999 [DOI] [PubMed] [Google Scholar]
- Towle et al., 1998. Towle VL, Syed I, Berger C, Grzesczcuk R, Milton J, Erickson RK, Cogen P, Berkson E, Spire JP. Identification of the sensory/motor area and pathologic regions using ECoG coherence. Electroencephalogr Clin Neurophysiol 106: 30–39, 1998 [DOI] [PubMed] [Google Scholar]
- Van Gompel et al., 2008. Van Gompel JJ, Stead SM, Giannini C, Meyer FB, Marsh WR, Fountain T, So E, Cohen-Gadol A, Lee KH, Worrell GA. Phase I trial: safety and feasibility of intracranial electroencephalography using hybrid subdural electrodes containing macro- and microelectrode arrays. Neurosurg Focus 25: E23, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling et al., 2003. Wendling F, Bartolomei F, Bellanger JJ, Bourien J, Chauvel P. Epileptic fast intracerebral EEG activity: evidence for spatial decorrelation at seizure onset. Brain 126: 1449, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetjen et al., 2009. Wetjen NM, Marsh WR, Meyer FB, Cascino GD, So E, Britton JW, Stead SM, Worrell GA. Intracranial electroencephalography seizure onset patterns and surgical outcomes in nonlesional extratemporal epilepsy. J Neurosurg 110: 1147–1152, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell et al., 2008. Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, Meyer FB, Marsh R, Litt B. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain 131: 928–937, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell et al., 2004. Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain 127: 1496–1506, 2004 [DOI] [PubMed] [Google Scholar]
- Zaveri et al., 2000. Zaveri HP, Duckrow RB, Spencer SS. The effect of a scalp reference signal on coherence measurements of intracranial electroencephalograms. Clin Neurophysiol 111: 1293–1299, 2000 [DOI] [PubMed] [Google Scholar]
- Zaveri et al., 2009. Zaveri HP, Pincus SM, Goncharova II, Duckrow RB, Spencer DD, Spencer SS. Localization-related epilepsy exhibits significant connectivity away from the seizure-onset area. Neuroreport 20: 891–895, 2009 [DOI] [PubMed] [Google Scholar]
- Zeng et al., 2007. Zeng LH, Xu L, Rensing NR, Sinatra PM, Rothman SM, Wong M. Kainate seizures cause acute dendritic injury and actin depolymerization in vivo. J Neurosci 27: 11604–11613, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]