Abstract
Patterns of stereotyped muscle coactivation, clinically referred to as synergies, emerge following stroke and impair arm function. Although researchers have focused on cortical contributions, there is growing evidence that altered stretch reflex pathways may also contribute to impairment. However, most previous reflex studies have focused on passive, single-joint movements without regard to their coordination during volitional actions. The purpose of this study was to examine the effects of stroke on coordinated activity of stretch reflexes elicited in multiple arm muscles following multijoint perturbations. We hypothesized that cortical injury results in increased stretch reflexes of muscles characteristic of the abnormal flexor synergy during active arm conditions. To test this hypothesis, we used a robot to apply position perturbations to impaired arms of 10 stroke survivors and dominant arms of 8 healthy age-matched controls. Corresponding reflexes were assessed during volitional contractions simulating different levels of gravitational support, as well as during voluntary flexion and extension of the elbow and shoulder. Reflexes were quantified by average rectified surface electromyogram, recorded from eight muscles spanning the elbow and shoulder. Reflex coordination was quantified using an independent components analysis. We found stretch reflexes elicited in the stroke group were significantly less sensitive to changes in background muscle activation compared with those in the control group (P < 0.05). We also observed significantly increased reflex coupling between elbow flexor and shoulder abductor–extensor muscles in stroke subjects relative to that in control subjects. This increased coupling was present only during volitional tasks that required elbow flexion (P < 0.001), shoulder extension (P < 0.01), and gravity opposition (P < 0.01), but not during the “no load” condition. During volitional contractions, reflex amplitudes scaled with the level of impairment, as assessed by Fugl-Meyer scores (r2 = 0.63; P < 0.05). We conclude that altered reflex coordination is indicative of motor impairment level and may contribute to impaired arm function following stroke.
INTRODUCTION
Multijoint coordination is impaired following stroke and largely restricted by abnormal coupling of muscle actions within the paretic limb, clinically referred to as muscle synergies (Brunnström 1970). In particular, recovery of arm function is limited and often characterized by constrained patterns of muscle activation that result in loss of independent joint control. This is generally manifested by the abnormal coupling of elbow flexion with shoulder abduction-extension-external rotation and, to a lesser extent, the coupling of elbow extension with shoulder adduction-flexion-internal rotation (Bourbonnais et al. 1989; Dewald and Beer 2001). Abnormal muscle coactivation can lead to an apparent weakness of the elbow, which is dependent on the impaired ability to generate torques at the shoulder (Beer et al. 2007). For example, persons with stroke often have difficulty using the affected shoulder to actively support the weight of the arm against gravity, thus requiring additional proximal arm support to activate muscles that are more distal. Inability to actively support the arm against gravity influences function, as does the reduced capacity to direct voluntary muscle actions to targeted proximal or distal joints in isolation. Although voluntary commands originating from the cortex are likely to contribute to these impairments (Schwerin et al. 2008), the role of involuntary reflex pathways is less certain.
It is well documented that stretch reflexes are altered following stroke, often defined in the classical sense as spasticity (Lance 1980). As a result, many clinical approaches target abnormal stretch reflexes. Such approaches include the Brunnström Method (Brunnström 1970), the Proprioceptive Neuromuscular Facilitation Technique (Voss et al. 1985), and the Bobath Concept (Bobath 1977, 1990). Even though impaired stretch reflexes are a common clinical target, their contribution to abnormal motor function remains questionable (Burne et al. 2005; Sheean and McGuire 2009) and the efficacy of these clinical approaches has been equivocal (Kollen et al. 2009; Luke et al. 2004). Thus quantitative studies are needed to assess the contributions of impaired stretch reflexes to motor disabilities following stroke, to better justify the use of clinical approaches targeted at reflex impairment.
Most studies assessing stretch reflex sensitivity following stroke have quantified behavior during passive conditions, demonstrating increased muscle activity in response to imposed joint perturbations (Thilmann et al. 1991). Although these passive, single-joint investigations are useful for characterizing the abnormal state of spastic muscles about a single joint, they have not been shown to correlate with motor impairment or functional outcomes (Sommerfeld et al. 2004), leading to confusion regarding stretch reflex contributions to motor impairments following stroke. Understanding the role of stretch reflexes during active conditions may be more clinically relevant. Moreover, reflex contributions to multijoint coordination are likely to be magnified during active conditions, in which the spinal cord is vital for integrating descending motor commands with afferent feedback.
A few recent studies have assessed stretch reflex behavior following stroke during more functionally relevant conditions. Musampa et al. (2007) demonstrated that resting stretch reflex thresholds at the elbow are abnormally regulated after stroke and also influenced by neural coupling from changes in static shoulder position. Furthermore, these thresholds were correlated with abnormal muscle cocontraction observed during voluntary elbow movements. Sangani et al. (2007) demonstrated that elbow perturbations induce reflex mediated torques about the elbow and shoulder and that this coupling is altered by changes in voluntary drive (Sangani et al. 2009). Although each of these studies provides important evidence that abnormal stretch reflexes contribute to impaired motor coordination following stroke, each considered only the influence of elbow perturbations and the voluntary generation of elbow motions or torques. Such conditions make it difficult to fully assess how patterns of reflex excitability throughout the limb may contribute to impaired multijoint coordination during the many functional tasks that involve coordinated activity of the elbow and shoulder.
The objective of this study was to examine the effects of stroke on stretch reflexes elicited in multiple muscles spanning the elbow and shoulder during volitional, isometric contractions. We had two specific goals. The first was to quantify how changes in background muscle activity altered reflex sensitivity following stroke. The second was to examine the patterns of reflex coordination throughout the arm. In particular, we were interested in quantifying how patterns of reflex activation are influenced by volitional activities known to be impaired after stroke, including supporting the arm against gravity or producing isolated flexion and extension torques at the elbow and shoulder. We hypothesized that stroke subjects would exhibit increased reflex activation of the arm muscles coupling elbow flexors and shoulder extensors/abductors and that this coupling would be most prevalent during active conditions involving these muscle groups. This hypothesis was tested by quantifying stretch reflex activity during two tasks that are typically challenging to stroke subjects: supporting the arm against gravity and generating isolated torques at the shoulder or elbow.
METHODS
Subjects
Experiments were performed on the paretic arms of 10 adults with chronic stroke and the dominant arms of 8 unimpaired control subjects. The stroke subjects and control subjects were age-matched, with ages (mean ± SD) of 56.9 ± 9.3 and 61.3 ± 6.8 yr, respectively. All protocols were approved by the Northwestern University Institutional Review Board and required informed consent. Control subjects had no history of upper limb or neurological impairments. Stroke subjects underwent an evaluation by a licensed physical therapist to determine their eligibility. Stroke subjects (Table 1) were included if they had sustained a unilateral stroke as defined from chart review, had full passive range of motion of the shoulder and elbow without pain or shoulder subluxation, some spasticity in elbow as defined by a cumulative Ashworth score of >1, some voluntary control of elbow and shoulder movements, no receptive aphasia, and the ability to follow verbal and visual commands. Subjects were excluded if they had a history of unilateral neglect (spatial and motor), inability to provide informed consent, and significant medical complications. We recorded Fugl-Meyer (FM) scores (Fugl-Meyer et al. 1975) as a reliable clinical measure of arm motor impairment (Duncan et al. 1983).
Table 1.
Demographic information of chronic stroke subjects
| Subject Number | Age, yr | Gender | Years Since Onset | Lesion Type | Lesion Location | Arm Impairmenta | Spasticity F/Eb |
|---|---|---|---|---|---|---|---|
| 01 | 67 | F | 10 | Left ischemic | Cortical | 51 | 2/1 |
| 02 | 61 | M | 12 | Left ischemic | Cortical | 20 | 3/2 |
| 03 | 57 | F | 12 | Right hemorrhagic | Cortical | 53 | 3/2 |
| 04 | 62 | M | 6 | Right ischemic | Cortical | 31 | 3/1 |
| 05 | 50 | F | 8 | Left ischemic | Cortical | 37 | 2/0 |
| 06 | 65 | M | 8 | Right ischemic | Cortical/subcortical | 34 | 3/3 |
| 07 | 62 | F | 5 | Left ischemic | Cortical | 41 | 3/2 |
| 08 | 50 | F | 4 | Right ischemic | Cortical | 30 | 3/2 |
| 09 | 38 | M | 3 | Right ischemic | Cortical | 39 | 3/2 |
| 10 | 26 | F | 4 | Right hemorrhagic | Cortical | 45 | 3/3 |
Based on Fugl-Meyer scale (maximum score = 66).
Modified Ashworth score for the elbow (0 = normal function; 5 = severe spasticity). F, flexion; E, extension.
Equipment
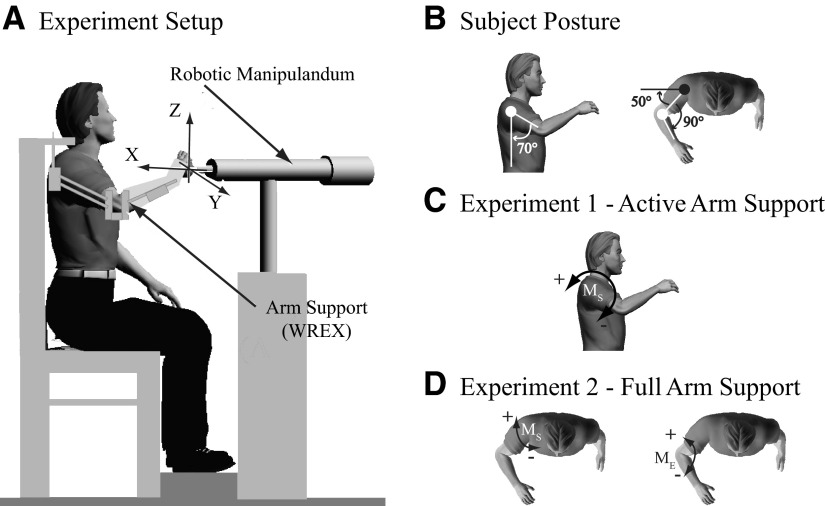
Details of the experimental setup have been previously provided (Perreault et al. 2008). In summary, subjects were seated with their trunk securely strapped to a rigid chair. Stretch reflexes were elicited using a 3 degree of freedom (DOF) robotic manipulator (HapticMaster; Moog-FCS Control Systems, Nieuw-Vennep, The Netherlands) to apply multidirectional displacement perturbations to the endpoint of the arm (Fig. 1A). The robot was configured as a stiff (50 kN/m) position servo and instrumented to measure endpoint forces (0.01 N resolution) and displacements (12 μm resolution). Subjects were attached to the robot using a custom rigid cast mounted to a gimbal at the end of the robot. Potentiometers embedded in the gimbal provided subjects visual feedback of their arm orientation. The nominal arm posture for these experiments positioned the hand directly in front of the glenohumeral joint, with joint angles of about 70° shoulder abduction, 50° shoulder flexion, 90° elbow flexion, and a neutral forearm angle (Fig. 1B).
Fig. 1.
Experimental setup. A: 3 degree-of-freedom (DOF) robotic manipulator used to apply displacement perturbations to the arm. Randomly timed sequence of ramp and hold displacement perturbations were applied to the arm along the coordinate axes while subjects (B) maintained arm posture of 70° of shoulder elevation, 50° of shoulder flexion, 90° of elbow flexion, and neutral forearm. An arm support device (Wilmington Robotic Exoskeleton [WREX]) provided graded weight support of the arm. C: during experiment 1, the arm was actively supported to oppose gravity (positive direction) or oppose support (negative direction). D: during experiment 2, the arm was supported by WREX. In the first full arm support condition, voluntary forces were directed along the shoulder joint to produce predominantly elbow moments (ME), elbow flexion moment (positive direction), and elbow extension moment (negative direction). In the second full arm support condition, voluntary forces were directed along the forearm to produce predominantly shoulder moments (MS), shoulder extension moment (positive direction), and shoulder flexion moment (negative direction).
Because many stroke patients have difficulty supporting their arm against gravity, arm support was provided by a customized version of the Wilmington Robotic Exoskeleton (WREX) (Rahman et al. 2001; Sanchez et al. 2006). This device has 4DOF, allowing for shoulder flexion/extension, shoulder abduction/adduction, internal/external rotation, and elbow flexion/extension. It can be sized for each subject and includes elastic bands that provide gradable levels of gravity compensation. A 1DOF load cell (Model MLP-200; Transducer Techniques, Temecula, CA), mounted to the base of the WREX elbow mechanism, was used to estimate the vertical arm support provided by these elements. All measures of the provided support were referenced to the shoulder, to indicate the shoulder abduction or adduction moments that the subjects were required to generate.
We recorded surface electromyograms (EMGs) from eight muscles that span the shoulder and elbow joints using bipolar surface electrodes (model 272; Noraxon USA, Scottsdale, AZ). The recorded muscles were the brachioradialis (BRD), biceps brachii (BI), long head (TRILONG) and lateral head (TRILAT) of triceps, anterior deltoid (AD), middle deltoid (MD), posterior deltoid (PD), and clavicular head of pectoralis (PC). EMGs were amplified by a Bortec AMT-16 system (Bortec Biomedical, Calgary, AB, Canada), which has a bandwidth of 10–1,000 Hz, an input impedance of 10 GΩ, and a common-mode rejection ratio of 115 dB at 60 Hz. The amplified signals were antialias filtered at 500 Hz using custom 5th-order Bessel filters and then sampled at 1,250 Hz with an 18-bit analog-to-digital converter (NI PCI-6289; National Instruments, Austin, TX). A common clock was used to synchronize data from the EMG and robotic systems.
Protocols
A series of maximum voluntary contractions (MVCs) were performed at the start of each experimental session. These data were later used to normalize EMGs recorded from each muscle. Standard muscle testing procedures were used to isolate the activity of each target muscle during these MVCs (Delagi and Perotto 1979).
Reflexes were elicited using a randomly timed series of ramp-and-hold perturbations applied to the arm. Perturbations were applied to the hand in six directions, corresponding to positive and negative directions along the axes shown in Fig. 1A. Each perturbation had a duration of 62.5 ms, which was sufficient to elicit consistent short- and long-latency reflexes (Lewis et al. 2005) and a velocity of 400 mm/s. These parameters corresponded to a perturbation amplitude of 25 mm. The endpoint displacement resulted in maximal elbow and shoulder joint displacements of about 7 and 4°, respectively. The corresponding joint velocities were about 110 and 60°/s for a typical subject. A ramp-and-hold position perturbation was elicited only after the subject maintained the required target force for 0.7 s. The hold time for the perturbation was uniformly distributed within the range of 1–1.25 s. The data collection for each trial lasted close to 10 s, which was ample time for subjects to reach a target force (±0 or ±5 N) and for the perturbation to be applied. Subjects were required to rest for a minimum of 30 s between each trial or longer as needed to avoid fatigue. In all experiments, subjects were instructed to maintain a constant effort and not to react to the perturbation, similar to instructions in previous studies (Burgess et al. 1995; Crago et al. 1976; Levin and Feldman 1994).
We used both the rising edge and the falling edge for each ramp-and-hold perturbation, to obtain close to 12 to 14 trials in each direction for averaging (Fig. 2). We quantified the resultant endpoint force and background muscle EMG activity prior to the rising and falling edges of each perturbation, to determine whether both edges could be used in our analyses. There was no significant change in the background EMG prior to the rising and falling edges of each perturbation (all P > 0.11), indicating that subjects were not able to intervene during the course of these assessments. We also found no statistical difference in the reflexes elicited by perturbations applied in a consistent direction, regardless of whether they were on the rising or the falling edge of the ramp (all P > 0.05 for all comparisons within each muscle and across all subjects). There were small differences in the endpoint force measured prior to the rising and falling edges of the perturbations (P < 0.05 for all subjects, conditions, and perturbation directions). However, the EMG results suggest that these were largely due to the intrinsic stiffness of the limb rather than changes in neural command, although contributions from muscles not monitored in this study cannot be discounted.
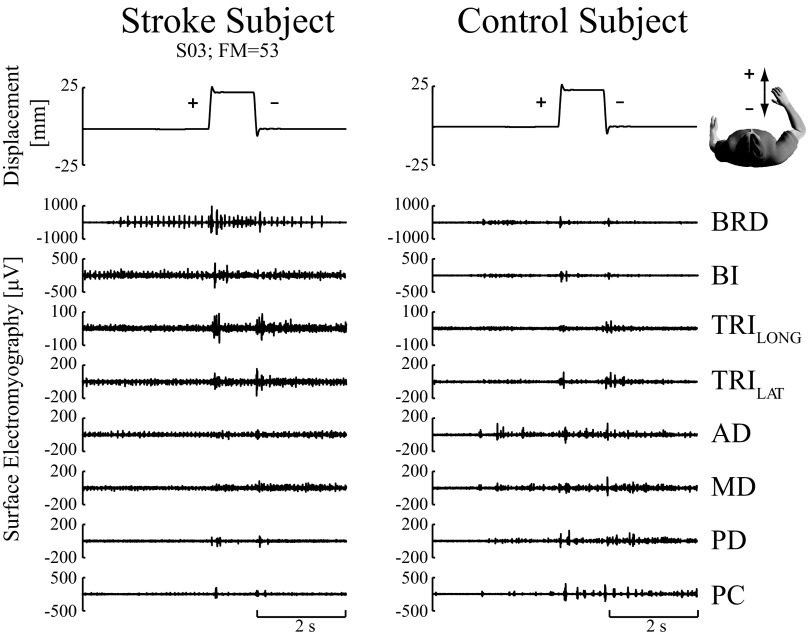
Fig. 2.
Representative raw stretch reflex electromyographic (EMG) responses. EMG responses from a representative stroke subject (S03) having a Fugl-Meyer (FM) score of 53 (left column) and a control subject (right column). Each was exerting a voluntary force of 5 Newtons (N) directed toward the glenohumeral joint (elbow flexion moment). Top traces correspond to a ramp-and-hold displacement perturbation along the X-axis, with a displacement of 25 mm and a velocity of 400 mm/s. The positive ramp corresponds to hand movement away from the body and the negative ramp corresponds to hand movement toward the body. The interval between the successive perturbations was 1 s. Raw EMGs in response to the displacement perturbations are shown for the elbow and shoulder muscles.
The experimental protocols required subjects to maintain a specified arm posture and endpoint force during each trial. Visual feedback was continuously provided to assist in these tasks. By use of a first-order infinite impulse response low-pass filter, with a cutoff frequency of 1 Hz, feedback was displayed on a liquid crystal display monitor, with a refresh rate of ≳40 Hz. Endpoint force was visualized as a solid sphere displayed in three-dimensional space and the associated force target was displayed as a translucent sphere that changed color when the subject was exerting the target endpoint force. Since the position of the hand and trunk were fixed in all experiments, the only postural degree of freedom that could be varied by the subject was the elevation of the arm (shoulder abduction). This was visualized by a rotating bar attached to the solid sphere. This bar was oriented horizontally when the subject elbow was at the desired elevation. The color and orientation of this bar changed to indicate deviations from the target posture. We monitored subject effort using a multichannel oscilloscope to display real-time changes in raw EMG activity of the spastic elbow flexor (primarily BRD and BI) and AD muscles. We found this to be especially helpful in detecting spontaneous changes in baseline EMG activity during “no load” trials when our stroke subjects often had a difficult time relaxing antigravity muscles. The background EMGs were also assessed off-line. Those that exceeded the average background activity by >3SDs above or 2SDs below were removed from further analysis. All subjects were able to maintain the desired endpoint forces and postures and reported no difficulties with the use of this display.
Seven different experimental conditions were tested, each corresponding to a different voluntary force required by the subject. These conditions were separated into two different experiments. The first assessed the ability to support the arm against gravity and the second assessed the ability to generate isolated torques about the elbow and shoulder when the arm was fully supported by the external orthosis. Details of each experiment are presented in the following text.
EXPERIMENT 1: ACTIVE ARM SUPPORT.
The first experiment assessed the influence of gravitational support, supplied by the WREX, on reflex coordination (Fig. 1C). The nominal condition corresponded to full gravitational support. In addition, we examined loading conditions that required the subjects to generate around 1.4 Nm of shoulder abduction (increased gravitational load) or shoulder adduction (decreased gravitational load). This load was used to elicit submaximal voluntary EMG activity of shoulder muscles within a manageable force range for all subjects.
EXPERIMENT 2: FULL ARM SUPPORT.
The second experiment assessed the influence of voluntary flexion and extension moments at the elbow and shoulder on reflex activity. Generating isolated torque at either the elbow or shoulder is known to be difficult following stroke (Beer et al. 1999; Dewald and Beer 2001), where involuntary coupling of elbow flexion and shoulder abduction/extension often becomes heightened. Our intent was to determine whether reflex excitability is also heightened during these tasks and whether the patterns of reflex activity mirror the abnormal volitional coactivity. During these experiments, the WREX provided full gravitational support and subjects were instructed to generate isolated flexion and extension moments about the shoulder and elbow. This was accomplished by having subjects exert endpoint forces directed along the axis of the forearm (shoulder moments) or along the line connecting the hand and the glenohumeral joint (elbow moments). Endpoint forces of ±5 N were used along each direction to elicit submaximal EMG activity of isolated shoulder and elbow muscles while maintaining force efforts of <15% MVC to minimize fatigue (Rohmert 1960) (Fig. 1D). These forces corresponded to elbow and shoulder moments of about 1.5 and 1.0 Nm, respectively, in our subject population. For baseline purposes, we also instructed subjects not to exert endpoint forces while their arm was fully supported by the WREX; this was referred to as the “no load” condition.
Data analysis
EMG data were processed as previously described (Perreault et al. 2008). Rectified electromyograms were used to quantify the magnitude of the stretch reflex and the background activity in each muscle. All measures were made relative to the background level of EMG by subtracting the mean activity 50 ms prior to perturbation onset from the reflex EMG. We normalized the EMG by that recorded during MVCs, to provide a measure of reflex behavior relative to maximum volitional activation. The MVC-normalized EMGs were used not only to quantify the magnitude of the elicited reflex EMG responses but also to examine the patterns of reflex activation across all muscles. We also examined unscaled EMGs to verify that changes in excitability were not merely due to normalization and to compare the magnitude of the elicited reflex EMG responses to those commonly reported in the literature. Only time periods within 100 ms after perturbation onset were considered in the reflex analysis.
Previous reports have shown that stretch reflex sensitivity increases after stroke (Dietz and Sinkjaer 2007). To determine whether this was evident with the multijoint perturbations used in our study, we compared the magnitude of the reflexes elicited in each muscle across our populations. In our stroke and age-matched control participants, we found the magnitude of reflex responses to be broad and typically without characteristic short-latency and long-latency bursts, as previously demonstrated in healthy young adults (Krutky et al. 2010; Perreault et al. 2008). Thus reflex magnitudes were quantified, within a single epoch, as the mean rectified EMG between 20 and 100 ms after perturbation onset. This period is likely to encompass both spinal and rapid supraspinal components, which we do not attempt to differentiate in this study. We found similarity in reflex responses between a short-latency window (20–50 ms) and a long-latency window (50–100 ms), using a series of linear regression models for each muscle and each subject. From the models we found the average relationship to be nearly one-to-one (regression slope: 0.9 ± 0.2) and significant (P values <0.0001; r2: 0.5 ± 0.1). Separate comparisons were made for each perturbation direction, although data from all force conditions were combined to provide a rough estimate across all activation levels. Reflex differences between groups for each perturbation direction were assessed using independent t-tests for each muscle and for each perturbation direction. For our parametric t-test, we transformed the reflex magnitudes to the logarithmic scale to meet the assumption of normality. We also used the Levene test to measure the equality of variance between groups (Levene 1960); in cases where we found this test to be significant (P < 0.05), we accounted for the unequal variances using an ANOVA with adjusted Welch F-ratio (Welch 1951). Results here and throughout the manuscript were analyzed using SPSS 16 statistical software (SPSS, USA) and were considered significant for P < 0.05. We used post hoc corrections of this P value to account for multiple comparisons.
Reflex magnitudes can change substantially with changes in background EMG (Matthews 1986; Stein et al. 1995) and we sought to examine how this sensitivity changes following stroke. Therefore we assessed this relationship in both groups using linear regression models to determine whether any observed increase in reflex sensitivity coincided with a proportional increase in voluntary activity prior to perturbation onset. Linear regression models were constructed for each muscle and perturbation direction; data were combined across all loading conditions and all subjects within each group to obtain a range of voluntary activation levels within each muscle. Comparisons across groups were made only for conditions in which stretch reflexes were consistently elicited in each group.
In addition to the individual muscle comparisons described earlier, we also examined the patterns of reflex activation across all muscles. This was done using an independent components analysis preceded by a principal components analysis (ICA/PCA), as detailed previously for this purpose (Krutky et al. 2010; Perreault et al. 2008). EMG data from 20 to 100 ms after perturbation onset were considered in this analysis. Prior to running the ICA/PCA algorithm, data were compressed by averaging the rectified EMG within each 10-ms window, creating 10 data points for the EMG measured in each muscle. Each independent component resulting from this analysis describes the relative activation of all recorded muscles. The identified components were used to determine not only whether the patterns of reflex coordination changed following stroke but also whether the relative activation of those patterns changed. Data from both experiments were included in this analysis to estimate the reflex coordination patterns applicable to all tested experimental conditions.
Changes in the patterns of reflex coordination were assessed by quantifying how well the independent components identified for one subject group could characterize the reflexes recorded in the alternate group. As will be shown in results, similar patterns of reflex coordination were identified for each group, suggesting that these were largely related to the selected perturbation directions. We therefore used a common set of independent components to examine changes in coordinated reflex activation across our subject populations. Because we were interested primarily in the relative activation of each reflex coordination pattern across the tested loading conditions and perturbation directions, the activation profiles for each independent component and each subject were normalized by the root-mean-squared activation computed across all experimental trials. These normalized activations were compared across the subject populations for each of the specific loading conditions described in the protocols for experiments 1 and 2. This was accomplished using a Welch-based ANOVA, since our group variances were statistically different. Game–Howell post hoc comparisons were made for each combination of perturbation direction and loading condition (Toothacker 1993).
Changes in the pattern of volitional activation could bias the patterns of reflex activation. Thus it was necessary to determine whether there were significant changes in the patterns of background muscle activity prior to perturbation onset. This was accomplished by defining an 8 × 1 vector of muscle activations summarizing the average EMG in each muscle across all trials in each of the tested load conditions. A dot product was used to compute the angle between these vectors for all subjects. A two-factor, Welch-based ANOVA was used to determine whether there were significantly larger differences in the computed angles across groups than within groups. The factors in this analysis were group (stroke, control, combined) and loading condition.
RESULTS
The perturbations used in these experiments elicited stretch reflexes in both subject groups. In general, perturbations elicited consistent responses in all subjects, with an average onset of 36.2 ± 8.6 ms across all muscles. In the control subjects, significant reflex responses were elicited in roughly 40% of the 336 possible conditions (8 muscles × 7 experiment conditions × 6 perturbation directions). In the stroke group, reflexes were elicited in close to 48% of the 336 possible conditions. The differences in number of responses between groups corresponded largely to the “no load” condition, where responses were elicited in 13% of the 48 possible conditions (8 muscles × 1 experiment condition × 6 perturbation directions) in the control group compared with 47% in the stroke group. Aside from the “no load” trials, most group differences were in the magnitude of the elicited responses, which will be described throughout this study.
Reflex characteristics within individual muscles
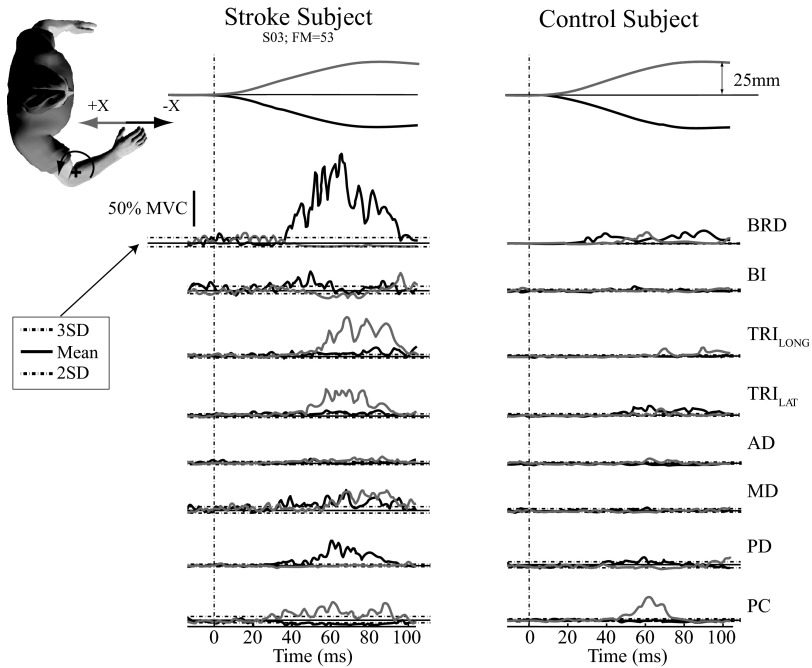
Stretch reflexes were significantly larger in stroke subjects relative to age-matched controls. Typical single-trial EMG responses from a stroke subject and a control subject are shown in Fig. 2. In this example, subjects exerted an elbow moment while the robot perturbed their arm along both directions of the X-axis. In this representative stroke subject (S03), large reflex responses were elicited from muscles spanning the elbow and shoulder joints, compared with the age-matched control. Only the time period within 20–100 ms after perturbation onset was considered for further analysis to avoid the possibility that voluntary interventions contributed to the observed responses. The reflexes elicited in each subject were averaged across like trials and normalized by the MVC recorded in each muscle to allow for comparisons across subjects. These average, normalized responses also were larger in the stroke subject compared with those in the age-matched control (Fig. 3). As shown in Table 2, the most significant differences in reflex amplitude between the control group and the stroke group were observed for the MVC-normalized EMGs. These differences were nearly all due to increased excitatory responses in the stroke group; only the PC muscle showed significantly larger inhibitory response in the stroke group. Table 2 also shows that there were significant differences in the raw EMGs, an indication that the larger reflexes in the stroke group were not simply due to differences in muscle strength across the populations. The most significant differences were observed in the elbow flexor muscles (BRD and BI; P < 0.001).
Fig. 3.
Typical maximum voluntary contraction (MVC)–normalized stretch reflex EMG responses. Stretch reflex EMG responses from a representative stroke subject (S03) having an FM score of 53 (left column) and an age-matched control subject (right column). Each was exerting a voluntary force of 5 N directed toward the glenohumeral joint (elbow flexion moment). EMGs are normalized to percentage of maximum voluntary contraction (%MVC). Thin and thick lines correspond to average reflex responses during positive and negative perturbations along the X-axis, respectively. The dashed vertical line corresponds to the perturbation onset. The thick horizontal lines correspond to average background EMG and dashed horizontal lines correspond to 3SDs above average background EMG and 2SDs below average background EMG.
Table 2.
Comparison of reflex magnitudes between stroke and control groups
| Perturbation Directions and Approximate Joint Displacements† |
|||||||
|---|---|---|---|---|---|---|---|
| +X |
−X |
+Y |
−Y |
+Z |
−Z |
||
| Muscle | Elbow Flex, Shoulder Ext Stroke/Control | Elbow Ext, Shoulder Flex Stroke/Control | Shoulder Ext Stroke/Control | Shoulder H. Flex Stroke/Control | Shoulder Abduction/ER Stroke/Control | Shoulder Adduction/IR Stroke/Control | |
| Scaled, %MVC | BRD | 4/1 | 38/7⇑ | 9/1⇑ | 4/1⇑ | 6/1⇑ | 12/1⇑ |
| BI | 2/1 | 11/3⇑ | 21/6⇑ | −2/0 | 5/1⇑ | 8/0⇑ | |
| TRILONG | 15/4⇑ | 6/1⇑ | 3/1⇑ | 7/3⇑ | 3/1 | 5/1⇑ | |
| TRILAT | 15/7⇑ | 8/3⇑ | 5/0⇑ | 5/2⇑ | 4/1⇑ | 6/3⇑ | |
| AD | 4/2⇑ | 1/0 | 3/3 | 1/1 | 0/0 | 3/1⇑ | |
| MD | 3/1⇑ | 3/2⇑ | 1/0 | 2/−2⇑ | 1/−1 | 1/−1⇑ | |
| PD | 3/0⇑ | 7/3⇑ | −1/0 | 8/6⇑ | 2/1 | 2/1 | |
| PC | 12/5⇑ | −2/0 | 16/9⇑ | −3/0⇓ | 1/1 | 7/1⇑ | |
| Unscaled, μV | BRD | 18/7 | 164/51⇑ | 44/8⇑ | 12/8 | 31/11 | 58/9⇑ |
| BI | 6/6 | 42/27⇑ | 94/43⇑ | −5/0 | 20/11 | 30/4⇑ | |
| TRILONG | 39/16⇑ | 12/5⇑ | 7/3 | 16/13 | 5/4 | 13/4⇑ | |
| TRILAT | 63/43⇑ | 18/18 | 11/−1 | 11/14 | 9/13 | 15/23 | |
| AD | 14/15 | 6/4 | 20/8⇑ | 6/6 | 1/2 | 10/9 | |
| MD | 7/8 | 9/10 | −1/6⇓ | 11/14 | 2/3 | 6/6 | |
| PD | 10/−2 | 34/27 | 1/−3 | 57/41⇑ | 6/9 | 11/7 | |
| PC | 37/32 | 3/−4 | 65/53 | 4/−6 | 10/5 | 20/12 | |
Absolute reflex magnitudes are summarized for the stroke group and, after the slash mark, the control group. Significant mean differences in magnitudes were defined at the Bonferroni-corrected 0.05 level and represented as either excitatory (⇑) or inhibitory (⇓) based on the reflex magnitude of the larger group's response. Negative differences correspond to larger responses (excitatory or inhibitory) in the control group compared with the stroke group.
Approximate joint displacements are characterized under each perturbation direction with the following abbreviations: Flex, flexion; Ext, extension; H. Flex, horizontal flexion; H. Ext, horizontal extension; ER, external rotation; IR, internal rotation.
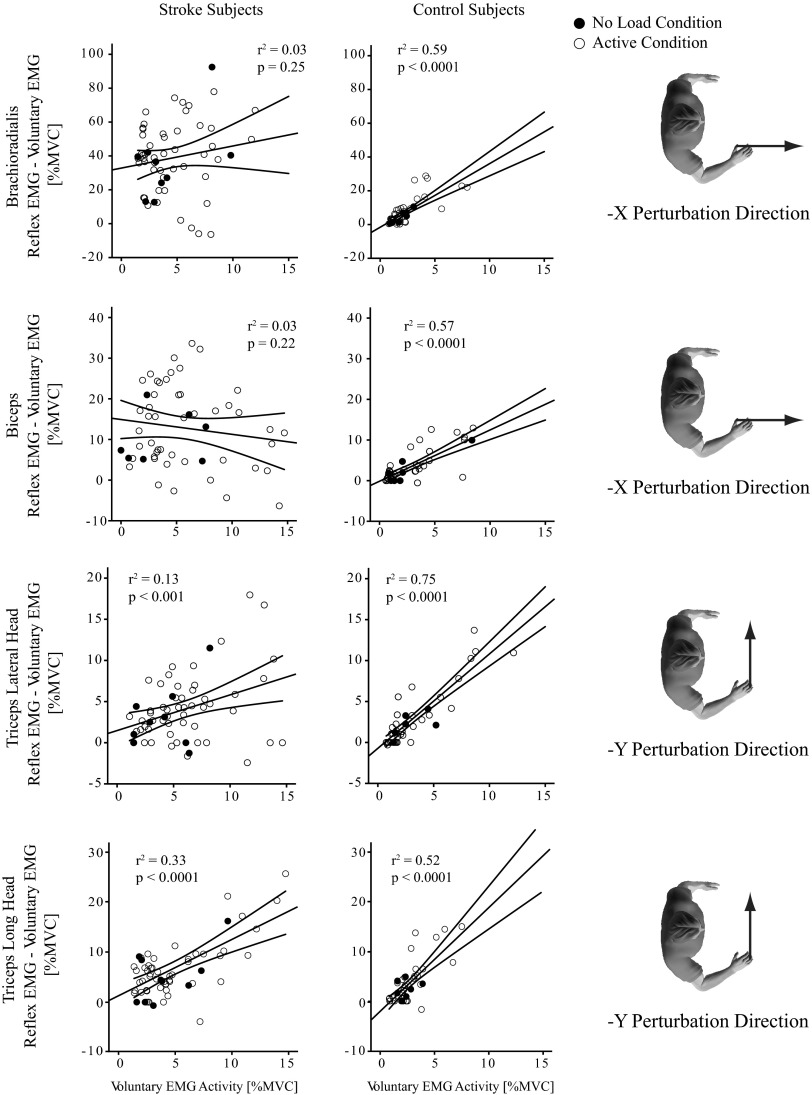
Stretch reflexes in the stroke group were not as sensitive to changes in background muscle activation as were those in the control group; rather, most remained high across a wide range of background EMG levels. This was assessed using linear regression (Fig. 4). Data from all loading conditions were considered to provide a range of background activations within each muscle. All muscles in the control group had a significant relationship between background and reflex EMG along the perturbation direction that elicited that largest response (Table 3). In contrast, this relationship was significant in only six of the eight tested muscles in the stroke group. For the six muscles that had a significant regression in both groups, the correlation coefficient was significantly higher in the control group (P < 0.0001), as was the slope of the relationship (P < 0.006). In contrast, the intercept was significantly higher in the stroke group (P = 0.02), where the normalized background EMG was generally larger across both the active and the “no load” condition. For similar normalized background EMGs, the greatest disparities between groups were observed in the elbow flexors. Linear regression models for the BRD and BI were not significant in the stroke group but were significant for controls (Fig. 4, top two rows). The elbow extensors (Fig. 4, bottom row) and shoulder muscles had significant regressions for both groups; however, offsets were highly variable and slopes were substantially reduced in the stroke group.
Fig. 4.
Linear regression between background EMG activity and amplitude of reflex EMG. Linear regression models were used to describe the relationship between reflex EMG and background EMG for 2 elbow flexors (BRD and BI) and an elbow extensor (TRILAT and TRILONG) muscle. Left column corresponds to the stroke group and right column to the control group. Data are pooled across all subjects. Data from active conditions are represented with open circles and data from conditions where subjects were instructed to remain relaxed (“no load” condition) are represented with black circles. Human figures on the right correspond to perturbation directions for each row of regression plots. Thin lines above and below the thick regression line define 95% confidence intervals (CIs).
Table 3.
Linear regression between background EMG activity and stretch reflex amplitude for each perturbation direction
| Group | Muscle | Perturbation Direction* | P Value | F-Statistic | Offset | Slope | r2 Value |
|---|---|---|---|---|---|---|---|
| Control | BRD | −X | <0.0001† | 69.70 | −1.59 | 3.77 | 0.59 |
| BI | −X | <0.0001† | 65.74 | −0.15 | 1.26 | 0.57 | |
| TRILONG | −Y | <0.0001† | 54.05 | −2.04 | 2.06 | 0.52 | |
| TRILAT | −Y | <0.0001† | 138.26 | −0.72 | 1.15 | 0.75 | |
| AD | −Z | <0.0001† | 61.83 | −1.18 | 1.42 | 0.56 | |
| MD | −X | <0.0001† | 74.11 | −0.83 | 0.95 | 0.60 | |
| PD | −X | <0.0001† | 43.92 | −0.06 | 1.62 | 0.47 | |
| PC | +Y | <0.0001† | 44.98 | 3.10 | 2.28 | 0.48 | |
| Stroke | BRD | −X | 0.22 | 1.54 | — | — | 0.03 |
| BI | −X | 0.25 | 1.33 | — | — | 0.03 | |
| TRILONG | −Y | <0.0001† | 29.91 | 1.26 | 1.13 | 0.33 | |
| TRILAT | −Y | <0.005† | 8.86 | 1.48 | 0.43 | 0.13 | |
| AD | −Z | <0.001† | 16.27 | 0.42 | 0.46 | 0.22 | |
| MD | −X | 0.02† | 6.09 | 1.44 | 0.31 | 0.10 | |
| PD | −X | <0.001† | 12.53 | 1.71 | 0.86 | 0.18 | |
| PC | +Y | <0.0001† | 17.62 | 5.74 | 1.01 | 0.23 |
Direction of perturbation was chosen for each muscle based on the maximum r2 value produced by the linear regression model for the control group.
Significant regressions were defined at the 0.05 level.
Reflex coordination across muscles
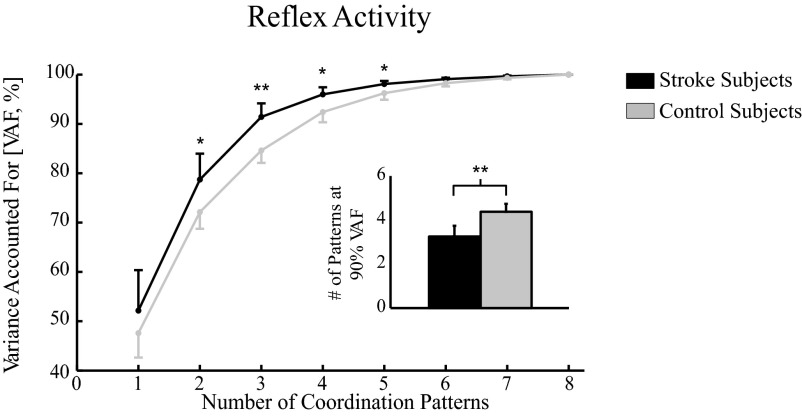
Significantly fewer reflex coordination patterns were needed to explain the EMG variance in the stroke group compared with the control group (Fig. 5). These were estimated from the data collected in both experiments, to provide a robust estimate of the reflex coordination patterns contributing to our entire data set. At most, eight reflex coordination patterns would be needed to account for all of the observed reflex variance, one for each muscle. However, >90% of the EMG variance across all muscles could be described by an average of only 3.4 ± 0.5 coordination patterns in the stroke group, whereas an average of 4.4 ± 0.5 patterns were needed to describe the same variance in the control group, a statistically significant difference (P < 0.005).
Fig. 5.
Comparing number of reflex coordination patterns between the stroke and control groups. Coordination patterns were estimated using independent component analysis/principal component analysis (ICA/PCA) on the entire set of reflex EMG data for stroke and control subjects. Bar graphs depict the total number of coordination patterns necessary to explain roughly 90% of EMG data variance accounted for (VAF). Average reflex EMGs from 20 to 100 ms following perturbation onset were included in this analysis; data within each 10-ms bin were averaged prior to processing.
The three dominant patterns for each group were similar, although they differed substantially in the amount of variance described (Fig. 6). Activation of muscles crossing the elbow was dominated by excitation of the BRD and BI, both contributing to elbow flexion; moreover, there was also significant excitation of the TRILAT, but to a much lesser degree. Shoulder activity was dominated by excitation of the PD and small but significant excitation of the MD; there was also inhibition of the PC in the stroke group. These actions would result in shoulder extension and some support against gravity. This first reflex coordination pattern was named the “elbow flexion and shoulder extension,” according to the net actions at the elbow and shoulder. This pattern accounted for 49.0 ± 2.2% of the total variance in the stroke group, but for only 22.7 ± 2.5% of the variance in the control group, a statistically significant difference (P < 0.0001). The second pattern (“elbow flexion and shoulder flexion pattern”), which consisted of primarily excitation of BI and PC and, to a lesser extent, excitation of AD and inhibition of PD muscles, accounted for a similar amount of variance in both groups (P = 0.19). The third pattern (“elbow extension pattern”) consisted of primarily TRI activation and, to a far lesser extent, generalized cocontraction at the shoulder. It accounted for significantly more variance in the control group than that in the stroke group (P < 0.0001). The fourth pattern, necessary only in the control group, consisted of mainly TRI and PD excitation.
Fig. 6.
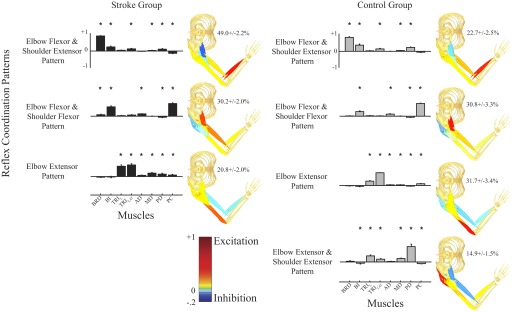
Summary of reflex coordination patterns for the stroke and control groups. Patterns on the left column describe reflex data collected from stroke subjects and those on the right column describe reflex data from the control subjects. Muscles in each coordination pattern are indicated at the bottom of each column. Bar heights in each pattern correspond to the relative reflex activation magnitudes in each muscle normalized to unity. Percentages on the right of each pattern indicate the relative variance described by that pattern; only data remaining after the initial PCA reduction are considered in the relative variance calculation. Statistical significance corresponds to values significantly different from chance (*P < 0.05).
Although fewer reflex coordination patterns were found in the stroke group, the first three reflex patterns in each group were similar. Similarity between groups was termed cross-prediction accuracy and was obtained by fitting the data in a subject from one group using the three coordination patterns estimated from the other group. The coordination patterns estimated for each of the control group subjects were able to explain 83.5 ± 6.1% of the reflex variance for each of the stroke group subjects and the coordination patterns estimated for the stroke group subjects were able to explain 78.5 ± 7.2% of the variance for the control group subjects. These differences were small and did not reach significance (P = 0.11). Thus we estimated a single set of three reflex coordination patterns to characterize the reflex responses observed in both experiments for all subjects. This set accounted for 82.0 ± 1.8% of the variance in the stroke group and 76.0 ± 2.0% of the variance in the control group. The use of this common set of reflex coordination patterns allowed us to compare the activation of these patterns across our subject pools.
Load-dependent activation of reflex coordination patterns
The activation of the elbow flexion and shoulder extension reflex pattern was larger in the stroke group compared with that in the control group during experimental conditions known to augment the abnormal voluntary flexion synergy. This was assessed by comparing the normalized reflex activations for each loading condition as well as for each perturbation that elicited the greatest activation of the muscles contributing to this pattern. To better clarify the functional consequence of these endpoint movements, we also include a qualitative description of the joint-based motions that correspond to the perturbation directions. In both groups, activation of the elbow flexion and shoulder extension pattern was greatest during perturbations along the −X axis, which extended the elbow and flexed the shoulder. In response to this perturbation, activation of this coordination pattern was significantly larger in the stroke group than that in the control group when subjects attempted to actively support their arm against gravity (P = 0.01) (Fig. 7A). A similar increased activation of this reflex coordination pattern was found when subjects attempted to actively generate elbow flexion moments (P < 0.001) and shoulder extension moments (P = 0.01) (Fig. 7B). The elbow flexor and shoulder flexor reflex pattern had the largest activation during perturbations along the +Y axis, which extended both the elbow and shoulder. In response to these perturbations, activation of the elbow flexor and shoulder flexor reflex pattern was significantly larger in the control group than that in the stroke group. These differences were significant only during the generation of voluntary shoulder flexion moments (Fig. 7B; P = 0.02). There were no significant between-group differences in the activation of the third reflex coordination pattern, corresponding to elbow extension; this pattern was activated most by perturbations along the +X direction, which flexed the elbow and extended the shoulder.
Fig. 7.
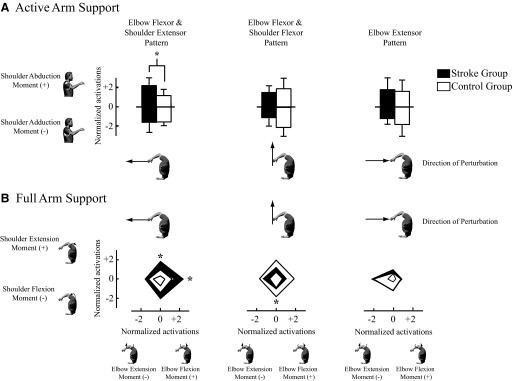
Normalized reflex activations during active and full arm support conditions in response to hand perturbations. A: between-group comparisons of coordination pattern activations during active arm support that opposed gravity (shoulder abduction moment) and opposed support (shoulder adduction moment). Statistical significance as defined by an asterisk corresponds to differences in reflex activation between groups (P < 0.05). B: between-group comparisons of reflex activations during conditions where subjects attempted to generate isolated shoulder moments and elbow moments. In both figures, reflex activations were normalized to the average root-mean-square activation computed across the 3 patterns and all force conditions for each subject. The robot-generated displacement perturbations in directions that produced the greatest responses in both groups for each pattern. The shaded regions correspond to SDs. Statistical significance as defined by an asterisk corresponds to differences in reflex activation between groups (P < 0.05).
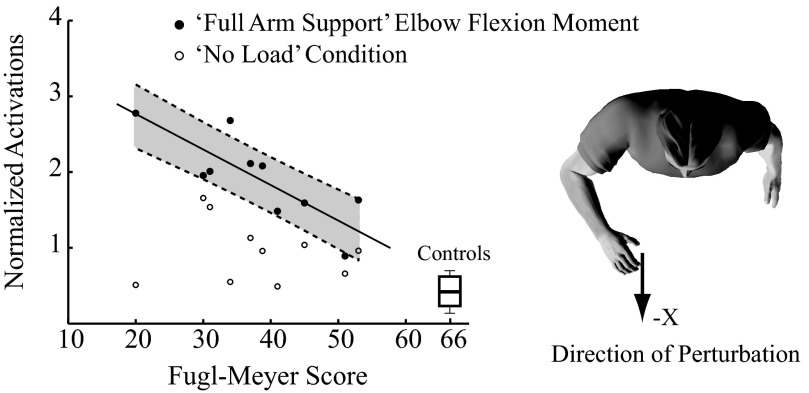
Activation of the most prominent reflex pattern in the stroke group—elbow flexion and shoulder extension—increased with increasing impairment, although this relationship appeared only during active elbow flexion (Fig. 8). Impairment was quantified using the FM score and reflex activation was considered only for the perturbation direction and the voluntary loading conditions that elicited the largest reflex activation in the stroke group relative to that in the control population. These conditions were for perturbations along the −X direction during voluntary elbow flexion, shoulder extension, and gravitation opposition. There was a linear relationship between the activation of this reflex coordination pattern during voluntary elbow flexion and arm FM score (r2 = 0.63; P = 0.03); subjects with lower FM scores had higher reflex activity. In contrast, subjects with lower levels of impairment and correspondingly higher FM scores had reflex activations similar to those of the control subjects. Importantly, this relationship between reflex activation and impairment level was true only for this active condition. No such relationship was found for the reflexes elicited during voluntary shoulder extension (P = 0.60) or gravity opposition (P = 0.58). There also was no significant relationship for the reflexes measured during the “no load” condition (P = 0.66).
Fig. 8.
Task-specific activation patterns vs. motor impairment level for the flexor coordination pattern. Normalized activations of the flexor pattern were plotted against FM scores during the “no load” condition when subjects were relaxed (open circles) and during voluntary elbow flexion (solid circles). For both conditions, the robot generated displacement perturbations in the −X direction, which produced the greatest responses in both groups. Shaded region in gray depicts the 95% CI of the linear model for the elbow “pull” condition. Boxplot represents the quartile range of normalized activations in the control subjects with designated maximum FM scores of 66. The black star indicates a significant regression (r2 = 0.63, P = 0.03) of elbow flexor torque on FM score.
Coordination of voluntary activity across muscles
Coordination of voluntary activity across muscles was similar between the stroke and control groups at the force levels studied. The similarity of voluntary muscle activation was assessed for the three volitional conditions that led to significant differences in reflex activation between the two groups. These were the conditions that required the subject to either 1) support the arm against gravity, 2) flex the elbow, or 3) extend the shoulder. Similarity was assessed by computing the angle between the vector of normalized muscle activations, as detailed in methods. During gravity opposition, the average similarity angle between groups was 29.1 ± 12.4°, which was not significantly different from that computed between stroke subjects (36.7 ± 8.4°; P = 0.17) or between control subjects (26.6 ± 3.6°; P = 1.0). Moreover, there were no significant differences in the between-group and within-group comparisons for the shoulder extension condition (stroke group, P = 0.21; control group, P = 0.53) or the elbow flexion condition (stroke group, P = 1.0; control group, P = 1.0). These results suggest that the increased activation of elbow flexion and shoulder extension reflex pattern in the stroke group was not solely due to differences in relative activation of the eight muscles prior to perturbation onset.
Although there were no significant differences in the coordination of muscle activity between the groups, the average level of activity was higher in the stroke subjects than that in the control subjects. Normalized background muscle activity was assessed for the three volitional conditions that led to significant differences in reflex activation between the two groups. Significant differences were seen in all muscles (all P < 0.01). During gravity opposition, the stroke subjects had an average increase in normalized background EMG of 195 ± 76% across all muscles. For the elbow flexion task, the stroke subjects had an increase of 218 ± 78% and for the shoulder extension task it was 197 ± 2%. These increases represent a heightened level of effort in the stroke group than that in the control group, which likely can be attributed to the constant loads used for both populations and the lower strength in the stroke group.
DISCUSSION
The purpose of this study was to examine the effect of stroke on the coordination of multijoint stretch reflexes during active conditions relevant to postural control. Specifically, we were interested in determining whether voluntary muscle activity altered the sensitivity of stretch reflexes following stroke and whether the patterns of reflex coordination mirror the abnormal synergies previously noted during voluntary isometric contractions. Our results show that stretch reflexes elicited in a specific muscle were less sensitive to changes in background activity within that muscle in the stroke group than they were in the control group. In the control group there was a significant relationship between background and reflex EMG within each muscle, whereas in the stroke subjects, the stretch reflexes remained high across a wide range of background EMG levels. Across muscles within the arm, a common pattern of reflex coordination coupling elbow flexion and shoulder extension was observed in both groups, although this pattern was substantially more active in the stroke subjects during conditions that required the subjects to actively support their arm against gravity or to exert isolated flexion torques at the shoulder or elbow. Furthermore, the activation of this reflex pattern increased with decreasing FM scores. Together, these findings suggest that abnormal reflex coupling may contribute to arm impairments and that the activation of these may serve as a biomarker for arm impairment following stroke.
Changes in reflex sensitivity following stroke
The stretch reflexes elicited by multijoint perturbations were generally larger and more variable in the stroke subjects than those in control subjects at all levels of activation. Regardless of whether the recorded reflex amplitudes were MVC-normalized or unscaled, the largest sensitivity was found in elbow flexor muscles of stroke subjects. As depicted in Table 2, the greatest difference in reflex sensitivity between groups involved the BRD muscle, where MVC-normalized group differences were as much as 31%. This finding of increased reflex sensitivity was consistent with previous single-joint investigations. A number of groups have reported increased reflexes in the elbow flexors when the elbow joint is passively stretched (Pisano et al. 2000; Schmit et al. 2000; Simons and Bingel 1971; Thilmann et al. 1990; Wolf et al. 1996). These increased reflex responses are accompanied by large variability in magnitudes across subjects (Starsky et al. 2005), which was clearly observed in our study and was probably explained by the broad range of FM motor impairment levels (see Table 1) in our volunteers. Increased elbow flexion reflexes during active conditions have also been previously reported (Bedingham and Tatton 1984; Dietz et al. 1991; O'Dwyer et al. 1996). Our study extends these single-joint results and suggests that these altered stretch reflex pathways are also evident with voluntary activation of multiple muscles during arm loading conditions relevant to posture control.
Stroke subjects exhibited reduced reflex sensitivity to changes in background muscle activity compared with that observed in the control subjects. In healthy humans, the stretch reflex activity has been shown to scale with background EMG (Cathers et al. 2004; Marsden et al. 1972), a finding replicated in this study (Fig. 4). This is in contrast to the stroke group that exhibited heightened and more variable reflex sensitivity at all levels of background muscle activity. The largest differences between the stroke and control groups were for the influence of background muscle activity on the reflexes elicited in the elbow flexors. In these muscles, slopes of the regression models were not significant in the stroke subjects but were highly significant for the control group. All other muscles had significant regressions for both groups, but the slopes were smaller and offsets were larger in the stroke subjects. This implies that sizes of the stretch reflexes were different to a lesser degree with changes in level of background muscle activity. Others have reported that with the presence of background muscle activity there were no differences between spastic and control reflexes for the elbow flexor muscles (Dietz et al. 1991; Lee et al. 1987; Powers et al. 1988). Our findings of reduced modulation are consistent with these previous reports; the reflexes were observed during volitional contractions of roughly 10% MVC, which tended to be similar between groups. Although these previous studies have been used to argue for a reduced contribution of stretch reflexes to impairments during active conditions, they were restricted to individual joints. Given that many of the impairments following stroke are related to the coordination between joints, we also sought to examine the coordination of stretch reflexes after stroke and to explore the possibility of heteronymous coupling between elbow and shoulder muscles during multidimensional perturbations of the arm while subjects simultaneously activated muscles spanning the elbow and shoulder.
Coordination of stretch reflexes following stroke
Coordination of multijoint reflexes involves highly adaptive responses during interactions between the arm and environmental mechanics (Krutky et al. 2010; Perreault et al. 2008). Our recent studies demonstrate the task specificity of multijoint stretch reflexes along with earlier work by Gielen et al. (1988) and Lacquaniti (1991) and exemplify the important role of multijoint stretch reflexes in normal motor control. The motor cortex contributes to reflex modulation (Kimura et al. 2006; Shemmell et al. 2009) during interactions with different mechanical environments. Thus cortical damage following stroke could result in altered coordinated activation of multijoint reflexes and associated loss of task-specific reflex function.
The prominence of the “elbow flexor and shoulder extensor” reflex pattern in stroke subjects was comparable to the abnormal voluntary flexor synergy, known to intensify during similar conditions of gravity compensation and shoulder and elbow moment generation. Reflex activation of the elbow flexors and, to a much lesser extent, shoulder extensors was greatest during arm loading conditions that augment the abnormal voluntary flexor synergy. A similar coactivation of elbow flexors and shoulder abductors/extensors has been observed during voluntary activation (Brunnström 1970) and is enhanced when actively supporting the arm against gravity (Beer et al. 2004, 2007), suggesting that similar mechanisms may contribute to the impairments in the coordination of voluntary and involuntary motor responses. Functionally, this raises the question of whether the observed reflex patterns might contribute to the observed abnormal coactivity of muscles at the elbow and shoulder during postural tasks involving gravity opposition and proximal joint control for distal object manipulation at the hand. Our conclusion is consistent with the recent work of Sangani and colleagues (Sangani et al. 2007, 2009) who suggested heteronymous reflex coupling between the elbow and shoulder joints in response to active-assist and passive elbow extension movements. In particular, they reported a simultaneous increase in elbow flexion and shoulder abduction reflex torque with elbow extension perturbations. However, their use of slower perturbations and longer time windows for analysis make it more difficult to separate reflex and voluntary contributions to the observed coupling. Nevertheless, both studies agree on the need to assess corrective responses during active conditions and on the similarity of impaired coordination seen in these corrective responses to that observed during voluntary force generation.
Changes in supraspinal control may contribute to the changes in reflex coordination observed in our stroke population. It has been suggested that descending motor commands rely more on spared brain stem structures (i.e., reticular pathways) that are less inhibited following disruption at the cortical level (Gracies 2005). Less inhibition of the reticular formation pathways could result in greater facilitation of elbow flexors, as was observed in the present study. Such a mechanism would be consistent with the work of Davidson and Buford (2004) who demonstrated that motor outputs to arm flexors were facilitated during stimulation of the medial pontomedullary reticular formation in nonhuman primates. From the same stimulation site, they also found poststimulus suppression of elbow extensors. Sprague et al. (1948) showed that regions of the reticulospinal tract also have an excitatory effect on spinal motor pathways, which could be unmasked after cortical inhibition. If these changes on spinal reflex excitability exhibit a similar flexion bias following stroke, they too could contribute to the observed results. Changes in the behavior of integrative spinal circuits, such as the propriospinal system (Pierrot-Deseilligny 1996), following stroke could also contribute to the observed results, although the influence of these changes on multijoint coordination has yet to be studied.
Our results were obtained for a single arm posture and a single set of perturbation parameters. Changes in each would undoubtedly lead to different reflex responses elicited in our subject populations. Musampa et al. (2007) demonstrated that reflex thresholds in the spastic elbow depend on the posture of the shoulder. Similar changes in arm posture may have led to different reflex coordination patterns in our study. However, since we were concerned primarily with differences in reflex behavior between our subject populations, consistent changes in joint angle are less likely to have influenced our main conclusions. Nevertheless, posture does alter spinal (Hyngstrom et al. 2007) and cortical (Mitsuhashi et al. 2007) excitability and assessing these influences within each of our subject populations could lead to a better understanding of how these changes influence the coordination of multijoint reflexes within each. Similarly, since we chose perturbation parameters and experimental conditions that elicited consistent responses in both populations, we do not expect that a change in these parameters would have a substantial effect on our conclusions. It is certainly possible to select perturbation parameters and experimental conditions that typically elicit reflexes in stroke subjects but not unimpaired subjects (Musampa et al. 2007; Nielsen et al. 2005). However, such a choice would not have allowed us to achieve our objective of comparing the reflexes that can be elicited in these populations.
Clinical correlates
A striking finding from this study was that the impairment level, as measured by the FM score, was correlated with the activation of the “elbow flexion and shoulder extension” reflex pattern. This result implies that the more severely impaired stroke survivors had greater activation of this reflex pattern. Although past studies have shown a correlation between the expressions of cortical pathways after stroke and recovery (Carey et al. 2006; Ward et al. 2003), attempts to correlate heightened reflex activity with spasticity (Alibiglou et al. 2008) and motor impairments (Sangani et al. 2007; Voerman et al. 2005) have not been successful. One explanation for these inconclusive results may be that these studies considered only reflexes elicited under passive conditions. Another possibility is that many of these studies used slower stretches and involved planar elbow–shoulder joint configurations. It is possible that in the present study, the consistent active conditions in three-dimensional space may have placed the motorneuron pool in a state more relevant to that used during functional tasks. Thus the reflex coupling observed in this study may be a marker of—and possibly a contributor to—arm impairment following stroke.
GRANTS
This work was supported by National Institutes of Health Grants K25 HD-044720 and RO1 NS-053813.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank E. Krepkovich for assistance with manuscript preparation, T. Haswell for assistance with technical issues, and all those who participated in the experiments.
REFERENCES
- Alibiglou et al., 2008. Alibiglou L, Rymer WZ, Harvey RL, Mirbagheri MM. The relation between Ashworth scores and neuromechanical measurements of spasticity following stroke (Abstract). J Neuroeng Rehabil 5: 18, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedingham and Tatton, 1984. Bedingham W, Tatton WG. Dependence of EMG responses evoked by imposed wrist displacements on pre-existing activity in the stretched muscles. Can J Neurol Sci 11: 272–280, 1984 [DOI] [PubMed] [Google Scholar]
- Beer et al., 2004. Beer RF, Dewald JPA, Dawson ML, Rymer WZ. Target-dependent differences between free and constrained arm movements in chronic hemiparesis. Exp Brain Res 156: 458–470, 2004 [DOI] [PubMed] [Google Scholar]
- Beer et al., 2007. Beer RF, Ellis MD, Holubar BG, Dewald JPA. Impact of gravity loading on post-stroke reaching and its relationship to weakness. Muscle Nerve 36: 242–250, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer et al., 1999. Beer RF, Given JD, Dewald JP. Task-dependent weakness at the elbow in patients with hemiparesis. Arch Phys Med Rehabil 80: 766–772, 1999 [DOI] [PubMed] [Google Scholar]
- Bobath, 1977. Bobath B. Treatment of adult hemiplegia. Physiotherapy 63: 310–313, 1977 [PubMed] [Google Scholar]
- Bobath, 1990. Bobath B. Adult Hemiplegia: Evaluation and Treatment. London: Heinemann Medical Books, 1990 [Google Scholar]
- Brunnström, 1970. Brunnström S. Movement Therapy in Hemiplegia: A Neurophysiological Approach. New York: Harper & Row, 1970 [Google Scholar]
- Burgess et al., 1995. Burgess PR, Cooper TA, Gottlieb GL, Latash ML. The sense of effort and two models of single-joint motor control. Somatosens Mot Res 12: 343–358, 1995 [DOI] [PubMed] [Google Scholar]
- Burne et al., 2005. Burne JA, Carleton VL, O'Dwyer NJ. The spasticity paradox: movement disorder or disorder of resting limbs? J Neurol Neurosurg Psychiatry 76: 47–54, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey et al., 2006. Carey LM, Abbott DF, Egan GF, O'Keefe GJ, Jackson GD, Bernhardt J, Donnan GA. Evolution of brain activation with good and poor motor recovery after stroke. Neurorehabil Neural Repair 20: 24–41, 2006 [DOI] [PubMed] [Google Scholar]
- Cathers et al., 2004. Cathers I, O'Dwyer N, Neilson P. Variation of magnitude and timing of wrist flexor stretch reflex across the full range of voluntary activation. Exp Brain Res 157: 324–335, 2004 [DOI] [PubMed] [Google Scholar]
- Crago et al., 1976. Crago PE, Houk JC, Hasan Z. Regulatory actions of human stretch reflex. J Neurophysiol 39: 925–935, 1976 [DOI] [PubMed] [Google Scholar]
- Davidson and Buford, 2004. Davidson AG, Buford JA. Motor outputs from the primate reticular formation to shoulder muscles as revealed by stimulus-triggered averaging. J Neurophysiol 92: 83–95, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delagi and Perotto, 1979. Delagi EF, Perotto A. Anatomical Guide for the Electromyographer. Springfield, IL: Charles C Thomas, 1979 [Google Scholar]
- Dewald and Beer, 2001. Dewald JP, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve 24: 273–283, 2001 [DOI] [PubMed] [Google Scholar]
- Dietz and Sinkjaer, 2007. Dietz V, Sinkjaer T. Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol 6: 725–733, 2007 [DOI] [PubMed] [Google Scholar]
- Dietz et al., 1991. Dietz V, Trippel M, Berger W. Reflex activity and muscle tone during elbow movements in patients with spastic paresis. Ann Neurol 30: 767–779, 1991 [DOI] [PubMed] [Google Scholar]
- Duncan et al., 1983. Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther 63: 1606–1610, 1983 [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer et al., 1975. Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med 7: 13–31, 1975 [PubMed] [Google Scholar]
- Gielen et al., 1988. Gielen CC, Ramaekers L, van Zuylen EJ. Long-latency stretch reflexes as co-ordinated functional responses in man. J Physiol 407: 275–292, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracies, 2005. Gracies JM. Pathophysiology of spastic paresis. II: Emergence of muscle overactivity. Muscle Nerve 31: 552–571, 2005 [DOI] [PubMed] [Google Scholar]
- Hyngstrom et al., 2007. Hyngstrom AS, Johnson MD, Miller JF, Heckman CJ. Intrinsic electrical properties of spinal motoneurons vary with joint angle. Nat Neurosci 10: 363–369, 2007 [DOI] [PubMed] [Google Scholar]
- Kimura et al., 2006. Kimura T, Haggard P, Gomi H. Transcranial magnetic stimulation over sensorimotor cortex disrupts anticipatory reflex gain modulation for skilled action. J Neurosci 26: 9272–9281, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollen et al., 2009. Kollen BJ, Lennon S, Lyons B, Wheatley-Smith L, Scheper M, Buurke JH, Halfens J, Geurts ACH, Kwakkel G. The effectiveness of the Bobath concept in stroke rehabilitation. What is the evidence? Stroke 40: e89–e97, 2009 [DOI] [PubMed] [Google Scholar]
- Krutky et al., 2010. Krutky MA, Ravichandran VJ, Trumbower RD, Perreault EJ. Interactions between limb and environmental mechanics influence stretch reflex sensitivity in the human arm. J Neurophysiol 103: 429–440, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacquaniti et al., 1991. Lacquaniti F, Borghese NA, Carrozzo M. Transient reversal of the stretch reflex in human arm muscles. J Neurophysiol 66: 939–954, 1991 [DOI] [PubMed] [Google Scholar]
- Lance, 1980. Lance J. Symposium Synopsis. Chicago, IL: Yearbook Medical, 1980 [Google Scholar]
- Lee et al., 1987. Lee WA, Boughton A, Rymer WZ. Absence of stretch reflex gain enhancement in voluntarily activated spastic muscle. Exp Neurol 98: 317–335, 1987 [DOI] [PubMed] [Google Scholar]
- Levene, 1960. Levene H. Robust tests for equality of variance. In: Contributions to Probability and Statistics: Essays in Honor of Howard Hotelling, edited by Olkin I. Palo Alto, CA: Stanford Univ. Press, 1960, vol. I, p. 278–292 [Google Scholar]
- Levin and Feldman, 1994. Levin MF, Feldman AG. The role of stretch reflex threshold regulation in normal and impaired motor control. Brain Res 657: 23–30, 1994 [DOI] [PubMed] [Google Scholar]
- Lewis et al., 2005. Lewis GN, Perreault EJ, Mackinnon CD. The influence of perturbation duration and velocity on the long-latency response to stretch in the biceps muscle. Exp Brain Res 163: 361–369, 2005 [DOI] [PubMed] [Google Scholar]
- Luke et al., 2004. Luke C, Dodd KJ, Brock K. Outcomes of the Bobath concept on upper limb recovery following stroke. Clin Rehabil 18: 888–898, 2004 [DOI] [PubMed] [Google Scholar]
- Marsden et al., 1972. Marsden CD, Merton PA, Morton HB. Servo action in human voluntary movement. Nature 238: 140–143, 1972 [DOI] [PubMed] [Google Scholar]
- Matthews, 1986. Matthews PB. Observations on the automatic compensation of reflex gain on varying the pre-existing level of motor discharge in man. J Physiol 374: 73–90, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi et al., 2007. Mitsuhashi K, Seki K, Akamatsu C, Handa Y. Modulation of excitability in the cerebral cortex projecting to upper extremity muscles by rotational positioning of the forearm. Tohoku J Exp Med 212: 221–228, 2007 [DOI] [PubMed] [Google Scholar]
- Musampa et al., 2007. Musampa NK, Mathieu PA, Levin MF. Relationship between stretch reflex thresholds and voluntary arm muscle activation in patients with spasticity. Exp Brain Res 181: 579–593, 2007 [DOI] [PubMed] [Google Scholar]
- Nielsen et al., 2005. Nielsen JB, Petersen NT, Crone C, Sinkjaer T. Stretch reflex regulation in healthy subjects and patients with spasticity. Neuromodulation 8: 49–57, 2005 [DOI] [PubMed] [Google Scholar]
- O'Dwyer et al., 1996. O'Dwyer NJ, Ada L, Neilson PD. Spasticity and muscle contracture following stroke. Brain 119: 1737–1749, 1996 [DOI] [PubMed] [Google Scholar]
- Perreault et al., 2008. Perreault EJ, Chen K, Trumbower RD, Lewis G. Interactions with compliant loads alter stretch reflex gains but not intermuscular coordination. J Neurophysiol 99: 2101–2113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny, 1996. Pierrot-Deseilligny E. Transmission of the cortical command for human voluntary movement through cervical propriospinal premotoneurons. Prog Neurobiol 48: 489–517, 1996 [DOI] [PubMed] [Google Scholar]
- Pisano et al., 2000. Pisano F, Miscio G, Del Conte C, Pianca D, Candeloro E, Colombo R. Quantitative measures of spasticity in post-stroke patients. Clin Neurophysiol 111: 1015–1022, 2000 [DOI] [PubMed] [Google Scholar]
- Powers et al., 1988. Powers RK, Marder-Meyer J, Rymer WZ. Quantitative relations between hypertonia and stretch reflex threshold in spastic hemiparesis. Ann Neurol 23: 115–124, 1988 [DOI] [PubMed] [Google Scholar]
- Rahman et al., 2001. Rahman T, Sample W, Seliktar R, Alexander M, Scavina M. An anti-gravity arm orthosis for people with muscular weakness. In: Integration of Assistive Technology in the Information Age, edited by Mokhtari M. Amsterdam: IOS Press, 2001, p. 31–36 [Google Scholar]
- Rohmert, 1960. Rohmert W. Ermittung von Erholungspausen fur statische Arbeit des Menschen. Int Z Angew Physiol einschl Arbeitsphysiol 18: 123–164, 1960 [PubMed] [Google Scholar]
- Sanchez et al., 2006. Sanchez RJ, Liu JY, Rao S, Shah P, Smith R, Rahman T, Cramer SC, Bobrow JE, Reinkensmeyer DJ. Automating arm movement training following severe stroke: functional exercises with quantitative feedback in a gravity-reduced environment. IEEE Trans Neural Syst Rehabil Eng 14: 378–389, 2006 [DOI] [PubMed] [Google Scholar]
- Sangani et al., 2007. Sangani SG, Starsky AJ, McGuire JR, Schmit BD. Multijoint reflexes of the stroke arm: neural coupling of the elbow and shoulder. Muscle Nerve 36: 694–703, 2007 [DOI] [PubMed] [Google Scholar]
- Sangani et al., 2009. Sangani SG, Starsky AJ, McGuire JR, Schmit BD. Multijoint reflex responses to constant-velocity volitional movements of the stroke elbow. J Neurophysiol 102: 1398–1410, 2009 [DOI] [PubMed] [Google Scholar]
- Schmit et al., 2000. Schmit BD, Dewald JP, Rymer WZ. Stretch reflex adaptation in elbow flexors during repeated passive movements in unilateral brain-injured patients. Arch Phys Med Rehabil 81: 269–278, 2000 [DOI] [PubMed] [Google Scholar]
- Schwerin et al., 2008. Schwerin S, Dewald JP, Haztl M, Jovanovich S, Nickeas M, MacKinnon C. Ipsilateral versus contralateral cortical motor projections to a shoulder adductor in chronic hemiparetic stroke: implications for the expression of arm synergies. Exp Brain Res 185: 509–519, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheean and McGuire, 2009. Sheean G, McGuire JR. Spastic hypertonia and movement disorders: pathophysiology, clinical presentation, and quantification. Phys Med Rehabil 1: 827–833, 2009 [DOI] [PubMed] [Google Scholar]
- Shemmell et al., 2009. Shemmell J, An JH, Perreault EJ. The differential role of motor cortex in stretch reflex modulation induced by changes in environmental mechanics and verbal instruction. J Neurosci 29: 13255–13263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons and Bingel, 1971. Simons DG, Bingel AG. Quantitative comparison of passive motion and tendon reflex responses in biceps and triceps brachii muscles in hemiplegic or hemiparetic man. Stroke 2: 58–66, 1971 [DOI] [PubMed] [Google Scholar]
- Sommerfeld et al., 2004. Sommerfeld DK, Eek EU, Svensson AK, Holmqvist LW, von Arbin MH. Spasticity after stroke: its occurrence and association with motor impairments and activity limitations. Stroke 35: 134–139, 2004 [DOI] [PubMed] [Google Scholar]
- Sprague et al., 1948. Sprague JM, Schreiner LH, Lindsley DB, Magoun HW. Reticulospinal influences on stretch reflexes. J Neurophysiol 11: 501–507, 1948 [DOI] [PubMed] [Google Scholar]
- Starsky et al., 2005. Starsky AJ, Sangani SG, McGuire JR, Logan B, Schmit BD. Reliability of biomechanical spasticity measurements at the elbow of people poststroke. Arch Phys Med Rehabil 86: 1648–1654, 2005 [DOI] [PubMed] [Google Scholar]
- Stein et al., 1995. Stein RB, Hunter IW, Lafontaine SR, Jones LA. Analysis of short-latency reflexes in human elbow flexor muscles. J Neurophysiol 73: 1900–1911, 1995 [DOI] [PubMed] [Google Scholar]
- Thilmann et al., 1990. Thilmann AF, Fellows SJ, Garms E. Pathological stretch reflexes on the “good” side of hemiparetic patients. J Neurol Neurosurg Psychiatry 53: 208–214, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilmann et al., 1991. Thilmann AF, Fellows SJ, Garms E. The mechanism of spastic muscle hypertonus: variation in reflex gain over the time course of spasticity. Brain 114: 233–244, 1991 [PubMed] [Google Scholar]
- Toothacker, 1993. Toothacker L. Multiple Comparisons Procedures. Quantitative Applications in the Social Sciences. Thousand Oaks, CA: Sage Publications, 1993 [Google Scholar]
- Voerman et al., 2005. Voerman GE, Gregoric M, Hermens HJ. Neurophysiological methods for the assessment of spasticity: the Hoffmann reflex, the tendon reflex, and the stretch reflex. Disabil Rehabil 27: 33–68, 2005 [DOI] [PubMed] [Google Scholar]
- Voss et al., 1985. Voss DE, Ionta MK, Myers BJ, Knott M. Proprioceptive Neuromuscular Facilitation: Patterns and Techniques. Philadelphia, PA: Harper & Row, 1985 [Google Scholar]
- Ward et al., 2003. Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain 126: 1430–1448, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch, 1951. Welch BL. On the comparison of several mean values: an alternative approach. Biometrika 38: 330–336, 1951 [Google Scholar]
- Wolf et al., 1996. Wolf SL, Segal RL, Catlin PA, Tschorn J, Raleigh T, Kontos H, Pate P. Determining consistency of elbow joint threshold angle in elbow flexor muscles with spastic hypertonia. Phys Ther 76: 586–600, 1996 [DOI] [PubMed] [Google Scholar]