Abstract
One potential expression of altered motoneuron excitability following a hemispheric stroke is the spontaneous unit firing (SUF) of motor units at rest. The elements contributing to this altered excitability could be spinal descending pathways, spinal interneuronal networks, afferent feedback, or intrinsic motoneuron properties. Our purpose was to examine the characteristics of spontaneous discharge in spastic–paretic and contralateral muscles of hemiparetic stroke survivors, to determine which of these mechanisms might contribute. To achieve this objective, we examined the statistics of spontaneous discharge of individual motor units and we conducted a coherence analyses on spontaneously firing motor unit pairs. The presence of significant coherence between units might indicate a common driving source of excitation to multiple motoneurons from descending pathways or regional interneurons, whereas a consistent lack of coherence might favor an intrinsic cellular mechanism of hyperexcitability. Spontaneous firing of motor units (i.e., ongoing discharge in the absence of an ongoing stimulus) was observed to a greater degree in spastic–paretic muscles (following 83.2 ± 16.7% of ramp contractions) than that in contralateral muscles (following just 14.1 ± 10.5% of ramp contractions; P < 0.001) and was not observed at all in healthy control muscle. The average firing rates of the spontaneously firing units were 8.4 ± 1.8 pulses/s (pps) in spastic–paretic muscle and 9.6 ± 2.2 pps in contralateral muscle (P < 0.001). In 37 instances (n = 63 pairs), we observed spontaneous discharge of two or more motor units simultaneously in spastic–paretic muscle. Seventy percent of the dually firing motor unit pairs exhibited significant coherence (P < 0.001) in the 0- to 4-Hz bandwidth (average peak coherence: 0.14 ± 0.13; range: 0.01–0.75) and 22% of pairs exhibited significant coherence (P < 0.001) in the 15- to 30-Hz bandwidth (average peak coherence: 0.07 ± 0.06; range: 0.01–0.31). We suggest that the spontaneous firing was likely not attributable solely to enhanced intrinsic motoneuron activation, but attributable, at least in part, to a low-level excitatory synaptic input to the resting spastic–paretic motoneuron pool, possibly from regional or supraspinal centers.
INTRODUCTION
Individuals who have sustained a stroke often exhibit spasticity and muscular weakness that limit daily function. Clinically, spasticity in stroke is characterized by increased stretch reflex responses that can be recorded with the muscles at rest (Lance 1980), suggesting enhanced excitability of the motoneuron pool (Chung et al. 2008). In addition, stroke survivors often exhibit an impaired ability to relax the muscle, especially following externally imposed movements such as extending the joint (Lewek et al. 2007). Furthermore, their muscles often exhibit prolonged (spontaneous) firing of motor units, either at rest or following voluntary or reflex muscle activation (Lewek et al. 2007; Lukacs 2005; Mottram et al. 2007, 2009b).
One possible explanation for this abnormal motoneuron firing is that the motoneurons themselves become intrinsically more excitable in stroke and this increase in excitability could appear as spontaneous unit firing. In fact, previous observations suggest that intrinsic motoneuron properties such as the persistent inward current (PIC) may contribute to spontaneous unitary firing (Gorassini et al. 2004). The PIC is a depolarizing current, generated by voltage-sensitive Na+ and/or Ca2+ channels (termed Na and Ca PICs) that persist for many seconds after activation and thus promote long-lasting discharge of motoneurons (Hounsgaard and Kiehn 1989; Lee and Heckman 1998b; Li and Bennett 2003; Schwindt and Crill 1982). In fact, it has been suggested that the observed long-lasting reflexes in the chronic spinal cat and the tonic stretch reflex in the decerebrate cat are both mediated by plateau potentials in motoneurons (Eken et al. 1989; Nielsen and Hultborn 1993) and that sustained motoneuron firing in the chronic spinalized rat may result from the uncontrolled activation of Na and Ca PICs in the motoneuron (Li and Bennett 2003).
It appears that PICs do play a major role in the manifestations of spasticity in patients with spinal cord injury (SCI). For example, following a muscle spasm, motor units in that muscle often exhibit sustained firing at very low firing rates with low firing rate variability (Gorassini et al. 2004; Zijdewind and Thomas 2001) and this sustained firing is often difficult to stop (Zijdewind and Thomas 2001). Based on these types of observations, it has been suggested that on average, roughly 40% of the excitation to motoneurons during this spasm activity arises from PICs (i.e., changes intrinsic to the motoneuron), given that the extrinsic synaptic drive required to keep a motor unit firing during a muscle spasm is significantly lower (by 40%) than the levels required to recruit the motor units initially (Gorassini et al. 2004).
The situation may well be different in stroke survivors. Here, there may be a tonic increase in net excitatory synaptic input to spastic–paretic motoneurons, rendering them more depolarized and thus resting closer to firing threshold (Katz and Rymer 1989). An increase in such excitatory synaptic input, which could arise from either descending or segmental pathways (Burke and Ashby 1972; Burke et al. 1972; Hultborn 2003; Katz and Rymer 1989; Mazzaro et al. 2007), is consistent with the observed lower threshold for stretch reflexes (Chardon et al. 2008; Powers et al. 1988) that occurs following a stroke.
Recently, a rigorous comparison of discharge patterns of motor units in biceps muscles of spastic–paretic stroke survivors and in healthy controls during voluntary ramp contractions revealed no significant differences in the estimated size of the PIC across spastic–paretic, contralateral, and healthy control muscles (Mottram et al. 2009a). However, these authors did note that following the voluntary ramp contractions in spastic–paretic muscles, many motor units continued to fire spontaneously, often simultaneously.
In this recent study performed in our laboratory, the majority of these simultaneously firing motor unit pairs (91% of instances of dually firing motor unit pairs) exhibited common modulation, as shown by high coefficient of determination (r2) values from the linear regression fit through their firing profiles, together with visible comodulation in the rate fluctuations across motor unit pairs (Mottram et al. 2009a).
Accordingly, we hypothesized that the spontaneous firing of motor units in stroke survivors arises, at least in part, from a mechanism different from that proposed for patients with SCI. Furthermore, given our earlier findings (Mottram et al. 2009a), we also questioned whether the difficulty stroke survivors have in relaxing their muscles may be due, in part, to the presence of a tonic, low-level ionotropic drive to the motoneuron pool, which keeps a certain proportion of motoneurons close to firing threshold and thus more readily activated at rest.
In light of these findings, the purpose of our current study was to examine the incidence and source of spontaneous unitary firing in spastic–paretic and contralateral muscles of stroke survivors and in the analogous muscles of age- and sex-matched healthy control subjects. This task was accomplished by examining characteristics of the firing profiles of motor units that fired spontaneously, either at rest or following a voluntary ramp contraction with the elbow flexor muscles.
We also conducted coherence analyses on spontaneously firing motor unit pairs to determine whether they could be receiving common synaptic inputs. The presence of coherence between units might indicate a common driving source of excitation to these motoneurons, whereas a consistent lack of coherence might favor an intrinsic cellular mechanism of hyperexcitability, such as exaggerated PICs. Last, we examined whether the spontaneously firing units were modulated by additional sensory inputs such as those that might arise during flexing or extending the elbow.
Our findings were that there was a much greater incidence of spontaneous firing of motor units in spastic–paretic muscles (following 83% of ramp voluntary contractions) than that in contralateral muscles (following 14% of ramp voluntary contractions). Additionally, when subjects were asked to increase their voluntary drive to the motoneuron pool by increasing force of the elbow flexor muscles, the firing rates of the spontaneous units increased in concert with increases in force. Last, the majority of the spontaneously firing motor unit pairs in spastic–paretic muscle (70%) exhibited significant coherence in the 0- to 4-Hz band, with visible comodulation across unit pairs.
These results suggest that in contrast to a primary PIC-based mechanism that has been proposed to explain the spontaneous unitary firing in SCI patients, the prolonged firing of motor units in stroke survivors while muscles are nominally at rest is likely to result, at least in part, from an enhanced ionotropic input or common drive to the motoneuron pool. Some of these data were previously presented in abstract form (Mottram et al. 2007, 2009b).
METHODS
Eight stroke survivors (58.1 ± 9.8 yr; range, 48–77 yr) with a unilateral brain lesion resulting in spastic hemiparesis of >6 mo duration and eight age- and sex-matched healthy subjects (60.3 ± 7.7 yr; range, 53–73 yr) participated in the study. Demographic and clinical measures for the stroke subjects are detailed in Table 1. Clinical assessments included spasticity measures at the elbow using the Modified Ashworth Scale (0–4) (Gregson et al. 2000) and magnitude of the biceps tendon jerk (0–4+) (Litvan et al. 1996).
Table 1.
Subject characteristics
| Subject ID | Stroke Type | Sex | Age, yr | Ashworth | Fugl-Meyer | Chedoke | Lesion Location | Involved Limb |
|---|---|---|---|---|---|---|---|---|
| 1 | Hemorrhagic | M | 48 | 1+ | 24/66 | Stage 3 | No information | R |
| 2 | Ischemic | M | 68 | 2 | 27/66 | Stage 3 | Left cerebral cortex | R |
| 3 | Hemorrhagic | F | 57 | 1+ | 30/66 | Stage 3 | Encephalomalacia in the lateral aspect of the right basal ganglia | L |
| 4 | Ischemic | M | 77 | 3 | 24/66 | Stage 3 | Left parietal lobe | R |
| 5 | Hemorrhagic | M | 50 | 1+ | 16/66 | Stage 2 | No information | R |
| 6 | Hemorrhagic | M | 58 | 3 | 15/66 | Stage 3 | Left middle cerebral artery | R |
| 7 | Ischemic | F | 52 | 1+ | 14/66 | Stage 3 | Left middle cerebral artery distribution infarction | R |
| 8 | Ischemic | M | 55 | 1+ | 33/66 | Stage 3 | Left carotid artery | R |
Upper extremity impairment was assessed using the Fugl-Meyer scale and the Chedoke–McMaster assessment. The lower boundary for spasticity was an Ashworth score of 1+ and a tendon jerk score of ≥3+. Subjects were excluded if they were unable to sit comfortably in the testing chair and report that they were relaxed during the testing session (n = 1) or if they were unable to perform ramp isometric contractions with the elbow flexor muscles (n = 2). All subjects were withdrawn from antispasticity medications for ≥2 wk prior to testing. This is because drugs such as baclofen have been shown to suppress generation of plateau potentials in motoneurons (Svirskis and Hounsgaard 1998) and to reduce the initial steep portion of firing rate modulation in human motoneurons (Hornby et al. 2004). All procedures were performed in accordance with the Declaration of Helsinki and approved by the Institutional Review Board at Northwestern University. Prior to participation in the study, all subjects gave written informed consent.
Experimental arrangement
Subjects were seated comfortably in a chair with their forearm, wrist, and fingers secured in a cast. The arm was abducted 30–40° from the sagittal plane and the elbow flexed to 90°. The casted forearm was fixed to a ring–mount interface attached to a 6 degrees-of-freedom load cell (FT-4227; ATI, Roseville, CA). The load cell apparatus was connected to a plastic elbow rest mounted on a steel table.
Forces about the elbow joint were recorded on-line using a Power 1401 A/D converter and Spike2 (Version 5.12) software (Cambridge Electronics Design [CED], Cambridge, UK) and the elbow force (resultant of Fx and Fz) was displayed on a computer monitor.
Motor unit action potentials in the biceps brachii were recorded with Teflon-coated double-stranded wires (bifilar 50-μm diameter; California Fine Wire Company, Grover Beach, CA). The double-stranded wire was inserted into a 27-gauge hypodermic needle and the tip was bent back to form a barb. The recording ends of the electrode were cut, so the pair served as a differential recording electrode. The electrodes were connected to preamplifiers located at the muscle that connected to an eight-channel amplifier system (Delsys, Boston, MA). Single motor unit recordings were amplified (×1,000–2,000), band-pass filtered (20–2,000 Hz), displayed on a computer monitor, and digitized for later analyses.
Surface electromyograms (EMGs) of the biceps brachii and triceps brachii were monitored simultaneously with the unitary recordings. This was for the purpose of monitoring background EMG during the spontaneous unitary firing and to record unitary firing in the surface EMG channels, when possible. Active surface differential EMG electrodes (Delsys) were placed on the biceps brachii short and long heads and on the triceps brachii, away from the innervation zone, to minimize signal cancellation (Merletti et al. 2001). All surface EMG signals were led to the same preamplifiers as the intramuscular EMG recordings (Delsys), were amplified (×1,000–2,000), band-pass filtered (20–450 Hz), displayed on a computer monitor, and digitized for later analyses.
Experimental procedures
Each stroke survivor participated in one to three sessions for the contralateral muscle and one to five sessions for the spastic–paretic muscle, with testing sessions separated by >1 wk. The order of testing was randomized. Control subjects participated in one to two sessions for the matched limb to ensure adequate trials for comparison with their spastic–paretic counterpart. On average, subjects performed 49 ± 26 isometric ramp contractions per session for the spastic–paretic muscle, 60 ± 31 isometric ramp contractions per session for the contralateral muscle, and 40 ± 12 isometric ramp contractions per session for the control muscle.
After being seated comfortably in the chair, the subject was instructed to relax. Subjects were encouraged to close their eyes, allow their head to rest on the headrest, and to report to the experimenter if they were unable to relax and rest comfortably. Surface EMG and motor unit signals were then recorded for ≤5 min with the subject at rest. Single motor unit potentials were monitored on-line and on a digital oscilloscope during data collection. Up to three fine wire electrodes were inserted in widely separated locations of the biceps brachii muscle. Motor units that were firing for >10s while the subject was at rest (i.e., not produced by voluntary effort) were considered spontaneously active (Zijdewind and Thomas 2001). If no unitary discharge was noted while the subject was at rest, the subject was asked to perform an isometric voluntary ramp contraction with the elbow flexor muscles to a force level that allowed recruitment of up to two motor units. Subjects viewed their elbow flexion force on the computer monitor in front of them and were instructed to make the rate of increase in force similar to the rate of relaxation. In addition, some subjects performed a small flexion (sometimes with a mild cocontraction of triceps brachii) movement, followed by return to rest position. On completion of the isometric voluntary ramp contraction or the small flexion movement or force, subjects were instructed to relax and rest quietly. Motor units that continued to fire for >10 s following completion of the voluntary ramp contractions or the small flexion forces were considered “spontaneously” active (i.e., there was an ongoing discharge in the absence of an ongoing stimulus). From here forward, italics will be used to differentiate those units that continued to discharge following removal of a known stimulus from those that fired without a known input. On occasion, additional motor units were recruited at termination of the voluntary ramp contractions, while the subject was supposedly at rest. If these units continued to fire for >10 s, these units were also considered “spontaneously” active.
To evaluate whether the firing behavior (firing rate or variability in firing rate) of these spontaneously active motor units could be influenced by additional inputs, subjects were instructed to generate mild flexion or extension forces of the forearm. All trials were separated by ≥30 s to avoid frequency-dependent facilitation of the motor units or a reduction in the level of estimated synaptic input required to recruit a unit (Gorassini et al. 2002). Sessions lasted 2–3 h depending on the number of motor units that were isolated and considered spontaneously active.
Data analysis
Force, intramuscular EMG, and surface EMG were recorded on-line and subsequently digitized (A/D converter, 16-bit resolution) and analyzed off-line using the Spike2 (version 5.12) data-analysis system (CED). The surface and intramuscular EMG signals were digitized at 2,013 and 18,000 Hz, respectively. The elbow force signals (resultant of Fx and Fz) were digitized at 200 Hz.
Action potentials discharged by single motor units in biceps brachii were discriminated using a computerized, spike-sorting algorithm (Spike2, version 5.12; CED). To ensure discrimination accuracy, the interspike intervals (ISIs) and waveforms of identified motor units were visually examined for every trial.
The incidence (% of trials) of “spontaneous” unitary discharge that occurred following the formal triangular isometric voluntary ramp contractions was determined for each limb type. (The small flexion forces were not included in this analysis.) The duration of spontaneous unitary firing for all units recorded (i.e., those without prior input and those following the formal ramp contractions or small flexion forces) was determined. In addition, for each trial of spontaneous unitary discharge, the mean firing rate was determined from the ISIs using custom-designed software written in Matlab 7.1 (The MathWorks, Natick, MA). These ISIs were detrended prior to determining the SD and coefficient of variation (CV) for the firing rate [(SD of firing rate/mean firing rate) × 100] and then converted to instantaneous frequencies. For each unit that fired tonically, an ISI histogram was plotted and a histogram skew calculated using Matlab 7.1 (The MathWorks). The percentage of instances in which spontaneously firing units were modulated by imposing voluntary flexion or extension of the elbow was also determined.
Correlations in the frequency domain were estimated from the coherence spectrum calculated between the discharge times of two firing motor units that discharged spontaneously in parallel. The method used was similar to that developed by Rosenberg et al. (1989) and this was implemented using Matlab 7.1 (The MathWorks). The discriminated motor unit data were divided into contiguous, nonoverlapping epochs of 2.5 s that comprised 512 bins. Each 5-ms bin was assigned a value of 1 when it contained a discharge and 0 when it did not. The time-series data from each disjoint section were transformed to the frequency domain, resulting in a frequency resolution of 0.39 Hz (sampling frequency = 200 samples/s and window size = 512). Auto- and cross-spectra were estimated by averaging over the disjoint sections and coherence estimates for the two concurrently recorded motor unit signals were computed. The sample coherence indicates the degree of linear correlation in the frequency domain between two signals on a scale of zero to one. Significant peaks were identified as values that exceeded the 95% confidence intervals (CIs) relative to the zero value (Moritz et al. 2005; Rosenberg et al. 1989).
Based on earlier published studies that examined common oscillations for a pair of motor units, two frequency bands in the coherence spectrum were analyzed: 0–4 Hz (De Luca and Erim 1994; Myers et al. 2004) and 15–30 Hz (Farmer et al. 1993a,b). The highest coherence (peak value) was calculated for both frequency bands. Coherence estimates were obtained for 37 instances of simultaneously firing motor units that discharged spontaneously. In many of these 37 instances, more than two motor units discharged spontaneously in parallel. Thus the coherence estimates for all potential combinations of two such motor units (n = 63) firing in parallel were determined. The percentage of pairs of simultaneously firing motor units that exhibited significant coherence was also determined.
In addition to the coherence analyses, a cross-correlation histogram was constructed for each pair of spontaneously firing motor units to see whether there was a narrow central peak. A narrow central peak in the cross-correlation histogram could be derived from branched common input from corticospinal neurons (Farmer et al. 1993b; Semmler et al. 2004). For each spontaneously firing motor unit pair, a cross-correlation histogram with a bin width of 1 ms was constructed by comparing each discharge time of the reference motor unit with all discharge times of the other unit within ±100 ms (Christou et al. 2007; Nordstrom et al. 1992; Semmler et al. 2004).
Statistical analysis
Because no spontaneous unitary activity was observed in the healthy control subjects, no statistical analyses were conducted for this muscle group. Instead, paired Student's t-tests (SPSS version 15.0) were used to compare the incidence of spontaneous unitary firing between spastic–paretic and contralateral limbs. Because one stroke survivor did not exhibit spontaneous unitary firing in the contralateral muscle, unpaired Student's t-tests (SPSS version 15.0) were used to compare the dependent variables across the spastic–paretic and contralateral muscles. Dependent variables included the duration of spontaneous unitary firing, the mean firing rate, the SD, and CV for the firing rate, the histogram skew, and the percentage of instances that spontaneously firing units were modulated. Spontaneously firing motor units that discharged in parallel were observed only in the spastic–paretic muscle, so the percentage of pairs that exhibited significant coherence could not be compared across muscle types. Instead, within the spastic–paretic muscle, the coherence was considered significant above the 95% CI from zero (Farmer et al. 1997; Rosenberg et al. 1989). Data are reported as means ± SD within the text and displayed as means ± SE in the figures.
RESULTS
To assess the origins of spontaneous firing in spastic–paretic muscles of stroke survivors, we monitored the firing characteristics of such motor units in the biceps brachii muscle in both spastic–paretic and contralateral limbs of stroke survivors and in matched muscles of age- or sex-matched control subjects. Specifically, we monitored motor unit spontaneous firing in the biceps brachii muscle following voluntary isometric ramp contractions or following small flexion forces with the elbow flexor muscles in all subjects. We also monitored spontaneous unitary firing that was not preceded by any known input (i.e., the subjects were instructed to relax), using both surface EMG and intramuscular recordings.
Following voluntary isometric ramp contractions with elbow flexor muscles in spastic–paretic stroke survivors, we observed a much greater incidence of “spontaneous” unitary firing in the spastic–paretic (following 83.2 ± 16.7% of ramp contractions) than that in the contralateral (following 14.1 ± 10.5% of ramp contractions) muscle of stroke survivors (P < 0.001; Table 2). Figure 1 shows an example of “spontaneous” unitary firing in the spastic–paretic biceps brachii muscle of a stroke survivor (Fig. 1, top) that continued for about 20 s following completion of the formal voluntary ramp contraction with the elbow flexor muscles (Fig. 1, bottom). This discharge continued despite verbal cueing from the investigator to relax the muscle and the subject's report that he was relaxed. We did not observe any spontaneous unitary firing either at rest or following voluntary contractions in the matched control muscle.
Table 2.
Firing rate characteristics from averaged values for each of the eight spastic–paretic stroke survivors during spontaneous unit firing
| Factor | Spastic–Paretic | Contralateral |
|---|---|---|
| Percentage postramp sustained firing incidence, % | 83.2 ± 16.7 | 14.1 ± 10.5 (<0.001) |
| Number of motor units analyzed | 171 | 34 |
| Firing duration, s | 70.7 ± 50.2 | 42.9 ± 36.9 (0.001) |
| Minimum | 13.7 | 10.5 |
| Maximum | 269 | 205.8 |
| Firing rate, pps | 8.4 ± 1.8 | 9.6 ± 2.2 (<0.001) |
| Minimum | 4.8 | 6.9 |
| Maximum | 17.5 | 17.8 |
| SD of firing rate, pps | 0.017 | 0.018 (0.04) |
| Minimum | 0.005 | 0.004 |
| Maximum | 0.029 | 0.032 |
| CV in firing rate, % | 13.4 ± 4.0 | 16.9 ± 4.3 (<0.001) |
| Minimum | 6.4 | 7.3 |
| Maximum | 26.9 | 25.1 |
| Peak coherence 0- to 4-Hz bandwidth of simultaneously firing unit pairs (37 instances, 63 pairs) | 0.14 ± 0.13 (<0.001) | NA |
| Minimum | 0.01 | NA |
| Maximum | 0.75 | NA |
| Peak coherence 15- to 30-Hz bandwidth of simultaneously firing unit pairs (37 instances, 63 pairs) | 0.07 ± 0.06 (<0.001) | NA |
| Minimum | 0.01 | NA |
| Maximum | 0.31 | NA |
Values are means ± SD. P values compared with spastic–paretic limb are in parentheses; peak coherence P values are expressed in parentheses and are determined by testing against the hypothesis of zero coherence. Spontaneous unit firing was not observed in the control limb. NA, not applicable.
Fig. 1.
“Spontaneous” unitary firing in the spastic–paretic biceps brachii muscle of a stroke survivor (top) continued for about 20 s following completion of the formal voluntary ramp contraction with the elbow flexor muscles (bottom). This discharge continued despite verbal cueing from the investigator to relax the muscle and the subject's report that he was relaxed.
In total, 171 motor units that fired spontaneously were analyzed for the spastic–paretic muscles and 34 spontaneously firing motor units were analyzed for the contralateral muscles (Table 2). This selection was equivalent to analyzing 51% of motor units and 47% of motor units that exhibited spontaneous unitary firing following the voluntary ramp contractions for the spastic–paretic and contralateral muscles, respectively. The remaining 49% of spontaneously firing units in spastic–paretic muscles and 53% of spontaneously firing units in contralateral muscles were not analyzed, due to noise in the signal, or additional units that were recruited alongside the primary unit, making accurate discrimination of the primary unit difficult.
Of the 171 motor units analyzed for the spastic–paretic muscles, 12.3 ± 0.2% of the units fired spontaneously without prior input (i.e., there was no intramuscular or surface EMG activity recorded in the elbow flexor musculature at the start of the recording session and this was followed by an onset of EMG activity while the subject was overtly at rest). We found that 63.4 ± 0.3% of the units continued to fire or began firing “spontaneously” following the ramp contractions; 14.2 ± 0.1% of the units fired “spontaneously” following the generation of small voluntary flexion forces with the elbow flexor muscles, whereas 10.2 ± 1.0% fired “spontaneously” for reasons unknown to the experimenters. (Here, intramuscular or surface EMG activity was recorded immediately at the start of the recording session, despite subject's report of being at rest.)
Of the 34 motor units analyzed from contralateral muscles, 17.1 ± 0.2% of the units fired spontaneously without prior input; 76.6 ± 0.2% of the units continued to fire or fired “spontaneously” following the formal ramp contractions; 0 ± 0% of the units fired “spontaneously” following small flexion forces with the elbow flexor muscles; and 6.4 ± 0.1% fired “spontaneously” for reasons unknown to the experimenters.
The duration of firing for all spontaneously firing units (n = 171 spastic–paretic muscle; n = 34 contralateral muscle) was longer for the spastic–paretic muscle (70.7 ± 50.2 s) than that for the contralateral muscle (42.9 ± 36.9 s; P = 0.001; Table 2). The average firing rates of the spontaneously firing units were lower in the spastic–paretic muscle (8.4 ± 1.8 pulses/s [pps]) than those in the contralateral muscle (9.6 ± 2.2 pps; Table 2; P < 0.001), as was the average variability in firing rate (CV) for the spastic–paretic muscle (13.4 ± 4.0%) than that for the contralateral muscle (16.9 ± 4.3%; Table 2; P < 0.001). Despite the lower firing rates and variability in firing rates for the spastic–paretic muscle, the histogram skew of the motor unit firing patterns during the spontaneous firing did not differ across spastic–paretic (1.3 ± 0.35) and contralateral muscles (1.4 ± 0.44; P = 0.11). This similar histogram skew is also portrayed as a fairly similar range of variability in firing rates across the spastic–paretic muscle (CV range: 6.4–26.9%) and the contralateral muscle of stroke survivors (CV range: 7.3–25.1%).
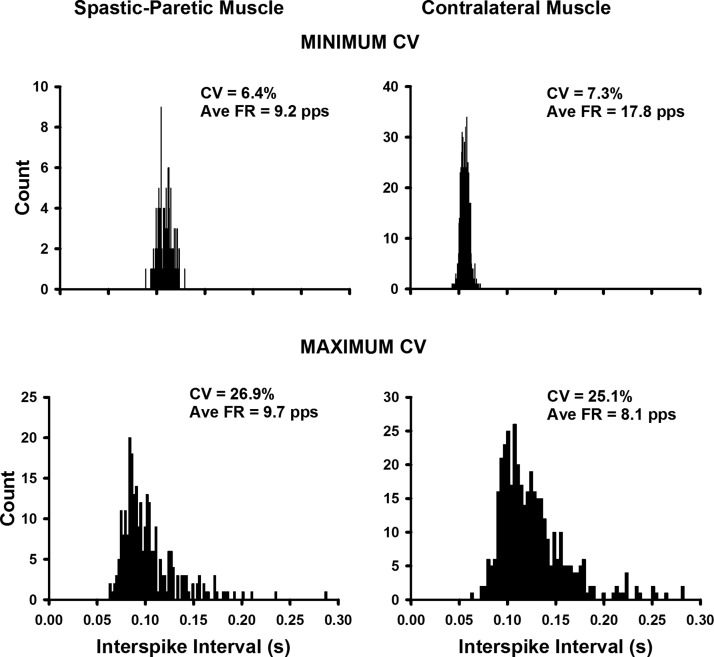
Figure 2 portrays representative ISI histograms, each derived from one motor unit, that show the low (top left) and high (bottom left) range for the variability in firing rate (CV) observed in the spastic–paretic muscle. The top right and bottom right panels denote the low and high ranges, respectively, that were observed in the contralateral muscle of two stroke survivors. This large range of variability in firing rates for the spastic–paretic and contralateral muscles also held true for within-muscle comparisons, suggesting that mechanisms other than PICs were contributing to the spontaneous unitary firing.
Fig. 2.
The range of variability in firing rates was similar for spastic–paretic (left) and contralateral (right) muscles. Representative interspike interval histograms show the low (top left) and high (bottom left) range for the variability in firing rate {CV, expressed as: [(SD of the firing rate/mean firing rate) × 100]} observed in the spastic–paretic muscle. The top right and bottom right panels denote the low and high ranges respectively that were observed in the contralateral muscle of 2 stroke survivors. The respective average firing rate values are also shown for each representative histogram. Note the similar shapes of the histograms for spastic–paretic and contralateral muscles for both the minimum and maximum CV values. CV, coefficient of variation in firing rate; Ave FR, average firing rate.
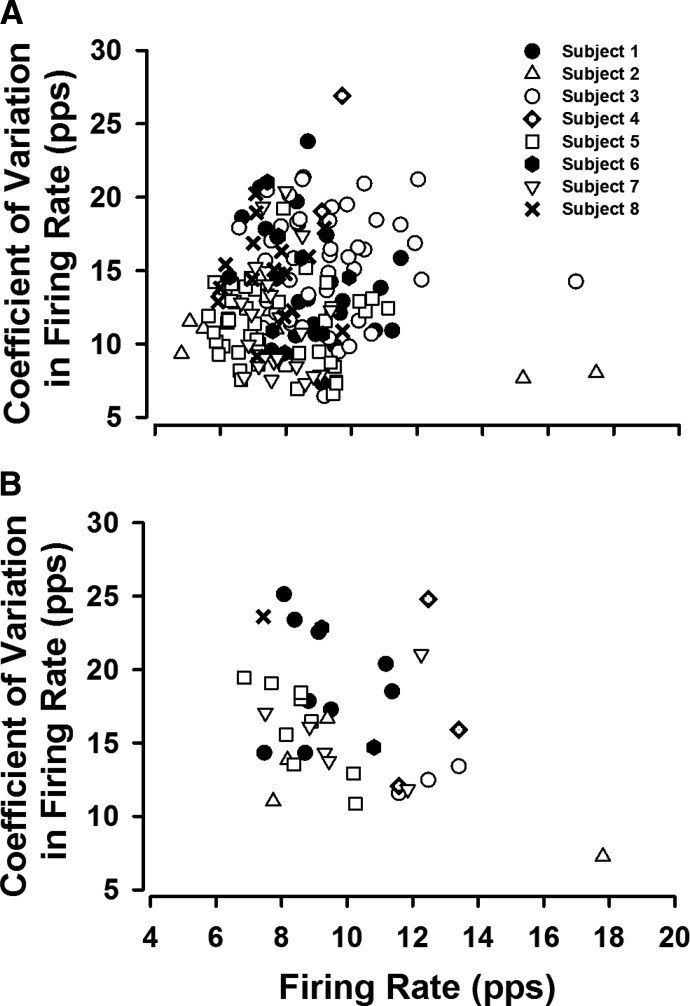
Figure 3 portrays the CV in firing rate (%) as a function of the firing rate (pps) for the spastic–paretic muscle (Fig. 3A) and contralateral muscle (Fig. 3B) for each of the eight subjects. Each symbol represents the average value from one spontaneously firing motor unit that fired without a known input or following a voluntary ramp contraction or small flexion force performed with the elbow flexor muscles.
Fig. 3.
The range in firing rates and variability in firing rates (CV) for the spontaneously firing units were large for the spastic–paretic and contralateral muscles, suggesting that the source of spontaneous unit firing was not solely due to persistent inward currents (PICs). The range of variability in firing rate (CV) in the spastic–paretic muscle for each of the 8 subjects is shown in A, with the corresponding firing rate values. The same relation is shown in B for the contralateral muscle. Each symbol represents the average value of one spontaneously firing motor unit that fired without a known input or following a voluntary ramp contraction or small flexion force performed with the elbow flexor muscles. Each of the 8 subjects is represented by a different symbol. The firing rates ranged from 4.8 to 17.5 pulses/s (pps) for spastic–paretic muscle, whereas the CV in firing rates ranged from 6.4 to 26.9%. These values were fairly similar to values for contralateral muscle. Firing rate range: 6.9–17.9 pps; variability in firing rate range: 7.3–25.1%.
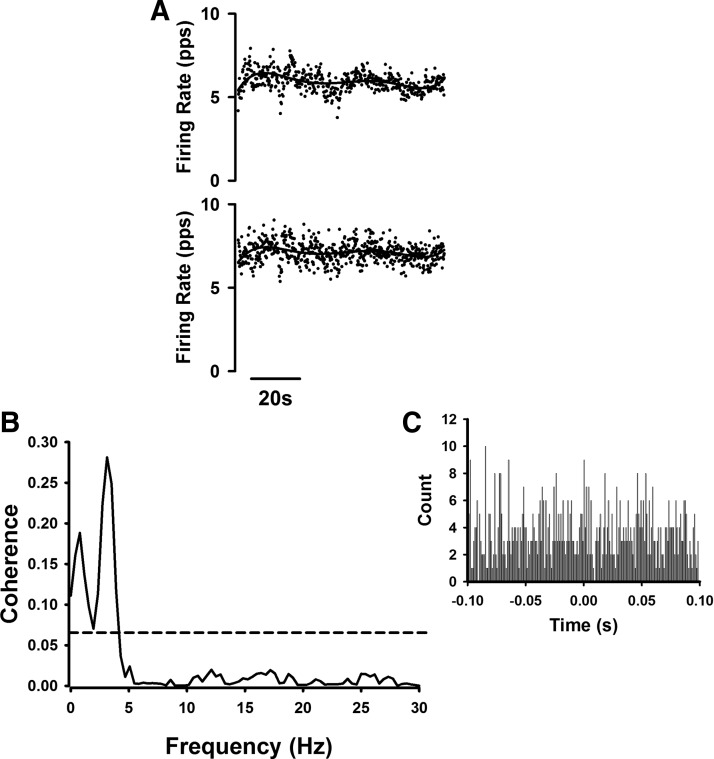
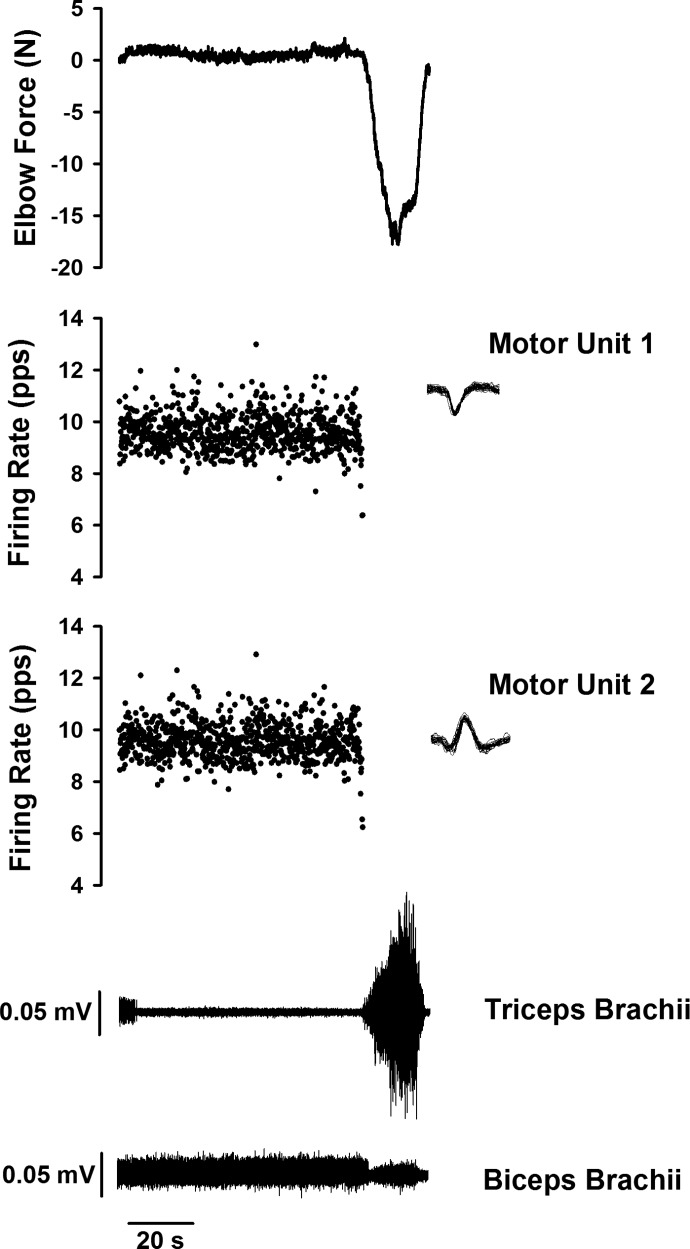
In 37 trials from the spastic–paretic muscle, we observed unitary discharge of two or more motor units that discharged simultaneously without a known input or following the ramp contractions. There was often observable comodulation of these dually firing unit pairs. An example of this comodulation is portrayed in Fig. 4A (top), which shows the force of the elbow flexor muscles during and following a voluntary ramp contraction performed with the elbow flexor muscles. Note that a motor unit that was recruited during the voluntary ramp contraction continued to fire “spontaneously” following the contraction (Fig. 4A, middle) and a second motor unit was recruited following the ramp contraction (Fig. 4A, bottom), despite verbal cueing to relax the muscle.
Fig. 4.
A motor unit from the biceps brachii muscle of a spastic–paretic stroke survivor (middle, A) was recruited during a voluntary ramp contraction performed with the elbow flexor muscles (top, A). The unit continued to fire “spontaneously” following the contraction (middle, A) and a second motor unit was recruited following the ramp contraction (bottom, A), despite verbal cueing from the investigator to relax the muscle. The 2 units fired simultaneously for about 172 s. Overlaid waveforms for motor unit 1 and motor unit 2 are shown above their respective firing profiles. B: coherence in the frequency domain of the same pair of “spontaneously” firing motor units following the voluntary ramp contraction. The dashed line shows the 95% confidence interval of a lack of correlation across unit pairs; any coherence values above this line are considered significant coherence from zero. Note the significant peak coherence in the 0- to 4-Hz bandwidth and the lack of significant coherence in the 15- to 30-Hz bandwidth. C: the cross-correlation histogram for the same pair of “spontaneously” firing motor units. Note the lack of a central peak in the cross-correlation histogram.
Correlations in the frequency domain were estimated from the coherence spectrum derived from the motor unit pairs that discharged spontaneously. Coherence analyses revealed that 70.0% of the dually spontaneously firing motor unit pairs exhibited significant coherence (P < 0.001) in the 0- to 4-Hz bandwidth (average peak coherence: 0.14 ± 0.13; range: 0.01–0.75; Table 2) and 22% of the dually spontaneously firing motor unit pairs exhibited significant coherence (P < 0.001) in the 15- to 30-Hz bandwidth (average peak coherence: 0.07 ± 0.06; range: 0.01–0.31; Table 2). Correlations in the time domain were estimated from the cross-correlation histogram derived from each of the motor unit pairs that discharged spontaneously. These analyses revealed no central peaks in any of the histograms.
Figure 4B shows the coherence in the frequency domain of the same pair of “spontaneously” firing motor units following the voluntary ramp contraction. Values above the dashed line indicate significant coherence from zero. Note the significant peak coherence in the 0- to 4-Hz bandwidth and the lack of significant coherence in the 15- to 30-Hz bandwidth. Figure 4C shows the cross-correlation histogram for the same pair of “spontaneously” firing motor units. Note the lack of a central peak in the cross-correlation histogram.
Figure 5A portrays the firing profiles of two motor units that fired for about 80 s following a voluntary ramp contraction performed with elbow flexor muscles of a spastic–paretic stroke survivor. Note that the lines fit through the firing profiles reveal the slow common modulation between the two units. Figure 5B shows the coherence in the frequency domain of the same pair of “spontaneously” firing motor units following the voluntary ramp contraction. As in Fig. 4B, values above the dashed line indicate significant coherence from zero. Note the significant peak coherence in the 0- to 4-Hz bandwidth (0.28) and the lack of significant coherence in the 15- to 30-Hz bandwidth. The inset above and to the right of the coherence plot in Fig. 5C shows the cross-correlation histogram for the same pair of “spontaneously” firing motor units. Note the lack of a central peak in the cross-correlation histogram.
Fig. 5.
A “spontaneously” firing motor unit pair showed strong coherence at the 0- to 4-Hz bandwidth, yet no coherence at the 15- to 30-Hz bandwidth and no central peak in its cross-correlation histogram. The instantaneous firing frequency profiles of 2 motor units, which fired for about 80 s following a voluntary ramp contraction performed with the elbow flexor muscles of a spastic–paretic stroke survivor, are shown in A. Note that the lines fit through the firing profiles reveal the slow common modulation between the 2 units. B: coherence in the frequency domain of the same pair of “spontaneously” firing motor units following the voluntary ramp contraction. The dashed line shows the 95% confidence interval of a lack of correlation across unit pairs; any coherence values above this line are considered significant coherence from zero. Note the significant peak coherence in the 0- to 4-Hz bandwidth (0.28) and the lack of significant coherence in the 15- to 30-Hz bandwidth. The inset above and to the right of the coherence plot (C) shows the cross-correlation histogram for the same pair of “spontaneously” firing motor units. Note the lack of a central peak in the cross-correlation histogram.
Two techniques were introduced to determine whether the spontaneously firing units could be modulated (i.e., influenced by additional synaptic inputs). One technique was to use voluntary triceps brachii activation to assess its effect on spontaneous firing of motor units; the second technique was to assess responses during voluntary activation of the biceps brachii and other elbow flexor muscles.
In all instances of triceps brachii activation in the spastic–paretic muscle (n = 40 instances; seven subjects), the spontaneously firing units ceased firing. Additionally, none of the units silenced by triceps brachii activation returned to “spontaneous” firing, despite continued recordings for ≤5 min. An example of such modulation via triceps activation is shown in Fig. 6, where the top panel shows the force of the elbow flexor muscles while a spastic–paretic stroke survivor was at rest and during activation of the triceps brachii muscle. The two middle panels show two motor units firing spontaneously in parallel and the two bottom panels show the surface EMG of the triceps brachii and biceps brachii muscles, respectively. The two units began firing without a known prior input. After about 80 s of firing, the subject was asked to activate the triceps brachii muscle, which caused both motor units to cease firing.
Fig. 6.
Activation of the triceps brachii muscle by a spastic–paretic stroke survivor caused 2 spontaneously firing motor units to cease firing. The top panel shows the force of the elbow flexor muscles while a spastic–paretic stroke survivor was at rest and during activation of the triceps brachii muscle. The 2 middle panels show the instantaneous firing frequency of 2 motor units firing spontaneously in parallel, with their corresponding overlaid waveforms to the top right of the respective firing profiles. The 2 bottom panels show the surface electromyograms (EMGs) of the triceps brachii and biceps brachii muscles, respectively. The 2 units began firing spontaneously without a known prior input. After about 80 s of firing, the subject was asked to activate the triceps brachii muscle, which caused both motor units to cease firing.
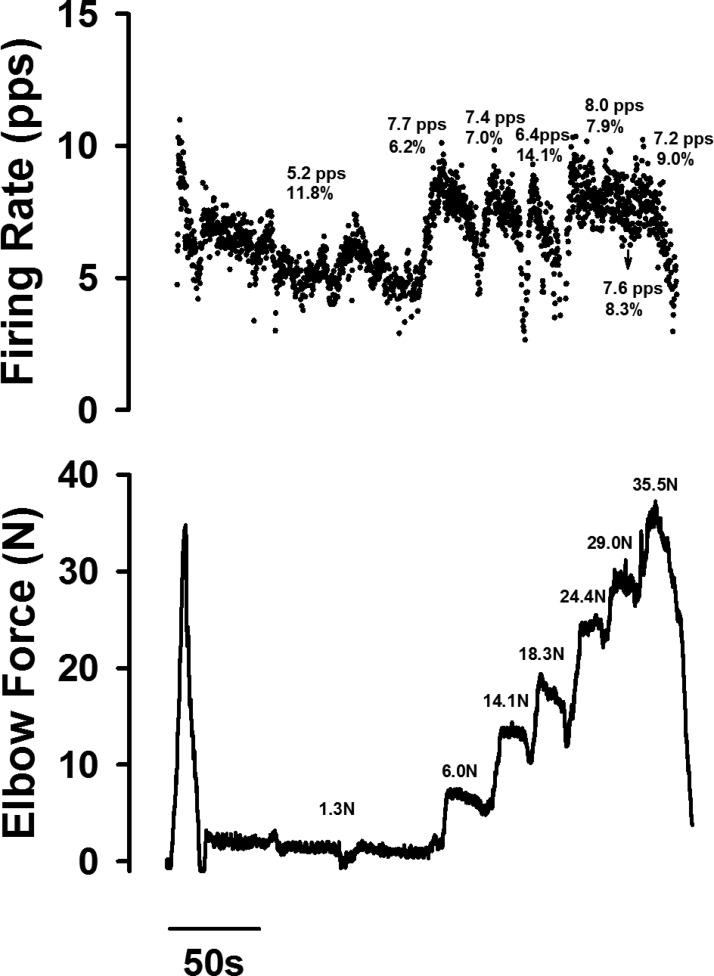
The spontaneously firing motor units were also modulated by voluntary activation of the elbow flexor muscles (i.e., the second technique). In eight examples, drawn from four spastic–paretic stroke survivors, the same spontaneously firing motor unit was monitored during increases in force with the elbow flexor muscles, as shown in Fig. 7. Figure 7 portrays the instantaneous firing frequency of a single motor unit (Fig. 7, top) during and following a voluntary ramp contraction performed with the involved elbow flexor muscles of a spastic–paretic stroke survivor (Fig. 7, bottom). The motor unit continued to fire for about 130 s following the voluntary contraction. Following this period of “spontaneous” unitary firing, the subject was asked to slowly increase force of the elbow flexor muscles. The motor unit firing rate increased from 5.2 to 7.2 pps (Fig. 7, top) as the voluntary force exerted with the elbow flexor muscles increased from 1.3 to 35.5 N (Fig. 7, bottom). In all eight instances that this activity was monitored, the firing rates and variability in firing rates were modulated with increases in force.
Fig. 7.
The instantaneous firing frequency of a single motor unit of a spastic–paretic stroke survivor increased with increases in force of the elbow flexor muscles. The instantaneous firing frequency of a single motor unit (top) is shown during and following a voluntary ramp contraction performed with the involved elbow flexor muscles of a spastic–paretic stroke survivor (bottom). The motor unit continued to fire for about 130 s following the voluntary contraction. Following this period of “spontaneous” unitary firing, the subject was asked to slowly increase force of the elbow flexor muscles. The motor unit firing rate increased from 5.2 to 7.2 pps (top) as the voluntary force exerted with the elbow flexor muscles increased from 1.3 to 35.5 N (bottom). In the bottom panel, the corresponding force values are shown in Newtons. In the top panel, the corresponding firing rate values (pps) and CV in firing rate values (%) are also shown.
DISCUSSION
The primary objective of this study was to examine the incidence and source of spontaneous firing of motor units in the spastic–paretic and contralateral biceps muscles of stroke survivors and in matched muscle of healthy control subjects. This task was accomplished by examining the firing profiles of motor units that fired spontaneously, either at rest or following a voluntary isometric ramp contraction with the elbow flexor muscles. We hypothesized that the observed spontaneous firing of motor units in stroke survivors is generated by a mechanism different from that of patients following a spinal cord injury.
In an attempt to examine the origins of spontaneous unitary firing, we conducted coherence analyses on spontaneously firing motor unit pairs to determine whether they were receiving common synaptic inputs. The presence of measurable coherence between units might indicate a common driving source of excitation to multiple motoneurons, whereas a consistent lack of coherence might favor an intrinsic cellular mechanism of hyperexcitability. A cross-correlation histogram was also constructed for each pair of spontaneously firing motor units to see whether there was a narrow central peak, which might favor the contributions of a branched common input from corticospinal neurons (Farmer et al. 1993b; Semmler et al. 2004). Last, we examined whether the spontaneously firing units were modulated by additional inputs such as activating elbow flexors or extensors.
In support of our hypothesis, we did indeed observe motor unit firing behaviors that were consistent with increased depolarizing synaptic inputs to the motoneuron pool. First, motor units from the biceps brachii muscle of stroke survivors continued to fire “spontaneously” following completion of voluntary isometric ramp contractions. This “spontaneous” firing was more common and lasted longer in spastic–paretic muscle than that in contralateral muscle. Second, the majority of the simultaneously firing motor unit pairs in spastic–paretic muscle exhibited significant coherence in the 0- to 4-Hz bandwidth, with a smaller portion of units exhibiting coherence in the 15- to 30-Hz bandwidth. Third, units that were firing spontaneously were also influenced by descending drive. This suggests that the units were not driven solely by an intrinsic cellular mechanism, but were influenced by extrinsic synaptic inputs such as a common low-level depolarizing synaptic input to the motoneuron pool as the most parsimonious explanation. Other explanations are certainly feasible, however, and are discussed in the following text.
Spontaneous firing of motor units in stroke survivors
POTENTIAL SOURCES.
1. Common drive. In 37 trials in spastic–paretic muscle, we observed unitary discharge of two or more motor units that discharged spontaneously without a known input or following the ramp contractions, often with observable comodulation of these unit pairs. Coherence analyses revealed significant coherence in the 0- to 4-Hz bandwidth (average peak coherence: 0.14 ± 0.13), similar to previously observed coherence values of 0.153 ± 0.12 during weak isometric contractions performed by stroke survivors (Farmer et al. 1993a). The more common low-frequency peak at 0–4 Hz likely represents processes involved in nonvoluntary brain stem drive, such as breathing (Pettersen and Westgaard 2005; Pettersen et al. 2005; Westgaard et al. 2006), and thus is unlikely to be related to the inability to terminate a contraction. In contrast to the strong coherence at 0–4 Hz, only a small portion of the unit pairs (22%) exhibited significant coherence in the 15- to 30-Hz bandwidth.
In addition, cross-correlation analyses, a measure of motor-unit synchronization, revealed no obvious central peaks, suggesting that short-term synchronization was not a major source of the common input provided to the motoneurons.
The 1- to 12- and 16- to 32-Hz coherence spectra may reflect periodicities in the firing of branched monosynaptic inputs to motoneurons or, alternatively, they may reflect activity in presynaptic inputs that are themselves periodically correlated (Farmer et al. 1993a; Sears and Stagg 1976). Furthermore, significant values of coherence in the range of 1–12 Hz may be generated by activity in inputs separate from those generating 16- to 32-Hz coherence (Farmer et al. 1993a): low-frequency common drive (0–4 Hz) persists after cortical or capsular stroke (Farmer et al. 1993b), suggesting a noncorticospinal origin, whereas activity in the 15- to 30-Hz band appears to be driven by the motor cortex (Conway et al. 1995).
Our observations of strong coherence in the 0- to 4-Hz bandwidth in the majority of spontaneously firing motor unit pairs, with lower coherence in a smaller portion of the units in the 15- to 30-Hz bandwidth and no obvious central peaks in the cross-correlation histograms, suggest that the source of spontaneous motor unit firing in the majority of stroke survivors is not attributable to short-term motor unit synchronization or branched common input to motoneurons, yet rather more likely to separate activity in presynaptic inputs that are themselves periodically correlated.
2. Persistent inward current (PIC). Persistent inward currents may be activated below action potential threshold and may thereby enhance motoneuron recruitment for a given synaptic input (Li et al. 2004). Following this, PICs may promote sustained discharge of the motoneuron (Bennett et al. 1998; Hounsgaard and Kiehn 1989; Lee and Heckman 1998b). As such, PICs could feasibly contribute to the spontaneous discharge of motoneurons observed in the spastic–paretic and contralateral muscles of stroke survivors who participated in this study.
The paired motor unit analysis technique conducted by Gorassini and colleagues (see Gorassini et al. 2002, 2004 for methods of this technique) indeed provides evidence that a motoneuron PIC is involved during prolonged muscle spasms in SCI patients. Here, the spontaneous sustained firing of motor units could continue for minutes, at very low discharge rates (average 5.2 ± 1.6 pps) and with extremely low spike-to-spike variability (CV = 5.4 ± 1.6%).
In contrast to patients with SCI, it is less likely that the spontaneous and comodulated unit activity at “rest” in stroke survivors is due primarily to an increase in PIC activation. First, a recent study from our laboratory discounted a major role for enhanced PICs in mediating increased excitability of spastic–paretic motoneurons (Mottram et al. 2009a). Second, the average firing rates of the spontaneously firing units in spastic–paretic (8.4 ± 1.8 pps) and contralateral (9.6 ± 2.2 pps) muscles of stroke survivors were much higher than the values observed in spontaneously firing motor units of SCI patients (average 5.2 ± 1.6 pps, Gorassini et al. 2004; 6.1 ± 1.1 pps, Zijdewind and Thomas 2001). Third, the average variability in firing rates of spontaneously firing units (CV) for the spastic–paretic (13.4 ± 4.0%) and contralateral muscles (16.9 ± 4.3%) was up to threefold greater than that observed in spontaneously firing motor units of patients with SCI (5.4 ± 1.6%; Gorassini et al. 2004). These findings suggest that the “spontaneous” and comodulated unit activity in stroke survivors was likely attributable to a tonic depolarizing synaptic drive, originating from descending or regional inputs.
3. Depolarizing synaptic drive to the motoneuron pool. As stated in the introduction, we questioned whether the difficulty stroke survivors have in relaxing their muscles may be due, in part, to the presence of a tonic, low-level ionotropic drive to the motoneuron pool. An increase in such excitatory synaptic input might arise from regional excitatory interneurons (spinal interneuronal networks) or from descending pathways (Burke et al. 1972; Hultborn 2003; Katz and Rymer 1989), including vestibulospinal or reticulospinal pathways.
For example, it has been suggested that net tonic excitation of motoneuron pools in spasticity may be due, in part, to the interruption of an inhibitory corticoreticulospinal pathway (Andrews et al. 1973a,b). This may result in enhanced activity in excitatory projections from the pontine reticular formation and brain stem to the spinal cord (Kline et al. 2007), bringing motoneurons closer to their discharge thresholds. Furthermore, the lateral vestibulospinal pathway has been shown to be important in the development of increased excitability in axial and limb extensor alpha motoneurons that display typical features of spastic hypertonia (Lance 1981).
4. Combinations of PIC and depolarizing synaptic drive. The observed coherence across dually spontaneously firing motor unit pairs does not exclude the presence of PICs in motoneurons. Instead, the observed coherence suggests that these spontaneously firing motor units are still responsive to other inputs—here as a common input to each motoneuron of the pair. Thus it remains possible that a combination of the PIC and a depolarizing descending drive contributed to the slow, regular spontaneous unit activity observed. In fact, subthreshold activation of the PIC consistently occurs with synaptic excitation of the motoneuron (as opposed to intracellular current injection) due to the predominantly dendritic location of the PIC channels (reviewed by Heckman et al. 2003). Second, the regenerative activation of the Na PIC cannot occur without some tonic, depolarizing bias current to the motoneuron (Li et al. 2004). It follows that the high number of spontaneously active motor units whose firing rates were tightly comodulated strongly suggest the presence of a common low-level depolarizing synaptic drive to the motoneuron pool.
The inability of patients with SCI to voluntarily stop spontaneous motor unit activity (Zijdewind and Thomas 2001) suggests a primarily PIC-based mechanism (Gorassini et al. 2004). In contrast, in all instances of triceps brachii activation in the spastic–paretic muscle the units ceased firing, suggesting that the units were influenced by descending inputs. Second, although the firing behavior of a spontaneously firing unit did not change during a maximal voluntary contraction of the thenar muscles performed by a patient with a spinal cord injury (see Fig. 8 A–C in Zijdewind and Thomas 2001), spastic–paretic stroke survivors in the current study exhibited spontaneous firing rates that were modulated with increases in force of the elbow flexor muscles (Fig. 7).
EVIDENCE FOR A DIFFUSE MONOAMINERGIC SYSTEM.
In an earlier study, we argued that enhanced monoaminergic drive to the spinal cord did not seem to be pivotal after stroke and thus that the effects of monoamines are not sufficient to generate lateralized PICs in spastic–paretic stroke survivors (Mottram et al. 2009a). Nonetheless, since the effects of monoamines are bilateral (Holstege and Kuypers 1987), they may explain some of the changes that occur in the contralateral muscle of stroke survivors (Mirbagheri et al. 2008; Suresh et al. 2005). Indeed, application of monoamines serotonin (5-HT) and norepinephrine (NE) not only substantially depolarizes the resting membrane potential of motoneurons (Harvey et al. 2006; Powers and Binder 2001) but also lowers the action potential threshold (Krawitz et al. 2001) and PIC threshold (Bennett et al. 1998; Lee and Heckman 1998a; Lee et al. 2003). It follows that the effects of tonic ionotropic input could be enhanced by the reduced resting membrane potential − threshold difference and also by amplification from the hyperpolarized PIC (Lee and Heckman 1996, 2000). Perhaps the monoamine effects on spasticity are best measured by their influence on the contralateral muscle, as shown by the “spontaneous” firing of motor units following about 14% of the voluntary ramp contractions performed with the contralateral elbow flexor muscles of stroke survivors in the current study.
Clinical implications
Our current observations suggest that the spontaneous firing of motor units in stroke survivors arises from a mechanism different from that proposed for patients with a spinal cord injury. Instead of the primarily PIC-based mechanism proposed for patients with spinal cord injury, the spontaneous firing of motor units in spastic–paretic stroke survivors may be due, in part, to a tonic, low-level ionotropic drive to the motoneuron pool that keeps a certain proportion of motoneurons close to firing threshold and thus more readily activated at rest.
Therapeutic interventions to reduce such a tonic synaptic depolarizing drive to the resting motoneuron pool in spastic–paretic stroke survivors or to reduce resting membrane potential of motoneurons will allow the clinician to more effectively target spasticity when treating these patients.
GRANTS
This work was supported by the Brinson Foundation Post-Doctoral Fellowship awarded to C. J. Mottram and National Institutes of Health Grants 2T32-HD-007418-16 and 5R24-HD-050821-04 to W. Z. Rymer and NS-062200 to R. K. Powers.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank J. Moore, T. DeMott, and C. Marchand for assistance with data collection; J. Madoff for technical assistance; Drs. C. J. Heckman, R. K. Powers, and E. A. Christou for assistance with data interpretation; and Dr. Francois Meyer for assistance with coherence analyses and interpretation.
REFERENCES
- Andrews et al., 1973b. Andrews C, Knowles L, Hancock J. Control of the tonic vibration reflex by the brain stem reticular formation in the cat. J Neurol Sci 18: 217–226, 1973b [DOI] [PubMed] [Google Scholar]
- Andrews et al., 1973a. Andrews C, Knowles L, Lance JW. Corticoreticulospinal control of the tonic vibration reflex in the cat. J Neurol Sci 18: 207–216, 1973a [DOI] [PubMed] [Google Scholar]
- Aymard et al., 2000. Aymard C, Katz R, Lafitte C, Lo E, Penicaud A, Pradat-Diehl P, Raoul S. Presynaptic inhibition and homosynaptic depression: a comparison between lower and upper limbs in normal human subjects and patients with hemiplegia. Brain 123: 1688–1702, 2000 [DOI] [PubMed] [Google Scholar]
- Barzi and Zehr, 2008. Barzi Y, Zehr EP. Rhythmic arm cycling suppresses hyperactive soleus H-reflex amplitude after stroke. Clin Neurophysiol 119: 1443–1452, 2008 [DOI] [PubMed] [Google Scholar]
- Bennett et al., 1998. Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2023–2037, 1998 [DOI] [PubMed] [Google Scholar]
- Bennett et al., 2001a. Bennett DJ, Li Y, Harvey PJ, Gorassini M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol 86: 1972–1982, 2001a [DOI] [PubMed] [Google Scholar]
- Bennett et al., 2001b. Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol 86: 1955–1971, 2001b [DOI] [PubMed] [Google Scholar]
- Buchanan and Kasicki, 1999. Buchanan JT, Kasicki S. Segmental distribution of common synaptic inputs to spinal motoneurons during fictive swimming in the lamprey. J Neurophysiol 82: 1156–1163, 1999 [DOI] [PubMed] [Google Scholar]
- Burke et al., 1971a. Burke D, Andrews CJ, Gillies JD. Reflex response to sinusoidal stretch in spastic man. Brain 94: 455–470, 1971a [DOI] [PubMed] [Google Scholar]
- Burke and Ashby, 1972. Burke D, Ashby P. Are spinal “presynaptic” inhibitory mechanisms suppressed in spasticity? J Neurol Sci 15: 321–326, 1972 [DOI] [PubMed] [Google Scholar]
- Burke et al., 1971b. Burke D, Gillies JD, Lance JW. Hamstrings stretch reflex in human spasticity. J Neurol Neurosurg Psychiatry 34: 231–235, 1971b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke et al., 1972. Burke D, Knowles L, Andrews C, Ashby P. Spasticity, decerebrate rigidity and the clasp-knife phenomenon: an experimental study in the cat. Brain 95: 31–48, 1972 [DOI] [PubMed] [Google Scholar]
- Chardon et al., 2008. Chardon MK, Suresh NL, Rymer WZ. Reflex threshold estimation in spastic muscles: a new method. Program No. 76.4/NN12. 2008 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience, 2008. Online [Google Scholar]
- Christou et al., 2007. Christou EA, Rudrof T, Enoka JA, Meyer F, Enoka RM. Discharge rate during low-force isometric contractions influences motor unit coherence below 15 Hz but not motor unit synchronization. Exp Brain Res 178: 285–295, 2007 [DOI] [PubMed] [Google Scholar]
- Chung et al., 2008. Chung SG, van Rey E, Bai Z, Rymer WZ, Roth EJ, Zhang LQ. Separate quantification of reflex and nonreflex components of spastic hypertonia in chronic hemiparesis. Arch Phys Med Rehabil 89: 700–710, 2008 [DOI] [PubMed] [Google Scholar]
- Conway et al., 1995. Conway BA, Halliday DM, Farmer SF, Shahani U, Maas P, Weir AI, Rosenberg JR. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J Physiol 489: 917–924, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone et al., 2003. Crone C, Johnsen LL, Biering-Sorensen F, Nielsen JB. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain 126: 495–507, 2003 [DOI] [PubMed] [Google Scholar]
- Datta and Stephens, 1990. Datta AK, Stephens JA. Synchronization of motor unit activity during voluntary contraction in man. J Physiol 422: 397–419, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca and Erim, 1994. De Luca CJ, Erim Z. Common drive of motor units in regulation of muscle force. Trends Neurosci 17: 299–305, 1994 [DOI] [PubMed] [Google Scholar]
- Eken et al., 1989. Eken T, Hultborn H, Kiehn O. Possible functions of transmitter-controlled plateau potentials in alpha motoneurones. Prog Brain Res 80: 257–267, 1989 [DOI] [PubMed] [Google Scholar]
- Farmer et al., 1993a. Farmer SF, Bremner FD, Halliday DM, Rosenberg JR, Stephens JA. The frequency content of common synaptic inputs to motoneurones studied during voluntary isometric contraction in man. J Physiol 470: 127–155, 1993a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer et al., 1997. Farmer SF, Halliday DM, Conway BA, Stephens JA, Rosenberg JR. A review of recent applications of cross-correlation methodologies to human motor unit recording. J Neurosci Methods 74: 175–187, 1997 [DOI] [PubMed] [Google Scholar]
- Farmer et al., 1993b. Farmer SF, Swash M, Ingram DA, Stephens JA. Changes in motor unit synchronization following central nervous lesions in man. J Physiol 463: 83–105, 1993b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorassini et al., 2002. Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: reduction of motor unit recruitment thresholds by repeated contractions. J Neurophysiol 87: 1859–1866, 2002 [DOI] [PubMed] [Google Scholar]
- Gorassini et al., 2004. Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain 127: 2247–2258, 2004 [DOI] [PubMed] [Google Scholar]
- Gregson et al., 2000. Gregson JM, Leathley MJ, Moore AP, Smith TL, Sharma AK, Watkins CL. Reliability of measurements of muscle tone and muscle power in stroke patients. Age Ageing 29: 223–228, 2000 [DOI] [PubMed] [Google Scholar]
- Halliday et al., 1998. Halliday DM, Conway BA, Farmer SF, Rosenberg JR. Using electroencephalography to study functional coupling between cortical activity and electromyograms during voluntary contractions in humans. Neurosci Lett 241: 1–4, 1998 [DOI] [PubMed] [Google Scholar]
- Halliday and Rosenberg, 1999. Halliday DM, Rosenberg JR. Time and frequency domain analysis of spike train and time series data. In: Modern Techniques in Neuroscience Research, edited by Windhorst U, Johansson H. New York: Springer-Verlag, 1999, p. 503–543 [Google Scholar]
- Harris-Warrick and Cohen, 1985. Harris-Warrick RM, Cohen AH. Serotonin modulates the central pattern generator for locomotion in the isolated lamprey spinal cord. J Exp Biol 116: 27–46, 1985 [DOI] [PubMed] [Google Scholar]
- Harvey et al., 2006. Harvey PJ, Li X, Li Y, Bennett DJ. 5-ht2 Receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 96: 1158–1170, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman et al., 2003. Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci 26: 688–695, 2003 [DOI] [PubMed] [Google Scholar]
- Holstege and Kuypers, 1987. Holstege JC, Kuypers HGJM. Brainstem projections to spinal motoneurones: an update. Neuroscience 23: 809–821, 1987 [DOI] [PubMed] [Google Scholar]
- Hornby et al., 2004. Hornby TG, Heckman CJ, Harvey RL, Rymer WZ. Changes in voluntary torque and electromyographic activity following oral baclofen. Muscle Nerve 30: 784–795, 2004 [DOI] [PubMed] [Google Scholar]
- Hounsgaard and Kiehn, 1989. Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol 414: 265–282, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn, 2003. Hultborn H. Changes in neuronal properties and spinal reflexes during development of spasticity following spinal cord lesions and stroke: studies in animal models and patients. J Rehabil Med 35, Suppl. 41: 46–55, 2003 [DOI] [PubMed] [Google Scholar]
- Katz and Rymer, 1989. Katz RT, Rymer WZ. Spastic hypertonia: mechanisms and measurement. Arch Phys Med Rehabil 70: 144–155, 1989 [PubMed] [Google Scholar]
- Kilner et al., 2002. Kilner JM, Alonso-Alonso M, Fisher R, Lemon RN. Modulation of synchrony between single motor units during precision grip tasks in humans. J Physiol 541: 937–948, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood and Sears, 1978. Kirkwood PA, Sears TA. The synaptic connexions to intercostal motoneurons as revealed by their average common excitation potential. J Physiol 275: 103–134, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline et al., 2007. Kline TL, Schmit BD, Kamper DG. Exaggerated interlimb neural coupling following stroke. Brain 130: 159–169, 2007 [DOI] [PubMed] [Google Scholar]
- Krawitz et al., 2001. Krawitz S, Fedirchuk B, Dai Y, Jordan LM, McCrea DA. State-dependent hyperpolarization of voltage threshold enhances motoneurone excitability during fictive locomotion in the cat. J Physiol 532: 271–281, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy et al., 2009. Lamy JC, Wargon I, Mazevet D, Ghanim Z, Pradat-Diehl P, Katz R. Impaired efficacy of spinal presynaptic mechanisms in spastic stroke patients. Brain 132: 734–748, 2009 [DOI] [PubMed] [Google Scholar]
- Lance, 1980. Lance JW. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology 30: 1303–1313, 1980 [DOI] [PubMed] [Google Scholar]
- Lance, 1981. Lance JW. Disordered muscle tone and movement. Clin Exp Neurol 18: 27–35, 1981 [PubMed] [Google Scholar]
- Lee and Heckman, 1996. Lee RH, Heckman CJ. Influence of voltage-sensitive dendritic conductances on bistable firing and effective synaptic current in cat spinal motoneurons in vivo. J Neurophysiol 76: 2107–2110, 1996 [DOI] [PubMed] [Google Scholar]
- Lee and Heckman, 1998a. Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol 80: 572–582, 1998a [DOI] [PubMed] [Google Scholar]
- Lee and Heckman, 1998b. Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol 80: 583–593, 1998b [DOI] [PubMed] [Google Scholar]
- Lee and Heckman, 2000. Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci 20: 6734–6740, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al., 2003. Lee RH, Kuo JJ, Jiang MC, Heckman CJ. Influence of active dendritic currents on input–output processing in spinal motoneurons in vivo. J Neurophysiol 89: 27–39, 2003 [DOI] [PubMed] [Google Scholar]
- Lewek et al., 2007. Lewek MD, Hornby TG, Dhaher YY, Schmit BD. Prolonged quadriceps activity following imposed hip extension: a neurophysiological mechanism for stiff-knee gait? J Neurophysiol 98: 3153–3162, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li and Bennett, 2003. Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol 90: 857–869, 2003 [DOI] [PubMed] [Google Scholar]
- Li et al., 2004. Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91: 767–783, 2004 [DOI] [PubMed] [Google Scholar]
- Litvan et al., 1996. Litvan I, Mangone CA, Werden W, Bueri JA, Estol CJ, Garcea DO, Rey RC, Sica RE, Hallett M, Bartko JJ. Reliability of the NINDS myotatic reflex scale. Neurology 47: 969–972, 1996 [DOI] [PubMed] [Google Scholar]
- Lukacs, 2005. Lukacs M. Electrophysiological signs of changes in motor units after ischaemic stroke. Clin Neurophysiol 116: 1566–1570, 2005 [DOI] [PubMed] [Google Scholar]
- Mazzaro et al., 2007. Mazzaro N, Nielsen JF, Grey MJ, Sinkjaer T. Decreased contribution from afferent feedback to the soleus muscle during walking in patients with spastic stroke. J Stroke Cerebrovasc Dis 16: 135–144, 2007 [DOI] [PubMed] [Google Scholar]
- McPherson et al., 2008. McPherson JG, Ellis MD, Heckman C, Dewald JP. Evidence for increased activation of persistent inward currents in individuals with chronic hemiparetic stroke. J Neurophysiol 100: 3236–3243, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merletti et al., 2001. Merletti R, Rainoldi A, Farina D. Surface electromyography for noninvasive characterization of muscle. Exerc Sport Sci Rev 29: 20–25, 2001 [DOI] [PubMed] [Google Scholar]
- Mirbagheri et al., 2008. Mirbagheri MM, Alibiglou L, Thajchayapong M, Rymer WZ. Muscle and reflex changes with varying joint angle in hemiparetic stroke (Abstract). J Neuroeng Rehabil 5: 6, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz et al., 2005. Moritz CT, Christou EA, Meyer FG, Enoka RM. Coherence at 16–32 Hz can be caused by short-term synchrony of motor units. J Neurophysiol 94: 105–118, 2005 [DOI] [PubMed] [Google Scholar]
- Mottram et al., 2009a. Mottram CJ, Suresh NL, Heckman CJ, Gorassini MA, Rymer WZ. Origins of abnormal excitability in biceps brachii motoneurons of spastic–paretic stroke survivors. J Neurophysiol 102: 2026–2038, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram et al., 2009b. Mottram CJ, Wallace CL, Chikando CN, Meyer FG, Rymer WZ. Spontaneously firing motor unit pairs in the spastic biceps brachii of stroke survivors are co-modulated. Program No. 659.17/CC9. 2009 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience, 2009b. Online [Google Scholar]
- Mottram et al., 2007. Mottram CJ, Wallace CL, Chikando CN, Rymer WZ. Mechanisms contributing to spontaneous firing of motor units in the spastic biceps brachii of stroke survivors. Program No. 726.10. 2007 Abstract Viewer/Itinerary Planner. San Diego, CA: Society for Neuroscience, 2007. Online [Google Scholar]
- Myers et al., 2004. Myers LJ, Erim Z, Lowery MM. Time and frequency domain methods for quantifying common modulation of motor unit firing patterns (Abstract). J Neuroeng Rehabil 1: 2, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen and Hultborn, 1993. Nielsen J, Hultborn H. Regulated properties of motoneurons and primary afferents: new aspects on possible spinal mechanisms underlying spasticity. In: Spasticity: Mechanisms and Management, edited by Thilmann AF. Berlin: Springer-Verlag, 1993, p. 177–191 [Google Scholar]
- Nordstrom et al., 1992. Nordstrom MA, Fuglevand AJ, Enoka RM. Estimating the strength of common input to human motoneurons from the cross-correlogram. J Physiol 453: 547–574,1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person and Kudina, 1972. Person RS, Kudina LP. Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr Clin Neurophysiol 32: 471–483, 1972 [DOI] [PubMed] [Google Scholar]
- Pettersen et al., 2005. Pettersen V, Bjorkoy K, Torp H, Westgaard RH. Neck and shoulder muscle activity and thorax movement in singing and speaking tasks with variation in vocal loudness and pitch. J Voice 19: 623–634, 2005 [DOI] [PubMed] [Google Scholar]
- Pettersen and Westgaard, 2005. Pettersen V, Westgaard RH. The activity patterns of neck muscles in professional classical singing. J Voice 19: 238–251, 2005 [DOI] [PubMed] [Google Scholar]
- Pierucci et al., 2009. Pierucci M, Di Matteo V, Benigno A, Crescimanno G, Esposito E, Di Giovanni G. The unilateral nigral lesion induces dramatic bilateral modification on rat brain monoamine neurochemistry. Ann NY Acad Sci 1155: 316–323, 2009 [DOI] [PubMed] [Google Scholar]
- Powers and Binder, 2001. Powers RK, Binder MD. Input–output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol 143: 137–263, 2001 [DOI] [PubMed] [Google Scholar]
- Powers et al., 1988. Powers RK, Marder-Meyer J, Rymer WZ. Quantitative relations between hypertonia and stretch reflex threshold in spastic hemiparesis. Ann Neurol 23: 115–124, 1988 [DOI] [PubMed] [Google Scholar]
- Rosenberg et al., 1989. Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol 53: 1–31, 1989 [DOI] [PubMed] [Google Scholar]
- Schwindt and Crill, 1982. Schwindt PC, Crill WE. Factors influencing motoneuron rhythmic firing: results from a voltage-clamp study. J Neurophysiol 48: 875–890, 1982 [DOI] [PubMed] [Google Scholar]
- Sears and Stagg, 1976. Sears TA, Stagg D. Short-term synchronization of intercostal motoneurone activity. J Physiol 263: 357–381, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki et al., 2003. Seki K, Perlmutter SI, Fetz EE. Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nat Neurosci 6: 1309–1316, 2003 [DOI] [PubMed] [Google Scholar]
- Semmler et al., 2003. Semmler JG, Kornatz KW, Enoka RM. Motor-unit coherence during isometric contractions is greater in a hand muscle of older adults. J Neurophysiol 90: 1346–1349, 2003 [DOI] [PubMed] [Google Scholar]
- Semmler et al., 2004. Semmler JG, Sale MV, Meyer FG, Nordstrom MA. Motor unit coherence and its relation with synchrony are influenced by training. J Neurophysiol 92: 3320–3331, 2004 [DOI] [PubMed] [Google Scholar]
- Suresh et al., 2005. Suresh NL, Ellis MD, Moore J, Heckman H, Rymer WZ. Excitatory synaptic potentials in spastic human motoneurons have a short rise-time. Muscle Nerve 32: 99–103, 2005 [DOI] [PubMed] [Google Scholar]
- Svirskis and Hounsgaard, 1998. Svirskis G, Hounsgaard J. Transmitter regulation of plateau properties in turtle motoneurons. J Neurophysiol 79: 45–50, 1998 [DOI] [PubMed] [Google Scholar]
- Westgaard et al., 2006. Westgaard RH, Bonato P, Westad C. Respiratory and stress-induced activation of low-threshold motor units in the human trapezius muscle. Exp Brain Res 175: 689–701, 2006 [DOI] [PubMed] [Google Scholar]
- Zijdewind and Thomas, 2001. Zijdewind I, Thomas CK. Spontaneous motor unit behavior in human thenar muscles after spinal cord injury. Muscle Nerve 24: 952–962, 2001 [DOI] [PubMed] [Google Scholar]