Abstract
The activity in the primary motor cortex (M1) reflects the direction of movements, but little is known about physiological changes in the M1 during generation of bilateral isometric forces in different directions. Here, we used transcranial magnetic stimulation to examine motor evoked potentials (MEPs), short-interval intracortical inhibition (SICI), and interhemispheric inhibition (IHI) in the left first dorsal interosseous (FDI) during isometric index finger abduction while the right index finger remained at rest or performed isometric forces in different directions (abduction or adduction) and in different postures (prone and supine). Left FDI MEPs were suppressed during bilateral compared with unilateral forces, with a stronger suppression when the right index finger force was exerted in the adduction direction regardless of hand posture. IHI targeting the left FDI increased during bilateral compared with unilateral forces and this increase was stronger during right index finger adduction despite the posture of the right hand. SICI decreased to a similar extent during both bilateral forces in both hand postures. Thus generation of index finger isometric forces away from the body midline (adduction direction), regardless of the muscle engaged in the task, down-regulates corticospinal output in the contralateral active hand to a greater extent than forces exerted toward the body midline (abduction direction). Transcallosal inhibition, but not GABAergic intracortical circuits, was modulated by the direction of the force. These findings suggest that during generation of bimanual isometric forces the M1 is driven by “extrinsic” parameters related to the hand action.
INTRODUCTION
Previous studies have investigated force control during bimanual isometric voluntary contraction in healthy humans (Carson 1995; Diedrichsen et al. 2003; Rinkenauer et al. 2001; Steglich et al. 1999). It has been demonstrated that the coordination dynamics of isometric forces are affected by the direction of the bimanual forces (Carson 1995). For example, at increasing pacing frequencies, the stability advantage present during bilateral isometric activation of homologous muscles is markedly diminished, whereas bilateral activation of nonhomologous muscles results in a more stable pattern (Carson 1995). These results contrast the effects observed during bimanual free joint movements (Carson et al. 1994; Kelso 1984; Mechsner et al. 2001). At present, the mechanisms involved in the control of bilateral isometric forces exerted in different directions remain poorly understood.
In nonhuman primates, the primary motor cortex (M1) is involved in the generation of isometric forces (Ashe 1997; Cheney and Fetz 1980; Evarts 1969; Georgopoulos et al. 1982, 1992; Sergio and Kalaska 2003; Taira et al. 1996). It has been demonstrated that the activity in M1 neurons also reflects the direction of static (Kalaska and Hyde 1985; Kalaska et al. 1989; Sergio and Kalaska 2003) and dynamic (Taira et al. 1996) isometric forces. For example, Kalaska and colleagues (1989) examined the activity in M1 cells while monkeys held a manipulandum against loads operating in eight different directions. These authors demonstrated that cells in M1 were broadly tuned to the direction of static forces and showed different degrees of sensitivity to loads. In humans, a previous study has also suggested that the M1 encodes for the direction of force outputs during isometric conditions (Cros et al. 2007). It has been demonstrated that coupling of bilateral isometric forces is greatly attenuated in patients with lesions of the corpus callosum, suggesting that force coupling takes place at a cortical level (Diedrichsen et al. 2003). Therefore, we hypothesized that changes in corticospinal excitability during isometric voluntary contraction in one hand will reflect the direction of the force exerted by the contralateral hand.
We examined corticospinal output in the active first dorsal interosseous muscle (FDI) while the right index finger exerted isometric forces in different directions (i.e., abduction and adduction). Transcallosal inhibition and corticomuscular coherence between the sensorimotor cortex and a contralateral active muscle are increased during bilateral forces of different amplitude compared with unilateral forces (Perez et al. 2009; MA Perez, S Soteropoulos, SN Baker, unpublished observations). Therefore in our protocol both hands exerted different force amplitudes. Because corticospinal output from one M1 is partially controlled by callosal inputs and by local intracortical circuits, we examined transcallosal inhibition (Ferbert et al. 1992) and short-interval intracortical inhibition (Kujirai et al. 1993) by using paired-pulse transcranial magnetic stimulation (TMS) protocols. Corticospinal excitability was also examined with the right hand positioned in both prone and supine postures to determine whether the physiological effects were related to the muscle group engaged in the task or to the direction of the force.
METHODS
Subjects
Twelve right-handed healthy volunteers (5 female, 7 male) with an average age of 23.1 ± 2.3 yr participated in the study. Handedness was confirmed by the Edinburgh inventory (Oldfield 1971) (mean laterality index = 81; range = 60–100). We tested right-handed subjects since interhemispheric inhibitory output is stronger from the dominant to the nondominant M1 (Bäumer et al. 2007; Netz et al. 1995). All subjects gave their informed consent to the experimental procedure, which was approved by the local ethics committee. The study was performed in accordance with the Declaration of Helsinki.
Experimental sessions
Subjects were seated in an armchair with both arms flexed at the elbow by 90°. The left hand was always positioned in prone posture (palm down), whereas the right hand was positioned in prone or supine (palm up) posture. The left and right index fingers were attached to a custom two-axis load cell, which measures the forces exerted by the subject (Fig. 1B). During testing subjects performed (Fig. 1A) 10% of left maximal isometric index finger abduction (ABD), whereas the right index finger remained at rest (baseline) or performed 30% of maximal isometric ABD or adduction (ADD). For reasons of clarity, we will refer to the direction of the force exerted by the right index finger as ABD and ADD and to the posture of the right index finger as prone and supine. Throughout this study the term “ABD” refers to force exerted by the index finger toward the body midline, whereas the term “ADD” refers to force exerted by the index finger away from the body midline. When the right index finger performed ABD in the prone position the FDI muscle was acting as an agonist to the task, whereas when the right index finger performed ABD in the supine position the FDI muscle was acting as an antagonist to the task. The opposite occurred when the right index finger performed ADD in both hand postures. At the start of the experiment, all subjects performed two to three brief maximal voluntary contractions (MVCs; 3–5 s) with the left and right index fingers into ABD or ADD separately, with 30 s rest between contractions. The maximal forces were used to set targets for subsequent submaximal contractions. During maximal contractions subjects were verbally encouraged to perform maximally and visual feedback was provided (Gandevia 2001). Custom software was written to acquire signals from the load cell and to display visual feedback corresponding to rest and 10% and 30% of each subject's maximal left and right index finger ABD and ADD force in real time (LabVIEW; National Instruments, Santa Ana, CA). Subjects were instructed to respond to the GO signal (target signal) presented on a computer monitor by moving a cursor to a target box. Figure 1A illustrates the location of the target box (gray bar), showing that at 10% of force there is a smaller distance between the target signal (black bar) and target box compared with 30% of force. All 10% forces were completed with the nondominant arm. Additional verbal feedback was provided to the subjects to ensure that both hands performed the correct task at all times.
Fig. 1.
Experimental setup. A: diagram showing the visual display presented to all subjects during testing of unilateral and bilateral isometric index finger forces. Subjects were instructed on a monitor to perform 10% of left maximal isometric index finger abduction (10% ABD), whereas the right index finger remained at rest (0% Rest) or performed 30% of maximal isometric index finger abduction (30% ABD) or adduction (30% ADD) while motor evoked potentials (MEPs), interhemipheric inhibition (IHI), and short-interval intracortical inhibition (SICI) were tested in the left index finger. The condition in which the right hand remained at rest was used as baseline. The black vertical bar is the cursor that subjects were instructed to move by performing left and right isometric forces. The “GO” signal (gray box located to the left or to the right of the cursor) was the target to which subjects had to move the cursor. The distance between cursor and target is related to the magnitude of force required to accomplish each task, normalized to the maximal voluntary effort determined in each participant. B: schematic of the experimental setup showing the posture of both hands during testing. The left hand was always positioned in prone posture (palm down), whereas the right hand was positioned in prone or supine (palm up) posture. Then, when the right index finger performed ABD in the prone position the first dorsal interosseous (FDI) muscle was acting as an agonist to the task, whereas when the right index finger performed ABD in the supine position the FDI muscle was acting as an antagonist to the task.
Electromyogram and force recordings
Electromyograms (EMGs) were recorded bilaterally from the FDI muscle by surface electrodes secured to the skin over the belly of each muscle (Ag–AgCl, 10 mm diameter). The signals were amplified, filtered (20–1,000 Hz), and collected at 2 kHz for off-line analysis (CED 1401 with Signal software; Cambridge Electronic Design, Cambridge, UK). The ABD and ADD forces exerted at the proximal interphalangeal joint of the index finger were measured bilaterally by two load cells (range: ±25 pounds, voltage: ±5 V, high-sensitivity transducer: 0.045 V/N; Honeywell, Columbus, OH). Force was sampled at 200 Hz and stored in a computer for off-line analysis.
Transcranial magnetic stimulation
Transcranial magnetic stimulation (TMS) was delivered to the optimal scalp position for activation of the left and right FDI muscles in each respective testing session. Motor evoked potentials (MEPs) were elicited by transcranial magnetic stimuli delivered from a Magstim 200 stimulator (Magstim, Carmathenshire, Wales, UK) through a figure-of-eight shaped coil (loop diameter: 8 cm; type number: SP15560) with a monophasic current waveform. The coil was held tangential to the scalp with the handle pointing backward and 45° away from the midline to activate the corticospinal system preferentially transynaptically via horizontal corticocortical connections (Di Lazzaro et al. 2004). The TMS coil was held to the head of the subject by a coil holder (Magstim). Measures of motor cortical excitability included resting and active motor thresholds (RMT and AMT, respectively), MEPs, short-interval intracortical inhibition (SICI), and interhemispheric inhibition (IHI) from left M1 to right M1. Because of the length of the physiological measurements and to avoid fatigue, all measurements were completed in three to five testing sessions. All TMS measurements were completed during 10% of left ABD, whereas the right index finger remained at rest or completed 30% of ABD or ADD (in prone or supine posture).
Motor evoked potentials
According to the International Federation of Clinical Neurophysiology guidelines (Rossini et al. 1994; Rothwell et al. 1999), RMT was defined as the minimal stimulus intensity required to induce MEPs >50 μV peak-to-peak amplitude in at least five of ten consecutive trials in the relaxed muscle and the AMT was defined as the minimal stimulus intensity able to evoke MEPs >200 μV peak-to-peak amplitude in at least five of ten consecutive trials during 10% of left ABD. The intensity of the TMS pulses used for testing was adjusted in each subject so that the MEP in the left FDI was about 2 mV, measured during 10% of left ABD. Subjects performed ten sets of three contractions. A set consisted of 10- to 15-s contractions in each of the three conditions, separated by 10 s of rest. During each contraction, TMS was delivered three times at 4- to 5-s intervals over the nondominant hemisphere, to give a total of 30 trials of each condition.
Interhemispheric inhibition
Interhemispheric inhibition (IHI) was tested using a randomized conditioning-test design reported previously (Ferbert et al. 1992). During all testing, a conditioning stimulus (CS) was given to the left M1 10 ms before a test stimulus (TS) was given to the right M1. A suprathreshold CS was set at an intensity of the RMT that elicited an amount of inhibition of roughly 50% of each individual maximal inhibition. In all subjects, the intensity used for the CS ranged from 39 to 71% of the stimulator output (unadjusted; see Table 1). The same stimulation intensity was used for the CS in all conditions tested. The Test MEP elicited by the TS was adjusted to produce a MEP of about 2 mV during a unilateral force (2.0 ± 0.8 mV) and the same stimulation intensity was used for all conditions tested (unadjusted; see Table 1). IHI measurements were also completed when the intensity of the TS was adjusted to maintain the size of the Test MEP and the size of the MEP elicited by the CS similar across conditions. Here, the intensity used for the CS ranged from 33 to 100% of the stimulator output (adjusted; see Table 1). The unadjusted measurements were completed with the right hand in both prone and supine postures and the adjusted measurements were completed in the prone posture.
Table 1.
Interhemispheric inhibition (IHI): stimulation parameters
| Parameter | Prone | Supine |
|---|---|---|
| Unadjusted | ||
| TS | 44.8 ± 4 (102 ± 5 RMT) | 43.6 ± 4 (98 ± 6 RMT) |
| CS | 50.7 ± 10 (117 ± 16 RMT) | 52.1 ± 8 (121 ± 11 RMT) |
| Prone |
|||
|---|---|---|---|
| Baseline | ABD | ADD | |
| Adjusted | |||
| TS | 45.3 ± 6 | 47.5 ± 8 | 50.3 ± 9 (F = 11.3, P < 0.001) |
| CS | 80.6 ± 10 | 50.5 ± 10 | 77.5 ± 10 (F = 14.2, P < 0.001) |
| Test MEP | 2.6 ± 0.6 mV | 2.3 ± 0.8 mV | 2.2 ± 0.9 mV (F = 1.1, P = 0.3) |
| MEP elicited by the CS | 3.2 ± 1.9 mV | 3.4 ± 1.8 mV | 3.3 ± 1.8 mV (F = 1.6, P = 0.2) |
Mean stimulus intensity (±SD) used for the test stimulus (TS) and conditioning stimulus (CS) during IHI testing when measurements were completed without adjusting (Unadjusted) or adjusting (Adjusted) the size of the Test MEP and the MEP elicited by the CS. In the unadjusted condition, the same stimulus intensity was used across conditions, with the right hand tested in both prone and supine postures. In the adjusted condition, the intensity values of the TS and CS were changed to acquire a Test MEP of around 2 mV and an MEP elicited by the CS similar to the baseline condition with the right hand in prone posture. Note that there were no differences in the size of the Test MEP and in the MEP elicited by the CS when IHI was tested across conditions (Baseline = right index finger remained at rest; ABD = 30% of right index finger abduction; and ADD = 30% of right index finger adduction).
IHI was measured by expressing the size of the conditioned MEP as a percentage of the size of the Test MEP [(Conditioned MEP × 100)/(Test MEP)] in each of the conditions tested. Subjects performed seven sets of three contractions in each condition. A set consisted of 10- to 15-s contractions in each of the conditions, separated by 10 s of rest. A total of 21 Test MEPs and 21 Conditioned MEPs were tested in each condition.
Short-interval intracortical inhibition
Short-interval intracortical inhibition (SICI) was tested using the method described by Kujirai et al. (1993). A CS was set at an intensity of around 70% of AMT. In all subjects, the intensity used for the CS ranged from 20 to 35% of the stimulator output (unadjusted; see Table 2). This low-intensity stimulus can test SICI independently of the effects on short-intracortical facilitation (SICF) at low contraction levels (Ortu et al. 2008). The same stimulation intensity was used for the CS in all conditions tested. The TS was adjusted to produce a MEP of about 2 mV during unilateral forces. SICI measurements were also tested by adjusting the size of the Test MEP to produce a MEP of about 2 mV across conditions (adjusted; see Table 2). Test stimuli were delivered 2.5 ms after CS, an optimal interstimulus interval for eliciting SICI, and to avoid a mixture of the two phases of inhibition (Fisher et al. 2002).
Table 2.
Short-interval intracortical inhibition (SICI): stimulation parameters
| Parameter | Prone | Supine |
|---|---|---|
| Unadjusted | ||
| TS | 45.0 ± 7.0 (120 ± 7 AMT) | 44.4 ± 4.7 (118.2 ± 6 AMT) |
| CS | 26.4 ± 4.1 (71 ± 7 AMT) | 27.0 ± 3.7 (72.9 ± 6 AMT) |
| Prone |
|||
|---|---|---|---|
| Baseline | ABD | ADD | |
| Adjusted | |||
| TS | 44.0 ± 3.7 | 46.7 ± 4.0 | 48.1 ± 5.0 (F = 19.5, P < 0.001) |
| Test MEP | 2.3 ± 0.4 mV | 2.4 ± 0.4 mV | 2.3 ± 0.5 mV (F = 1.7, P = 0.6) |
Mean stimulus intensity (±SD) used for the test stimulus (TS) and conditioning stimulus (CS) during SICI testing when measurements were completed without adjusting (Unadjusted) or adjusting (Adjusted) the size of the Test MEP. In the unadjusted condition, the same stimulus intensity was used across conditions, with the right hand tested in both prone and supine postures. In the adjusted condition, the intensity of the TS was changed to acquire a Test MEP of around 2 mV, with the right hand in prone posture. Note there were no differences in the size of the Test MEP when SICI was tested across conditions (Baseline = right index finger remained at rest; ABD = 30% of right index finger abduction; and ADD = 30% of right index finger adduction).
SICI was calculated by expressing the size of the conditioned MEP as a percentage of the size of the Test MEP [(Conditioned MEP × 100)/(Test MEP)] in all conditions tested. Subjects performed seven sets of three contractions in each condition. An illustration of changes inA set consisted of 10- to 15-s contractions in each of the conditions, separated by 10 s of rest. A total of 21 Test MEPs and 21 Conditioned MEPs were tested in each condition.
Data analysis
Normal distribution was tested by the Shapiro–Wilk test and homogeneity of variances by the Brown–Forsythe test. Two-way factorial ANOVA was performed to determine the effect of the direction of the right index finger voluntary activity (ABD, ADD) and posture (prone, supine) on MEP size, SICI, IHI, mean rectified EMG activity, and force. Tukey post hoc analysis was used to test for significant comparisons. Paired t-test was used to compare measurements during unilateral and bilateral isometric voluntary contractions as needed. One-way ANOVA was performed to compare the intensity for TS and CS, Test MEP, and the size of MEP elicited by the CS across adjusted conditions. Mean rectified EMG activity in the FDI and force amplitude were measured in the left and right sides 100 ms prior to TMS stimulus artifact. Significance was set at P < 0.05. Group data are presented as means ± SDs in the text. Pearson correlation analysis was used to test correlations as needed.
RESULTS
MEPs
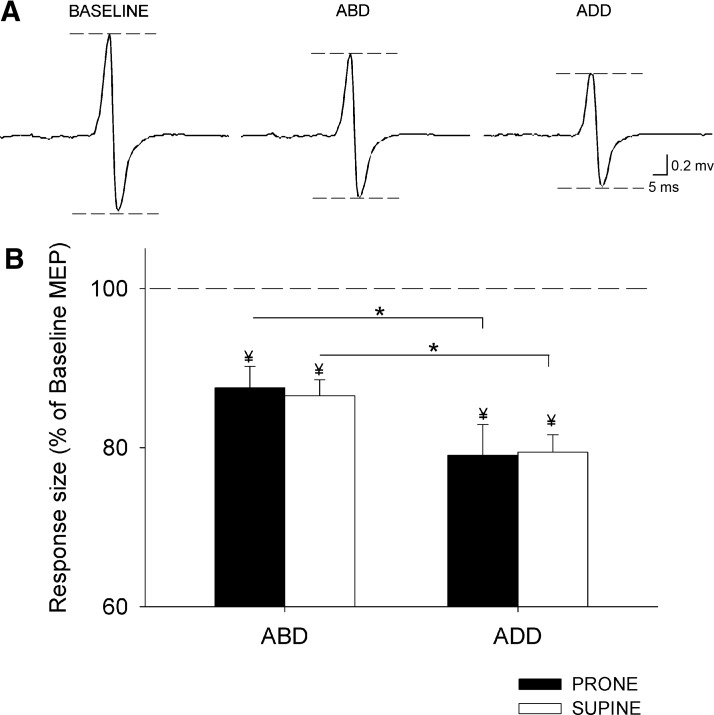
Figure 2A illustrates left FDI MEPs recorded in a single subject during unilateral and bilateral isometric forces. A factorial ANOVA showed a significant effect of direction (F = 28.7, P < 0.001) but not posture (F = 0.2, ns) nor their interaction (F = 0.7, ns; n = 12) on the size of MEPs evoked in the left FDI. We found a larger suppression of left FDI MEPs during ADD than that during ABD in prone (ADD = 79 ± 13% of baseline MEP and ABD = 87 ± 10% of baseline MEP, P < 0.001; Fig. 2B) and supine (ADD = 79.4 ± 9% of baseline MEP and ABD = 86 ± 7% of baseline MEP, P < 0.001; Fig. 2B) postures. No differences were observed between postures during each contraction (ABD, P = 0.7; ADD, P = 0.9). These results indicate that left FDI MEPs were smaller when the right index finger force was exerted in the ADD direction (away from the body midline) and that this was independent of the right FDI muscle being used as an agonistic or antagonistic muscle to the task. No effects of direction, posture, nor their interaction were observed on mean rectified EMG activity in the left FDI (F = 0.7, ns; F = 1.4, P = 0.3; F = 1.1 P = 0.4) and in the force exerted by the left (F = 2.4, P = 0.2; F = 1.3, P = 0.15; F = 0.6, ns) and right FDI (F = 0.2, ns; F = 0.6, ns; F = 1.8, P = 0.2).
Fig. 2.
Motor evoked potentials (MEPs). A: MEPs recorded from the left FDI of a representative subject during 10% of left ABD, whereas the right index finger remained at rest (baseline) or performed 30% of ABD or ADD. The specific action completed by the right index finger is indicated as Baseline, ABD, and ADD. In this example the right hand was positioned in prone posture. B: group data (n = 12). The abscissa shows the action completed by the right index finger in prone (black bars) and supine (white bars) postures. The ordinate shows left FDI MEP amplitudes as a percentage of the baseline left FDI MEPs measured with the right index finger at rest. The horizontal dashed line represents the left FDI MEP baseline measure with the right index finger at rest. Note the larger attenuation of the left FDI MEPs during both right hand postures during right ADD compared with right ABD contractions. Error bars indicate SEs. *P < 0.05. Note the left FDI MEP amplitudes were suppressed with respect to the baseline in all conditions (¥ indicates significant difference with respect to baseline).
Paired t-test showed that left FDI MEPs were significantly suppressed during bilateral isometric voluntary contraction compared with a unilateral contraction in prone (rest vs. ABD, P < 0.001; rest vs. ADD, P < 0.001) and supine (rest vs. ABD, P < 0.001; rest vs. ADD, P < 0.001) postures.
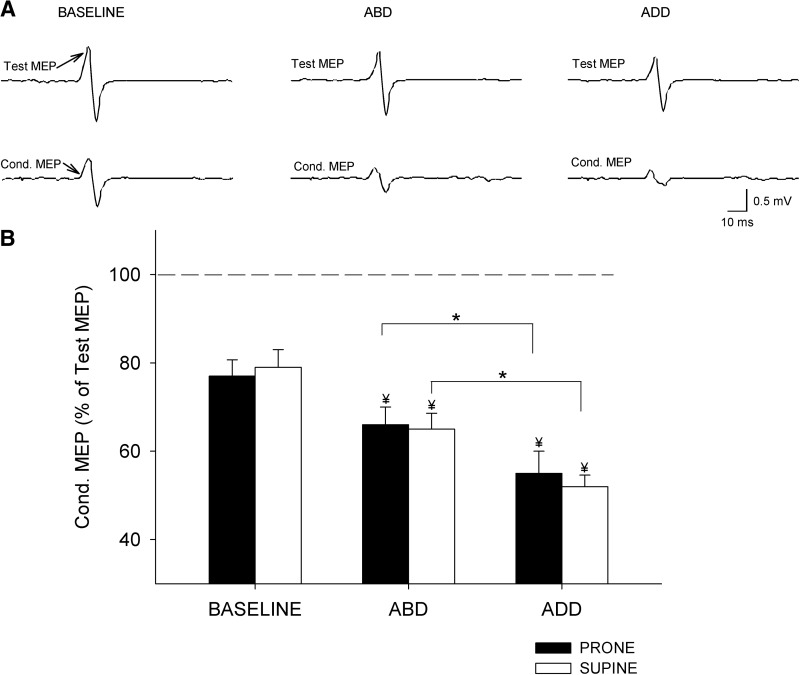
IHI
An illustration of changes in IHI recorded in a single subject during unilateral and bilateral isometric forces is shown in Fig. 3A. A factorial ANOVA showed a significant effect of direction (F = 61.9, P < 0.001) but not posture (F = 2.2, P = 0.16) nor their interaction (F = 0.1, ns; n = 12) on IHI. Our analysis revealed a larger increase in IHI during ADD than that during ABD in prone (ADD = 55 ± 15% and ABD = 66 ± 12%, P < 0.01; Fig. 3B) and supine (ADD = 52 ± 8% and ABD = 65 ± 11%, P < 0.001; Fig. 3B) postures. We found no differences between postures during each contraction (ABD, P = 0.8; ADD, P = 0.7). Compared with a unilateral contraction, the magnitude of IHI was significantly increased during ABD (P < 0.001) and ADD (P < 0.001) in both hand postures. Overall, our results indicate that IHI was stronger when the right FDI exerted force in the ADD direction (away from the body midline) independent of the right-hand posture. No effects of direction, posture, nor their interaction were observed on mean rectified EMG activity in the left FDI (F = 1.5, P = 0.2; F = 0.5, ns; F = 0.3, ns) and in the force exerted by the left (F = 1.1, P = 0.2; F = 1.6, P = 0.1; F = 0.4, ns) and right FDI (F = 0.8, ns; F = 2.3, P = 0.1; F = 0.3, ns).
Fig. 3.
Interhemispheric inhibition (IHI). A: IHI recorded from the left FDI of a representative subject during 10% of left ABD, whereas the right index finger remained at rest (baseline) or performed 30% of ABD or ADD. The actions by the right index finger are indicated as Baseline, ABD, and ADD. In this example the right hand was positioned in prone posture. Test MEP and conditioned MEP (Cond. MEP) are indicated by arrows. B: group data (n = 12). The abscissa shows the conditions tested during the assessment of IHI contractions in prone (black bars) and supine (white bars) postures. The ordinate indicates the magnitude of the conditioned MEP expressed as a percentage of the Test MEP [(Conditioned MEP × 100)/(Test MEP)] during bilateral isometric forces. The horizontal dashed line represents the size of the Test MEP. Note that IHI was increased to a larger extent during ADD forces regardless of the right hand posture. Error bars indicate SEs. *P < 0.05. Also note that IHI was significantly increased with respect to the baseline in all conditions tested (¥ indicates significant difference with respect to baseline).
When adjusting for the size of the Test MEP and the MEP elicited by the CS, IHI [(Conditioned MEP × 100)/(Test MEP)] was significantly increased during ADD (44 ± 14%, P < 0.01) compared with ABD (65 ± 11%, P < 0.01). Compared with a unilateral contraction (54 ± 12%), IHI was significantly decreased during ABD (P < 0.001) and increased during ADD (P < 0.001). Mean rectified EMG activity in the left FDI and force (F = 2.2, P = 0.2; F = 1.4, P = 0.5) exerted by the left FDI was similar across conditions.
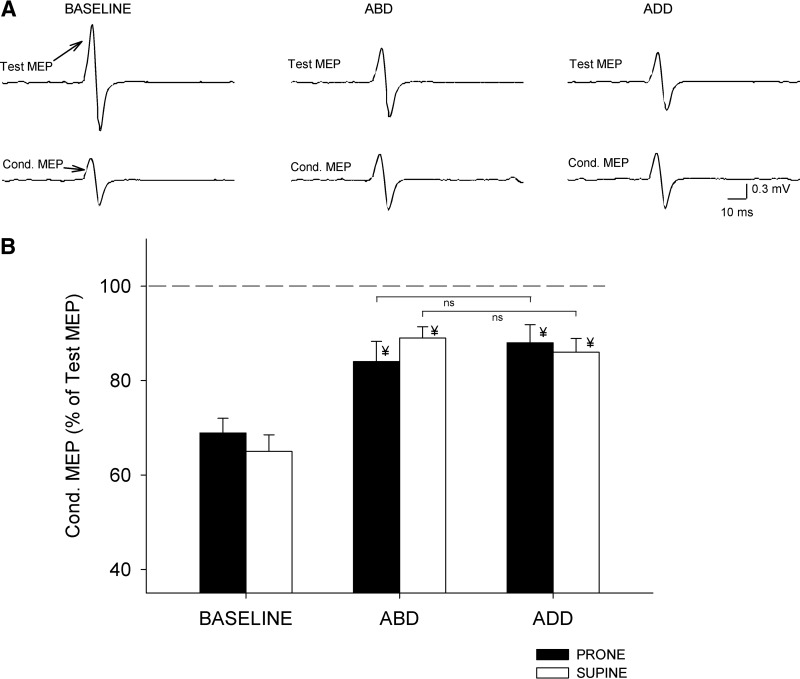
SICI
An illustration of changes in SICI recorded in a single subject during unilateral and bilateral isometric forces is shown in Fig. 4A. A factorial ANOVA showed no effect of direction, posture, nor their interaction (F = 0.8, ns; F = 0.87, ns; F = 0.4, ns; n = 12) on SICI. The magnitude of SICI was similar during right ADD and ABD in prone (ADD = 88 ± 10% and ABD = 84 ± 12%, P = 0.4; Fig. 4B) and supine (ADD = 86 ± 10% and ABD = 89 ± 8%, P = 0.3; Fig. 4B) postures. No differences were observed between postures during each contraction (ABD, P = 0.3; ADD, P = 0.4). Compared with a unilateral contraction SICI was significantly decreased during ABD (P < 0.001) and ADD (P < 0.001) in both hand postures. These results indicate that the magnitude of SICI was not modulated by the direction of the right index finger force in either hand posture. No effects of direction, posture, nor their interaction were observed on mean rectified EMG activity in the left FDI (F = 2.4, P = 0.1; F = 0.6, ns; F = 0.3, ns) and in the force exerted by the left (F = 2.0, P = 0.3; F = 1.4, P = 0.1; F = 0.7, ns) and right FDI (F = 2.2, P = 0.4, F = 0.2, ns; F = 1.5, P = 0.3).
Fig. 4.
Short-interval intracortical inhibition (SICI). A: SICI recorded from the left FDI of a representative subject during 10% of left ABD, whereas the right index finger remained at rest (baseline) or performed 30% of ABD or ADD. The actions by the right index finger are indicated as Baseline, ABD, and ADD. In this example the right hand was positioned in prone posture. Test MEP and conditioned MEP (Cond. MEP) are indicated by arrows. B: group data (n = 12). The abscissa shows all conditions tested during the assessment of SICI in prone (black bars) and supine (white bars) postures. The ordinate indicates the magnitude of the conditioned MEP expressed as a percentage of the Test MEP [(Conditioned MEP × 100)/(Test MEP)] during bilateral activation. The horizontal dashed line represents the size of the Test MEP. Note that the magnitude of SICI was decreased to a similar extent during both bilateral forces in both right hand postures. Error bars indicate SEs. *P < 0.05. Also note that SICI was significantly decreased with respect to the baseline in all conditions tested (¥ indicates significant difference with respect to baseline).
When SICI measurements were completed by maintaining the size of the Test MEP similar in all conditions, SICI [(Conditioned MEP × 100)/(Test MEP)] was similar during ABD (87 ± 6%) and ADD (84 ± 7%, P = 0.4). SICI was significantly decreased during ABD (P < 0.001) and ADD (P < 0.001) compared with a unilateral contraction (64 ± 5%). No differences were observed between SICI measured during both bilateral conditions (P = 0.7). Mean rectified EMG activity in the left FDI and force (F = 1.5, P = 0.4; F = 1.8, P = 0.3) exerted by the left FDI was similar across conditions.
Correlation analysis
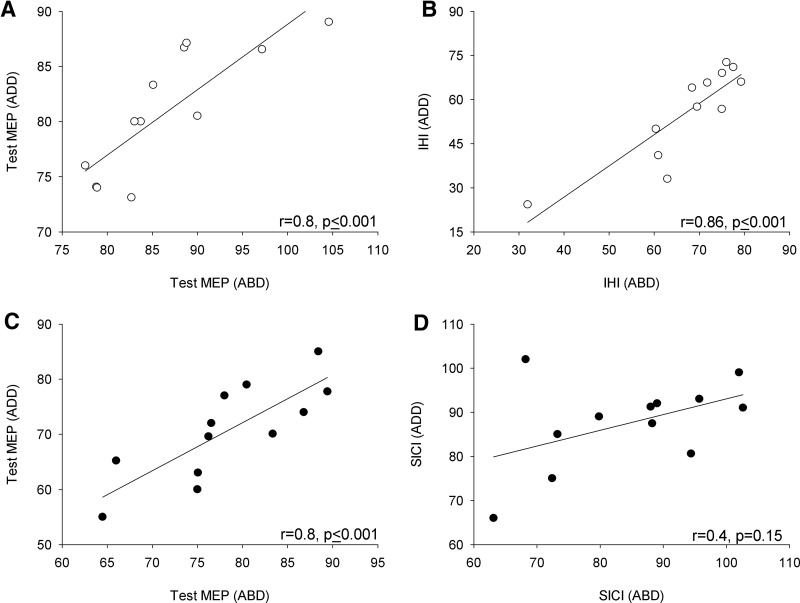
We found a significant correlation between the size of left FDI MEPs during right index finger ABD and ADD in prone (r = 0.9, P < 0.001) and supine (r = 0.91, P < 0.001) postures. This indicated that subjects who had larger left FDI MEPs during right ABD also had larger MEPs during right ADD, regardless of the muscle group engaged in the task. The size of the Test MEP measured during right ABD and ADD during IHI (prone: r = 0.82, P < 0.001; Fig. 5A, supine: r = 0.76, P < 0.01) and SICI (prone: r = 0.8, P < 0.001; Fig. 5C, supine: r = 0.78, P < 0.01) were correlated. A significant correlation was found between IHI during right ABD and ADD in the prone (r = 0.86, P < 0.001; Fig. 5B) and supine (r = 0.81, P < 0.001) postures. Note that the correlation shown in Fig. 5B is also present without including a subject who showed very strong levels of IHI in both conditions (r = 0.8, P < 0.01; n = 11). No correlations were present between SICI measurements (prone: r = 0.4, P = 0.15; Fig. 5D, supine: r = 0.3, P = 0.2). Additionally, the changes in size of MEPs (unilateral force minus bilateral force) and IHI (unilateral force minus bilateral force) were correlated during right ABD (prone: r = 0.78, P < 0.01; supine r = 0.61, P = 0.02) and ADD (prone: r = 0.58, P = 0.03; supine: r = 0.64, P = 0.01).
Fig. 5.
Relationship between IHI and SICI measurements. Graphs A and C show the magnitude of the Test MEP during bilateral isometric forces during assessment of IHI and SICI, respectively. We indicate the actions by the right index finger as ABD (abscissa) and ADD (ordinate). Note that subjects who had larger left FDI Test MEPs during right ABD also had larger Test MEPs during right ADD during IHI and SICI measurements. Graphs B and D show the magnitude of IHI and SICI [(Conditioned MEP × 100)/(Test MEP)], respectively, during bilateral isometric forces. The action completed by the right index finger is indicated as ABD (abscissa) and ADD (ordinate). Note that changes in SICI during both bilateral forces were not associated with the other measurements.
DISCUSSION
In the present study, we examined the effect of bilateral isometric index finger forces in different directions on motor cortical function. Our main findings are 1) left FDI MEPs were suppressed during bilateral compared with unilateral forces, with a stronger suppression during right ADD regardless of the right-hand posture; 2) IHI targeting the left FDI was increased during bilateral compared with unilateral forces and this increase was stronger during right ADD despite the posture of the right hand; and 3) SICI was decreased to a similar extent during both bilateral forces in both hand postures. Our findings indicate that generation of isometric forces away from the body midline down-regulate corticospinal output in the contralateral active hand to a greater extent than forces exerted toward the body midline, regardless of the muscle group engaged in the task. Transcallosal inhibition, but not GABAergic intracortical circuits mediating SICI, was modulated by the direction of the isometric force.
Direction of bilateral isometric forces drives corticospinal output
Our finding of a suppression of MEP size in the left voluntary active FDI muscle during contralateral isometric forces agrees with a previous study demonstrating a decrease in MEP size during bilateral compared with unilateral contraction of wrist flexor muscles (Stinear and Byblow 2004a). We found that the MEP suppression was more prominent when the right index finger force was exerted away from the body midline (ADD direction), even if the right FDI muscle was used as an agonist or antagonist to the task. To our knowledge this is the first demonstration that the direction of the volitional activity is an important factor that drives corticospinal excitability during bilateral isometric forces in intact humans. This finding is in agreement with the view that the M1 is concerned with the generation of actions in terms of an “extrinsic” space related to the hand motion (Duque et al. 2005; Georgopoulos et al. 1982; Kakei et al. 1999; Mechsner et al. 2001; Post et al. 2009). Previous results have also demonstrated that the preference to move both hands in symmetry (toward or away from the body midline) is independent of muscular constraints, suggesting that perceptual cues play an important role in the control of bimanual free joint movements (Mechsner et al. 2001; Müller et al. 2009). In agreement, our results suggest that changes in corticospinal excitability during bilateral isometric forces are independent of muscular constraints and relate to the direction of the volitional activity.
Previous studies have shown that a unimanual isometric voluntary contraction of roughly 30% of force resulted in an increase in MEP size in the contralateral resting hand (Hess et al. 1986; Perez and Cohen 2008; Stedman et al. 1998). Our findings demonstrate that a similar amount of force decreased the MEP size when the contralateral hand is voluntarily active. These results might shed light on how unimanual and bimanual isometric forces are controlled (Carson 2005). A possibility is that an increased inhibitory effect during bilateral forces enables a flexible context-dependent degree of bimanual coupling (Rokni et al. 2003) and might contribute to suppress unwanted muscle activity (Duque and Ivry 2009; Giovannelli et al. 2009).
Mechanisms involved in the control of bilateral forces in different directions
We found that IHI targeting the left voluntary active FDI was increased during bilateral compared with unilateral forces. This result supports the findings by Diedrichsen et al. (2003), suggesting that coupling of bilateral isometric forces of different amplitudes takes place in part through the corpus callosum. Our findings also agree with the results by Giovanelli et al. (2009) that demonstrated an increase in transcallosal inhibition (measured by the ipsilateral silent period) during bilateral compared with unilateral index finger isometric forces, although the same authors did not find changes in IHI measured by the paired-pulse TMS protocol as we demonstrated here. The differences might be partially related to the methodologies used in both studies. Giovanelli et al. (2009) tested IHI during maximal isometric voluntary contractions, which involves different changes at the cortical and spinal cord level compared with a lower level of force (Taylor and Gandevia 2008) as performed in the present study. This can also relate to the fact that the mechanisms mediating these two transcallosal inhibitory effects might differ (Chen et al. 2003; Perez and Cohen 2009).
We found that IHI was stronger when the right force was exerted away from the body midline compared with the other conditions. One possible interpretation is that stronger IHI contributed to the larger suppression in left FDI MEPs. This is supported by a correlation between changes in IHI and in MEP size, suggesting that subjects with stronger left FDI MEP suppression were those who showed stronger IHI. Since IHI can be influenced by changes occurring in both M1s (Daskalakis et al. 2002), we propose that the M1 controlling the index finger activated in opposite directions drives information about force direction to the contralateral hemisphere. A difference in properties of corticomotoneuronal cells related to movements of opposite direction has been described (Cheney and Fetz 1980). Indeed, a previous study showed that more cortical cells are activated with movements directed away from the body than toward the body (Georgopoulos et al. 1982). Interestingly, in our study ADD (activation away from the body midline) resulted in a stronger IHI than ABD (activation toward the body midline). Although care must be taken with this interpretation, since we measured forces only into ABD and ADD, any directional deviation away from pure ABD and ADD might have affected our results.
Even though IHI may be a purely cortical phenomenon (Di Lazzaro et al. 1999; Ferbert et al. 1992; Meyer et al. 1995), its estimation is based on discharge of spinal motoneurons and in the generation of a MEP, which likely show a nonlinear relationship between “excitability” and their level of activity during voluntary contractions (Devanne et al. 1997; Matthews 1999). Therefore the state of the muscle is critical during IHI assessment. In this regard, we noticed a disparity in IHI (measured with and without adjustments) only when the right FDI was acting as a primary mover and the intensity of the conditioning pulse was substantially reduced to match conditions. This observation favors the view that stimulus intensity is a critical factor for IHI estimation during voluntary activity (Perez and Cohen 2008).
Another mechanism that might have contributed to our results is SICI. The activity of intracortical circuits decreases during muscle activation (Ortu et al. 2008; Zoghi and Nordstrom 2007) and less intracortical inhibition in one hemisphere might result from an increase in IHI from the contralateral hemisphere (Kukaswadia et al. 2005; Perez and Cohen 2008). We found less SICI and increments in IHI during both bilateral forces, suggesting that this might be the case, although the disinhibitory effect on SICI was similar during both bilateral tasks. This result indicates that it is unlikely that the effect of IHI on SICI was the only factor contributing to changes in SICI. It is also unlikely that factors such as handedness or the degree of force have affected our results since comparable isometric force tasks exerted similar changes in corticospinal excitability in both hemispheres (Stinear et al. 2001; Stinear et al. 2004a,b). The present experiments cannot address the precise mechanism for the lack of changes in SICI. However, our results indicate that the decrease in SICI observed in the right M1 is not an epiphenomenon of the information from the contralateral M1, supporting the view that both M1s show activity related to the bilateral task (Cardoso de Oliviera et al. 2001; Donchin et al. 1998).
Functional implications
An important question that emerges from our results is why do bilateral forces in different directions modulate M1 function to a different extent? Regarding free joint finger movements, there is a natural preference toward moving both hands in symmetry, either toward or away from the body midline (Swinnen 2002) and this is independent of muscular constraints (Mechsner et al. 2003). We speculate that in our task the presence of less inhibition when both index fingers contracted toward the body midline indicates that a weaker inhibitory effect needs to be overcome to complete the task, which might contribute to perform the task more efficiently (Perez et al. 2007); however, caution must be taken in extrapolating the present results to more dynamic tasks, since movements and isometric forces are controlled by different processes (Ashe 1997). Another important aspect to consider is that in our motor task ABD and ADD forces are related to the body midline of the individual. Therefore it is unclear whether these results will extrapolate to motor outputs with hand-centered anatomical actions.
In summary, we found that the direction of the isometric index finger forces, regardless of the muscle group engaged in the task, is a variable that drives corticospinal excitability during bilateral forces. This information might help in the design of bilateral training strategies aimed at enhancing motor function after injury (van Delden et al. 2009).
GRANTS
This work was funded by the National Institutes of Health Grant R00 NS-06201 to M. A. Perez.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the participants of the study for generous commitment of time.
REFERENCES
- Ashe, 1997. Ashe J. Force and the motor cortex. Behav Brain Res 87: 255–269, 1997 [DOI] [PubMed] [Google Scholar]
- Bäumer et al., 2007. Bäumer T, Dammann E, Bock F, Klöppel S, Siebner HR, Münchau A. Laterality of interhemispheric inhibition depends on handedness. Exp Brain Res 180: 195–203, 2007 [DOI] [PubMed] [Google Scholar]
- Cardoso de Oliveira et al., 2001. Cardoso de Oliveira S, Gribova A, Donchin O, Bergman H, Vaadia E. Neural interactions between motor cortical hemispheres during bimanual and unimanual arm movements. Eur J Neurosci 14: 1881–1896, 2001 [DOI] [PubMed] [Google Scholar]
- Carson, 1995. Carson RG. The dynamics of isometric bimanual coordination. Exp Brain Res 105: 465–476, 1995 [DOI] [PubMed] [Google Scholar]
- Carson, 2005. Carson RG. Neural pathways mediating bilateral interactions between the upper limbs. Brain Res Brain Res Rev 49: 641–662, 2005 [DOI] [PubMed] [Google Scholar]
- Carson et al., 1994. Carson RG, Byblow WD, Goodman D. The dynamical substructure of bimanual coordination. In: Interlimb Coordination: Neural, Dynamical, and Cognitive Constraints, edited by Swinnen S, Heuer H, Massion J, Casaer P. San Diego, CA: Academic Press, 1994, p. 319–337 [Google Scholar]
- Chen et al., 2003. Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol 89: 1256–1264, 2003 [DOI] [PubMed] [Google Scholar]
- Cheney and Fetz, 1980. Cheney PD, Fetz EE. Functional classes of primate corticomotoneuronal cells and their relation to active force. J Neurophysiol 44: 773–791, 1980 [DOI] [PubMed] [Google Scholar]
- Cros et al., 2007. Cros D, Soto O, Chiappa KH. Transcranial magnetic stimulation during voluntary action: directional facilitation of outputs and relationships to force generation. Brain Res 1185: 103–116, 2007 [DOI] [PubMed] [Google Scholar]
- Daskalakis et al., 2002. Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol 543: 317–326, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanne et al., 1997. Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res 114: 329–338, 1997 [DOI] [PubMed] [Google Scholar]
- Diedrichsen et al., 2003. Diedrichsen J, Hazeltine E, Nurss WK, Ivry RB. The role of the corpus callosum in the coupling of bimanual isometric force pulses. J Neurophysiol 90: 2409–2418, 2003 [DOI] [PubMed] [Google Scholar]
- Di Lazzaro et al., 2004. Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol 115: 255–266, 2004 [DOI] [PubMed] [Google Scholar]
- Di Lazzaro et al., 1999. Di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp Brain Res 124: 520–524, 1999 [DOI] [PubMed] [Google Scholar]
- Donchin et al., 1998. Donchin O, Gribova A, Steinberg O, Bergman H, Vaadia E. Primary motor cortex is involved in bimanual coordination. Nature 395: 274–278, 1998 [DOI] [PubMed] [Google Scholar]
- Duque and Ivry, 2009. Duque J, Ivry RB. Role of corticospinal suppression during motor preparation. Cereb Cortex 19: 2013–2024, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque et al., 2005. Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG. Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cereb Cortex 15: 588–593, 2005 [DOI] [PubMed] [Google Scholar]
- Evarts, 1969. Evarts EV. Activity of pyramidal tract neurons during postural fixation. J Neurophysiol 32: 375–385, 1969 [DOI] [PubMed] [Google Scholar]
- Ferbert et al., 1992. Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol 453: 525–546, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher et al., 2002. Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res 143: 240–248, 2002 [DOI] [PubMed] [Google Scholar]
- Gandevia, 2001. Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001 [DOI] [PubMed] [Google Scholar]
- Georgopoulos et al., 1992. Georgopoulos AP, Ashe J, Smyrnis N, Taira M. The motor cortex and the coding of force. Science 256: 1692–1695, 1992 [DOI] [PubMed] [Google Scholar]
- Georgopoulos et al., 1982. Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci 2: 1527–1537, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannelli et al., 2009. Giovannelli F, Borgheresi A, Balestrieri F, Zaccara G, Viggiano MP, Cincotta M, Ziemann U. Modulation of interhemispheric inhibition by volitional motor activity: an ipsilateral silent period study. J Physiol 587: 5393–5410, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess et al., 1986. Hess CW, Mills KR, Murray NM. Magnetic stimulation of the human brain: facilitation of motor responses by voluntary contraction of ipsilateral and contralateral muscles with additional observations on an amputee. Neurosci Lett 71: 235–240, 1986 [DOI] [PubMed] [Google Scholar]
- Kakei et al., 1999. Kakei S, Hoffman DS, Strick PL. Muscle and movement representations in the primary motor cortex. Science 285: 2136–2139, 1999 [DOI] [PubMed] [Google Scholar]
- Kalaska et al., 1989. Kalaska JF, Cohen DA, Hyde ML, Prud'homme M. A comparison of movement direction-related versus load direction-related activity in primate motor cortex, using a two-dimensional reaching task. J Neurosci 9: 2080–2102, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaska and Hyde, 1985. Kalaska JF, Hyde ML. Area 4 and area 5: differences between the load direction-dependent discharge variability of cells during active postural fixation. Exp Brain Res 59: 197–202, 1985 [DOI] [PubMed] [Google Scholar]
- Kelso, 1984. Kelso JA. Phase transitions and critical behavior in human bimanual coordination. Am J Physiol 246: 1000–1004, 1984 [DOI] [PubMed] [Google Scholar]
- Kujirai et al., 1993. Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol 471: 501–519, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukaswadia et al., 2005. Kukaswadia S, Wagle-Shukla A, Morgante F, Gunraj C, Chen R. Interactions between long latency afferent inhibition and interhemispheric inhibitions in the human motor cortex. J Physiol 563: 915–924, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews, 1999. Matthews PB. The effect of firing on the excitability of a model motoneurone and its implications for cortical stimulation. J Physiol 518: 867–882, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechsner et al., 2001. Mechsner F, Kerzel D, Knoblich G, Prinz W. Perceptual basis of bimanual coordination. Nature 414: 69–73, 2001 [DOI] [PubMed] [Google Scholar]
- Meyer et al., 1995. Meyer BU, Roricht S, Grafin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain 118: 429–440, 1995 [DOI] [PubMed] [Google Scholar]
- Müller et al., 2009. Müller K, Kleiser R, Mechsner F, Seitz RJ. Perceptual influence on bimanual coordination: an fMRI study. Eur J Neurosci 30: 116–124, 2009 [DOI] [PubMed] [Google Scholar]
- Netz et al., 1995. Netz J, Ziemann U, Hömberg V. Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res 104: 527–533, 1995 [DOI] [PubMed] [Google Scholar]
- Oldfield, 1971. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971 [DOI] [PubMed] [Google Scholar]
- Ortu et al., 2008. Ortu E, Deriu F, Suppa A, Tolu E, Rothwell JC. Effects of volitional contraction on intracortical inhibition and facilitation in the human motor cortex. J Physiol 586: 5147–5159, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez et al., 2009. Perez MA, Butler JE, Taylor JL. Transcallosal inhibition between proximal arm muscles during isometric voluntary contractions. Soc Neurosci Abstr 663, 2009 [Google Scholar]
- Perez and Cohen, 2008. Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci 28: 5631–5640, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez and Cohen, 2009. Perez MA, Cohen LG. Interhemispheric inhibition between primary motor cortices: what have we learned? J Physiol 587: 725–726, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez et al., 2007. Perez MA, Wise SP, Willingham DT, Cohen LG. Neurophysiological mechanisms involved in transfer of procedural knowledge. J Neurosci 27: 1045–1053, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post et al., 2009. Post M, Bakels R, Zijdewind I. Inadvertent contralateral activity during a sustained unilateral contraction reflects the direction of target movement. J Neurosci 29: 6353–6357, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkenauer et al., 2001. Rinkenauer G, Ulrich R, Wing AM. Brief bimanual force pulses: correlations between the hands in force and time. J Exp Psychol Hum Percept Perform 27: 1485–1497, 2001 [PubMed] [Google Scholar]
- Rokni et al., 2003. Rokni U, Steinberg O, Vaadia E, Sompolinsky H. Cortical representation of bimanual movements. J Neurosci 23: 11577–11586, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini et al., 1994. Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, DimitrijeviĆ MR, Hallett M, Katayama Y, Lücking CH, Maertens de Noordhout AL, Marsden CD, Murray NMF, Rothwell JC, Swash M, Tomberg C. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91: 79–92, 1994 [DOI] [PubMed] [Google Scholar]
- Rothwell et al., 1999. Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl 52: 97–103, 1999 [PubMed] [Google Scholar]
- Sergio and Kalaska, 2003. Sergio LE, Kalaska JF. Systematic changes in motor cortex cell activity with arm posture during directional isometric force generation. J Neurophysiol 89: 212–228, 2003 [DOI] [PubMed] [Google Scholar]
- Stedman et al., 1998. Stedman A, Davey NJ, Ellaway PH. Facilitation of human first dorsal interosseous muscle responses to transcranial magnetic stimulation during voluntary contraction of the contralateral homonymous muscle. Muscle Nerve 21: 1033–1039, 1998 [DOI] [PubMed] [Google Scholar]
- Steglich et al., 1999. Steglich C, Heuer H, Spijkers W, Kleinsorge T. Bimanual coupling during the specification of isometric forces. Exp Brain Res 129: 302–316, 1999 [DOI] [PubMed] [Google Scholar]
- Stinear et al., 2001. Stinear CM, Walker KS, Byblow WD. Symmetric facilitation between motor cortices during contraction of ipsilateral hand muscles. Exp Brain Res 139: 101–105, 2001 [DOI] [PubMed] [Google Scholar]
- Stinear and Byblow, 2004a. Stinear JW, Byblow WD. Modulation of human cervical premotoneurons during bilateral voluntary contraction of upper-limb muscles. Muscle Nerve 29: 506–514, 2004a [DOI] [PubMed] [Google Scholar]
- Stinear and Byblow, 2004b. Stinear JW, Byblow WD. An interhemispheric asymmetry in motor cortex disinhibition during bimanual movement. Brain Res 1022: 81–87, 2004b [DOI] [PubMed] [Google Scholar]
- Swinnen, 2002. Swinnen SP. Intermanual coordination: from behavioural principles to neural-network interactions. Nat Rev Neurosci 3: 348–359, 2002 [DOI] [PubMed] [Google Scholar]
- Taira et al., 1996. Taira M, Boline J, Smyrnis N, Georgopoulos AP, Ashe J. On the relations between single cell activity in the motor cortex and the direction and magnitude of three-dimensional static isometric force. Exp Brain Res 109: 367–376, 1996 [DOI] [PubMed] [Google Scholar]
- Taylor and Gandevia, 2008. Taylor JL, Gandevia SC. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J Appl Physiol 104: 542–550, 2008 [DOI] [PubMed] [Google Scholar]
- van Delden et al., 2009. van Delden AL, Peper CL, Harlaar J, Daffertshofer A, Zijp NI, Nienhuys K, Koppe P, Kwakkel G, Beek PJ. Comparing unilateral and bilateral upper limb training: the ULTRA-stroke program design. BMC Neurol 9: 57, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghi and Nordstrom, 2007. Zoghi M, Nordstrom MA. Progressive suppression of intracortical inhibition during graded isometric contraction of a hand muscle is not influenced by hand preference. Exp Brain Res 177: 266–274, 2007 [DOI] [PubMed] [Google Scholar]