Abstract
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterized by pain and hypersensitivity in the relative absence of colon inflammation or structural changes. To assess the role of P2X receptors expressed in colorectal dorsal root ganglion (c-DRG) neurons and colon hypersensitivity, we studied excitability and purinergic signaling of retrogradely labeled mouse thoracolumbar (TL) and lumbosacral (LS) c-DRG neurons after intracolonic treatment with saline or zymosan (which reproduces 2 major features of IBS—persistent colorectal hypersensitivity without inflammation) using patch-clamp, immunohistochemical, and RT-PCR techniques. Although whole cell capacitances did not differ between LS and TL c-DRG neurons and were not changed after zymosan treatment, membrane excitability was increased in LS and TL c-DRG neurons from zymosan-treated mice. Purinergic agonist adenosine-5′-triphosphate (ATP) and α,β-methylene ATP [α,β-meATP] produced inward currents in TL c-DRG neurons were predominantly P2X3-like fast (∼70% of responsive neurons); P2X2/3-like slow currents were more common in LS c-DRG neurons (∼35% of responsive neurons). Transient currents were not produced by either agonist in c-DRG neurons from P2X3−/− mice. Neither total whole cell Kv current density nor the sustained or transient Kv components was changed in c-DRG neurons after zymosan treatment. The number of cells expressing P2X3 protein and its mRNA and the kinetic properties of ATP- and α,β-meATP-evoked currents in c-DRG neurons were not changed by zymosan treatment. However, the EC50 of α,β-meATP for the fast current decreased significantly in TL c-DRG neurons. These findings suggest that colorectal hypersensitivity produced by intracolonic zymosan increases excitability and enhances purinergic signaling in c-DRG neurons.
INTRODUCTION
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterized by abdominal pain, hypersensitivity, and changes in bowel activity in the relative absence of inflammation or other structural changes (Camilleri 2005; Gershon and Tack. 2007; Spiller 2007). Despite continuing investigation, the pathogenic mechanisms of IBS remain unclear (Drossman et al. 1997).
Several endogenous mediators have been implicated in gastrointestinal hypersensitivity in general and IBS in particular, including serotonin (Gershon and Tack. 2007; Mawe et al. 2006), proteases (Cenac et al. 2007; Coelho et al. 2002; Kirkup et al. 2003), and adenosine-5′-triphosphate (ATP). ATP has been of long-term interest as a signaling molecule in the gut (Burnstock 2001, 2008). ATP is released from a wide variety of cells (North 2002), including colonic epithelium in response to colorectal distension (Shinoda et al. 2009; Wynn et al. 2003). ATP and its metabolites can interact with a large number of ligand-gated ion channels (P2X receptors) and G-protein-coupled receptors of the P2Y family. P2X receptors are expressed on sensory neuron cell bodies in dorsal root ganglia (DRG) (Burnstock 2006), including visceral sensory DRG neurons (Dang et al. 2008; Wynn et al. 2003). ATP also contributes to colorectal mechanotransduction by activating pelvic nerve afferents (Wynn et al. 2003), a role that is enhanced after colon inflammation (Wynn et al. 2004). It also has been reported that P2X receptor antagonists reverse visceral hypersensitivity, further supporting the contribution of ATP to visceral sensory mechanisms (Xu et al. 2008). Finally, ATP is implicated in human colon pathology by increased expression of P2X3 receptors in inflammatory bowel disease (Yiangou et al. 2001).
We previously documented that colorectal mechanotransduction and zymosan-produced colon hypersensitivity, which develops and persists in the absence of a neutrophil-based colorectal inflammation, are attenuated and do not develop, respectively, in P2X3−/− mice (Shinoda et al. 2009). These outcomes further support a role for purinergic signaling in colorectal hypersensitivity. In the present study, we examined purinergic responses and P2X receptor expression in colorectal DRG (c-DRG) neurons after intracolonic saline or zymosan treatment (replicating the visceral hypersensitivity in IBS patients), hypothesizing that the robust colon hypersensitivity produced would be associated with increased cell excitability and enhanced purinergic sensitivity.
METHODS
Animals
Adult male C57BL/6 (Taconic, Germantown, NY) and congenic P2X3 knockout (backcrossed onto the C57BL/6 genetic background for ≥10 generations) mice (20–30 g) were used in these experiments. All procedures were approved by the Institutional Animal Care and Use Committee, University of Pittsburgh.
Cell labeling
Mice were anesthetized (2% isoflurane; Hospira, Lake Forest, IL), the colon exposed surgically (lower abdominal incision, ∼1 cm in length), and 50 mg/ml (in 100% DMSO) of 1,1′-dioctadecyl-3,3,3′,3-tetramethylindocarbocyanine methanesulfonate (DiI; Molecular Probes, Eugene, OR) was injected into one to three sites within the descending colon wall (∼1.5 cm from the anal verge) using a 30-gauge needle (∼6 μl per site). Visible leakage of DiI was removed with a cotton swab. The incision was closed (5–0 polyglactin sutures; Ethicon, Somerville, NJ), and mice were subsequently returned to their home cages until use 14–21 days later.
Intracolonic treatments
Mice were anesthetized with ketamine/xylazine (87.5/12.5 mg/kg ip) and 0.1 ml of saline or of a suspension of 30 mg/ml zymosan (derived from Saccharomyces cerevisiae; Sigma, St. Louis, MO) in saline was administered transanally via a 22-gauge, 24-mm-long stainless-steel feeding needle. Saline or zymosan was given daily for three consecutive days; lumbosacral (LS) and thoracolumbar (TL) DRG were harvested on day 4. This treatment protocol results in a robust and long-lasting colon hypersensitivity to balloon distension (Shinoda et al. 2009). We checked each mouse postmortem for leakage of DiI by examining the colon and surrounding tissues after removal of DRG. No labeling in any tissue other than the colon was noted.
Cell dissociation and culture
The mouse colon is innervated by pelvic and lumbar splanchnic nerves with cell bodies contained in LS and TL DRG, respectively. Because there are significant differences between the LS and TL innervations of pelvic organs (Brierley et al. 2005; Dang et al. 2008; Xu and Gebhart. 2008), c-DRG neurons from both innervations were studied. Mice were killed via CO2 inhalation and T11–-L1 or L6–S2 DRG removed rapidly and incubated at 37°C in 5% CO2 for 40 min in serum free, advanced Dulbecco's modified eagle medium (DMEM)/F12 containing 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA), collagenase (type 4; 2 mg/ml; Worthington Biochemical, Lakewood, NJ), and trypsin (1 mg/ml; Worthington). After washing three times with advanced DMEM/F12 containing 10% fetal bovine serum (Sigma; without enzymes), DRG were submerged in DMEM containing 10% fetal bovine serum, 1% penicillin/streptomycin, 625 μM l-glucose, 4.5 g/l d-glucose, 110 mg/l sodium pyruvate and triturated gently to encourage cell dissociation. Neurons were plated on poly-d-lysine-coated coverslips (Becton Dickinson Labware, Bedford, MA) and incubated at 37°C in 5% CO2. Only c-DRG neurons (i.e., DiI-containing DRG neurons) were studied. All recordings were performed within 24 h after plating.
Solutions and electrophysiological recordings
Coverslips with cells were transferred to a recording chamber (1 ml) and superfused continuously (2 ml/min) with external solution containing (in mM): 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose. The pH was adjusted to 7.4 with NaOH (305 mosM). Neurons that innervated the colon were identified by DiI content using a rhodamine filter. Fire-polished micropipettes with tip resistances of 2–4 MΩ were used for current- and voltage-clamp recordings. The uncompensated series resistance was generally <10 MΩ. The pipette was filled with an internal solution consisting of (in mM): 130 KCl, 0.2 CaCl2, 4 NaCl, 10 EGTA, 10 HEPES, 0.5 Na triphosphate, and 2 MgATP. The pH was adjusted to 7.25 using KOH (290 mosM). After establishing the whole cell configuration, the voltage was clamped at −70 mV using an Axopatch 200B amplifier, digitized at 1 kHz (Digidata 1350), and controlled by Clampex software (pClamp 10.0; all from Axon Instruments, Foster City, CA). Cell capacitance and resting membrane potential (RMP) were obtained from the Axopatch 200B amplifier. Recordings began 3 min after establishing whole cell configuration to ensure stable recording conditions. Only cells that had a RMP more negative than −40 mV and generated action potentials with a distinct overshoot >0 mV in response to depolarizing current injections were studied. Spontaneous activity was counted during a 1 min period before electrical or chemical stimulation. A series of 5-ms current pulses in 50-pA increments (1 s apart) was injected to determine action potential (AP) threshold, which was taken as the greatest membrane potential in the absence of an AP; the minimum current required to evoke an AP was considered rheobase. To examine firing patterns in c-DRG neurons, suprathreshold current (2 × rheobase) was injected for 500 ms and the number of APs counted. Drugs were applied using a fast-step SF-77B superfusion system (Warner Instruments, Hamden, CT) with a three-barrel pipette placed in close proximity (100 μm) to the cell, allowing complete solution exchange within 25 ms (Dang et al. 2005a). Purinergic agonists [ATP and α,β-methylene ATP (α,β-meATP); Sigma] were applied for 2 s, whereas antagonists were superfused for 30 s before the application of agonists. A washout period of 4 min was allowed between agonist applications. Drugs and chemicals were prepared fresh from stock solutions on the day of the experiment; experiments were performed at room temperature (21–23°C). We used current increases >20 pA or voltage changes >4 mV to identify responses to purinergic agonists because these levels exceeded baseline variability by a factor of two. To determine the kinetics of response onset, we measured the time from 10 to 80% of peak amplitude.
To isolate voltage-gated K+ (Kv) currents and minimize contamination by Na+ and Ca2+, NaCl was replaced with an equimolar amount of choline chloride, and CdCl2 (50 μM) was included in the external solution (pH was adjusted to 7.4 with Tris-base). Cells were held at −70 mV and subjected to either a −100 or −30 mV prepulse potential for 400 ms before outward currents were elicited by stepping the membrane potential from −60 to 40 mV for 400 ms in 10 mV increments. Inactivating Kv currents were isolated by subtracting the outward currents after −30 mV prepulse from those after −100 mV. The −30 mV prepulse for evoking the noninactivating currents was chosen based on the steady-state inactivation/availability curve of K+ currents in a pilot study. Series resistance was compensated by >80%, and leak currents were subtracted using the on-line P/4 protocol. Data were sampled at 10 kHz and low-pass filtered at 5 kHz.
Immunohistochemistry
Mice were anesthetized with ketamine/xylazine as in the preceding text and transcardially perfused with saline followed by a cold fixative containing 4% paraformaldehyde in 0.2% picric acid and 0.1 M phosphate buffer (pH 7.4). T11–L1 or L6–S2 DRG were removed rapidly and immersed in the same fixative for 4 h at 4°C. Postfixed DRG were cryoprotected for 12 h in 0.1 M phosphate buffered saline (PBS) containing 20% sucrose, then embedded in Tissue-Tek (Sakura Finetechnical, Tokyo, Japan) at −20°C and cut on a cryostat (10 μm). DRG sections were reacted with rabbit anti-P2X3 polyclonal antiserum (1:1000; Neuromics, Northfield, MN) overnight at 4°C. After rinsing with 0.01 M PBS, all samples were reacted with AlexaFluor488-conjugated goat anti-rabbit IgG (Invitrogen) at a dilution of 1:200 in 0.01 M PBS for 2 h at room temperature. After rinsing with 0.01 M PBS, sections were coverslipped in mounting medium (Dako, Carpinteria, CA) and examined under a fluorescence microscope. Using appropriate filters, double (DiI and AlexaFluor488)-labeled cells were identified and analyzed using ImageJ software (National Institutes of Health). Cells twofold or more intense than average background were considered positive for P2X3 immunoreactivity. No specific labeling was observed in the absence of primary antibody or in DRG from P2X3−/− mice. The percentage of P2X3 immunoreactive, DiI-labeled c-DRG neurons was calculated based on neuron counts in five randomly chosen sections separated by 100 μm to avoid double counting of cells. Histological analysis was performed blind with respect to treatment to colon.
Single-cell reverse transcription polymerase chain reaction (RT-PCR)
DiI-labeled c-DRG neurons (dissociated and cultured as in the preceding text) were collected with large-bore (∼50 μm) glass pipettes and expelled into microcentrifuge tubes containing reverse-transcriptase (RT) mix (Nealen et al. 2003). Cell lysis and RT reactions consisted of incubation at 65°C for 1.5 min, at room temperature for 2 min, at 37°C for 20 min after administrating 20 U SuperScript II (Invitrogen), and at 65°C for 10 min. For each experiment, negative controls were performed by omitting reverse transcriptase or using a cell-free bath aspirate as template. The first-strand complementary deoxyribonucleic acid (cDNA) from a c-DRG neuron was used as template in a polymerase chain reaction (PCR) containing 1xGoTaq reaction buffer (Promega, Madison, WI), 10 μM outer primers, 0.2 mM dNTPs, and 0.2 μl GoTaq DNA polymerase (Promega); primer sequences are listed in Table 1A. Reactions consisted of initialization at 95°C for 10 min, 35 cycles at 94°C/30 s, 52°C/30 s and 72°C/30 s before a final extension step at 72°C for 10 min. Each initial PCR product served as template in a subsequent PCR reaction using a nested primer pair. Each PCR product in a subsequent PCR reaction was electrophoresed on 2% agarose–ethidium bromide gels and photographed. Only neurons producing detectable amplification of a housekeeping gene (GAPDH) were analyzed further.
Table 1.
P2X3, P2X2, or both P2X3 and P2X2 mRNA expressed colon senory neurons
|
A. DNA primers used for PCR amplification | |||
|---|---|---|---|
| Target Gene/Predicted Size | Outer Primers | Nested Primers | Genbank No. |
| P2X3 | GCTCCGTAGAAGAAGATGGAGA | TGTCCTAAGAGGATCCTGTACC | NM_145526 |
| 251 bp, 141 bp | CTGTGTGACCATGTTAGGGATG | GGCATCTAGCACATAGAAGTGG | |
| P2X2 | CTCTTCAGTAACCATGTCCACG | GAAGATAGGCATCTTGCTCTGG | NM_153400 |
| 233 bp, 113bp | CCGGAAGACAGCTCTAATTTGG | GGGATCCTATGAGGAGTTCTGT | |
|
B. Colon sensory neurons expressing P2X3, P2X2 or both P2X3 and P2X2 mRNA | ||||
|---|---|---|---|---|
| P2X3 Only, % | Both P2X3 and P2X2, % | P2X2 Only, % | Neither P2X3 or P2X2, % | |
| Saline | ||||
| LS | 33/59; 55.9 | 20/59; 33.9 | 3/59; 5.1 | 3/59; 5.1 |
| TL | 31/54; 57.4 | 20/54; 37.0 | 3/54; 5.6 | 3/54; 5.6 |
| Zymosan | ||||
| LS | 25/58; 43.1 | 29/58; 50.0 | 2/58; 3.4 | 2/58; 3.4 |
| TL | 33/55; 60.0 | 18/55; 32.7 | 1/55; 1.8 | 3/55; 5.5 |
Sequence of P2X3, and P2X2 mRNA are indicated with 5′ at let. a, adenosine; c, cytosine; g, guanosine; t, thymidine; Outer primers were used for the first round of PCR; nested primers were used for the second round. Sequences used for primer design are indicated at right. bp, base pairs.
Colon sensory neurons expressing P2X3, P2X2 or both P2X3 and P2X2 mRNA are shown. The ratios of mRNA expression are calculated per total number of GAPDH mRNA-positive neurons in each group. LS, lumbosacral; TL, thoracolumbar.
Data analysis
Data are presented as means ± SE. The software packages pClamp 10.0 (Axon Instruments) and Prism 4 (Graph Pad, San Diego, CA) were used for data analysis. Dose–response curves were fit to sigmoidal functions using the following equation: Y = A/[1 + 10(logEC50 – X)B], where X is the logarithm of agonist concentration and Y is the response and starts at 0 and ends at A with a sigmoidal shape. B is the Hill slope. Desensitization kinetics were fitted with a standard exponential equation: Y = K0 + K1 × exp(−t/τ), where Y is the current amplitude at time t, K0 is the amplitude of the sustained component, and τ is the time constant. K0 and K1 represent the contribution to current amplitude from the fast and slow components of the current, respectively. For dichotomous variables, χ2 or unconditional exact tests were used. Statistical analyses were performed using Student's t-test and one- or two-way (repeated-measures) ANOVA where appropriate. Results were considered statistically significant when P ≤ 0.05.
RESULTS
Cell size
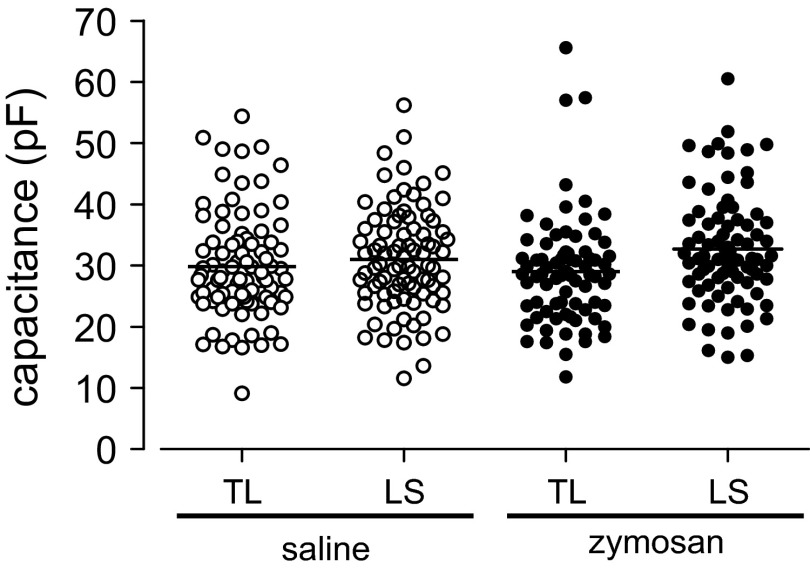
In previous studies, rat LS and TL colon (Gold and Traub 2004) and urinary bladder (Dang et al. 2005a; Yoshimura and De Groat. 1999) neurons were reported to differ in size and to increase after organ inflammation (Dang et al. 2008). Mean whole cell capacitances (as an index of cell size) did not significantly differ between mouse LS (33.5 ± 1.3 pF) and TL (32.96 ± 1.7 pF) c-DRG neurons and were not changed after zymosan treatment (Fig. 1).
Fig. 1.
Distribution of whole cell capacitance in thoracolumbar (TL) and lumbosacral (LS) colorectal dorsal root ganglion (c-DRG) neurons from saline- and zymosan-treated mice. c-DRG neurons from TL and LS DRG and from saline- and zymosan-treated mice exhibited the same distribution of capacitances.
c-DRG neuron excitability
ACTIVE AND PASSIVE MEMBRANE PROPERTIES.
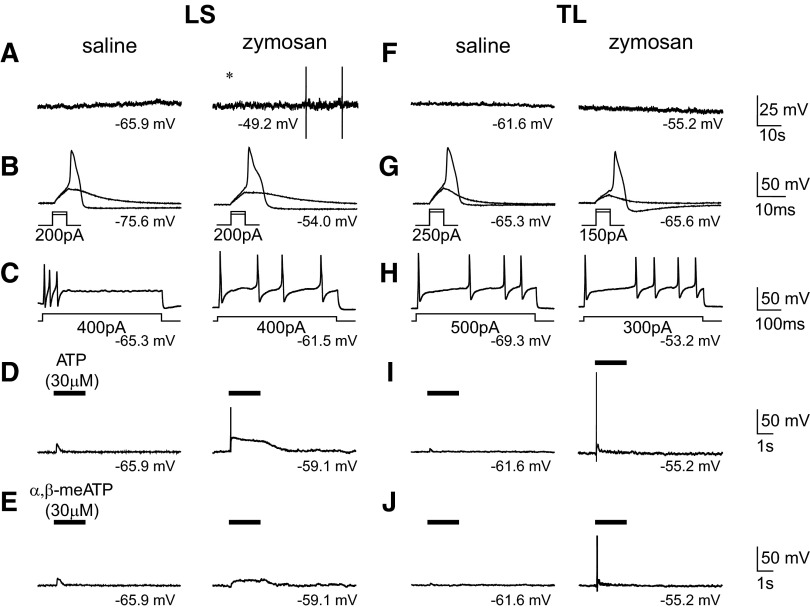
Zymosan treatment was associated with a significantly depolarized RMP and enhanced membrane excitability in both LS and TL c-DRG neurons (Fig. 2). No c-DRG neurons from saline-treated mice exhibited spontaneous activity (LS: 0/35; TL: 0/37), but a significant proportion of LS neurons (6/37, 16%; unconditional exact test, P < 0.05) from zymosan-treated mice were spontaneously active (Table 2). Zymosan treatment did not affect action potential (AP) threshold, rheobase, number of APs evoked by suprathreshold current injection (2 X rheobase) or input resistance.
Fig. 2.
Passive and active properties of c-DRG neurons from saline- and zymosan-treated mice examined in current-clamp mode. Examples illustrate that neither LS (A, left) nor TL (F, left) neurons were spontaneously active in control mice, but ∼1/6 of LS neurons from zymosan-treated mice exhibited spontaneous activity (A, right). The excitability of LS and TL neurons as well as the number of TL neurons in which action potentials were produced by ATP (e.g., I, right; see also Table 1) was increased by zymosan treatment. Resting membrane potentials are indicated under each trace.
Table 2.
Passive and active electrical properties of LS and TL colon neurons from zymosan-treated and control (saline-treated) mice
| Property | Treatment | TL | LS | |
|---|---|---|---|---|
| A. Current injection | ||||
| Whole cell capacitance, pF | Control | 33.0 ± 1.7 (37) | 33.5 ± 1.3 (35) | |
| Zymosan | 28.9 ± 1.2 (25) | 34.8 ± 1.3 (37) | ||
| Series resistance, mohm | Control | 6.0 ± 0.3 (37) | 6.5 ± 0.3 (35) | |
| Zymosan | 6.3 ± 0.5 (25) | 6.3 ± 0.3 (37) | ||
| Resting membrane potential, mV | Control | *−63.5 ± 1.4 (37) | −64.3 ± 1.4 (35)* | |
| Zymosan | *−58.4 ± 1.9 (25) | −57.9 ± 1.6 (37)* | ||
| Spontaneous activity (frequency) | Control | 0/37 | 0/35* | |
| Zymosan | 1/25 | 6/37* | ||
| AP threshold, mV | Control | −27.6 ± 1.2 (31) | −28.9 ± 1.2 (24) | |
| Zymosan | −29.8 ± 1.3 (16) | −29.4 ± 1.4 (26) | ||
| Rheobase, pA | Control | 247.3 ± 13.0 (37) | 227.1 ± 21.7 (24) | |
| Zymosan | 237.5 ± 23.5 (16) | 211.5 ± 16.5 (26) | ||
| AP@2× rheobase (frequency) | Control | 2.8 ± 0.8 (31) | 4.4 ± 0.9 (23) | |
| Zymosan | 4.8 ± 1.4 (16) | 5.4 ± 1.1 (26) | ||
| Input resistance, mohm | Control | −32.4 ± 3.6 (32) | −40.1 ± 3.3 (26) | |
| Zymosan | −28.8 ± 3.8 (16) | −35.9 ± 3.7 (26) | ||
| B. ATP (30 μM) | ||||
| Number of cells which induce AP, % | Control | *2.9 (1/34) | 5.7 (2/35) | |
| Zymosan | *20 (4/20) | 16.8 (5/30) | ||
| Number of cells depolarized, % | Control | 50 (17/34) | 52.6 (20/38) | |
| Zymosan | 75 (15/20) | 67.7 (21/31) | ||
| Depolazation, mV | Control | 16.8 ± 2.7 (17) | 13.9 ± 1.5 (20) | |
| Zymosan | 16.9 ± 1.7 (15) | 17.1 ± 1.8 (21) | ||
| C. α,β-meATP (30 μM) | ||||
| Number of cells which induce AP, % | Control | 0 (0/32) | 0 (0/31) | |
| Zymosan | 10 (2/20) | —* | 0 (0/25) | |
| Number of cells depolarized, % | Control | 50 (16/32) | 25.8 (8/31)* | |
| Zymosan | 70 (14/20) | —* | 60 (15/25)* | |
| Depolazation, mV | Control | 15.7 ± 2.0 (16) | 9.5 ± 1.5 (8) | |
| Zymosan | 17.2 ± 1.7 (15) | 11.3 ± 1.5 (15) | ||
Values are means ± SE, or percentages. Number of neurons are in parentheses.
P < 0.05. AP, action potential, ATP, adenosine-5′-triphosphate; α,β-meATP, α,β-m ethylene ATP.
Effect of purinergic agonists
In current clamp mode, ATP (30 μM) depolarized one-half of LS (20/38, 53%) and TL (17/34, 50%) c-DRG neurons from saline-treated mice, triggering APs in 6% of LS and 3% of TL neurons (Fig. 2, D and I; Table 2). Zymosan treatment did not change the magnitude of depolarization produced by ATP in either LS (21/31; 68%) or TL (15/20; 75%) c-DRG neurons compared with control (Table 2). Zymosan treatment did, however, significantly increase the proportion of TL c-DRG neurons in which APs were triggered by ATP (4/20, 20% vs. 1/34, 3% in control; unconditional exact test, P < 0.05).
α,β-meATP, a selective homomeric P2X3 and heteromeric P2X2/3 receptor agonist (North 2002), was applied to the same neurons previously challenged with ATP. The proportion of TL c-DRG neurons from saline-treated mice depolarized by α,β-meATP (30 μM) was significantly greater than the proportion of LS neurons (LS: 8/31, 26%. TL: 16/32, 50%. χ2 test, P < 0.05); α,β-meATP did not trigger APs in either LS or TL c-DRG neurons (Fig. 2, E and J, Table 2). In zymosan-treated mice, the proportion of LS c-DRG neurons depolarized by α,β-meATP was significantly increased (15/25, 60% vs. 8/31, 26% in control; χ2 test, P < 0.01). α,β-meATP (30 μM) also produced a greater depolarization in TL c-DRG neurons from control mice compared with LS counterparts (TL: 15.7 ± 2.0 mV; LS: 9.5 ± 1.5 mV. Student's t-test, P < 0.05; Table 2). While the fraction of responsive cells increased, the depolarization produced by α,β-meATP did not change after zymosan treatment in either TL or LS c-DRG neurons.
Whole cell Kv currents
Several currents may contribute to changes in neuron excitability (e.g., inward rectifying potassium currents, voltage-sensitive sodium or calcium currents). Accordingly, we examined whether Kv channels also were involved in the zymosan-produced increase in membrane excitability of c-DRG neurons. Total whole cell Kv current density was not changed in either TL or LS c-DRG neurons after zymosan treatment, and neither was the sustained nor transient Kv component (−30 mV prepulse; Fig. 3).
Fig. 3.
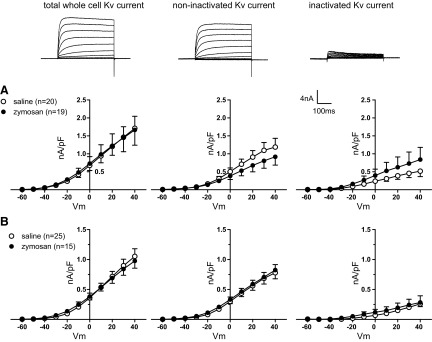
Whole cell Kv current in TL (A) and LS (B) colonic DRG neurons. Total whole cell Kv current (left), noninactivating (middle), and inactivating (right) Kv current by −30 mV prepulse were not changed after zymosan treatment.
Characterization of P2X receptor-mediated currents in c-DRG neurons
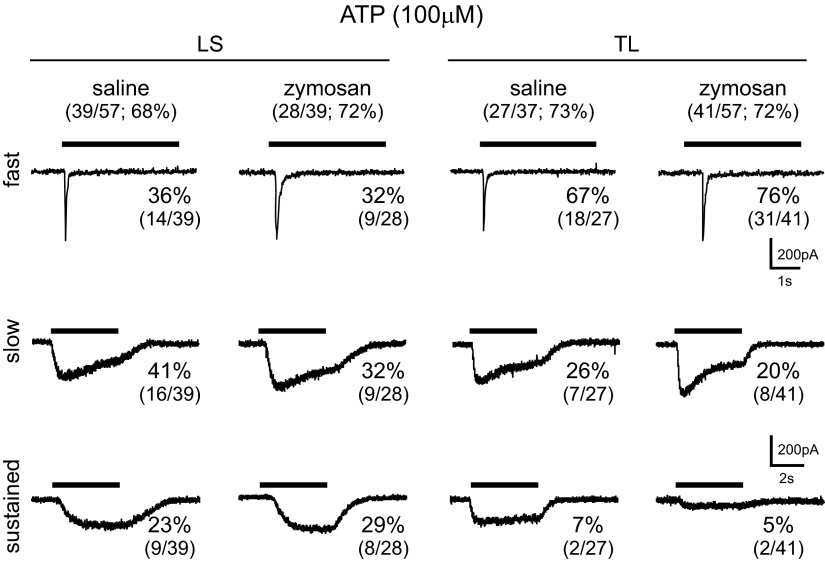
LS c-DRG NEURONS.
To evaluate functionally purinergic receptors on c-DRG neurons, we applied ATP (100 μM), which triggered inward currents in most LS neurons tested (saline-treated, 39/57, 68%; zymosan-treated, 28/39, 72%). Based on the kinetics of current decay, we differentiated fast, slow, and sustained currents in neurons from saline- and zymosan-treated mice (Fig. 4). In saline-treated mice, 14/39 (36%), 16/39 (41%), and 9/39 (23%) LS c-DRG neurons expressed fast, slow, and sustained current kinetics, respectively. Similar proportions of LS c-DRG neurons from zymosan-treated mice, 9/28 (32%), 9/28 (32%), and 8/28 (32%) expressed fast, slow, and sustained current kinetics, respectively. The 10–80% rise time and desensitizing time constants of fast and slow current kinetics generated by ATP did not differ between saline- and zymosan-treated mice in LS c-DRG neurons (Table 3).
Fig. 4.
ATP-activated inward currents in both LS and TL c-DRG neurons were characterized based on desensitization kinetics as fast, slow, and sustained.
Table 3.
Current kinetics for LS and TL colon sensory neurons from saline- and zymosan-treated mice
| ATP (100 μM) |
α,β-meATP (100 μM) |
||||||
|---|---|---|---|---|---|---|---|
| Current | Treatment | 10–80% Rise Time, ms | tau. ms | Current Density, pA/pF | 10–80% Rise Time, ms | tau. ms | Current Density, pA/pF |
| LS | |||||||
| Fast | Saline | 36.4 ± 6.5 | 96.8 ± 15.4 | 15.5 ± 3.1 | 27.3 ± 6.2 | 94.8 ± 20.6 | 21.9 ± 3.0 |
| Zymosan | 49.1 ± 9.9 | 127.7 ± 9.5 | 13.8 ± 3.2 | 30.1 ± 4.4 | 77.2 ± 10.5 | 22.0 ± 3.3 | |
| Slow | Saline | 224.1 ± 29.4 | N/A | 18.7 ± 4.6 | 258.5 ± 11.0 | N/A | 16.3 ± 3.9 |
| Zymosan | 281.1 ± 36.3 | N/A | 20.1 ± 4.7 | 264.9 ± 22.8 | N/A | 17.3 ± 3.6 | |
| TL | |||||||
| Fast | Saline | 53.5 ± 11.0 | 100.1 ± 15.7 | 17.3 ± 2.6 | 44.6 ± 7.2 | 84.1 ± 8.9 | 20.5 ± 2.9 |
| Zymosan | 68.8 ± 9.4 | 110.1 ± 9.7 | 17.4 ± 3.3 | 34.9 ± 4.6 | 83.4 ± 6.6 | 24.0 ± 2.0 | |
| Slow | Saline | 220.6 ± 31.1 | N/A | 25.0 ± 4.0 | 358.0 ± 54.0 | N/A | 13.7 ± 8.6 |
| Zymosan | 224.6 ± 25.6 | N/A | 33.4 ± 10.4 | 349.1 ± 26.0 | N/A | 16.2 ± 3.4 | |
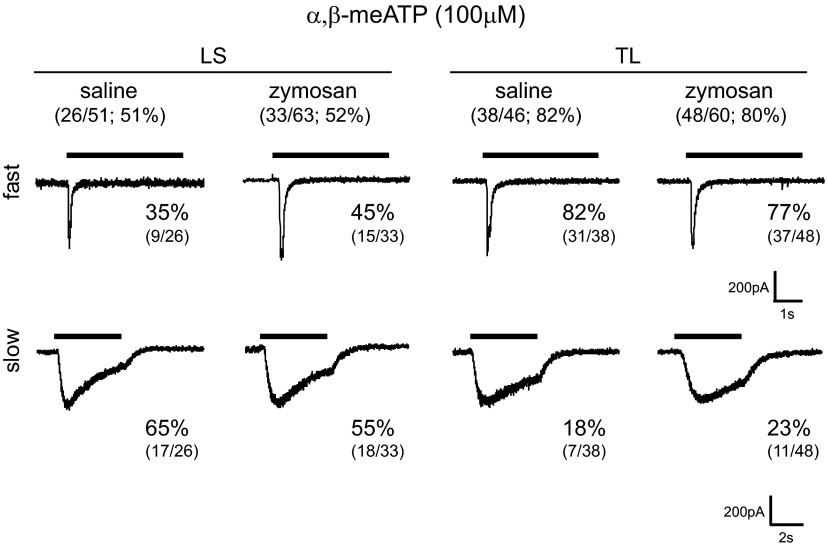
We also examined the effects of α,β-meATP (100 μM), which produced inward currents in LS c-DRG neurons (saline-treated, 26/51, 51%; zymosan-treated, 33/63, 52%) (Fig. 5). Based on the current kinetics described in the preceding text, we identified fast (9/26, 36%) and slow (17/26, 65%) currents in LS c-DRG neurons from saline-treated mice and similar proportions of fast (15/33, 45%) and slow (18/33, 55%) currents in LS c-DRG neurons from zymosan-treated mice. The activation and desensitization kinetics were similar to those obtained in response to ATP and did not differ between neurons from saline- and zymosan-treated mice (Table 3).
Fig. 5.
α,β-Methylene adenosine-5′-triphosphate (α,β-meATP)-activated inward currents in both LS and TL c-DRG neurons were characterized based on desensitization kinetics as fast and slow. In both LS and TL c-DRG neurons, α,β-meATP produced currents with kinetics similar to that triggered by ATP.
TL c-DRG NEURONS.
Similar to LS neurons, ATP (100 μM) produced inward currents in most TL c-DRG neurons tested (saline-treated, 27/37, 73%; zymosan-treated, 41/57, 72%). In saline-treated mice, 18/27 (67%), 7/27 (26%), and 2/27 (7%) TL c-DRG neurons expressed fast, slow, and sustained current kinetics, respectively. The relative proportion of these kinetic properties did not differ in neurons from zymosan-treated mice [fast (31/41, 76%), slow (8/41, 20%), and sustained (2/41, 5%) currents; Fig. 4]. Similar to LS c-DRG neurons, the 10–80% rise time and desensitizing time constants of fast and slow current kinetics generated by ATP did not differ between saline- and zymosan-treated mice in TL neurons (Table 3).
A high proportion of TL c-DRG neurons tested (saline-treated, 38/46, 82%; zymosan-treated, 48/60, 80%) responded to α,β-meATP (Fig. 5). Like ATP, α,β-meATP triggered similar proportions of fast and slow currents in TL neurons from saline-treated (fast, 31/38, 82%; slow, 7/38, 18%) and zymosan-treated (fast, 37/48, 77%; slow, 11/48, 23%) mice. As described for LS c-DRG neurons, the activation and desensitization kinetics were similar to those obtained in response to ATP, 10–80% rise time, and desensitizing time of fast and slow current kinetics generated by α,β-meATP did not differ between saline- and zymosan-treated mice in TL neurons (Table 3).
Effects of P2X3 deletion and purinergic antagonists on P2X-dependent currents
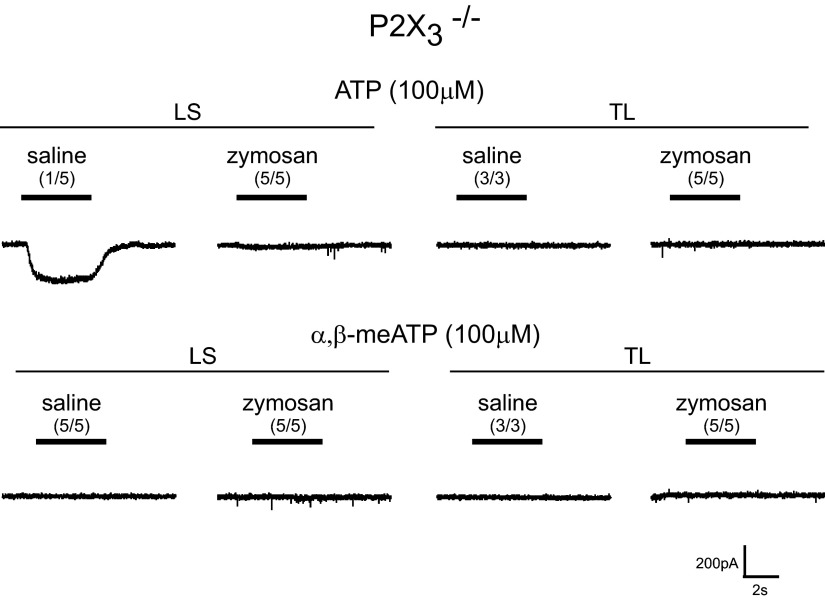
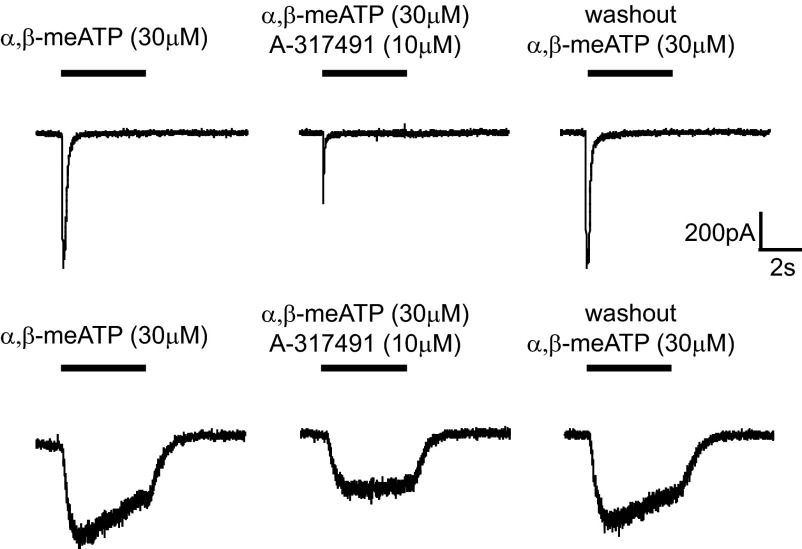
To establish that the fast current triggered by ATP and α,β-meATP is dependent on P2X3 receptors, we examined c-DRG neurons from P2X3−/− mice. The genetic status of the mice was established by polymerase chain reaction for the P2X3 gene. In LS and TL c-DRG neurons from P2X3−/− mice, a transient current was not produced either by ATP or α,β-meATP (Fig. 6). However, we did observe a sustained current in response to ATP in 1/5 neurons (3 mice were used for harvesting cells; 1 cell from 1 mouse, 2 cells each from 2 mice). To further examine P2X-dependent currents, we applied the potent and selective homomeric P2X3 and heteromeric P2X2/3 receptor antagonist A-317491 (Jarvis et al. 2002) to c-DRG neurons from saline-treated mice. In the presence of A-317491 (10 μM), both ATP- and α,β-meATP-mediated fast and slow currents were reversibly attenuated (Fig. 7).
Fig. 6.
ATP- and α,β-meATP-activated fast and slow inward currents are absent in P2X3−/− mice; a sustained current, however, was present.
Fig. 7.
Antagonism of purinergic-activated fast and slow inward currents by co-application of the P2X3, 2/3 receptor antagonist, A-317491.
P2X receptor dose–response relationship
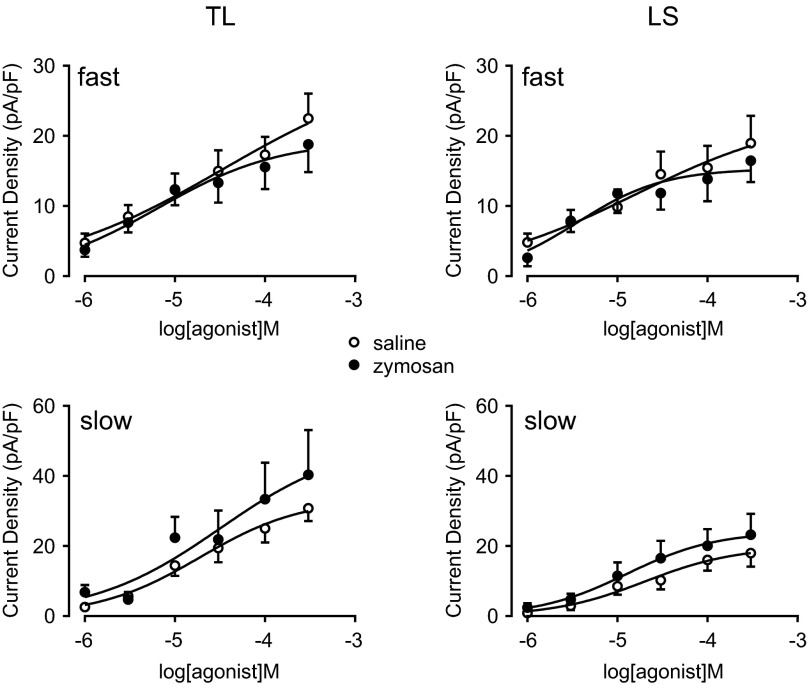
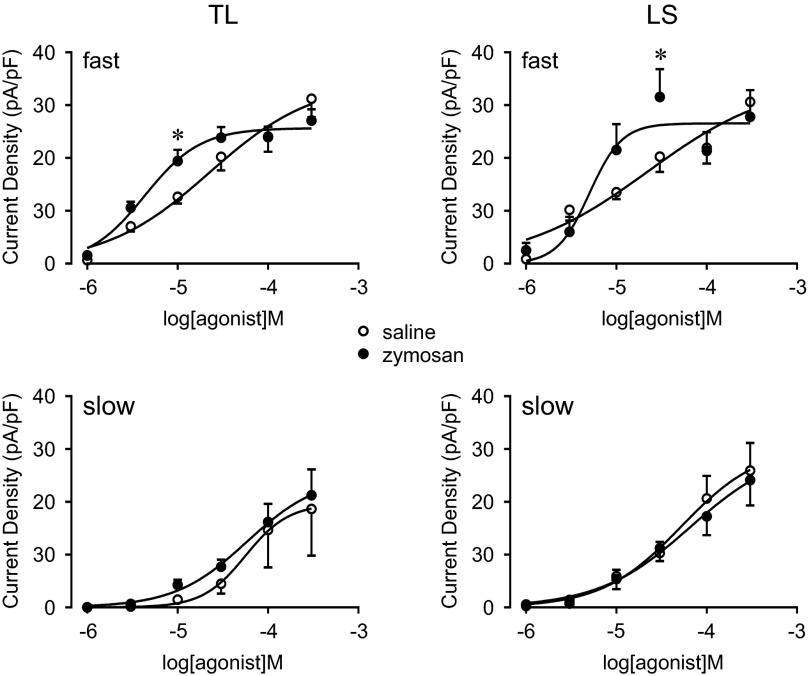
We next determined the dose-response relationship for both agonists by applying either ATP or α,β-meATP in increasing concentrations. In TL and LS c-DRG neurons, both ATP- and α,β-meATP-activated currents were concentration-dependent for both fast and slow currents (Figs. 8 and 9). The dose-response curves of fast but not slow currents induced by α,β-meATP shifted to the left in c-DRG neurons from zymosan-treated mice. The logEC50 for α,β-meATP to induce the fast current in LS c-DRG neurons from zymosan-treated mice (−5.30 ± 0.19) showed a tendency toward a decrease compared with that from saline-treated mice (−4.65 ± 0.45; Student's t-test, P = 0.18). When analyzed using two way ANOVA followed by the Holm-Sidak multiple comparison test, a significant increase in the fast current density was detected at 30 μM α,β-meATP in LS c-DRG neurons from zymosan-treated mice (P < 0.05). For fast currents in TL c-DRG neurons, logEC50 values for α,β-meATP (zymosan-treated; −5.37 ± 0.09. saline-treated; −4.65 ± 0.30. Student's t-test, P < 0.05) were significantly lower after zymosan treatment compared with saline treatment, and the current density at 10 μM α,β-meATP was significantly increased in TL c-DRG neurons from zymosan-treated mice (P < 0.05, 2 way ANOVA followed by the Holm-Sidak multiple comparison test).
Fig. 8.
Concentration–response relationship for ATP. Current responses were generated for kinetically distinct currents. Zymosan treatment did not affect the peak current of either the fast or slow component in TL or LS c-DRG neurons.
Fig. 9.
Concentration–response relationship for α,β-meATP. Current responses were generated for kinetically distinct currents. In TL c-DRG neurons, zymosan treatment significantly lowered the EC50 and increased the peak current of the fast component induced by 10 μM α,β-meATP. *P < 0.05.
P2X immunohistochemistry and mRNA expression in c-DRG neurons
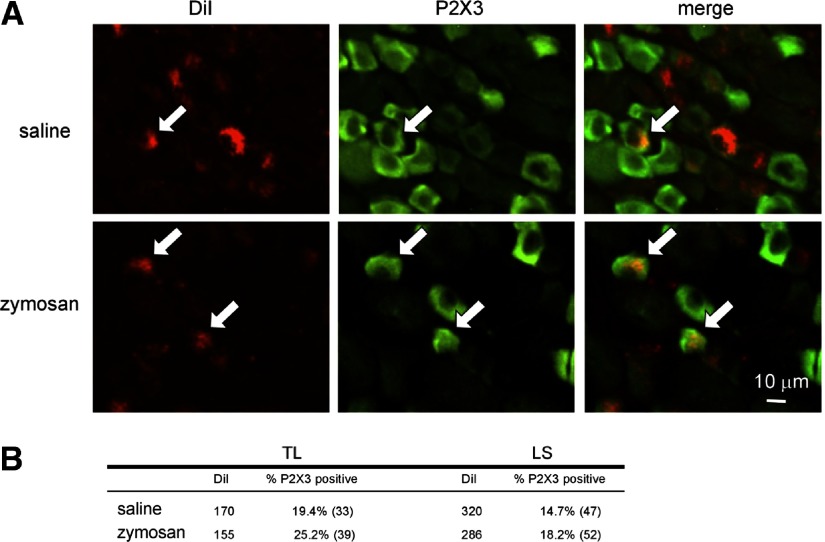
DiI-positive c-DRG neurons co-labeled for P2X3 were present in both TL and LS DRG (Fig. 10). There was no significant difference in P2X3 immunoreactivity between LS (47/320; 15%) and TL (33/170; 19%) c-DRG neurons and zymosan treatment did not alter these proportions (LS: 52/286, 18%; TL: 39/155; 25%).
Fig. 10.
A: P2X3 immunoreactivity of neurons innervating the mouse colon (identified by content of the retrograde tracer, 1,1′-dioctadecyl-3,3,3′,3-tetramethylindocarbocyanine methanesulfonate). B: the number of P2X3 receptor-positive c-DRG neurons was unchanged by zymosan treatment.
To evaluate expression of P2X2 and P2X3 in LS and TL c-DRG neurons, we performed single-cell RT-PCR on DiI-labeled c-DRG neurons (Table 1A). Table 1B shows the percentage of c-DRG neurons expressing only P2X3, only P2X2 and both P2X3 and P2X2 transcripts. Transcripts for P2X2 and/or P2X3 were present in nearly all colon neurons examined with about half of the cells only containing P2X3 transcripts. While purinergic agonist triggered fast currents more frequently in TL than LS c-DRG neurons, the percentage of cells only expressing P2X3 did not differ between these two populations under control conditions and was not changed by zymosan treatment.
DISCUSSION
These findings add to a growing literature demonstrating the importance of peripheral sensitization and P2X receptors in visceral hypersensitivity. The novel aspect of the present report relates to the IBS-like colorectal insult produced by intracolonic instillation of zymosan, which is characterized by long-lasting colorectal hypersensitivity in the absence of colorectal inflammation assessed either by neutrophilic infiltration (myeloperoxidase assay) or histologically (Jones et al. 2005; Shinoda et al. 2009). Significantly, spontaneous action potential discharges of c-DRG neurons were increased as has been reported after significant insult and inflammation of visceral organs (Bielefeldt et al. 2002; Dang et al. 2004, 2005b; Yoshimura and De Groat. 1999). This enhanced excitability has been attributed to decreased transient outward potassium currents (Dang et al. 2004; Ibeakanma et al. 2009) and increases in voltage-gated sodium channel 1.8 protein expression (King et al. 2009) and its current density (Beyak et al. 2004; Bielefeldt et al. 2002), lowered input resistance, AP threshold (Moore et al. 2002), and rheobase (Ibeakanma et al. 2009) in DRG neurons innervating visceral organs.
In the present study, consistent with the absence of significant colonic insult, we did not see changes in neuron size or potassium currents that have previously been described in animal models of visceral hypersensitivity associated with inflammation (Dang et al. 2004; Yoshimura and De Groat. 1999). We found that zymosan had no effect on voltage-gated K+ currents, suggesting that voltage-gated K+ currents do not play a role in the mechanism(s) underlying zymosan-produced neuron excitability. Because of the present focus on purinergic receptors, we did not specifically investigate other currents that may contribute to changes in excitability, such as inward rectifying potassium currents or voltage-sensitive sodium or calcium currents. Zymosan treatment significantly increased the number of TL c-DRG neurons firing APs in response to ATP application. Because ATP-induced depolarization was not significantly changed by zymosan, the increase is likely the result of a decrease in RMP rather than a change in ATP current responses.
Recent studies have documented the importance of purinergic signaling in visceral sensory physiology. Colon distension increases ATP release into the colon lumen, which is further increased after induction of experimental colitis (Wynn et al. 2004). P2X3−/− mice have blunted basal responses to colorectal distension and do not develop hypersensitivity after zymosan treatment (Shinoda et al. 2009). The data presented here suggest that enhanced purinergic signaling contributes to the hypersensitivity, which has not previously been studied in c-DRG neurons.
Purinergic currents in c-DRG neurons
Using teased fiber recordings, we previously demonstrated significant differences between LS and TL colon sensory pathways (Brierley et al. 2004). Consistent with anatomic and functionally distinct roles, we document here differences in responses to purinergic agonists; most TL c-DRG neurons exhibited fast currents, whereas LS c-DRG neurons had similar proportions of fast and slow purinergic responses. The P2X purinergic identity of the currents was further confirmed by the absence of the fast current in c-DRG neurons from P2X3−/− mice and also by reversible inhibition of ATP- and α,β-meATP-mediated fast and slow currents by the P2X3-P2X2/3 antagonist A-317491. This suggests that the slow current is primarily due to activation of heteromeric P2X2/3 receptors, whereas the fast current is mediated by homomeric P2X3 receptors.
The single-cell PCR results for P2X3 and P2X2 transcripts revealed a greater frequency of expression in c-DRG neurons than percentages of neurons giving fast and slow responses to purinergic agonists, not surprisingly revealing greater sensitivity of the former. DiI-positive c-DRG neurons co-labeled for P2X3 were present in both TL and LS DRG. The proportions of co-labeled c-DRG neurons were less than reported by others (Brierley et al. 2005; Robinson et al. 2004). The discrepancy may be due to use of a different retrograde tracer (e.g., fast blue) or, more likely, due to the limited number of DiI injections made into the colon wall in the present experiments. In contrast, immunohisto-chemical evaluation of P2X3 in c-DRG neurons revealed less sensitivity than the functional whole cell results. The present electrophysiological results (purinergic responses in about 2/3 of the cells) are in line with prior publications (Chen and Gebhart. 2009; Dang et al. 2005a) suggesting that differences in the sensitivities of RNA-based or immunohistochemical assays are responsible for these discrepancies.
Purinergic responses and colorectal hypersensitivity
We noted a significant shift in the concentration-response relationship for the fast purinergic current; this may suggest a potential mechanism to explain why P2X3−/− mice do not develop zymosan-induced colon hypersensitivity (Shinoda et al. 2009). Although we did not detect any changes in kinetic properties of ATP (100 μM)- and α,β-meATP (100 μM)-evoked currents in TL or LS c-DRG neurons or the proportions or current density of P2X3-like fast current and slow P2X2/3-like currents after zymosan treatment, the EC50 for the α,β-meATP-produced fast current in TL c-DRG neurons was significantly lower. These findings differ from those produced by frank organ inflammation (e.g., cystitis) (Dang et al. 2008), which affect current kinetics and the relative contribution of fast and slow purinergic currents. Importantly, changes in excitability and purinergic responses were seen in TL and LS neurons innervating the colon, suggesting that both pelvic and splanchnic pathways participate in visceral hypersensitivity.
Although the mechanism(s) for the leftward shift of P2X3-agonists concentration-response curves in c-DRG neurons after zymosan treatment is unclear, it is noteworthy that P2X3 function can be upregulated by calcitonin gene-related peptide (CGRP) and nerve growth factor (NGF), both of which are known to participate in visceral pain in IBS models (Barreau et al. 2008; Bourdu et al. 2005). CGRP stimulates trafficking of P2X3 receptors from intracellular stores to the cell membrane via protein-kinase-A- and protein-kinase-C-dependent mechanisms (Fabbretti et al. 2006), and NGF rescues P2X3 from an inhibitory control of conductance by C-terminal Src inhibitory kinase-dependent phosphorylation (D'Arco et al. 2009). It would be of interest to study whether these mechanisms underlie the increased P2X3-mediated responses in c-DRG neurons after zymosan treatment.
In summary, mild colonic insult enhanced membrane excitability and shifted the dose-response relationship to P2X3 agonists in TL c-DRG neurons, thereby contributing to the development of colon hypersensitivity. Responses to purinergic agonists differed between TL and LS c-DRG neurons, providing further evidence that these afferent pathways are indeed physiologically distinct. The properties of both TL and LS c-DRG neurons changed after zymosan treatment, arguing against a selective role of splanchnic pathways in the development of hypersensitivity during and after visceral insult. While we cannot exclude contribution of central P2X3 receptors as previously reported (Shinoda et al. 2009), the present results suggest that purinergic signaling contributes to peripheral sensitization. (Table 4)
Table 4.
EC50 of ATP and α,β-meATP-produced currents in LS and TL colon neurons fron saline- and zymosan-treated mice
| ATP, μM |
α,β-meATP, μM |
||
|---|---|---|---|
| Current | Treatment | logEC50 | logEC50 |
| LS | |||
| Fast | Saline | −4.80 ± 1.31 (53) | −4.65 ± 0.45 (41)]P=0.18 |
| Zymosan | −5.45 ± 0.28 (36) | −5.30 ± 0.19 (42) | |
| Slow | Saline | −4.72 ± 0.32 (43) | −4.29 ± 0.42 (54) |
| Zymosan | −4.50 ± 1.26 (39) | −4.19 ± 0.62 (55) | |
| TL | |||
| Fast | Saline | −4.47 ± 1.51 (95) | −4.65 ± 0.30 (119)]* |
| Zymosan | −5.19 ± 0.50 (90) | −5.37 ± 0.09 (153) | |
| Slow | Saline | −4.72 ± 0.32 (30) | −4.25 ± 0.34 (34) |
| Zymosan | −4.50 ± 1.26 (32) | −4.23 ± 0.42 (50) |
Number of data points for a does-response curve construction are in parentheses.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-19912. P2X3 knockout mice were graciously provided by Dr. Debra A. Cockayne, Neurobiology Unit, Roche Bioscience, Palo Alto, CA.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank M. Gold and L. Fan for assistance with single cell RT-PCR and M. Burcham for preparation of the graphics.
REFERENCES
- Barreau et al., 2008. Barreau F, Salvador-Cartier C, Houdeau E, Bueno L, Fioramonti J. Long-term alterations of colonic nerve-mast cell interactions induced by neonatal maternal deprivation in rats. Gut 57: 582–590, 2008 [DOI] [PubMed] [Google Scholar]
- Beyak et al., 2004. Beyak MJ, Ramji N, Krol KM, Kawaja MD, Vanner SJ. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol 287: G845–G855, 2004 [DOI] [PubMed] [Google Scholar]
- Bielefeldt et al., 2002. Bielefeldt K, Ozaki N, Gebhart GF. Experimental ulcers alter voltage-sensitive sodium currents in rat gastric sensory neurons. Gastroenterology 122: 394–405, 2002 [DOI] [PubMed] [Google Scholar]
- Bourdu et al., 2005. Bourdu S, Dapoigny M, Chapuy E, Artigue F, Vasson MP, Dechelotte P, Bommelaer G, Eschalier A, Ardid D. Rectal instillation of butyrate provides a novel clinically relevant model of noninflammatory colonic hypersensitivity in rats. Gastroenterology 128: 1996–2008, 2005 [DOI] [PubMed] [Google Scholar]
- Brierley et al., 2005. Brierley SM, Carter R, Jones W, 3rd, Xu L, Robinson DR, Hicks GA, Gebhart GF, Blackshaw LA. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J Physiol 567: 267–281, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley et al., 2004. Brierley SM, Jones RC, 3rd, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127: 166–178, 2004 [DOI] [PubMed] [Google Scholar]
- Burnstock, 2001. Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci 22: 182–188, 2001 [DOI] [PubMed] [Google Scholar]
- Burnstock, 2006. Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol Ther 110: 433–454, 2006 [DOI] [PubMed] [Google Scholar]
- Burnstock, 2008. Burnstock G. The journey to establish purinergic signalling in the gut. Neurogastroenterol Motil 20 Suppl 1: 8–19, 2008 [DOI] [PubMed] [Google Scholar]
- Camilleri, 2005. Camilleri M. Mechanisms in IBS: something old, something new, something borrowed. Neurogastroenterol Motil 17: 311–316, 2005 [DOI] [PubMed] [Google Scholar]
- Cenac et al., 2007. Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, ndrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, Sharkey KA, Ferraz JG, Shaffer E, Vergnolle N. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest 117: 636–647, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen and Gebhart, 2009. Chen X, Gebhart GF. Differential purinergic signaling in bladder sensory neurons of naïve and bladder inflamed mice. Pain 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho et al., 2002. Coelho AM, Vergnolle N, Guiard B, Fioramonti J, Bueno L. Proteinases and proteinase-activated receptor 2: a possible role to promote visceral hyperalgesia in rats. Gastroenterology 122: 1035–1047, 2002 [DOI] [PubMed] [Google Scholar]
- D'Arco et al., 2009. D'Arco M, Giniatullin R, Leone V, Carloni P, Birsa N, Nair A, Nistri A, Fabbretti E. The C-terminal Src inhibitory kinase (Csk)-mediated tyrosine phosphorylation is a novel molecular mechanism to limit P2X3 receptor function in mouse sensory neurons. J Biol Chem 284: 21393–21401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang et al., 2004. Dang K, Bielefeldt K, Gebhart GF. Gastric ulcers reduce A-type potassium currents in rat gastric sensory ganglion neurons. Am J Physiol Gastrointest Liver Physiol 286: G573–G579, 2004 [DOI] [PubMed] [Google Scholar]
- Dang et al., 2005a. Dang K, Bielefeldt K, Gebhart GF. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci 25: 3973–3984, 2005a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang et al., 2005b. Dang K, Bielfeldt K, Lamb K, Gebhart GF. Gastric ulcers evoke hyperexcitability and enhance P2X receptor function in rat gastric sensory neurons. J Neurophysiol 93: 3112–3119, 2005b [DOI] [PubMed] [Google Scholar]
- Dang et al., 2008. Dang K, Lamb K, Cohen M, Bielefeldt K, Gebhart GF. Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons. J Neurophysiol 99: 49–59, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossman et al., 1997. Drossman DA, Whitehead WE, Camilleri M. Irritable bowel syndrome: a technical review for practice guideline development. Gastroenterology 112: 2120–2137, 1997 [DOI] [PubMed] [Google Scholar]
- Fabbretti et al., 2006. Fabbretti E, D'Arco M, Fabbro A, Simonetti M, Nistri A, Giniatullin R. Delayed upregulation of ATP P2X3 receptors of trigeminal sensory neurons by calcitonin gene-related peptide. J Neurosci 26: 6163–6171, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon and Tack, 2007. Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132: 397–414, 2007 [DOI] [PubMed] [Google Scholar]
- Gold and Traub, 2004. Gold MS, Traub RJ. Cutaneous and colonic rat DRG neurons differ with respect to both baseline and PGE2-induced changes in passive and active electrophysiological properties. J Neurophysiol 91: 2524–2531, 2004 [DOI] [PubMed] [Google Scholar]
- Ibeakanma et al., 2009. Ibeakanma C, Miranda-Morales M, Richards M, Bautista-Cruz F, Martin N, Hurlbut D, Vanner S. Citrobacter rodentium colitis evokes post-infectious hyperexcitability of mouse nociceptive colonic dorsal root ganglion neurons. J Physiol 587: 3505–3521, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis et al., 2002. Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, Van BT, Cartmell J, Bianchi B, Niforatos W, Kage K, Yu H, Mikusa J, Wismer CT, Zhu CZ, Chu K, Lee CH, Stewart AO, Polakowski J, Cox BF, Kowaluk E, Williams M, Sullivan J, Faltynek C. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci USA 99: 17179–17184, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones et al., 2005. Jones RC, 3rd, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci 25: 10981–10989, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King et al., 2009. King DE, Macleod RJ, Vanner SJ. Trinitrobenzenesulphonic acid colitis alters Na 1.8 channel expression in mouse dorsal root ganglia neurons. Neurogastroenterol Motil 21: 880–e64, 2009 [DOI] [PubMed] [Google Scholar]
- Kirkup et al., 2003. Kirkup AJ, Jiang W, Bunnett NW, Grundy D. Stimulation of proteinase-activated receptor 2 excites jejunal afferent nerves in anesthetized rats. J Physiol 552: 589–601, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawe et al., 2006. Mawe GM, Coates MD, Moses PL. Review article: intestinal serotonin signalling in irritable bowel syndrome. Aliment Pharmacol Ther 23: 1067–1076, 2006 [DOI] [PubMed] [Google Scholar]
- Moore et al., 2002. Moore BA, Stewart TM, Hill C, Vanner SJ. TNBS ileitis evokes hyperexcitability and changes in ionic membrane properties of nociceptive DRG neurons. Am J Physiol Gastrointest Liver Physiol 282: G1045–G1051, 2002 [DOI] [PubMed] [Google Scholar]
- Nealen et al., 2003. Nealen ML, Gold MS, Thut PD, Caterina MJ. TRPM8 mRNA is expressed in a subset of cold-responsive trigeminal neurons from rat. J Neurophysiol 90: 515–520, 2003 [DOI] [PubMed] [Google Scholar]
- North, 2002. North RA. Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002 [DOI] [PubMed] [Google Scholar]
- Robinson et al., 2004. Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil 16: 113–124, 2004 [DOI] [PubMed] [Google Scholar]
- Shinoda et al., 2009. Shinoda M, Feng B, Gebhart GF. Peripheral and central P2X receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology 137: 2096–2104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller, 2007. Spiller R. Clinical update: irritable bowel syndrome. Lancet 369: 1586–1588, 2007 [DOI] [PubMed] [Google Scholar]
- Wynn et al., 2004. Wynn G, Ma B, Ruan HZ, Burnstock G. Purinergic component of mechanosensory transduction is increased in a rat model of colitis. Am J Physiol Gastrointest Liver Physiol 287: G647–G657, 2004 [DOI] [PubMed] [Google Scholar]
- Wynn et al., 2003. Wynn G, Rong W, Xiang Z, Burnstock G. Purinergic mechanisms contribute to mechanosensory transduction in the rat colorectum. Gastroenterology 125: 1398–1409, 2003 [DOI] [PubMed] [Google Scholar]
- Xu et al., 2008. Xu GY, Shenoy M, Winston JH, Mittal S, Pasricha PJ. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut 57: 1230–1237, 2008 [DOI] [PubMed] [Google Scholar]
- Xu and Gebhart, 2008. Xu L, Gebhart GF. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. J Neurophysiol 99: 244–253, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiangou et al., 2001. Yiangou Y, Facer P, Baecker PA, Ford AP, Knowles CH, Chan CL, Williams NS, Anand P. ATP-gated ion channel P2X(3) is increased in human inflammatory bowel disease. Neurogastroenterol Motil 13: 365–369, 2001 [DOI] [PubMed] [Google Scholar]
- Yoshimura and De Groat, 1999. Yoshimura N, De Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci 19: 4644–4653, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]