Abstract
Astrocytic inwardly rectifying K+ currents (IKIR) have an important role in extracellular K+ homeostasis, which influences neuronal excitability, and serum extravasation has been linked to impaired KIR-mediated K+ buffering and chronic hyperexcitability. Head injury induces acute impairment in astroglial membrane IKIR and impaired K+ buffering in the rat hippocampus, but chronic spontaneous seizures appear in the perilesional neocortex—not the hippocampus—in the early weeks to months after injury. Thus we examined astrocytic KIR channel pathophysiology in both neocortex and hippocampus after rostral parasaggital fluid percussion injury (rpFPI). rpFPI induced greater acute serum extravasation and metabolic impairment in the perilesional neocortex than in the underlying hippocampus, and in situ whole cell recordings showed a greater acute loss of astrocytic IKIR in neocortex than hippocampus. IKIR loss persisted through 1 mo after injury only in the neocortical epileptic focus, but fully recovered in the hippocampus that did not generate chronic seizures. Neocortical cell-attached recordings showed no loss or an increase of IKIR in astrocytic somata. Confocal imaging showed depletion of KIR4.1 immunoreactivity especially in processes—not somata—of neocortical astrocytes, whereas hippocampal astrocytes appeared normal. In naïve animals, intracortical infusion of serum, devoid of coagulation-mediating thrombin activity, reproduces the effects of rpFPI both in vivo and at the cellular level. In vivo serum infusion induces partial seizures similar to those induced by rpFPI, whereas bath-applied serum, but not dialyzed albumin, rapidly silenced astrocytic KIR membrane currents in whole cell and cell-attached patch-clamp recordings in situ. Thus both acute impairment in astrocytic IKIR and chronic spontaneous seizures typical of rpFPI are reproduced by serum extravasation, whereas the chronic impairment in astroglial IKIR is specific to the neocortex that develops the epileptic focus.
INTRODUCTION
The mechanistic etiology of human post-traumatic epilepsy remains elusive. Although seizures can result from neuronal or network abnormalities, they can also be precipitated by impaired homeostasis of the extracellular space. Normal neuronal activity results in extrusion of neurotransmitters and K+, whose decreased extracellular clearance depolarizes neuronal membranes and collapses inhibitory electrochemical gradients, producing a potent endogenous proepileptic stimulus (D'Ambrosio 2004; Delgado-Escueta et al. 1999).
Astrocytes play a major role in the homeostasis of the extracellular space, and it has been hypothesized (Pollen and Trachtenberg 1970) that injury-induced astroglial reaction involves changes in membrane properties that impair K+ homeostasis and cause post-traumatic epilepsy (PTE). Astrocytic homeostatic functions rely on proper membrane resistance, potential (Vm), electrochemical gradients, and K+ permeability to drive the clearance of neurotransmitters by electrogenic transporters (Brew and Attwell 1987), and K+ by spatial buffering or uptake (Orkand et al. 1966). Astrocytic KIR channels are ideally suited for Vm regulation and passive control of extracellular K+ because 1) their large K+ conductance clamps Vm close to EK, 2) their inward rectification facilitates influx of K+ while offering higher resistance to its efflux (Hagiwara and Takahashi 1974; Karwoski et al. 1989; Newman et al. 1984), 3) their pore conductance increases with increasing [K+]o (Hille 2001), and 4) their buffering activity in situ complements that of active pumps (D'Ambrosio et al. 2002). Several lines of evidence support this notion. Pharmacological blockade of glial KIR channels results in impaired extracellular K+ regulation and in neuronal hypersynchrony (Ballanyi et al. 1987; D'Ambrosio et al. 1998, 2002; Kofuji and Newman 2004). Astrocyte-specific inactivation of the Tsc1 gene results in epilepsy (Uhlmann et al. 2002) and loss of astrocytic KIR current (IKIR) (Jansen et al. 2005) in the mouse. KIR gene knockout results in impaired K+ buffering and dysregulation of neuronal activity in the retina (Kofuji et al. 2000) and brain stem (Neusch et al. 2006) and stress-induced seizures with impaired buffering of both glutamate and K+ in the hippocampus (Djukic et al. 2007). RNA interference of KIR gene expression in culture diminishes astrocytic uptake of both glutamate and potassium (Kucheryavykh et al. 2008), and glial KIR channel genes have been linked to seizure susceptibility in humans (Buono et al. 2004) and mice (Ferraro et al. 2004). Therefore pathologies of astrocytic KIR channels may play an important role in human epileptogenesis. Indeed, MRI confirms the coexistence of epileptic foci and foci of reactive astroglia in humans (Messori et al. 2005), and decreased astrocytic IKIR and impaired extracellular K+ homeostasis have been well documented in human surgical tissue (Bordey and Sontheimer 1998; Bordey and Spencer 2004; Hinterkeuser et al. 2000; Jauch et al. 2002; Schröder et al. 2000).
In particular, a role for astrocytic K+ buffering in PTE was indicated by the decrease in astroglial IKIR and impaired K+ regulation observed acutely after fluid percussion injury in the rat (D'Ambrosio et al. 1999), a model that is mechanically identical to human contusive closed head injury and shares with it all pathological features that have been studied (Thompson et al. 2005), including intraparenchymal and subdural hemorrhage, which are risk factors for human PTE (Temkin 2003). Recently, serum extravasation was linked to impaired KIR-mediated K+ buffering and PTE. In vivo exposure of naïve neocortex to blood serum or bovine serum albumin, and blood–brain barrier (BBB) failure, were both shown to induce chronic hyperexcitability, impaired buffering of K+, and a decrease in astrocytic KIR4.1 mRNA (Cacheaux et al. 2009; Ivens et al. 2007; Seiffert et al. 2004).
Fluid percussion injury (FPI) induces widespread serum extravasation (Hoshino et al. 1996; Tanno et al. 1992), acute impairment in astroglial IKIR and K+ buffering in the hippocampus (D'Ambrosio et al. 1999), and chronic spontaneous recurrent partial seizures (CRSPSs) of both neocortical and limbic origin (D'Ambrosio et al. 2004, 2005, 2009), suggesting a role for serum induced astrocytic pathophysiology in FPI-induced PTE. However, the first epileptic focus to develop after FPI is in the neocortex, whereas the hippocampus generates seizures only several months later (D'Ambrosio et al. 2005, 2009). To better understand the mechanistic links among head injury, serum extravasation, astroglial pathophysiology, and epilepsy, we used PTE induced by rostral parasaggital FPI (rpFPI) in the rat. We performed whole cell and cell-attached patch-clamp recordings from astrocytes in situ before and after the onset of seizures and used confocal imaging to examine the changes in expression and distribution of one type of KIR channel. In addition, we delivered blood serum to naïve brain slices and astrocytes in situ while blocking neuronal and synaptic activity and to the naïve neocortex in vivo. We show that head injury induces a pro-epileptic pathology of astrocytic KIR channels that persist specifically in the cells' processes in the perilesional epileptic focus but recovers in the hippocampus and that astroglial IKIR impairment and spontaneous seizures similar to those induced by FPI are reproduced by applications of blood serum, but not dialyzed albumin, to naïve astrocytes in situ or to the naïve cerebral cortex in vivo.
METHODS
All procedures were approved by the University of Washington Institutional Animal Care and Use Committee. All reagents were purchased from Sigma (St. Louis, MO) unless otherwise noted. All data are expressed as means ± SE unless otherwise specified.
rpFPI has been described in detail previously (D'Ambrosio et al. 2004, 2005). Briefly, male Sprague-Dawley rats (postnatal days 30–35; Charles Rivers, Hollister, CA) were anesthetized with 4% halothane, intubated, and mechanically ventilated on 1.5% halothane and 30% O2 and air. Core temperature was maintained at 37°C with a heat pad. A 3 mm burr hole was drilled 2 mm posterior to Bregma and 3 mm from the midline over the right convexity. FPI was delivered to the intact dura at 3.25 ± 0.1 atm, as measured by a pressure transducer (Measurement Specialities, Hampton, VA). Injured animals had righting times >10 min. Acute (≤1 wk) mortality rate was ∼10%.
Electrocorticography
A random subset of animals used for patch-clamp recording at 1 mo after rpFPI (7/21) was selected for 24 h of video-Electrocorticography recording (D'Ambrosio et al. 2004, 2005). Briefly, seven animals were anesthetized as per FPI, and a montage of five epidural electrodes was implanted 5–7 days before video-ECoG acquisition. A reference electrode was placed midline on the frontal bone, and two electrodes per parietal bone were placed at coordinates bregma 0 and −6.5 mm, 4 mm from the midline. Video-ECoG recordings were obtained, as previously described (D'Ambrosio et al. 2004, 2005), 1–4 days before death for patch-clamp study. All seven animals presented chronic seizures consistent with our previous work. Sixteen additional animals underwent video-ECoG recordings after intraparenchymal injection of artificial cerebrospinal fluid (ACSF) or diluted rat serum (DRS). These animals received the same five-electrode montage as those used for patch-clamp analysis, but the tip of a fine glass micropipette (tip diameter <10 μm, tapered from a shank diameter of ∼30 μm 700 μm from the tip) was lowered ∼500 μm beneath the dura though a burr hole into the frontal parietal neocortex at stereotaxic coordinates bregma −2 mm, lateral 3 mm. Recordings (24 h) were acquired weekly from week 1 to week 8 after implantation.
Brain slice preparation
At 22–26 h (1 day) or 28–35 days (1 mo) after injury, rpFPI- and age-matched naïve rats were anesthetized with halothane and transcardially perfused for 90 s with an ice-cold heparin-supplemented (4 U/ml) cutting solution containing (in mM) 206 sucrose, 3.1 KCl, 2 MgCl2, 26 NaHCO3, 1 CaCl2, 1.25 KH2PO4, 10 glucose, and 1 kynurenic acid (KYNA). Rats were decapitated, and their brains were rapidly removed into an oxygenated iced cutting solution lacking heparin. Brains were immersed in ice-cold oxygenated cutting solution, and 350 μm coronal slices were cut with a Vibratome (Ted Pella, Redding, CA). Acute slices were transferred to a 35°C 95% O2-5% CO2-bubbled holding solution containing ACSF composed of (in mM) 120 NaCl, 3.1 KCl, 2 MgCl2, 26 NaHCO3, 2 CaCl2, 1.25 KH2PO4, 10 glucose, and 1 KYNA. It has been shown that glial channel activity can be affected by the spontaneous activity of neighboring neurons (Marrero et al. 1989). This possible confound was minimized by inhibiting neuronal and synaptic activity with KYNA as previously described (D'Ambrosio et al. 1999, 2002). A subset of experiments (Fig. 1) was performed in the presence of 17.5 μM tamoxifen, which has been reported to diminish in situ gap junctional coupling in cardiac astrocytes (Verrecchia and Herve 1997). Although the uncoupling efficacy of tamoxifen has not been verified in astrocytes, it has been reported to have no effect on astrocytic KIR currents (Smitherman and Sontheimer 2001), and it exhibited no effects on KIR currents in our pilot experiments. Slices were incubated at 35°C for 30 min, after which the bath was allowed to cool to 25°C and for 1 h before use.
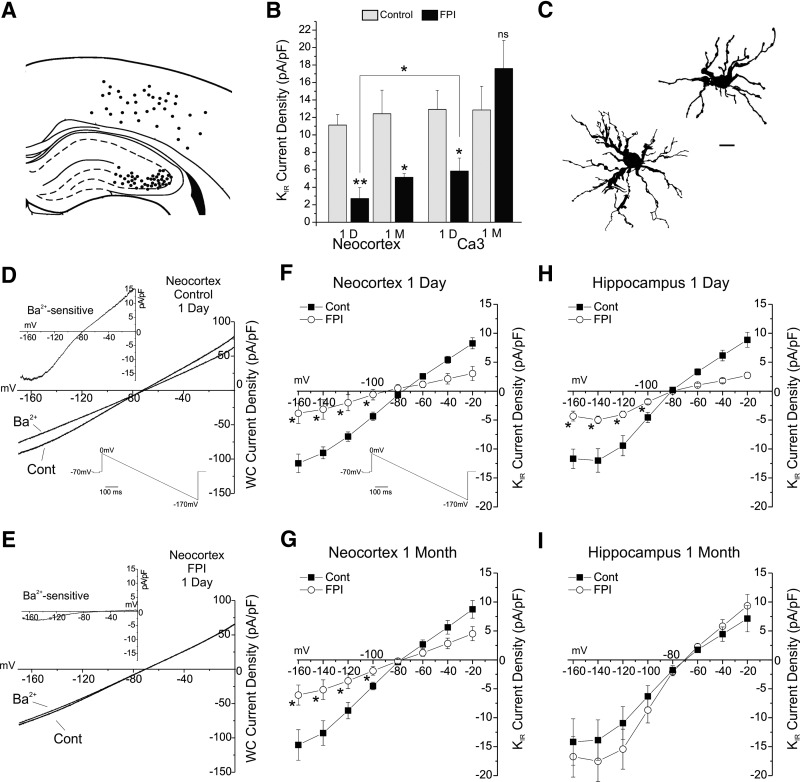
Fig. 1.
Astrocytic KIR current loss after rostral parasaggital fluid percussion injury (rpFPI) persists chronically in the neocortex but not in the hippocampus. A: locations of the astrocytes sampled for in situ whole cell evaluation of KIR current. Patched cells from Bregma −0.5 to −3.5 mm are referred to a single anterior-posterior coordinate. B: group analysis of whole cell voltage-clamp recordings satisfying voltage-clamp quality control. Recordings were obtained from neocortical and CA3 astrocytes 1 day and 1 mo after FPI and in age-matched controls. KIR current density (δIKIR) is detected by its sensitivity to Ba2+ (40 μM) and was measured at −140 mV during voltage ramps from 0 to 170 mV. Ba2+-sensitive δIKIR is significantly decreased compared with age-matched controls in both neocortex and CA3 1 day after injury, but only in the neocortex 1 mo later. The acute loss in astrocytic δIKIR is more severe in the neocortex than in the hippocampus (*P < 0.025, **P < 0.0025). C: camera lucida images of confirmed isolated astrocytes filled with biocytin during patch-clamp recordings in brain slices obtained from FPI animals. Scale bar = 10 μm. D and E: representative whole cell current-voltage traces obtained with downward voltage ramps in neocortical astrocytes 1 day after rpFPI (E) and in age-matched controls (D), before (Cont) and after Ba2+ (40 μM) application, showing the acute loss in δIKIR. Top left insets in each panel show I-V plots of the Ba2+-sensitive δIKIR. F–I: cumulative I-V plots of the Ba2+-sensitive δIKIR measured during downward voltage ramps in the subset of astrocytes shown in B that were confirmed to be isolated. The δIKIR are plotted at 20 mV intervals from −160 to −20 mV. Ba2+-sensitive whole cell δIKIR was depressed 1 day after injury in both neocortex (F) and CA3 (H) and remained depressed at 1 mo only in neocortex (G), but not in CA3 where it recovered to preinjury values (I). Asterisks in F–I indicate statistical significance at the P < 0.01 level and, for clarity, are shown for voltage range from −160 to −120 mV only. Bottom right insets in D and F show the voltage ramp protocol.
Patch-clamp recordings
Slices were gently transferred to a submersion recording chamber constantly perfused with modified oxygenated ACSF composed of (in mM) 120 NaCl, 4.35 KCl, 1 MgCl2, 26 NaHCO3, 2 CaCl2, 10 glucose, and 1 KYNA at a rate of 1–2 ml/min. All electrophysiological recordings were performed at 35°C unless noted otherwise. Glial cells were visually selected for recordings at 800× magnification with a Nikon E600FN microscope equipped with infrared DIC optics and 40× immersion objective. Whole cell and cell-attached patch-clamp recordings were obtained using an Axopatch 200B (Molecular Devices, Sunnyvale, CA). Patch pipettes had resistance of 4.5–6 MΩ and were filled with either 5 mg/ml biocytin or 1 mg/ml of rhodamine dextrane for cell type confirmation and (in mM) 140 K-gluconate, 1 MgCl2, 2 Na2-ATP, 0.3 NaGTP, 10 HEPES, and 0.5 EGTA, adjusted to a final pH of 7.2 with NaOH. The theoretical whole cell K+ reversal potential (EK) was –92 mV. For whole cell voltage-clamp recordings, series resistance (Rs) was compensated at ∼75% (lag time, 10–15 μs) and monitored during the experiment. Recordings were filtered at 2–5 KHz, digitized at 5–20 KHz (Digidata 1322A, Molecular Devices), and acquired on a Pentium 4 computer with Clampex 9 (Molecular Devices). Cell membrane capacitance (CM) and input resistance (RIN) were measured in voltage clamp (VC) using ±5 mV steps (125 ms) from the holding potential of –70 mV. CM was determined by integrating the capacitive current for the time of the capacitive transient, and RIN was estimated from the steady-state current response. Reported currents are not leak-subtracted. To ensure the VC was adequate to measure changes in slow nonregenerative currents like IKIR in situ, whole cell recordings were only considered if compensated Rs was ≤7% of RIN (Rs was 6.0 ± 0.2% of RIN; n = 101). Thus recordings from tens of coupled cells (low RIN) or with high RS, which both tend to result in poor VC, were not used. In addition, we used quasi-steady-state ramps to probe IKIR, and we provide independent analysis of in situ astrocytes confirmed to be isolated as shown by morphological analysis of biocytin-filled cells and membrane capacitance consistent with isolated cells (D'Ambrosio et al. 1998). To minimize the effects of possible run down, a uniform recording protocol was used in each set of experiments, and its execution time was consistent among recordings. Electrophysiological phenotypes of cells were classified on the basis of whole cell current responses to 750 ms upward voltage ramps from −170 to +100 mV. Inward currents were analyzed using 750 ms downward ramps from 0 to −180 mV and series of 18 voltage steps (−10 mV; 200 ms) from a potential of 0 mV. IKIR was measured during downward voltage ramps as the difference between the whole cell currents before and after blockade with bath-applied 40 μM Ba2+. Current density (δI) was calculated by dividing membrane current by the membrane capacitance. Channel conductance and open probability (Po) were studied in cell-attached patch-clamp configuration in situ, using the same pipette solution used for whole cell voltage-clamp experiments. Cell type was confirmed by subsequent break-in for whole cell recordings and biocytin filling. Because this work was not focused on the study of possible subconductance states of the KIR channels, we estimated conductance and Po of the channels from Gaussian fits to normalized all-points histograms of the current response to series of applied voltage commands (Vcom) ranging +70-0 mV after visual examination for quality of the traces. Single channel conductance is reported as slope conductance (+70-0 mV) to account for a variable unknown cell Vm. To pharmacologically determine the class of channels in the cell-attached patch, the pipette solution was exchanged for an otherwise identical solution containing 100 μM BaCl2 using a 2PK+ pipette perfusion kit (ALA Scientific Instruments, Westbury, NY). In these experiments, a mean patch current was obtained by integrating the current over each sweep and dividing by the sweep duration, to facilitate comparison among patches containing variable numbers of channels. The effect of Ba2+ was evaluated in series of 50 s sweeps at Vcom = +70 mV (over the course of ∼15 min) by comparing the mean patch currents obtained for the four sweeps immediately preceding the onset of blockade (control) with the last four sweeps obtained during Ba2+ blockade. Open probabilities were estimated for patches containing a single channel, no more than two nonidentical channels or no more than four channels of uniform conductance. Channel conductance was estimated for these patches and for patches containing larger numbers of channels with approximately equal conductance such that the several peaks in all points histograms were well resolved. Finally, although a count of stable conductances could be estimated for a wider set of patches, one third of the patches were unsuitable for analysis because of the presence of a mixture of channels with differing conductances. Patch-clamp data were analyzed with Clampfit 9 (Axon Instruments), and data were graphed and plotted with Origin 5.0 (MicroCal, Northampton, MA).
Albumin preparation and dialysis
Dialyzed and undialyzed albumin solutions were prepared using 99% pure bovine serum albumin (BSA, Sigma, cat.# A3059) and always prepared at the same time and stored in the refrigerator for the same time before use. Undialyzed BSA (0.4 mM) in ACSF was maintained at 4°C for 48 h before use. Dialyzed albumin was obtained from a solution of 30 g of BSA in 150 ml of ultrafiltered water (pH = 7). This was placed in dialysis membranes (25 kDa molecular-weight cut-off; Spectra/Por 7, Spectrum Laboratories, Rancho Dominguez, CA), and dialyzed at 4°C first against 4 liters of ultrafiltered water (pH = 7) for 24 h and then against 4 liters of ACSF (bath solution) for an additional 24 h. Final BSA concentration was determined by evaporatating of the resulting solution and weighing the dehydrated crystals. Dialyzed BSA was diluted to 26 mg/ml (0.4 mM) with ACSF.
Serum application
To determine the cellular effects of serum in brain slices, rats were decapitated under deep halothane anesthesia and rapidly exsanguinated. Heterologous blood was obtained from siblings. Autologous blood was collected in limited quantities from the punctured heart of the rat providing brain slices for electrophysiology. Whole blood was allowed to coagulate, diluted with an equal volume of oxygenated ACSF with KYNA 1 mM and without tamoxifen, and spun at 3,000 rpm for 10 min at 4°C, and the supernatant serum was collected, resulting in an approximately threefold dilution of blood serum. DRS (autologous or heterologous) was bubbled with 95% O2-5% CO2 and loaded into a syringe pump. An agar bridge prepared from 1% agarose in ACSF was used for these experiments, and no change was observed in pipette potential during DRS application. Serum exposure experiments were conducted at room temperature. Both complex and passive astrocytes were recorded for this experiment, but it is unlikely that NG2+ macroglia, representing just 5–8% of total glia in normal tissue (Dawson et al. 2003), contributed significantly to these data. For the in vivo experiments on the epileptogenesis induced by serum exposure, 300 μl of blood was obtained from the tail vein of the same animal, allowed to coagulate, and was diluted three- or sixfold with ACSF with total K+ of 2 mM (1.75 mM KCl). DRS was spun at 3,000 rpm for 10 min at 4°C, and the supernatant serum was collected. The autologous DRS was bubbled with 95% O2-5% CO2 and loaded into a primed subcutaneous osmotic minipump (Durect, Cupertino, CA). Animals received primed minipumps loaded with either low-potassium ACSF or DRS and were infused for 6–7 days at 0.25 μl/h through a glass micropipette in the animals' headsets.
Histochemistry
For visualization of biocytin-filled glia, slices were fixed overnight in 4% paraformaldehyde in PBS (pH 7.4; 4°C). They were washed (3 × 10 min) in 0.1 M sodium phosphate (pH 7.5; PB), quenched for 30 min in PB containing 1% H2O2 and 20% methanol, and washed three more times (10 min) in PB. After permeabilization in 0.3% Triton X-100 in PB for 2 h, sections were incubated overnight at room temperature with the Vectastain ABC reagent (PK-4000 or PK-6700, Vector Laboratories, Burlingame, CA) diluted 1:50 in PB. Slices were washed extensively (3 × 10 min, 6 × 1 h, and overnight) in PBT, equilibrated with Tris-buffered (0.01 M; pH 7.5) saline (TBS; 3 × 10 min), and developed with 0.01% H2O2, 0.5 mg/ml diaminobenzidine (DAB), and 0.02% ammonium nickel(II) sulfate in TBS. Stained sections were mounted on gelatin-coated slides, dehydrated through graded ethanol, cleared in xylene, and coverslipped in Permount. For localization of serum extravasation, rats were transcardially perfused with 50 ml ice-cold PBS and 200 ml 4% paraformaldehyde/4% sucrose under deep halothane anesthesia 5–6 h after rpFPI. Brains were removed and postfixed in 4% paraformaldehyde/4% sucrose for 12 h at 4°C and transferred to a solution of 30% sucrose in PB. After equilibration with the sucrose solution, brains were rapidly frozen in dry ice/isopentane, and 30 μm sections were prepared using a sliding microtome. Sections were washed in PB (3 × 10min), quenched in 1% H2O2, and further rinsed in 0.1 M PB (2 × 20 min). Slices were incubated for 60 min at room temperature in a blocking solution containing 0.3% Triton X-100 and 5% normal goat serum in PB and then overnight at 4°C with Axell horseradish peroxidase–conjugated goat anti-rat albumin antibody (1:1,250; Accurate Chemical and Scientific, Westbury, NY) in blocking solution. After rinses in blocking solution (2 × 20 min) and PB (2 × 20 min), sections were incubated with 0.0125% DAB for 10 min and developed with 0.0125% DAB/0.0015% H2O2 in PB. Stained sections were rinsed three times for 10 min in PB, mounted, dehydrated, cleared, and coverslipped.
Densitometry
Densitometric assessment of albumin extravasation in hippocampus and neocortex was carried out using coronal sections collected from rats killed 6 h after rpFPI (Bregma −2 to −4 mm) and stained as described for albumin. Control and FPI sections were stained together, and the reaction was stopped before saturation. Photomicrographs (24-bit RGB) were acquired using a Nikon Optiphot-2 microscope equipped with a 2× objective and a Spot IIe camera. Images were acquired under uniform lighting conditions using identical exposure times and were white balanced and flatfield corrected. These images were converted to 8-bit grayscale before analysis using ImageJ (http://rsb.info.nih.gov/ij/). For each section, hippocampal regions of interest (ROIs) were drawn to include the entire region bounded by but excluding the pyramidal and granule cell layers, carefully omitting obvious high and low transmittance artifacts. Neocortical ROIs of similar (±5%) area were drawn to sample continuous neocortical regions of high immunoreactivity in the perilesional cortex, also omitting high and low transmittance artifacts. The ranges of pixel values obtained from hippocampal and neocortical ROIs defined in this manner did not differ in either control (62 ± 11 vs. 58 ± 9; P = 0.50, Wilcoxon) or in injured (109 ± 16 vs. 100 ± 13; P = 0.55, Wilcoxon) tissue. Immunoreactivities are reported for each rat as the area-weighted median hippocampal or neocortical optical density (http://rsbweb.nih.gov/ij/docs/examples/calibration) determined from sections taken at Bregma −2, −3, and −4 mm.
HPLC quantification of adenosine nucleotides
Tissue content of ATP and ADP was measured in naïve rats at 30–35 (1 day control) or 58–65 days of age (1 mo control) and at 12 h, 1 day, 3 days, and 1 mo after FPI from rats injured at 30–35 days of age. Tissue was obtained from frontal-parietal neocortex and hippocampus beneath the rpFPI site (Bregma −0.5 to −3.5 mm; 0–6 mm lateral). Injury, perfusion, and brain slice preparation were as per electrophysiological experiments, except that the ipsilateral frontal parietal neocortex and hippocampus were dissected from each coronal slice for separate analysis. After 1 h incubation in a 95%O2-5%CO2-bubbled holding solution, hippocampal or neocortical sections were frozen in liquid nitrogen, crushed, and homogenized in 1.8 ml 70°C 0.5 mM EGTA in 60% methanol. Homogenates were centrifuged at 10,000g for 10 min at 5°C. Pellets were stored at −80°C for subsequent protein assay, and supernatants were filtered through a 0.22 μm pore-size filter and immediately used for HPLC determination of nucleotide content. ATP and ADP were determined in 200 μl samples of tissue homogenate supernatant by anion-exchange HPLC at 254 nm (Kratos SF773 detector). Samples were injected onto an APS-2 Hypersil column (250 × 4.6 mm) with guard column (10 × 4 mm, 5 μm; ThermoElectron) held at 35°C with a sleeve column heater (Restek, Bellefonte, PA) and eluted isocratically with 5 mM KH2PO4 (pH 4.15) for 5 min, followed by a linear gradient to 750 mM KH2PO4 (pH 4.15) at a constant flow rate of 1.5 ml/min. Absorbance chromatograms were digitally acquired and analyzed using Baseline 810 Chromatography Workstation v 3.3 (Waters, Milford, MA). Nucleotide levels were determined by comparison of sample peak areas to concentration standards that were run throughout each experiment. Tissue pellets were solubilized in 0.5 M NaOH (Fischer Scientific, Pittsburgh, PA)/0.1% sodium dodecylsulfate (Fluka, Buchs, Switzerland), and protein was determined using a Coomassie Plus assay kit (Pierce, Rockford, IL). Nucleotide concentrations were expressed as nmoles per milligram protein.
Confocal microscopy
Rats were killed using isofluorane, followed by transcardial perfusion with 50 ml of PBS at pH 7.4, and perfused with 150 ml fixation buffer (4% formalin, 4% sucrose in PBS). Brains were fixed overnight in the same fixation buffer and cryoprotected in 30% sucrose/PBS. Frozen coronal brain sections (20 μm) were collected in the region of injury ranging from bregma 0 to bregma −3.5. Thawed, air-dried sections were postfixed with acetone, rinsed in PBS, and blocked in PBS containing 5% goat serum and 1% BSA. Kir4.1 was immunodetected with a polyclonal rabbit antisera (Alomone Lab, Jerusalem, Israel) previously characterized as immuno-specific for Kir4.1 on the basis of positive labeling in normal, but not Kir4.1 knockout mouse brain (Djukic et al. 2007). Glial fibrillary acid protein (GFAP) was detected using mouse monoclonal anti-GFAP (Covance, Emeryville, CA). Primary antibodies were detected using goat anti-rabbit Alexa-488 and goat anti-mouse Alexa-568 secondary antibodies (Molecular Probes/Invitrogen, Carlsbad, CA). Sections were treated with Vectashield anti-fade mounting medium (Vector Laboratories, Burlingame, CA). Single- or double-labeled laser confocal microscopy was performed using a Leica DMR/TCS-SP (Leica Microsystems, Bannockburn, IL). For each experiment, injured and control tissue sections were immunostained, and images were acquired under identical conditions. All image processing and formatting (Photoshop, Adobe Systems, San Jose, CA) were limited to linear brightness and contrast adjustments that were performed identically on experimental and control images.
Assays of serum thrombin activity
Thrombin/prothrombin activity was determined in samples of DRS by the clinical laboratory at Harborview Medical Center (Seattle, WA) using an assay based on the ability of a serum sample to restore clotting in prothrombin-deficient plasma. The sample to be analyzed was combined with prothrombin-deficient plasma (Precision Biologic, Dartmouth, Nova Scotia, Canada), and the clotting cascade was initiated by addition of rabbit brain thromboplastin (Diagnostica Stago). Clotting time was determined using a STAR analyzer (Diagnostica Stago), and enzymatic activity is reported as percent normal plasma activity.
Statistical analyses
Nonparametric statistical tests were used when feasible, and the Mann-Whitney test was used unless noted otherwise. Parametric tests were conducted on data transformed as needed to satisfy their distributional requirements. ANOVA-based comparison of differently scaled whole cell (real valued) and cell attached (both real and integer valued) data required data transformation. For this purpose, the classes of data to be compared (whole cell and cell attached) were separately aggregated (control and FPI) and ranked (ties each assigned the appropriate mean rank), and each rank was divided by the aggregate SD of the ranks. These normalized ranks were used for analysis. Maple 9 (Maplesoft, Waterloo, Ontario, Canada) was used to compute Fisher's exact probabilities. All other statistics were computed with SPSS 12.0 (SPSS, Chicago, IL).
RESULTS
Whole cell astrocytic KIR current is lost after head injury
Previous work using epidural grid ECoG showed the perilesional neocortex to generate CRSPSs from 2 to 4 wk onward (D'Ambrosio et al. 2004, 2005, 2009), whereas depth electrode recordings showed the hippocampus to be silent in the early months after injury (D'Ambrosio et al. 2005). Thus these regions were specifically targeted for study of the role of astrocytes in epileptogenesis.
We examined the electrophysiology of astrocytes in the perilesional neocortex and underlying hippocampus in slices obtained 1 day and 1 mo after rpFPI and in age-matched controls. All glial cells targeted for patch clamp had clearly visible rounded or oval cell bodies ∼10–12 μm in diameter. Neurons were readily identifiable on the basis of spontaneous excitatory postsynatic currents (EPSCs) or inhibitory postsynaptic currents (IPSCs), depolarization-induced action potentials, and the morphological properties of the biocytin-filled cells. Glial cells lacked EPSCs or IPSCs, did not produce action potentials after depolarization, and presented consistent morphology at the microscopic analysis of biocytin-filled cells. Nine of 317 glial cells patched for determination of the effect of rpFPI on astrocytic whole cell KIR current density (δIKIR) were identified as microglia and excluded from subsequent analysis. In agreement with previous reports for intact microglia (Abraham et al. 2001; Bordey and Spencer 2003; Boucsein et al. 2000; Soltys et al. 2005), these cells were all depolarized (Vm = −26 ± 5 mV, n = 9), exhibited significantly higher RIN (840 ± 300 MΩ; P < 0.001) and lower Cm (17 ± 6 pF P < 0.001) than confirmed astrocytes (n = 81; RIN = 76 ± 5 MΩ, Cm = 29 ± 2 pF) that met our inclusion criteria for δIKIR determination, and displayed morphological features of either activated or ramified microglia. Remaining glial cells were identified as macroglia. They all exhibited Vm < −50 mV (Boucsein et al. 2000) and the three electrophysiological profiles (linear, inwardly rectifying, and complex) previously described in the hippocampus (D'Ambrosio et al. 1998). Oligodendrocytes most commonly displayed a complex electrophysiological profile and could be identified on the basis of their polygonal cell bodies and comparatively fine, straight, and directed processes bearing periodic “cup-like” swellings (D'Ambrosio et al. 1998), and were excluded from further analysis. Astrocytes used in this study were characterized by hyperpolarized membranes, fibrous or protoplasmic morphologies often with some processes extending toward blood vessels, and varying degrees of cell-to-cell coupling (D'Ambrosio et al. 1998).
Neocortical astrocytes were significantly depolarized (−68 ± 1 mV; n = 9) with respect to uninjured control (−72 ± 0.5 mV; n = 10; P = 0.01) at 1 day after injury, but no differences were detected at 1 mo (Table 1). The effect of rpFPI on whole cell δIKIR (Fig. 1) was assessed by its sensitivity to external barium (DiFrancesco et al. 1984). We examined the effect of rpFPI on IKIR currents 1 day and 1 mo after injury using 1) whole cell recordings that satisfied stringent quality control of the VC and 2) the subset of these whole cell recordings in which astrocytes were confirmed isolated.
Table 1.
Passive properties of neocortical and hippocampal astrocytes in whole cell recordings obtained from injured and control rats 1 day and 1 mo after injury
|
n |
Cm, pF |
Vm, mV |
Rin, MΩ |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Naïve | FPI | Naïve | FPI | P | Naïve | FPI | P | Naïve | FPI | P |
| 1 day cortex | 10 | 9 | 32 ± 4 | 34 ± 4 | 0.74 | −72 ± 0.5 | −68 ± 1 | 0.01* | 57 ± 8 | 78 ± 13 | 0.16 |
| 1 day CA3 | 11 | 9 | 28 ± 4 | 39 ± 7 | 0.21 | −72 ± 0.6 | −72 ± 1 | 0.97 | 80 ± 11 | 77 ± 12 | 0.85 |
| 1 mo Cortex | 8 | 9 | 27 ± 6 | 26 ± 5 | 0.99 | −71 ± 0.7 | −71 ± 2 | 0.60 | 74 ± 21 | 83 ± 21 | 0.53 |
| 1 mo CA3 | 12 | 13 | 25 ± 4 | 23 ± 4 | 0.34 | −69 ± 0.8 | −70 ± 1 | 0.35 | 74 ± 11 | 78 ± 10 | 0.72 |
Values are means ± SE.
Statistically significant. Cm membrane capacitance; Vm, membrane potential; RIN, input resistance.
Astrocytes with complex electrophysiology were rarer in the neocortex than noncomplex ones, resulting in too few complex cells with confirmed astrocytic morphology to allow separate statistical comparisons of the effect of rpFPI on δIKIR. Thus the astrocytes used for this experiment were characterized by a noncomplex electrophysiological profile, and NG2+ macroglia were excluded from analysis on the basis of their complex electrophysiological profile.
Whole cell recordings controlled for quality of VC (n = 80; Fig. 1B) showed that, 1 day after injury, neocortical astrocytic δIKIR decreased ∼65% from 11.1 ± 1.2 pA/pF in control (n = 10) to 2.7 ± 1.2 pA/pF in FPI (n = 8; P = 0.002), whereas CA3 astrocytic δIKIR decreased ∼40% from 12.4 ± 2.7 pA/pF in control (n = 11) to 5.2 ± 0.4 pA/pF in FPI (n = 9; P = 0.017). The rpFPI-induced loss in astrocytic δIKIR was more severe in the neocortex than in the hippocampus (P = 0.012). One month after rpFPI, neocortical astrocytic δIKIR was 5.9 ± 1.5 pA/pF (n = 9), significantly below the age-matched control value of 12.9 ± 2.2 pA/pF (n = 8; P = 0.012). In contrast, 1 mo after rpFPI, CA3 astrocytic δIKIR fully recovered to 17.6 ± 3.2 pA/pF (n = 13; P = 0.83) versus control levels of 12.8 ± 2.7 pA/pF (n = 12). These findings were confirmed by separate analysis of the subset of whole cell recordings in situ in which astrocytes were confirmed to be isolated by morphological analysis of biocytin-filled cells (n = 60; Fig. 1, F–I). One day after injury, δIKIR measured at −140 mV in isolated neocortical astrocytes decreased from 10.7 ± 1 pA/pF in control (n = 7) to 3.3 ± 1.7 pA/pF in FPI (n = 6; P = 0.015), whereas δIKIR in isolated CA3 astrocytes decreased from 12 ± 2 pA/pF in control (n = 7) to 5.5 ± 0.7 pA/pF in FPI (n = 6; P = 0.003). One month after rpFPI, δIKIR in neocortical astrocytes was 5.2 ± 1.7 pA/pF (n = 8), significantly below the age-matched control value of 12.6 ± 2.1 pA/pF (n = 6; P = 0.014). In contrast, 1 mo after rpFPI, CA3 astrocytic δIKIR fully recovered to 17.5 ± 3.5 pA/pF (n = 11; P = 0.47) versus control levels of 13.9 ± 3.5 pA/pF (n = 9).
Somatic astrocytic KIR current increases chronically after head injury
The injury-induced loss in whole cell IKIR could result from a change in number, conductance, or open probability (Po) of KIR channels. We examined each of these parameters in neocortical astrocytes in situ by cell-attached patch-clamp recordings (Fig. 2). These can only be obtained from the somata because of the small diameter of the astrocytic processes. KIR channels were identified based on four defining features. First, EREV, extrapolated from I-V plots of single channel currents and assuming astroglial Vm = −70 mV was −22 mV (Fig. 2D), in perfect agreement with the theoretical EK computed for 35°C and 66 mM intracellular K+, the [K+] measured directly in neocortical macroglia in situ (Ballanyi et al. 1987). Second, Po of individual channels increased with depolarizing voltage commands (Fig. 2, A and F). Third, the observed range of conductances (30–70 pS; Fig. 2E) was compatible with the expected heterogeneous expression of astrocytic KIR channels in situ. Fourth, channels displayed sensitivity to extracellular Ba2+ (100 μM) delivered through the patch pipette (Fig. 2, B and C). The average patch current (Vcom = +70 mV) decreased significantly from 5.4 ± 3.0 pA in control to 0.32 ± 0.14 pA in Ba2+ (n = 4; P = 0.004; paired t-test after logarithmic transformation). rpFPI induced no detectable change in the channel EREV (Fig. 2D) or conductance (Fig. 2E) of the currents carried through 19 control and 27 rpFPI KIR channels. Mean conductance was 52.3 ± 2.1 pS (n = 13) for controls and 52.2 ± 1.2 pS (n = 19) for rpFPI at 1 day and 59.2 ± 3.0 pS (n = 6) for controls and 57.1 ± 2.2pS (n = 9) for rpFPI at 1 mo after injury. Po of the same channels displayed identical magnitude and voltage dependence in control and FPI channels (Fig. 2, A and F). Therefore neither EREV, conductance, nor Po of somatic cell-attached channels could account for the loss in whole cell δIKIR 1 day after rpFPI.
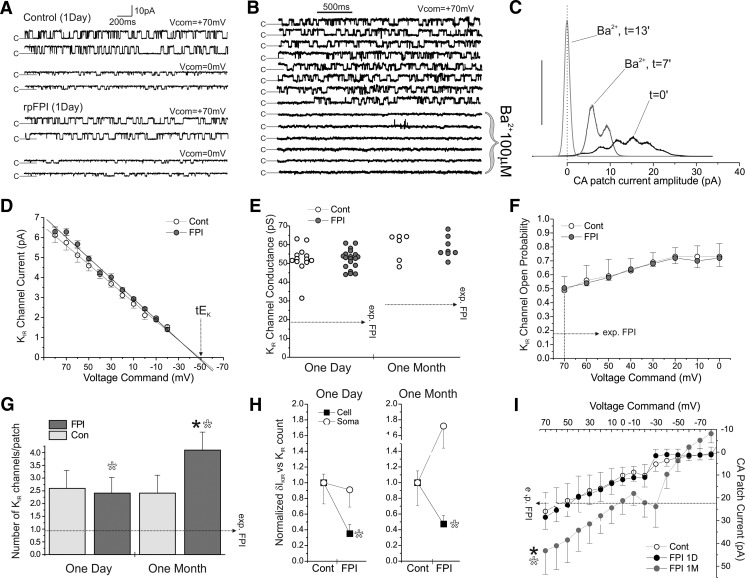
Fig. 2.
Neocortical astrocytic somata present no change in KIR current at 1 day and a compensatory increase at 1 mo after rpFPI. Cell-attached recordings from astrocytic somata do not show loss of IKIR, suggesting the IKIR loss observed in whole cell is from the impairment of astrocytes' processes. A: channel gating (c = closed) during cell-attached recordings from control and rpFPI neocortical astrocytic somata at Vcom of +70 and 0 mV. B: segments of consecutive sweeps obtained over 15 min from a cell-attached patch containing 1 active KIR channel before and during Ba2+ (100 μM) application through the pipette. Channel opening is potently blocked by Ba2+ as expected for KIR channels. C: all-points histograms of KIR channel activity during application of 100 μM Ba2+ through the pipette to a patch bearing multiple channels. Peaks at t = 0 indicate opening of multiple channels before Ba2+ application. Peaks are left-shifted and consolidated over time, indicating Ba2+ blockade of all channels on the patch. At t = 13 min, all channels are permanently closed. Y scale in arbitrary units. D: current-voltage plots for control and rpFPI channels recorded in somatic cell-attached patches show the same reversal potential (Vcom ≈ −48mV) consistent with a theoretical EK (tEK) in cell-attached of ≈ −22 mV, assuming cell Vm ≈ −70 mV, [K+]i = 66 mM, and [K+]pipette = 140 mM. E: slope conductance of cell-attached KIR channels from control and FPI rats 1 day and 1 mo after FPI. No injury-induced changes in channel conductance are observed. F: voltage dependence of Po in channels from control and 1 day FPI patches. No injury-induced changes in channel Po are observed. G: numbers of KIR channels found in cell-attached patches obtained 1 day and 1 mo after injury. No changes are observed 1 day after rpFPI, when the whole cell δIKIR deficit is most severe, whereas an increase in number of KIR channels is observed 1 mo after injury. Filled asterisk indicates statistical significant difference of rpFPI (1 mo) compared with a pooled control incorporating all other groups (P = 0.027). Hollow asterisks indicate statistical significance of the 2-way ANOVA test shown in H. H: summary of the 2-way ANOVA indicating a statistically significant difference between the effects of rpFPI on whole cell δIKIR (Cell) and on cell-attached KIR channel count (Soma). Cell values are normalized to the mean of the control groups. Asterisks indicate statistical significance: 1 day, P = 0.025; 1 mo, P = 0.002. I: I-V plot of mean cell-attached patch currents (corrected for leak current through the gigaseal) recorded during applied potentials from +70 to −70 mV in control and FPI at 1 day and 1 mo after injury. Currents measured 1 day after FPI are similar to control but are increased at 1 mo after FPI. Filled asterisk indicates P < 0.05 compared with controls. Empty asterisk indicates the significant difference (treatment × technique interaction) in the effect of rpFPI on somatic cell-attached vs. whole cell inward currents measured in the same cells (P = 0.003). Horizontal dotted arrows (exp. FPI) in E–I indicate the expected changes required to fully account for the whole cell KIR deficit after FPI.
The number of active KIR channels in the patch was estimated by the number of discrete conductance states that could be resolved in all-points histograms. Patches from controls age-matched to the 1 day and 1 mo time points contained 2.6 ± 0.7 (n = 13) and 2.4 ± 0.7 (n = 11) active channels, respectively. There was no change in number of channels 1 day after injury (2.4 ± 0.6 channels/patch; n = 23; P = 0.9), and the increase to 4.1 ± 0.7 (n = 16; P = 0.027) at 1 mo after injury was significant compared with a pooled control (n = 47) incorporating the other groups (Fig. 2G). We also examined the effect of rpFPI on somatic cell-attached currents (Fig. 2I; Vcom = +70 mV), which were nearly identical in FPI (n = 27) versus controls (n = 46) at 1 day after injury and significantly elevated at 1 mo (P = 0.04). After completing cell-attached recordings, patches were ruptured for the acquisition of whole cell downward inward currents.
Although whole cell measurements in situ indicated a global loss in IKIR in isolated neocortical astrocytes at both intervals after rpFPI, cell-attached measurements indicated that both the count of somatic KIR channels and the amplitude of somatic inward currents were unchanged at 1 day and increased at 1 mo after injury. This discrepancy was studied using two-way ANOVA, focusing on the interaction between treatment (control vs. rpFPI) and technique (whole cell vs. cell-attached). ANOVA detected significant measurement × treatment interactions at both 1 day (F = 5.37; 1 df; P = 0.025) and 1 mo (F = 11.1; 1 df; P = 0.002) after FPI for whole cell IKIR (Fig. 1) versus cell-attached KIR channel count (Fig. 2H). In a subset of cells that yielded both cell-attached and whole cell currents, a separate two-way ANOVA detected significant measurement × treatment interaction (F = 9.8; 1 df; P = 0.003) at 1 mo (n = 27) but not at 1 day after FPI (n = 26), for whole cell inward current versus cell-attached patch current. Thus both the inward currents and the number of somatic KIR channels measured in the soma in cell-attached patches vary independently of the corresponding inward currents and IKIR measured in whole cell. Because whole cell IKIR cannot decrease without a decrease in IKIR in either soma or processes, and we observed either no change or an increase in IKIR in the soma, these data point to rpFPI-induced loss of astrocytic IKIR predominantly in the astrocytes' processes.
Confocal imaging indicates loss of KIR 4.1 in astrocytic processes
Although many KIR channels could potentially contribute the observed injury-induced changes in astroglial electrophysiology, numerous studies have shown the inwardly rectifying K+ channel, KIR4.1, to be prominent in astrocytic processes and to play a role in astrocytic K+ buffering. We therefore examined the astrocytic expression of KIR4.1 in the ipsilateral hippocampi and neocortices of rpFPI rats 1 mo after injury and age-matched naives. In the hippocampus, no differences in KIR4.1 staining were found in injured versus naïve tissue 1 mo after injury (Fig. 3, J and M). The stratum pyramidale is rich in astrocytic processes that wrap pyramidal neuron somata, which are devoid of KIR4.1 immunoreactivity. In the naïve neocortex, KIR4.1 immunoreactivity colocalized with GFAP in most cases (Fig. 3, A–C) and was apparent in both processes and somata of cells with morphological characteristics of astrocytes. In the perilesional neocortices of injured rats, KIR4.1 immunoreactivity remained evident in the somata of astrocytes and substantially diminished beyond the most proximal processes (Fig. 3, D–F).
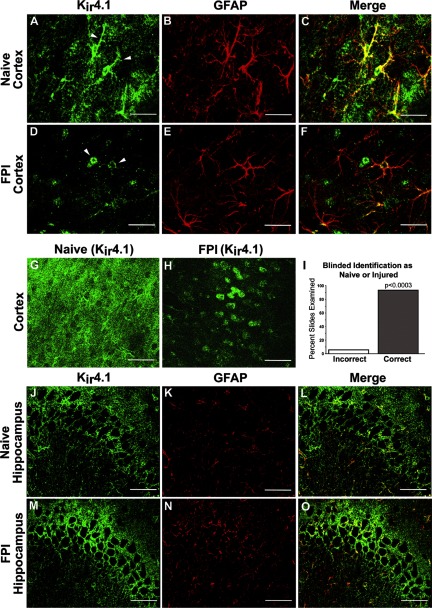
Fig. 3.
FPI induces chronic KIR4.1 mislocalization in cortical but not hippocampal astrocytes. Confocal microscopic images of KIR4.1 (green) and glial fibrillary acid protein (GFAP; red) immunofluorescence were obtained from regions of neocortex (A–H) and CA3 hippocampus (J–O), corresponding to the regions from which astroglial patch-clamp recordings were obtained (see Fig. 1A). Controls are shown in A–C, G, and J–L. Sections obtained at 1 mo after FPI are shown in D–F, H, and M–O. G, H, and all right-most images are merged images showing both KIR4.1 and GFAP immunoreactivity. A–C: naïve control cortex. KIR4.1 was expressed prominently in GFAP-positive astrocytic processes (arrowheads), as well as in astrocytic cell bodies. D–F: perilesional rpFPI cortex 1 mo after injury. Compared with controls, KIR4.1 immunoreactivity in injured cortical astrocytes (D–F) appears more prominent in swollen-appearing astrocytic cell bodies (arrowheads), whereas KIR4.1 immunoreactivity in GFAP-positive processes was markedly reduced. G and H: lower-magnification images of naïve (G) and injured cortex (H) similarly show that FPI was associated with an apparent loss of KIR4.1 immunoreactivity in finely ramifying processes. I: results of a histological analysis carried out under double-blind conditions that confirmed that the patterns of KIR4.1 expression in the somata and processes of astrocytes (D, F, and H) were distinct in naive and perilesional FPI neocortices. P = exact binomial probability. J–O: in contrast to the injury-induced Kir4.1 mislocalization observed in neocortex, the expression pattern of Kir4.1 in the CA3 region of hippocampus of injured rats 30 days after injury (M–O) was comparable to controls (J–L). Scale bars: C, F, L, and O: 50 μm; G and H: 100 μm.
The contrasting distributions of KIR4.1 immunoreactivity in sections from age-matched naïve and 1 mo postFPI rats permitted the reliable identification of the perilesional neocortex on the basis of blind evaluation KIR4.1 immunofluorescence alone (Fig. 3I). For the blind analysis, one investigator randomized 16 coronal sections (8 naïve and 8 FPI). Another investigator, who was blinded to the identity of the tissue, mounted them on the fluorescent microscope at 20× magnification and selected a field within the perilesional frontal neocortex. A third investigator, who was blind to the identity of the tissue and was not allowed to explore beyond the frontal perilesional cortex, evaluated the field. After all fields were evaluated, the blind was broken, and 15 of 16 sections were correctly identified (P < 0.0003, exact binomial probability).
Thus in agreement with the electrophysiological data, confocal imaging studies confirmed a striking and consistent mislocalization of KIR4.1 in neocortical, but not hippocampal, astrocytes 1 mo after head injury.
Serum induces acute shut-down of astrocytic KIR currents in situ
Serum extravasation is a reliable feature of FPI (Hoshino et al. 1996; Tanno et al. 1992), and exposure of the brain parenchyma to serum has been shown to result in chronic neuronal hyperexcitability and diminished astroglial K+ homeostasis (Ivens et al. 2007; Seiffert et al. 2004). To determine whether FPI-induced acute astrocytic IKIR loss is because of an effect of serum, we examined the effects of threefold DRS in whole cell with neuronal and synaptic activity suppressed by 1 μM TTX and 1 mM KYNA. Astrocytic inward currents were monitored during upward voltage ramps (−170 to +100 mV) repeated every 20 s during DRS exposure and by voltage steps (−170 to −0 mV) obtained before and during serum exposure. Although untreated astrocytes (n = 4) presented stable inward currents over the course of 12 min (97 ± 2% of initial values), these were reliably reduced during heterologous (n = 8) or autologous (n = 3) threefold DRS application (Fig. 4E). In four cells surviving 10 min of DRS exposure, inward currents were reduced to 84 ± 1% (P = 0.002, paired t-test) of their initial values. Therefore, in naïve tissue, DRS exposure reproduces the astrocytic KIR impairment observed 1 day after rpFPI.
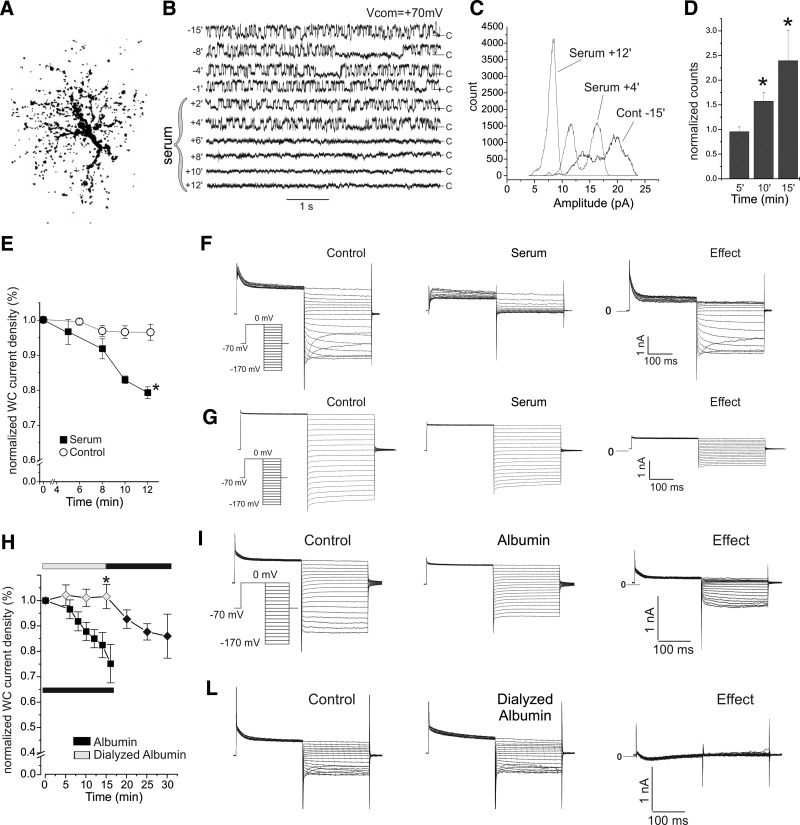
Fig. 4.
Serum, but not dialyzed albumin, induces acute loss of neocortical astrocyte KIR channel activity under conditions of blocked neuronal and synaptic activity. A: brightest point projection of an astrocyte with complex electrophysiological profile filled in situ with rhodamine dextran after cell-attached recordings. B: the activity of a single KIR channel, recorded from a neocortical astrocytic soma in a brain slice, is shown in sweeps obtained at a command potential of +70 mV before (t = −15 to 0 min) and during (t = 0–2 min) exposure to threefold diluted rat serum (DRS). Channel activity, stable over the course of 15 min before DRS application, ceased abruptly after 4 min in DRS. C: all-points histograms obtained at a command potential of +70 mV from a somatic cell-attached patch from another neocortical astrocyte, in situ, displayed numerous stable conductance states before DRS application, and just 1 (closed) afterward. The increase in peak amplitude and decrease in the width of the histogram during DRS exposure indicate a progressive shut-down of the channels in the patch. After 12 min of DRS exposure, all channel activity has ceased, resulting in an all-points histogram with a single, narrow high-amplitude peak. D: group analysis of peak amplitudes in all-points histograms obtained in cell-attached recordings after 5–15 min of DRS exposure normalized to preserum control amplitudes. Asterisks indicate P < 0.05. E: group analysis of the effect of exposure of neocortical astrocytes in situ to threefold DRS. DRS induces a time-dependent loss of whole cell δIKIR during upward voltage ramps. Plot shows time course of δIKIR measured at −140 mV and normalized to pretreatment values. Slices were treated with artificial cerebrospinal fluid (ACSF; control) or threefold DRS (serum) both with kynurenic acid (KYNA; 1 mM) and TTX (1 μM) to prevent epileptiform activity. F and G: whole cell current response to descending voltage steps in 2 in situ astrocytes before (control) and after (serum) ∼12 min of exposure to DRS. KIR current was lost (effect) in both complex (F) and noncomplex (G) astrocytes. Inset: voltage-clamp commands. H: group analysis of the effect of exposure to 0.4 mM whole bovine serum albumin (BSA; 99% purity; filled squares), or 0.4 mM dialyzed albumin (gray diamonds), in KYNA (1 mM). Whole BSA induces a serum like time-dependent loss of whole cell δIKIR during upward voltage ramps in naïve neocortical astrocytes that is not reproduced by dialyzed albumin (asterisk indicates P < 0.05). Note the positive effect of whole albumin applied to the same cells after the ineffective exposure to dialyzed albumin. Plot shows time course of δIKIR measured at −140 mV and normalized to pretreatment values. Black and gray bars indicate the exposure to whole albumin or dialyzed albumin, respectively. I: whole cell current response to descending voltage steps in an in situ astrocyte before (control) and after (albumin) 15 min of exposure to 99% pure albumin in KYNA (1 mM). KIR current was lost (effect). Inset: voltage-clamp protocol. Note the abolished KIR current in the bottom panel. J: whole cell current recordings during voltage clamp (VC) protocols identical to that used in G in an in situ astrocyte before (control) and after (dialyzed albumin) 15 min of exposure to dialyzed albumin in KYNA (1 mM). KIR current was not affected (effect).
To study possible contributors to the effect of serum, we used similar VC protocols in astrocytes in situ during perfusion with ACSF supplemented with either whole or dialyzed BSA at physiological concentration (0.4 mM). Whole BSA (n = 11) induced a progressive and rapid loss in astrocytic inward currents (75 ± 8% of initial values at 16 min). Conversely, astrocytes treated with dialyzed BSA (n = 8) displayed stable inward currents over the course of 15 min (P < 0.05 compare with undialyzed BSA; Mann-Whitney). Subsequent administration of whole BSA rapidly induced loss in inward currents (Fig. 4H). Therefore the effect of BSA on astrocytic inward currents is attributable to a soluble contaminant with molecular weight <25 kDa that copurifies with albumin.
Because thrombin has been implicated in PTE, thrombin was assayed in our DRS preparations. It was undetectable in all DRS preparations tested (n = 6), as expected for a derivative of clotted blood.
To understand whether the acute loss of IKIR results from a direct interaction of serum components with KIR channels, or requires cytoplasmic signal transduction, we studied neocortical astrocytes in naïve brain slices during bath application of threefold DRS with synaptic activity suppressed by 1 mM KYNA. We first examined the effect of threefold DRS on single KIR channels in cell-attached recordings (Vcom = +70mV) from neocortical astrocytic somata in situ, such that the extracellular face of the channels was inaccessible to bath-applied serum. We measured the peak amplitude (maximized when 1 conductance state dominates) of all points histograms of the current sweeps to measure channel function and compare serum effects on patches bearing varying numbers of channels. DRS invariably resulted in a progressive leftward shift in the all-point histograms, consistent with diminishing patch conductance (n = 7; Fig. 4C). By 15 min of DRS exposure, peak amplitude increased to 2,300 ± 700 samples (n = 5; P = 0.043, Wilcoxon) versus control values of 1,500 ± 400 samples. In patches bearing single KIR channels (n = 2), activity ceased abruptly after a period of DRS exposure (Fig. 4B). These data indicate that serum-induced loss of astroglial whole cell IKIR is likely mediated via cytoplasmic signaling.
Serum is sufficient to induce FPI-like spontaneous seizures in vivo
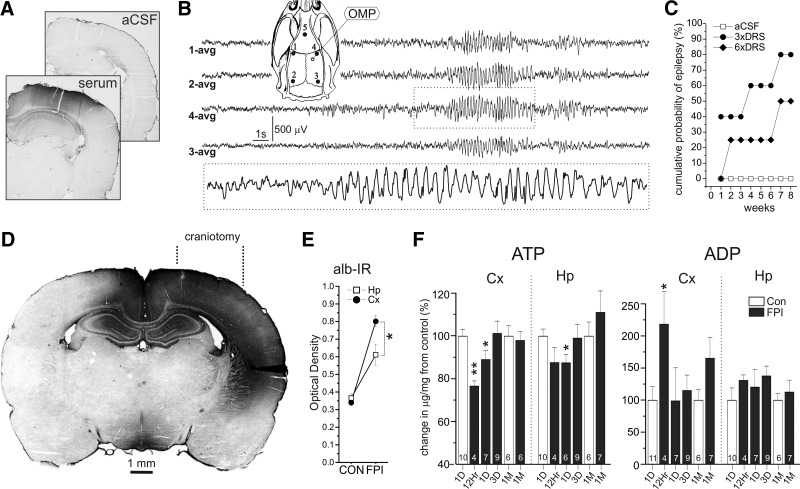
To determine whether serum may play a role in FPI-induced epilepsy, we performed video-ECoG monitoring in 16 naïve rats after continuous intracortical infusion of autologous DRS (n = 9) or ACSF (n = 7) into the frontal parietal neocortex. Serum was injected intracranially through a glass micropipette (tip diameter ∼10 μm) connected to a subcutaneous osmotic minipump delivering either ACSF or diluted serum for 6 days, a temporal window consistent with opening of the BBB after FPI (Hoshino et al. 1996). Intraparenchymal albumin was visualized immunohistochemically to determine the extent of serum infusion and verify that the fine glass micropipette did not induce blood extravasation (Fig. 5A). Animals were recorded from week 1 to week 8 after the treatment. We observed seizures (Fig. 5B) electrographically identical to focal (grade 1) and focal-onset spreading (grade 2) seizures induced by rpFPI (D'Ambrosio et al. 2004, 2005, 2009). DRS-induced seizures were first detected by the epidural electrode closer to the delivery site. In epileptic animals seizure frequency ranged 1–10 events per day. The cumulative probability that rats developed epilepsy, as defined by the occurrence of at least two seizures, increased over time after treatment, and was dose dependent, reaching 50 and 80% for animals receiving serum diluted six- and threefold, respectively (Fig. 5C). No animal injected with ACSF developed any seizure activity over the 8 wk of monitoring.
Fig. 5.
Serum infusion in vivo induces chronic recurrent spontaneous seizures and demonstration of a greater severity of injury suffered by the neocortex than the underlying hippocampus. A: albumin immunoreactivity marks the area affected by 6 days of in vivo infusion of either threefold autologous DRS or ACSF into the neocortical parenchyma of naïve rats, through a glass micropipette. Infusion of DRS results in prominent albumin immunoreactivity throughout much of the surrounding neocortex and dorsal hippocampus (serum), whereas there was no detectable albumin extravasation after infusion of ACSF. B: a representative grade 2 focal seizure recorded 2 wk after infusion of threefold DRS. Dotted box delimits the portion of ECoG shown at higher temporal resolution. The numbers next to each ECoG trace indicate the electrode (inset) by which the trace was recorded and the average reference (avg). Inset: schematic of the rat skull and 5-electrode epidural ECoG montage (1–5) used to detect serum induced neocortical seizures. Empty circle represents the site of insertion of the glass micropipette for intraparenchymal infusion of DRS or ACSF by osmotic minipump (OMP) in vivo. C: time course of epileptogenesis after intracortical infusion of ACSF, threefold DRS or sixfold DRS for 6 days. Whereas seizures were not observed in any ACSF infused animal, those receiving DRS developed epilepsy in a dose-dependent fashion. D: albumin immunoreactivity (alb-IR) marks extensive blood–brain barrier compromise and serum extravasation in the neocortex and hippocampus 6 h after rpFPI. Craniotomy indicates site of rpFPI delivery. E: densitometric analysis of the effect of injury on alb-IR in neocortex (Cx) and hippocampus (Hp). The FPI-induced increase in albIR was ∼50% greater in neocortex than hippocampus (P < 0.05, Wilcoxon). C: changes in tissue ATP and ADP content in the ipsilateral rostral parietal neocortex (Cx) and hippocampus (Hp) over time after injury. The neocortex suffers greater disruption of energy metabolism than the hippocampus. Numbers at the base of each column indicate group size. Asterisks in E and F indicate statistical significance (*P < 0.05, **P < 0.005) compared with age-matched controls.
Neocortex, not hippocampus, receives the greater damage and serum extravasation after FPI
Injury-induced serum extravasation was mapped using albumin immunohistochemistry. At 5–6 h after rpFPI, prominent albumin immunoreactivity was evident in the ipsilateral and medial-contralateral neocortex and hippocampus (Fig. 5D), matching the mapping of chronic neocortical hyperexcitability and of reactive astrocytosis 2 mo after rpFPI (D'Ambrosio et al. 2004). Densitometric analysis in five control and five rpFPI rats showed injury-induced increase in optical density by 136% in the neocortex (P = 0.009) and 67% in the hippocampus (P = 0.009). The increase in optical density in the neocortex was ∼50% greater than in the hippocampus (P < 0.05; Fig. 5E). We also assessed tissue energetics in frontal-parietal neocortical and hippocampal sections from beneath the rpFPI site by HPLC (Fig. 5F). Compared with age-matched controls, neocortical ATP levels were significantly reduced by 22 and 11% at 12 (P < 0.005) and 24 h (P < 0.05) after injury, respectively, whereas ADP showed a significant 120% elevation at 12 h after injury (P < 0.05). Conversely, hippocampal ATP levels were decreased by just 12% at 12 h after injury, and no significant changes in ADP were detected. Therefore the acute disruption of energy metabolism was more severe in the neocortex.
DISCUSSION
This is the first comparison of the acute versus chronic effects of head injury on astroglial electrophysiology in a developing epileptic focus, and the findings are unexpected. After acute astroglial IKIR impairment in both neocortex and hippocampus, this impairment recovered fully in hippocampal astrocytes by 1 mo after injury but persisted in astrocytes in the perilesional neocortical epileptic focus. Also, this is the first study to show that a major loss of astrocytic KIR current persists in the cells' processes, not their somata, which points to mechanisms of channel mislocalization. The data also support and expand on the previous finding that serum extravasation induces chronic hyperexcitability and impaired astroglial K+ buffering (Ivens et al. 2007; Seiffert et al. 2004), by showing that DRS infusion, in brain tissue ex vivo and naïve animals in vivo, reproduces, in a dose-dependent fashion, both the acute electrophysiological changes in astrocytic IKIR 1 day after rpFPI and the induction of CRSPSs similar to those induced by rpFPI.
Acute and chronic effects of head injury
Whole cell recordings were obtained in situ from neocortical and hippocampal astrocytes in brain slices from rpFPI animals with epilepsy confirmed by video-ECoG and from age-matched controls. The data showed rpFPI induced an acute large functional impairment of astroglial IKIR that was greater in the neocortex than in the hippocampus, and persisted only in the neocortical epileptic focus (Fig. 1). Patch-clamp recordings in situ are necessary to assess IKIR in situ, and we took several precautions to ensure the quality of voltage clamp: we excluded extensively coupled astrocytes, characterized by low RIN, and all recordings in which compensated Rs was >7% of RIN. In addition, we performed parallel analyses of all qualifying recordings (Fig. 1B) and of the subset of astrocytes that were confirmed isolated after biocytin filling (Fig. 1, D–I), with similar results. The measured IKIR loss is likely an underestimate of the actual brain pathophysiology in vivo, especially in the neocortex, because severely injured astrocytes do not survive (Hill-Felberg et al. 1999) or are less likely to yield successful patch-clamp recordings.
The large loss of whole cell IKIR observed both acutely and chronically in uncoupled neocortical astrocytes (Fig. 1, F and G) was not observed in cell-attached patches obtained from astrocytic somata 1 day after injury (Fig. 2). The somatic IKIR and the number of KIR channels recorded in cell-attached patches both actually increased at 1 mo after injury, whereas whole cell IKIR remained depressed (Fig. 2, G–I). Because whole cell IKIR cannot decrease in the face of unchanged or increased somatic IKIR without a decrease in the remaining cellular compartments, the loss of IKIR observed in whole cell must occur in the astrocytic processes, which, in normal tissue, are reported to be enriched with KIR channels (Hibino et al. 2004; Higashi et al. 2001; Kofuji et al. 2000; Neusch et al. 2006; Newman 1984; Rojas and Orkand 1999; Thomzig et al. 2001). Although the large size (∼15 μm in diameter) of vitreal endfeet of retinal Muller cells permits direct electrophysiological recordings (Newman 1984), such recordings are not possible in CNS parenchyma. The processes of astrocytes in situ taper steeply to reach submicrometer diameter around neuronal, synaptic, and vascular structures where KIR channels are mostly located, which prevents direct patch-clamp recordings from them. However, in agreement with the conclusions of our electrophysiological measurements, confocal imaging of KIR4.1 channel protein showed that the processes of neocortical astrocytes are profoundly depleted of KIR4.1 channels chronically after injury, whereas their somata are not. These pathological astrocytes provide a reliable basis for the blind identification of the neocortical epileptic focus (Fig. 3I). Conversely, hippocampal astrocytes, which suffer a milder acute loss in IKIR 1 day after injury, fully recover by 1 mo after injury (Fig. 1B), and their KIR4.1-rich processes surround CA3 pyramidal neurons indistinguishably from control tissue (Fig. 3, J–O).
Although KIR4.1 channels clearly contribute to the persistent IKIR pathology in neocortical astrocytes we observed, the astrocytic IKIR we studied in situ is likely carried by several channel types, which likely also contribute to injury-induced IKIR pathology. The IKIR we studied was susceptible to blockade by 40–100 μM extracellular Ba2+ and exhibited inward rectification to a different degree in individual cells; therefore it was likely carried by multiple channels such as KIR 2.x, KIR 3.x, KIR 4.1, heteromeric KIR 4.1/5.1, KIR 6.x, and/or TWIK channels, which all have been immunocytochemically shown to exist on macroglial membranes (Butt and Kalsi 2006; Lesage et al.1996; Patel et al. 2000). The varying single channel conductances we observed in cell-attached patches in situ (Fig. 2E) also suggest the presence of diverse KIR channels types on astrocytic somata. However, they provide no clues as to their identities because the properties of molecularly identified channels have only been examined in expression systems and in cultured astrocytes. Although the depletion of KIR4.1 immunoreactivity in astrocytic processes is consistent with whole cell data, impotant changes in other channel types cannot be ruled out. Further work will be needed to determine which other channel types may be contributing to the rpFPI-induced chronic changes in the level and distribution of IKIR.
The observed increase in somatic IKIR at 1 mo, but not at 1 day, after injury (Fig. 2, G–I) may represent a compensatory response of the astrocytic somata to chronic pathology of the processes to restore normal cellular Vm and RIN, as indeed was observed (Table 1). An increase in KIR channel density in astrocytic somata could partially compensate for failing spatial buffering by promoting the uptake of KCl, but at the cost of collateral pro-epileptic changes. Because uptake into the soma would require diffusion of extracellular K+ from distal sites, the speed of buffering would be reduced (Karwoski et al. 1989; Newman et al. 1984), whereas KCl accumulation would result in increased water uptake, cellular swelling, and shrinkage of the extracellular space (Dietzel et al. 1989; Hochman et al. 1995). It is also possible that the increased somatic expression in IKIR may result from its impaired transport to the processes. Indeed, KIR 4.1 is expressed in neocortical astrocytes chronically after injury, but it is predominantly localized in the somata (Fig. 3). A third possibility is that astrocytic processes that wrap blood vessels, and are therefore most exposed to serum after BBB failure, are simply more damaged than somata. Future work is needed to elucidate the specific mechanisms that result in the persistent IKIR loss in the processes.
Astrocytic IKIR in post-traumatic epileptogenesis
The IKIR dysfunction persisted only in the perilesional neocortex (Fig. 1) that developed an epileptic focus (D'Ambrosio et al. 2009), and not in the underlying hippocampus that sustained a milder metabolic injury (Fig. 5F), less serum extravasation (Fig. 5E), and milder acute astrocytic IKIR loss (Fig. 1B), and that does not initiate seizures by 1 mo after injury (D'Ambrosio et al. 2005). Thus post-traumatic epileptogenesis may require a more prolonged and/or severe astroglial KIR dysfunction than was induced in the hippocampus by the head injury used in this study. An alternative hypothesis is that additional mechanisms of epileptogenesis are at work in the perilesional neocortex and contribute to the development of seizures, which, in turn, contribute to the persistence of neocortical astrocytic IKIR loss. These seizures may result from greater blood extravasation, greater astrocytic IKIR loss, thrombin extravasation (Lee et al. 1997; Maggio et al. 2008), extracellular K+ and glutamate accumulation (Katayama et al. 1990), or the many other acute neuronal/synaptic changes described in the post-traumatic brain (Cohen et al. 2007; D'Ambrosio and Perucca 2004). In particular, the observed transient deficit in energy metabolism (Fig. 5F) could exacerbate virtually all other deficits and promote epileptogenesis. Although further work will be needed to elucidate the mechanisms of consolidation of the astroglial KIR hypofunction in the neocortex, our data suggest that the post-traumatic neocortical and hippocampal epileptogenesis after rpFPI may be mechanistically distinct. Neocortial epileptogenesis likely involves mechanisms unleashed as a direct result of the initiating trauma, including a severe extravasation of serum sufficient to trigger an epileptogenic cascade that includes shut-down of astrocytic KIR channels (Fig. 4) and the development of spontaneous seizures similar to those induced by rpFPI (Fig. 5, B and C). Conversely, the later emergence of a hippocampal epileptic focus (D'Ambrosio et al. 2005), after full recovery from its initial milder injury, may reflect a kindling-like phenomenon because of the relentless activity of the early neocortical focus, which propagates seizures to the hippocampus. Further work will be needed to test this hypothesis.
The consequences of astrocytic IKIR loss for the excitability, function, and survival of neurons are pernicious. The resulting astroglial membrane depolarization and decreased permeability to extracellular K+ disrupt the homeostatic control of the surrounding extracellular space and promote neuronal hypersynchronization, hyperexcitability, and seizures. Spatial buffering of extracellular K+, which requires both Vm close to EK and large membrane K+ conductance (Newman et al. 1984; Orkand et al. 1966), and clearance of extracellular glutamate, by electrogenic transporters that strongly depend on a hyperpolarized Vm (Brew and Attwell 1987), are both compromised by the persistent IKIR hypofunction (D'Ambrosio 2004; Djukic et al. 2007; Olsen and Sontheimer 2008). Also, by offering higher resistance to extracellular flow of neuronal and synaptic currents, KIR-depleted astrocytes increase ephaptic depolarization and synchronization of neuronal membranes (Dietzel et al. 1989; Dudek et al. 1986; Grundfest and Magnes 1951). This multiple hit is both ictogenic, in that it lowers the threshold for seizure precipitation, and epileptogenic, in that it can promote the permanent pathophysiological changes in synaptic drive, neuronal excitability, and network connectivity observed in acquired epilepsy (Albensi and Janigro 2003; D'Ambrosio 2004; Zha et al. 2005). Indeed, conditional knockout of KIR4.1, eliminating just one of the several KIR channels expressed in astrocytes, is sufficient to result in stress-sensitive epilepsy in the mouse (Djukic et al. 2007). Thus the early loss of astrocytic IKIR after rpFPI is likely a very important mechanism contributing to post-traumatic epileptogenesis.
Pro-epileptic effects of FPI are partially reproduced by serum
Recent studies have linked BBB opening and serum extravasation to chronic neocortical hyperexcitability, altered extracellular K+ clearance, and decrease in KIR 4.1 mRNA (Ivens et al. 2007; Seiffert et al. 2004). Our data support a role for this mechanism in FPI-induced epileptogenesis. The data showed intraparenchymal infusion of threefold and sixfold diluted autologous serum for 6 days is sufficient to induce CRSPSs that are electrically similar to those induced by rpFPI in naïve rats in a dose-dependent fashion (Fig. 5, A–C). Because serum extravasation is prominent after rpFPI in the incipient epileptic focus (Fig. 5, D and E), we conclude that serum extravasation through a leaky BBB is sufficient to initiate the proepileptic loss of astrocytic IKIR and post-traumatic epileptogenesis. However, both incidence of epilepsy and seizure frequency were much lower than those induced by rpFPI (D'Ambrosio et al. 2004, 2005, 2009). These differences may reflect a more severe and/or widespread extravasation of serum after head injury or the operation of additional injury-induced epileptogenic mechanisms. Indeed, direct effects of trauma on neuronal and synaptic function have been reported (Cohen et al. 2007; D'Ambrosio and Perucca 2004) that are likely to act synergistically with the astroglial impairment induced by serum. Furthermore, in addition to the chronic effects of serum extravasation on K+ buffering (Ivens et al. 2007; Seiffert et al. 2004), we now show that DRS application induces a rapid (<15min) shut-down of membrane KIR channels in neocortical astrocytes in situ (Fig. 4, E and F), which is expected to happen immediately after post-traumatic BBB failure. The ability of bath-applied DRS to rapidly modulate KIR channels in cell-attached patches (Fig. 4, B–D) indicates that serum component(s) do not act by extracellular channel blockade but through the cytoplasm, pointing to receptors and pathways of modulation of KIR channels as possible therapeutic targets.
Blood serum is a complex mixture incorporating many bioactive components, and the components mediating the acute pro-epileptic effects we describe remain to be identified. However, two candidate serum components suggested by the literature, thrombin (Lee et al. 1997; Maggio et al. 2008) and albumin (Ivens et al. 2007; Seiffert et al. 2004), have been ruled out. Thrombin was undetectable in all DRS preparations, as expected for a derivative of clotted blood. Thus although thrombin may still contribute to post-traumatic epileptogenesis, it does not mediate the loss of astrocytic IKIR and in vivo epileptogenesis we describe after DRS infusion. BSA did induce acute astrocytic IKIR decrease similar to DRS, but this activity was attributable to a dialyzable impurity with molecular weight <25 kDa. The dialysis-induced loss of albumin's activity was not caused by time-dependent degradation because no such loss of activity was observed in identically treated, but undialyzed, albumin. A number of apparent actions of albumin on various cells, including astrocytes, have been attributed to incompletely characterized impurities in BSA (Alexander et al. 1998; Bünemann and Pott 1993; Kreps et al. 1993; Nadal et al. 1997), and vascular physiologists have found dialysis to be necessary to prevent irreversible vasocostriction (Duling et al. 1981) in a tissue that should not be sensitive to the presence of native albumin. It is worth noting that the bioactive impurity in commercially prepared BSA need not be identical to the similarly active component in rat serum, and further work will be needed to identify the serum component(s) responsible for astroglial IKIR reduction and epileptogenesis.
Conclusions
We delineated a sequence of events linking rpFPI to serum extravasation and loss of astrocytic IKIR, which contributes to epileptogenesis. Exposure of the brain parenchyma to serum, as occurs in the immediate aftermath of head injury, results in an acute and rapid loss of astroglial IKIR, and in CRSPSs that are similar to those induced by rpFPI. The post-traumatic impairment of astrocytic IKIR predominantly affects the astrocytic processes and becomes chronic in the neocortical epileptic focus but not in the hippocampus that fully recovers from the milder injury.
GRANTS
This work was supported by Veteran's Affairs Office of Research and Development Medical Research Service grants to D. G. Cook and National Institute of Neurological Disorders and Stroke Grants NS-053928, NS-40823 to R. D'Ambrosio, and T32 NS-007144.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Nancy Temkin for advice on statistical analysis.
REFERENCES
- Abrahám et al., 2001. Abrahám H, Losonczy A, Czéh G, Lázár G. Rapid activation of microglial cells by hypoxia, kainic acid, and potassium ions in slice preparations of the rat hippocampus. Brain Res 906: 115–126, 2001 [DOI] [PubMed] [Google Scholar]
- Albensi and Janigro, 2003. Albensi BC, Janigro D. Traumatic brain injury and its effects on synaptic plasticity. Brain Inj 17: 653–663, 2003 [DOI] [PubMed] [Google Scholar]
- Alexander et al., 1998. Alexander JS, Patton WF, Christman BW, Cuiper LL, Haselton FR. Platelet-derived lysophosphatidic acid decreases endothelial permeability in vitro Am J Physiol Heart Circ Physiol 274: H115–H122, 1998 [DOI] [PubMed] [Google Scholar]
- Annegers et al., 1998. Annegers JF, Hauser WA, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injuries. N Engl J Med 338: 20–24, 1998 [DOI] [PubMed] [Google Scholar]
- Ballanyi et al., 1987. Ballanyi K, Grafe P, ten Bruggencate G. Ion activities and potassium uptake mechanisms of glial cells in guinea-pig olfactory cortex slices. J Physiol 382: 159–174, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordey and Sontheimer, 1998. Bordey A, Sontheimer H. Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res 32: 286–303, 1998 [DOI] [PubMed] [Google Scholar]
- Bordey and Spencer, 2003. Bordey A, Spencer DD. Chemokine modulation of high-conductance Ca(2+)-sensitive K(+) currents in microglia from human hippocampi. Eur J Neurosci 18: 2893–2898, 2003 [DOI] [PubMed] [Google Scholar]
- Bordey and Spencer, 2004. Bordey A, Spencer DD. Distinct electrophysiological alterations in dentate gyrus versus CA1 glial cells from epileptic humans with temporal lobe sclerosis. Epilepsy Res 59: 107–122, 2004 [DOI] [PubMed] [Google Scholar]
- Boucsein et al., 2000. Boucsein C, Kettenmann H, Nolte C. Electrophysiological properties of microglial cells in normal and pathologic rat brain slices. Eur J Neurosci 12: 2049–2058, 2000 [DOI] [PubMed] [Google Scholar]
- Brew and Attwell, 1987. Brew H, Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature 327: 707–709, 1987 [DOI] [PubMed] [Google Scholar]
- Bünemann and Pott, 1993. Bünemann M, Pott L. Membrane-delimited activation of muscarinic K current by an albumin-associated factor in guinea-pig atrial myocytes. Pfluegers 425: 329–334, 1993 [DOI] [PubMed] [Google Scholar]
- Buono et al., 2004. Buono RJ, Lohoff FW, Sander T, Sperling MR, O'Connor MJ, Dlugos DJ, Ryan SG, Golden GT, Zhao H, Scattergood TM, Berrettini WH, Ferraro TN. Association between variation in the human KCNJ10 potassium ion channel gene and seizure susceptibility. Epilepsy Res 58: 175–183, 2004 [DOI] [PubMed] [Google Scholar]
- Butt and Kalsi, 2006. Butt AM, Kalsi A. Inwardly rectifying potassium channels (Kir) in central nervous system glia: a special role for Kir4.1 in glial functions. J Cell Mol Med 10: 33–44, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacheaux et al., 2009. Cacheaux LP, Ivens S, David Y, Lakhter AJ, Bar-Klein G, Shapira M, Heinemann U, Friedman A, Kaufer D. Transcriptome profiling reveals TGF-beta signaling involvement in epileptogenesis. J Neurosci 29: 8927–8935, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen et al., 2007. Cohen A, Pfister BJ, Schwarzbach E, Grady MS, Goforth PB, Satin LS. Injury-induced alterations in CNS electrophysiology. Prog Brain Res 161: 143–169, 2007 [DOI] [PubMed] [Google Scholar]
- D'Ambrosio, 2004. D'Ambrosio R. The role of glial membrane ion channels in seizures and epileptogenesis. Pharmacol Ther 103: 95–108, 2004 [DOI] [PubMed] [Google Scholar]
- D'Ambrosio et al., 2004. D'Ambrosio R, Fairbanks JP, Fender JS, Born DE, Doyle DL, Miller JW. Post-traumatic epilepsy following fluid percussion injury in the rat. Brain 127: 304–314, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio et al., 2005. D'Ambrosio R, Fender JS, Fairbanks JP, Simon EA, Born DE, Doyle DL, Miller JW. Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain 128: 174–188, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio et al., 2002. D'Ambrosio R, Gordon DS, Winn HR. Differential role of KIR channel and Na+/K+-pump in the regulation of extracellular K+ in rat hippocampus. J Neurophysiol 87: 87–102, 2002 [DOI] [PubMed] [Google Scholar]
- D'Ambrosio et al., 2009. D'Ambrosio R, Hakimian S, Stewart T, Verley DR, Fender JS, Eastman CL, Sheerin AH, Gupta P, Diaz-Arrastia R, Ojemann J, Miller JW. Functional definition of seizure provides new insight into post-traumatic epileptogenesis. Brain 132: 2805–2821, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio et al., 1999. D'Ambrosio R, Maris DO, Grady MS, Winn HR, Janigro D. Impaired K(+) homeostasis and altered electrophysiological properties of post-traumatic hippocampal glia. J Neurosci 19: 8152–8162, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio and Perucca, 2004. D'Ambrosio R, Perucca E. Epilepsy after head injury. Curr Opin Neurol 17: 731–735, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio et al., 1998. D'Ambrosio R, Wenzel J, Schwartzkroin PA, McKhann GM, II, Janigro D. Functional specialization and topographic segregation of hippocampal astrocytes. J Neurosci 18: 4425–4438, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson et al., 2003. Dawson M, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci 24: 476–488, 2003 [DOI] [PubMed] [Google Scholar]
- Dietzel et al., 1989. Dietzel I, Heinemann U, Lux HD. Relations between slow extracellular potential changes, glial potassium buffering, and electrolyte and cellular volume changes during neuronal hyperactivity in cat brain. Glia 2: 25–44, 1989 [DOI] [PubMed] [Google Scholar]
- DiFrancesco et al., 1984. DiFrancesco D, Ferroni A, Visentin S. Barium-induced blockade of the inward rectifier in calf Purkinje fibres. Pfluegers 402: 446–453, 1984 [DOI] [PubMed] [Google Scholar]
- Delgado-Escueta et al., 1999. Delgado-Escueta AV, Wilson WA, Olsen RW, Porter RJ. Glia and epilepsy. In: Jasper's Basic Mechanisms of the Epilepsies, (3rd ed.) edited by Delgado-Escueta Av, Jasper HH. Philadelphia, PA: Lippincott Williams and Wilkins, 1999, p. 561–591 [Google Scholar]
- Djukic et al., 2007. Djukic B, Caspe KB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci 27: 11354–11365, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek et al., 1986. Dudek FE, Snow RW, Taylor CP. Role of electrical interactions in synchronization of epileptiform burst. In: Basic Mechanisms of the Epilepsies: Molecular and Cellular Approachs, edited by Delgado-Escueta AV, Ward AA, Woodbury DM, Porter RJ. New York: Raven Press, 1986 [Google Scholar]
- Ferraro et al., 2004. Ferraro TN, Golden GT, Smith GG, Martin JF, Lohoff FW, Gieringer TA, Zamboni D, Schwebel CL, Press DM, Kratzer SO, Zhao H, Berrettini WH, Buono RJ. Fine mapping of a seizure susceptibility locus on mouse Chromosome 1: nomination of Kcnj10 as a causative gene. Mamm Genome 15: 239–251, 2004 [DOI] [PubMed] [Google Scholar]
- Grundfest and Magnes, 1951. Grundfest H, Magnes J. Excitability changes in dorsal roots produced by electrotonic effects from adjacent afferent activity. Am J Physiol 164: 502–508, 1951 [DOI] [PubMed] [Google Scholar]
- Hagiwara and Takahashi, 1974. Hagiwara S, Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol 18: 61–80, 1974 [DOI] [PubMed] [Google Scholar]
- Hibino et al., 2004. Hibino H, Fujita A, Iwai K, Yamada M, Kurachi Y. Differential assembly of inwardly rectifying K+ channel subunits, Kir4.1 and Kir5. 1, in brain astrocytes. J Biol Chem 279: 44065–44073, 2004 [DOI] [PubMed] [Google Scholar]
- Higashi et al., 2001. Higashi K, Fujita A, Inanobe A, Tanemoto M, Doi K, Kubo T, Kurachi Y. An inwardly rectifying K(+) channel, Kir4.1, expressed in astrocytes surrounds synapses and blood vessels in brain. Am J Physiol Cell Physiol 281: C922–C931, 2001 [DOI] [PubMed] [Google Scholar]
- Hille, 2001. Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates, 2001 [Google Scholar]
- Hill-Felberg et al., 1999. Hill-Felberg SJ, McIntosh TK, Oliver DL, Raghupathi R, Barbarese E. Concurrent loss and proliferation of astrocytes following lateral fluid percussion brain injury in the adult rat. J Neurosci Res 57: 271–279, 1999 [DOI] [PubMed] [Google Scholar]
- Hinterkeuser et al., 2000. Hinterkeuser S, Schröder W, Hager G, Seifert G, Blümcke I, Elger CE, Schramm J, Steinhäuser C. Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Eur J Neurosci 12: 2087–2096, 2000 [DOI] [PubMed] [Google Scholar]
- Hochman et al., 1995. Hochman DW, Baraban SC, Owens JW, Schwartzkroin PA. Dissociation of synchronization and excitability in furosemide blockade of epileptiform activity. Science 270: 99–102, 1995 [DOI] [PubMed] [Google Scholar]
- Hoshino et al., 1996. Hoshino S, Kobayashi S, Nakazawa S. Prolonged and extensive IgG immunoreactivity after severe fluid-percussion injury in rat brain. Brain Res 711: 73–83, 1996 [DOI] [PubMed] [Google Scholar]
- Ivens et al., 2007. Ivens S, Kaufer D, Flores LP, Bechmann I, Zumsteg D, Tomkins O, Seiffert E, Heinemann U, Friedman A. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain 130: 535–547, 2007 [DOI] [PubMed] [Google Scholar]
- Jansen et al., 2005. Jansen LA, Uhlmann EJ, Crino PB, Gutmann DH, Wong M. Epileptogenesis and reduced inward rectifier potassium current in tuberous sclerosis complex-1-deficient astrocytes. Epilepsia 46: 1871–1880, 2005 [DOI] [PubMed] [Google Scholar]
- Jauch et al., 2002. Jauch R, Windmüller O, Lehmann TN, Heinemann U, Gabriel S. Effects of barium, furosemide, ouabaine and 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) on ionophoretically-induced changes in extracellular potassium concentration in hippocampal slices from rats and from patients with epilepsy. Brain Res 925: 18–27, 2002 [DOI] [PubMed] [Google Scholar]
- Karwoski et al., 1989. Karwoski CJ, Lu HK, Newman EA. Spatial buffering of light-evoked potassium increases by retinal Müller (glial) cells. Science 244: 578–580, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama et al., 1990. Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg 73: 889–900, 1990 [DOI] [PubMed] [Google Scholar]
- Kofuji et al., 2000. Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA, et al. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci 20: 5733–5740, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji and Newman, 2004. Kofuji P, Newman EA. Potassium buffering in the central nervous system. Neuroscience 129: 1045–1056, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreps et al., 1993. Kreps DM, Whittle SM, Hoffman JM, Toews ML. Lysophosphatidic acid mimics seruminduced sensitization of cyclic AMP accumulation. FASEB J 7: 1376–1380, 1993 [DOI] [PubMed] [Google Scholar]
- Kucheryavykh et al., 2008. Kucheryavykh YV, Shuba YM, Antonov SM, Inyushin MY, Cubano L, Pearson WL, Kurata H, Reichenbach A, Veh RW, Nichols CG, Eaton MJ, Skatchkov SN. Complex rectification of Müller cell Kir currents. Glia 56: 775–790, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al., 1997. Lee KR, Drury I, Vitarbo E, Hoff JT. Seizures induced by intracerebral injection of thrombin: a model of intracerebral hemorrhage. J Neurosurg 87: 73–78, 1997 [DOI] [PubMed] [Google Scholar]
- Lesage et al., 1996. Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romey G, Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J 15: 1004–1011, 1996 [PMC free article] [PubMed] [Google Scholar]
- Maggio et al., 2008. Maggio N, Shavit E, Chapman J, Segal M. Thrombin induces long-term potentiation of reactivity to afferent stimulation and facilitates epileptic seizures in rat hippocampal slices: toward understanding the functional consequences of cerebrovascular insults. J Neurosci 28: 732–736, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero et al., 1989. Marrero H, Astion ML, Coles JA, Orkand RK. Facilitation of voltage-gated ion channels in frog neuroglia by nerve impulses. Nature 339: 378–380, 1989 [DOI] [PubMed] [Google Scholar]
- Messori et al., 2005. Messori A, Polonara G, Carle F, Gesuita R, Salvolini U. Predicting posttraumatic epilepsy with MRI: prospective longitudinal morphologic study in adults. Epilepsia 46: 1472–1481, 2005 [DOI] [PubMed] [Google Scholar]
- Nadal et al., 1997. Nadal A, Fuentes E, Pastor J, McNaughton PA. Plasma albumin induces calcium waves in rat cortical astrocytes. Glia 19: 343–351, 1997 [DOI] [PubMed] [Google Scholar]
- Neusch et al., 2006. Neusch C, Papadopoulos N, Müller M, Maletzki I, Winter SM, Hirrlinger J, Handschuh M, Bähr M, Richter DW, Kirchhoff F, Hülsmann S. Lack of the Kir4.1 channel subunit abolishes K+ buffering properties of astrocytes in the ventral respiratory group: impact on extracellular K+ regulation. J Neurophysiol 95: 1843–1852, 2006 [DOI] [PubMed] [Google Scholar]
- Newman, 1984. Newman EA. Regional specialization of retinal glial cell membrane. Nature 309: 155–157, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman et al., 1984. Newman EA, Frambach DA, Odette L. Control of extracellular potassium levels by retinal glial cell K+ siphoning. Science 225: 1174–1175, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen and Sontheimer, 2008. Olsen ML, Sontheimer H. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem 107: 589–601, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkand et al., 1966. Orkand RK, Nicholls JG, Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol 29: 788–806, 1966 [DOI] [PubMed] [Google Scholar]
- Patel et al., 2000. Patel AJ, Maingret F, Magnone V, Fosset M, Lazdunski M, HonorÉ E. TWIK-2, an inactivating 2P domain K+ channel. J Biol Chem 275: 28722–28730, 2000 [DOI] [PubMed] [Google Scholar]
- Pollen and Trachtenberg, 1970. Pollen DA, Trachtenberg MC. Neuroglia: gliosis and focal epilepsy. Science 167: 1252–1253, 1970 [DOI] [PubMed] [Google Scholar]
- Rojas and Orkand, 1999. Rojas L, Orkand RK. K+ Channel density increases selectively in the endfoot of retinal glial cells during development of Rana catesbiana. Glia 25: 199–203, 1999 [DOI] [PubMed] [Google Scholar]
- Schröder et al., 2000. Schröder W, Hinterkeuser S, Seifert G, Schramm J, Jabs R, Wilkin GP, Steinhäuser C. Functional and molecular properties of human astrocytes in acute hippocampal slices obtained from patients with temporal lobe epilepsy. Epilepsia 41 Suppl. 6: S181–S184, 2000 [DOI] [PubMed] [Google Scholar]
- Seiffert et al., 2004. Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, Friedman A. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci 24: 7829–7836, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smitherman and Sontheimer, 2001. Smitherman KA, Sontheimer H. Inhibition of glial Na+ and K+ currents by tamoxifen. J Membr Biol 181: 125–135, 2001 [DOI] [PubMed] [Google Scholar]
- Soltys et al., 2005. Soltys Z, Orzylowska-Sliwinska O, Zaremba M, Orlowski D, Piechota M, Fiedorowicz A, Janeczko K, Oderfeld-Nowak B. Quantitative morphological study of microglial cells in the ischemic rat brain using principal component analysis. J Neurosci Methods 146: 50–60, 2005 [DOI] [PubMed] [Google Scholar]
- Tanno et al., 1992. Tanno H, Nockels RP, Pitts LH, Noble LJ. Breakdown of the blood-brain barrier after fluid percussive brain injury in the rat. Part 1. Distribution and time course of protein extravasation. J Neurotrauma 9: 21–32, 1992 [DOI] [PubMed] [Google Scholar]
- Temkin, 2003. Temkin NR. Risk factors for posttraumatic seizures in adults. Epilepsia 44 Suppl. 10: 18–20, 2003 [DOI] [PubMed] [Google Scholar]
- Thompson et al., 2005. Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK. Lateral fluid percussion injury: a 15-year review and evaluation. J Neurotrauma 22: 42–75, 2005 [DOI] [PubMed] [Google Scholar]
- Thomzig et al., 2001. Thomzig A, Wenzel M, Karschin C, Eaton MJ, Skatchkov SN, Karschin A, Veh RW. Kir6.1 is the principal pore-forming subunit of astrocyte but not neuronal plasma membrane K-ATP channels. Mol Cell Neurosci 18: 671–690, 2001 [DOI] [PubMed] [Google Scholar]
- Uhlmann et al., 2002. Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, Yamada K, Gutmann DH. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol 52: 285–296, 2002 [DOI] [PubMed] [Google Scholar]
- Verrecchia and Herve, 1997. Verrecchia F, Herve J. Reversible inhibition of gap junctional communication by tamoxifen in cultured cardiac myocytes. Pfluegers 434: 113–116, 1997 [DOI] [PubMed] [Google Scholar]
- Zha et al., 2005. Zha XM, Green SH, Dailey ME. Regulation of hippocampal synapse remodeling by epileptiform activity. Mol Cell Neurosci 29: 494–506, 2005 [DOI] [PubMed] [Google Scholar]