Abstract
Neurons in the hand representation of primary somatosensory cortex (area 3b) are known to have discretely localized receptive fields; and these neurons form modules that can be visualized histologically as distinct digit and palm representations. Despite these indicators of the importance of local processing in area 3b, widespread interactions between stimuli presented to locations across the hand have been reported. We investigated the relationship of neuron firing rate with distance from the site of maximum activation in cortex by recording from a 100-electrode array with electrodes spaced 400 μm apart, implanted into the area 3b hand representation in anesthetized owl monkeys. For each stimulated location on the hand, the electrode site where neurons had the highest peak firing rate was defined as the peak activation site. The lesser firing rates of neurons at all other electrode sites in the grid were compared with the firing rates of neurons at the peak activation site. On average, peak firing rates of neurons decreased rapidly with distance away from the peak activation site. The effect of distance on the variance of firing rates was highly significant (P < 0.0001). However, individual neurons retained high firing rates for distances over 3 mm. The clear decline in firing rate with distance from the most activated location indicates that local processing is emphasized in area 3b, while the distance of neurons with reduced but maintained firing rates ≤3–4 mm from the site of best activation demonstrated widespread activation in primary somatosensory cortex.
INTRODUCTION
The emphasis on modular processing in the somatosensory system has been recently supplemented by concepts of more widespread interactions, including surround modulation and stimulus integration. In the primary somatosensory cortex, area 3b in primates, there is good evidence that local or modular processing occurs within individual representations of the parts of the hand, via neurons with small, discrete receptive fields (e.g., DiCarlo et al. 1998; Iwamura et al. 1983; Pons et al. 1987; Sur 1980; Sur et al. 1985). As reported by Jain et al. (1998) and Qi and Kaas (2004), the representations of individual digits and pads of the palm can be visualized in area 3b of monkeys as myelin-dense regions surrounded by myelin-light septa. These histologically visible representations of the digits and palm pads are relatively consistent across individual monkeys and do not change in adults that have experienced a sensory loss that alters the somatotopy of the hand representation (Jain et al. 1998). The presence of such delimiting septa is consistent with the evidence that neurons in the hand representation in area 3b normally have small excitatory receptive fields confined to single digits at least during development.
In contrast, other observations point to widespread interactions between neurons distributed across the hand representation in area 3b. We reported that cross-correlations between pairs of neurons demonstrate the existence of functional connections between neurons separated by ≥3 mm in cortex (Reed et al. 2008), and firing rates of area 3b neurons are modulated by stimuli outside their receptive fields (e.g., Reed et al. 2010). Further evidence that even a discrete tactile stimulus produces widespread activation in primary somatosensory cortex has been long known. For example, 2-deoxyglucose metabolic labeling combined with neurophysiological mapping studies in macaque monkeys suggested that widespread increases in metabolic activity following repetitive, precisely controlled tactile stimulation can occur even when conventional receptive field mapping techniques fail to detect activation (Juliano and Whitsel 1987). In addition, the spatial and temporal activation patterns across large regions of primary somatosensory cortex have been explored using optical imaging techniques in New World squirrel monkeys (e.g., Simons et al. 2007; Tommerdahl et al. 2002). Notably responses to single-site flutter stimulation results in widespread cortical activity that shrinks in size after a few seconds of continued stimulation (Tommerdahl et al. 2002). We tested responses to indentation rather than flutter stimulation; however, flutter stimulation presented for the same duration as our indentation stimuli (0.5 s) resulted in spatially widespread cortical activation over the 3-mm imaged radius in primary somatosensory cortex (Simons et al. 2007). While our stimulus and methods differ from these previous studies, their results and our previous studies (Reed et al. 2008, 2010) provide reason to expect widespread activation in response to single-site stimulation.
This phenomenon of widespread activation beyond that expected based on receptive field mapping is not unique to the primary somatosensory cortex. Research continues to show that somatosensory cortex has similarities to other sensory cortices, like visual cortex. While a review of the extensive literature on primary visual cortex is beyond our scope, we highlight a particularly relevant study. Using optical imaging in macaque monkeys, Grinvald et al. (1994) reported as a primary finding that 350 ms after onset of a 1 × 1° stimulus, a cortical region larger than 6 × 6 mm was activated. Thus activity in primary visual cortex quickly spread beyond the distance expected based on receptive field mapping.
These results are compatible with other physiological studies specific to the cortical forelimb representation that have determined spatial characteristics for stimulus interactions when one stimulus is presented inside the receptive field and a second stimulus is presented nearby (e.g., Burton et al. 1998; DiCarlo and Johnson 1998, 2000, 2002; Friedman et al. 2008; Gardner and Costanzo 1980a,b; Greek et al. 2003; Laskin and Spencer 1979; Mountcastle and Powell 1959; Sripati et al. 2006). Although the proximity of the second stimulus to the first varies in these studies, the dominant finding is that the presentation of the second stimulus results in inhibition the strength of which is maximal at the receptive field center and decreases with distance from center.
Instead of using optical imaging or recording with a single electrode and studying the responses of a single neuron to the presentation of paired stimuli, in the present study, we recorded from 100 electrodes simultaneously while a stimulus was presented to a single location. After recordings were collected for that stimulus location, other locations were stimulated. In this way, we examined the spatial extent of excitation rather than the extent of inhibition. More specifically, we examined peak firing rates of single neurons and multi-neuron clusters in response to a stimulus delivered to a single site (instead of paired stimuli used in the previously referenced studies) and determined the spatial spread of above-baseline activity across cortical distance using recordings from an array of 100 regularly spaced electrodes implanted in the lissencephalic cortex of New World owl monkeys. We asked two main questions in this study. First, what is the relationship of neuron firing rate to distance from the site of maximum activation? Specifically, for each location on the hand that we stimulated, we determined which electrode in the 100-electrode array recorded the highest peak firing rate in response to the stimulation. This electrode became known as the site of peak activation. Neurons at this site in the cortex would be activated by near-threshold stimuli at the location of our 1-mm-diam stimulus probe and have their minimal receptive field at that skin location (e.g., Merzenich et al. 1983; Wu and Kaas 2003; Xerri et al. 1999). If local processing is emphasized in area 3b, the peak firing rates of neurons in response to a suprathreshold stimulus should decrease rapidly at distances away from the site of peak activation. If widespread integration is emphasized in 3b, peak firing rates should be maintained above baseline at distances away from the site of peak activation. Second, do firing rates differ when neurons at sites across digits are compared versus sites within a single digit? This second question arose from questions about the distributions of lateral, intrinsic connections within the hand representation in area 3b. While some research indicates that connections are denser within individual digit representations, connecting digit representations to those of the palm (Fang et al. 2002); others report preferential connections across distal digit representations of different digits rather than within single digit representations (Negyessy et al. 2009). This issue of the fine details of intrinsic connectivity within the primary somatosensory cortex hand representation has not been well-studied, in part due to technical issues; thus we determined if the firing rates of neurons in area 3b showed differences in the relationship to the distance from the site of peak activation when recordings were selected from across digit locations versus within a single digit.
Here we show that on average, peak firing rates of neurons decrease substantially with cortical distances away from the site of peak activity; however, some individual neurons retain high firing rates for great distances (>3 mm). The trends in the firing rates and their relationship to the distance from the site of peak activation do not differ significantly when recordings from stimulation sites across digits were compared with recordings from stimulation sites within a single digit. These results demonstrate the co-existence of neurons dominated by discrete local processing along with the ability of stimuli to cause widespread spatial activation in primary somatosensory cortex.
METHODS
Preparation for recording
Five adult owl monkeys (Aotus nancymaae) were prepared for electrophysiological recordings in the left hemisphere of primary somatosensory cortex. Three monkeys were males (cases 1, 2, and 5 each 1 kg) and two were females (case 3, 1.2 kg; case 4, 1.5 kg). All procedures followed the guidelines established by the National Institutes of Health and the Animal Care and Use Committee at Vanderbilt University and have been described previously (Reed et al. 2008, 2010). In brief, each monkey was given a ketamine injection (10–30 mg/kg im) for initial sedation, and anesthesia was induced with 2–4% halothane gas and then maintained with propofol (10 mg · kg−1 · h−1 iv) during surgery. The monkey was secured in a stereotaxic device for surgery and throughout the experiment. After anesthetizing the animal, paralysis was induced with 1–3 ml vecuronium bromide and maintained by intravenous vecuronium bromide (0.1–0.3 mg · kg−1 · h−1) mixed with 5% dextrose and lactated Ringer solution. Electrocardiograms (ECGs) and electroencephalograms (EEGs) were monitored. The skull and dura overlying primary somatosensory cortex were removed. The pneumatic inserter for the 100-electrode “Utah” array (see following text) was set to a depth of 600 μm to aim for the electrode tips to be placed within layer 3. After the insertion of the electrode array, the opening was covered with 1% agar mixed with Ringer solution to provide electrode stability and prevent desiccation. Following surgical procedures, supplemental anesthesia during recordings was provided by 0.3 mg · kg−1 · h−1h propofol. Similar methods have been described elsewhere (Samonds et al. 2003; Xu et al. 2003; Zhou et al. 2008). In one monkey (case 3), 1.2 mg/kg sufentanil was added to the lactated Ringer solution for slow infusion during the surgical procedures to stabilize anesthetic depth. Following delivery of this amount of sufentanil, the monkey was maintained under propofol anesthesia and vecuronium bromide paralysis without supplemental sufentanil during extracellular recordings. All monkeys were maintained approximately within sleep stage 2 during the recording experiments, as estimated by inspection of the EEG and ECG.
Stimulation procedures
Each neurophysiological recording consisted of a block of tactile stimuli presented to a single location on the hand. Independent force- and position-feedback controlled motor systems (300B, Aurora Scientific, Aurora, ON, Canada) delivered stimuli in the majority of recordings. The lever arm of the motor was tipped with a round Teflon probe 1 mm in diameter; thus a small contact surface was stimulated. Each stimulus block consisted of square wave pulses that indented the skin 0.5 mm for 0.5 s, followed by 2.0 s off of the skin, repeated for 255–300 s (100–120 trials). We used the minimal ramp time allowed by the stimulation equipment, which had a length step response time of 1.3 ms. For one monkey, case 4, an alternative tactile stimulator, a Chubbuck stimulator (Chubbuck 1966), was used. This stimulator also had a contact surface 1 mm in diameter. The fingernails were glued (cyanoacrylic) to Teflon screws fixed in plasticine to keep the hand in place during stimulus blocks. Adhesive was removed from the fingernails using acetone.
Data acquisition
As we have described previously (Reed et al. 2008, 2010), all recordings were made using the 100-electrode “Utah” array, consisting of a 10 × 10 grid of silicon spike electrodes, with electrodes spaced 400 μm apart. The Bionics data acquisition system (a legacy version of the system now offered by Blackrock Microsystems, Salt Lake City, UT) captured recordings, amplifying signals on each channel by 5,000 and band-pass filtering between 250 Hz and 7.5 kHz. Waveforms were sampled at 30 kHz for 1.5-ms windows, and the threshold for each electrode was automatically set for 3.25 times the mean activity (Samonds et al. 2003).
Histology
Following data collection, animals were perfused with saline followed by fixative, and the brains were prepared for histological analysis as described previously (Jain et al. 2001). Electrode locations were identified by evaluating 40-μm sections of flattened cortex processed for myelin. Details can be found in Reed et al. (2008).
Data analysis
SPIKE SORTING.
The details of the spike sorting procedures have been described previously (Reed et al. 2008). Recorded signals were sorted off-line with an automatic spike classification program based on the t-distribution expectation maximization algorithm (Shoham et al. 2003), which is part of the data acquisition system. The recordings for a given stimulus block were sorted together to standardize sorting across recordings. We used a second spike sorter program, Plexon off-line sorter (Plexon, Dallas, TX) to verify the quality of unit isolation such that single units had refractory periods ≥1.2 ms; P values ≤0.05 for multivariate ANOVA related to cluster separation; and distinct waveform amplitudes and shapes when compared with other activity on the same electrode (Nicolelis et al. 2003). Single- and multiunits were categorized separately. Typically, our multiunit recordings were of multiple neuron clusters that could not be isolated into individual units, but the waveforms had typical neuron-like negative and positive deflections.
PEAK FIRING RATE.
As described in Reed et al. (2008, 2010), spike trains were smoothed with a spike density function to determine peak firing rate using Matlab (The Mathworks, Natick, MA). A spike density function was produced by convolving the spike train from each trial with a function resembling a postsynaptic potential specified by τg, the time constant for the growth phase, and τd, the time constant for the decay phase as
Based on physiological data from excitatory synapses, τg was set to 1 ms (e.g., Mason et al. 1991; Moore and Nelson 1998) and τd was set to 5 ms (Mason et al. 1991; Moore and Nelson 1998; Veredas et al. 2005) as described (Reed et al. 2008).
For excitatory responses, the peak firing rate was determined as the maximum of the spike density function within the response time window, and the average baseline firing rate (calculated over a 500-ms window prior to stimulation onset) was subtracted from this value. This peak firing rate value was required to be greater than a threshold value, which was the average baseline firing rate plus 2 SD of this baseline with a minimum value of 5 spike/s. To determine the significance of this response, the nonparametric Mann-Whitney U test and the parametric Student's t-test with two tails were performed and results compared. Both tests resulted in the same categorizations of significance (α = 0.05). If no excitatory response was detected in the response window, responses were examined for possible suppressive effects. However, responses that showed inhibition only were not included in this analysis. While not the focus of this study, excitatory response latencies were calculated using Matlab and determined from spike density function histograms as the initial time when the rate met the half-height over the threshold value of the peak firing rate within the response time window, as described in Reed et al. (2010).
PEAK ACTIVATION.
For each recording session, the maximum peak firing rate was determined, and the electrode at which this maximum peak firing rate occurred was the site of peak activation in the cortex. If more than one unit was isolated at the site of peak activation, only the unit with the maximum peak firing rate was classified as the peak activation. The electrode spacing of rows and columns in the 100-electrode array is a known quantity, 400 μm. Thus we calculated the distance between 1) the site of peak activation to 2) each electrode with a significant response to the stimulation in a given recording. Distance = 400 × √ [(x1 − x2)2 + (y1 − y2)2]; where the row coordinates of the electrodes are indicated by x and the column coordinates are indicated by y. (Subscript 1 indicates the coordinates for the site of peak activation. Subscript 2 indicates the coordinates for the site of the electrode with a significant but weaker response to the given stimulation.) In plots comparing the peak firing rate to the distance from the peak activation, the site of peak activation was assigned a distance value of zero.
SUMMARY ANALYSIS.
A data set was compiled in Excel (Microsoft) containing the identifying information for all of the neurons recorded from each case. In addition to the raw distance and firing rate values, we calculated the percent of the maximum peak firing rate of each responding electrode during each recording condition. Thus the percentage was 100% for the electrode assigned to be the site of peak activation and was some value <100% for all of the other responding electrodes. Thus the firing rate values for each responding electrode in the 100-electrode array were normalized within each recording file. While the stimuli of interest were presented to one hand site at a time, we were interested in possible differences between the stimulus representations across digits or palm sites compared with within a single digit. There are reasons to suspect that there may be some differences as described in the introduction. Thus we classified all of the recordings within each monkey as part of the “across digit analysis” or part of the “within digit analysis” using dummy coding.
The data were imported into SPSS 17.0 (SPSS, Chicago, IL) for analysis of the relationship between the electrode distance from the site of peak activation to the peak firing rate and percent of the maximum peak firing rate. We used SPSS to determine if factors of interest contributed to differences in firing rate values (and latency values) with nonparametric Kruskal-Wallis analysis (as Kolmogorov-Smirnov 1-sample distribution tests showed that the data were significantly different from a normal distribution). Factors of interest were the distance (of the given electrode) from the site of peak activation, the type of unit isolation (single- or multiunit), the monkey (of 5 cases), and whether the stimulation sites included in the group were taken from across digits or within a single digit. We performed Kruskal-Wallis tests for all five monkeys combined as well as for each monkey individually. When the factor of interest had only two levels, we used a two-tailed Mann-Whitney U test. We also performed univariate ANOVA using the general linear model to assess the contributions to the variance in the peak firing rate values using those same factors for comparison, noting that the assumptions of this analysis were not all supported (supplemental information).1
We plotted the data in SPSS to examine trends in groups of data. Scatter plots were produced to show the dependent variable, peak firing rate (or the percentage value) versus the independent variable, electrode distance from the site of the peak activation. The plots were produced for single units and multi-units separately and for groups taken from across digits or within a single digit.
RESULTS
We recorded 513 single unit (SU) responses and 747 multiunit (MU) responses for analysis in this experiment from the area 3b hand representation in the five monkey cases. (case 1 = 17 SU, 78 MU; case 2 = 46 SU, 84 MU; case 3 = 397 SU, 492 MU; case 4 = 10 SU, 34 MU; case 5 = 43 SU, 59 MU.) The exclusion of case 5 (with a long-standing injury to digit 3) did not significantly change the trends in the compiled results; therefore we included all five monkeys in the group data analysis. However, we also analyzed data for each monkey individually (plots shown in supplementary Fig. S1). Results using the univariate ANOVA can be found in the supplemental information (Tables S1–S4). When examining data from individual monkeys, we did not find a strong relationship between the excitatory response latency and the cortical distance from the site of peak activation; however, these results are included in supplemental information (Figs. S2 and S3). Peak firing rates were collected over the first 50 ms following stimulus onset; longer-latency responses were excluded from this analysis. We excluded recordings from electrodes outside the area 3b hand representation. (A schematic of the electrode placement in monkeys 1–3 is illustrated in Fig. 2 of Reed et al. 2010.) To appreciate the trends in the data, we plotted the peak firing rate data versus distance from the site of peak activation. For these plots, data were separated into SUs and MUs and into categories for across digits and within digits.
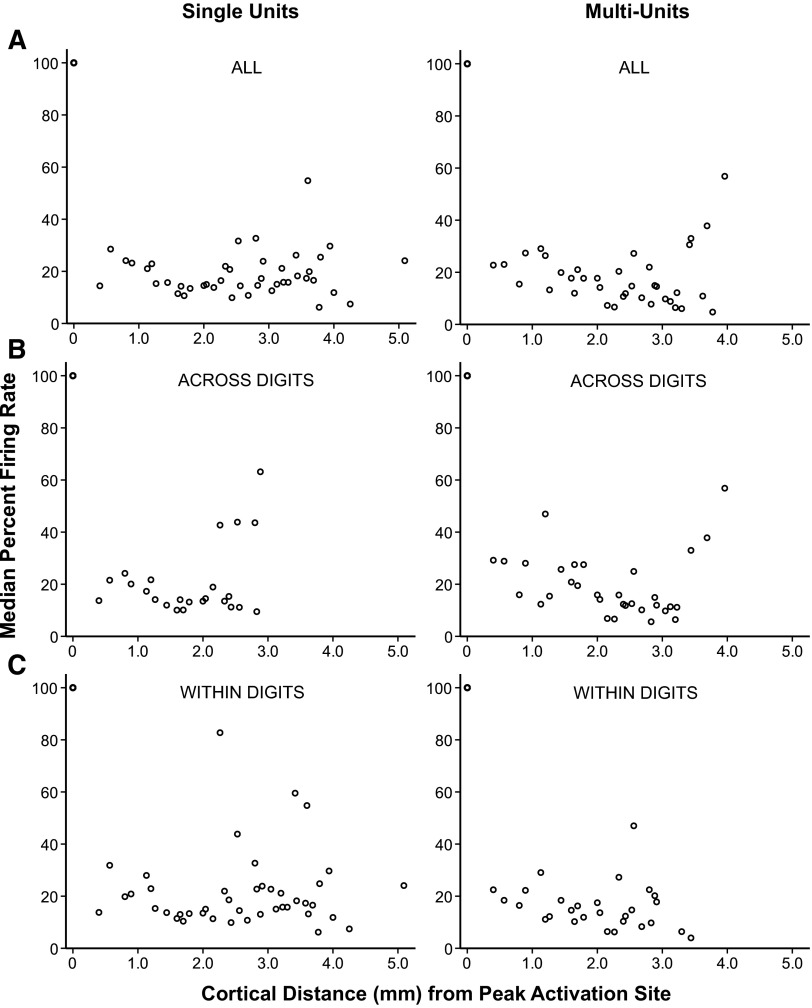
Fig. 2.
Relationship of the percent of maximum firing rate to the electrode distance from the peak activation. The y axis shows the dependent variable, percent of maximum firing rate, in which the peak firing rates were normalized for each recording based on the peak firing rate at the site of the peak activation. The x axis shows the electrode distance (mm) from the site of peak activation, which was placed at the 0 location. All recordings were thus normalized to 100% and appear as 1 data marker. Left: results from single units. Right: results from multiunits. Conventions are the same as in Fig. 1. A: results from all recordings from all 5 monkey cases. B: results from single-site recordings, but only recordings from across distal digit locations or across palm locations for a group termed “across digits.” On the distal digits, 1 series of comparisons was taken from the distal-most locations and a 2nd series of comparisons was taken from the middle-distal locations. C: results from single-site recordings within a single digit (distal, middle, and proximal sites within a digit) for a group termed “within digits.” The schematics of the owl monkey hand show the locations stimulated by the single probe (●), collapsed across all 5 monkeys. →, the direction of the statistical comparisons as not all points were compared with all other points for the across digits and within digits categories. The values compared depended on the responsive neurons and locations stimulated within each monkey case. Visual inspection as well as statistical analysis indicates that there is no difference in the cortical spread of activation when stimulating locations across digits vs. within digits for both single and multiunits. The firing rate normalized to the value of the peak activation drops dramatically for some neurons but remains high for other neurons even at cortical distances far from the peak activation site.
Nonparametric analysis of firing rates
As expected, the distance of recorded neurons from the site of peak activation significantly affected the variance in their peak firing rates (H = 267.62, df = 43, P < 0.0001) and the percent of the firing rate compared with the maximum peak firing rate within the array (H = 329.19, df = 43, P < 0.0001). Figure 1 shows the relationship of the electrode distance from the site of peak activation with the peak firing rate at each electrode location from which responsive SUs and MUs were recorded. The data for all single site stimulation locations in all five monkeys is shown. SUs and MUs showed similar trends; however, the raw firing rates of many of the MUs were higher than those of SUs (U = 150,623.50, n = 513 SUs, n = 747 MUs, P < 0.0001). The two scatter plots depict similar trends in which the peak firing rate decreased with distance from the site of the peak activation; however, the peak firing rates remained high at large distances across the electrode array. The full data sets (Fig. 1) provide several important results. First, most of the peak firing rates are greatly reduced for neurons at electrodes 400 μm away and farther from the neurons at the electrode with the highest peak firing rate.
Fig. 1.
Relationship of peak firing rate to the electrode distance from the peak activation. The y axis shows the dependent variable, peak firing rate (spike/s). The x axis shows the electrode distance (mm) from the site of peak activation, which was placed at the 0 location. Left: results from single units. Right: results from multiunits. The results from recordings from all 5 monkey cases are plotted to compare peak firing rates prior to normalizing to the peak of activation. The schematic of the owl monkey hand shows the locations stimulated by the single probe (●), collapsed across all 5 monkeys (not every site was stimulated in all 5 monkeys). Visual inspection as well as statistical analysis indicates that overall firing rates were lower in single- vs. multiunits when different hand locations were stimulated. The average firing rate at the peak activation site varied for different neurons, but drops as cortical distance from the peak activation site increases. More multiunits than single units maintained higher firing rates at sites beyond the peak activation. However, even for single units, some firing rates over 2 mm beyond the peak activation site were maintained as high as firing rates only 0.4 mm away from the peak activation.
Converting the peak firing rates of each neuron to the percentage relative to the magnitude of the peak activation of that neuron (Fig. 2, Supplementary Table S2) normalized the data. The normalized data show that several neurons (SUs and MUs) maintained firing rates close to the magnitude of the peak activation for distances >3 mm away from the site of the peak activation. Normalizing the firing rates removed the differences between firing rates of SUs and MUs (U = 189,866, P = 0.784). The overall trends were similar when locations across the distal digits were grouped, when locations within a single digit were grouped, and when all of the data were plotted together. All of the subsequent results are reported only for peak firing rates normalized as a percent of the peak activation.
Figure 3 shows the medians of the peak firing rates after the data were converted to the percentage of the peak activation. By viewing the medians at each distance location, the average trends in the data can be observed. The medians of the normalized peak firing rates decreased greatly at distances starting 400 μm away from the site of peak activation; however, these values were maintained above the spontaneous baseline firing rates at locations distant from the site of peak activation (Fig. 3).
Fig. 3.
Summary of median percent firing rate relationship to the electrode distance from the peak activation. Left: results from single units. Right: results from multiunits. The full data sets are plotted in Fig. 2. A: scatter plots of the median values of the peak firing rate converted to the percentage relative to the peak activation at each electrode distance location, summarized across all 5 monkey cases. B: scatter plots including only those single-site recordings that were recorded across distal digit sites for a group termed across digits. C: scatter plots including only those single-site recordings that were recorded within a single digit (distal, middle, and proximal sites within a digit) for a group termed within digits. Median values of the firing rate normalized to the value of the peak activation indicate similar patterns for all data categories (single vs. multiunits and across vs. within digits) such that on average the firing rates of neurons drop dramatically at 0.4 mm away from the peak activation site, increase slightly for neurons around 1 mm away. Median normalized firing rates are variable at cortical distances beyond 2 mm due in part to lower numbers of responding neurons recorded at those distances.
The schematic in Fig. 4A shows summarized results of the normalized peak firing rate percentages for each distance measured in cortex are shown for SUs from all five monkeys. The distances are duplicated in the negative direction to help visualize the activity present in cortex as a maximal peak at the center of peak activation, dropping sharply at adjacent sites, and then activity increases variably at distances beyond the peak activation site. As Fig. 4B shows, variable activity at distances >2 mm beyond the peak activation site is related to the low numbers of single neurons with significant activation recorded at these distances. The array samples a fixed region of ∼4 × 4 mm; however, few neurons were recorded with increased firing rates at long distances away from the center of activation. Of the total numbers of single neurons we collected, 66.67% were located within 2 mm of the peak activation site and 33.33% were located between 2 and 4 mm away from the peak activation site. Within 2 mm of the peak activation site, 54.62% of the single neurons were classified as responding. Between 2 and 4 mm of the peak activation site, 25.35% of the single neurons were classified as responding.
Fig. 4.
Summary of percent firing rate relationship to the electrode distance from the peak activation averaged across monkeys. A: data from only single units were plotted with a straight line interpolating between the points indicating the peak firing rate converted to the percentage relative to the peak activation site on the y axis and the cortical distance based on electrode spacing (mm) from the peak activation site on the x axis. Each data point is the average percent firing rate across all 5 monkeys for a given distance away from the peak activation site. Error bars are ±1 SE. The data were duplicated to better depict the spread of activation across directions in cortex, shown here in 2 dimensions. As described in Fig. 3, the general pattern appears to be a decrease in firing rates at the nearest electrode recording sites to the peak activation (0.4 mm) with an increase in firing rates recorded from electrodes beyond 0.4 mm at values well below the firing rate at the site of peak activation. B: the number of single neurons recorded (y axis) at each cortical distance (mm) from the peak activation site (x axis) is charted to show the number of single units (SUs) responding to the stimulus at that distance (dark gray bars) compared with the total number of SUs recorded at that distance (light gray bars). Thus the variability in the firing rate and the error bars shown in A are due to the low numbers of neurons firing above spontaneous to stimuli presented to a hand location that most strongly activates sites over 2 mm away.
Figure 4 summarizes these normalized firing rates to depict the average pattern of activation found in primary somatosensory cortex (5 monkeys) during single site stimulation, showing variable but rather high firing activity relative to the peak activation at cortical sites between 2 and 4 mm away. Thus in all formats, these data provide quantitative evidence for both the importance of local, modular processing and the ability for a single stimulus to activate a large extent of cortex, including cortex well beyond the borders of an individual digit representation.
Normalized firing rates showed weak but significant differences for individual monkeys (H = 42.72, df = 4, P < 0.0001). Therefore we performed Kruskal-Wallis analysis for data from each monkey to explore the differences.
Nonparametric analysis of normalized firing rates for each monkey
Nonparametric analysis of the data from individual monkeys showed that generally the data from each monkey followed the group trends; however, this was not universal. Supplementary Fig. S1 shows scatter plots of the normalized (percent) firing rate in relation to the cortical distance from the site of peak activation for each monkey individually. The effect of distance on normalized firing rate was significant for all five cases (case 1: H = 38.92, df = 25, P = 0.038; case 2: H = 80.85, df = 20, P < 0.0001; case 3: H = 179.64, df = 43, P < 0.0001; case 4: H = 29.19, df = 12, P = 0.004; case 5: H = 42.59, df = 27, P = 0.029).
Similarly, there were no significant differences between normalized firing rates of SUs and MUs for any of the five monkeys (case 1: P = 0.053; case 2: P = 0.262; case 3: P = 0.808; case 4: P = 0.178; case 5: P = 0.567).
In general, the grouping of the data based on whether the single-site stimuli were from within digits or across digits did not affect the normalized firing rates (case 1: U = 315.0, P = 0.706; case 2: U = 323.0, P = 0.406; case 4: U = 172.0, P = 0.259; case 5: U = 196.0, P = 0.365). However, for case 3, the mean rank of normalized firing rates grouped for stimuli presented within a digit was lower than that of the normalized firing rates grouped for stimuli presented across digits (U = 30057.5, P = 0.002). Yet the parametric analysis of the interaction of the distance and the spatial relationship of the stimulus groups did not reveal a significant effect on normalized firing rates [F(80, 1177) = 1.277, P = 0.058; Supplementary Table S2]. Thus the relationship between normalized firing rates and cortical distance from the site of peak activation did not appear to differ among representations within a digit and those across digit representations.
DISCUSSION
While the organization and receptive field properties of neurons of primary somatosensory cortex indicate the importance of local processing, surround modulation and other stimulus interactions have been reported to occur, implying the existence of more widespread activation in cortex than small, discrete receptive fields suggest. We used an array of 100 regularly spaced electrodes (400 μm apart) to examine the magnitudes of peak firing activity for neurons recorded across the hand representation of primary somatosensory cortex, area 3b, when stimulated at single, but varied locations on the hand. While stimuli at single skin locations cause widespread activation in primary somatosensory cortex over distances as much as 3 mm away from the site of peak activation, the drop off in peak firing rates with distance from the site where neurons had the highest peak firing rate indicates that local processing is emphasized in area 3b.
This main finding supports the concept of the importance of local processing within the primary somatosensory cortex hand representation. Thus most of the activation is within a small volume of cortex when a single skin location is stimulated. In addition, SUs had lower firing rates than MUs, and more MUs showed variability and higher firing rates at locations away from the peak activation. This likely reflects the variable numbers of neurons that are included in the MU samples from each electrode array. More importantly, SU and MU results demonstrate similar relationships between peak firing rate and distance from the site of peak activation (Fig. 1, see also Table S1). Thus conclusions based on either data set are basically the same.
While a definite peak of activation occurs in cortex in response to single-site stimulation, the normalized results add further support to the conclusion that many neurons are activated in response to the stimulation, and these neurons are spread across relatively large distances in the cortex, as much as 3–4 mm away (Fig. 2, Table S2). As this distance corresponds to the length of the hand representation in area 3b of owl monkeys, which is on the order of ∼4 × 2.5 mm, the entire hand representation is likely activated by most suprathreshold stimuli. Interestingly, we found no difference in the cortical spread of activation when stimulating locations across digits versus within digits (Figs. 2 and 3, Table S2), and this finding will be further discussed.
Figure 4 summarizes average normalized (percent) firing rates across cortical distance for single neurons to depict the general activation pattern across primary somatosensory cortex. The decrease in firing rates at the nearest electrode recording sites to the peak activation may be related to lateral inhibitory effects, while the increase in firing rates recorded from electrodes beyond 0.4 mm may reflect weak facilitative effects that extend beyond the traditional receptive field. Note we recorded from a smaller number of single neurons with spontaneous activity or driven activity in the regions 2–4 mm beyond the peak activation site; and fewer single neurons at these distances were classified as responsive. These lower sample sizes contribute to the variability in the firing rates at distances 2–4 mm away from the peak activation site.
These results of both focal increases in cortical activity coupled with weak widespread activation are supported by previous recording experiments (e.g., Burton et al. 1998; DiCarlo et al. 1998; Gardner and Costanzo 1980a,b; Juliano and Whitsel 1987; Sripati et al. 2006) and optical imaging experiments (e.g., Simons et al. 2007; Tommerdahl et al. 2002). Some intrinsic signal optical imaging experiments of the zones of significantly increased activity in the hand region of area 3b of squirrel monkeys after stimulation of a single digit reveal a less extensive spread of activation (not across the entire hand) but demonstrate the activation of a region larger than the representation of a single digit (e.g., Chen et al. 2009; Friedman et al. 2008). Intrinsic signal optical imaging may not reveal the full extent of the region where a stimulus increases neural activity, while electrophysiological recording with multi-electrode arrays allows fine temporal and spatial resolution of a relatively large region of cortex (4 × 4 mm), more than covering the hand representation of area 3b.
Our results in the hand representation of primary somatosensory cortex in owl monkeys complement previous findings in the rat whisker representation in primary somatosensory cortex. In an impressive study, Frostig et al. (2008) examined the spread of cortical activity by recording from multi-electrode arrays implanted into the barrel cortex of rats while stimulating specific whiskers. They found that single whisker stimulation resulted in peak activity occurring in neurons within the corresponding whisker barrel in cortex; however, they also found that the activity spreads across a cortical region ≤19.6 mm2 in size (Frostig et al. 2008). Our results apply to supragranular layers, while the findings of Frostig et al. (2008) applied to supragranular and granular layers. Similar but more limited results have been repeatedly observed for the barrel field of rats (e.g., Ebner and Armstrong-James 1990; Ghazanfar and Nicolelis 1999; Moore and Nelson 1998; Petersen and Diamond 2000).
Anatomical correlates
With anatomical investigations accompanying their physiological recordings, Frostig et al. (2008) found that the spread of activity in the barrel field of rats after single whisker stimulation corresponded approximately to the size of the spread of horizontal connections radiating from the sites of peak activity. In addition to these findings in rodents, there are reasons to believe that horizontal connections are underlying contributors to the widespread activation (over 3 mm) we found in the primary somatosensory cortex in monkeys. First, horizontal collaterals of pyramidal cells in layers 3 and 5 extend 3–6 mm in area 3b of macaque monkeys (De Felipe et al. 1986). This distance is consistent with other measures of the horizontal spread of intrinsic connections in the hand representation of area 3b of macaque monkeys (e.g., Burton and Fabri 1995; Manger et al. 1997) and with more limited results in the smaller hand representation in New World owl and squirrel monkeys (e.g., Fang et al. 2002). Nevertheless horizontal connections within area 3b appear to span the entire representation of five digits and join the representations of the digits and palm.
Horizontal connections are not the only possible contributors to the patterns of peak firing rates we found in this study. Thalamocortical axons have been traced in owl monkeys (Garraghty et al. 1989) and macaque monkeys (Garraghty and Sur 1990), revealing that these arbors can span across cortical columns and can have secondary branches terminating away from the main branch. In addition, adjacent thalamic neurons can project to targets ≤1.5 mm apart in area 3b of macaque monkeys (Rausell and Jones 1995). Convergence and divergence of thalamocortical connections to area 3b have been described in macaque monkeys (Padberg et al. 2009), and it is noteworthy that some of the same neurons in the ventroposterior lateral nucleus of the thalamus projected to two separate representations in area 3b. Thus thalamocortical connections are likely to be major contributors to the magnitude of the peak activation and may contribute to the activation at distances within 2 mm away from the peak activation. Neuron firing rates in area 3b are also influenced by feedback connections from other somatosensory representations, especially from areas 3a, 1, 2, S2, and PV (e.g., Burton and Fabri 1995; Burton et al. 1995; Cusick et al. 1989; Darian-Smith et al. 1993; Krubitzer and Kaas 1990; Qi et al. 2002; Wu and Kaas 2003). These feedback connections should produce slightly delayed peaks of activation.
Across digits versus within digits
Traditional anatomical connection studies have not studied the intrinsic connections within the primary somatosensory cortex hand representation in primates in fine detail, in part because tracer injection volumes tend to be large, obscuring labeling in the tissue surrounding the injection site. When the intrinsic connections have been examined, the results have conflicted between studies. In particular, Fang et al. (2002) reported that tracer injections in New World monkeys resulted in preferential labeling that connected digits with adjoining palm representations. However, Negyessy et al. (2009) presented results indicating that small tracer injections resulted in preferential labeling that connected neighboring distal digit regions rather than connecting regions within single digit representations. We sought to explore functional differences in the spread of activity in area 3b that may correspond to the density of anatomical connections across neighboring digits versus within a single digit. However, when results were grouped for stimuli presented across digits versus within digits, the results were similar and did not show significant differences (Fig. 2, Table S2). Accordingly, our results did not support the contention that intrinsic cortical connections spread activation more effectively within or across representations of digits.
Functional implications
The trends in peak activation across distances in cortex, as depicted in Fig. 4A, correspond well with known effects of lateral inhibition to sharpen responses in one zone while dampening responses in the surrounding zone. This inhibitory effect appears to weaken at the distances beyond 2 mm from the site of peak activation, resulting in higher, but variable, firing rates. However, we also recorded from fewer neurons at distances beyond 2 mm from the site of peak activation (Fig. 4B). This is not a problem due to the configuration of the multi-electrode array; rather this appears to reflect cortical organization. Low numbers of neurons fired above spontaneous rates to stimuli presented to a hand location that most strongly activated sites over 2 mm away.
The coexistence of the definite site of peak activation in cortex with the distant spread of activation levels in cortex when a single site is stimulated implies that when stimuli are presented to multiple sites, stimulus interactions will occur based on the spread of activation. Stimulus interactions ranging from facilitative to suppressive have been reported for paired or multipoint stimuli presented to the monkey hand in studies using optical imaging in primary somatosensory cortex (Chen et al. 2003; Friedman et al. 2008) and in studies recording from single neurons and multi-neuron clusters (Friedman et al. 2008; Reed et al. 2010). Such stimulus interactions likely underlie processes of surround modulation and may be important for processing stimulus information from one part of the hand in the context of stimulus information from other parts of the hand.
The potential overlap of spreading activation may have a role in associative learning and plasticity as Frostig et al. (2008) recently suggested. Several studies have shown that paired tactile stimulation, particularly long-term co-stimulation or behaviorally relevant co-stimulation results in cortical reorganization in primates (e.g., Blake et al. 2005; Recanzone et al. 1992; Wang et al. 1995; Xerri et al. 1996, 1999) and rats (e.g., Godde et al. 1996; Xerri et al. 1996); and affects human performance (e.g., Godde et al. 1996; Höffken et al. 2007; Kalisch et al. 2007; Pilz et al. 2004) and short-term cortical reorganization (e.g., Höffken et al. 2007; Pilz et al. 2004). While the current study investigated responses to stimulation of a single site with a small contact area (1 mm diam), our findings of widespread activation in monkeys, like those in rats (Frostig et al. 2008), suggest that a higher level of tactile processing occurs in area 3b than may have been expected based on the discrete processing regions.
In conclusion, our results are consistent with the traditional views of the somatotopic organization of the primary somatosensory cortex in primates and the importance of localized processing as the results consistently revealed the presence of a distinct site of peak activation when a single site was stimulated on the hand. However, we also found that activation spreads away from this site of peak activation, generally around 3 mm. Thus the presence of sites of peak activation with spreading excitation may underlie contextual modulation, and this higher-level processing occurs as early as primary somatosensory cortex in primates.
GRANTS
This work was supported by funding from the James S. McDonnell Foundation to J. H. Kaas and National Institutes of Health Grants NS-16446 to J. H. Kaas, F31-NS-053231 to J. L. Reed, EY-014680-03 to A. B. Bonds, and T32-GM-07347 to M. J. Burish.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank J. Haitas for assistance developing the Matlab code for data analysis, Drs. Zhiyi Zhou and Melanie Bernard for performing surgical manipulations, and Dr. Omar Gharbawie and C. Camalier for assisting with data collection from three of the five monkeys.
Present address of P. Pouget: INSERM—Université Pierre et Marie Curie Neurologie et Thérapeutique, Expérimentale Hôpital de la Salpêtrière, 47 boulevard de l'Hôpital, 75651 Paris CEDEX 13, France.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Blake et al., 2005. Blake DT, Strata F, Kempter R, Merzenich MM. Experience-dependent plasticity in S1 caused by noncoincident inputs. J Neurophysiol 94: 2239–2250, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton and Fabri, 1995. Burton H, Fabri M. Ipsilateral intracortical connections of physiologically defined cutaneous representations in area 3b and 1 of macaque monkeys: projections in the vicinity of the central sulcus. J Comp Neurol 355: 508–538, 1995 [DOI] [PubMed] [Google Scholar]
- Burton et al., 1995. Burton H, Fabri M, Alloway K. Cortical areas within the lateral sulcus connected to cutaneous representations in areas 3b and 1: a revised interpretation of the second somatosensory area in macaque monkeys. J Comp Neurol 355: 539–562, 1995 [DOI] [PubMed] [Google Scholar]
- Burton et al., 1998. Burton H, Sinclair RJ, Whang K. Vibrotactile stimulus order effects in somatosensory cortical areas of rhesus monkeys. Somato Mot Res 15: 316–324, 1998 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2003. Chen LM, Friedman RM, Roe AW. Optical imaging of a tactile illusion in area 3b of the primary somatosensory cortex. Science 302: 881–885, 2003 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2009. Chen LM, Friedman RM, Roe AW. Optical imaging of digit topography in individual awake and anesthetized squirrel monkeys. Exp Brain Res 196: 393–401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubbuck, 1966. Chubbuck JG. Small motion biological stimulator. Appl Phys Lab Tech Digest 5: 18–23, 1966 [Google Scholar]
- Cusick et al., 1989. Cusick CG, Wall JT, Felleman DJ, Kaas JH. Somatotopic organization of the lateral sulcus of owl monkeys: area 3b, S-II, and a ventral somatosensory area. J Comp Neurol 282: 169–190, 1989 [DOI] [PubMed] [Google Scholar]
- Darian-Smith et al., 1993. Darian-Smith C, Darian-Smith I, Burman K, Ratcliffe N. Ipsilateral cortical projections to areas 3a, 3b, and 4 in the macaque monkey. J Comp Neurol 335: 200–213, 1993 [DOI] [PubMed] [Google Scholar]
- De Felipe et al., 1986. De Felipe J, Conley M, Jones EG. Long-range focal collateralization of axons arising from corticocortical cells in monkey sensory-motor cortex. J Neurosci 6: 3749–3766, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo and Johnson, 2000. DiCarlo JJ, Johnson KO. Spatial and temporal structure of receptive fields in primate somatosensory area 3b: effects of stimulus scanning direction and orientation. J Neurosci 20: 495–510, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo and Johnson, 2002. DiCarlo JJ, Johnson KO. Receptive field structure in cortical area 3b of the alert monkey. Behav Brain Res 135: 167–178, 2002 [DOI] [PubMed] [Google Scholar]
- DiCarlo et al., 1998. DiCarlo JJ, Johnson KO, Hsiao SS. Structure of receptive fields in area 3b of primary somatosensory cortex in the alert monkey. J Neurosci 18: 2626–2645, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner and Armstrong-James, 1990. Ebner FF, Armstrong-James MA. Intracortical processes regulating the integration of sensory information. Prog Brain Res 86: 129–141, 1990 [DOI] [PubMed] [Google Scholar]
- Fang et al., 2002. Fang P-C, Jain N, Kaas JH. Few intrinsic connections cross the hand-face border of area 3b of New World monkeys. J Comp Neurol 454: 310–319, 2002 [DOI] [PubMed] [Google Scholar]
- Friedman et al., 2008. Friedman RM, Chen LM, Roe AW. Responses of areas 3b and 1 in anesthetized squirrel monkeys to single- and dual-site stimulation of the digits. J Neurophysiol 100: 3185–3196, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostig et al., 2008. Frostig RD, Xiong Y, Chen-Bee CH, Kvšňák E, Stehberg J. Large-scale organization of rat sensorimotor cortex based on a motif of large activation spreads. J Neurosci 28: 13274–13284, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner and Costanzo, 1980a. Gardner EP, Costanzo RM. Spatial integration of multiple-point stimuli in primary somatosensory cortical receptive fields of alert monkeys. J Neurophysiol 43: 420–443, 1980a [DOI] [PubMed] [Google Scholar]
- Gardner and Costanzo, 1980b. Gardner EP, Costanzo RM. Temporal integration of multiple-point stimuli in primary somatosensory cortical receptive fields of alert monkeys. J Neurophysiol 43: 444–468, 1980b [DOI] [PubMed] [Google Scholar]
- Garraghty et al., 1989. Garraghty PE, Pons TP, Sur M, Kaas JH. The arbors of axons terminating in middle cortical layers of somatosensory area 3b in owl monkeys. Somatosens Motor Res 6: 401–411, 1989 [DOI] [PubMed] [Google Scholar]
- Garraghty and Sur, 1990. Garraghty PE, Sur M. Morphology of single intracellularly stained axons terminating in area 3b of macaque monkeys. J Comp Neurol 294: 583–593, 1990 [DOI] [PubMed] [Google Scholar]
- Ghazanfar and Nicolelis, 1999. Ghazanfar AA, Nicolelis MAL. Spatiotemporal properties of layer V neurons of the rat primary somatosensory cortex. Cereb Cortex 9: 348–361, 1999 [DOI] [PubMed] [Google Scholar]
- Godde et al., 1996. Godde B, Spengler F, Dinse HR. Associative pairing of tactile stimulation induces somatosensory cortical reorganization in rats and humans. Neuroreport 8: 281–285, 1996 [DOI] [PubMed] [Google Scholar]
- Greek et al., 2003. Greek KA, Chowdhury SA, Rasmusson DD. Interactions between inputs from adjacent digits in somatosensory thalamus and cortex of the raccoon. Exp Brain Res 151: 364–371, 2003 [DOI] [PubMed] [Google Scholar]
- Grinvald et al., 1994. Grinvald A, Lieke EE, Frostig RD, Hildesheim R. Cortical point-spread function and long-range lateral interactions revealed by real-time optical imaging of macaque monkey primary visual cortex. J Neurosci 14: 2545–2568, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höffken et al., 2007. Höffken O, Viet M, Knossalla F, Lissek S, Bliem B, Ragert P, Dinse HR, Tegenthoff M. Sustained increase of somatosensory cortex excitability by tactile coactivation studied by paired median nerve stimulation in humans correlates with perceptual gain. J Physiol 584.2: 463–471, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamura et al., 1983. Iwamura Y, Tanaka M, Sakamoto M, Hikosaka O. Functional subdivisions representing different finger regions in area 3b or the first somatosensory cortex of the conscious monkey. Exp Brain Res 51: 315–326, 1983 [Google Scholar]
- Jain et al., 1998. Jain N, Catania KC, Kaas JH. A histologically visible representation of the fingers and palm in primate area 3b and its immutability following long-term deafferentations. Cereb Cortex 8: 227–236, 1998 [DOI] [PubMed] [Google Scholar]
- Jain et al., 2001. Jain N, Qi H-X, Kaas JH. Long-term chronic multichannel recordings from sensorimotor cortex and thalamus of primates. Prog Brain Res 130: 1–10, 2001 [PubMed] [Google Scholar]
- Juliano and Whitsel, 1987. Juliano SL, Whitsel BL. A combined 2-deoxyglucose and neurophysiological study of primate somatosensory cortex. J Comp Neurol 263: 514–525, 1987 [DOI] [PubMed] [Google Scholar]
- Kalisch et al., 2007. Kalisch T, Tegenthoff M, Dinse HR. Differential effects of synchronous and asynchronous multifinger coactivation on human tactile performance. BMC Neurosci 8: 58, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer and Kaas, 1990. Krubitzer LA, Kaas JH. The organization and connections of somatosensory cortex in marmosets. J Neurosci 10: 952–974, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin and Spencer, 1979. Laskin SE, Spencer WA. Cutaneous masking. II. Geometry of excitatory and inhibitory receptive fields of single units in somatosensory cortex of the cat. J Neurophysiol 42: 1061–1082, 1979 [DOI] [PubMed] [Google Scholar]
- Manger et al., 1997. Manger PR, Woods TM, Muñoz A, Jones EG. Hand/face border as a limiting boundary in the body representation in monkey somatosensory cortex. J Neurosci 17: 6338–6351, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason et al., 1991. Mason A, Nicoll A, Stratford K. Synaptic transmission between individual pyramidal neurons of the rat visual cortex in vitro. J Neurosci 11: 72–84, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich et al., 1983. Merzenich MM, Kaas JH, Wall J, Nelson RJ, Sur M, Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience 8: 33–55, 1983 [DOI] [PubMed] [Google Scholar]
- Moore and Nelson, 1998. Moore CI, Nelson SB. Spatio-temporal subthreshold receptive fields in the vibrissa representation of rat primary somatosensory cortex. J Neurophysiol 80: 2882–2892, 1998 [DOI] [PubMed] [Google Scholar]
- Mountcastle and Powell, 1959. Mountcastle VB, Powell TPS. Neural mechanisms subserving cutaneous sensibility, with special reference to the role of afferent inhibition in sensory perception and discrimination. Bull Johns Hopkins Hosp 105: 201–232, 1959 [PubMed] [Google Scholar]
- Negyessy et al., 2009. Negyessy L, Friedman R, Chen LM, Dillenburger BC, Palmer CT, Jákli B, Cserey G, Roe AW. Intrinsic and extrinsic connections of fingertip representation in the somatosensory cortex of the squirrel monkey. Soc Neurosci Abstr 65612, 2009 [Google Scholar]
- Nicolelis et al., 2003. Nicolelis MAL, Dimitrov D, Carmena JM, Crist R, Lehew G, Kralik JD, Wise SP. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc Natl Acad Sci USA 100: 11041–11046, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padberg et al., 2009. Padberg J, Cerkevich C, Engle J, Rajan AT, Recanzone G, Kaas JH, Krubitzer L. Thalamocortical connections of parietal somatosensory cortical fields in macaque monkeys are highly divergent and convergent. Cereb Cortex 19: 2038–2064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen and Diamond, 2000. Petersen RS, Diamond ME. Spatial-temporal distribution of whisker-evoked activity in rat somatosensory cortex and the coding of stimulus location. J Neurosci 20: 6135–6143, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz et al., 2004. Pilz K, Veit R, Braun C, Godde B. Effects of co-activation on cortical organization and discrimination performance. Neuroreport 15: 2669–2672, 2004 [DOI] [PubMed] [Google Scholar]
- Pons et al., 1987. Pons TP, Wall JT, Garraghty PE, Cusick CG, Kaas JH. Consistent features of the representation of the hand in area 3b of macaque monkeys. Somatosens Res 4: 309–331, 1987 [DOI] [PubMed] [Google Scholar]
- Qi and Kaas, 2004. Qi H-X, Kaas JH. Myelin stains reveal an anatomical framework for the representation of the digits in somatosensory area 3b of macaque monkeys. J Comp Neurol 447: 172–187, 2004 [DOI] [PubMed] [Google Scholar]
- Qi et al., 2002. Qi H-X, Lyon DC, Kaas JH. Cortical and thalamic connections of the parietal ventral somatosensory area in marmoset monkeys (Callithrix jacchus). J Comp Neurol 443: 168–182, 2002 [DOI] [PubMed] [Google Scholar]
- Rausell and Jones, 1995. Rausell E, Jones EG. Extent of intracortical arborization of thalamocortical axons as a determinant of representational plasticity in monkey somatic sensory cortex. J Neurosci 15: 4270–4288, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone et al., 1992. Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization in cortical area 3b of owl monkeys trained in a frequency-discrimination task. J Neurophysiol 67: 1031–1056, 1992 [DOI] [PubMed] [Google Scholar]
- Reed et al., 2008. Reed JL, Pouget P, Qi H-X, Zhou Z, Bernard MR, Burish MJ, Haitas J, Bonds AB, Kaas JH. Widespread spatial integration in primary somatosensory cortex. Proc Natl Acad Sci USA 105: 10233–10237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed et al., 2010. Reed JL, Qi H-X, Zhou Z, Bernard MR, Burish MJ, Bonds AB, Kaas JH. Response properties of neurons in primary somatosensory cortex of owl monkeys reflect widespread spatiotemporal integration. J Neurophysiol 103: 2139–2157, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samonds et al., 2003. Samonds JM, Allison JD, Brown HA, Bonds AB. Cooperation between Area 17 neuron pairs enhances fine discrimination of orientation. J Neurosci 23: 2416–2425, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham et al., 2003. Shoham S, Fellows MR, Normann RA. Robust, automatic spike sorting using mixtures of multivariate t-distributions. J Neurosci Methods 127: 111–122, 2003 [DOI] [PubMed] [Google Scholar]
- Simons et al., 2007. Simons SB, Chiu J, Favorov OV, Whitsel BL, Tommerdahl M. Duration-dependent response of SI to vibrotactile stimulation in squirrel monkey. J Neurophysiol 97: 2121–2129, 2007 [DOI] [PubMed] [Google Scholar]
- Sripati et al., 2006. Sripati AP, Yoshioka T, Denchev P, Hsiao SS, Johnson KO. Spatiotemporal receptive fields of peripheral afferents and cortical area 3b and 1 neurons in the primate somatosensory system. J Neurosci 26: 2101–2114, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur, 1980. Sur M. Receptive fields of neurons in areas 3b and 1 of somatosensory cortex in monkeys. Brain Res 198: 465–471, 1980 [DOI] [PubMed] [Google Scholar]
- Sur et al., 1985. Sur M, Garraghty PE, Bruce CJ. Somatosensory cortex in macaque monkeys: laminar differences in receptive field size in areas 3b and 1. Brain Res 342: 391–395, 1985 [DOI] [PubMed] [Google Scholar]
- Tommerdahl et al., 2002. Tommerdahl M, Favorov O, Whitsel BL. Optical imaging of intrinsic signals in somatosensory cortex. Behav Brain Res 135: 83–91, 2002 [DOI] [PubMed] [Google Scholar]
- Veredas et al., 2005. Veredas FJ, Vico FJ, Alonso JM. Factors determining the precision of the correlated firing generated by a monosynaptic connection in the cat visual pathway. J Physiol 567.3: 1057–1078, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 1995. Wang X, Merzenich MM, Sameshima K, Jenkins WM. Remodelling of hand representation in adult cortex determined by timing of tactile stimulation. Nature 378: 71–75, 1995 [DOI] [PubMed] [Google Scholar]
- Wu and Kaas, 2003. Wu CW-H, Kaas JH. Somatosensory cortex of prosimian galagos: physiological recording, cytoarchitecture, and cortiocortical connections of anterior parietal cortex and cortex of the lateral sulcus. J Comp Neurol 457: 263–292, 2003 [DOI] [PubMed] [Google Scholar]
- Xerri et al., 1996. Xerri C, Coq JO, Merzenich MM, Jenkins WM. Experience-induced plasticity of cutaneous maps in the primary somatosensory cortex of adult monkeys and rats. J Physiol 90: 277–287, 1996 [DOI] [PubMed] [Google Scholar]
- Xerri et al., 1999. Xerri C, Merzenich MM, Jenkins W, Santucci S. Representational plasticity in cortical area 3b paralleling tactual-motor skill acquisition in adult monkeys. Cereb Cortex 9: 264–276, 1999 [DOI] [PubMed] [Google Scholar]
- Xu et al., 2003. Xu X, Collins CE, Kaskan PM, Khaytin I, Kaas JH, Casagrande VA. Optical imaging of visually evoked responses in prosimian primates reveals conserved features of the middle temporal visual area. Proc Natl Acad Sci USA 101: 2566–2571, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al., 2008. Zhou Z, Bernard MR, Bonds AB. Deconstruction of spatial integrity in visual stimulus detected by modulation of synchronized activity in cat visual cortex. J Neurosci 28: 3759–3768, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.