Abstract
Many analgesic drugs, including μ-opioids, cannabinoids, and the novel nonopioid analgesic improgan, produce antinociception by actions in the rostral ventromedial medulla (RVM). There they activate pain-inhibiting neurons, termed “off-cells,” defined by a nociceptive reflex-related pause in activity. Based on recent functional evidence that neuronal P450 epoxygenases are important for the central antinociceptive actions of morphine and improgan, we explored the convergence of opioid and nonopioid analgesic drug actions in RVM by studying the effects of the P450 epoxygenase inhibitor CC12 on the analgesic drug-induced activation of these off-cells and on behavioral antinociception. In rats lightly anesthetized with isoflurane, we recorded the effects of intraventricular morphine and improgan, with and without CC12 pretreatment, on tail flick latency and activity of identified RVM neurons: off-cells, on-cells (pronociceptive neurons), and neutral cells (unresponsive to analgesic drugs). CC12 pretreatment preserved reflex-related changes in off-cell firing and blocked the analgesic actions of both drugs, without interfering with the increase in spontaneous firing induced by improgan or morphine. CC12 blocked suppression of evoked on-cell firing by improgan, but not morphine. CC12 pretreatment had no effect by itself on RVM neurons or behavior. These data show that the epoxygenase inhibitor CC12 works downstream from receptors for both μ-opioid and improgan, at the inhibitory input mediating the off-cell pause. This circuit-level analysis thus provides a cellular basis for the convergence of opioid and nonopioid analgesic actions in the RVM. A presynaptic P450 epoxygenase may therefore be an important target for development of clinically useful nonopioid analgesic drugs.
INTRODUCTION
Both opioid and nonopioid analgesic drugs act in the brain stem to stimulate modulatory circuits capable of dampening spinal nociceptive transmission. Although different analgesic drug classes bind to distinct receptors, many details of the postreceptor transduction mechanisms leading to analgesia remain unknown (Christie et al. 2000; Ingram 2000). Recently, pharmacological and molecular studies demonstrated a critical role for brain cytochrome P450 epoxygenase activity in the analgesic action of the μ-opioid agonist morphine (Conroy et al. 2010). An important pathway for arachidonic acid metabolism, P450 epoxygenase was subsequently shown to be required for the antinociceptive effects of a novel nonopioid analgesic, improgan (Hough et al. 2009a). These findings provided a pharmacological explanation for the earlier report that the P450 epoxygenase inhibitor CC12 {[4(5)-[(4-iodobenzyl)thiomethyl]-1H-imidazole]} was able to block both morphine and improgan antinociception (Hough et al. 2007; Stadel et al. 2008). Although these results suggested that P450 enzymes represent a point of convergence of both opioid and nonopioid analgesic mechanisms in the brain stem, no neurophysiological studies of P450 inhibitors on identified brain stem pain-modulating neurons have yet been performed. Such studies are needed to map pharmacological findings onto functionally identifiable neural elements.
μ-Opioid agonists and improgan produce their antinociceptive effects at least in part by actions in the rostral ventromedial medulla (RVM), the spinally projecting output relay of an important brain stem pain-modulating system. The RVM receives a dense input from the midbrain periaqueductal gray (PAG) and projects via the dorsolateral funiculus to the dorsal horn of the spinal cord, where it modulates nociceptive transmission (Fields et al. 2006; Heinricher et al. 2009). Direct local application of μ-agonists or improgan in the RVM produces antinociception, and inactivation of the RVM attenuates or blocks the antinociceptive effects of both drugs (Heinricher and Ingram 2008; Nalwalk et al. 2004).
μ-Opioids have been shown to act both pre- and postsynaptically in the RVM, whereas the membrane mechanisms of improgan antinociception are as yet unknown. Nevertheless, both drugs activate a physiologically defined population of RVM neurons, referred to as “off-cells,” and suppress the firing of a second set, the “on-cells” (Heinricher and Ingram 2008; Heinricher et al. 2010). These two cell classes support bidirectional nociceptive modulation from the RVM: off-cells are defined by an abrupt cessation of activity (“off-cell pause”) associated with nociceptive withdrawal responses, and these neurons have a net antinociceptive effect. on-cells are defined by an activation during nociceptive withdrawals (“on-cell burst”), and they exert a net pronociceptive influence. Activation of off-cells is sufficient to produce behaviorally measurable antinociception, and preventing this activation attenuates the antinociceptive effect of systemically administered morphine (Heinricher and Ingram 2008). By contrast, on-cells contribute to hypersensitivity in inflammatory and neuropathic pain models (Heinricher et al. 2009). Suppression of on-cell firing by analgesic drugs likely contributes to antinociception, but is not by itself sufficient to produce potent analgesia (Heinricher et al. 1999, 2001b).
The present experiments investigated the cellular basis for the postulated convergence of opioid and nonopioid analgesic drug actions in the RVM using a circuit-level, in vivo electrophysiological analysis of the effects of the P450 epoxygenase inhibitor CC12 (Stadel et al. 2008) on opioid- and improgan-sensitive pain-modulating neurons in the RVM. We hypothesize that P450 inhibitors will prevent activation of RVM off-cells by analgesic drugs.
METHODS
Animals, surgical preparation, and nociceptive testing
All experimental procedures followed the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain and were approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University. Male Sprague–Dawley rats (275–325 g; Taconic Farms) were maintained under isoflurane anesthesia throughout the experiments, which generally required 3–5 h. After initial induction (4%, humidified O2, 1.5 L/min), isoflurane concentration was reduced to 3% and the rats were placed in a stereotaxic instrument for surgical preparation. Small craniotomies and openings in the dura were made to allow placement of electrodes in the RVM and infusion cannulae in the lateral ventricle. Following surgery, the animals were maintained with 1.00–1.25% isoflurane, a concentration that allows a stable baseline tail flick latency and is sufficient to prevent signs of discomfort. With this approach, animals did not show spontaneous movement and noxious stimulation such as pinch elicited only a brief withdrawal reflex. Body temperature was maintained at close to 37°C by use of a circulating water pad. A stabilization period of ≥30 min was required before initiating the drug protocol, and isoflurane concentration was not altered during the protocol.
Tail flick (TF) latency was used as a measure of nociceptive responsiveness, as described previously (Barbaro et al. 1989; Neubert et al. 2004). Each trial consisted of a linear increase in temperature at about 1.8°C/s from a holding temperature of 34°C, until the reflex occurred, or to a maximum of 53°C at 10.6 s. Trials were carried out at 5-min intervals throughout the experiment. The holding temperature obviates any concern that apparent effects on reflex latency could be attributed to changes in skin temperature. Previous work has shown that response latencies and neuronal response properties are stable for a period of several hours with this testing protocol (Edelmayer et al. 2009; Heinricher et al. 2004, 2010; Kincaid et al. 2006; Martenson et al. 2009).
RVM recording
A gold- and platinum-plated stainless steel recording microelectrode (FHC, Bowdoinham, ME or Microprobe, Gaithersburg, MD) was placed in the RVM using landmarks. RVM neurons were classified as previously described (Barbaro et al. 1989; Fields et al. 1983). Spike waveforms were monitored and stored for off-line analysis (Spike2; CED, Cambridge, UK) to ensure that the unit under study was unambiguously discriminated throughout the experiment. off-cells were characterized by an abrupt pause in any ongoing activity that began just prior to the TF. on-cells were identified by a sudden burst of activity beginning just prior to the TF. Both off- and on-cells typically responded to noxious pinch of any region of the body, with inhibition and excitation, respectively. neutral-cells were identified by a lack of TF-related change in activity. Because reflex-related on-cell activation is not normally detectable when a neuron is already spontaneously active and because a reflex-related off-cell pause can be seen only when a neuron is active, we attempted to test neurons during both active and silent periods during the characterization phase.
Protocol and data analysis
We determined the effects of CC12 on the ongoing and reflex-related discharges of RVM neurons and on the responses of these neurons to improgan and morphine. CC12 hydrochloride (Hough et al. 2007) was dissolved in saline, with pH adjusted to 4.5–5. Improgan base (Hough et al. 2000) was dissolved in dilute HCl, neutralized to pH 5.5 to 6, and diluted with saline. Morphine sulfate was dissolved in saline. All compounds were given by intracerebroventricular (icv) injection (8 μl, coordinates relative to bregma: −0.8 anterior–posterior, 1.5 lateral, 3.3 ventral to surface). Injections were made into the lateral ventricle over a period of about 4 min and only one protocol was performed per animal.
In the first set of experiments, the effects of CC12 by itself on TF latency and RVM cell activity were determined. Following three predrug baseline TF trials, CC12 (460 nmol, sufficient to reverse the antinociceptive effects of improgan) or pH-matched saline was infused into the lateral ventricle. TF latency and cell activity were then monitored for an additional 70 min. In the second set of experiments, CC12 (460 nmol) or pH-matched saline was infused into the lateral ventricle following three predrug baseline TF trials. An additional three TF trials were performed, and improgan (580 nmol) was then given icv. Finally, in the third set of experiments, CC12 (150–460 nmol/8 μl) or saline was injected followed by morphine (7.5–30 nmol). Averages of the three baseline trials and the three pretreatment trials were compared with postimprogan/morphine time points.
Up to three cell parameters were analyzed so that we could identify drug effects on both ongoing (i.e., spontaneous) activity and reflex-related responses of RVM neurons.
1 Ongoing activity. Because off-cells and on-cells often show irregular alternations between periods of silence and activity, cell activity integrated over the 30 s prior to each withdrawal trial was used as an index of overall ongoing firing.
2 on-cell TF-related burst. Average firing rate in the 3-s period beginning 0.5 s before the TF was recorded for all TF trials. For trials in which the TF was inhibited by improgan or morphine, this parameter was calculated around the mean predrug TF latency. This approach, rather than counting the number of spikes or duration of the reflex-related burst, was necessary because a burst as such can be identified only in cases in which the neuron is inactive at the time of heat onset.
3 off-cell pause. Reflex- or heat-related inhibition was calculated as the firing rate in the 3 s beginning 0.5 s before the reflex (or mean predrug reflex latency in cases with no flick) expressed as a percentage of firing rate in the 10 s immediately before heat onset. A value of 100% therefore indicates no slowing during the heat stimulus, a value of 0% that firing was completely inhibited over this 3-s interval. In addition, duration of the reflex-related pause was determined for the subset of trials that fell at a time when the off-cell was active at heat onset and for which there was a tail flick.
Cell data are presented only for those experiments in which we were able to follow and reliably identify the cell under study for the entire protocol.
Data are presented as mean + SE. The average of the predrug baseline was compared with postinjection time points. Wilcoxon's signed ranks or Mann–Whitney U tests were used for statistical analysis of firing rates. ANOVA with repeated measures (followed by Dunnett's test for comparison with baseline or Bonferroni test for comparisons between groups) or a t-test for correlated means was used to compare pre- and postdrug TF latencies and pause parameters. P < 0.05 was considered statistically significant.
Histology
At the conclusion of the experiments, recording sites were marked with an electrolytic lesion and lateral ventricle infusion sites by injection of pontamine sky blue dye. Animals were killed with an overdose of isoflurane and perfused intracardially with physiological saline followed by 10% formalin. Recording and infusion sites were histologically verified. The RVM was defined as the nucleus raphe magnus and adjacent reticular formation at the level of the facial nucleus. Recording sites were located within the RVM as in previous publications from this laboratory (Heinricher and Roychowdhury 1997; Heinricher and Tortorici 1994).
RESULTS
Effects of CC12 on TF response and RVM neuronal activity
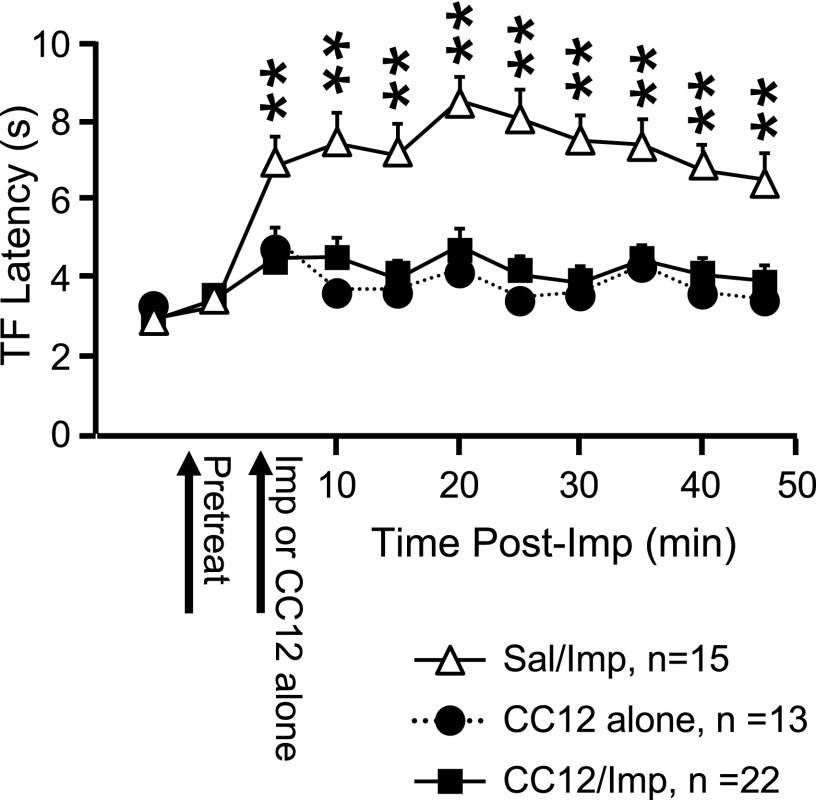
By itself, CC12 produced a modest increase in TF latency lasting about 5 min (Fig. 1, which also shows the effect of CC12 pretreatment on improgan antinociception), consistent with previous work in awake, behaving animals (Hough et al. 2007). Ongoing discharges of off- and on-cells were unaffected by CC12. For off-cells, mean ongoing firing was 16.4 ± 7.2 and 16.6 ± 7.3 spikes/s before and after CC12 injection (n = 6), respectively. For on-cells, mean ongoing firing was 6.9 ± 5.1 and 6.9 ± 5.2 spikes/s before and after CC12 injection (n = 6), respectively. Reflex-related changes in firing of off- or on-cells were also not altered by CC12 (Fig. 2, right, group data).
Fig. 1.
Analgesic action of intracerebroventricularly (icv) administered improgan is blocked by pretreatment with CC12 {[4(5)-[(4-iodobenzyl)thiomethyl]-1H-imidazole]}. Tail flick (TF) latency as a function of time following improgan injection. Saline or CC12 and improgan (Imp) were injected at times indicated. In CC12-alone group, CC12 was injected at 2nd arrow. **P < 0.01 compared with CC12/improgan animals. (Two-way repeated-measures ANOVA, followed by Bonferroni posttest for comparisons among groups. Significant effect of drug F(2,45) = 19.9, P < 0.0001; time F(9,405) = 16.3, P < 0.0001; and drug × time interaction F(18,405) = 5.9, P < 0.0001.) Mean + SE.
Fig. 2.
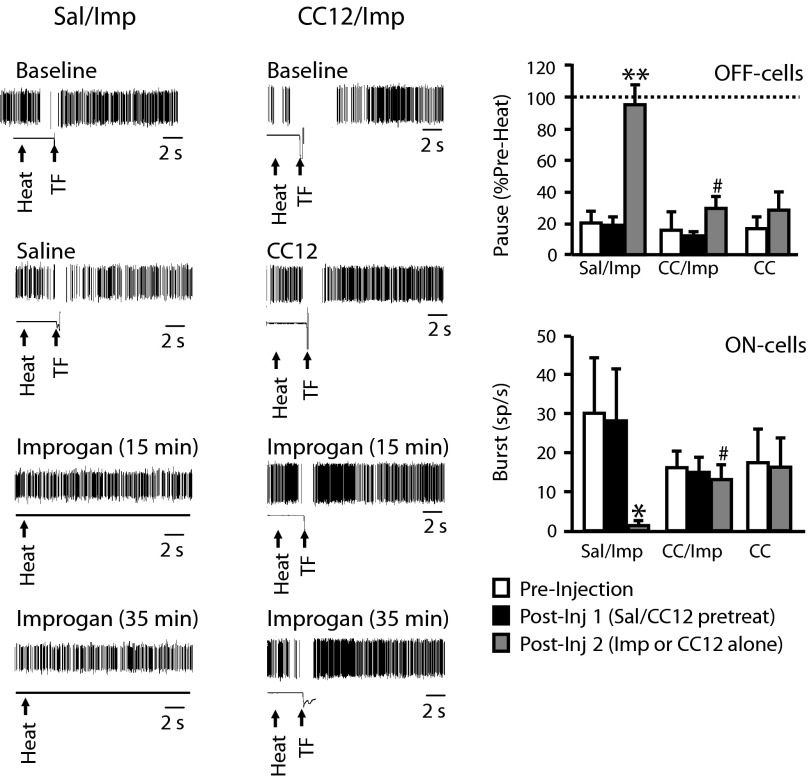
Improgan effects on reflex-related off-cell pause and on-cell burst are blocked by CC12. Left: individual TF trials shown on an expanded time base (10-s sweep) document block of off-cell pause following improgan administration in an animal pretreated with saline (left, Sal/Imp) and maintained pause in animal pretreated with CC12 (right, CC12/Imp). Spike train and output of tail position monitor shown, with heat onset and TF time indicated by arrows. Ongoing activity of these 2 neurons is shown in Fig. 3. Right: group data. off-cell pause and on-cell burst in preinjection baseline, following pretreatment with saline or CC12 (CC) and after improgan (or CC12-alone). Pause is defined as firing rate at time of the reflex as percentage of rate immediately prior to heat onset. A value of 100% indicates no slowing during the heat stimulus. Different time points compared using repeated-measures ANOVA followed by Dunnett's test for comparison with preinjection baseline or with t-test for correlated means with only 2 time points (CC12-alone). For Sal/Imp group: F(2,14) = 33.4, P < 0.0001; for CC/Imp group: F(2,8) = 0.74, P = 0.51; for CC12-alone group, t5 = 2.5, P = 0.053. Postimprogan time point for Sal/Imp and CC12/Imp compared using t-test for unpaired means, t11 = 5.1, P = 0.003. Burst defined as firing rate (in spikes/s) at time of the reflex. Different time points compared using Friedman's (FR) ANOVA by ranks with significant result followed by comparison of postinjection times with preinjection baseline, or by Wilcoxon's test with only 2 time points (CC12-alone). For Sal/Imp group: Fr (2) = 10.3, P = 0.006; for CC/Imp group: Fr (2) = 2.89, P = 0.24; for CC12-alone group, z = 0.74, P = 0.46. Postimprogan time point for Sal/Imp and CC12/Imp compared using Mann–Whitney, U = 2.0, P = 0.01. For improgan or CC12-alone injection, average of 10- to 20-min postinjection trials is given. Mean + SE, *P < 0.05 compared with predrug baseline. #P < 0.05 compared with saline-pretreated animals receiving improgan. No difference among groups in predrug baseline [off: F(2,16) = 0.091, P = 0.91, on: Kruskal–Wallis (KW) (2) = 1.04, P = 0.59] or after saline/CC12 injection [off: F(2,16) = 1.07, P = 0.37; on KW (2) = 0.48, P = 0.79]; 5–8 cells/group.
Effects of CC12 on behavioral and neuronal responses to improgan
IMPROGAN ANTINOCICEPTION.
As shown previously in awake, behaving animals (Hough et al. 2007), CC12 pretreatment significantly attenuated the antinociceptive actions of improgan (Fig. 1). The antagonism of the behavioral effect of improgan by CC12 was complete, since no differences in latencies were observed between the CC12-alone and CC12/Improgan groups at any time point.
IMPROGAN ACTIVATION OF OFF-CELLS AND SUPPRESSION OF ON-CELL FIRING.
We were able to successfully record the effects of CC12 pretreatment on the neuronal responses to improgan in a subset of the animals described above (5–8 cells/class/group). We previously showed that improgan causes off-cells to become continuously active, with an increase in firing rate and elimination of the reflex-related pause. on-cell firing is suppressed following icv improgan administration (Heinricher et al. 2010).
As shown in the examples in Fig. 2, the effects of improgan on the off-cell reflex-related pause were blocked by CC12. Although the pause evident in the predrug baseline (top row) was neither blocked nor potentiated by either saline (left column, second row) or CC12 (right column, second row), injection of improgan did not eliminate the pause when given subsequent to CC12 (postimprogan trials in third and fourth rows). Thus off-cells continued to pause at the time of the TF in animals that received CC12 prior to improgan, whereas these neurons fired without slowing during tail heat in animals pretreated with saline (Fig. 2, right, group data). The duration of the reflex-related pause was not significantly reduced following improgan in CC12-pretreated subjects (predrug baseline: 18.0 ± 12.0 s; postimprogan: 4.8 ± 2.4 s, P > 0.05). Finally, there was no change after improgan in the latency from the final spike before each TF to the reflex (predrug baseline: 631 ± 177 ms between final spike and reflex; postimprogan: 588 ± 184 ms, P > 0.05). As already noted, CC12 by itself had no effect on the off-cell pause (Fig. 2, right).
CC12 also prevented improgan suppression of the on-cell burst (Fig. 2, right, group data). on-cells continued to show a reflex-related burst in animals that received CC12 prior to improgan, whereas there was no discharge during tail heating in animals that received saline prior to improgan. CC12 by itself had no effect on the on-cell burst (Fig. 2, right).
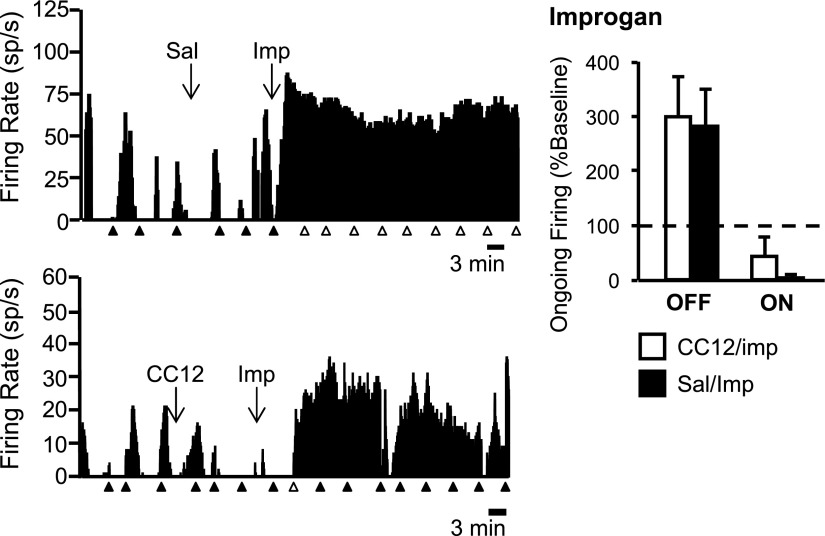
By contrast with its effects on reflex-related activity, CC12 did not antagonize improgan's effects on the ongoing activity of off- or on-cells. Ratemeter records illustrating typical off-cell responses to improgan following saline and CC12 pretreatment are shown in Fig. 3, with both neurons exhibiting a strong activation following improgan injection. Group data confirm the inability of CC12 to block improgan-induced changes in ongoing activity of either off- or on-cells (Fig. 3, right).
Fig. 3.
Effects of improgan on ongoing off- and on-cell firing are not blocked by CC12. Left: ratemeter records illustrate ongoing activity of 2 off-cells in predrug baseline, after icv injection of saline (Sal, top) or CC12 (bottom) and following subsequent icv injection of improgan (Imp). Ongoing activity was increased to >500% of predrug rate in both cases. Triangles indicate TF trials. Filled triangles indicate that there was a withdrawal; open triangles, no behavioral response within 10.6-s cutoff; 1-s bins. Individual TF trials for these 2 cells shown on expanded time base in Fig. 2. Right: group data. Ongoing firing of off- and on-cells following icv Imp expressed as percentage of predrug baseline. In neither case was the response to Imp blocked by CC12 pretreatment. Firing rates from saline-pretreated (Sal) animals compared with CC12-pretreated animals using Mann–Whitney U test (off-cells: U = 16, P = 0.56; on-cells: U = 18, P = 0.66). Mean + SE. Improgan: 5–8 cells/group; morphine: 5–8 cells/group.
neutral-cells do not respond to improgan (Heinricher et al. 2010) and remained unresponsive following CC12 pretreatment (n = 5; data not shown).
In sum, CC12 interfered with improgan's effects on the reflex-related changes in off- and on-cell firing, but did not block effects on ongoing (i.e., spontaneous) activity.
Effects of CC12 on behavioral and neuronal responses to morphine
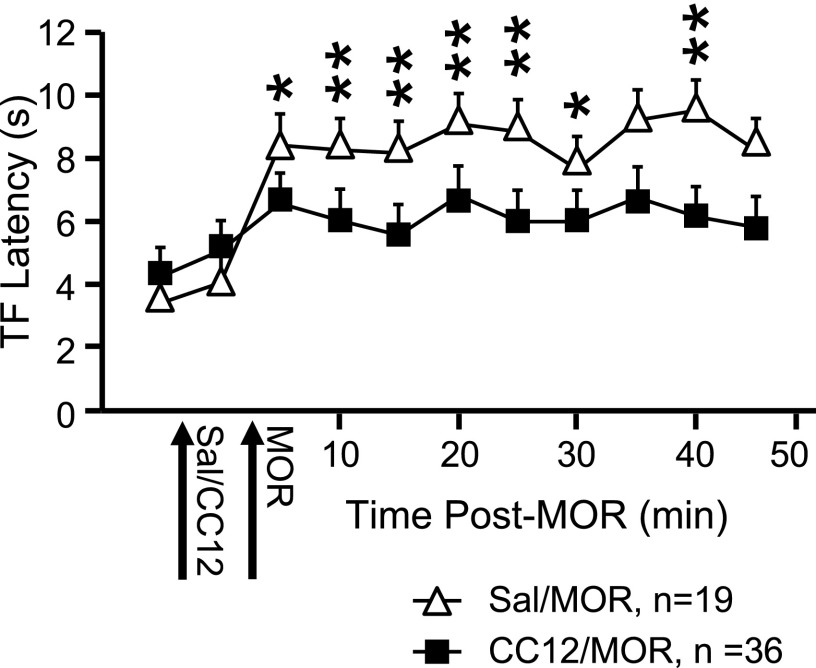
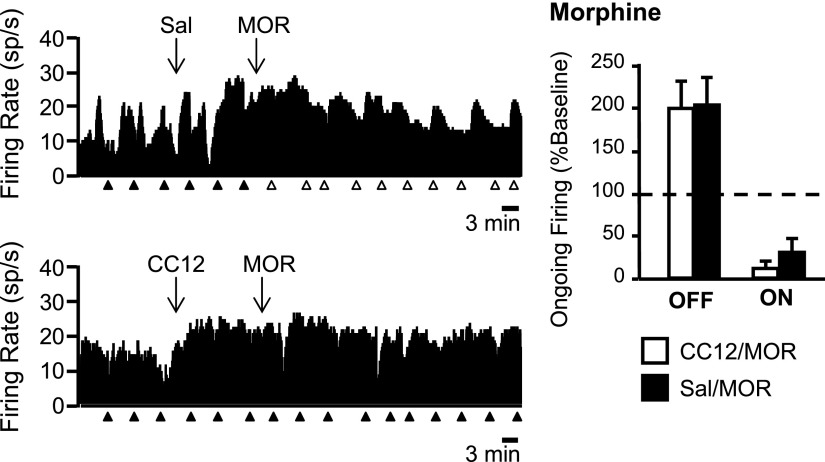
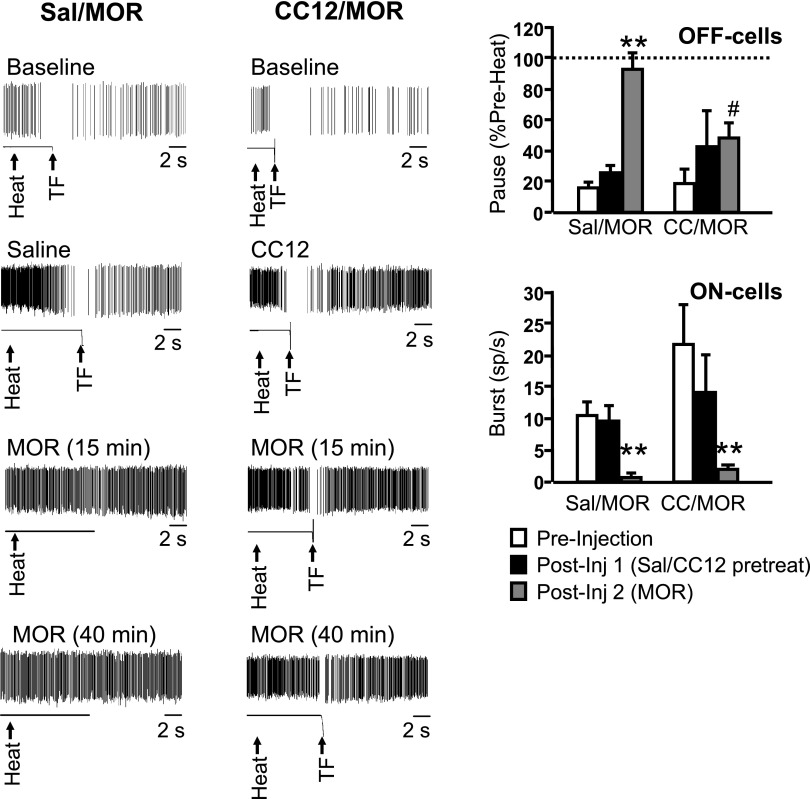
Because CC12 attenuates the analgesic actions of opioids, as well as improgan, in awake, behaving animals (Hough et al. 2007), we next determined whether CC12 interfered with the responses of off- and on-cells to morphine. As in awake animals, CC12 attenuated the antinociceptive effect of morphine (Fig. 4). As found with improgan, the effects of morphine on the ongoing activity of off- and on-cells were not blocked by CC12 (Fig. 5). However, and also as with improgan, CC12 antagonized the blockade of the reflex-related off-cell pause by morphine (Fig. 6). Unlike with improgan, CC12 did not interfere with morphine's suppression of the reflex-related on-cell burst (Fig. 6).
Fig. 4.
Analgesic action of icv morphine (MOR) is significantly reduced by pretreatment with CC12. TF latency as a function of time following MOR injection. Saline or CC12 and MOR are injected at times indicated. *P < 0.05, **P < 0.01 compared with CC12-pretreated animals [2-way ANOVA with repeated measures and Bonferroni posttest. Significant effect of drug F(1,56) = 12.8, P = 0.0007; time F(10,560) = 36.7, P < 0.0001; and drug × time interaction F(10,560) = 9.7, P < 0.0001]. Mean + SE.
Fig. 5.
Effects of morphine on ongoing off- and on-cell firing are not blocked by CC12. Left: ratemeter records illustrate ongoing activity of 2 off-cells in predrug baseline, after icv injection of saline (Sal, top) or CC12 (bottom) and following subsequent icv injection of morphine (MOR). Ongoing activity was increased following morphine to 146 and 272% of baseline in the saline- and CC12-pretreated experiments, respectively. Triangles indicate TF trials. Filled triangles indicate that there was a withdrawal; open triangles, no behavioral response within 10.6-s cutoff; 1-s bins. Individual TF trials for these 2 cells shown on expanded time base in Fig. 6. Right: group data. Ongoing firing of off- and on-cells following icv improgan expressed as percentage of predrug baseline. In neither case was the response to morphine blocked by CC12 pretreatment. Firing rates from saline-pretreated (Sal) animals compared with CC12-pretreated animals using Mann–Whitney U test (off-cells: U = 33, P = 0.53; on-cells: U = 77.5, P = 0.27). Mean + SE; 8–17 cells/group.
Fig. 6.
Morphine effects on reflex-related off-cell pause but not on-cell burst are blocked by CC12. Left: individual TF trials shown on an expanded time base (10-s sweep) document blockade of off-cell pause by morphine in an animal pretreated with saline (left, Sal/MOR), but not in an animal pretreated with CC12 (right, CC12/MOR). Spike train and output of tail position monitor shown, with heat onset and TF time indicated by arrows. Same neurons as in Fig. 5. Right: group data. off-cell pause and on-cell burst in preinjection baseline, following pretreatment with saline or CC12 (CC), and after subsequent morphine injection. Pause defined as firing rate at time of the reflex as percentage of firing rate immediately prior to heat onset. A value of 100% indicates no slowing during the heat stimulus. Different time points compared using repeated-measures ANOVA followed by Dunnett's test for comparison with preinjection baseline. For Sal/MOR group: F(2,14) = 29.9, P < 0.0001; for CC/MOR group: F(2,18) = 1.03, P = 0.38. Postmorphine time point for Sal/MOR and CC12/MOR compared using t-test for unpaired means: t16 = 2.8 P = 0.013. Burst defined as firing rate (in spikes/s) at time of the reflex. Different time points compared using Friedman's ANOVA by ranks with significant result followed by comparison of postinjection times with preinjection baseline. For Sal/MOR group: Fr (2) = 16.54, P = 0.0003; for CC/MOR group: Fr (2) = 23.29, P < 0.0001. Postmorphine time point for Sal/MOR and CC12/MOR compared using Mann–Whitney U test: U = 67.5, P = 0.2134. For morphine, average of the 10–20 min postmorphine trials is given. Mean + SE, **P < 0.01 compared with predrug period. #P < 0.05 compared with saline-pretreated animals. No difference between groups in predrug baseline (off: t16 = 0.38, P = 0.71; on: U = 67.5, P = 0.22) or after saline/CC12 injection (off: t16 = 0.64, P = 0.53; on: U = 85, P = 0.69). In addition, the duration of the reflex-related pause was not significantly reduced by morphine in CC12-pretreated animals (predrug baseline: 9.0 ± 3.8 s; postmorphine: 3.0 ± 2.1 s, P > 0.05); 8–17 cells/group.
In sum, CC12 blocked the effects of improgan on the reflex-related changes in firing of both off- and on-cells. CC12 blocked the effects of morphine on the reflex-related off-cell pause, but not the on-cell burst. CC12 did not interfere with improgan or morphine's effects on the ongoing activity of either cell class. Table 1 summarizes the effects of improgan and morphine in the presence and absence of CC12 pretreatment on the ongoing and reflex-initiated changes in off- and on-cell firing.
Table 1.
Modulation of RVM off- and on-cell activity by improgan and morphine, and effect of CC12 on response to each drug
|
off-Cell Activity |
on-Cell Activity |
|||
|---|---|---|---|---|
| Treatment | Ongoing | Reflex–Pause | Ongoing | Reflex–Burst |
| CC12 alone | No effect | No effect | No effect | No effect |
| Imp | Activation | Elimination | Inhibition | Inhibition |
| Imp/CC12 | Activation | Blocks Imp | Inhibition | Blocks Imp |
| Mor | Activation | Elimination | Inhibition | Inhibition |
| Mor/CC12 | Activation | Blocks Mor | Inhibition | Inhibition |
The table represents a summary of results with improgan, morphine, and CC12. Imp, improgan; Mor, morphine.
DISCUSSION
Experiments with P450-deficient mice and a variety of pharmacological inhibitors recently demonstrated that P450 epoxygenase activity is required for opioid antinociception (Conroy et al. 2010). This suggested an explanation for the earlier finding that CC12 antagonizes morphine antinociception without blocking opioid receptors, since CC12 was subsequently found to inhibit several P450 epoxygenase enzymes (Hough et al. 2007; Stadel et al. 2008). The reports that CC12 also interferes with improgan and cannabinoid antinociception suggested that all three classes of analgesic compounds converge on a common, CC12-sensitive mechanism (Hough et al. 2007; Salussolia et al. 2007; Stadel et al. 2008).
That possibility was examined here using a circuit-level analysis of the responses of identified RVM pain-modulating neurons to morphine and improgan and the modulation of these effects by CC12. Our experiments in anesthetized animals confirmed the broad function of CC12 as a central “analgesic antagonist,” since both morphine and improgan antinociception were blocked by CC12 pretreatment. Because opioids, improgan, and cannabinoids all activate RVM off-cells (Heinricher and Ingram 2008; Heinricher et al. 2010; Meng and Johansen 2004), one candidate mechanism for antagonism of the antinociceptive effect of all three drug classes was that CC12 suppresses firing of RVM off-cells or even all RVM neurons. This was ruled out in the present experiments, since CC12 had no effect on basal firing in RVM and failed to prevent the increase in spontaneous firing displayed by off-cells following morphine and improgan. Further, because CC12 modulated RVM circuitry only in the presence of analgesic drugs, these data significantly advance our understanding of three distinct problems: 1) opioid antinociceptive mechanisms in the RVM, 2) cellular sites and mechanisms of action of improgan in the RVM, and 3) the relationship between activity of RVM neurons and behavioral antinociception.
Opioid antinociception
Table 1 summarizes four actions of morphine in the RVM and the effect of CC12 on each. By itself, morphine increased ongoing activity of off-cells and suppressed that of on-cells. The reflex-related off-cell pause and on-cell burst were both blocked. CC12 prevented morphine's suppression of the off-cell pause without altering the drug's effects on either the on-cell burst or the ongoing activity of either class.
Pharmacologically, these findings indicate that the target for CC12 is interposed in only a subset of μ-opioid actions affecting the RVM. The mechanisms underlying off- and on-cell responses to morphine are known to be different (Heinricher and Ingram 2008). However, only the reflex-related disinhibition of off-cells was prevented by CC12. Opioid suppression of ongoing and reflex-related firing of on-cells, known to reflect a postsynaptic action, was not blocked by CC12. This pattern is most easily explained if the CC12 target is part of a signal transduction pathway(s) linking presynaptic opioid-receptor activation to inhibition of γ-aminobutyric acid (GABA) release, but not in the pathways linking postsynaptic opioid-receptor activation to membrane hyperpolarization. A differential involvement of the P450 pathway would add to other well-documented differences in signal transduction mechanisms for pre- and postsynaptically located μ-opioid receptors in the PAG–RVM system (Chieng and Christie 1994; Ingram 2000; Vaughan et al. 1997).
Physiologically, these data point to a differential regulation of ongoing discharge and reflex-related changes in off-cell activity. Morphine activates off-cells in both the presence and the absence of noxious stimuli; i.e., there is increased ongoing activity as well as elimination of the nociceptive pause. Because the direct membrane effect of μ-opioid agonists in the RVM is hyperpolarizing, and because off-cell firing is unaffected by direct iontophoretic application of morphine, changes in both aspects of off-cell discharge are recognized to be indirect (Heinricher et al. 1992; Pan et al. 1990). The present results show that ongoing firing and nociception-evoked changes in firing can be dissociated pharmacologically. This could happen if ongoing and reflex-related activity were regulated by different opioid-sensitive inhibitory inputs, with CC12 targeting only the latter. Alternatively, icv-administered opioids could activate (disinhibit) an excitatory input to off-cells from some other brain area that contributes to the increase in ongoing firing. The source of such an input would need to be identified, but the PAG for example supports opioid analgesia and sends excitatory connections to off-cells (Vanegas et al. 1984).
Functionally, the primacy of the off-cell in the antinociceptive effects of RVM action has been amply demonstrated (Heinricher and McGaraughty 1998; Heinricher et al. 2001a,b). These new data point specifically to the off-cell pause as an all-or-nothing gate that permits behavioral responses to a noxious stimulus. By contrast, the increase in ongoing activity of these neurons is insufficient to produce potent antinociception. However, the balance of ongoing activity in the off-cell and on-cell populations is likely important for setting overall nociceptive gain (Jinks et al. 2007; Kincaid et al. 2006).
Improgan antinociception
Neither improgan's receptor nor its membrane actions have been identified. However, improgan is known to act directly in the RVM to produce antinociception (Nalwalk et al. 2004). Further, icv-administered improgan activates RVM off-cells and this activation is necessary for improgan antinociception (Heinricher et al. 2010; Nalwalk et al. 2004). It was therefore of great interest to assess the ability of CC12 to inhibit improgan-evoked changes in off-cell activity. As with morphine, CC12 selectively prevented improgan's ability to eliminate the reflex-related off-cell pause, without blocking improgan's effect on ongoing firing (Table 1). CC12's antagonism of improgan's behavioral antinociception is thus specifically related to its ability to preserve reflex-related changes in off-cell firing.
The effect of CC12 on the on-cell response to improgan was not the same as its effect on opioid inhibition of these neurons (Table 1). As discussed earlier, CC12 was unable to block morphine's effects on either ongoing or reflex-related on-cell discharge. By contrast, CC12 interfered with improgan's ability to inhibit the on-cell burst, although as with morphine, improgan's suppression of ongoing activity was unchanged by CC12. This differential block of the effects of improgan and morphine by CC12 has implications for connections within the RVM. on- and off-cell populations exhibit reciprocal spontaneous and reflex-related firing and on-cells are directly inhibited by opioids. Early concepts of the RVM therefore postulated that on-cells were opioid-sensitive inhibitory interneurons that mediated the off-cell pause. Subsequent work showed this view to be incorrect (Cleary et al. 2008; Heinricher et al. 1999). The present finding that the on-cell response to CC12 and improgan mirrored that of off-cells suggests that the effects of improgan on on-cells are secondary to changes in off-cell firing, i.e., that off-cells inhibit on-cells. Consistent with this, off-cells often have local axonal arborizations within the RVM and at least some off-cells are GABAergic (Mason and Fields 1989; Winkler et al. 2006).
Finally, CC12 was originally developed as an improgan receptor antagonist and was only later found to be a P450 epoxygenase inhibitor (Hough et al. 2007; Stadel et al. 2008). The fact that CC12 failed to block all of improgan's effects in the RVM shows conclusively that this compound is not an improgan receptor antagonist, at least if all of improgan's effects in the RVM are mediated by a single receptor.
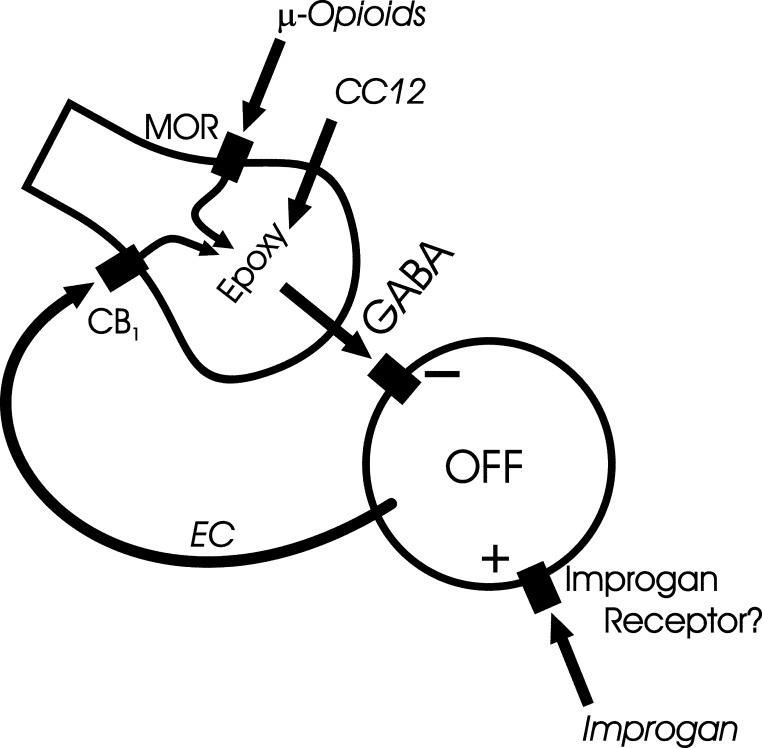
An epoxygenase model for opioid, cannabinoid, and improgan antinociception in the RVM
A new model for RVM antinociceptive mechanisms is put forward in Fig. 7. This model centers on the off-cell and the GABAergic input responsible for the off-cell pause. This model incorporates earlier evidence that μ-opioid and cannabinoid receptor 1 (CB1) agonists act presynaptically to inhibit GABA release from terminals mediating the off-cell pause (Heinricher et al. 1991, 1992; Meng and Johansen 2004; Pan et al. 1990; Vaughan et al. 1999) and that morphine engages an excitatory input to off-cells (Heinricher et al. 2001b). It also brings together the observations that improgan, which lacks affinity for known cannabinoid receptors, produces antinociception dependent on CB1 receptors (Hough et al. 2009b; Nalwalk et al. 2006) and that CC12 blocks antinociception produced by both CB1-receptor agonists and improgan (Hough et al. 2007). Finally, taken together with recent evidence implicating P450 epoxygenase activity in μ-opioid analgesic action (Conroy et al. 2010), the present findings with CC12 suggest a P450 link between presynaptic μ-opioid receptor activation and inhibition of GABA release onto off-cells.
Fig. 7.
Convergence of opioid and improgan effects on a CC12-sensitive target in the GABAergic terminal mediating the reflex-related off-cell pause in the rostral ventromedial medulla (RVM). Presynaptic actions of the μ-opioid agonist morphine, known to suppress γ-aminobutyric acid (GABA)–mediated inhibition in the RVM, are blocked by CC12. Improgan antinociception involves an endocannabinoid mechanism. This would be consistent with a direct action of improgan on off-cells, leading to retrograde activation of presynaptic cannabinoid receptor 1 (CB1) on the opioid- and cannabinoid-sensitive GABA terminal. Both opioid and CB1 receptors are postulated to signal via a CC12-sensitive transduction mechanism, likely a P450 epoxygenase, inhibiting release of GABA responsible for the reflex-related off-cell pause.
The model proposes that CC12 exerts its effects presynaptically, inhibiting an epoxygenase necessary for both opioid- and cannabinoid-induced inhibition of GABA release. Improgan is suggested to activate off-cells directly, which in turn causes release of an endocannabinoid. Retrograde activation of presynaptic CB1 receptors then inhibits release of GABA from terminals mediating the off-cell pause. Although endocannabinoid-mediated retrograde modulation of GABAergic activity has not been investigated within the RVM, it is documented to occur in the hippocampus and elsewhere, including the PAG, another important substrate for improgan and opioid antinociception (Drew et al. 2009; Freund et al. 2003; Lovinger 2008).
This proposed model of RVM analgesic action is consistent with 1) the improgan-evoked increase in ongoing off-cell firing, which is not blocked by CC12; 2) opioid- and improgan-induced elimination of the off-cell pause, which is blocked by CC12; and 3) CC12 inhibition of μ-opioid-, improgan-, and cannabinoid-mediated antinociception (Hough et al. 2007). Certainly many additional studies will be needed to verify the diverse elements in this circuit.
Conclusion
The recognition that the GABAergic terminal mediating the off-cell pause is the target for CC12 makes understanding the molecular mode of action of this compound a high priority. CC12 is now recognized to be an inhibitor of several brain cytochrome P450 epoxygenases (Stadel et al. 2008). Together with recent evidence that supraspinal opioid receptors activate analgesic circuits through a P450–epoxygenase signaling pathway (Conroy et al. 2010), our data are consistent with a model in which CC12 blocks an epoxygenase product that couples presynaptic μ-opioid and cannabinoid receptor activation to suppression of GABA release. Testing the responses of off-cells to more specific manipulations of epoxygenase and epoxygenase products will be needed to fully answer this question. These data also demonstrate that drugs acting on P450 epoxygenases are valuable new tools for discerning brain stem antinociceptive mechanisms.
GRANTS
This work was supported by National Institute on Drug Abuse Grants DA-03816 to L. B. Hough and DA-022492 to M. M. Heinricher.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. James Phillips (Curragh Chemistries, Cleveland, OH) for providing the sample of CC12.
REFERENCES
- Barbaro et al., 1989. Barbaro NM, Heinricher MM, Fields HL. Putative nociceptive modulatory neurons in the rostral ventromedial medulla of the rat display highly correlated firing patterns. Somatosens Mot Res 6: 413–425, 1989 [DOI] [PubMed] [Google Scholar]
- Chieng and Christie, 1994. Chieng B, Christie MJ. Hyperpolarization by opioids acting on mu-receptors of a sub-population of rat periaqueductal gray neurones in vitro. Br J Pharmacol 113: 121–128, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie et al., 2000. Christie MJ, Connor M, Vaughan CW, Ingram SL, Bagley EE. Cellular actions of opioids and other analgesics: implications for synergism in pain relief. Clin Exp Pharmacol Physiol 27: 520–523, 2000 [DOI] [PubMed] [Google Scholar]
- Cleary et al., 2008. Cleary DR, Neubert MJ, Heinricher MM. Are opioid-sensitive neurons in the rostral ventromedial medulla inhibitory interneurons? Neuroscience 151: 564–571, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conroy et al., 2010. Conroy JL, Fang C, Gu J, Zeitlin SO, Yang W, Yang J, Vanalstine MA, Nalwalk JW, Albrecht PJ, Mazurkiewicz JE, Snyder-Keller A, Shan Z, Zhang SZ, Wentland MP, Behr M, Knapp BI, Bidlack JM, Zuiderveld OP, Leurs R, Ding X, Hough LB. Opioids activate brain analgesic circuits through cytochrome P450/epoxygenase signaling. Nat Neurosci 13: 284–286, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew et al., 2009. Drew GM, Lau BK, Vaughan CW. Substance P drives endocannabinoid-mediated disinhibition in a midbrain descending analgesic pathway. J Neurosci 29: 7220–7229, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmayer et al., 2009. Edelmayer RM, Vanderah TW, Majuta L, Zhang ET, Fioravanti B, De Felice M, Chichorro JG, Ossipov MH, King T, Lai J, Kori SH, Nelsen AC, Cannon KE, Heinricher MM, Porreca F. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol 65: 184–193, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields et al., 2006. Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: Wall and Melzack's Textbook of Pain (5th ed.), edited by McMahon S, Koltzenburg M. London: Elsevier, 2006, p. 125–142 [Google Scholar]
- Fields et al., 1983. Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci 3: 2545–2552, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund et al., 2003. Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83: 1017–1066, 2003 [DOI] [PubMed] [Google Scholar]
- Heinricher et al., 1991. Heinricher MM, Haws CM, Fields HL. Evidence for GABA-mediated control of putative nociceptive modulating neurons in the rostral ventromedial medulla: iontophoresis of bicuculline eliminates the off-cell pause. Somatosens Mot Res 8: 215–225, 1991 [DOI] [PubMed] [Google Scholar]
- Heinricher and Ingram, 2008. Heinricher MM, Ingram SL. The brainstem and nociceptive modulation. In: The Senses, A Comprehensive Reference: Pain, edited by Bushnell MC, Basbaum AI. San Diego, CA: Academic Press, 2008, vol. 5, p. 593–626 [Google Scholar]
- Heinricher et al., 2010. Heinricher MM, Martenson ME, Nalwalk JW, Hough LB. Neural basis for improgan antinociception. Neuroscience 169: 1414–1420, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher et al., 2004. Heinricher MM, Martenson ME, Neubert MJ. Prostaglandin E2 in the midbrain periaqueductal gray produces hyperalgesia and activates pain-modulating circuitry in the rostral ventromedial medulla. Pain 110: 419–426, 2004 [DOI] [PubMed] [Google Scholar]
- Heinricher and McGaraughty, 1998. Heinricher MM, McGaraughty S. Analysis of excitatory amino acid transmission within the rostral ventromedial medulla: Implications for circuitry. Pain 75: 247–255, 1998 [DOI] [PubMed] [Google Scholar]
- Heinricher et al., 1999. Heinricher MM, McGaraughty S, Farr DA. The role of excitatory amino acid transmission within the rostral ventromedial medulla in the antinociceptive actions of systemically administered morphine. Pain 81: 57–65, 1999 [DOI] [PubMed] [Google Scholar]
- Heinricher et al., 2001a. Heinricher MM, McGaraughty S, Tortorici V. Circuitry underlying antiopioid actions of cholecystokinin within the rostral ventromedial medulla. J Neurophysiol 85: 280–286, 2001a [DOI] [PubMed] [Google Scholar]
- Heinricher et al., 1992. Heinricher MM, Morgan MM, Fields HL. Direct and indirect actions of morphine on medullary neurons that modulate nociception. Neuroscience 48: 533–543, 1992 [DOI] [PubMed] [Google Scholar]
- Heinricher and Roychowdhury, 1997. Heinricher MM, Roychowdhury S. Reflex-related activation of putative pain facilitating neurons in rostral ventromedial medulla (RVM) depends upon excitatory amino acid transmission. Neuroscience 78: 1159–1165, 1997 [DOI] [PubMed] [Google Scholar]
- Heinricher et al., 2001b. Heinricher MM, Schouten JC, Jobst EE. Activation of brainstem N-methyl-d-aspartate receptors is required for the analgesic actions of morphine given systemically. Pain 92: 129–138, 2001b [DOI] [PubMed] [Google Scholar]
- Heinricher et al., 2009. Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev 60: 214–225, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher and Tortorici, 1994. Heinricher MM, Tortorici V. Interference with GABA transmission in the rostral ventromedial medulla: disinhibition of off-cells as a central mechanism in nociceptive modulation. Neuroscience 63: 533–546, 1994 [DOI] [PubMed] [Google Scholar]
- Hough et al., 2000. Hough LB, Nalwalk JW, Chen Y, Schuller A, Zhu Y, Zhang J, Menge WM, Leurs R, Timmerman H, Pintar JE. Improgan, a cimetidine analog, induces morphine-like antinociception in opioid receptor-knockout mice. Brain Res 880: 102–108, 2000 [DOI] [PubMed] [Google Scholar]
- Hough et al., 2007. Hough LB, Nalwalk JW, Phillips JG, Kern B, Shan Z, Wentland MP, de Esch IJ, Janssen E, Barr T, Stadel R. CC12, a high-affinity ligand for [3H]cimetidine binding, is an improgan antagonist. Neuropharmacology 52: 1244–1255, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough et al., 2009a. Hough LB, Nalwalk JW, Yang J, Conroy JL, Vanalstine MA, Shan Z, Zhang S-Z, Wentland MP, Knapp BI, Bidlack JM, Zuiderveld OP, Leurs R, Yang W, Ding X. Brain P450/epoxygenase activity is required for the antinociceptive effects of improgan, a non-opioid analgesic Soc Neurosci Abstr 7578, 2009a [Google Scholar]
- Hough et al., 2009b. Hough LB, Svokos K, Nalwalk JW. Non-opioid antinociception produced by brain stem injections of improgan: significance of local, but not cross-regional, cannabinoid mechanisms. Brain Res 1247: 62–70, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram, 2000. Ingram SL. Cellular and molecular mechanisms of opioid action. Prog Brain Res 129: 483–492, 2000 [DOI] [PubMed] [Google Scholar]
- Jinks et al., 2007. Jinks SL, Carstens EE, Antognini JF. Glutamate receptor blockade in the rostral ventromedial medulla reduces the force of multisegmental motor responses to supramaximal noxious stimuli. Neurosci Lett 426: 175–180, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid et al., 2006. Kincaid W, Neubert MJ, Xu M, Kim CJ, Heinricher MM. Role for medullary pain facilitating neurons in secondary thermal hyperalgesia. J Neurophysiol 95: 33–41, 2006 [DOI] [PubMed] [Google Scholar]
- Lovinger, 2008. Lovinger DM. Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol 184: 435–477, 2008 [DOI] [PubMed] [Google Scholar]
- Martenson et al., 2009. Martenson ME, Cetas JS, Heinricher MM. A possible neural basis for stress-induced hyperalgesia. Pain 142: 236–244, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason and Fields, 1989. Mason P, Fields HL. Axonal trajectories and terminations of on- and off-cells in the cat lower brainstem. J Comp Neurol 288: 185–207, 1989 [DOI] [PubMed] [Google Scholar]
- Meng and Johansen, 2004. Meng ID, Johansen JP. Antinociception and modulation of rostral ventromedial medulla neuronal activity by local microinfusion of a cannabinoid receptor agonist. Neuroscience 124: 685–693, 2004 [DOI] [PubMed] [Google Scholar]
- Nalwalk et al., 2006. Nalwalk JW, Svokos K, Hough LB. Cannabinoid-improgan cross-tolerance: improgan is a cannabinomimetic analgesic lacking affinity at the cannabinoid CB1 receptor. Eur J Pharmacol 549: 79–83, 2006 [DOI] [PubMed] [Google Scholar]
- Nalwalk et al., 2004. Nalwalk JW, Svokos K, Taraschenko O, Leurs R, Timmerman H, Hough LB. Activation of brain stem nuclei by improgan, a non-opioid analgesic. Brain Res 1021: 248–255, 2004 [DOI] [PubMed] [Google Scholar]
- Neubert et al., 2004. Neubert MJ, Kincaid W, Heinricher MM. Nociceptive facilitating neurons in the rostral ventromedial medulla. Pain 110: 158–165, 2004 [DOI] [PubMed] [Google Scholar]
- Pan et al., 1990. Pan ZZ, Williams JT, Osborne PB. Opioid actions on single nucleus raphe magnus neurons from rat and guinea-pig in vitro. J Physiol 427: 519–532, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salussolia et al., 2007. Salussolia CL, Nalwalk JW, Hough LB. Improgan-induced hypothermia: a role for cannabinoid receptors in improgan-induced changes in nociceptive threshold and body temperature. Brain Res 1152: 42–48, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadel et al., 2008. Stadel R, Yang J, Nalwalk JW, Phillips JG, Hough LB. High affinity binding of [3H]-cimetidine to a heme-containing protein in rat brain. Drug Metab Dispos 36: 1–31, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas et al., 1984. Vanegas H, Barbaro NM, Fields HL. Midbrain stimulation inhibits tail-flick only at currents sufficient to excite rostral medullary neurons. Brain Res 321: 127–133, 1984 [DOI] [PubMed] [Google Scholar]
- Vaughan et al., 1997. Vaughan CW, Ingram SL, Connor MA, Christie MJ. How opioids inhibit GABA-mediated neurotransmission. Nature 390: 611–614, 1997 [DOI] [PubMed] [Google Scholar]
- Vaughan et al., 1999. Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol 127: 935–940, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler et al., 2006. Winkler CW, Hermes SM, Chavkin CI, Drake CT, Morrison SF, Aicher SA. Kappa opioid receptor (KOR) and GAD67 immunoreactivity are found in off and neutral cells in the rostral ventromedial medulla. J Neurophysiol 96: 3465–3473, 2006 [DOI] [PubMed] [Google Scholar]