Abstract
DEG/ENaC channels have been broadly implicated in mechanosensory transduction, yet many questions remain about how these proteins contribute to complexes that sense mechanical stimuli. In C. elegans, two DEG/ENaC channel subunits are thought to contribute to a gentle touch transduction complex: MEC-4, which is essential for gentle touch sensation, and MEC-10, whose importance is less well defined. By characterizing a mec-10 deletion mutant, we have found that MEC-10 is important, but not essential, for gentle touch responses in the body touch neurons ALM, PLM, and PVM. Surprisingly, the requirement for MEC-10 in ALM and PLM is spatially asymmetric; mec-10 animals show significant behavioral and physiological responses to stimulation at the distal end of touch neuron dendrites, but respond poorly to stimuli applied near the neuronal cell body. The subcellular distribution of a rescuing MEC-10::GFP translational fusion was found to be restricted to the neuronal cell body and proximal dendrite, consistent with the hypothesis that MEC-10 protein is asymmetrically distributed within the touch neuron process. These results suggest that MEC-10 may contribute to only a subset of gentle touch mechanosensory complexes found preferentially at the proximal dendrite.
INTRODUCTION
The senses of touch, hearing, and balance depend on sensory neurons that generate receptor potentials in response to mechanical force. Most, if not all, mechanosensory neurons sense force using ion channels that are directly mechanically gated. The structural subunits of these channels appear to come primarily from one of two protein superfamilies: the TRP (transient receptor potential) channels and the DEG/ENaC (degenerin/epithelial Na+ channel) channels (Garcia-Anoveros and Corey 1997; Goodman et al. 2004). TRP channels are nonspecific cation channels composed of subunits with six transmembrane α-helices. At least some TRP channels appear to be sufficient by themselves to produce touch- or stretch-evoked currents (Christensen and Corey 2007). In addition, TRP channels can be activated by G protein signaling, which has been implicated in other sensory transduction processes including taste, vision, and olfaction (Kahn-Kirby and Bargmann 2006). In contrast, DEG channel subunits have two transmembrane α-helices and form channels permeable to sodium and, in some cases, calcium (Bounoutas and Chalfie 2007). Relatively little is known about how DEG channels are activated by mechanical or other stimuli.
Perhaps the best-studied case of DEG channel–mediated mechanosensation involves the gentle body touch neurons of C. elegans. Three gentle touch neurons (ALML, ALMR, and AVM) have processes extending from the midbody to the pharynx and are required for escape responses to light mechanical stimulation to the anterior body (Chalfie et al. 1985). Two additional neurons (PLML and PLMR) have processes extending from the tail to the midbody and are required for escape responses to posterior gentle touch. Screens for mutants defective in gentle touch avoidance have identified over a dozen mec genes whose products are specifically required for the function of these neurons (Chalfie and Au 1989). Among the mec genes are two that encode DEG/ENaC channel proteins, MEC-4 (Driscoll and Chalfie 1991) and MEC-10 (Huang and Chalfie 1994), and two that encode DEG channel accessory subunits, MEC-2 (Huang et al. 1995) and MEC-6 (Chelur et al. 2002). Additional mec genes encode extracellular or intracellular structures thought to be important for coupling external forces to channel gating; however, the mechanisms by which this might occur are not known (Bounoutas and Chalfie 2007; Goodman and Schwarz 2003).
The importance of each of the mec genes for mechanosensation in the gentle touch neurons has been investigated at the cellular level through in vivo imaging and electrophysiology. Wild-type C. elegans exhibit robust calcium transients in the gentle touch neurons in response to mechanical stimulation; null mutations in mec-4, mec-2, and mec-6 abolish these responses (Suzuki et al. 2003). Likewise, mec-4, mec-2, and mec-6 null mutant neurons lack mechanoreceptor potentials measured by electrophysiology (O'Hagan et al. 2005). Previously characterized mec-10 alleles are missense mutations (Huang and Chalfie 1994) that reduce, but do not eliminate, mechanoreceptor potentials evoked by mechanical stimulation (O'Hagan et al. 2005). Recently, analysis of a mec-10 deletion allele showed that MEC-10, along with a second DEG/ENaC protein known as DEGT-1, is required for harsh touch responses in the ALMs (Chatzigeorgiou et al. 2010). However, the effect of the mec-10 deletion allele on gentle touch responses has not been reported.
In addition to the gentle body touch neurons, MEC-10 is expressed in several additional neurons, where its function has not been established. The PVM neurons express not only mec-10, but also most of the other mec genes (Huang and Chalfie 1994), and their overall morphology is very similar to that of the gentle touch neurons. However, unlike the gentle touch neurons, PVM is not sufficient to mediate an escape response to gentle touch and its role in mechanosensory behavior in general is not known (Chalfie and Sulston 1981; Chalfie et al. 1985). Unlike the gentle touch neurons, PVM expresses another DEG channel gene, unc-8, which has been hypothesized to encode a stretch receptor potentially involved in proprioception (Tavernarakis and Driscoll 1997). Another class of neurons expressing mec-10 are the FLPs, which play a role in escape responses to nose touch. The FLPs have highly branched multidendritic arbors that surround the animal's head, which are thought to be mechanosensory (Huang and Chalfie 1994). mec-4 is not expressed in the FLPs, although these neurons do express the TRP channel OSM-9, which is required for nose touch responses by the polymodal ASH neurons (Colbert et al. 1997). Finally, mec-10 is expressed in the PVD neurons, which have been implicated in responses to harsh body touch (Way and Chalfie 1989). Similar to the FLPs, the PVDs have multidendritic arbors that cover the animal's body. Likewise, the PVDs do not express MEC-4, but express OSM-9, a TRP channel that is involved in mechanosensation in other C. elegans neurons (Colbert et al. 1997). MEC-10 and DEGT-1 have been shown to be required for harsh touch responses in PVD (Chatzigeorgiou et al. 2010).
In this study, we have investigated the role of MEC-10 in the C. elegans gentle touch mechanosensory neurons. By analyzing a loss-of-function mec-10 deletion mutant, we find that MEC-10 is important for responses to gentle touch applied near the mechanoreceptor neuron's cell body, but is less important for touch applied near the distal end of the touch receptor process. Consistent with this, MEC-10::GFP translational fusions are restricted to the ALM cell body and proximal dendrite, whereas MEC-4::GFP fusions are distributed in puncta all along the ALM process. These results suggest that the touch neurons may contain both MEC-10–dependent and MEC-10–independent mechanotransduction complexes, with the MEC-10–containing complexes specifically important for gentle touch near the cell body.
METHODS
C. elegans strains
Wild-type C. elegans (strain N2) were grown at 21°C using standard methods.
Strains used.
AQ906 bzIs17[pmec-4::YC2.12; lin-15(+)], AQ908 mec-4(u253); bzIs17, AQ1413 mec-10(tm1552); bzIs17, AQ2150 mec10(tm1552) mec-4(u253) bzIs17, AQ2268 mec-10(tm1552); bzIs17; ljEx228[pmec-4::mec-10; pmyo-2::GFP], AQ2436 mec-10(tm1552) mec-4(u253); bzIs17; ljEx228[pmec-4::mec-10(+); pmyo-2::GFP], ZB154 zdIs5[mec-4::GFP] I, ZB2672 bzEx[mec-10::GFP; pmyo-2::GFP].
Transgenic lines
For cloning, we used the MultiSite Gateway Three Fragment Vector Construction Kit. Individual PCR products were cloned into the appropriate pDONOR vector generating pENTRY clones. Subsequently, pENTRY vectors with the promoter and gene of interest as well as an unc-54 3′UTR-containing vector were recombined with a pDEST vector to generate an Expression vector. To generate the pmec-10::mec-10(+) transgene, a 4.4-kb (kilobase) mec-10 genomic DNA was amplified using Phusion High- Fidelity DNA Polymerase F-530S from adult genomic DNA using the primers 5′-GTA CAA AAT TCA AAA AAT GAA TCG-3′ and 5′-GAA ATA AGA AAT TTA TTT TCCG-3′. A 1-kb mec-4 promoter region was obtained from plasmid pIR13, a gift from I. Rabinowitch from the Schafer lab. The pmec-4::mec10 construct was injected in mec-10(tm1552); bzIs17 animals at 70 ng/μl with pmyo-2::GFP at 20 ng/μl to generate array ljEx228[pmec-4::mec-10; pmyo-2::GFP].
Behavioral assays
For gentle body touch assays, animals were touched by stroking an eyelash hair across the worm's body at different positions. The stimulus was applied at the following positions: anterior body position 3 = behind the terminal bulb of the pharynx, position 2 = between the pharynx and the midbody, and position 1 = at the midbody, −1 = control outside the receptive field; posterior body position 3 = near the anus position, position 2 = halfway between the anus and the vulva, and position 1 near the vulva. Animals were stimulated in each position and were scored for whether they reversed direction, with a 3-min interval between each stimulus. In some animals, stimuli were applied in a proximal-to-distal direction and in other animals in a distal-to-proximal direction. In all, 100 animals were assayed from each genotype.
Calcium imaging
Optical recordings were performed essentially as previously described (Kerr 2006; Kerr et al. 2000) on a Zeiss Axioskop 2 upright compound microscope equipped with a Dual View beam splitter and a Uniblitz Shutter. Fluorescence images were acquired using MetaVue 6.2. Filter-dichroic pairs were excitation, 400–440; excitation dichroic 455; cyan fluorescent protein (CFP) emission, 465–495; emission dichroic 505; yellow fluorescent protein (YFP) emission, 520–550. Individual adult worms (∼24 h past L4) were glued with Nexaband S/C cyanoacrylate glue to pads composed of 2% agarose in extracellular saline (in mM: 145 NaCl, 5 KCl, 1 CaCl2, 5 MgCl2, 20 d-glucose, and 10 HEPES buffer; pH 7.2). Worms used for calcium imaging had similar levels of cameleon expression in sensory neurons, as inferred from initial fluorescence intensity. Acquisitions were taken at 28 Hz (35-ms exposure time) with 4 × 4 or 2 × 2 binning, using a ×63 Zeiss Achroplan water-immersion objective.
Gentle body touch stimulation
Gentle body touch stimulation was performed as previously described (Suzuki et al. 2003), with a standard probe displacement of about 10 μm. Positions for anterior body touch stimulation were defined as follows: 3 = behind terminal bulb, 2 = midway between the ALM cell body and terminal bulb, 1 = within 10 μm from ALM cell body, −1 = >10 μm posterior of the ALM cell body. For posterior body touch the points of stimulation were 3 = within 10 μm from the vulva, 2 = midway between the vulva and the tip of the tail, 1 = within 10 μm of PLM cell body. For PVM imaging, 3 = 200 μm anterior of the ALM cell body, 2 = 10 μm anterior of the vulva, 1 = within 10 μm of the PVM cell body, −1 = >10 μm posterior of PVM cell body. Individual worms were stimulated at all sites of interest, with a 5-min interval between each stimulation. Some animals were stimulated at more proximal positions first and then at more distal positions, whereas others were stimulated in the converse order. Some worms were probed only once at one location to exclude a potential artifact due to desensitization of the neuron.
GFP fusions to MEC-4 and MEC-10
We used the mec-4::GFP strain ZB154 zdIs5[pmec-4GFP] I, described previously (Clark and Chiu 2003). For the mec-10::GFP strain, we used a pmec-10::mec-10::GFP plasmid created by introducing a Pst I–Bam HI fragment, including the mec-10 promoter and coding sequences except for those encoding the last three AAs, into the pPD95.77 vector, which includes enhanced GFP. The mec-10(tm1552)::GFP strain was constructed in the same way, amplifying the same mec-10 genomic fragment from tm1552 DNA. Plasmid DNA (50 mg/ml) was coinjected with myo-2::GFP cotransformation marker DNA (50 mg/ml) to generate strain ZB2672.
RESULTS
Effects of mec-10 mutations on anterior gentle touch responses
To investigate the contribution of MEC-10 to gentle touch, we studied three mutant alleles: e1515, a recessive missense mutation affecting a highly conserved region near the first transmembrane domain (Huang and Chalfie 1994); u20, a missense mutation that encodes a functional channel with altered reversal potential; and a deletion, tm1552 (Zhang et al. 2008), which removes exon 5 and part of exon 6 (Supplemental Fig. S1).1 To determine the effects of these mutations on gentle touch avoidance behavior, we scored the reversal responses of mec-10 mutants to a light eyelash stroke across the anterior body (Fig. 1). We observed that when mec-10(tm1552) animals were touched near the midbody, they exhibited a strong touch-insensitive (Mec) phenotype. Interestingly, when the animals were stimulated at locations along the ALM process that were more distal to the cell body, we observed that mec-10 mutants showed significantly greater touch sensitivity. This spatial asymmetry in the severity of the mec-10 touch phenotype contrasted with mec-4 null mutants, which were strongly touch-insensitive throughout the anterior body (Fig. 1). A similar phenotype was observed for the other two mec-10 mutations (Fig. 1, Supplemental Fig. S2), indicating that the stronger proximal defect is related to the function of the MEC-10 protein rather than to specific features of particular mutant alleles.
Fig. 1.
mec-10 is important, but not essential, for anterior gentle touch avoidance. Animals were touched with an eyelash at the indicated positions; escape responses (reversals) were scored as described. mec-10(tm1552) and mec-10(u20) animals were significantly less responsive than wild-type at all positions (***P < 0.001) according to the Student's t-test (n = 100 for each genotype over 5 different days). One-way ANOVA demonstrated a statistically significant difference in the responses of mec-10(tm1552) and mec-10(u20) animals at different positions along the anteroposterior axis (P < 0.001).
The distal dendrites of the ALM and AVM anterior touch receptors are closer to their synaptic outputs in the nerve ring than their proximal dendrites; thus one explanation for the more severe proximal defect in mec-10 mutants is that weak proximal responses propagate less effectively to ALM/AVM synapses than weak distal responses (Savage et al. 1989). Alternatively, mec-10 mutations might have asymmetric defects in touch response per se. To distinguish between these possibilities, we measured calcium transients evoked in ALM by mechanical stimulation across the anterior body. We used a transgenic line, bzIs17, which expresses the calcium-sensitive protein cameleon from the touch-neuron-specific mec-4 promoter, to image touch-evoked calcium transients in the ALM cell body. As observed previously, wild-type animals generated calcium transients of similar size in response to gentle stimulation at various points along the anterior cell body (Suzuki et al. 2003). When we conducted similar experiments in a mec-10(tm1552) or mec-10(e1515) background, we observed that the magnitude of the touch-evoked calcium influx was significantly reduced compared with wild-type (Fig. 2). Moreover, as in the behavioral experiments, we observed a spatial asymmetry in the magnitude of the ALM response to touch: responses to stimulations near the ALM cell body were more strongly defective than to those applied near the head (Fig. 2, Supplemental Fig. S2). Recordings from mec-10(u20) mutants showed altered response dynamics compared with those from wild-type and other mutant strains, presumably because of altered properties of the mutant mechanoreceptor complex; however, the u20 mutant responses were nonetheless stronger to distal stimuli than to proximal ones. For all mutants, the asymmetry in the severity of the touch response defect was observed not only across the population of animals tested, but also in individual animals (Fig. 2E, Supplemental Fig. S2C). Thus mec-10 mutants appear to be less sensitive to gentle touch stimuli applied near the ALM cell body than to stimuli applied to the distal dendrite.
Fig. 2.
mec-10 mutants have reduced touch-evoked calcium transients in ALM touch neurons. A: stimulus positions for imaging experiments. Animals expressing cameleon in touch neurons were given a 1-s gentle (buzz) stimulus at the indicated position, as described in methods. B–E: averaged calcium responses of wild-type (B), mec-10(tm1552) (C), mec-10(u20) (D), and mec-4(u253) (E) mutants. Each red trace represents the average percentage change in R/R0, where R is the fluorescence emission ratio at a given time point and R0 is its initial value. The number of individual recordings averaged for each trace were n = 27, 23, 22, and 12 (wild-type, positions 3, 2, 1, and −1, respectively); n = 20, 25, 25, and 8 (mec-10(tm1552), positions 3, 2, 1, and −1, respectively); n = 20 [mec-10(u20), all positions]; and n = 10, 10, 8, and 8 (mec-4, positions 3, 2, 1, and −1, respectively). Gray shading indicates SE of the mean response. Scale bars are indicated in the top left. The green bar indicates the time of the stimulus. Decreases in the u20 ratio signal (e.g., at position −1) are not accompanied by reciprocal changes in yellow fluorescent protein (YFP) and cyan fluorescent protein (CFP) emission intensities, suggesting that they are probably motion artifacts. F: scatterplot of peak calcium responses for each genotype. Red lines indicate the mean response at each of the 4 stimulus points; error bars indicate SE. Every other line indicates the response for a single animal. Half the animals were stimulated from anterior to posterior and half from posterior to anterior. One-way ANOVA demonstrated a statistically significant difference in the responses of mec-10 but not mec-4 animals at different positions along the anteroposterior axis (P < 0.001).
The mec-10(tm1552) allele is recessive for behavioral and calcium imaging phenotypes
To interpret the mec-10(tm1552) mutant phenotype, it is important to determine whether the mutant allele leads to a loss or gain of MEC-10 function. Although the tm1552 allele is a sizable deletion, the deleted gene retains the capacity to encode a transcript that links exon 3 in-frame to exon 7 (details in Supplemental Fig. S1). If translated, the deletion transcript would encode a protein lacking 153 amino acids of the extracellular domain, including highly conserved regions known to be critical for function in the DEG/ENaC protein MEC-4 (Hong et al. 2000).
To assess whether mec-10(tm1552) is a loss-of-function allele, we conducted dominance tests assaying both behavioral and calcium imaging phenotypes. We observed that mec-10(tm1552)/+ heterozygotes were indistinguishable from wild-type homozygotes in their sensitivity to gentle touch (Fig. 3). Likewise, the touch-evoked calcium transients in mec-10(tm1552)/+ heterozygotes expressing the bzIs17 cameleon transgene were comparable to those seen in wild-type bzIs17 animals (Fig. 4). We also generated transgenic extrachromosomal arrays expressing the wild-type mec-10(+) allele under the control of its own promoter or the touch neuron-specific pmec-4 promoter. These extrachromosomal arrays rescued the touch-insensitive phenotype of the mec-10(tm1552) homozygote at all points of stimulation (Figs. 3 and 4, Supplemental Fig. S3). These results show that the mec-10(tm1552) allele is fully recessive and thus most likely represents a loss-of-function allele.
Fig. 3.
mec-10(tm1552) is a recessive allele and acts in the touch neurons. Animals were touched with an eyelash at the indicated positions; escape responses (reversals) were scored as described. mec-10(tm1552)/+ heterozygous animals showed wild-type touch sensitivity at all positions. An extrachromosomal array carrying a pmec-4::mec-10(+) transgene rescued the phenotype of the mec-10(tm1552) homozygote at all positions; statistical significance (**P < 0.01 at position 3; ***P < 0.001 at positions 1 and 2) is according to the Student's t-test (n = 100 for each genotype over 5 different days).
Fig. 4.
mec-10(tm1552) dominance tests assayed by calcium imaging. A: stimulus positions for imaging experiments. Animals expressing cameleon in touch neurons were given a 1-s gentle (buzz) stimulus at the indicated position, as described in methods. B–D: averaged calcium responses of wild-type (B), mec-10(tm1552)/+ (C), mec-10(tm1552) (D, red trace), and mec-10(tm1552); ljEx228[pmec-4::mec-10; pmyo-2::GFP] (D, green trace) animals. Each trace represents the average percentage change in R/R0, where R is the fluorescence emission ratio at a given time point and R0 is its initial value. The number of individual recordings averaged for each trace were n = 13, 13, 13, and 13 (wild-type, positions 3, 2, 1, and −1, respectively); n = 13, 13, 13, and 13 [+/mec-10(tm1552) heterozygous positions 3, 2, 1, and −1, respectively]; and n = 13, 13, 13, and 13 [mec-10(tm1552) homozygous, positions 3, 2, 1, and −1, respectively]. Gray shading indicates SE of the mean response. Scale bars are indicated in the top right. The green bar indicates the time of the stimulus. E: scatterplot of peak calcium responses for each genotype. Red lines indicate the mean response at each of the 4 stimulus points; error bars indicate SE. Every other line indicates the response for a single animal. Statistical significance (***P < 0.0005; **P < 0.005) is according to the Mann–Whitney rank-sum test (asterisks at stimulus points indicate comparisons to wild-type; asterisks on bars indicate comparisons to the rescue line). Half the animals were stimulated from anterior to posterior and half from posterior to anterior.
Gentle touch phenotype of a mec-10 mec-4 double mutant
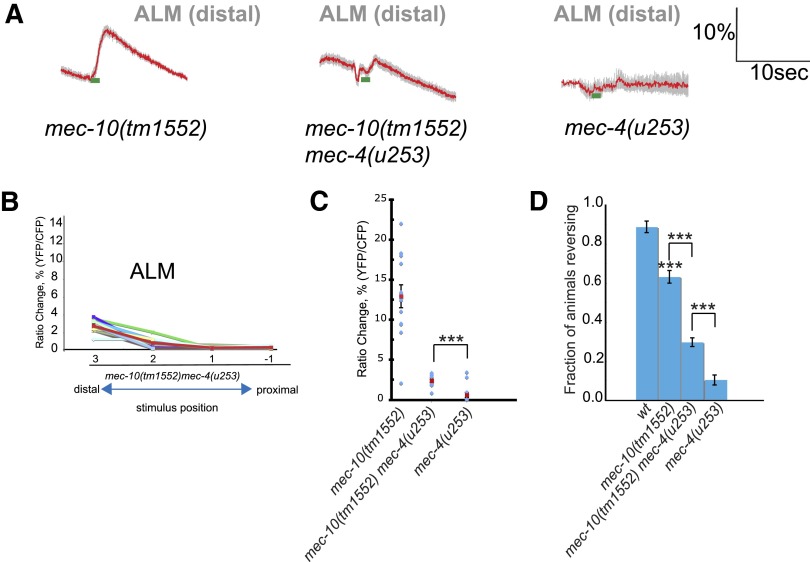
The observation that significant gentle touch responses are seen in mec-10 but not mec-4 loss-of-function mutants suggested that touch neuron mechanosensory complexes, particularly those in the distal dendrite, require MEC-4 but not MEC-10. This model would predict that the gentle touch response remaining in mec-10(tm1552) deletion mutants would be dependent on MEC-4 and thus absent in a mec-4 null mutant background. To test this possibility, we generated a mec-10(tm1552) mec-4(u253) double mutant and assayed its response to gentle touch by behavior and calcium imaging. We observed that these double mutant animals, like mec-4(u253) single mutants, were significantly less sensitive to anterior gentle touch at all stimulus positions as measured in behavioral assays (Fig. 5). However, at the most distal position, the double mutant did show measurable sensitivity to gentle touch, in contrast to the mec-4 single mutant, which was completely touch-insensitive. When we measured touch-evoked calcium transients in ALM, we observed a similar result: mec-10(tm1552) mec-4(u253) double mutants showed responses that were small but significant compared with baseline and to the mec-4(u253) single mutant. Thus the sensitivity to gentle touch remaining in mec-10(tm1552) animals is largely but not completely dependent on mec-4. The source of the mec-4– and mec-10–independent touch response in ALM is not known; potentially, loss of both proteins could enhance the normally negligible activity of another ALM-expressed DEG/ENaC channel (e.g., Chatzigeorgiou et al. 2010). Alternatively, indirect touch activation of ALM by other neurons (e.g., ADE/PDE through the PVR interneuron) might be enhanced.
Fig. 5.
Touch phenotype of mec-10 mec-4 double mutants. A: averaged calcium responses of mec-10(tm1552), mec-10(tm1552) mec-4(u253), and mec-4(u253) animals to stimulation of the ALM distal dendrite. Animals were stimulated at position 3 as described in Figs. 2 and 4. Each trace represents the average percentage change in R/R0, where R is the fluorescence emission ratio at a given time point and R0 is its initial value. In all, 13 animals were imaged for each genotype. B: avoidance behavior in response to gentle anterior touch. Animals were touched with an eyelash at the indicated positions; escape responses (reversals) were scored as described. Over 200 animals were tested for each genotype; statistical significance (P < 0.0005) is according to the Student's t-test. C: scatterplot of peak calcium responses for mec-10(tm1552) mec-4(u253) animals with different stimulus positions. Red lines indicate the mean response at each of the 4 stimulus points (see Figs. 2 and 4); error bars indicate SE. Every other line indicates the response for a single animal. Half the animals were stimulated from anterior to posterior and half from posterior to anterior. D: scatterplot of peak calcium responses for distal stimulation of each genotype. Each point indicates peak calcium response for stimulation at position 3 as in A. The double mutant showed significantly more response than mec-4 (u253) according to the Mann–Whitney rank-sum test (***P < 0.0005).
Effects of mec-10 mutations on PLM posterior touch receptor responses
C. elegans respond to gentle touch on the posterior body by accelerating forward away from the stimulus; this response is dependent on the left and right PLM neurons. To assess the role of mec-10 in the PLMs, we assayed avoidance behavior in response to posterior touch. We used an eyelash to apply gentle stimuli to various points on the posterior half of the body and assayed whether the animals exhibited a forward escape response. We observed that mec-10(tm1552) mutants were partially, but not completely, defective in posterior touch avoidance, in contrast to mec-4(u253) mutants that were more strongly touch-insensitive. Moreover, we found that mec-10(tm1552) mutants were strongly defective in responding to stimuli in areas near the tail (i.e., proximal to the PLM cell body), but responded better to stimuli applied in areas nearer the midbody and further from the PLM cell body (Fig. 6). As with anterior touch, the mec-10 phenotypes were all fully recessive in mec-10(tm1552)/+ heterozygotes and were rescued by the pmec-4::mec-10(+) transgene at all points of stimulation. Thus mec-10(tm1552) showed a partial Mec phenotype, more severe at cell body–proximal stimulus points, for both posterior and anterior touch.
Fig. 6.
Effect of mec-10 on posterior gentle touch avoidance. Animals were touched at the indicated positions; forward accelerations were scored as escape responses. One-way ANOVA demonstrated a statistically significant difference in the responses of mec-10(tm1552) animals at different positions along the anteroposterior axis (P < 0.001). All mec-10(tm1552) responses were significantly lower than those of wild-type (P < 0.0005). Expression of genomic mec-10(+) under the mec-4 promoter significantly rescues gentle touch in all positions of stimulation (n >100 per position of stimulation).
We also assayed the effect of mec-10 on touch-evoked calcium transients in PLM (Fig. 7). In wild-type animals, stimuli applied anywhere between the midbody and the tail robustly evoked calcium transients in PLM. mec-10(tm1552) mutants showed a reduction in the magnitude of touch-evoked calcium transients compared with wild-type and the severity of this defect was significantly greater in response to cell body–proximal stimulation than to distal stimulation (Fig. 7). As previously observed in ALM, this asymmetry in the severity of the touch response defect was observed in individual mutants as well as across the population of tested animals (Fig. 7F). Similar results were observed with the mec-10(e1515) and mec-10(u20) alleles (Supplemental Fig. S4). Thus in PLM mec-10 is also important, but not essential, for gentle touch mechanosensation and its function appears to be more important in proximal regions of the dendrite than in distal regions.
Fig. 7.
mec-10(tm1552) mutants have reduced touch-evoked calcium transients in PLM touch neurons. A: stimulus positions for imaging experiments. Animals were given a 1-s gentle (buzz) stimulus at the indicated position, as described in methods. B–E: averaged calcium responses of wild-type (B), mec-10(tm1552) (C), mec-4(u253) null mutants (D), and mec-10(tm1552); ljEx228[pmec-4::mec-10 pmyo-2::GFP] rescue animals (E). Each trace represents the average percentage change in R/R0. The numbers of individual recordings averaged for each trace were n = 22, 22, and 22 (wild-type, positions 3, 2, and 1, respectively); n = 22, 22, and 22 (mec-10, positions 3, 2, and 1, respectively); n = 22, 22, and 22 (mec-10 rescue, positions 3, 2, and 1, respectively); and n = 10, 10, and 10 (mec-4, positions 3, 2, and 1, respectively). Gray shading indicates SE of the mean response. Scale bars are indicated in the top left. The green bar indicates the time of the stimulus. F: scatterplot of peak calcium responses for each genotype. Red lines indicate the mean response at each of the stimulus points; error bars indicate SE. Every other line indicates the response for a single animal. Half the animals were stimulated from anterior to posterior and half from posterior to anterior. Statistical significance (***P < 0.001; **P < 0.01, *P < 0.05) is according to the Mann–Whitney rank-sum test.
Effects of mec-10 mutations on the potential touch receptor neuron PVM
We also investigated the function of MEC-10 in the PVM neuron. PVM shares the morphology of the gentle body touch neurons ALM, AVM, and PLM and, like these neurons, expresses MEC-10, MEC-4, and other mechanosensory genes. However, unlike these other neurons, PVM is neither necessary nor sufficient for gentle touch avoidance and its role in touch-regulated behaviors has not been characterized (Chalfie et al. 1985).
To determine whether PVM responds to mechanical stimuli, we used the bzIs17 line to image touch-induced calcium transients in PVM. We observed that in wild-type animals, PVM generated calcium transients in response to gentle touch stimuli, similar in displacement and speed to those that activated ALM and PLM (Fig. 8). However, whereas touch stimuli applied between the midbody and the PVM cell body robustly evoked calcium transients in PVM, stimuli applied near the tail did not. Thus the receptive field of PVM correlated closely with the position of the PVM dendrite and was distinct from that of the other gentle body touch neurons. In mec-10(tm1552) mutants, the touch-evoked calcium transient was reduced but not eliminated; however, unlike in the other touch neurons, no clear spatial asymmetry was observed in the severity of the phenotype (Fig. 8). As in ALM, neither the mec-4 null mutant nor a mec-10(tm1552); mec-4 double mutant had any detectable response to gentle touch. Thus PVM appears to be a primary gentle touch mechanoreceptor that shares many of the same genetic requirements for touch sensitivity with those (i.e., ALM, AVM, and PLM) involved in the gentle touch escape response.
Fig. 8.
mec-10 mutants have reduced touch-evoked calcium transients in PVM touch neurons. A: stimulus positions for imaging experiments. Animals were given a 1-s gentle (buzz) stimulus at the indicated position, as described in methods. B–D: averaged calcium responses of (B) wild-type, (C) mec-10(tm1552), and (D) mec-4(u253) mutants. Each red trace represents the average percentage change in R/R0, where R is the fluorescence emission ratio at a given time point and R0 is its initial value. The number of individual recordings averaged for each trace were n = 24, 20, 26, and 11 (wild-type, positions 3, 2, 1, and −1, respectively); n = 19, 18, 17, and 11 (mec-10, positions 3, 2, 1, and −1, respectively); and n = 10, 10, 8, and 8 (mec-10; mec-4, positions 3, 2, 1, and −1, respectively). Gray shading indicates SE of the mean response. Scale bars are indicated in the top left. The green bar indicates the time of the stimulus. E: scatterplot of peak calcium responses for each genotype. Red lines indicate the mean response at each of the 4 stimulus points; error bars indicate SE. Every other line indicates the response for a single animal. Statistical significance (***P < 0.001; **P < 0.01) is according to the Mann–Whitney rank-sum test.
Localization patterns of mec-10 and mec-4 gene fusions in touch neurons
The spatial asymmetry of the mec-10 touch phenotype raised the possibility that the MEC-10 protein might be asymmetrically localized within the touch neuron dendrites. However, it is also possible that MEC-10 is normally localized throughout the dendrite, but in the mec-10 mutant MEC-4–containing mechanoreceptors become restricted to the proximal dendrite. To address this question, we generated transgenic lines expressing a full-length gene fusion between the mec-10 and GFP coding regions under the control of the touch-neuron–specific pmec-4 promoter. The full-length fusion transgene rescued the gentle touch defect of the mec-10(tm1552) deletion (Supplemental Fig. S5), suggesting its product is functionally localized within the touch neurons. We compared the fluorescence patterns of these animals with animals expressing a full-length mec-4::GFP fusion in the same cells. We observed that MEC-4::GFP fluorescence was distributed in a punctate pattern throughout the ALM and PLM dendrites (Fig. 9), as reported previously (Cueva et al. 2007; Emtage et al. 2004). In contrast, MEC-10::GFP fluorescence was concentrated in the touch neuron cell bodies and the most proximal regions of the dendrites (Fig. 9; Supplemental Fig. S6). A mutant fusion protein consisting of the mec-10(tm1552) deleted polypeptide fused to GFP showed a similar distribution to the wild-type MEC-10::GFP in touch neurons (Fig. 9C). RNAi of the degt-1 harsh touch DEG/ENaC channel did not alter the pattern of MEC-10::GFP fluorescence in ALM (Supplemental Fig. S6), consistent with the possibility that MEC-10 protein involved in gentle touch is asymmetrically distributed. These results suggest that MEC-10 protein may be selectively localized to mechanotransduction complexes near the touch neuron cell bodies.
Fig. 9.
Localization of MEC-4 and MEC-10 protein fusions in body touch neurons. Shown are images of MEC-4::GFP (A), MEC-10::GFP (B), and MEC-10(tm1552)::GFP (C) fusions expressed in ALM. MEC-4::GFP normally is distributed in punctae along the touch receptor process. MEC-10::GFP and MEC-10(tm1552)::GFP are found only in the cell body in about 70% of observations; in the rest some staining can be observed in the posterior-projecting dendrite. Similar results were seen using anti-GFP antibodies or antibodies against the MEC-4 and MEC-10 proteins (which stain only overexpressing strains due to the low abundance of the MEC-4/MEC-10 proteins).
DISCUSSION
MEC-10 is important but probably not essential for gentle touch response
In recent years, DEG/ENaC channel family members have emerged as central contributors to sensory transduction, although how distinct subunits and complexes contribute to differential perception is poorly understood. In C. elegans, the DEG/ENaC channel genes mec-4 and mec-10 were identified in screens for mechanosensory (Mec) defects in gentle touch receptor neurons; although mec-4 appears essential for gentle touch sensation in these neurons, the importance of mec-10 has not been clearly defined. In this study, we found that although mec-10 mutations impair the responsiveness of the gentle body touch mechanoreceptors, they do not completely abolish touch responses in these neurons. Indeed, in response to stimulations distal to the cell body, mec-10 animals exhibit escape behavior over 60% of the time and the calcium transients evoked in the cell body are nearly half the magnitude of those in wild-type animals. This phenotype contrasts with that of mec-4, mec-2, and mec-6 null mutants, which have stronger Mec behavioral phenotypes and whose body touch neurons have no detectable response to gentle touch as measured by calcium imaging (Suzuki et al. 2003) or electrophysiology (O'Hagan et al. 2005). Although we cannot be completely certain the mec-10(tm1552) deletion allele is a null, based on molecular criteria as well as the results of dominance tests it almost certainly results in a severe loss of mec-10 function. Our results therefore suggest that MEC-10 is not an essential component of the gentle touch mechanosensory complex.
Standard models hypothesize that the mechanosensory complex in the gentle touch neurons consists of a core channel composed of MEC-4 and MEC-10, along with MEC-2 and MEC-6 as accessory subunits. However, our results suggest that homomeric complexes containing only MEC-4 channel subunits may be capable of functioning as touch receptors. In support of this hypothesis, coexpression of dominantly active MEC-4 with MEC-2 and MEC-6 yields functional channels in Xenopus oocytes, and the addition of MEC-10 actually reduces expressed currents in this heterologous system (Goodman et al. 2002). Likewise, whereas MEC-4 is required for neurodegeneration induced by gain-of-function alleles of mec-10, MEC-10 is not required for mec-4(gf)-induced degeneration (Huang and Chalfie 1994). These findings all indicate that MEC-4 can form functional channels without MEC-10 and are consistent with the existence of homomeric mechanotransduction channels. Alternatively, it is possible that MEC-4 may be able to form a gentle touch mechanotransducer by associating with a different, unidentified DEG/ENaC channel subunit. The possibility of a DEG/ENaC protein participating in multiple heteromeric complexes in the same cell is supported by recent evidence that MEC-10 associates with the DEGT-1 protein to form a harsh touch mechanoreceptor in the ALM neurons (Chatzigeorgiou et al. 2010). Either of these models suggests a heterogeneous population of mechanosensory complexes might exist in the touch neurons, with only a subset including MEC-10 as a subunit.
Asymmetric requirements for MEC-10 in the gentle touch neurons
We observed an unexpected asymmetry in the requirement for MEC-10 in the ALM and PLM touch neurons. Specifically, in both behavioral and calcium imaging experiments, we observed that mec-10 null animals were significantly more defective in responding to touch stimuli administered near the neuronal cell body than to stimuli administered at the distal end of the dendrite. Interestingly, this spatial asymmetry in phenotypic strength resembles what was previously described for weak alleles of mec-7 (Savage et al. 1989). Since the imaging experiments measured calcium transients in the cell body, the fact that mec-10 disproportionately affected responses to stimuli near the cell body is unlikely to reflect a defect in propagation of the mechanoreceptor potential along the process. Rather, MEC-10 may contribute differentially to mechanoreceptor complexes in different regions of the dendrite. Specifically, mechanoreceptor complexes in the cell body–proximal region of the dendrite may require both MEC-4 and MEC-10 subunits, whereas mechanoreceptor complexes in the distal region may require MEC-4 but not MEC-10. This is consistent with the subcellular distribution of the MEC-10::GFP reporter, which was restricted to regions near the touch neuron cell body.
These results may explain the apparent disparity between the relatively mild Mec phenotype observed in this work and a previous electrophysiological study (O'Hagan et al. 2005), which reported that strong defects in PLM mechanoreceptor potentials were recorded from mec-10 point mutants. In the latter study, receptor potentials were measured in response to stimuli applied within one length constant of the PLM cell body, about 90 μm. This position, corresponding approximately to stimulus point 1 in Figs. 5 and 6, was in the cell body–proximal region of the dendrite, where we observed the strongest requirement for mec-10. Thus all data appear to be consistent with the possibility that MEC-10 is an important subunit of cell body–proximal mechanosensory complexes but less important for distal complexes.
PVM is a gentle touch mechanosensory neuron
This study also provides insight into the function of the PVM neuron in C. elegans. Although PVM resembles the gentle touch avoidance neurons (ALM, PLM, and AVM) in morphology and gene expression profile, cell ablation studies have failed to provide evidence for a mechanosensory function for this neuron. Our calcium imaging results show that PVM responds to mechanosensory stimuli of similar intensity to those that activate the known gentle touch neurons. Moreover, the receptive field of PVM defined by these experiments is distinct from that of any of the gentle touch neurons and correlates precisely with the location of PVM's dendrite. Thus PVM is likely to be a primary mechanosensory neuron responding to gentle touch.
Since PVM is neither necessary nor sufficient for touch-activated escape responses, what role might it play in touch-controlled behaviors? One possibility is that it might mediate longer-term responses to mechanical stimulation. Repeated or prolonged touch or tap has been implicated in a variety of types of behavioral modulation or plasticity (Rankin and Broster 1992; Wicks and Rankin 1996; Zhao et al. 2003). The timescales of these effects suggest the involvement of monoamine or neuropeptide modulators released from neurons in mechanosensory circuits (Kindt et al. 2007; Sanyal et al. 2004). Since PVM is known to express at least two neuropeptide genes (flp-8 and flp-20; Kim and Li 2004), it is a plausible candidate for mediating long-term effects of touch on behavior. Future studies of PVM and PVM-expressed neuropeptides may help to characterize the broader effects of mechanosensory information on C. elegans behavior and to dissect their neural and molecular mechanisms.
GRANTS
This work was supported in part by grants from the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank M. Chalfie and the Caenorhabditis Genetics Center for strains.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Bounoutas and Chalfie, 2007. Bounoutas A, Chalfie M. Touch sensitivity in Caenorhabditis elegans. Pflügers Arch 454: 691–702, 2007 [DOI] [PubMed] [Google Scholar]
- Chalfie and Au, 1989. Chalfie M, Au M. Genetic control of differentiation of the Caenorhabditis elegans touch receptor neurons. Science 243: 1027–1033, 1989 [DOI] [PubMed] [Google Scholar]
- Chalfie and Sulston, 1981. Chalfie M, Sulston J. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev Biol 82: 358–370, 1981 [DOI] [PubMed] [Google Scholar]
- Chalfie et al., 1985. Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S. The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci 5: 956–964, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzigeorgiou et al., 2010. Chatzigeorgiou M, Yoo S, Watson JD, Lee WH, Spencer WC, Kindt KS, Hwang SW, Miller DM, 3rd, Treinin M, Driscoll M, Schafer WR. Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat Neurosci 13: 861–868, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelur et al., 2002. Chelur DS, Ernstrom GG, Goodman MB, Yao CA, Chen L, O'Hagan R, Chalfie M. The mechanosensory protein MEC-6 is a subunit of the C. elegans touch-cell degenerin channel. Nature 420: 669–673, 2002 [DOI] [PubMed] [Google Scholar]
- Christensen and Corey, 2007. Christensen AP, Corey DP. TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci 8: 510–521, 2007 [DOI] [PubMed] [Google Scholar]
- Clark and Chiu, 2003. Clark SG, Chiu C. C. elegans ZAG-1, a Zn-finger-homeodomain protein, regulates axonal development and neuronal differentiation. Development 130: 3781–3794, 2003 [DOI] [PubMed] [Google Scholar]
- Colbert et al., 1997. Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in C. elegans. J Neurosci 17: 8259–8269, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueva et al., 2007. Cueva JG, Mulholland A, Goodman MB. Nanoscale organization of the MEC-4 DEG/ENaC sensory mechanotransduction channel in Caenorhabditis elegans touch receptor neurons. J Neurosci 27: 14089–14098, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll and Chalfie, 1991. Driscoll M, Chalfie M. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature 349: 588–593, 1991 [DOI] [PubMed] [Google Scholar]
- Emtage et al., 2004. Emtage L, Gu G, Hartwieg E, Chalfie M. Extracellular proteins organize the mechanosensory channel complex in C. elegans touch receptor neurons. Neuron 44: 795–807, 2004 [DOI] [PubMed] [Google Scholar]
- Garcia-Anoveros and Corey, 1997. Garcia-Anoveros J, Corey DP. The molecules of mechanosensation. Annu Rev Neurosci 20: 567–594, 1997 [DOI] [PubMed] [Google Scholar]
- Goodman et al., 2002. Goodman MB, Ernstrom GG, Chelur DS, O'Hagan R, Yao CA, Chalfie M. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature 415: 1039–1042, 2002 [DOI] [PubMed] [Google Scholar]
- Goodman et al., 2004. Goodman MB, Lumpkin EA, Ricci A, Tracey WD, Kernan M, Nicolson T. Molecules and mechanisms of mechanotransduction. J Neurosci 24: 9220–9222, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman and Schwarz, 2003. Goodman MB, Schwarz EM. Transducing touch in Caenorhabditis elegans. Annu Rev Physiol 65: 429–452, 2003 [DOI] [PubMed] [Google Scholar]
- Hong et al., 2000. Hong K, Mano I, Driscoll M. In vivo structure–function analyses of Caenorhabditis elegans MEC-4, a candidate mechanosensory ion channel subunit. J Neurosci 20: 2575–2588, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang and Chalfie, 1994. Huang M, Chalfie M. Gene interactions affecting mechanosensory transduction in Caenorhabditis elegans. Nature 367: 467–470, 1994 [DOI] [PubMed] [Google Scholar]
- Huang et al., 1995. Huang M, Gu G, Ferguson EL, Chalfie M. A stomatin-like protein necessary for mechanosensation in C. elegans. Nature 378: 292–295, 1995 [DOI] [PubMed] [Google Scholar]
- Kahn-Kirby and Bargmann, 2006. Kahn-Kirby AH, Bargmann CI. TRP channels in C. elegans. Annu Rev Physiol 68: 719–736, 2006 [DOI] [PubMed] [Google Scholar]
- Kerr et al., 2000. Kerr R, Lev-Ram V, Baird G, Vincent P, Tsien RY, Schafer WR. Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans. Neuron 26: 583–594, 2000 [DOI] [PubMed] [Google Scholar]
- Kerr. Kerr RA. Imaging the activity of neurons and muscles. WormBook, edited by. The C. elegans research community, 2006. Wormbook, doi/10.1895/wormbook.1.113.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim and Li, 2004. Kim K, Li C. Expression and regulation of an FMRFamide-related neuropeptide gene family in Caenorhabditis elegans. J Comp Neurol 475: 540–550, 2004 [DOI] [PubMed] [Google Scholar]
- Kindt et al., 2007. Kindt KS, Quast KB, Giles AC, De S, Hendrey D, Nicastro I, Rankin CH, Schafer WR. Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron 55: 662–676, 2007 [DOI] [PubMed] [Google Scholar]
- O'Hagan et al., 2005. O'Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci 8: 43–50, 2005 [DOI] [PubMed] [Google Scholar]
- Rankin and Broster, 1992. Rankin CH, Broster BS. Factors affecting habituation and recoveryfrom habituation in the nematode Caenorhabditis elegans. Behav Neurosci 106: 239–249, 1992 [DOI] [PubMed] [Google Scholar]
- Sanyal et al., 2004. Sanyal S, Wintle RF, Kindt KS, Nuttley WM, Arvan R, Fitzmaurice P, Bigras E, Merz DC, Hebert TE, van der Kooy D, Schafer WR, Culotti JG, Van Tol HH. Dopamine modulates the plasticity of mechanosensory responses in Caenorhabditis elegans. EMBO J 23: 473–482, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage et al., 1989. Savage C, Hamelin M, Culotti JG, Coulson A, Albertson DG, Chalfie M. mec-7 is a β-tubulin gene required for the production of 15-protofilament microtubules in Caenorhabditis elegans. Genes Dev 3: 870–881, 1989 [DOI] [PubMed] [Google Scholar]
- Suzuki et al., 2003. Suzuki H, Kerr R, Bianchi L, Frokjaer-Jensen C, Slone D, Xue J, Gerstbrein B, Driscoll M, Schafer WR. In vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron 39: 1005–1017, 2003 [DOI] [PubMed] [Google Scholar]
- Tavernarakis and Driscoll, 1997. Tavernarakis N, Driscoll M. Molecular modeling of mechanotransduction in the nematode Caenorhabditis elegans. Annu Rev Physiol 59: 659–689, 1997 [DOI] [PubMed] [Google Scholar]
- Way and Chalfie, 1989. Way JC, Chalfie M. The mec-3 gene of Caenorhabditis elegans requires its own product for maintained expression and is expressed in three neuronal cell types. Genes Dev 3: 1823–1833, 1989 [DOI] [PubMed] [Google Scholar]
- Wicks and Rankin, 1996. Wicks SR, Rankin CH. The integration of antagonistic reflexes revealed by laser ablation of identified neurons determines habituation kinetics of the Caenorhabditis elegans tap withdrawal response. J Comp Physiol A Sens Neural Behav Physiol 179: 675–685, 1996 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2008. Zhang W, Bianchi L, Lee WH, Wang Y, Israel S, Driscoll M. Intersubunit interactions between mutant DEG/ENaCs induce synthetic neurotoxicity. Cell Death Differ 15: 1794–1803, 2008 [DOI] [PubMed] [Google Scholar]
- Zhao et al., 2003. Zhao B, Khare P, Feldman L, Dent JA. Reversal frequency in Caenorhabditis elegans represents an integrated response to the state of the animal and its environment. J Neurosci 23: 5319–5328, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.