Abstract
After experimental status epilepticus, many dentate granule cells born into the postseizure environment migrate aberrantly into the dentate hilus. Hilar ectopic granule cells (HEGCs) have also been found in persons with epilepsy. These cells exhibit a high rate of spontaneous activity, which may enhance seizure propagation. Electron microscopic studies indicated that HEGCs receive more recurrent mossy fiber innervation than normotopic granule cells in the same animals but receive much less inhibitory innervation. This study used hippocampal slices prepared from rats that had experienced pilocarpine-induced status epilepticus to test the hypothesis that an imbalance of synaptic excitation and inhibition contributes to the hyperexcitability of HEGCs. Mossy fiber stimulation evoked a much smaller GABAA receptor–mediated inhibitory postsynaptic currents (IPSC) in HEGCs than in normotopic granule cells from either control rats or rats that had experienced status epilepticus. However, recurrent mossy fiber-evoked excitatory postsynaptic currents (EPSCs) of similar size were recorded from HEGCs and normotopic granule cells in status epilepticus–experienced rats. HEGCs exhibited the highest frequency of miniature excitatory postsynaptic currents (mEPSCs) and the lowest frequency of miniature inhibitory postsynaptic currents (mIPSCs) of any granule cell group. On average, both mEPSCs and mIPSCs were of higher amplitude, transferred more charge per event, and exhibited slower kinetics in HEGCs than in granule cells from control rats. Charge transfer per unit time in HEGCs was greater for mEPSCs and much less for mIPSCs than in the normotopic granule cell groups. A high ratio of excitatory to inhibitory synaptic function probably accounts, in part, for the hyperexcitability of HEGCs.
INTRODUCTION
A unique feature of temporal lobe epilepsy is the anatomical reorganization of the dentate gyrus (reviews: Nadler 2003, 2009). One component of this reorganization is the seizure-induced enhancement of granule cell replication. Dentate granule cells are unusual in that they continue to be born and differentiate throughout life. After pilocarpine-induced status epilepticus, most of the newly born granule cells migrate into the granule cell body layer and differentiate. However, an estimated 21–25% of these cells, accounting for ∼1% of the total granule cell population, migrate aberrantly into the dentate hilus (Kron et al. 2010; Walter et al. 2007). Only a few granule cells are located in the hilus normally (Jiao and Nadler 2007; Marti-Subirana et al. 1986; Scharfman et al. 2003). Hilar ectopic granule cells (HEGCs) survive for at least months after status epilepticus (Jessberger et al. 2007b; Jiao and Nadler 2007; McCloskey et al. 2006), and some percentage of newborn granule cells continue to migrate aberrantly even after the replication rate normalizes (Bonde et al. 2006). Thus the fraction of granule cells that is ectopically located may increase with time after the initial insult. Granule cell neurogenesis may be enhanced in humans with temporal lobe epilepsy as well. Some findings support the hypothesis that seizures induce neurogenesis in young patients (Siebzehnrubl and Blümcke 2008), and HEGCs have been found in tissue resected from persons with epilepsy (Houser et al. 1992; Parent et al. 2006).
The increased frequency and duration of spontaneous seizures with time after status epilepticus in rats with neuronal death and mossy fiber sprouting (the “progression of seizures”) has been linked to enhanced granule cell neurogenesis (Jung et al. 2004, 2006). Seizure-related neurogenesis also seems to disrupt hippocampus-dependent learning (Jessberger et al. 2007a; Pekcec et al. 2008). It is uncertain whether these adverse outcomes relate to postseizure-generated granule cells that migrate normally, aberrantly, or both. Many HEGCs burst spontaneously (Scharfman et al. 2000; Zhan and Nadler 2009), and they are active during experimental limbic seizures (Scharfman et al. 2002). In addition, the nucleus of HEGCs is indented, unlike that of normal granule cells, consistent with a high rate of activity (Dashtipour et al. 2001). These findings suggest that HEGCs contribute to circuit hyperexcitability. HEGCs may thus be important for seizure propagation through the dentate gyrus.
Electron microscopic studies suggest that one reason for the hyperexcitability of HEGCs may be a relative excess of excitatory innervation. The somata and proximal apical dendrites of these cells are contacted by numerous boutons having the typical ultrastructure of mossy fiber boutons, synaptic terminals of dentate granule cell axons, and some have been positively identified as such by retrograde labeling with biocytin or by ZnT3 immunocytochemistry (Dashtipour et al. 2001; Pierce et al. 2005). HEGCs are more densely innervated by other granule cells than normotopic granule cells in the same animals (Pierce et al. 2005). Furthermore, the somata and proximal dendrites of HEGCs seem practically devoid of inhibitory innervation, as evidenced by the apparent lack of symmetric synapses (Dashtipour et al. 2001). This finding is consistent with the location of HEGCs at a considerable distance from most dentate basket cells, which innervate the somata and proximal dendrites of normotopic granule cells.
If HEGCs receive a high ratio of excitatory to inhibitory innervation relative to normotopic granule cells, this difference should be reflected in a similarly high ratio of evoked and miniature excitatory to inhibitory synaptic currents. This study used whole cell patch-clamp recording to show such a difference.
METHODS
Pilocarpine-induced status epilepticus
Male Sprague-Dawley rats (150–200 g; Zivic Laboratories, Pittsburgh, PA) received a single injection of pilocarpine hydrochloride (340–380 mg/kg, ip) 30 min after pretreatment with scopolamine methyl bromide and terbutaline hemisulfate (both 2 mg/kg, ip). Status epilepticus, defined as a continuous limbic motor seizure of stage 2 or higher (Racine 1972), was allowed to self-terminate after 6–8 h. Rats treated in this way develop extensive and consistent hilar lesions followed by consistently robust mossy fiber sprouting, the accumulation of HEGCs, and spontaneous seizures (Jiao and Nadler 2007; Sloviter et al. 2003). Some rats pretreated with methylscopolamine and terbutaline and then injected with pilocarpine exhibited only a few brief behavioral seizures but not status epilepticus. They were used as controls to account for any possible action of pilocarpine not mediated by status epilepticus. Histological tests showed no evidence of neuronal degeneration or mossy fiber sprouting in these animals (Okazaki et al. 1999), and their electrophysiological responses were not significantly different from those of age-matched untreated rats (Hardison et al. 2000; Molnár and Nadler 1999; Okazaki and Nadler 2001; Okazaki et al. 1999). All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved in advance by the Duke University Institutional Animal Care and Use Committee.
Hippocampal slice preparation
Hippocampal slices were prepared 10–40 wk after pilocarpine administration. Control rats were studied 97 ± 66 days and rats that had developed status epilepticus 93 ± 67 (SD) days after pilocarpine administration. Animals were decapitated under deep ether anesthesia, and the brain was removed to ice-cold high-Mg2+ artificial cerebrospinal fluid (high-Mg2+ ACSF; in mM): 112 NaCl, 25 NaHCO3, 3.1 KCl, 1.8 CaCl2, 11.2 MgSO4, 0.4 KH2PO4, 1 ascorbic acid, and 10 d-glucose, equilibrated with 95% O2-5% CO2. Transverse 420-μm-thick slices of the caudal hippocampus were prepared with a vibratome, incubated at 34°C in high-Mg2+ ACSF for 30 min, and maintained in standard ACSF (122 mM NaCl, 1.2 mM MgSO4) at room temperature (22–24°C).
Electrophysiology
A slice was submerged in a recording chamber mounted on the stage of a Nikon Eclipse E600FN microscope equipped with far infrared-differential interference contrast optics, a CCD camera, and a 40× water-immersion objective. The slice was superfused with standard ACSF at room temperature (22–24°C) and a rate of 3 ml/min. Criteria for selecting HEGCs were 1) soma located within the hilus and of a size and shape indistinguishable from granule cells in the cell body layer and 2) no more than three dendrites emerged from the soma. In rats studied after status epilepticus, numerous cells scattered throughout the dentate hilus met these criteria, comprising an average of 62% of the total hilar neuron population (Jiao and Nadler 2007). Recorded HEGCs were located in all parts of the hilus except for the deep region adjacent to the end of area CA3c. Cell identity was confirmed by intracellular dialysis with biocytin and subsequent visualization of cellular morphology (Zhan and Nadler 2009). Results obtained from putative HEGCs were included in this study only if cellular morphology appeared identical to previous descriptions of HEGCs (Dashtipour et al. 2001; Scharfman et al. 2000, 2003): small (8–12 μm diam) soma located within the dentate hilus, one to two apical dendrite(s) penetrating into or directed toward the dentate molecular layer, and axon with giant boutons in area CA3 and extensive branches within the hilus. Examples of HEGCs recorded in this study are shown in Fig. 1. More than 90% of the recorded hilar neurons were confirmed to be HEGCs; data obtained from hilar neurons not confirmed to be HEGCs were discarded. The normotopic dentate granule cells selected for recording were located in the granule cell body layer at the apex of the granule cell arch or in the supragranular blade close to the apex.
Fig. 1.
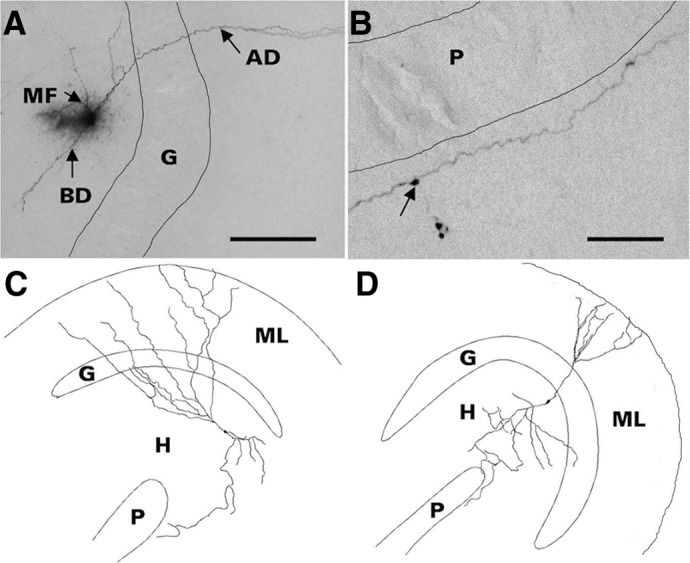
Morphology of representative hilar ectopic granule cells (HEGCs). Cells were filled with biocytin during whole cell patch-clamp recording, fixed slices were cut into serial 60-μm-thick sections, biocytin was visualized with nickel-intensified avidin-horseradish peroxidase-diaminobenzidine (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA), and cell morphology was reconstructed with use of Neurolucida (MicroBrightField, Williston, VT). Criteria for confirmation of HEGC identity included hilar location of the soma, apical dendrite(s) penetrating into or directed toward the dentate molecular layer, and axon with giant boutons in area CA3 and extensive branches within the hilus. A: the apical dendrite (AD) of this HEGC crosses the granule cell body layer (G) and penetrates the molecular layer. The mossy fiber (MF) branches within the hilus. This cell also has a basal dendrite (BD) directed into the hilus. Scale bar, 500 μm. B: HEGC mossy fiber courses through stratum lucidum of area CA3 adjacent to the pyramidal cell body layer (P). Arrow indicates a giant bouton from which a filopodium originates. Scale bar, 200 μm. C: reconstructed morphology of an HEGC from which miniature inhibitory postsynaptic currents (mIPSCs) were recorded. D: reconstructed morphology of an HEGC from which miniature excitatory postsynaptic currents (mEPSCs) were recorded. The apical dendrites of both cells reached the outer edge of the dentate molecular layer (ML) and the cell in D had a short basal dendrite directed into the hilus (H). The main branch of the mossy fiber reached stratum lucidum of area CA3.
Borosilicate patch electrodes used for whole cell recording had tip resistances of 4.5–6.5 MΩ. Recordings were made with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). Series resistances (<20 MΩ) were compensated 75%. Recordings were rejected if the series resistance varied by >20%. Voltage-clamp recordings were filtered at 2 kHz, digitized at 20 kHz, and stored for analysis off-line with pClamp8.1 (Molecular Devices) or MiniAnalysis (Synaptosoft, Decatur, GA) software.
To record compound synaptic currents evoked by mossy fiber stimulation, the recording electrode was filled with an internal solution that contained (in mM) 120 cesium gluconate, 10 HEPES, 2 MgATP, 1 EGTA, 5 creatine phosphate, 20 U/ml creatine phosphokinase, 10 QX-314 (N-ethyl lidocaine) and 1% (wt/vol) biocytin, pH 7.25–7.30 and 293–297 mosm. The liquid junction potential was determined to be 10 mV with use of the method described by Neher (1992), and this value was subtracted from all membrane potentials. The monopolar stimulating electrode was a 25-μm-diam nichrome wire insulated to the tip with a polymerized polyvinyl resin. It was placed in stratum lucidum at the junction of areas CA3b and CA3c. Rectangular electrical stimuli of 100-μs duration were delivered every 30 s at an intensity (500–800 μA) that evoked an antidromic population spike of just-maximal amplitude in a portion of the granule cell body layer close to where whole cell patch-clamp recordings were made. Under each experimental condition, 10 stimuli were delivered, and the responses were averaged. First, the mossy fiber–evoked GABAA receptor–mediated inhibitory postsynaptic current (IPSC) was recorded at a holding potential of 0 mV. The IPSC was defined as the outward current obtained by subtracting electronically the averaged response recorded in the presence of 30 μM bicuculline from the averaged response recorded before addition of bicuculline to the superfusion medium. Activation of postsynaptic GABAB receptors was prevented by the use of a cesium-based internal solution that contained QX-314 but not GTP. Then the holding potential was changed to −30 mV. The N-methyl-d-aspartate (NMDA) receptor–mediated excitatory postsynaptic current (EPSC) was defined as the inward current obtained by subtracting electronically the averaged response recorded in the presence of 50 μM d-2-amino-5-phosphonopentanoate (d-AP5) and 30 μM bicuculline from the averaged response recorded in the presence of bicuculline alone. Finally, the holding potential was changed to −80 mV. The AMPA/kainate receptor–mediated EPSC was defined as the inward current obtained by subtracting electronically the averaged response recorded in the presence of 10 μM 2,3-dihydroxy-6-nitro-7-sulfamyl-benzo(F)quinoxaline-2,3-dione (NBQX), 50 μM d-AP5, and 30 μM bicuculline from the averaged response recorded in the presence of d-AP5 and bicuculline alone.
To record miniature synaptic currents, the recording electrode was filled with an internal solution that contained (in mM) 126 CsCl, 10 HEPES, 5 EGTA, 2 MgATP, 1 MgCl2, 0.1 CaCl2, 5 creatine phosphate, 20 U/ml creatine phosphokinase, and 1% (wt/vol) biocytin, pH 7.25–7.30 and 293–297 mosm. Miniature excitatory postynaptic currents (mEPSCs) were recorded in the presence of 30 μM bicuculline and 1 μM TTX, whereas miniature inhibitory postsynaptic currents (mIPSCs) were recorded in the presence of 10 μM NBQX, 50 μM d-AP5, and 1 μM TTX. Miniature events were recorded for 2.5 min at a holding potential of −70 mV. The amplitude threshold for detecting these events was 5 pA, the smallest event that could be distinguished reliably from electrical noise. After MiniAnalysis had identified the putative miniature events automatically, each record was examined manually to exclude false positives. Interevent intervals, peak amplitudes, 10–90% rise times, decay time constants (τ), and charge transfers per event from all recorded granule cells in each group were combined, and between-group differences were analyzed for statistical significance by the Kolmogorov-Smirnov test. Between-group differences in event frequency were analyzed by one-way ANOVA followed by the Newman-Keuls post hoc test.
Grouped data are expressed as mean ± SE unless otherwise indicated.
Materials
TTX, d-AP5, and NBQX were obtained from Tocris Bioscience (Ellisville, MO). Bicuculline methiodide, MgATP, creatine phosphate, creatine phosphokinase, d-gluconic acid lactone, cesium hydroxide (99.9%; 50% by weight), CsCl, HEPES, EGTA, biocytin, pilocarpine hydrochloride, (−)scopolamine methyl bromide, and terbutaline hemisulfate were purchased from Sigma (St. Louis, MO). QX-314 was obtained from Alomone Laboratories (Jerusalem, Israel).
RESULTS
Membrane properties of HEGCs and normotopic dentate granule cells
Resting membrane potential was assessed on breakin, and input resistance and membrane capacitance were determined after intracellular dialysis with the CsCl-based internal solution. In agreement with our previous report (Zhan and Nadler 2009), HEGCs had a significantly less polarized resting membrane potential than normotopic granule cells [dentate granule cells from control rats (CGCs): −76 ± 0 mV, n = 15; normotopic granule cells from rats subjected to pilocarpine-induced status epilepticus (GC-SEs): −74 ± 1 mV, n = 16; HEGCs: −69 ± 1 mV, n = 17; P < 0.001 compared with CGCs and P < 0.005 compared with GC-SEs by Newman-Keuls test after 1-way ANOVA yielded P < 0.001]. There was no significant between-group difference in input resistance (CGC: 190 ± 20 MΩ; GC-SE: 180 ± 20 MΩ; HEGC: 200 ± 20 MΩ) or membrane capacitance (CGC: 21 ± 2 pF; GC-SE: 23 ± 3 pF; HEGC: 27 ± 3 pF).
Mossy fiber stimulation evokes small IPSCs, but normal-size recurrent EPSCs, in HEGCs
Stimulation of the mossy fibers at the CA3b–CA3c border evoked a GABAA receptor–mediated compound IPSC in HEGCs, GC-SEs, and CGCs (Fig. 2). Both the peak amplitude of and charge transferred by mossy fiber–evoked IPSCs were much smaller in HEGCs than in either normotopic granule cell population. In HEGCs, the average size of the mossy fiber–evoked IPSC, measured by the charge transferred, was an order of magnitude smaller than the mossy fiber-evoked IPSC in GC-SEs (HEGC: 1265 ± 355 fC, GC-SE: 11,276 ± 2863 fC; P < 0.001 by Newman-Keuls test). The largest mossy fiber–evoked IPSCs were recorded from GC-SEs. The average charge transferred was 45% greater than in CGCs (GC-SE: 11,276 ± 2863 fC, CGC: 6143 ± 600 fC; P < 0.05 by Newman-Keuls test). Because peak IPSC amplitudes did not differ significantly (P > 0.4 by Newman-Keuls test), the greater charge transferred to GC-SEs was accounted for mainly by a more prolonged response (cf. left and middle of Fig. 2, top).
Fig. 2.
Mossy fiber stimulation evokes a small GABAA receptor–mediated IPSC in HEGCs and a large GABAA receptor–mediated IPSC in GC-SEs. Top: responses recorded from a representative normotopic dentate granule cell from a control rat (CGC), a normotopic granule cell from a rat subjected to pilocarpine-induced status epilepticus (GC-SE), and an HEGC. The mossy fiber-evoked IPSC, NMDA receptor–mediated EPSC, and AMPA/kainate (KA) receptor–mediated EPSC were recorded sequentially. *Stimulus artifact. Bottom left: the mossy fiber-evoked IPSC was smallest in HEGCs whether measured by amplitude or charge transfer. IPSC charge transfer was greatest in GC-SEs largely because of the longer response duration. *P < 0.05 compared with CGC; **P < 0.001 compared with GC-SE and P = 0.025 compared with CGC by Newman-Keuls test after 1-way ANOVA yielded P < 0.001. ***P < 0.01 compared with CGC or GC-SE by Newman-Keuls test after 1-way ANOVA yielded P = 0.003. Bottom right: the charge transfer ratio of AMPA/kainate receptor–mediated EPSC (AMPA) to GABAA receptor–mediated IPSC (GABA) was significantly greater in HEGCs than in GC-SEs. However, the charge transfer ratios of NMDA receptor–mediated EPSC (NMDA) to AMPA/kainate receptor–mediated EPSC were not significantly different. *P = 0.01 by 2-tailed t-test.
Mossy fiber stimulation evoked an AMPA/kainate receptor–mediated EPSC only in granule cells from rats that had experienced status epilepticus (Fig. 2). This finding can probably be explained by the sprouting of mossy fibers after status epilepticus and the subsequent formation of functional synapses with other granule cells (Nadler 2003, 2009). Mossy fiber stimulation evoked AMPA/kainate receptor–mediated EPSCs of similar size in HEGCs (mean: 1489 fC, range: 164–5127 fC) and GC-SEs (mean: 2184 fC, range: 266–9127 fC). There was also no significant between-group difference in the ratio between the sizes of NMDA receptor– and AMPA/kainate receptor–mediated components of the evoked EPSC.
Because AMPA/kainate receptor–mediated EPSCs were about the same size in HEGCs and GC-SEs, whereas the GABAA receptor–mediated IPSC was much smaller in HEGCs, the ratio between the two responses was an order of magnitude greater in HEGCs (Fig. 2; P = 0.01 by Student's t-test).
Although mossy fiber stimulation did not evoke an AMPA/kainate receptor–mediated EPSC in granule cells from control rats, it did evoke a small NMDA receptor–mediated response (Fig. 2, top left). Most likely, glutamate released at mossy fiber synapses on nearby interneurons overflowed those synapses and activated extrasynaptic NMDA receptors on the recorded cell.
Electrical stimulation in stratum lucidum at the Ca3b–CA3c border could possibly activate projections to dentate granule cells other than recurrent mossy fibers, especially when using a large stimulus current. We tested the specificity of our stimuli by varying the position of the stimulating electrode. Moving the stimulating electrode perpendicular to the pyramidal cell body layer by as little as 50 μm abolished both the antidromic response and any evoked compound EPSC. This observation suggests that the electrical stimuli activated predominantly mossy fibers. They probably also activated some CA3 pyramidal cells located close to the electrode. The firing of CA3 pyramidal cells can evoke an excitatory synaptic response in dentate granule cells through a disynaptic pathway that involves hilar mossy cells (Scharfman 1994). If this pathway contributed significantly to compound EPSCs in this study, a compound EPSC should have been recorded in CGCs. No such response was observed in those cells. Furthermore, little synaptic excitation could have been relayed through hilar mossy cells to granule cells from rats that had experienced pilocarpine-induced status epilepticus, because status epilepticus induced by the method used in this study consistently kills >90% of mossy cells (Jiao and Nadler 2007). It is possible that status epilepticus causes CA3 pyramidal cells to form monosynaptic feedback connections with dentate granule cells. If so, synapses made by axons of CA3 pyramidal cells could have accounted for some portion of the excitatory synaptic response evoked by stimulating in stratum lucidum. However, there is currently no evidence to support the formation of these synapses.
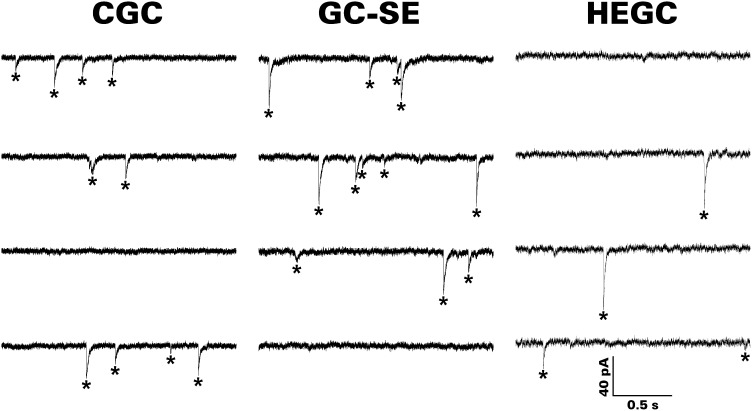
mEPSC frequency is greatest in HEGCs
Spontaneous EPSCs were recorded from dentate granule cells with a CsCl-based internal solution in the presence of bicuculline and TTX at a holding potential of −70 mV (Fig. 3). mEPSCs were recorded most frequently from HEGCs. The interval between these events was less than one half as great compared with GC-SEs and only one third as great compared with CGCs (Fig. 4; Table 1; P < 0.001 by Kolmogorov-Smirnov test in each case). Otherwise, mEPSCs recorded from HEGCs resembled those recorded from GC-SEs. Compared with CGCs, a substantially greater average charge was transferred by each event (P < 0.001 by Kolmogorov-Smirnov test for both GC-SEs and HEGCs). In both HEGCs and GC-SEs, the mean peak amplitude was greater than control, and on average, the events decayed more slowly (P < 0.001 by Kolmogorov-Smirnov test in each instance). The mean rise time was longer than control only in HEGCs, however (P < 0.001 by Kolmogorov-Smirnov test). Thus mEPSCs were recorded most frequently from HEGCs, and in those cells, they were relatively large and exhibited relatively slow kinetics.
Fig. 3.
mEPSCs are recorded more frequently from HEGCs than from either GC-SEs or CGCs; mEPSC amplitude is generally greater in HEGCs and GC-SEs than in CGCs. The traces shown are from representative experiments. Recordings were made with a CsCl-based internal solution, and the membrane potential was clamped at −70 mV. Individual events that met our criterion are indicated by asterisks.
Fig. 4.

Cumulative probability plots show lower interevent intervals (higher frequency) of mEPSCs in HEGCs and differences from control in mEPSC amplitude, rise time, decay time constant, and charge transfer per event. Results were computed from the number of events given in Table 1, which also presents the grouped data. Averaged mEPSCs recorded from a representative CGC, GC-SE, and HEGC are shown at the top left.
Table 1.
Properties of mEPSCs in dentate granule cells after pilocarpine-induced status epilepticus
| CGC | GC-SE | HEGC | |
|---|---|---|---|
| Frequency, Hz | 0.16 ± 0.02 | 0.22 ± 0.03 | 0.38 ± 0.08*† |
| Interevent interval, s | 6.0 ± 0.4 | 4.6 ± 0.3 | 2.0 ± 0.1‡§ |
| Amplitude, pA | 8.48 ± 0.18 | 9.86 ± 0.24‡ | 10.38 ± 0.35‡ |
| 10–90% rise time, ms | 1.89 ± 0.07 | 2.19 ± 0.08 | 2.52 ± 0.08‡ |
| τ, ms | 2.22 ± 0.09 | 3.51 ± 0.17‡ | 4.15 ± 0.16‡ |
| Charge transfer, fC | 22.83 ± 0.93 | 38.73 ± 2.23‡ | 57.74 ± 3.42‡ |
Values are means ± SE. mEPSC frequency was determined for 9 (CGC) or 10 (GC-SE and HEGC) cells per group. Other parameters were determined for 268 (CGC), 397 (GC-SE), or 785 (HEGC) events recorded from the indicated number of cells. mEPSCs were recorded in the presence of 30 μM bicuculline and 1 μM TTX for 2.5 min at 22–24°C. Recordings were made with a CsCl-based internal solution at a holding potential of −70 mV.
Significantly different from CGC at P < 0.05 (Newman-Keuls test after 1-way ANOVA yielded P < 0.025).
Significantly different from GC-SE at P < 0.05 (Newman-Keuls test).
Significantly different from CGC at P < 0.001 (Kolmogorov-Smirnov test).
Significantly different from GC-SE at P < 0.001 (Kolmogorov-Smirnov test). mEPSC, miniature excitatory posysynaptic current; CGC, normotopic granule cells from control rats; GC-SE, normotopic granule cells from rats subjected to pilocarpine-induced status epilepticus; HEGC, hilar ectopic granule cells.
Between-group comparisons of mean interevent interval and charge transfer per event showed an inverse relationship between these properties (Table 1). The more frequently mEPSCs were recorded from these granule cell populations, the larger they were. Thus the total charge transferred per unit time in HEGCs was on average 2.6 times as great as in GC-SEs and 6.1 times as great as in CGCs.
To determine whether there was a selective increase in fast- or slow-rising mEPSCs in HEGCs, we constructed histograms that included the events recorded from every cell (Fig. 5, top). There was no between-group difference in the proportion of mEPSCs with 10–90% rise times <1 ms (CGC: 18.3%; GC-SE: 17.6%; HEGC: 17.7%). However, 16.4% of the mEPSCs recorded from HEGCs had 10–90% rise times >4 ms compared with 8.6% in GC-SEs and 4.5% in CGCs. These differences were statistically significant (P < 0.001 by χ2 test comparing HEGCs to each of the other granule cell groups).
Fig. 5.
Histograms of 10–90% rise times for all mEPSCs and mIPSCs recorded in this study. The numbers of events included are given in Tables 1 and 2. In HEGCs, slowly rising mEPSCs (>4 ms) constituted a significantly higher percentage of total mEPSCs than in the normotopic granule cell groups, and there was no between-group difference in the percentage of rapidly rising mEPSCs (<1 ms). Slowly rising mIPSCs constituted a significantly higher percentage of total mIPSCs than in the normotopic granule cell groups, but rapidly rising mIPSCs constituted a significantly smaller percentage of the total in both HEGCs and GC-SEs than in CGCs.
mIPSC frequency in HEGCs is very low
Spontaneous IPSCs were recorded from dentate granule cells with a CsCl-based internal solution in the presence of NBQX, d-AP5, and TTX at a holding potential of −70 mV (Fig. 6). mIPSCs were recorded from GC-SEs and CGCs about an order of magnitude more frequently than from HEGCs (Fig. 7; Table 2). The interval between these events was about four times as great in HEGCs compared with CGCs and about three times as great compared with CG-SEs (P < 0.001 by Kolmogorov-Smirnov test in each case). Recordings made at 34–35°C produced similar differences in interevent intervals (HEGC: 1.51 ± 0.09 s, GC-SE: 0.93 ± 0.07 s, CGC: 0.56 ± 0.01 s, n = 496, 716, and 2418 mIPSCs recorded from 5–9 cells, respectively; P < 0.001 for all between-group comparisons by Kolmogorov-Smirnov test). However, each mIPSC recorded from HEGCs transferred on average 58% more charge than the average mIPSC in CGCs (Table 2; P < 0.001 by Kolmogorov-Smirnov test). The mean charge transferred by mIPSCs recorded from GC-SEs was intermediate between mean values from the other groups, significantly larger (P < 0.005 by Kolmogorov-Smirnov test) than in CGCs but significantly smaller (P < 0.001 by Kolmogorov-Smirnov test) than in HEGCs. These differences can be explained, in part, by a small (10–11%), but statistically significant (P < 0.001 by Kolmogorov-Smirnov test), increase in the mean peak amplitude of mIPSCs recorded from HEGCs and GC-SEs compared with those recorded from CGCs. mIPSCs recorded from HEGCs, but not those recorded from GC-SEs, decayed significantly more slowly on average than those recorded from CGCs (P < 0.001 by Kolmogorov-Smirnov test). This finding largely explains the greater charge transfer per event in HEGCs than in GC-SEs. Mean rise times were significantly longer in GC-SEs than in CGCs and longer still in HEGCs (P < 0.001 by Kolmogorov-Smirnov test for all between-group comparisons). Thus mIPSCs were recorded from HEGCs with low frequency, but in those cells, these events were relatively large and exhibited relatively slow kinetics.
Fig. 6.
mIPSCs are much less frequently recorded from HEGCs than from either GC-SEs or CGCs; mIPSC amplitude is generally greater in HEGCs and GC-SEs than in CGCs. The traces shown are from representative experiments. Recordings were made with a CsCl-based internal solution, and the membrane potential was clamped at −70 mV. Individual events that met our criterion are indicated by asterisks. In the 2nd segment of the CGC trace, the 1st asterisk marks 2 overlapping mIPSCs.
Fig. 7.
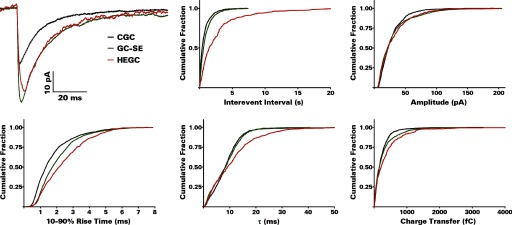
Cumulative probability plots show greater intervent intervals (lower frequency) of mIPSCs in HEGCs and differences from control in mIPSC amplitude, rise time, decay time constant, and charge transfer per event. Results were computed from the number of events given in Table 2, which also presents the grouped data. Averaged mIPSCs recorded from a representative CGC, GC-SE, and HEGC are shown at the top left.
Table 2.
Properties of mIPSCs in dentate granule cells after pilocarpine-induced status epilepticus
| CGC | GC-SE | HEGC | |
|---|---|---|---|
| Frequency, Hz | 1.44 ± 0.21 | 1.19 ± 0.25 | 0.34 ± 0.06*† |
| Interevent interval, s | 0.69 ± 0.02 | 0.91 ± 0.04‡ | 2.69 ± 0.22‡§ |
| Amplitude, pA | 27.61 ± 0.55 | 30.24 ± 0.85‡ | 30.78 ± 1.40‡ |
| 10–90% rise time, ms | 1.71 ± 0.03 | 2.05 ± 0.04‡ | 2.39 ± 0.07‡§ |
| τ, ms | 8.66 ± 0.16 | 8.44 ± 0.17 | 10.80 ± 0.46‡§ |
| Charge transfer, fC | 226 ± 7 | 271 ± 10¶ | 358 ± 25‡§ |
Values are means ± SE. mIPSC frequency was determined for 6 (CGC and GC-SE) or 7 (HEGC) cells per group. Other parameters were determined for 1,293 (CGC), 1,073 (GC-SE), or 362 (HEGC) events recorded from the indicated number of cells. mIPSCs were recorded in the presence of 10 μM NBQX, 50 μM D-AP5, and 1 μM TTX for 2.5 min at 22–24°C. Recordings were made with a CsCl-based internal solution at a holding potential of −70 mV.
Significantly different from CGC at P < 0.01 (Newman-Keuls test after 1-way ANOVA yielded P < 0.005).
Significantly different from GC-SE at P < 0.01 (Newman-Keuls test).
Significantly different from CGC at P < 0.001 (Kolmogorov-Smirnov test).
Significantly different from GC-SE at P < 0.001 (Kolmogorov-Smirnov test).
Significantly different from CGC at P < 0.005 (Kolmogorov-Smirnov test). mIPSC, miniature inhibitory posysynaptic current. See Table 1 for other abbreviations.
Comparisons of mean interevent interval and charge transfer per event showed a direct relationship between these properties (Table 2). The less frequently mIPSCs were recorded from these granule cell populations, the larger they were. Thus the relatively large size of individual mIPSCs in HEGCs compensated to some degree for their infrequency. Nevertheless, the total charge transferred per unit time was still only 37–38% as great as in GC-SEs or CGCs.
Histograms of mIPSC rise times showed between-group differences in the proportions of both rapidly rising and slowly rising events (Fig. 5, bottom). The proportion of mIPSCs having a 10–90% rise time <1 ms was much smaller in granule cells from survivors of status epilepticus (CGC: 31.8%; GC-SE: 15.4%; HEGC: 13.5%; P < 0.001 for both GC-SEs and HEGCs compared with CGCs by χ2 test). However, only HEGCs had an unusually large proportion of mIPSCs with 10–90% rise times >4 ms (CGC: 6.3%; GC-SE: 7.8%; HEGC: 13.0%; P < 0.001 compared with CGCs and P = 0.005 compared with GC-SEs by χ2 test).
Comparison of mEPSCs and mIPSCs shows a much higher ratio of synaptic excitation to synaptic inhibition in HEGCs than in normotopic granule cells
The frequency and charge transfer/s of mEPSCs or mIPSCs were calculated for each granule cell from which recordings were made and the group ratios were compared (Fig. 8). HEGCs exhibited far higher mEPSC/mIPSC ratios by either measure. Compared with granule cells from control rats, the frequency ratio was 10.3 times as great and the charge transfer/s ratio was 16.1 times as great. Compared with GC-SEs, the frequency ratio was 5.9 times as great and the charge transfer/s ratio was 6.8 times as great.
Fig. 8.
Analysis of miniature synaptic events shows an excess of excitation over inhibition in HEGCs compared with the normotopic granule cell groups. mEPSCs and mIPSCs were recorded from different cells. The numbers of cells from which each were recorded are given in Tables 1 and 2. Event frequency and charge transfer/s were computed for each granule cell studied, and the results obtained from all cells in each group were averaged. The mean mEPSC frequency or charge transfer/s was divided by the mean mIPSC frequency or charge transfer/s. The mEPSC/mIPSC ratio for both measures was much greater in HEGCs.
DISCUSSION
Our results show a marked difference between HEGCs and normotopic dentate granule cells in the balance between afferent synaptic excitation and synaptic inhibition. In HEGCs, this balance strongly favors synaptic excitation, whether assessed by mossy fiber–evoked compound synaptic responses or by miniature synaptic events. mEPSCs are larger and more frequently recorded than in CGCs. Although mIPSCs are also larger than control, they are recorded much less frequently. The resulting >60% reduction in mIPSC charge transfer per unit time correlates with a relatively small mossy fiber–evoked compound IPSC. Thus both enhanced synaptic excitation and reduced synaptic inhibition contribute to the high synaptic excitation/inhibition ratio in HEGCs. GC-SEs share with HEGCs most differences from control in the properties of miniature synaptic events. However, these differences are generally more pronounced in HEGCs. In addition, the frequencies of mEPSCs and mIPSCs recorded from GC-SEs differ only subtly or insignificantly from control compared with the several-fold differences observed in HEGCs. Finally, mossy fiber stimulation evokes a significantly larger compound IPSC in GC-SEs than in CGCs. These observations may be explained at least partly by seizure-related loss of innervation followed by axon sprouting, changes in the expression and composition of postsynaptic receptors, the unique anatomical location of HEGCs, and the different ages of granule cells in the three experimental groups. Model-dependent variables, including the time between slice preparation and the last spontaneous seizure and the extent of seizure-related brain damage and axon sprouting, may also influence some of our outcome measures. We have not assessed these variables, however.
Excitatory synaptic responses in HEGCs
Pilocarpine-induced status epilepticus destroys the great majority of hilar mossy cells (Buckmaster and Jongen-Rêlo 1999; Jiao and Nadler 2007; Sloviter et al. 2003), an average of 95% when performed by the method used in this study (Jiao and Nadler 2007). The associational–commissural axons of mossy cells provide excitatory innervation to the proximal third of the granule cell apical dendrite (Ribak et al. 1985). When these connections degenerate or fail to form because of the seizure-induced death of mossy cells, granule cell mossy fibers sprout collaterals that establish synapses on the denervated (GC-SEs) or noninnervated (HEGCs) dendritic region of other granule cells, thus replacing the synapses that had been lost. Both GC-SEs and HEGCs are synaptic targets of recurrent mossy fibers. In addition, HEGCs and those GC-SEs that were born either after or within 5 wk before status epilepticus have been identified as the source of those fibers (Kron et al. 2010). Both granule cell types receive mossy fiber synapses on their apical dendrite(s), with HEGCs having a greater number than GC-SEs (Pierce et al. 2005). Mossy fibers also contact the soma of HEGCs (Dashtipour et al. 2001). Moreover, granule cell basal dendrites are innervated predominantly by excitatory afferent projections (Thind et al. 2008), at least some of which are mossy fibers (Ribak et al. 2000). Granule cells normally have a basal dendrite during their early development, but it either retracts or evolves into an apical dendrite as the cell migrates into the cell body layer and differentiates (Shapiro and Ribak 2005). HEGCs and those GC-SEs that entered their final division after or within 5 wk before status epilepticus often retain a hilar basal dendrite (Jessberger et al. 2007b; Kron et al. 2010; Shapiro and Ribak 2005; Walter et al. 2007). Thus HEGCs may be expected to receive more excitatory innervation, on average, than normotopic granule cells and, in particular, more innervation from recurrent mossy fibers.
mEPSCs were recorded about twice as frequently from HEGCs as from either normotopic granule cell population. This finding is consistent with HEGCs having a greater number of excitatory synapses than other granule cells, although it could also be explained by a greater mean probability of action potential–independent glutamate release. The total dendritic length of HEGCs is no greater than that of normotopic granule cells (M. C. Cameron, R.-Z. Zhan, and J. V. Nadler, unpublished observations). Membrane capacitance is also not significantly different. Therefore the greater mEPSC frequency observed in HEGCs probably cannot be explained by their having a larger membrane surface available for innervation. HEGCs have many mossy fiber synapses but presumably few associational–commissural synapses, whereas GCGs have many associational–commissural synapses but few or no mossy fiber synapses. It is possible that recurrent mossy fiber synapses release glutamate more reliably than associational–commissural synapses. In that case, however, one might expect mEPSC frequency in GC-SEs also to be greater than control. This result was not obtained, in agreement with previous work (Epsztein et al. 2005). It is also possible that more mEPSCs were counted in recordings from HEGCs because their mean peak amplitude was larger, causing a smaller percentage of mEPSCs to be undetected because they were obscured by electrical noise. However, the mean peak amplitude of mEPSCs recorded from GC-SEs was also greater than control and not significantly different from that of mEPSCs recorded from HEGCs. However, mEPSC frequency was significantly less than in HEGCs and not significantly different from control. Thus between-group differences in peak mEPSC amplitude did not correlate with differences in mEPSC frequency. Although further studies are needed, the relatively high frequency of mEPSCs recorded from HEGCs seems to be explained best by a greater density of excitatory synapses compared with normotopic granule cell populations, that is, more excitatory synapses per unit dendritic length. The greater excitatory synaptic density may be related not only to HEGCs having a greater number of recurrent mossy fiber synapses than GC-SEs, but possibly also to their receiving innervation from neurons that do not project to normotopic granule cells. Again, further studies are needed to assess this possibility.
The establishment of mossy fiber synapses with HEGCs and GC-SEs most likely explains the larger average size of mEPSCs in these groups. Mean peak amplitude, decay time constant, and charge transfer per event were significantly greater than in CGCs, with no significant difference between them. Unitary postsynaptic responses at recurrent mossy fiber synapses can be quite large relative to unitary responses produced by activation of other excitatory pathways (Molnár and Nadler 1999; Simmons et al. 1997). In part, these findings can be explained by the proximity of these synapses to the soma and by the simultaneous or overlapping release of glutamate from the multiple active zones of each synaptic bouton. In addition, recurrent mossy fiber synapses differ from the perforant path and associational–commissural synapses normally present on dentate granule cells in that released glutamate activates postsynaptic kainate and AMPA receptors (Epsztein et al. 2005). In GC-SEs, AMPA and kainate receptors mediate synaptic transmission at largely separate populations of recurrent mossy fiber synapses. Each population contributes about one half the total recurrent mossy fiber innervation. Importantly, kainate receptor–mediated synaptic responses exhibit slower kinetics than AMPA receptor–mediated responses. Thus the activation of kainate receptors contributes to postsynaptic responses in GC-SEs, but not in CGCs, and would be expected to do so in HEGCs as well. Kainate receptor activation at recurrent mossy fiber synapses could account for the slower average decay of mEPSCs in both GC-SEs and HEGCs. It could also account for the longer average rise time of mEPSCs in HEGCs than in CGCs. However, GC-SEs did not differ significantly from control in this regard. Possibly, kainate receptor activation contributes to mEPSCs more prominently in HEGCs than in GC-SEs. Rise times are also influenced by the location of the synapse relative to the recording electrode at the soma. All else being equal, the greater the distance between the synapse and the soma, the longer the apparent rise time. The apical dendrite(s) of most HEGCs extends from within the dentate hilus to the outer edge of the molecular layer, and some HEGCs also have an elongated basal dendrite (Scharfman et al. 2000, 2003; Zhan and Nadler 2009). Thus the average distance of excitatory synapses from the soma is probably greater for HEGCs than for normotopic granule cells.
Inhibitory synaptic responses in HEGCs
Electron micrographs suggested that the somata and proximal dendrites of HEGCs are practically devoid of inhibitory innervation, as evidenced by the apparent lack of symmetric synapses (Dashtipour et al. 2001). This finding is consistent with the location of HEGCs at a considerable distance from most dentate basket cells, which innervate the somata and proximal dendrites of normotopic granule cells. In addition, we estimate that pilocarpine-induced status epilepticus kills ∼70–80% of hilar interneurons in our animals (Jiao and Nadler 2007). In contrast, status epilepticus kills few interneurons located at the hilar border of the granule cell body layer and apparently none in the molecular layer. The most vulnerable inhibitory neurons are the large multipolar somatostatin/neuropeptide Y-immunoreactive HIPP cells (Buckmaster and Jongen-Rêlo 1999). Basket cells and HIPP cells are the major interneuron populations that contribute to mossy fiber–evoked inhibition of dentate granule cells. We therefore predicted that HEGCs receive little inhibition of this type. Our results confirmed this prediction.
Another mechanism that might explain, in part, the relatively small mossy fiber–evoked IPSCs is that the inhibitory projections to HEGCs are more likely to be transected during slice preparation than those of normotopic granule cells. The coincidence of a small evoked IPSC with a low mIPSC frequency argues more strongly for a deficit of inhibitory synapses, however. In addition, a higher probability of inhibitory afferent transection would predict that HEGCs exhibit a lower frequency of sIPSCs (spontaneous inhibitory postsynaptic currents recorded in the absence of TTX) than GC-SEs, even if the spontaneous firing rate of inhibitory interneurons increases after status epilepticus (Shao and Dudek 2005). Our recordings of sIPSCs did not support this prediction (Zhan and Nadler 2009).
Recordings of mIPSCs further supported the hypothesis that HEGCs receive much less synaptic inhibition than normotopic granule cells. mIPSC frequency and charge transfer per unit time were much lower in HEGCs than in normotopic granule cells. Because spontaneous inhibitory events recorded from dentate granule cells in control rats originate mainly from GABA projections that terminate on or close to the soma (Soltesz et al. 1995), the rarity of these projections probably explains the low frequency of mIPSCs. However, the mean charge transfer per event was 58% greater than in CGCs. Both a high mean peak amplitude and slow kinetics contributed to the enhanced charge transfer. The scarcity of inhibitory synapses on and near the soma suggests that the mean distance of these synapses from the soma is greater for HEGCs than for either normotopic granule cell population. Thus greater dendritic filtering may account for the relatively slow kinetics of mIPSCs recorded from these cells. Cell age may also be a factor. The earliest inhibitory synaptic currents to appear in adult-generated dentate granule cells exhibit slow rise and decay (Espósito et al. 2005; Karten et al. 2006; Overstreet-Wadiche et al. 2005). These responses seem to be generated by dedicated inputs that produce a relatively low concentration of GABA at postsynaptic receptors (Markwardt et al. 2009). These dedicated inputs probably originate from neurogliaform cells (Karayannis et al. 2010). Because HEGCs continue to be produced for months at least after status epilepticus (Bonde et al. 2006), the birthdates of the HEGCs recorded in this study could not be estimated. Some of the recorded HEGCs could have been young enough to retain the slow inhibitory synaptic currents characteristic of immature granule cells. Differences in the number, clustering, and composition of postsynaptic GABAA receptors may also contribute to differences in mIPSC properties. Further studies are needed to evaluate these possibilities.
The mean mIPSC amplitude, rise time, and charge transfer were also significantly increased beyond control in GC-SEs, but the mean rise time and charge transfer were less than in HEGCs. As in HEGCs, mIPSCs were recorded less frequently than in CGCs, but this effect of status epilepticus was much smaller than in HEGCs and only achieved statistical significance when interevent intervals were compared. Previous studies of granule cell mIPSCs during the chronic epileptic phase after status epilepticus produced diverse results (Cohen et al. 2003; Kobayashi and Buckmaster 2003; Shao and Dudek 2005; Sun et al. 2007a,b). Our finding that GC-SEs have larger mIPSCs than CGCs replicates previous findings, although Shao and Dudek (2005) reported greater mean amplitude and charge transfer per event only 4–7 days after status epilepticus not in the chronic epileptic phase. Greater mIPSC amplitude may be explained by a higher density of postsynaptic GABAA receptors (Gibbs et al. 1997; Nusser et al. 1998; Otis et al. 1994). Similarly, all studies have shown at least some long-term deficit in mIPSC frequency, although the decrease was not statistically significant in the study of Cohen et al. (2003). In contrast, the effect of status epilepticus on rise and decay times has varied considerably. Different outcomes may relate to different recording conditions, analytical methods, animal model, survival times, and/or proportion of each interneuron population that was lost.
HIPP cells project to the distal dendrites of dentate granule cells (Han et al. 1993). Their degeneration after status epilepticus therefore predicts a loss of slowly rising mIPSCs, resulting in a downward shift in the mean rise time. In contrast, we found a small increase in mean rise time associated with loss of some of the fastest rising events. Although a loss of slowly rising mIPSCs was found shortly after status epilepticus in an electrical stimulation model of epilepsy, there seemed to have been substantial recovery before spontaneous seizures appeared (Sun et al. 2007a). No change in rise time was reported previously in pilocarpine-treated (Cohen et al. 2003; Kobayashi and Buckmaster 2003) or kainic acid–treated (Shao and Dudek 2005) animals. These unexpected findings may be explained by replacement of the HIPP cell→granule cell synapses, loss of mIPSCs from inhibitory synapses located more proximal to the soma, changes in the composition of GABAA receptors, and/or the young age of some recorded GC-SEs. The lost synapses may be replaced by axon sprouting from surviving HIPP cells (Zhang et al. 2009). In this way, the initial loss of slowly rising mIPSCs would be compensated. Furthermore, the dentate basket cell circuit seems dysfunctional in pilocarpine-treated rats (Zhang and Buckmaster 2009). Basket cells receive less excitatory drive, and transmission failure at basket cell→granule cell synapses is increased. Basket cell dysfunction may explain why fewer rapidly rising mIPSCs were recorded from GC-SEs than from CGCs. The increased mean rise time we observed could be explained by a greater loss of mIPSCs associated with basket cell synapses than with HIPP cell synapses. Studies of epilepsy models showed increased expression by dentate granule cells of the α4 subunit of the GABAA receptor at the expense of the more abundant α1 subunit (Brooks-Kayal et al. 1998; Nishimura et al. 2005; Peng et al. 2004). Many of these α4 subunits are inserted in the postsynaptic membrane of inhibitory synapses (Sun et al. 2007b). Studies of recombinant receptors indicate that α4βxγ2 GABAA receptors activate more slowly than α1βxγ2 GABAA receptors (Lagrange et al. 2007). Thus a longer mean rise time could involve a change in the subunit composition of synaptic GABAA receptors. Finally, an unknown percentage of GC-SEs recorded in this and previous studies was generated during and after status epilepticus. Because the time course for the transition from slowly rising to more rapidly rising mIPSCs during granule cell differentiation after seizures is not known in detail, the mean mIPSC rise time in some recorded GC-SEs may have been prolonged because the cell was still immature in that respect. However, we did not observe a significant difference in the percentage of slowly rising (10–90% rise time >4 ms) mIPSCs between GC-SEs and CGCs.
Mossy fiber–evoked compound IPSCs were significantly larger in GC-SEs than in CGCs, despite the degeneration of most hilar interneurons and dysfunction of the basket cell circuit. Their relatively slow decay supports the hypothesis that reverberating excitation among granule cells allows inhibitory interneurons to be driven repeatedly by a single mossy fiber volley rather than just once. Other mechanisms that may contribute to this overcompensation include the sprouting of inhibitory axons (Andre et al. 2001; Davenport et al. 1990; Mathern et al. 1995; Wittner et al. 2001; Zhang et al. 2009), enhanced interneuron excitability (Shao and Dudek 2005), and increased mossy fiber innervation of basket cells (Sloviter et al. 2006). Along with enhanced tonic GABA inhibition (Zhan and Nadler 2009), larger inhibitory feedback responses may contribute to the normalization of granule cell excitability with time after status epilepticus (Buckmaster and Dudek 1997; Sloviter et al. 2006; Uruno et al. 1994; Wilson et al. 1998; Wu and Leung 2001).
Possible implications for the role of HEGCs in promoting excitability of the granule cell network
The high ratio of excitatory to inhibitory synaptic function shown in this study probably accounts, in part, for the hyperexcitability of HEGCs. Excitatory synaptic activity may drive the bursting and action potential firing of these cells with little opposition from synaptic inhibition. HEGCs become tightly integrated into dentate gyrus circuitry. The progression of seizures after status epilepticus has been linked to enhanced granule cell neurogenesis (Jung et al. 2004, 2006). It has been suggested that postseizure-generated granule cells that migrate normally into the granule cell body layer are less excitable than preexisting granule cells (Jakubs et al. 2006). If so, the postseizure-generated granule cells associated with seizure progression must be HEGCs. To the extent that hyperexcitable HEGCs enhance synchronous granule cell firing during seizures, their unique innervation may be important for impairing the filtering function of the dentate gyrus and thus facilitating seizure propagation through the limbic circuit (Gabriel et al. 2004; Hardison et al. 2000; Okazaki and Nadler 2001; Patrylo and Dudek 1998).
GRANTS
This study was supported by National Institute of Neurological Disorders and Stroke Grants NS-38108 and NS-61849.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Y. Jiao, X. Yuan, and D. A. Evenson for technical assistance and K. Gorham for clerical help. M. C. Cameron and M. M. Okazaki also contributed to this work.
Present address of R.-Z. Zhan: Institute of Physiology, Shandong University School of Medicine, 44 Wenhua Xi Road, Jinan, Shandong, China.
REFERENCES
- Andre et al., 2001. Andre V, Marescaux C, Nehlig A, Fritschy JM. Alterations of hippocampal GABAergic system contribute to spontaneous recurrent seizures in the rat lithium pilocarpine model of temporal lobe epilepsy. Hippocampus 11: 452–468, 2001 [DOI] [PubMed] [Google Scholar]
- Bonde et al., 2006. Bonde S, Ekdahl CT, Lindvall O. Long-term neuronal replacement in adult rat hippocampus after status epilepticus despite chronic inflammation. Eur J Neurosci 23: 965–974, 2006 [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal et al., 1998. Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med 4: 1166–1172, 1998 [DOI] [PubMed] [Google Scholar]
- Buckmaster and Dudek, 1997. Buckmaster PS, Dudek FE. Network properties of the dentate gyrus in epileptic rats with hilar neuron loss and granule cell axon reorganization. J Neurophysiol 77: 2685–2696, 1997 [DOI] [PubMed] [Google Scholar]
- Buckmaster and Jongen-Rêlo, 1999. Buckmaster PS, Jongen-Rêlo AL. Highly specific neuron loss preserves lateral inhibitory circuits in the dentate gyrus of kainate-induced epileptic rats. J Neurosci 19: 9519–9529, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen et al., 2003. Cohen AS, Lin DD, Quirk GL, Coulter DA. Dentate granule cell GABAA receptors in epileptic hippocampus: enhanced synaptic efficacy and altered pharmacology. Eur J Neurosci 17: 1607–1616, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashtipour et al., 2001. Dashtipour K, Tran PH, Okazaki MM, Nadler JV, Ribak CE. Ultrastructural features and synaptic connections of hilar ectopic granule cells in the rat dentate gyrus are different from those of granule cells in the granule cell layer. Brain Res 890: 261–271, 2001 [DOI] [PubMed] [Google Scholar]
- Davenport et al., 1990. Davenport CJ, Brown WJ, Babb TL. Sprouting of GABAergic and mossy fiber axons in dentate gyrus following intrahippocampal kainate in the rat. Exp Neurol 109: 180–190, 1990 [DOI] [PubMed] [Google Scholar]
- Epsztein et al., 2005. Epsztein J, Represa A, Jorquera I, Ben-Ari Y, Crépel V. Recurrent mossy fibers establish aberrant kainate receptor-operated synapses on granule cells from epileptic rats. J Neurosci 25: 8229–8239, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espósito et al., 2005. Espósito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci 25: 10074–10086, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel et al., 2004. Gabriel S, Njunting M, Pomper JK, Merschhemke M, Sanabria ERG, Eilers AQ, Kivi A, Zeller M, Meencke H-J, Cavalheiro EA, Heinemann U, Lehmann T-N. Stimulus and potassium-induced epileptiform activity in the human dentate gyrus from patients with and without hippocampal sclerosis. J Neurosci 24: 10416–10430, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs et al., 1997. Gibbs JW, Shumate MD, Coulter DA. Differential epilepsy-associated alterations in postsynaptic GABA (A) receptor function in dentate granule and CA1 neurons. J Neurophysiol 77: 1924–1938, 1997 [DOI] [PubMed] [Google Scholar]
- Han et al., 1993. Han Z-S, Buhl E, Lörinczi Z, Somogyi P. A high degree of spatial selectivity in the axonal and dendritic domains of physiologically-identified local-circuit neurons in the dentate gyrus of the rat hippocampus. Eur J Neurosci 5: 395–410, 1993 [DOI] [PubMed] [Google Scholar]
- Hardison et al., 2000. Hardison JL, Okazaki MM, Nadler JV. Modest increase in extracellular potassium unmasks effect of recurrent mossy fiber growth. J Neurophysiol 84: 2380–2389, 2000 [DOI] [PubMed] [Google Scholar]
- Houser et al., 1992. Houser CR, Swartz BE, Walsh GO, Delgado-Escueta AV. Granule cell disorganization in the dentate gyrus: possible alterations of neuronal migration in human temporal lobe epilepsy. Epilepsy Res Suppl 9: 41–48, 1992 [PubMed] [Google Scholar]
- Jakubs et al., 2006. Jakubs K, Nanobashvili A, Bonde S, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O. Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron 52: 1047–1059, 2006 [DOI] [PubMed] [Google Scholar]
- Jessberger et al., 2007a. Jessberger S, Nakashima K, Clemenson GD, Mejia E, Mathews E, Ure K, Ogawa S, Sinton CM, Gage FH, Hsieh J. Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J Neurosci 27: 5967–5975, 2007a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger et al., 2007b. Jessberger S, Zhao C, Toni N, Clemenson GD, Li Y, Gage FH. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J Neurosci 27: 9400–9407, 2007b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao and Nadler, 2007. Jiao Y, Nadler JV. Stereological analysis of GluR2-immunoreactive hilar neurons in the pilocarpine model of temporal lobe epilepsy: correlation of cell loss with mossy fiber sprouting. Exp Neurol 205: 569–582, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung et al., 2004. Jung K-H, Chu K, Kim M, Jeong S-W, Song Y-M, Lee S-T, Kim J-Y, Lee SK, Roh J-K. Continuous cytosine-b-D-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Eur J Neurosci 19: 3219–3226, 2004 [DOI] [PubMed] [Google Scholar]
- Jung et al., 2006. Jung K-H, Chu K, Lee S-T, Kim J, Sinn D-I, Kim J-M, Park D-K, Lee J-J, Kim SU, Kim M, Lee SK, Roh J-K. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis 23: 237–246, 2006 [DOI] [PubMed] [Google Scholar]
- Karayannis et al., 2010. Karayannis T, Elfant D, Huerta-Ocampo I, Teki S, Scott RS, Rusakov DA, Jones MV, Capogna M. Slow GABA transient and receptor desensitization shape synaptic responses evoked by hippocampal neurogliaform cells. J Neurosci 30: 9898–9909, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten et al., 2006. Karten YJ, Jones MA, Jeurling SI, Cameron HA. GABAergic signaling in young granule cells in the adult rat and mouse dentate gyrus. Hippocampus 16: 312–320, 2006 [DOI] [PubMed] [Google Scholar]
- Kobayashi and Buckmaster, 2003. Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci 23: 2440–2452, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron et al., 2010. Kron MM, Zhang H, Parent JM. The developmental stage of dentate granule cells dictates their contribution to seizure-induced plasticity. J Neurosci 30: 2051–2059, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange et al., 2007. Lagrange AH, Botzolakis EJ, Macdonald RL. Enhanced macroscopic desensitization shapes the response of α4 subtype-containing GABAA receptors to synaptic and extrasynaptic GABA. J Physiol 578: 655–676, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwardt et al., 2009. Markwardt SJ, Wadiche JI, Overstreet-Wadiche LS. Input-specific GABAergic signaling to newborn neurons in adult dentate gyrus. J Neurosci 29: 15063–15072, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti-Subirana et al., 1986. Marti-Subirana A, Soriano E, Garcia-Verdugo JM. Morphological aspects of the ectopic granule-like cellular populations in the albino rat hippocampal formation: a Golgi study. J Anat 144: 31–47, 1986 [PMC free article] [PubMed] [Google Scholar]
- Mathern et al., 1995. Mathern GW, Babb TL, Pretorius JK, Leite JP. Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J Neurosci 15: 3990–4004, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey et al., 2006. McCloskey DP, Hintz TM, Pierce JP, Scharfman HE. Stereological methods reveal the robust size and stability of ectopic hilar granule cells after pilocarpine-induced status epilepticus in the adult rat. Eur J Neurosci 24: 2203–2210, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár and Nadler, 1999. Molnár P, Nadler JV. Mossy fiber-granule cell synapses in the normal and epileptic rat dentate gyrus studied with minimal laser photostimulation. J Neurophysiol 82: 1883–1894, 1999 [DOI] [PubMed] [Google Scholar]
- Nadler, 2003. Nadler JV. The recurrent mossy fiber pathway of the epileptic brain. Neurochem Res 28: 1649–1658, 2003 [DOI] [PubMed] [Google Scholar]
- Nadler, 2009. Nadler JV. Sprouting in epilepsy. In: Encyclopedia of Basic Epilepsy Research, edited by Schwartzkroin PA. Oxford, UK: Elsevier, 2009, vol. 3, p. 1143–1148 [Google Scholar]
- Neher, 1992. Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol 207: 123–131, 1992 [DOI] [PubMed] [Google Scholar]
- Nishimura et al., 2005. Nishimura T, Schwarzer C, Gasser E, Kato N, Vezzani A, Sperk G. Altered expression of GABAA and GABAB receptor subunit mRNAs in the hippocampus after kindling and electrically induced status epilepticus. Neuroscience 134: 691–704, 2005 [DOI] [PubMed] [Google Scholar]
- Nusser et al., 1998. Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of synaptic GABAA receptors underlies potentiation at hippocampal inhibitory synapses. Nature 395: 172–177, 1998 [DOI] [PubMed] [Google Scholar]
- Okazaki et al., 1999. Okazaki MM, Molnár P, Nadler JV. Recurrent mossy fiber pathway in rat dentate gyrus: synaptic currents evoked in presence and absence of seizure-induced growth. J Neurophysiol 81: 1645–1660, 1999 [DOI] [PubMed] [Google Scholar]
- Okazaki and Nadler, 2001. Okazaki MM, Nadler JV. Glutamate receptor involvement in dentate granule cell epileptiform activity evoked by mossy fiber stimulation. Brain Res 915: 58–69, 2001 [DOI] [PubMed] [Google Scholar]
- Otis et al., 1994. Otis TS, De Koninck Y, Mody I. Lasting potentiation of inhibition is associated with an increased number of gamma-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc Natl Acad Sci USA 91: 7698–7702, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet-Wadiche et al., 2005. Overstreet-Wadiche L, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. J Neurophysiol 94: 4528–4532, 2005 [DOI] [PubMed] [Google Scholar]
- Parent et al., 2006. Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol 59: 81–91, 2006 [DOI] [PubMed] [Google Scholar]
- Patrylo and Dudek, 1998. Patrylo PR, Dudek FE. Physiological unmasking of new glutamatergic pathways in the dentate gyrus of hippocampal slices from kainate-induced epileptic rats. J Neurophysiol 79: 418–429, 1998 [DOI] [PubMed] [Google Scholar]
- Pekcec et al., 2008. Pekcec A, Fuest C, Mühlenhoff M, Gerardy-Schahn R, Potschka H. Targeting epileptogenesis-associated induction of neurogenesis by enzymatic depolysialylation of NCAM counteracts spatial learning dysfunction but fails to impact epilepsy development. J Neurochem 105: 389–400, 2008 [DOI] [PubMed] [Google Scholar]
- Peng et al., 2004. Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci 24: 8629–8639, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce et al., 2005. Pierce JP, Melton J, Punsoni M, McCloskey DP, Scharfman HE. Mossy fibers are the primary source of afferent input to ectopic granule cells that are born after pilocarpine-induced seizures. Exp Neurol 196: 316–331, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine, 1972. Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32: 281–284, 1972 [DOI] [PubMed] [Google Scholar]
- Ribak et al., 1985. Ribak CE, Seress L, Amaral DG. The development, ultrastructure and synaptic connections of the mossy cells of the dentate gyrus. J Neurocytol 14: 835–857, 1985 [DOI] [PubMed] [Google Scholar]
- Ribak et al., 2000. Ribak CE, Tran PH, Spigelman I, Okazaki MM, Nadler JV. Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. J Comp Neurol 428: 240–253, 2000 [DOI] [PubMed] [Google Scholar]
- Scharfman, 1994. Scharfman HE. EPSPs of dentate granule cells during epileptiform bursts of dentate hilar “mossy” cells and area CA3 pyramidal cells in disinhibited rat hippocampal slices. J Neurosci 14: 6041–6057, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman et al., 2000. Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci 20: 6144–6158, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman et al., 2003. Scharfman HE, Sollas AE, Berger RE, Goodman JH, Pierce JP. Perforant path activation of ectopic granule cells that are born after pilocarpine-induced seizures. Neuroscience 121: 1017–1029, 2003 [DOI] [PubMed] [Google Scholar]
- Scharfman et al., 2002. Scharfman HE, Sollas AL, Goodman JH. Spontaneous recurrent seizures after pilocarpine-induced status epilepticus activate calbindin-immunoreactive hilar cells of the rat dentate gyrus. Neuroscience 111: 71–81, 2002 [DOI] [PubMed] [Google Scholar]
- Shao and Dudek, 2005. Shao L-R, Dudek FE. Changes in mIPSCs and sIPSCs after kainate treatment: evidence for loss of inhibitory input to dentate granule cells and possible compensatory responses. J Neurophysiol 94: 952–960, 2005 [DOI] [PubMed] [Google Scholar]
- Shapiro and Ribak, 2005. Shapiro LA, Ribak CE. Integration of newly born dentate granule cells into adult brains: hypotheses based on normal and epileptic rodents. Brain Res Rev 48: 43–56, 2005 [DOI] [PubMed] [Google Scholar]
- Siebzehnrubl and Blümcke, 2008. Siebzehnrubl FA, Blümcke I. Neurogenesis in the human hippocampus and its relevance to temporal lobe epilepsies. Epilepsia 49 Suppl. 5: 55–65, 2008 [DOI] [PubMed] [Google Scholar]
- Simmons et al., 1997. Simmons ML, Terman GW, Chavkin C. Spontaneous excitatory currents and κ-opioid receptor inhibition in dentate gyrus are increased in the rat pilocarpine model of temporal lobe epilepsy. J Neurophysiol 78: 1860–1868, 1997 [DOI] [PubMed] [Google Scholar]
- Sloviter et al., 2003. Sloviter RS, Zappone CA, Harvey BD, Bumanglag AV, Bender RA, Frotscher M. “Dormant basket cell” hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat. J Comp Neurol 459: 44–76, 2003 [DOI] [PubMed] [Google Scholar]
- Sloviter et al., 2006. Sloviter RS, Zappone CA, Harvey BD, Frotscher M. Kainic acid-induced recurrent mossy fiber innervation of dentate gyrus inhibitory interneurons: possible anatomical substrate of granule cell hyperinhibition in chronically epileptic rats. J Comp Neurol 494: 944–960, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz et al., 1995. Soltesz I, Smetters DK, Mody I. Tonic inhibition originates from synapses close to soma. Neuron 14: 1273–1283, 1995 [DOI] [PubMed] [Google Scholar]
- Sun et al., 2007a. Sun C, Mtchedlishvili Z, Bertram EH, Erisir A, Kapur J. Selective loss of dentate hilar interneurons contributes to reduced synaptic inhibition of granule cells in an electrical stimulation-based animal model of temporal lobe epilepsy. J Comp Neurol 500: 876–893, 2007a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun et al., 2007b. Sun C, Mtchedlishvili Z, Erisir A, Kapur J. Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the α4 subunit of GABAA receptors in an animal model of epilepsy. J Neurosci 27: 12641–12650, 2007b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thind et al., 2008. Thind KK, Ribak CE, Buckmaster PS. Synaptic input to dentate granule cell basal dendrites in a rat model of temporal lobe epilepsy. J Comp Neurol 509: 190–202, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uruno et al., 1994. Uruno K, O'Connor MJ, Masukawa LM. Alterations of inhibitory synaptic responses in the dentate gyrus of temporal lobe epileptic patients. Hippocampus 4: 583–593, 1994 [DOI] [PubMed] [Google Scholar]
- Walter et al., 2007. Walter C, Murphy BL, Pun RYK, Spieles-Engemann AL, Danzer SC. Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells. J Neurosci 27: 7541–7552, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson et al., 1998. Wilson CL, Khan SU, Engel J, Isokawa M, Babb TL, Behnke EJ. Paired pulse suppression and facilitation in human epileptogenic hippocampal formation. Epilepsy Res 31: 211–230, 1998 [DOI] [PubMed] [Google Scholar]
- Wittner et al., 2001. Wittner L, Maglóczky Z, Borhegyi Z, Halász P, Tóth S, Erőss L, Szabó Z, Freund T. Preservation of perisomatic inhibitory input of granule cells in the epileptic human dentate gyrus. Neuroscience 108: 587–600, 2001 [DOI] [PubMed] [Google Scholar]
- Wu and Leung, 2001. Wu K, Leung LS. Enhanced but fragile inhibition in the dentate gyrus in vivo in the kainic acid model of temporal lobe epilepsy: a study using current source density analysis. Neuroscience 104: 379–396, 2001 [DOI] [PubMed] [Google Scholar]
- Zhan and Nadler, 2009. Zhan R-Z, Nadler JV. Enhanced tonic GABA current in normotopic and hilar ectopic dentate granule cells after pilocarpine-induced status epilepticus. J Neurophysiol 102: 670–681, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang and Buckmaster, 2009. Zhang W, Buckmaster PS. Dysfunction of the dentate basket cell circuit in a rat model of temporal lobe epilepsy. J Neurosci 29: 7846–7856, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 2009. Zhang W, Yamawaki R, Wen X, Uhl J, Diaz J, Prince DA, Buckmaster PS. Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy. J Neurosci 29: 14247–14256, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]