Abstract
In voluntary control, supraspinal motor systems select the appropriate response and plan movement mechanics to match task constraints. Spinal circuits translate supraspinal drive into action. We studied the interplay between motor cortex (M1) and spinal circuits during voluntary movements in wild-type (WT) mice and mice lacking the α2-chimaerin gene (Chn1−/−), necessary for ephrinB3-EphA4 signaling. Chn1−/− mice have aberrant bilateral corticospinal systems, aberrant bilateral-projecting spinal interneurons, and disordered voluntary control because they express a hopping gait, which may be akin to mirror movements. We addressed three issues. First, we determined the role of the corticospinal system in adaptive control. We trained mice to step over obstacles during treadmill locomotion. We compared performance before and after bilateral M1 ablation. WT mice adaptively modified their trajectory to step over obstacles, and M1 ablation increased substantially the incidence of errant steps over the obstacle. Chn1−/− mice randomly stepped or hopped during unobstructed locomotion but hopped over the obstacle. Bilateral M1 ablation eliminated this obstacle-dependent hop selection and increased forelimb obstacle contact errors. Second, we characterized the laterality of corticospinal action in Chn1−/− mice using pseudorabies virus retrograde transneuronal transport and intracortical microstimulation. We showed bilateral connections between M1 and forelimb muscles in Chn1−/− and unilateral connections in WT mice. Third, in Chn1−/− mice, we studied adaptive responses before and after unilateral M1 ablation. We identified a more important role for contralateral than ipsilateral M1 in hopping over the obstacle. Our findings suggest an important role for M1 in the mouse in moment-to-moment adaptive control, and further, using Chn1−/− mice, a role in mediating task-dependent selection of mirror-like hopping movements over the obstacle. Our findings also stress the importance of subcortical control during adaptive locomotion because key features of the trajectory remained largely intact after M1 ablation.
INTRODUCTION
A feature shared by voluntary movements is that they are adapted to motor task demands on a moment-to-moment basis. Supraspinal motor systems select the appropriate motor response and plan movement mechanics to match task constraints. Spinal motor circuits translate supraspinal drive into action. The focus of our study is the interplay between the motor cortex (M1)—key to adaptive motor control in cats, monkeys, and humans—and spinal circuits, during voluntary motor control in the mouse. Mouse motor control studies to date have largely focused on the role of intrinsic spinal circuitry in controlling stereotypic hind leg locomotion (Goulding 2009). To begin to elucidate higher level motor control in the mouse, we combined study of an adaptive locomotor task (Drew et al. 2004), where mice step over obstacles, with a developmental genetic approach. Supraspinal motor systems, especially M1 and the corticospinal tract (CST), are thought to modify the stereotypic cyclic behavior of central pattern generators (CPGs) in the spinal cord, to adapt their output in flexible ways to perform this task (Armstrong 1988).
We studied wild-type (WT) mice and mice lacking the gene for the protein α2-Chimaerin (Chn1−/−), necessary for ephrinB3-EphA4 signaling (Beg et al. 2007; Iwasato et al. 2007; Shi et al. 2007; Wegmeyer et al. 2007). EphrinB3 is expressed and developmentally regulated in many CNS regions (Benson et al. 2005), in particular, along the spinal midline (Kullander et al. 2001). The CST, spinal circuits, and locomotor control have been extensively studied in Chn1−/−, ephrin B3, and Eph A4 mouse mutants. These mice have three key characteristics. First, they have an aberrant bilateral CST and M1 hind leg motor map (Beg et al. 2007; Coonan et al. 2001). Second, one spinal interneuron class (EphA4; Kullander et al. 2003) and possibly other spinal interneurons (Beg et al. 2007) have aberrant bilateral-projecting axons. Third, they express a hopping gait (Beg et al. 2007; Kullander et al. 2003), indicating disordered voluntary control of motor behavior. Hopping in these mice may be akin to mirror movements in patients with genetic defects involving CST axon guidance (Srour et al. 2010) and developmental impairments producing bilateral CSTs in hemiplegic cerebral palsy (Brouwer and Ashby 1991; Carr et al. 1993; Farmer et al. 1991). Study of hopping in these mutant mice, especially in the context of voluntary limb control, offers the opportunity to examine mirror-like responses in a mouse model.
Our study addressed three topics. First, we developed an adaptive locomotor task for the mouse, like that of the cat (Drew 1988), where animals modified the trajectory of the forepaw to step over an obstacle. By comparing performance before and after bilateral M1 ablation, we determined the role of the corticospinal (CS) system in trajectory modification to step over obstacles in WT mice and either to step or hop over obstacles in Chn1−/− mice. We found that mice modified their trajectory to step over obstacles and that this was under both M1 and brain stem control. Using Chn1−/− mice, we found a key role for M1 in mediating task-dependent selection of mirror-like hopping over the obstacle. Second, because Chn1−/− mice express mirror-like hopping movements thought to be associated with bilateral CSTs, we compared the laterality of CS system motor actions in WT and Chn1−/− mice using retrograde transneuronal transport of pseudorabies virus and intracortical microstimulation. The key difference was a strongly bilateral CST circuit with distal forelimb muscles in Chn1−/− mice. Third, we used unilateral M1 lesions in Chn1−/− mice to distinguish the contributions of bilateral CST drive versus a more lateralized drive from the intact M1, acting on bilateral spinal interneuronal circuits, in obstructed locomotion. We found a preferential role for the contralateral CST, acting on bilaterally organized spinal circuits, rather than the combined actions of the ipsilateral and contralateral tracts in adapting responses in Chn1−/− mice. Our findings suggest important roles for M1 in moment-to-moment adaptive limb control in the mouse and, using Chn1−/− mice, in mediating task-dependent selection of mirror-like hopping movements over the obstacle. However, our findings also stress the importance of parallel subcortical control during adaptive locomotion because features of the trajectory remained largely intact after M1 ablation.
METHODS
Experiments were conducted on adult male and female C57/BL6 mice and Chn1−/− (Beg et al. 2007). The Chn1−/− mice carry a gene-trap insertion in the chimaerin-1 gene that results in selective loss of the α2-chimaerin protein isoform, leaving the α1 isoform produced from the same gene intact. All procedures were approved by the institutional animal care and use committees of Columbia University, NYS Psychiatric Institute, and the City College of the City University of New York.
Locomotor training and testing
Animals were acclimated to the treadmill and testing environment. Animals were accustomed to unobstructed running on the treadmill, initially at a slow speed (6 cm/s). Gradually the speed was increased (≤20 cm/s), within the same and across sequential training sessions. After the animals became accustomed to unobstructed stepping, we typically mounted two obstacles to the treadmill belt (1 small, 5 mm) and (1 large, 10 mm). Depending on the speed, they experienced an obstacle approximately once every 5 s. At first, animals (WT and Chn1−/−) tended to pause briefly as the obstacle approached. With practice, they stepped (or hopped) over the obstacle without breaking stride. Animals were trained daily with the two obstacle heights, typically for 1 wk, whereupon we videotaped performance using a commercial digital video camcorder (Canon ZR960) for subsequent analysis.
Kinematic analyses
We conducted a kinematic analysis of unobstructed and obstructed locomotor performance after animals had experienced at least 1 wk of training. We assessed the effects of M1 ablation on locomotion by comparing performance the day before (and/or the morning of) ablation with performance beginning the day after the lesion. Because we used a short-acting anesthetic (ketamine) and the ablation procedure was also short (∼30 min), the animals were fully ambulatory within several hours after surgery. Videotaped recordings were digitized. For assessments of response type (step or hop) and for monitoring errors in clearing the obstacle, we viewed either single video frames (30 Hz) or single video fields (60 Hz). A hop was defined as a bilaterally symmetrical forelimb or hindlimb movement that began synchronously and typically ended synchronously. For kinematic analysis, we analyzed video data at 60 Hz, as we did for a previous kinematic study of obstructed locomotion in the cat (Friel et al. 2007). Digitized video files were converted to the AVI format and analyzed using the program MaxTraq (Innovision). The paw tip was marked on sequential video fields. X and Y coordinates of the paw tip and obstacle were exported and graphed (KaleidoGraph). For measurement of step length, we computed the difference between the X coordinate at lift off and landing. For measurement of step height, we imported the tip coordinates into Matlab (Mathworks) and used a spline interpolation. We determined the maximal Y value of the interpolated trace. We combined kinematic data across the two treadmill speeds examined (6.66 and 10 cm/s) for WT and Chn1−/− mice because the values were not systematically different. Only steps in which the animal's leading forepaw cleared the obstacle were analyzed. Steps in which there was contact between the leading forepaw and the obstacle were termed errant steps and were analyzed separately as a simple tally.
M1 ablations
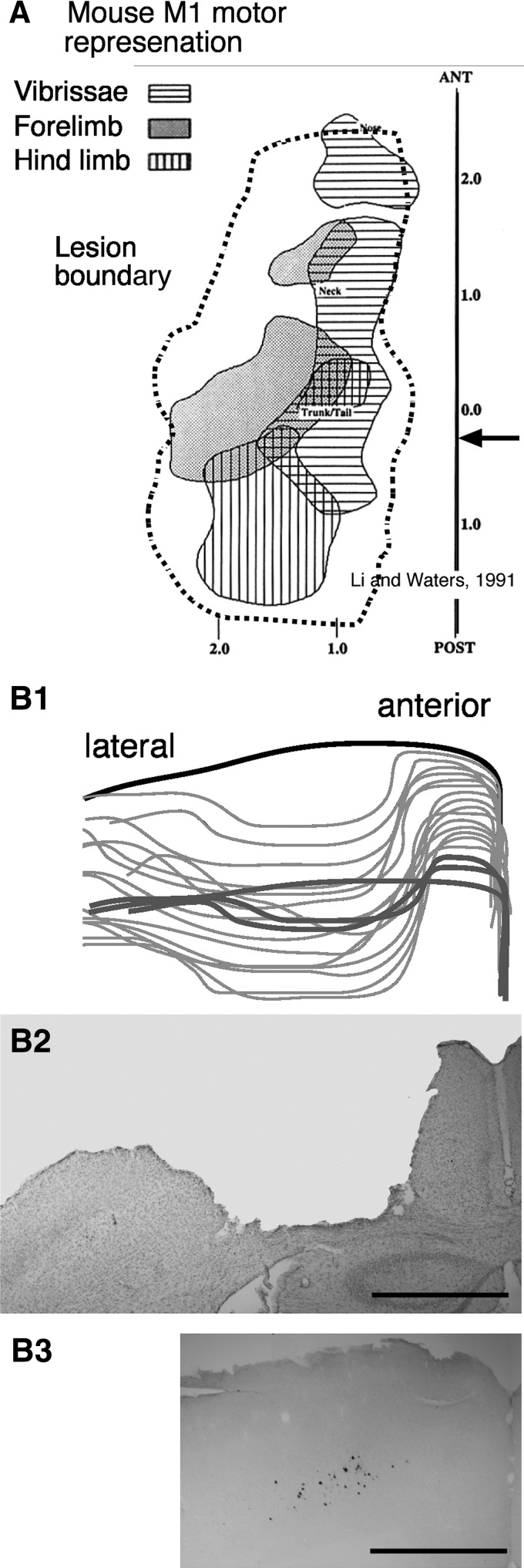
Animals were anesthetized with ketamine/xylazine mix (100/10 mg/kg body weight; ip). They were placed in a stereotaxic frame. Body temperature was maintained at 39°C by a heating plate. The skin over the skull was cut along the midline. The coordinates of bregma were obtained. The coordinates of the forelimb and hindlimb regions of M1 were marked on the bone surface relative to bregma, according to the intracortical microstimulation (ICMS) maps and horseradish peroxidase (HRP)-retrograde labeling data of Li and Waters (1991). The craniotomy was made sufficiently large to ablate M1. Figure 1 shows a representative lesion (dashed gray line), reconstructed from a series of coronal sections (Fig. 1B1), in relation to the published mouse M1 motor map (Fig. 1A) (Li and Waters 1991). Figure 1B2 (arrow in Fig. 1A) shows a coronal section just caudal to bregma from a representative lesion. Figure 1B3 shows the distribution of corticospinal tract neurons that were transneuronally labeled by injecting pseudorabies virus into a contralateral forelimb muscle (from a different animal). The location of these neurons matches the location of the lesion site. To make the lesion, the dura was excised and the cortex was aspirated to the white matter, which could be distinguished visually from gray matter. The cavity was filled with gelfoam, and the craniotomy was covered with dental acrylic cement. The wound margins were apposed and sutured. Bilateral lesions were made symmetrically. Unilateral lesions were made in the right or left hemisphere. Animals were administered an analgesic and were noted to be ambulatory within 1–3 h after surgery. We began retesting the animals the day after surgery. It should be noted that performance changes (assessed quantitatively with hopping) expressed on the first day after M1 ablation did not change significantly over the subsequent 5–6 days of testing (see results).
Fig. 1.
Motor cortex (M1) ablation. A: M1 motor representation is shown at the top (reproduced from Li and Waters 1991). The dashed region corresponds to a representative lesion boundary. Scale is in millimeters. B1: tracings of the lesioned cortex from anterior to the lesion (thick black line), through the lesion (think gray lines), and at the caudal pole of the lesion (thick gray lines). B2: a representative Nissl-stained section through part of the lesioned M1 (arrow in A). Calibration for B1 and B2 (shown in B2) is corrected for 20% shrinkage because of histological processing. B3: PRV-labeled cells (see Fig. 4) are shown, also sized to correspond to the lesion figure above. The lesioned cortex is where pseudorabies virus (PRV)-labeled corticospinal tract (CST) neurons are located. Note that M1 neurons retrogradely labeled from spinal cord occupy a wider mediolateral territory, corresponding to that of the lesioned cortex, than implied by the PRV-labeled neurons (calibration: 1 mm).
ICMS
For ICMS motor mapping, anesthesia was induced with a ketamine/xylazine mix as above and maintained using intraperitoneal ketamine injections to render the animal unresponsive to paw pinch. Ketamine anesthesia maintains muscle tone in rodents and therefore is well suited for motor mapping studies. Animals were placed in a stereotaxic frame. Body temperature was maintained at 39°C by a heating pad. A craniotomy was made over the forelimb area of M1. Electrode penetrations were made perpendicular to the pial surface and ∼0.3 mm apart. In all animals, the region sampled was the same, from 1 to 2.0 mm lateral to bregma and ≤1.0 mm rostral to bregma. We examined the forelimb area broadly across all animals, with the goal of assessing the thresholds for evoking contralateral and ipsilateral responses. We used low-impedance tungsten microelectrodes (Microprobe; 0.5 MOhm impedance; 0.081 mm shaft diameter, 1–2 μm tip diam). Motor effects produced by microstimulation occurred at the lowest stimulus currents at the same depths where we recorded multiunit activity with the largest amplitude spikes (typically 0.8−1.0 mm below the pial surface).
Stimuli (45 ms duration train, 330 Hz, 0.2 ms biphasic; every 2 s) were delivered using a constant current stimulator (A-M Systems). The threshold is defined as the lowest current that consistently produced a motor effect (>50% of trials). For a given site, we started at a low current and first determined the threshold for evoking a contralateral response. Then, as current amplitude increased, the ipsilateral response threshold was determined. The thresholds were examined in reverse through the loss of the responses with decreasing currents. We randomized placement of the electrode to prevent biasing our results by anesthesia level or other state-dependent changes. A maximal current of 100 μA was used. For each penetration, the type of motor effect produced by a threshold stimulus was determined on the basis of the evoked phasic kinematic change; adjacent joints were stabilized. Limb posture was the same for all experiments—with the shoulder and elbow extended and the wrist plantarflexed. For each penetration, we noted the contralateral and ipsilateral motor threshold (μA). If no response was evoked below 100 μA, the penetration was considered nonresponsive. We tallied the number of sites evoking movements.
Retrograde transneuronal tracing using pseudorabies virus
In WT mice and Chn1−/− mice, multiple injections of pseudorabies virus (PRV) were made unilaterally into biceps and the wrist extensor compartment (4 × 2.5 μl). We used two types of the Bartha strains: 152 (titer of 2.28 × 108 pfu/ml) (Smith et al. 2000) and 614 (titer of 8.1 × 108 pfu/ml) (Banfield et al. 2003). PRV was generously provided by Dr. Lynn Enquist (Princeton University, Princeton, NJ).
Mice were administered an overdose of anesthetic and perfused with saline followed by 4% paraformaldehyde. The brain was dissected, postfixed for 2 h, and transferred to 20% sucrose in buffered saline (pH 7.4). Frozen sections (40 μm) were cut serially and collected in a 0.1 M PBS solution (pH 7.4). Ipsilateral and contralateral sides were distinguished by piercing the cortex with a 27-gauge needle through the brain on one side. The region of the sensory-motor cortex was removed as a block. The tissue was cut in the coronal plane into 40 μm sections. All sections were collected.
For visualization of PRV, we immunostained the sections with an antibody to PRV (rabbit anti-PRV antibody, Abcam Ab3534). Sections were first incubated in a mixture of 1× PBS with 3% hydrogen peroxide and 10% methanol for 10 min to block endogenous peroxidase activity. After rinsing sections in 1× PBS, sections were incubated for 1 h at room temperature in blocking buffer (3% donkey serum in 1× PBS with 0.2% Tween 20, pH 7.4). The sections were next incubated at 4°C overnight in PBS containing primary anti-PRV antibody (1:1,000). After rinsing, sections were incubated for 2 h at room temperature in blocking buffer containing 0.2% anti-rabbit secondary antibody conjugated to peroxidase (1:200; pH 7.4). After rinsing, sections were incubated with the chromogen diaminobenzidine (DAB; Sigma) for 6–30 min. To examine cell morphology, we also used a fluorophore to label cells infected with PRV-152. After incubating the sections for 1 h at room temperature in blocking buffer (3% donkey serum in 1× PBS with 0.2% Tween 20, pH 7.4), the sections were incubated with rabbit anti-green fluorescence protein (anti-GFP; 1:1,000; Invitrogen) overnight at 4°C. After rinsing with 1× PBS, sections were incubated for 2 h at room temperature with FITC-conjugated donkey anti-rabbit antibody (1:500; Jackson Immunoresearch Laboratories). All sections were mounted on gelatin-coated slides and air dried. For fluorescence labeling, sections were coverslipped with Vectashield (Vector Laboratories). For peroxidase staining, sections were dehydrated before coverslipping.
To quantify PRV labeling, we randomly selected and processed (as above) sections through the sensory-motor cortex. We counted all labeled neurons on each selected section. At first, three sections were analyzed per animal. We observed that there were fewer labeled neurons in the Chn1−/− mice. We were concerned that our assessment of unilateral and bilateral labeling might be compromised by this difference. To compensate for this, we randomly selected and processed more sections from Chn1−/− mice. Although we analyzed more sections from Chn1−/− animals, we also sampled from a larger selection of 78 sections compared with 53 sections from WT animals, giving an overall sampling rate that was identical for both groups of approximately one section counted for every three sampled.
We computed a normalized laterality index for every labeled coronal section. The index is normalized to the contralateral cell counts using the following equation (C − I)/C, where C and I represent contralateral and ipsilateral cell counts, respectively. A laterality index of 1 indicates only contralateral labeling, 0 indicates a bilaterally symmetrical labeling, and negative values indicate more ipsilateral than contralateral labeling. We constructed a frequency distribution of the laterality index for all sections in all animals that enabled us to compare labeled neurons in WT and Chn1−/− mice. We also measured the depth of labeling from the surface of the brain to verify that only layer 5 pyramidal neurons were labeled. Based on studies by Li and Waters, labeling within the agranular lateral cytoarchitectonic field at a depth of 0.7–0.8 mm (deep layer 5) corresponds to the location of labeled pyramidal neurons.
PRV survival times and retrograde synaptic transfer
We used survival time after PRV injection to estimate monosynaptic and disynaptic retrograde transport, as others have done using transneuronal viral tracing (Rathelot and Strick 2006, 2009). After 24 and 48 h, there were no PRV-labeled neurons in the cervical spinal cord and brain (n = 2 mice for each time point). After 72 h (n = 4 mice), motoneurons were labeled in locations consistent with rodent biceps and wrist motor pools (McKenna et al. 2000). Interneurons in the same or nearby sections were also labeled at this survival time. In addition, neurons in the reticular formation and vestibular nuclei were labeled, but no neurons were detected in cortex or red nucleus. We interpret these findings to mean that, at 72 h, neurons that are monosynaptically connected to motoneurons, and motoneurons themselves, are labeled. We did not capture the time when only motoneurons were labeled. Finally, at 96 h, labeling was present in layer 5 neurons in M1 and red nucleus. We interpret the PRV labeling pattern at 96-h survival to reflect a predominantly disynaptic connections with motoneurons (Kuypers 1981). Importantly, in M1 labeling was restricted to layer 5, where corticospinal tract neurons are located. Thus this pattern is consistent with restricting labeling to spinal projection neurons and not local neurons presynaptic to spinal projection neurons (i.e., layer 3). Had layer 3 neurons also been labeled, it would indicate passage of the virus across an additional synapse (Rathelot and Strick 2009). It is important to note that we are not using this technique to elucidate the number of synapses from M1 to motoneuron. Rather, we use PRV labeling in WT and Chn1−/− mice, with identical survival times, to determine whether the CS system in one hemisphere in the mutant mouse are part of a bilateral circuit to forelimb muscles.
Statistical analyses
Standard statistical tests including Student's t-test and ANOVA were conducted using Microsoft Excel (Microsoft) and Statview (SAS).
RESULTS
Performance parameters and M1 control of adaptive locomotion in WT mice
FOREPAW TRAJECTORIES DURING UNOBSTRUCTED AND OBSTRUCTED LOCOMOTION.
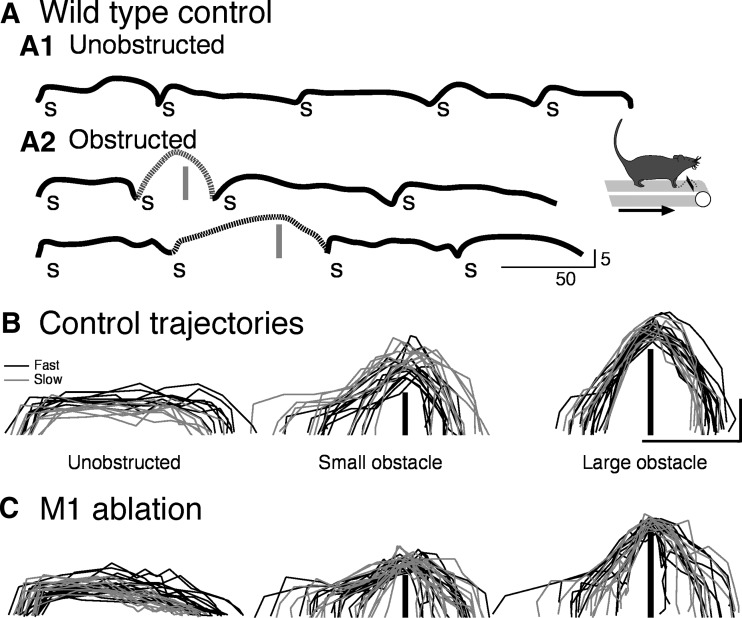
We developed an adaptive motor task to examine cortical motor control in mice, similar to the one developed by Drew (1988). Mice were trained on a treadmill, and subsequently obstacles were inserted onto the treadmill belt, requiring the mice to step over these obstacles as they approached. WT mice responded by maintaining contact with one forelimb on the treadmill belt, termed the trailing limb, while stepping over the obstacle with the other, termed the leading limb. Figure 2A presents representative right paw paths for intact WT mice, walking from left to right. The inset in Fig. 2 shows schematically the obstacle on the treadmill belt and the direction of locomotion. Figure 2A1 shows a sequence of steps during unobstructed locomotion and, in Fig. 2A2, two sequences of steps as the animal approached and cleared the obstacle. Each step is marked by the letter “s.” The paths of the steps over the obstacle are dotted. The trajectory of the leading limb was modified by the presence of the obstacle, heightened sufficiently to clear it without contact. This is a task-dependent change in the animals motor performance, driven by obstacle stimulus information, as in the cat (Drew 1988).
Fig. 2.
Representative forepaw trajectories of wild-type (WT) mice. A: right forelimb trajectories of 1 intact WT mouse during a sequence of unobstructed steps (A1) and unobstructed and obstructed steps (A2). “s” indicates an individual step. Obstacles are indicated by vertical gray bars. Inset: schematically the direction of locomotion and the obstacle. Speed of locomotion in A and B was 6.66 cm/s. B and C: trajectories for unobstructed (left) and obstructed (middle and right) stepping are shown before (B) and after (C) bilateral M1 ablation. The gray lines are steps while the treadmill moved at 6.66 cm/s (termed slow) and the black lines are at 10 cm/s (termed fast). The obstacles are shown by the vertical black lines in the middle and right panels of B and C. Calibrations in A–C: 5 mm vertical height; 50 mm horizontal distance.
Figure 2B shows representative forepaw trajectories for single steps at two treadmill speeds (black, 10 cm/s; gray, 6.66 cm/s). Only steps in which the animal did not contact the obstacle were analyzed for trajectory characteristics. During unobstructed locomotion (Fig. 2B, left), the forepaw is lifted rapidly to the maximal height, maintained at this height for most of the step, and placed back onto the treadmill belt. We focus on the leading forelimb because its motion over the obstacle must reflect feed-forward control. By contrast, motion of the trailing forelimb (and hindlimbs) likely reflect a combination of feed-forward control and feedback/corollary signaling from the motion of the leading forelimb.
During obstructed locomotion (Fig. 2B, middle and right columns), the location of lift-off before the obstacle is variable, as is the initial form of the trajectory. Some of the trajectories appeared to start as a typical unobstructed step and then the paw was raised farther to clear the obstacle. This was especially the case for the small obstacle, and more so for slow than fast steps. As the foot approached the obstacle, the trajectory became more stereotypic, especially for the higher obstacle (Fig. 2B, right column), because of the need for the trajectory to conform to the mechanical constraint of obstacle height and shape. The peak trajectories were not different for the two treadmill speeds.
Comparison of the trajectories for the two obstacles shows that the animal steps higher over the higher obstacle. Figure 3A (intact) quantifies this relationship by graphing the maximal height of the trajectory as the animals stepped over small and large obstacles (paired t-test; each P < 0.0001; n = 5 animals). Comparison of the heights of steps over the small and large obstacles shows that the animals adopted the strategy to modify the trajectory adaptively, to scale the height of the step to that of the obstacle, rather than adopting a strategy of a stereotypically high trajectory that clears both the small and large obstacle. These findings show that WT mice are capable of incorporating information about the occurrence and size of the upcoming obstacle to modify forepaw trajectory adaptively to step over the obstacle.
Fig. 3.
Obstructed locomotor performance in WT mice before and after bilateral M1 ablation. A: scaling of step height for small and large obstacles (mean ± SE) in intact WT mice (n = 5) and, for the same cohort, after bilateral M1 ablation. Note that the lesion significantly reduced maximal step height over both the small (P < 0.001) and large (P < 0.04) obstacle. B: error rates (mean ± SE) for clearing the obstacle before (left) and after (right) bilateral M1 ablation.
EFFECTS OF BILATERAL M1 ABLATION ON LOCOMOTOR PERFORMANCE.
To determine cortical control of adaptive locomotion in WT mice, we ablated M1 bilaterally. We examined animals the day after M1 ablation, during their first postlesion exposure to the obstacles, to minimize the influences of lesion-adaptive changes. The lesion included both the forelimb and hindlimb regions from which motor responses can be evoked at low current thresholds (see Fig. 1A for representative lesion) (Li and Waters 1991). Figure 2C shows postlesion data for the same mouse as in Fig. 2B. As for prelesion responses, only steps in which the animal did not contact the obstacle were analyzed. Data are from the first postlesion day, during the initial exposure to the task after ablation (see methods). Across animals, the peak height and length of unobstructed trajectories were not significantly different before and after M1 ablation (preablation height: 5.1 ± 0.34 mm; postablation height: 4.7 ± 0.52 mm; P = 0.14; preablation length: 70.1 ± 5.9 mm; postablation length: 66.9 ± 3.4 mm; P = 0.73).
During obstructed locomotion, the temporal–spatial relation between the modified step and the obstacle was not changed. For most steps, lift off was consistently related to the occurrence of the obstacle and the form of the trajectory was similar after, as before, the lesion. Nevertheless, there may be subtle differences before and after the lesion that were undetected. However, there was a small and significant reduction in the amplitude of the maximal height of the steps over both the small and large obstacles, as seen in the trajectory plots (Fig. 2C, middle and right panels). Scaling, which is defined as the relationship between response amplitude (step height) and obstacle height, was preserved after bilateral M1 ablation, although significantly reduced. This is shown in Fig. 3A. Response amplitude to small and large obstacles remained significantly different after the lesion (M1x; P = 0.006), and the mean ratios of maximal trajectory height for small and large obstacles were not different (prelesion: 1.58 ± 0.05; postlesion: 1.56 ± 0.07; paired t-test; P = 0.197), indicating that scaling was preserved. However, there were significant reductions in the amplitude of the modified trajectory to both the small and large obstacles (P < 0.001 and P < 0.04 for small and large, respectively). Scaling, measured as millimeter response amplitude per millimeter obstacle height, for the low targets was 1.79 ± 0.05 before M1 ablation and 1.44 ± 0.009 after ablation (P < 0.007). For the large targets, scaling was 1.31 ± 0.03 for the small targets before the lesion and 1.19 ± 0.04 after (P < 0.044). We interpret this not as simple muscle weakness, but rather a sensory-motor impairment because the animals were still capable of making larger amplitude responses to a larger obstacle. There was a 5.4-fold increase in errant steps (from 5.2 to 28.8%; paired t-test; T = 5.242, P = 0.034), where the leading limb contacted the obstacle (Fig. 3B). Again, this is not consistent with weakness. Error rate was actually somewhat larger when the animals stepped over the small target (6.5 times higher) than the large target (4.2 times higher); again, showing that the errors are not caused by weakness. Moreover, we do not think that this only reflects the scaling reduction, which would reduce the safety margin for clearing the obstacle, because after the lesion, the animals often disregarded the obstacle and stepped on it. To summarize the results of bilateral M1 ablation in WT mice, our findings show moment-to-moment M1 control of adaptive modification of the trajectory to step over the obstacle. With a significant reduction in the scaled amplitude of the step over the obstacle and increased stepping errors after ablation, the response is no longer matched to obstacle stimulus information and thus less adaptive for the animal. However, substantial trajectory control was preserved after bilateral M1 ablation. On nonerrant trails (i.e., no obstacle contact), the form of the trajectory was not changed by M1 ablation, and the scaling factor was preserved (albeit reduced), suggesting the importance of subcortical control of spinal motor circuits.
CS system in Chn1−/− mice is part of a bilateral motor circuit to forelimb muscles
Motor control studies in mice with mutations in genes for Chn1−/−, ephrinB3, and EphA4 have focused, on the one hand, on the bilateral CST projections (Beg et al. 2007; Coonan et al. 2001), and, on the other, the lumbar spinal cord control of the hind legs and aberrant hindlimb locomotor behavior (Akay et al. 2006; Beg et al. 2007; Shi et al. 2007; Wegmeyer et al. 2007). To determine more directly functional CST connections for control of lead limb stepping over the obstacle, we used PRV to elucidate the laterality of connections from forelimb muscles to M1 and ICMS of the forelimb area of M1 to evoke forelimb movements.
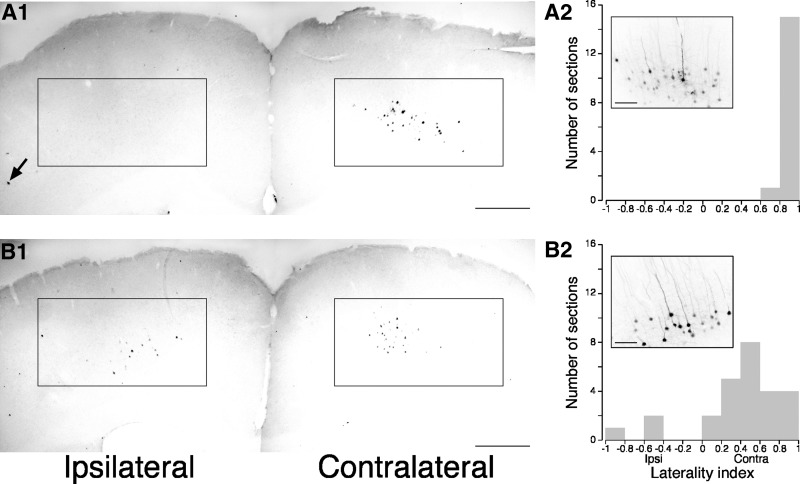
PRV was injected unilaterally into the biceps and wrist/digit extensor compartment in WT and Chn1−/− mice (see methods). These are the most common muscles activated by ICMS. We counted PRV-labeled neurons in layer 5 of M1 contralateral and ipsilateral to the side of injection. This was the only layer in which PRV-labeled neurons were located. The labeled neurons had the classical pyramidal morphology, with a prominent apical dendrite (Fig. 4, A2 and B2, insets). In WT animals (n = 5), PRV almost exclusively resulted in labeling of neurons in contralateral but not ipsilateral cortex. By contrast, in Chn1−/− mice (n = 4), cortical neurons were labeled bilaterally. Figure 4A1 shows a representative montage at low magnification of the contralateral (right) and ipsilateral (left) M1 in a WT mouse. In this section, we counted 48 contralateral cortical neurons and 1 ipsilateral cortical neuron (arrow). For all WT mice, the depth locations of PRV-labeled neurons from the pial surface did not differ between the two sides (0.6 ± 0.03 to 0.8 ± 0.04 mm ipsilaterally and 0.7 ± 0.04 to 0.9 ± 0.04 mm contralaterally). Labeled neurons were specific to the lateral agranular (i.e., M1) field, where ICMS thresholds for evoking forelimb movements are lowest, suggesting selective labeling via a retrograde motor path from forelimb muscles. Overall, labeled neurons occupied a region 1.3 ± 0.14–1.6 ± 0.12 mm from the midline laterally on the ipsilateral side and 0.9 ± 0.04–1.9 ± 0.07 mm contralaterally.
Fig. 4.
Pseudorabies transneuronal labeling in WT and Chn1−/− mice. Representative sections through M1 from a WT (A1) and Chn1−/− (B1) mouse. Boxes outline labeled cells in contralateral M1 in WT and Chn1−/− mice, ipsilateral M1 in Chn1−/− mice, and the absence of cells in ipsilateral M1 in WT mice. Note that there is an abundance of labeled ipsilateral neurons in the Chn1−/− mouse (B1), but only 1 in the WT mouse (A1; arrow). Insets in A2 and B2 show clusters of labeled neurons in M1 for WT and Chn1−/− mice. The laterality index for sections from the WT and Chn1−/− mice is shown in A2 and B2, respectively. Calibrations: A1 and B1, 500 μm; A2 and B2 insets, 100 μm.
Figure 4B1 shows a representative low-magnification montage of contralateral (right) and ipsilateral (left) M1 in a Chn1−/− mouse. In this section, we counted 40 contralateral cortical neurons and 19 ipsilateral cortical neurons. The inset shows a cluster of labeled neurons in ipsilateral M1. PRV-labeled neurons, as in WT mice, also had a pyramidal morphology. The range of depths of labeled neurons from the pia were similar to the WT mice. Also similar to WT mice, labeled neurons in Chn1 −/− mice were localized to M1 (lateral agranular field) and not somatic sensory or the medial agranular fields.
To quantify differences in bilateral labeling in WT and Chn1−/− mice, we counted all labeled neurons in coronal sections throughout M1 (see methods). Figure 4, A2 and B2, shows the laterality index distribution for labeled sections in WT and Chn1−/− mice. All of the sections sampled from WT animals showed a high degree of contralateral labeling, resulting in a laterality index close to 1 (0.96; maximal value is 1; see methods for description; Fig. 4A2). In contrast, sections from Chn1−/− mice had a significantly lower mean laterality index (0.39; Fig. 4B2; Mann-Whitney test comparing WT and Chn1−/−: P < 0.001). Moreover, there were more labeled neurons on the ipsilateral than contralateral side in several sections in the Chn1−/− mice, as shown by the negative laterality index values. Paired t-test also showed that there were significant differences between the mean contralateral and ipsilateral cell counts in WT (contralateral: 49.2 ± 14.3 neurons/mouse; ipsilateral: 2.6 ± 1.9 neurons/mouse; P < 0.05) and Chn1−/− (contralateral: 20.0 ± 3.7 neurons/mouse; ipsilateral: 8.9 ± 0.7 neurons/mouse; P < 0.05) mice. Labeled cortical neurons seemed to be less abundant in Chn1−/− than WT mice. These findings show that layer 5 neurons in M1 of both WT and Chn1−/− mice make oligosynaptic connections with forelimb muscles. Importantly, in WT mice, layer 5 neurons were sparsely connected to ipsilateral forelimb muscle, whereas in Chn1−/− mice, they were densely connected.
To further elucidate bilateral CS system control in Chn1−/− mice, we extended our earlier ICMS motor mapping experiments (Beg et al. 2007) in two ways. First, we examined the forelimb representation of M1. Previously, only the hindlimb M1 representation was examined because hind leg hopping was the focus. Plausibly, the foreleg representation has a different organization because the forelegs are engaged in a much broader motor repertoire than the hindlimbs. Second, we determined whether the ipsilateral motor representation in Chn1−/− mice is a redundant copy of the contralateral representation. We recently showed in the rat that the ipsilateral M1 forelimb motor representation depends on M1 in the opposite hemisphere for the expression of ipsilateral responses (Brus-Ramer et al. 2009). When M1 in the other hemisphere was reversibly inactivated or the medullary pyramid on the opposite side was ablated, ipsilateral responses could no longer be evoked by stimulation of the intact M1. Whereas some of this dependence is explained by transcallosal connections, most is caused by interactions at the spinal level where the ipsilateral CST from one side and the contralateral CST from the other side converge on common spinal circuits for the expression of ipsilateral responses (Fig. 5B, inset; see also Fig. 10).
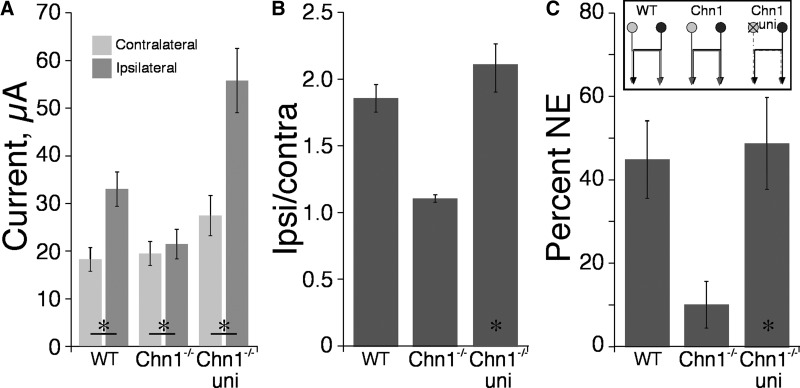
Fig. 5.
Effects of intracortical microstimulation (ICMS) on WT and Chn1−/− mice. A: threshold currents (mean ± SE) for evoking contralateral and ipsilateral forelimb responses in WT, Chn1−/−, and Chn1−/− with unilateral M1 ablation mice. B: ratio of ipsilateral to contralateral thresholds in WT, Chn1−/−, and Chn1−/− with unilateral M1 ablation mice. C: percentage of nonresponsive sites in WT, Chn1−/−, and Chn1−/− with unilateral M1 ablation mice. There were 5 mice for each group. Inset in C shows schematically CST projections in the 3 animal groups.
Fig. 10.
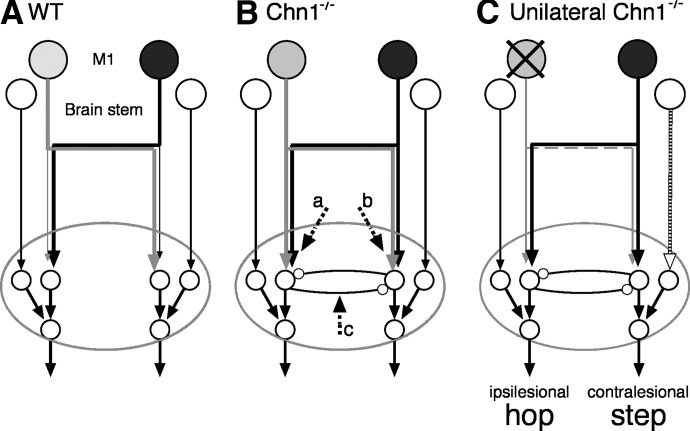
Schematic of proposed circuitry for trajectory modification for stepping/hopping over the obstacle. Each panel shows projections from M1 (filled neurons) and from the brain stem (large open neurons) and the termination field in the spinal cord (ellipse; small open neurons are spinal interneurons and motoneurons). A: WT mouse. Thin lines imply sparse and weak connections. B: Chn1−/−. C: Chn1−/− with unilateral M1 ablation (“X” over lesioned side). Note, because the limb on the ipsilesional side (left) did not show a significant increase in stepping, we conclude that control on this side favors hopping. By contrast, stepping increased significantly on the contralesional (right) side.
We conducted ICMS experiments on three animal groups, as shown schematically in Fig. 5C: 1) intact WT (Fig. 5A; n = 5) mice as controls; 2) intact Chn1−/− (n = 5; Fig. 5B) as controls; and 3) Chn1−/− mice with unilateral ablation of the nonstimulated M1 (n = 5; 3 also subjected to the behavioral experiments; 2 additional mice, ICMS only; see Fig. 5C; stimulate right side; n = 5). We stimulated M1 on one side and recorded evoked responses from the contralateral and ipsilateral limbs. We used three functional measures: 1) current threshold, 2) the ratio between ipsilateral and contralateral thresholds, and 3) percentage of sites where stimulation evoked a contralateral but not an ipsilateral response. The threshold for evoking a contralateral forelimb movement was not different across groups (Fig. 5A; ANOVA, F = 2.215, P = 0.152), although there were trends between groups, especially between the intact and lesioned Chn1−/− mice. Ipsilateral thresholds differed significantly between groups (F = 13.4, P = 0.0009).
WT mice have substantially higher thresholds for evoking ipsilateral responses than for evoking contralateral responses (Fig. 5A; 18.3 ± 2.5 vs. 33.0 ± 3.6 μA; P = 0.0003), as we observed in the rat (Brus-Ramer et al. 2009). The ipsilateral-contralateral threshold current ratio was 1.86 ± 0.10 (Fig. 5B). Whereas the ipsilateral and contralateral thresholds were very similar in intact Chn1−/− mice (19.48 ± 3.1 vs. 21.48 ± 3.13 μA; ipsilateral to contralateral ratio: 1.11 ± 0.03), they were significant on paired comparison (P = 0.0027; Fig. 5A). This suggests that the dense bilateral CSTs originating from M1 in Chn1−/− mice are effective in activating forelimb spinal motor circuits bilaterally and are concordant with the PRV findings showing that M1 in Chn1−/− mice is part of a bilateral motor circuit.
After unilateral M1 ablation in the Chn1−/− mice, ipsilateral thresholds become substantially elevated (Fig. 5, A and B; from 21.4 to 55.8 μA; P = 0.0017). Importantly, the number of sites in M1 from which ICMS evoked a contralateral but no ipsilateral response, which normally is very low in Chn1−/− mice (10. 2%; Fig. 5C), increased significantly to 49% after unilateral lesion (P = 0.013). The postlesion values were similar to WT mice (Fig. 5C). These findings suggest that, despite robust bilateral CST connections to forelimb muscle, the ipsilateral representation is not a redundant copy of the contralateral functions. Rather, it depends on M1, including the CST (Brus-Ramer et al. 2009), in the other hemisphere to evoke movement at low thresholds, suggesting that the motor function of the aberrant ipsilateral CS system in Chn1−/− mice are limited. This question is revisited later, in the context of emergence of lead stepping contralateral to a unilateral M1 ablation.
Adaptive locomotion in Chn1−/− mice
Chn1−/− mice, as previously reported (Beg et al. 2007), express a hopping phenotype, in which the actions of both forelimbs and/or hindlimbs are commonly bilaterally yoked (see methods for definition of hop). Figure 6A shows representative forelimb locomotor sequences of a Chn1−/− mouse. During unobstructed locomotion (Fig. 6A1), forelimb steps and hops (h) are interspersed in both sequences, with steps slightly outnumbering hops in these examples. Unobstructed forelimb hopping occurred randomly; we were unable to detect a trend that predicted an upcoming hop or a step. The hindlimbs hop nearly 100% of the time. This is similar to what has been reported for EphA4-null mutants (Akay et al. 2006).
Fig. 6.
Representative forepaw trajectories of Chn1−/− mice. A: right forelimb trajectories of 1 intact Chn1−/− mouse during a sequence of unobstructed steps (A1) and unobstructed and obstructed steps (A2). B: obstructed steps are shown by the dotted lines. “s” indicates a step and “h,” a hop. Obstacles are indicated by vertical gray bars. Inset: the direction of locomotion and the obstacle. Speed of locomotion in A and B was 6.66 cm/s. B and C: trajectories for unobstructed (left) and obstructed (middle and right) stepping are shown in B for a representative Chn1−/− mouse before bilateral M1 ablation and, in C, after bilateral M1 ablation. Steps and hops are shown. The gray lines are steps/hops while the treadmill moved at 6.66 cm/s (termed slow) and the black lines at 10 cm/s (termed fast). The obstacles are shown by the vertical black lines in the middle and right panels. Calibrations in A–C: 5 mm vertical height; 50 mm horizontal distance.
To test whether the CS system wiring abnormalities resulted in functional changes in locomotor behavior, we tested Chn1−/− mice in adaptive locomotion. Because the gait pattern during obstructed locomotion is lateralized in humans (Patla et al. 1996), cats (Drew et al. 2004; Friel et al. 2007), and WT mice (as above), we anticipated that the presence of an obstacle would be sufficient for the Chn1−/− animals to select a step, opposed to a hop, as they are clearly capable of during unobstructed locomotion (e.g., Fig. 6A1). Surprisingly, as the obstacle approached (Fig. 6A2), a different pattern emerged whereby the Chn1−/− mouse hopped over the obstacle on most trials (90%; Fig. 7). The trajectories in Fig. 6A2 also show that the height of the hop over the obstacle was higher than hops (or steps) during unobstructed locomotion (examined quantitatively below; Fig. 9). After the obstacle, the mouse returned to intermittent forelimb hopping. Immediately after the obstacle, steps or hops were evoked with equal frequency. As in WT mice, the obstacle produced a task-dependent change in locomotor performance whereby the trajectory was adaptively modified to clear the obstacle. Importantly, in Chn1−/− mice, the obstacle determined a response change, whereby intermittent forelimb stepping and hopping was converted to nearly obligatory hopping.
Fig. 7.
Incidence of forelimb (A) and hindlimb (B) hopping in Chn1−/− mice. The percentage of hopping (mean ± SE) in intact animals is show by the light gray bars, and after bilateral M1 ablation, by the dark gray bars.
Fig. 9.
Obstructed locomotor performance in Chn1−/− mice before and after bilateral M1 ablation. A: scaling of step and hop height for small and large obstacles (mean ±SE) in intact Chn1−/− mice (n = 5) and, for the same cohort, after bilateral M1 ablation. B: error rates (mean ± SE) for clearing the obstacle before (left) and after (right) bilateral M1 ablation. Only hop data are presented for the intact condition because steps were uncommon. Calibrations: 5 mm vertical height; 50 mm horizontal distance.
Frequency of hopping before and after M1 bilateral ablation in Chn1−/− mice
In the absence of supraspinal drive, the isolated spinal cord of Chn1−/−, ephrinB3, and EphA4 mutant mice expresses bilateral motor output, or “fictive hopping,” under the same in vitro conditions that WT mice show, alternating right-left output (Iwasato et al. 2007; Kullander et al. 2003; Wegmeyer et al. 2007). Bilateral autonomous spinal output has been proposed to be caused by aberrant bilateral projections between spinal interneurons (Kullander et al. 2003). Thus the bilateral output can be explained by the aberrant bilateral spinal circuitry. The question we were interested in pursuing is whether this spinal circuitry is recruited by the motor cortex, and its bilateral CST, to evoke a hop over the obstacle. To address this question, we used bilateral and unilateral M1 ablation.
Figure 7 plots the frequency of forelimb (A) and hindlimb (B) hopping before and after bilateral M1 ablation (n = 5 mice). Intact Chn1−/− mice express forelimb steps and hops at approximately equal frequency (51.6 ± 6.0%; Fig. 7A) during unobstructed locomotion. Remarkably, when animals encountered the obstacle, forelimb hopping rate increased to 89.0 ± 3.3% (paired t-test; P = 0.0075). A second cohort of six mice (described below) had an obstructed hopping rate of 91%. Together, Chn1−/− show a 76% increase in hopping over the obstacle than during unobstructed locomotion.
By contrast to forelimb hopping, hind leg hopping is normally high during unobstructed locomotion, 83.7 ± 3.6%, and only increased modestly, although significantly, when presented with the obstacle, 94.1 ± 1.6% (Fig. 7B; P = 0.025)—a 12% increase. These results show that the presence of the obstacle evokes a task-dependent change in which the execution of a pair of equally probable behaviors, steps or hops, switches to hopping, to the near exclusion of stepping.
When M1 was bilaterally ablated (Fig. 7, dark bars), forelimb hopping during unobstructed locomotion was reduced from 51.6 to 32.2% (P = 0.022, corrected for multiple comparisons). Now, presentation of the obstruction failed to evoke a further increase in hopping (i.e., 32.2 to 35.8%; P = 0.57). Importantly, M1 ablation eliminated the task-dependent hopping over the obstacle, so that when the animal encountered the obstacle, it hopped or stepped over it with equal frequency. Hind leg hopping was reduced overall after M1 ablation (P < 0.024, corrected for multiple comparisons) and, as the forelimb, there was no further increase during obstructed locomotion (Fig. 7B; P = 0.104). The reductions in hopping we observed on the first day after M1 ablation were maintained for the duration of testing. There was no significant change in the frequency of hopping over the 4-day postlesion testing period (F = 1.6; P = 0.206). These results indicate an essential role for M1 in the task-dependent switch to hop over the obstacle and a constitutive role for M1 in the hopping during unobstructed locomotion.
Emergence of lead stepping contralateral to unilateral M1 ablation in Chn1−/− mice
Bilateral M1 ablation eliminated the choice to hop over the obstacle. We next asked what happens to the choice to hop over the obstacle after unilateral M1 ablation? The strongly bilateral organization of the CS system in the Chn1−/− mouse, together with bilateral spinal circuitry, might confer redundancy, so that unilateral ablation would have little or no effect on the choice to hop or step over the obstacle. The contralateral CST from M1 on one side and the ipsilateral CST from M1 on the other side converge onto the same side of the spinal cord (Fig. 8B, inset, asterisk), thereby providing a route by which the ipsilateral path could replace lost contralateral functions. In this case, unilateral ablation would have no effect on hopping over the obstacle. Alternatively, the loss of contralateral control by M1 on one side might not be readily compensated by the remaining ipsilateral path. This would reveal a reduction in hopping, and, in consequence, an increase in stepping with one or the other forelimb.
Fig. 8.
The effects of unilateral M1 ablation in Chn1−/− mice. A: percent of forelimb hopping before and after unilateral M1 ablation (mean ± SE; n = 6 mice). B: percent of forelimb lead stepping on the ipsilesional and contralesional sides. Note that the small increase in lead stepping with the ipsilateral forepaw was not significant. Inset: the organization of bilateral CST inputs to the spinal cord. The schematic neuron on the left is meant to indicate the lesioned side.
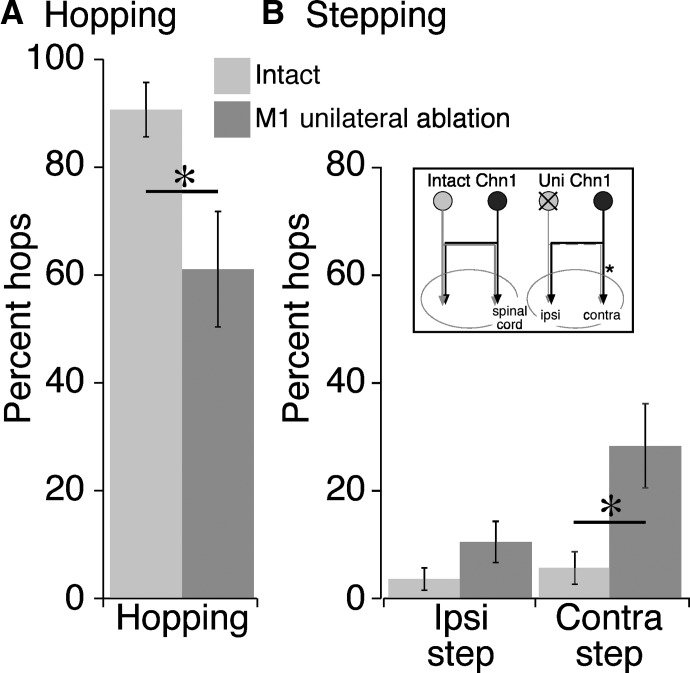
We unilaterally ablated M1 (n = 6 mice; 3 left M1, 3 right M1) over the same extent as for bilateral lesions. Unilateral ablation produced a significant reduction in hopping over the obstacle. Hopping during obstructed locomotion was 91% before unilateral M1 ablation and was 61% after the lesion (paired t-test: T = 2.59; P = 0.049; Fig. 8A). On average, this was a 31% reduction across animals (range, 16–90%). Unilateral ablation also produced a reduction in hopping during unobstructed locomotion (44% before lesion to 29% after; paired t-test, P = 0.007). We conclude that the dense ipsilateral CST projections, together with aberrant bilateral spinal circuitry, are no proxy for the lost contralateral projections.
We next analyzed the pattern of increased lead stepping over the obstacle that occurred as a consequence of the reduced hopping. Lead stepping is lateralized. We looked for changes in lead stepping after unilateral M1 lesion on the contralesional side (Fig. 8B, Uni Chn1, asterisk), where the spared ipsilateral CST projects, and the ipsilateral side, where the spared contralateral CST projects. On the contralesional side, in the presence of the spared ipsilateral CST, there was more than a threefold increase in lead stepping (Fig. 8B; T = 2.679; P = 0.04). By contrast, on the ipsilesional side, in the presence of the spared contralateral CST, lead stepping was not significantly changed. These findings suggest that the aberrant ipsilateral CST is less capable of selecting a hop over the obstacle than the contralateral projection. In discussion, we consider the implication of this finding in producing mirror-like movements and the role of brain stem pathways in controlling lead stepping after M1 ablation.
Forepaw trajectories in Chn1−/− mice during unobstructed and obstructed locomotion
We also examined the role of M1 in trajectory control in Chn1−/− mice. Representative forelimb trajectories from an intact Chn1−/− mouse are shown in Fig. 6B. During unobstructed locomotion, Chn1−/− mice, as EphA4−/− (Akay et al. 2006), have a shortened step distance compared with WT mice. Mean step length in the Chn1−/− mice was 44.1 ± 3.4 mm compared with 70.1 ± 5.9 mm in WT mice (P = 0.006). The trajectories of hops and steps were remarkably similar in height (hop: 6.1 ± 0.8 mm; step: 5.9 ± 0.7 mm; P = 0.86) and length (hop: 48.0 ± 3.2 mm; step: 44.1 ± 3.4 mm; P = 0.43). For obstructed locomotion, as above, we focused on the leading forelimb for the trajectory analysis. Only hops were examined because steps were sparse. As with stepping in WT mice, the amplitude of the hop was scaled to the height of the obstacle, as shown with individual trajectories (Fig. 6B, middle and right columns) and as mean trajectory amplitude for the group examined (n = 5; Fig. 9A; paired t-test, P = 0.0015). As for the WT animals, error rate was low (Fig. 9B).
Effects of bilateral M1 ablation on forepaw trajectories and errors
After bilateral M1 ablation, unobstructed forepaw trajectories were not remarkably different (Fig. 6C). Both steps and hops remained scaled after M1 ablation (Fig. 9A; hops: P < 0.0007; steps: P < 0.0006). Note that, because animals rarely stepped over the obstacle before bilateral M1 ablation, we cannot determine whether step height scaling was affected by the lesion. Interestingly, the absolute amplitudes of the postlesion steps over the obstacle were remarkably similar to those of the WT mice (low target—WT: 7.20 ± 0.05; Chn1−/−: 7.19 ± 0.025; high target—WT: 11.9 ± 0.37; Chn1−/−: 12.2 ± 0.36). After M1 ablation (Fig. 9B), error rate increased substantially [from 1.5 to 12.1% for hops (t = 2.872; P = 0.045) and to 17.7% for steps].
As with WT mice, our findings in the Chn1−/− show a role for M1 in the moment-to-moment control of adaptive stepping. Without M1, hopping was no longer selected as the typical response to clear the obstacle. Hopping amplitude was not changed after the lesion. Both hops and steps were less well adapted to clearing the obstacle after the lesion. From a kinematic perspective, the amplitude of postlesion steps were identical to those in the WT mouse and were the least well adapted after the lesion. Like WT mice, M1 in Chn1−/− mice plays little or no role in shaping the form of the trajectory or when the modified response occurs relative to the obstacle.
DISCUSSION
To study adaptive motor control in the mouse, we used a locomotor task in which animals modified their gait to step over an obstacle. This movement requires the task-dependent integration of obstacle sensory information with the timing and amplitude of the ongoing step cycle. Our task is similar to the one developed by Drew (1988) and used to study the kinematics and neural control of gait modification in the cat. Bringing adaptive locomotion to the mouse potentially enables dissection of voluntary control circuits in an animal that can be exploited by genetic manipulation. Adaptive locomotion shares features with reaching (Drew et al. 2004; Georgopoulos and Grillner 1989). For example, the modified forelimb step and paw placement are guided by obstacle height and location (Drew et al. 2004), like reaching, which is guided by target stimulus information (Georgopoulos 1986). Indeed, the same M1 neurons modulate their activity during both reaching and adaptive stepping (Drew et al. 2004). A plausible framework for voluntary control is that descending supraspinal motor pathways modify the activity of intrinsic spinal circuits that produce stereotypic and automatic behaviors (e.g., CPG), thereby adapting them to perform voluntary motor tasks in flexible and goal-directed ways (Brown 1914).
Feed-forward kinematic control is impaired after bilateral M1 lesion
For obstructed locomotion, we concentrated on the forelimb because it is under feed-forward control by obstacle sensory information (i.e., height) during obstacle avoidance. By contrast, the hindlimbs can be controlled by any combination of feed-forward, propriospinal, or forelimb feedback signals. Results from both the WT and Chn1−/− mice suggest an important role for M1 in the moment-to-moment control of the adaptive response. In Chn1−/− mice, the switch to hopping over the obstacle involves a signal operating over a single step cycle, because the step after the obstacle was not biased by prior hopping. The CS system mediates this switch, because bilateral ablation eliminated the conversion from stochastic hopping and stepping to nearly obligatory hopping over the obstacle. After bilateral M1 ablation in the WT mouse, the animals made substantially more stepping errors and showed a small scaling impairment, suggesting that the errors reflected impairment in feed-forward amplitude control.
However, our findings also stress the importance of subcortical control in adaptive locomotion. After bilateral M1 ablation, WT mice were capable of stepping over the obstacle. Trials in which stepping occurred without obstacle contact showed trajectories that were not substantially different from prelesion controls, except for the small amplitude reduction. Chn1−/− mice were capable of making appropriately scaled steps and hops after bilateral M1 ablation, with trajectories similar to prelesion. A likely subcortical candidate is the rubrospinal tract, which shares many functions with the CS system (Cheney et al. 1991; Martin and Ghez 1988) and has been shown to be important in recovery after CS system injury (Z'Graggen et al. 2000). Moreover, cats show a persistent impairment in obstructed locomotion only after combined CST and rubrospinal tract lesion (Jiang and Drew 1996). Rubrospinal neurons modulate their activity during obstructed locomotion in the cat (Lavoie and Drew 2002). There is a corticorubral projection from posterior parietal cortex (Fanardjian and Papoyan 1997), where neurons also modulate their activity during adaptive locomotion (McVea and Pearson 2007; Pearson and Gramlich 2010), that would be spared by the M1 lesion. Our findings point to principal subcortical control of the trajectory and shared, parallel, cortical, and subcortical control of response decision making and scaling (at least in the WT mouse).
Bilateral CST circuitry
Transneuronal retrograde labeling showed that Chn1−/− mice have bilateral CS projections from M1 to forelimb muscles, with about one half of the projection originating from ipsilateral cortex. This matches well with anterograde findings (Beg et al. 2007; Coonan et al. 2001). PRV-labeled neurons in WT and mutant mice were layer 5 pyramidal cells. Whereas many of these were likely CST neurons, some could have been corticoreticular neurons (Rho et al. 1997) that project to neurons with motoneuronal connections. Similar transport times would be expected for the direct CST path and the cortico-reticulospinal path because both are disynaptically linked to motoneurons (Kuypers 1981). Whereas we know that the CST has dense spinal terminations in the mouse (Bareyre et al. 2005), we do not know the relative density of the corticoreticulo-spinal path.
Our ICMS findings agree well with PRV tracing, showing strong bilateral muscle activation in the Chn1−/− mice. We were, however, surprised to observe a substantial elevation in the ipsilateral thresholds with unilateral M1 ablation in Chn1−/− mice. This shows that the ipsilateral responses are dependent on the contralateral CST projection from M1 in the other hemisphere, like the rat (Brus-Ramer et al. 2009). Our starting hypothesis was that the dense (Beg et al. 2007) and strong (e.g., Fig. 5) ipsilateral CST would confer independence, but this was not so. Indeed, in the context of our behavioral experiments in the unilateral lesioned Chn1−/− mice, we concluded that the ipsilateral CST projection, together with bilateral intrinsic circuitry, cannot replace the lost contralateral CST projection in evoking the hop over the obstacle. This finding points to less of a role for the ipsilateral CST in the feed-forward drive of spinal motor circuits and more for organizing ipsilateral motor synergies, such as in posture/balance or object stabilization during bimanual manipulation.
Cortically evoked hopping in Chn1−/− mice
The isolated lumbar cord in Chn1−/− mice (Iwasato et al. 2007; Wegmeyer et al. 2007), and also in EphA4/ephrinB3 mutants (Kullander et al. 2003), shows fictive hopping, as bilaterally synchronous ventral root output (Wegmeyer et al. 2007). The same conditions in WT mice produce right-left alternating responses. In the null mice, the EphA4 interneuron class (Kullander et al. 2003), and likely other classes of interneuron (Beg et al. 2007), have bilateral projections, which is the plausible explanation for synchronous output. Aberrant bilateral spinal motor circuits helps explain the high rate of hindlimb hopping (∼90%) during unobstructed locomotion. However, this model needs refinement for the cervical cord because forelimb hopping only occurred roughly 50% of the time during unobstructed locomotion. A similar hindlimb bias for hopping was reported by Akay et al. (2006) for EphA4 mutants. We speculate that the underlying anatomical circuit changes are the same at all spinal levels but that the threshold for recruiting cervical bilateral interneurons is higher than for lumbar interneurons, possibly because of greater independent forelimb use, steered by descending rather than intrinsic inputs. This recruitment seems to be under strong M1 control because M1 ablation reduced forelimb and hindlimb unobstructed hopping by similar percentages. We propose that, during unobstructed locomotion, CST “tone,” is low, resulting in the 50–80% forelimb-hindlimb hopping differences. Similar reasoning applies to obstructed locomotion, where CST tone is high during (i.e., greater pyramidal tract neuronal activity (Drew 1988; Karayannidou et al. 2009), resulting in the elevated (90–95% forelimb-hindlimb) hopping percentages. The dense CST spinal terminations are likely important in constitutive regulation of spinal motor circuit excitability.
What drives the aberrant bilateral spinal circuitry to evoke mirror-like hopping over the obstacle in Chn1−/− mice: the bilateral CST (Fig. 10B, arrows a, b), in particular the strong ipsilateral projection, or a unilateral projection that targets the aberrant bilateral spinal circuits (Fig. 10B, arrow c)? Our unilateral lesion data bare on this question. They show that stepping was not significantly increased on the ipsilesional side. From the perspective of CST control, this side receives only the spared contralateral CST (Fig. 10C, ipsilesional side). This implies that the spared contralateral CST is capable of mediating hopping. By contrast, stepping was significantly increased on the contralesional side. Again, from the perspective of CST control, this side receives only the spared ipsilateral CST (Fig. 10C, contralesional side). This implies that the spared ipsilateral CST is less capable of mediating hopping (i.e., because of the emergence of significant lead stepping) than its contralateral counterpart. We propose that the contralateral CST projection to aberrant bilateral spinal circuits (Fig. 10; arrow c) is more important than the ipsilateral CST in driving hopping over the obstacle. We propose that the emergence of lead stepping of the limb ipsilateral to M1 unilateral lesion is produced by brain stem pathways (e.g., rubrospinal) through access to a spinal network more lateralized than the one the CST accesses (Fig, 10C, black/white arrow), although rapid adaptation by a few spared CST axons cannot be completely ruled out.
It is not known whether mirror movements in humans are caused by bilateral CSTs, bilateral spinal circuitry, or a combination of the two. We do know that cats with a robust ipsilateral CST caused by an activity imbalance in the developing CSTs in each hemisphere (Friel and Martin 2007; Salimi et al. 2008), but no evidence for aberrant bilateral spinal motor circuits, do not hop over the obstacle during adaptive locomotion (Friel et al. 2007) or display other mirror-like movements. The genetic mirror movement disorders in humans (Brouwer and Ashby 1991; Carr et al. 1993; Farmer et al. 1991; Mayston et al. 1997; Srour et al. 2010; Vulliemoz et al. 2005) have bilateral CSTs and may also have aberrant bilateral spinal interneurons because the genetic impairments produce midline axon guidance defects in mice (Srour et al. 2010). In response to perinatal forebrain trauma, hemiplegic cerebral palsy patients develop a bilateral CST from the less impaired side (Eyre et al. 2007). These patients often express mirror movements (Muller et al. 1997). It is not known whether these patients develop stronger spinal commissural connections as a consequence of CST miswiring. Our unilateral ablation findings in Chn1−/− mice suggest that mirror movements may depend on cooperation between a bilateral CST and bilateral spinal motor circuits and not just bilateral CST projections. The question of the differential roles of a bilateral CST versus bilateral spinal circuits will not be answered until effective CS system-only and spinal-only conditional knockouts are developed.
Role of mouse M1 in response selection and motor set–dependent changes
The decision to hop or step for a given cycle during unobstructed locomotion in Chn1−/− mice was stochastic. Random response selection in obstructed locomotion converts to nearly obligatory hopping over the obstacle, and after bilateral M1 ablation, there is a return to random selection of hops and steps over the obstacle. This shows an important role for M1 in response selection: to hop or not to hop.
Elucidating cortical control of hopping in Chn1−/− mice may provide insight into gait modification in WT animals. The switch to preferential hopping over the obstacle may be analogous to modifying forepaw trajectory to step over the obstacle. In the cat, the trajectory of the forelimb during stepping is governed by a complex muscle synergy (Drew et al. 2008; Krouchev et al. 2006). In M1, the modified response is associated with differential activation of pyramidal tract neurons thought to control different sets of forelimb muscles (Drew et al. 2008). It is not known how this is implemented at the spinal level. The kinematically and electromyographically new response could occur by having the CST select a different spinal premotor circuit to produce the modified trajectory, as the CST must do in selecting the circuit for the hop over the obstacle in Chn1−/− mice. Through differential interneuronal circuit selection, M1 and the CST could contribute to the decision to produce a task-specific adapted response.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-36835 to J. Martin and grants from the Motor Neuron Center of Columbia University to. P. Scheiffele and J. Martin.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Lynn Enquist (Dept. Molecular Biology, Princeton University) for the generous gift of the two strains of Pseudorabies virus, X. Wu for histochemistry and histology, Dr. Kathleen Friel for help with some of the initial behavioral experiments, and A. Lyashechenko for help with some of the ICMS experiments. We also thank Dr. Trevor Drew for insights and comments on an earlier version of the manuscript.
REFERENCES
- Akay et al., 2006. Akay T, Acharya HJ, Fouad K, Pearson KG. Behavioral and electromyographic characterization of mice lacking EphA4 receptors. J Neurophysiol 96: 642–651, 2006 [DOI] [PubMed] [Google Scholar]
- Armstrong, 1988. Armstrong DM. The supraspinal control of mammalian locomotion. J Physiol 405: 1–37, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfield et al., 2003. Banfield BW, Kaufman JD, Randall JA, Pickard GE. Development of pseudorabies virus strains expressing red fluorescent proteins: new tools for multisynaptic labeling applications. J Virol 77: 10106–10112, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre et al., 2005. Bareyre FM, Kerschensteiner M, Misgeld T, Sanes JR. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat Med 11: 1355–1360, 2005 [DOI] [PubMed] [Google Scholar]
- Beg et al., 2007. Beg AA, Sommer JE, Martin JH, Scheiffele P. alpha2-Chimaerin is an essential EphA4 effector in the assembly of neuronal locomotor circuits. Neuron 55: 768–778, 2007 [DOI] [PubMed] [Google Scholar]
- Benson et al., 2005. Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci USA 102: 10694–10699, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer and Ashby, 1991. Brouwer B, Ashby P. Altered corticospinal projections to lower limb motoneurons in subjects with cerebral palsy. Brain 114: 1395–1407, 1991 [DOI] [PubMed] [Google Scholar]
- Brown, 1914. Brown TG. On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. J Physiol 48: 1–17, 1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brus-Ramer et al., 2009. Brus-Ramer M, Carmel JB, Martin JH. Motor cortex bilateral motor representation depends on subcortical and interhemispheric interactions. J Neurosci 29: 6196–6206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr et al., 1993. Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain 116: 1223–1247, 1993 [DOI] [PubMed] [Google Scholar]
- Cheney et al., 1991. Cheney PD, Fetz EE, Mewes K. Neural mechanisms underlying coricospinal and rubrospinal control of limb movements. Prog Brain Res 87: 213–252, 1991 [DOI] [PubMed] [Google Scholar]
- Coonan et al., 2001. Coonan JR, Greferath U, Messenger J, Hartley L, Murphy M, Boyd AW, Dottori M, Galea MP, Bartlett PF. Development and reorganization of corticospinal projections in EphA4 deficient mice. J Comp Neurol 436: 248–262, 2001 [PubMed] [Google Scholar]
- Drew, 1988. Drew T. Motor cortical discharge during voluntary gait modification. Brain Res 457: 181–187, 1988 [DOI] [PubMed] [Google Scholar]
- Drew et al., 2008. Drew T, Andujar JE, Lajoie K, Yakovenko S. Cortical mechanisms involved in visuomotor coordination during precision walking. Brain Res Rev 57: 199–211, 2008 [DOI] [PubMed] [Google Scholar]
- Drew et al., 2004. Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Prog Brain Res 143: 251–261, 2004 [DOI] [PubMed] [Google Scholar]
- Eyre et al., 2007. Eyre JA, Smith M, Dabydeen L, Clowry GJ, Patacchi E, Battini R, Guzzetta A, Cioni G. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system? Ann Neurol 62: 493–503, 2007 [DOI] [PubMed] [Google Scholar]
- Fanardjian and Papoyan, 1997. Fanardjian VV, Papoyan EV. Patterns of inputs to the parietal cortex efferent neurons from the motor cortex and cerebellum in the cat. Neuroscience 77: 965–974, 1997 [DOI] [PubMed] [Google Scholar]
- Farmer et al., 1991. Farmer SF, Harrison LM, Ingram DA, Stephens JA. Plasticity of central motor pathways in children with hemiplegic cerebral palsy. Neurology 41: 1505–1510, 1991 [DOI] [PubMed] [Google Scholar]
- Friel and Martin, 2007. Friel K, Martin JH. Bilateral activity-dependent interactions in the developing corticospinal system. J Neurosci 27: 11083–11090, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel et al., 2007. Friel KM, Drew T, Martin JH. Differential activity-dependent development of corticospinal control of movement and final limb position during visually-guided locomotion. J Neurophysiol 97: 3396–3406, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos, 1986. Georgopoulos AP. On reaching. Annu Rev Neurosci 9: 147–170, 1986 [DOI] [PubMed] [Google Scholar]
- Georgopoulos and Grillner, 1989. Georgopoulos AP, Grillner S. Visuomotor coordination in reaching and locomotion. Science 245: 1209–1210, 1989 [DOI] [PubMed] [Google Scholar]
- Goulding, 2009. Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci 10: 507–518, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasato et al., 2007. Iwasato T, Katoh H, Nishimaru H, Ishikawa Y, Inoue H, Saito YM, Ando R, Iwama M, Takahashi R, Negishi M, Itohara S. Rac-GAP alpha-chimerin regulates motor-circuit formation as a key mediator of ephrinB3/ephA4 forward signaling. Cell 130: 742–753, 2007 [DOI] [PubMed] [Google Scholar]
- Jiang and Drew, 1996. Jiang W, Drew T. Effects of bilateral lesions of the dorsolateral funiculi and dorsal columns at the level of the low thoracic spinal cord on the control of locomotion in the adult cat. I. Treadmill walking. J Neurophysiol 76: 849–866, 1996 [DOI] [PubMed] [Google Scholar]
- Karayannidou et al., 2009. Karayannidou A, Beloozerova IN, Zelenin PV, Stout EE, Sirota MG, Orlovsky GN, Deliagina TG. Activity of pyramidal tract neurons in the cat during standing and walking on an inclined plane. J Physiol 587: 3795–3811, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krouchev et al., 2006. Krouchev N, Kalaska JF, Drew T. Sequential activation of muscle synergies during locomotion in the intact cat as revealed by cluster analysis and direct decomposition. J Neurophysiol 96: 1991–2010, 2006 [DOI] [PubMed] [Google Scholar]
- Kullander et al., 2003. Kullander K, Butt SJ, Lebret JM, Lundfald L, Restrepo CE, Rydstrom A, Klein R, Kiehn O. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science 299: 1889–1892, 2003 [DOI] [PubMed] [Google Scholar]
- Kullander et al., 2001. Kullander K, Croll SD, Zimmer M, Pan L, McClain J, Hughes V, Zabski S, DeChiara TM, Klein R, Yancopoulos GD, Gale NW. Ephrin-B3 is the midline barrier that prevents corticospinal tract axons from recrossing, allowing for unilateral motor control. Genes Dev 15: 877–888, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers, 1981. Kuypers HGJM. Anatomy of the descending pathways. In: Handbook of Physiology, Neurophysiology, edited by Brookhart JM, Mountcastle VB. Bethesda, MD: American Physiological Society, 1981, vol. 2, p. 597–666 [Google Scholar]
- Lavoie and Drew, 2002. Lavoie S, Drew T. Discharge characteristics of neurons in the red nucleus during voluntary gait modifications: a comparison with the motor cortex. J Neurophysiol 88: 1791–1814, 2002 [DOI] [PubMed] [Google Scholar]
- Li and Waters, 1991. Li C-X, Waters RS. Organization of the mouse motor cortex studied by retrograde tracing and intracortical microstimulation (ICMS) mapping. Can J Neurol Sci 18: 28–38, 1991 [DOI] [PubMed] [Google Scholar]
- Martin and Ghez, 1988. Martin JH, Ghez C. Red nucleus and motor cortex: parallel motor systems for the initiation and control of skilled movement. Behav Brain Res 28: 217–223, 1988 [DOI] [PubMed] [Google Scholar]
- Mayston et al., 1997. Mayston MJ, Harrison LM, Quinton R, Stephens JA, Krams M, Bouloux PM. Mirror movements in X-linked Kallmann's syndrome. I. A neurophysiological study. Brain 120: 1199–1216, 1997 [DOI] [PubMed] [Google Scholar]
- McKenna et al., 2000. McKenna JE, Prusky GT, Whishaw IQ. Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: a retrograde carbocyanine dye analysis. J Comp Neurol 419: 286–296, 2000 [DOI] [PubMed] [Google Scholar]
- McVea and Pearson, 2007. McVea DA, Pearson KG. Stepping of the forelegs over obstacles establishes long-lasting memories in cats. Curr Biol 17: R621-R-623, 2007 [DOI] [PubMed] [Google Scholar]
- Muller et al., 1997. Muller K, Kass-Iliyya F, Reitz M. Ontogeny of ipsilateral corticospinal projections: a developmental study with transcranial magnetic stimulation. Ann Neurol 42: 705–711, 1997 [DOI] [PubMed] [Google Scholar]
- Patla et al., 1996. Patla AE, Rietdyk S, Martin C, Prentice S. Locomotor patterns of the leading and the trailing limbs as solid and fragile obstacles are stepped over: some insights into the role of vision during locomotion. J Motor Behav 28: 35–47, 1996 [DOI] [PubMed] [Google Scholar]
- Pearson and Gramlich, 2010. Pearson K, Gramlich R. Updating neural representations of objects during walking. Ann NY Acad Sci 1198: 1–9, 2010 [DOI] [PubMed] [Google Scholar]
- Rathelot and Strick, 2006. Rathelot JA, Strick PL. Muscle representation in the macaque motor cortex: an anatomical perspective. Proc Natl Acad Sci USA 103: 8257–8262, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathelot and Strick, 2009. Rathelot JA, Strick PL. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci USA 106: 918–923, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho et al., 1997. Rho MJ, Cabana T, Drew T. Organization of the projections from the pericruciate cortex to the pontomedullary reticular formation of the cat: a quantitative retrograde tracing study. J Comp Neurol 388: 228–249, 1997 [DOI] [PubMed] [Google Scholar]
- Salimi et al., 2008. Salimi I, Friel K, Martin JH. Pyramidal tract stimulation restores normal corticospinal tract connections and visuomotor skill after early postnatal motor cortex activity blockade. J Neurosci 28: 7426–7434, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi et al., 2007. Shi L, Fu WY, Hung KW, Porchetta C, Hall C, Fu AK, Ip NY. Alpha2-chimaerin interacts with EphA4 and regulates EphA4-dependent growth cone collapse. Proc Natl Acad Sci USA 104: 16347–16352, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith et al., 2000. Smith BN, Banfield BW, Smeraski CA, Wilcox CL, Dudek FE, Enquist LW, Pickard GE. Pseudorabies virus expressing enhanced green fluorescent protein: a tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc Natl Acad Sci USA 97: 9264–9269, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srour et al., 2010. Srour M, Riviere JB, Pham JM, Dube MP, Girard S, Morin S, Dion PA, Asselin G, Rochefort D, Hince P, Diab S, Sharafaddinzadeh N, Chouinard S, Theoret H, Charron F, Rouleau GA. Mutations in DCC cause congenital mirror movements. Science 328: 592, 2010 [DOI] [PubMed] [Google Scholar]
- Vulliemoz et al., 2005. Vulliemoz S, Raineteau O, Jabaudon D. Reaching beyond the midline: why are human brains cross wired? Lancet Neurol 4: 87–99, 2005 [DOI] [PubMed] [Google Scholar]
- Wegmeyer et al., 2007. Wegmeyer H, Egea J, Rabe N, Gezelius H, Filosa A, Enjin A, Varoqueaux F, Deininger K, Schnutgen F, Brose N, Klein R, Kullander K, Betz A. EphA4-dependent axon guidance is mediated by the RacGAP alpha2-chimaerin. Neuron 55: 756–767, 2007 [DOI] [PubMed] [Google Scholar]
- Z'Graggen et al., 2000. Z'Graggen WJ, Fouad K, Raineteau O, Metz GA, Schwab ME, Kartje GI. Compensatory sprouting and impulse rerouting after unilateral pyramidal tract lesion in neonatal rats. J Neurosci 20: 6561–6569, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]