Abstract
The median nerve N20 and P22 SEP components constitute the initial response of the primary somatosensory cortex to somatosensory stimulation of the upper extremity. Knowledge of the underlying generators is important both for basic understanding of the initial sequence of cortical activation and to identify landmarks for eloquent areas to spare in resection planning of cortex in epilepsy surgery. We now set out to localize the N20 and P22 using subdural grid recording with special emphasis on the question of the origin of P22: Brodmann area 4 versus area 1. Electroencephalographic dipole source analysis of the N20 and P22 responses obtained from subdural grids over the primary somatosensory cortex after median nerve stimulation was performed in four patients undergoing epilepsy surgery. Based on anatomical landmarks, equivalent current dipoles of N20 and P22 were localized posterior to (n = 2) or on the central sulcus (n = 2). In three patients, the P22 dipole was located posterior to the N20 dipole, whereas in one patient, the P22 dipole was located on the same coordinate in anterior-posterior direction. On average, P22 sources were found to be 6.6 mm posterior [and 1 mm more superficial] compared with the N20 sources. These data strongly suggest a postcentral origin of the P22 SEP component in Brodmann area 1 and render a major precentral contribution to the earliest stages of processing from the primary motor cortex less likely.
INTRODUCTION
Localization of early cortical activity is of paramount interest for the understanding of physiological function of sensory pathways and to validate tests such as somatosensory evoked potentials (SEPs) in neurologic diseases and landmarks for cortical mapping in neurosurgery. There is wide agreement about the cortical origin of the earliest cortical response following electrical stimulation of the median nerve, the N20, which arises from the primary somatosensory cortex (S1), Brodmann area 3b. In contrast, there is still debate about the origin of the second response, the P22, which has been assigned either to area 4 of the primary motor cortex (M1) in the precentral gyrus or to area 1 in the crown of the postcentral gyrus.
Allison and coworkers (1989) used subdural grid recordings in a collection of patients undergoing epilepsy surgery and concluded the P22 would most likely originate from area 1 by interpreting phase reversals. Scalp mapping studies and recordings from patients with cerebral lesions like Huntington's disease and from focal infarctions suggested area 4 as generator (Abbruzzese et al. 1990; Desmedt and Ozaki 1991; Desmedt et al. 1987; Mauguiere and Desmedt 1991; Rossini et al. 1989; Töpper et al. 1993). In a recent study using electroencephalographic (EEG) dipole source analysis, Jung et al. (2008) localized the P22 dipole source in area 4 but could not rule out area 1, which also contributed to the signal.
From a neurophysiologic point of view, both options appear reasonable: it has been suggested that the proximate, anatomic connections between the sensory and motor cortex may result in cortical loops that traverse cortex in somewhat the same way that the stretch reflexes traverse spinal cord (Burke et al. 1982; Phillips 1969). However, sequential activation of different cytoarchitectonic areas within the postcentral gyrus may (Bodegård et al. 2001; Hayashi et al. 1995) or may not account for the facts (Jones and Porter 1980). Although area 3b is only one cytoarchitectonic area of four within the postcentral gyrus, it is often used as synonym for S1; other areas within the postcentral gyrus are not considered to be “primary” somatosensory (Kaas 1983). Whereas the cortical representation of tactile objects is isomorphic up to area 3b (Hsiao et al. 1993; Phillips et al. 1988), area 1, the other possible origin of the P22, maybe involved in serial processing of higher order somatosensory function like feature and edge detection and detection of movement direction of objects touching the skin (Gardner 1988; Gardner and Constanzo 1980; Hyvärinen and Poranen 1978).
From a methodological point of view, neurophysiologic localization methods are more likely to render reliable results than imaging methods given the temporal resolution necessary to distinguish between activations separated by only 2 or 3 ms. With respect to localization of the P22, EEG should be advantageous over magnetoencephalography (MEG) because the generator is oriented more radially than the N20 generator (Wood et al. 1985), similar to the P40 source of the tibial nerve SEP, which cannot be as easily detected using MEG only (Yamada 2000).
In contrast to scalp recordings, grid recordings do not suffer from the distortions or artifacts of skull and other tissues that influence EEG. However, these are still brain surface recordings, and the estimation of underlying generators from inspection of surface potential distributions only is difficult. The old debate about the origin of the P20/N20 potential fields after median nerve stimulation—one tangential versus two radial dipoles—is a prominent example of how ambiguous interpretation of surface potentials can be (Allison et al. 1991a).
Dipole source analysis (Scherg 1990, 1992) is an alternative method of source localization; it can estimate the location and direction where polarity inversion would take place in depth recordings. Because there is no unique solution, it is necessary to apply constraints to the model, which may prove as powerful tool to localize sources without invasive measures. With respect to localization of median nerve SEP sources, dipole localization has been performed so far by using surface recordings only (Buchner et al. 1994; Jung et al. 2003, 2008). We have successfully used this technique in an anatomically difficult region, the parasylvian cortex, to estimate the source of pain evoked potentials from subdural grid recordings (Vogel et al. 2003), and the results were independently confirmed by another laboratory using depth recordings (Frot and Mauguière 2003).
We now propose to use these techniques to test the hypothesis that the P22 arises from Brodmann area 4.
METHODS
Subjects
This study was carried out in four patients (3 female, 1 male, 21–51 yr old) who had subdural grids implanted for surgical treatment of medically intractable seizures. Although typically more than one grid was implanted in the subdural space to identify the seizure onset, the recordings described here were derived from one 8 × 8 64-channel grid in each patient that was implanted over the medial frontoparietal convexity. These subdural electrode grids were implanted over the left hemisphere in three patients (patients p#01, p#03, and p#04) and over the right hemisphere in one patient (p#02). The following brain areas were the regions of seizure onset: Left medial and (dorso-)lateral prefrontal cortex (p#01 and p#03), right posterior parietal medial cortex (p#02) and right temporal lobe (p#04).
Neurological examination, including a standard sensory testing protocol (Lenz et al. 1993), disclosed no abnormality in any patient, and structural brain magnetic resonance images were normal. All seizure medications were discontinued for 36 h after the implantation of the electrodes. Therefore all subjects had substantial blood levels of these drugs at all points relevant to this study. No subject had any medical or psychiatric condition other than epilepsy or took mediations other than anti-epileptic drugs. The protocol was approved by the Institutional Review Board of the Johns Hopkins University, and all patients signed an informed consent.
Stimuli and electrocorticographic recording
SEPs were recorded by electrically stimulating the median nerve at the wrist contralateral to the side of grid implantation with an interstimulus-interval of 213 ms. The duration of the constant current square pulses was 300 μs, and the intensity was set at ∼15–20% above the motor threshold. Cortical electrical activities were recorded from subdural grid electrodes [electrocorticogram (ECoG)]. The electrodes consisted of platinum-iridium circular electrodes (2.3 mm diam) embedded in a transparent silastic sheet at evenly spaced 1-cm center-to-center intervals (Ad-Tech, Racine, WI). ECoG from subdural grid electrodes were amplified and band-pass filtered at 30–300 Hz with Grass amplifiers (12A5, Astro-Med, West Warwick, RI). All ECoG signals were referenced to a single intracranial (subdural) reference electrode chosen for its inactivity and distance from the active electrodes. The amplified ECoG signals were digitized at 1,000–2,500 Hz and recorded to computer hard disk along with stimulus markers for subsequent off-line analysis.
ECoG averaging and measurement of surface potentials
For each averaged potential, 2,018–5,200 responses were averaged time-locked to the stimulus, using a time window of 120 ms with a 20-ms prestimulus period. For each subject, averaged waveforms were obtained after confirming the reproducibility of results from two recording sessions. Peak latencies and amplitudes were measured from reproducible, averaged waveforms. Peak amplitudes were measured from the baseline value, which was defined as the averaged value during the prestimulus period. All latencies reported in this manuscript were measured as peak latencies.
Computer tomography and magnetic resonance image normalization and matching
Each patient underwent a structural magnetic resonance imaging (MRI) scan prior to grid implantation. After the implantation of the electrodes, the correct localization of the grids was verified by a computer tomography (CT) scan. To perform the EEG source analysis using the information of the individual brain shape, electrode positions had to be determined relative to the individual brain; hence a common coordinate system was needed for the CT and MRI scans (Talairach and Tournoux 1988) using Brainvoyager. Center of the Talairach x,y,z coordinate system is the anterior commissure. x: medial-lateral direction, negative values for the left hemisphere; y: anterior-posterior direction, positive values anterior; z: superior-inferior direction, positive values more cranial. Matching of the MRI and CT scans was achieved with the help of anatomical landmarks visible in both scans. These fiducials were the nasion, the inion and the preauricular points. On the basis of individual electrode coordinates in Talairach space, Brain Electrical Source Analysis software (BESA 5.1.8, Scherg 1992) calculated the best-fitting ellipsoid of each subject. A homogenous sphere served as head model for the source analysis of the evoked potentials recorded from the brain surface.
Dipole source analysis
After import of the averaged SEP data into the source analysis software, data were re-referenced off-line versus average reference for calculation of the global field power (GFP = spatial SD as a function of time) (Strik and Lehmann 1993). GFP is a measure of spatial variance, which collapses information from all scalp electrodes at each time point independent of the location of a particular activation maximum and thus yields a reference-free measure of the component structure. According to the GFP structure of the individual patients, a time window for the analysis of the N20 and P22 components was chosen. Given the expected proximity between the source localization of these two components and the temporal overlap of their electrical activity, we decided to start out with the fitting procedure using a regional source (RS). A regional source is a set of three dipoles with the same location but mutually perpendicular orientations representing electrical activity in a small volume of cortex irrespective of the net dipole orientation. In the fitting procedure, which is purely data-driven, a regional source has only 3 df making this a robust analysis without the possible bias of a set of constraints that may be needed if multiple dipoles are fitted simultaneously.
After the location was fitted, orientations for the N20 and P22 dipole were fitted according to the individual activation peaks. Because the third dipole of the regional source had no relevant activity left after these orientation fits, it was deleted from the model. The locations of remaining two components (N20, P22) were finally fitted separately keeping the orientations from the previous step, and the Talairach coordinates obtained after the final fit were taken as estimated source locations for the N20 and P22 dipoles. The quality of a fit can be estimated by the “goodness of fit” (GoF), which is the amount of data variance explained by the dipole model. The procedure is described in detail in previous publications (for tibial nerve SEP, see Baumgärtner et al. 1998; for median nerve SEP, see Jung et al. 2003) and is shown in the results for one example subject (see following text, Fig. 3).
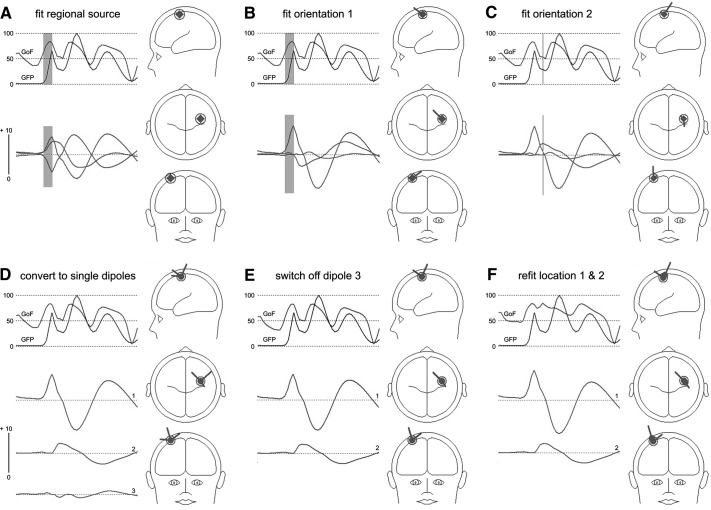
Fig. 3.
Brain Electrical Source Analysis software (BESA) strategy of source fitting (patient p#02). Top left: GFP and goodness of fit (GoF); bottom left: source activities (in nAm) over time; right: head model views with dipole sources. The time window comprises 5 till 50 ms post stimulus. A: a regional source (RS), consisting of 3 orthogonally bound dipoles, is fitted for best location and rotated to activity maximum within the N20 time window (B). C: orientation fit of 2nd RS component at peak of P22. D: conversion of RS to 3 single dipoles. E: the 3rd dipole in C does not contribute to the solution and is switched off. F: the remaining dipole 1 is fitted for location at N20 onset to peak (orientation fixed), dipole 2 is fitted for location at P22 onset to peak (orientation fixed). Dipoles 1 and 2 are sufficient to explain the data variance of this time window and represent the tangentially oriented N20 and radially oriented P22 components with clearly separated dipole source activities. Further details are given in the text (see results).
RESULTS
Grid locations
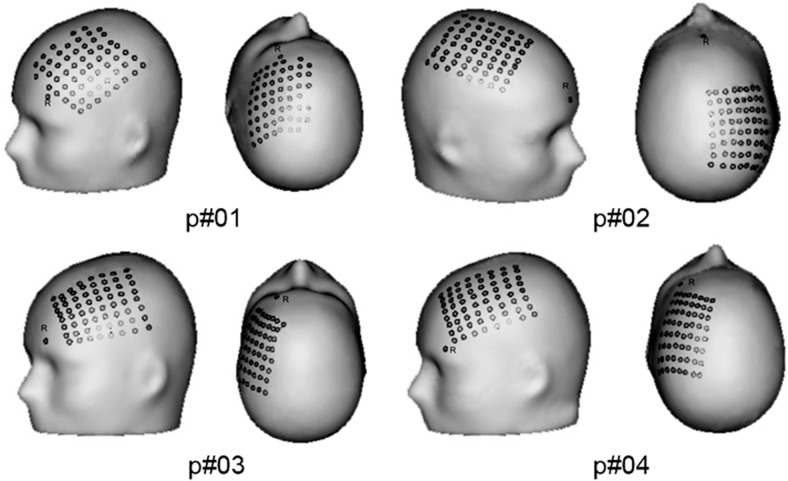
To get an impression of the size and location of the individual 8 × 8 grids, Fig. 1 shows the three-dimensional projection of the grids on the head surface of the four individual patients. According to the expected seizure focus, the major part of the grids covered the frontal lobe. In three patients, the grids extended at least one full vertical row of electrodes posteriorly to the central sulcus, including one patient (p#01) where the grid was angulated. In one patient (p#04), only five electrodes of the lower part of the grid were posterior to the central sulcus. “R” indicates the common reference. Missing electrodes (4 channels in p#01) indicate bad channels that were excluded from further analysis.
Fig. 1.
Subdural grid locations for 4 patients with views from side and from top. Note that patient p#02 had the grid on the right hemisphere. R, the reference electrode.
Surface ECoG signals and GFP
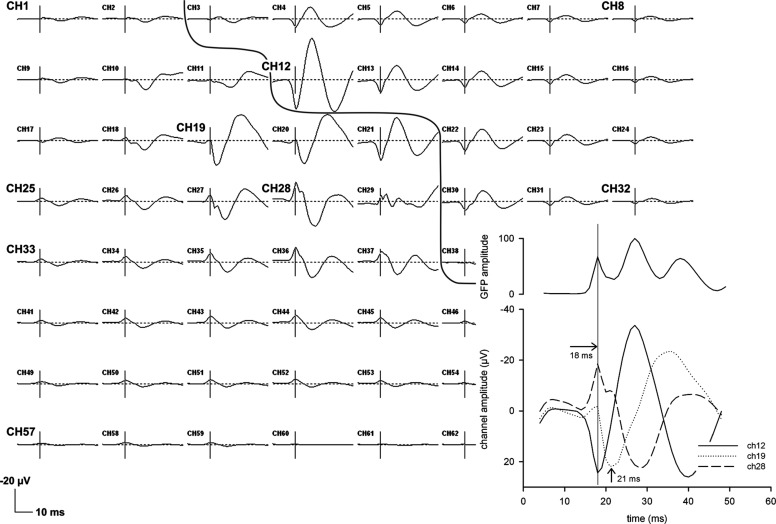
Figure 2 gives an example of the averaged SEP in one patient (p#02). Among channels 28, 20, and 12 (vertical direction on the grid), a clear phase reversal at 18 ms can be seen. The fact that the phase reversal occurs at the same time (18 ms) can be examined in the inset (channels 12 and 28). At that time, potentials are negative toward posterior positions (channels on the left/ inferior part of the grid) and positive toward anterior positions (channels on the right/ upper part of the grid), together forming the dipolar field of the N20/P20. The P22 has its maximum amplitude at electrode 19 and is separate from the N20/P20 potential both in space and time (see inset). A phase reversal cannot be seen, suggesting a single dipole with radial orientation as concluded from the surface potential distribution.
Fig. 2.
Original recording (patient p#02) of the 64 subdural electrodes (time window: 5 ms till 50 ms post stimulus, negativity upward) with respect to a common reference electrode (CH 65, outside the sketch). The arrangement of channels reflects the real positions within the grid. Because the grid was implanted on the right hemisphere, channels further on right represent anterior positions, channels further on the left represent posterior positions. The bended line passing through the center of the grid represents the central sulcus as determined intraoperatively. The marker is set at 18 ms, which is the individual peak latency of the N20 component. In this patient, the largest N20 phase reversal is between channels 28 (negativity) and 12 (positivity), the P22 maximum amplitude is at channel 19 (at 21 ms). Inset: the details are enlargened where latencies and polarities of these channels and the global field power (GFP) can be directly compared.
It is important to note, that, even though the data derived from subdural recordings, the distribution of the dipolar field of the N20 can be inspected almost across the entire grid (Fig. 2), whereas, in this patient, the P22 can only be detected at two electrodes (19 and 20; also see lower part of Fig. 6A), suggesting a radial dipole as source of this component.
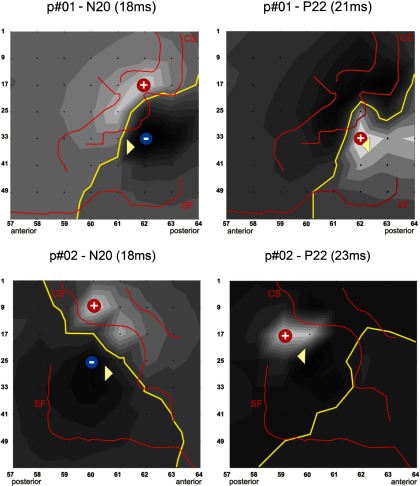
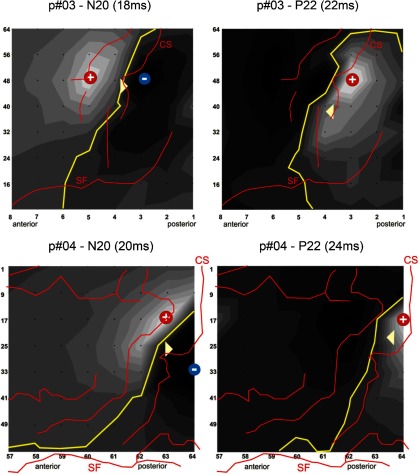
Fig. 6.
Estimation of source locations (triangles) in relation to the grid electrodes (black dots) on a flattened grid view and individual anatomy (red lines) as obtained from stimulation mapping and intraoperative photo shots for all 4 patients. Left: N20; right: P22. CS, central sulcus; SF, sylvian fissure; yellow line, line of phase reversal; +/−, sites of maximum polarity/amplitude; dark gray, negative; light gray, positive fields on the scalp. At the time of the N20 peak amplitude, clear dipolar fields across the central sulcus can be identified in all patients. For the P22, the maximum scalp positivity was located posterior to the central sulcus in 3 patients and on the central sulcus in 1 patient (p#04), in whom the lack of electrodes behind the central sulcus prevented localization of the source further posterior.
Source analysis
The fitting process with the regional source (RS) is shown for one subject (p#02) in detail in Fig. 3. As the first step, the RS (circle diamond symbol) is introduced into the head, and the location is fitted in the time window (onset-to-peak) of the N20 component, as shown in Fig. 3A. Two observations can be made. First, the RS was localized near the central sulcus, contralaterally to the stimulated hand (heads on the right of each panel). Second, the GoF, indicator of how much of the data variance is explained by the model used, paralleled the course of the GFP with its three major peaks visible in the whole time window shown (5 ms until 50 ms post stimulus, top left of each panel, fit window shaded in gray). The peak values of the GoF were between 75 and 83%, demonstrating that the RS explained almost the entire electric activity during the first 50 ms after stimulation. Without any fit of the orientations, the three single dipoles, which constitute the RS, show an overlapping activity pattern over time (source activity in nAm, shown on the bottom left).
The orientation fit for the first (N20) component in Fig. 3B resulted in a tangential orientation of one RS component picking up this activity with the positivity (flag of the dipole) pointing in anterior and slightly medial direction. Note that this procedure has no effect on the GoF because the location and composition of the RS is unchanged with its three dipoles in orthogonal orientations. However, the effect of the orientation fit resulted in a separation of the dipole activities because all of the activity into anterior-medial direction was now picked up by only one component. Observation of the two remaining dipole activities showed a second dipole activity exhibiting one peak (downward deflection) shortly after the large N20 component, whereas activity of the third dipole was very low. In C, a second orientation fit was performed on the time of the peak activity of the second dipole (cursor) while keeping the orientation of the first dipole (note: in this subject, there was no P22 peak in the GFP). As a result, the other two components showed a more radial orientation, the stronger component pointing upward and slightly posterior; the weak third component can hardly be detected as dipole in the head views.
In D, the RS was transformed into three single dipoles still bound together. The separate source activities and the different dipole orientations can now be examined in more detail. Dipoles 1 and 2 picked up an early, tangential component and a component with a different time course of activity (peaking slightly later than activity of dipole 1) and radial orientation. The third dipole of the former regional source was almost totally inactive. Because the third dipole contributed only marginally to the explanation of the data variance, it was deleted from the model in E, leaving two sources with clearly separated time courses of activity; dipole 1 picked up activity of the N20 component, and dipole 2 picked up activity of the P22 component. Deletion of dipole 3 lead to a decrease in the GoF of only 1.2%, and changes in the GoF between D and E can hardly be detected. The two remaining single dipoles, one with a tangential orientation (N20), the other with a radial orientation (P22) were then separately re-fitted for location (keeping the orientation fixed). The final result is shown in Fig. 3F, with two single dipoles for the N20 and P22 components with slightly different locations and clearly different orientations. Dipole source analysis of the first two cortical components as described in the preceding text yielded very similar results in all four patients.
The GFP, as derived from all 64 channels, was the basis for the source analysis. In Fig. 4, the time courses of the GFP (as “EEG signal”) and the GoF (quality criterion for data explanation) is shown in parallel for all subjects. Within the time window used for the analysis, the GoF was ∼80% [79.3 ± (SD) 2.3] for each of the four patients. The marked time window contains the onset of the N20 till peak of the P22. In one patient (p#02), the N20 GFP amplitude exceeded the P22; in the other three cases, it was vice versa. Only in one patient (p#04) were two clear peaks visible in the GFP, whereas in the other cases, one of the peaks appeared as a “shoulder” adjacent to the other, indicating the temporal overlap of the two components. Peak latencies of surface channels and GFP peaks of all subjects are given in Table 1 (left columns).
Fig. 4.
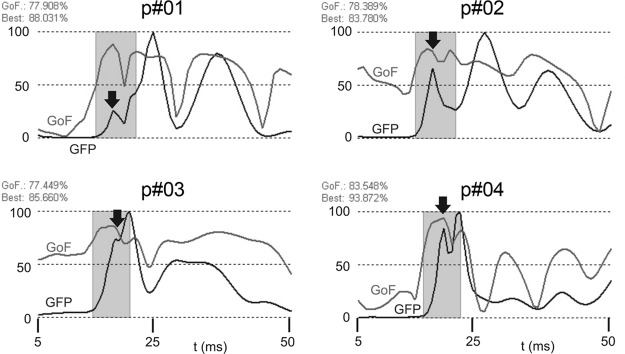
GFP (black) and GoF (gray) of the 4 patients. The time window for the fit procedures includes onset of the N20 to peak of the P22 (total time scale 5 till 50 ms post stimulus; the electrical stimulus was applied at t = 0). Eighty percent of the data were explained by the BESA model (GoF). Arrows indicate the 1st cortical component (N20).
Table 1.
Peak latencies
| Surface Channels, ms |
Global Field Power, ms |
Source Activity, ms |
||||
|---|---|---|---|---|---|---|
| Subject | N20 | P22 | N20 | P22 | N20 | P22 |
| p#01 | 18 | 21 | 18 | 21 | 18 | 21 |
| p#02 | 18 | 21 | 18 | n.p. | 18 | 21 |
| p#03 | 18 | 21 | 18 | 21 | 18 | 20 |
| p#04 | 20 | 22 | 20 | 23 | 20 | 22 |
| Average | 18.5 ± 1.0 | 21.3 ± 0.5 | 18.5 ± 1.0 | 21.7 ± 1.2 | 18.5 ± 1.0 | 21.0 ± 0.8 |
Averages are shown as means ± SD. n.p., no peak present.
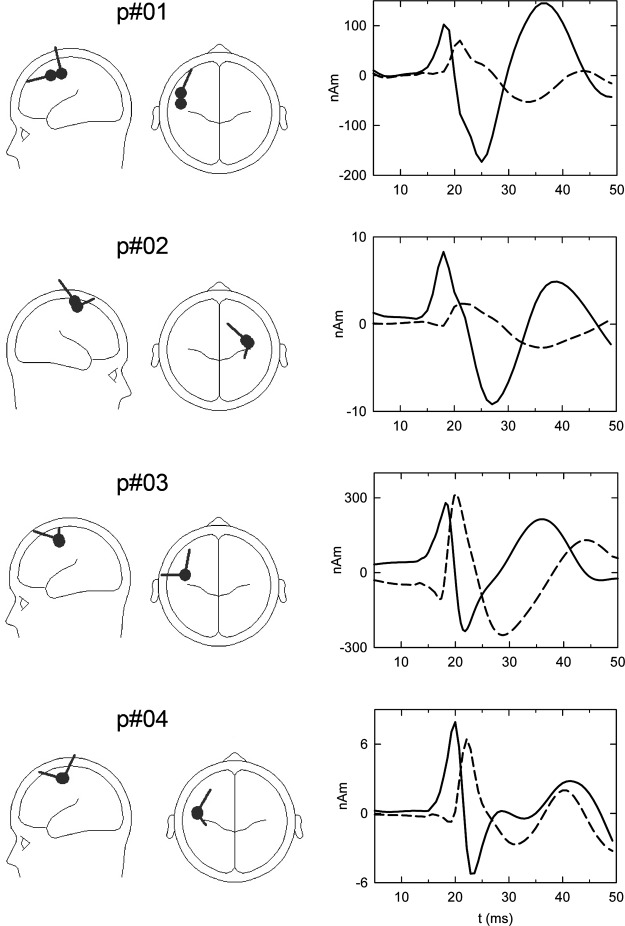
The source analyses for the four patients yielded a tangential source pointing anteriorly (frontal positivity in scalp distribution) in all subjects, and a radial source, almost strictly radially in two subjects (p#01 and p#04), slightly posteriorly or laterally in the remaining two, as displayed in the model heads in Fig. 5. The time courses for the dipole activities are displayed on the right side. The activity with the first peak (—) belongs to the tangential dipole in the heads, whereas the activity with the later peak (- - -) belongs to the radial dipole. The different time courses show good separation of the two components in each patient. The delay between the N20 and P22 source activities was 2 or 3 ms (cf. Table 1 for latencies of surface and depth activities). As to the dipole localizations, the radial (P22) source was situated either on the same spot as the tangential (N20) source or further posteriorly, in no case it was found anterior to the tangential source. On average, the P22 source was located 6.6 mm posterior to the N20 source. Individual and average coordinates of the source localizations as well as differences are given in Table 2.
Fig. 5.
Results of the individual dipole source analyses of the 4 patients. Left: standard head views showing the dipole localizations for the N20 (tangential) and the P22 (radial) sources. Right: corresponding source activities over time in a time window ranging from 5 ms till 50 ms post stimulus for the N20 (—) and P22 (- - -) dipole sources. Note the clear separation of the source activity waveforms and the close proximity of the sources in the head views. Compared with the N20 sources, P22 sources are localized further posterior or on the same y coordinate. (Note in all cases a more tangential component representing current flow can presumably be attributed to area 3b, and the radial component can be attributed to area 1).
Table 2.
Dipole coordinates
| p#01 | p#02 | p#03 | p#04 | Average | |
|---|---|---|---|---|---|
| N20 | |||||
| x | −51.0 | 33.2 | −41.6 | −49.1 | 43.7 ± 8.1* |
| y | 10.9 | −6.9 | −4.3 | −7.6 | −2.0 ± 8.7 |
| z | 51.2 | 60.3 | 54.0 | 58.5 | 56.0 ± 4.2 |
| P22 | |||||
| x | −51.0 | 36.3 | −41.5 | −49.7 | 44.6 ± 7.0 |
| y | −7.0 | −11.5 | −4.7 | −11.0 | −8.6 ± 3.3 |
| z | 52.1 | 67.1 | 50.7 | 58.4 | 57.1 ± 7.5 |
| Diff. | |||||
| x | 0.0 | −3.1 | 0.1 | −0.6 | −0.9 ± 1.5 |
| y | 17.9 | 4.6 | 0.4 | 3.4 | 6.6 ± 7.8 |
| z | −0.9 | −6.8 | 3.3 | 0.1 | −1.1 ± 4.2 |
| 3D distance | 17.9 | 8.8 | 3.3 | 3.5 | 8.4 ± 6.8 |
Averages are shown in mm, Talairach space, as means ± SD. Diff., difference in mm between N20 and P22 source coordinates;
for x coordinates, only absolute values were averaged.
Back projection of the dipoles on the recording grids (Fig. 6) demonstrated the following. 1) The N20 source (white triangle) was localized ∼0.5–1.0 cm posterior to the central sulcus in 2 cases (p#01 and p#02) and located on the central sulcus in the other two cases (p#03 and p#04). 2) Except in one patient (p#03), were N20 and P22 sources were localized both on the central sulcus, the P22 was localized on average 6.6 mm further posterior than the N20 in the other three patients (Fig. 6, Table 2). These findings together strongly suggest a P22 source in area 1 because of the posterior location and the radial orientation. The distance of 6.6 mm (on average) further provides evidence for area 1 rather than areas further posterior like e.g., areas 2 or 5. P22 sources were on average 1 mm more superficial compared with N20 sources.
In patient p#04 (Fig. 6B), The N20 source was localized on the central sulcus. The P22 source was localized further posterior than the N20 source; however, because the CS was bending away further posteriorly in that region, the P22 source was projected on the precentral gyrus. In our view, this is very likely to be an artifact (mislocalization) for both sources because the edge of the grid was located anterior to the central sulcus in the upper region (on top of area 4), and the sources cannot be localized outside—in this case posteriorly to—the grid. Interpolation of the spline map to the right side of the grid would very likely yield a maximum of the P22 positivity, and thus the source localization, further to the right of the sketch, which is posteriorly to the central sulcus.
Because we had expected that at least in some of the subjects the P22 would be located anterior to the generator of the N20 (Jung et al. 2008), we actively tested the possibility of a relevant contribution of a precentral source. We manually shifted the P22 dipole location found in our analysis further into anterior positions. In each subject, the P22 dipole was shifted 1–3 cm further anterior (in y-coordinate direction) while the GoF was monitored. This analysis yielded only decreases in the GoF. These were already evident, when sources were shifted anterior by only 1 cm and became worse at a distance of 2 or 3 cm anterior to the original position, where the GoF became similar to the value when the P22 was totally removed from the model (“switched off”; see Fig. 7) and only the N20 source was left active. To avoid any possible bias due to the fixed orientation of the P22 dipole, we also fitted its orientation freely after each shift. We further examined the combined time window of both N20 and P22 sources and the time window of just the P22 (shown in Fig. 7) but did not find an increase in the GoF in any case.
Fig. 7.
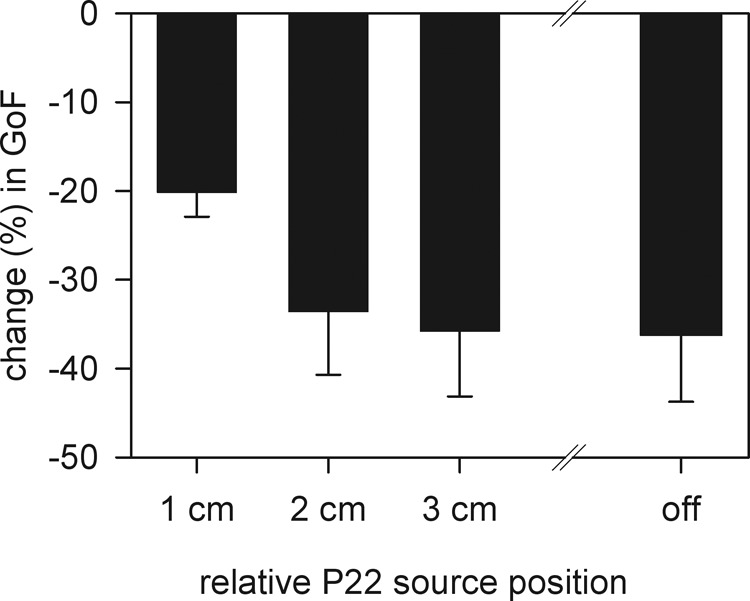
Effect of shifting the P22 source further anterior on the GoF. From the position of the P22 as found in the individual source analyses, the P22 dipole was shifted into anterior direction (precentral positions) by 1–3 cm. The GoF was reduced by 20% to 37% from its original value in the fit window for the P22 source. The condition “off” means that the P22 source was deleted from the model. Note that all GoF changes were negative. Error bars: SE.
DISCUSSION
The main findings in this study using median nerve stimulation together with subcortical grid recording and dipole source analysis are that the N20 source was localized posterior to or on the central sulcus and, relative to that location, the P22 was localized on average 7 mm further posterior. This direction and distance together with the radial orientation as found by the dipole source analysis makes the origin of the P22 highly likely to derive from the crown of the postcentral gyrus, in area 1. Active testing of the hypothesis that the P22 generator may be generated in area 4 yielded negative results.
Methodological considerations
GENERAL.
Source analysis from subdural grids is a refinement of dipole source analysis techniques from scalp recordings–bringing improvement in terms of accuracy of the localization results. This method has been validated in principle in the study of Vogel et al. (2003), where the cortical sources of auditory versus nociceptive stimuli were determined below and above the sylvian fissure in patients with implanted grids. In the present study, the requirement for spatial resolution was higher because it was expected that the median nerve N20 and P22 sources were located close to each other near the central sulcus. There is now general agreement about the generator of the N20, a tangential source with frontal positivity and parietal negativity that has been assigned to Brodmann area 3b in the anterior wall of the postcentral gyrus (e.g., Allison et al. 1991a; Arezzo et al. 1979; Deiber et al. 1986; Desmedt et al. 1987; Jung et al. 2003; Kakigi 1994; McCarthy et al. 1991; Tiihonen et al. 1989; Valeriani et al. 1998, 1999; review by Lee and Seyal 1998), the classical S1 (Kaas et al. 1983). The fact that the same localization was found in this study can be interpreted as a positive validation of the method of source analysis from subdural grids in this specific context. In agreement with previous studies, the N/P20 peak positivity was found to be anterior and the peak negativity posterior to the central sulcus in all subjects, and the line of phase reversal followed the central sulcus in most parts.
TECHNICAL.
Source analysis results are dependent from a vast number of factors that have differential impact depending on the recording setup, the head model and the algorithm chosen (Michel et al. 2004). For average reference recordings, source localization offsets have been described (“polar average reference effect”) when the electrode density was low and only the upper part of the head was covered (Junghöfer et al. 1999). In our recording, we had a high electrode density over the frontal (and partly parietal) lobe of one hemisphere without coverage of the contralateral part of the brain. These factors should ameliorate and deteriorate the results at the same time–the net effect in numbers is hardly possible to be appraised. In one of our patients (patient #4: Fig. 6B, bottom right), this effect, however, could be the reason why the P22 source was “dragged” toward the inner grid area.
Localization differences between a sphere, as used in our study, and realistic head models (boundary or finite element models; BEM, FEM) range between a few millimeters up to a centimeter and even more. This has major impact on source locations in deep brain regions and the temporal lobes, whereas differences in spherical parts of the brain–like in the region investigated here–appear to be minor (2–4 mm) (Buchner et al. 1995; Fuchs et al. 2002; Kristeva-Feige et al. 1997; van't Ent et al. 2001; Yvert et al. 1997; review by Fuchs et al. 2007).
More recently, the impact of anisotropy of the skull and brain tissue on source estimation has come to the attention of several researchers. Whereas the skull anisotropy had considerable influence with mislocalizations of 10 mm on average, white matter anisotropy had a negligible effect (2 mm displacement, <10° angle deviations) (Hallez et al. 2009; Lee et al. 2009; Wolters et al. 2006) and adds only to an improved resolution, if all other factors allow a resolution of ≤1 mm (Güllmar et al. 2010), which is probably not the case in our recordings.
Assuming that the effects described in the preceding text should affect the two neighboring N20 and P22 sources in a similar, systematic way, we still feel content our statement of a postcentral origin of the P22 is valid, because the P22 was always posterior to the N20 source, and the N20 generator is surely located behind the central sulcus, which was our anatomic anchor structure.
An important question is whether cortical reorganization as potential consequence of epilepsy may be a confounding factor for the source localizations. In principle, cortical reorganization can have an impact on dipole localizations, especially when large lesions are present that may directly displace the eloquent area or lead to mislocalization due to volume conduction changes (Akhtari et al. 2010; Ossenblok et al. 2003; Vatta et al. 2002). However, source locations determined following tactile hand stimulation in young patients with epilepsies where the focus was located in the central region but without any structural lesion were not significantly different from source locations in normal/asymptomatic hemispheres (Bast et al. 2007). Because the patients investigated in the present work did not have lesions in the MRI, and because the epileptic focus was distant from the central sulcus, a relevant impact of reorganization on the source analysis results appears unlikely.
Supporting data from grid recordings in humans and monkeys
The localization of the P22 source as postcentral generator confirms the suggestion of Allison. The conclusion of a postcentral origin of both N20 and P22–the so-called tangential + somatosensory radial model (Allison et al. 1991a)—was based on the following observations.
First, in two patients undergoing epilepsy surgery, both with normal SEP responses in the 20–35 ms range prior to surgery, one was operated on S1 with excision of the hand area of the somatosensory cortex, the other had an excision of the hand area of the motor cortex. After removal of the hand area of the somatosensory cortex, all median nerve SEP responses in the time range between 20 and 35 ms disappeared, whereas in the other patient they remained present (Allison et al. 1991b).
Second, excision experiments performed in monkeys with analysis of the human N20/P22 homologues N10/P12 yielded the same results. In addition, removal of smaller cortex volumes from the crown of the postcentral gyrus only (presumably area 1) resulted in loss of P12 with the N10-P10 component still present (Allison et al. 1991b).
Third, Buchner et al. (1996) found the P22 abolished in a patient with a lesion in the crown of the postcentral gyrus.
Clinical data challenging the finding of a postcentral P22 generator
A challenge to the assumption that the generator of the P22 is located posteriorly to the motor cortex comes from the lesion studies by Mauguière and Desmedt (1991). In a selection of patients with cerebral lesions of various origins, these authors (Mauguière and Desmedt 1991; see also Mauguière et al. 1983) found a correlation between amplitudes of the precentral scalp component P22 and motor function and between postcentral N20 scalp amplitude and sensory function, and a differential affection of the two components by precentral and postcentral lesions. Whereas slowly evolving pathologies in the central region appeared to leave N20, P22, and later components relatively unchanged (probably due to cortical reorganization, cf. case 22), sudden events like stroke were associated with the changes described in the preceding text. In this clinical pioneer study, the authors used a limited number of electrodes on the scalp, and termed both frontal P20 (the positive counterpart of the parietal N20) and P22 as one component (P22) (cf. Fig. 1 in Mauguière et al. 1983: the wide spread frontal P20 is visible at electrodes 5, 7, and 8, whereas the slightly later occurring P22 is only visible at electrode 3 after right median nerve stimulation). Interpretations of differences in amplitudes of SEP components between one lesioned hemisphere and the corresponding unaffected hemisphere is rendered difficult through the fact that amplitudes can be considerably asymmetric in healthy subjects already, both between pre- and postcentral components of the same hemisphere and between hemispheres as well (Allison et al. 1991a; Jung et al. 2003; Sörös et al. 1999; Theuvenet et al. 2005). Instead of interhemispheric comparison of SEP components, a better control condition may be the same hemisphere that can be examined in patients before and after elective surgery. In these rare cases, the findings supplied strong evidence for a P22 generator behind the central sulcus (cf. Allison 1991b).
The impact of a large lesion on conductivity properties of the brain and skull may also be responsible for possible distortions or cancellations of electrical fields on the scalp that do not properly reflect functionality of the brain tissue. In a study with parallel recording from subdural grids and scalp electrodes in a realistic head model calculated using finite elements, current source density mapping of the surface showed distortions of the electric field above the grid, especially at the edges (Zhang et al. 2008) demonstrating that even only little to moderate inhomogenieties induce changes that may result in localization errors, especially when relying on scalp channels only.
“Long-loop” reflexes
Whatever the distinct contribution of cutaneous and muscle afferents to cortical potentials, the P22 generator is of particular interest because it may be part of the pathway involved in long latency reflexes (Desmedt 1978). In monkey, motor cortical pyramidal cells responsive to wrist movements generate spikes, which lead to facilitation of electromyographic (EMG) activity in the stretched muscles as has been identified by analyzing spike triggered EMG activity (Cheney and Fetz 1984). Furthermore, selective lesions of monkey motor cortex lead to decreases in long latency activity in muscles acting across the primate hand, although not in other species or muscles (Lenz et al. 1983a,b; Ghez and Shinoda 1978). These findings strongly suggest that somatic sensory inputs from low threshold, rapidly conducting afferents form a functionally significant input to motor cortex. However, the route of short latency somatosensory input from the periphery to the motor cortex is unclear because the part of the thalamus receiving input from the dorsal column nuclei does not project directly to motor cortex and the areas of sensory cortex receiving this input do not project to motor cortex (Jones and Porter 1980; Tracey et al. 1980; Wiesendanger and Miles 1982).
We could not identify the motor cortex as an area giving substantial contribution to the generation of the P22 component even though we looked for it by shifting the dipole further anterior. Therefore we can only conclude that its contribution is minor, possibly as part of the remaining 20% data variance not explained by our model or because the motor cortex is activated later.
Contradictive findings of dipole source analyses from scalp recordings
Using dipole source analysis in normal brains of healthy subjects, a previous study (Jung et al. 2008) has determined area 4 as probable generator site of the P22; this is in contrast to our present findings with the P22 surface maximum positivity and dipole localization strictly posterior to or at least directly on top of the N20 source. A possible reason for this discrepancy could be that the results obtained by Jung et al. (2008) derived from scalp recordings using 32 channels as opposed by recordings from 64 channels from the brain surface used in the present study, the latter offering a (locally) higher spatial resolution with less volume conduction that may blur signals on the head surface. A notable difference is the orientation of the P22 dipoles which is more radial in the present study as compared with the results found by Jung et al. (2008). Assuming that the positive field originating from a postcentral P22 generator on the scalp surface was not strictly radial, but pointing more anteriorly, the projected field on the scalp would merge with the frontal field of the N20 dipole (the P20) typically still present when the P22 shows its maximum activity on the scalp. This overlap of scalp potentials could lead to a localization error toward frontal regions because the N/P20 is located further anterior and because changes in conduction properties between the different tissues could further distort the electrical field in the same direction. The inverse modeling may erroneously lead to a back-projection of the source toward the gyrus anterior to the central sulcus (e.g., area 4), like it is the case for the tibial nerve P40 source across the interhemispheric fissure, a phenomenon called paradoxical lateralization. However, in case of the P40 scalp surface field on the hemisphere ipsilaterally to stimulation, the dipole source was correctly assigned to the contralateral hemisphere (Baumgärtner et al. 1998).
Depth recordings
Further evidence for a postcentral origin of the P22 comes from the recordings by Barba et al. (2004, 2008). In patients with depth recordings in the postcentral gyrus, a small P22 can be inspected as superficial positive dip 3 ms following the larger N20 component with a depth negativity, which is, however, larger and wider than the smaller superficial component. Although this could be interpreted as phase reversal from surface to depth, the asymmetric structure may leave doubt whether the two components are generated by a single radial dipole in the crown of the postcentral gyrus.
In addition to the clinical observations by Mauguière et al. (1983) and Mauguière and Desmedt (1991), depth recordings in epilepsy patients by Balzamo and coworkers (Balzamo et al. 2004) may point to area 4 as early generator following median nerve stimulation. In one patient with recording in the postcentral gyrus, they found two negative components at 22 and 26 ms that they assigned to areas 3b and 3a. In a second patient with a single electrode track through the omega hand region, a cortical landmark in the hand areas of primary motor cortices (Yousry et al. 1995), they identified a phase reversal of the N/P20 across the omega (dipole in area 3b), followed by a large negativity in the anterior wall of the central sulcus, e.g., area 4(?) 6 ms later. Assignment of this negativity to area 4 is not easy to explain because a primary source from that region should have positive polarity.
Possible sources could be the negativity from underneath the M1 cortex wall, primary muscle afferent input to area 3a, or a far spread negativity from underneath the crown or S1 (less likely). A spread of S1 activity from area 3b is unlikely as it should be positive anterior to the central sulcus. In a third patient, a precentral lateral/superficial positivity is recorded with a deep and steep phase reversal at 25 ms. If these results point to area 4, one should ask why it is not seen in surface recordings. An explanation could be the depth inside the central sulcus below the dipole in area 3b, the activity of which may act as a cover from scalp sensors. Furthermore, the latency of this component does not really fit with the surface time of the P22 or the temporal delay of 5 ms from the preceding N20 instead of 2 or 3 ms. A putative generator in area 4 in the depth should yield a dipolar field on the surface rather than a radial field, which is evident in our recordings from the brain surface.
From single wires it is hard to conclude on exact source locations without steep phase reversals because even depth recordings can show considerable volume conduction. For example, projections from sources in the postcentral gyrus can be visible up to the presupplementary motor area far away in the frontal lobe (Barba et al. 2005; Kanovsky et al. 2003). In the studies mentioned in the previous paragraph, the only consistent finding is the tangential generator in area 3b. Other potentials have been identified, the origin of which is uncertain. There is high variability between the findings from single patients and from depth recordings: Each subject yields slightly different results. This may be due to the different extent of the lesions and/or variability in cytoarchitectonic anatomy in the region of the central sulcus (Geyer et al. 1999, 2000).
Conclusion
Consistent findings all point to area 1 as generator site for the P22: our subdural recordings with postcentral positivity on the surface and posterior dipole localization are in accordance with subdural recordings in other patients (Allison et al. 1991a; Buchner et al. 1996; Sonoo et al. 1991) and monkeys (Hayashi et al. 1995; McCarthy et al. 1991), controlled lesion studies in humans and monkeys (Allison et al. 1991b), and dipole localization studies in humans (Buchner et al. 1991) as well as dipole localization analyses from high-density electrode grids in monkeys (Hayashi et al. 1995). The differential assignment of the generators of these early cortical potentials (N/P20 and P22) to subdivisions of the postcentral gyrus opens up the possibility of separate functional testing of Brodmann areas 3b and 1.
GRANTS
This study was supported by National Institute of Neurological Disorders and Stroke Grant NS-38493 to F. A. Lenz and Deutsche Forschungsgemeinschaft Grant Tr236/13-4.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank M. Dettling for expert help with graphics work.
REFERENCES
- Abbruzzese et al., 1990. Abbruzzese G, Dall'Agata D, Morena M, Reni L, Favale E. Abnormalities of parietal and prerolandic somatosensory evoked potentials in Huntington's disease. Electroencephalogr Clin Neurophysiol 77: 340–346, 1990 [DOI] [PubMed] [Google Scholar]
- Akhtari et al., 2010. Akhtari M, Mandelkern M, Bui D, Salamon N, Vinters HV, Mathern GW. Variable anisotropic brain electrical conductivities in epileptogenic foci. Brain Topogr 23: 292–300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison et al., 1989. Allison T, McCarthy G, Wood CC, Darcey TM, Spencer DD, Williamson PD. Human cortical potentials evoked by stimulation of the median nerve. I. Cytoarchitectonic areas generating short-latency activity. J Neurophysiol 62: 694–710, 1989 [DOI] [PubMed] [Google Scholar]
- Allison et al., 1991a. Allison T, McCarthy G, Wood CC, Jones SJ. Potentials evoked in human and monkey cerebral cortex by stimulation of the median nerve. A review of scalp and intracranial recordings. Brain 114: 2465–2503, 1991a [DOI] [PubMed] [Google Scholar]
- Allison et al., 1991b. Allison T, Wood CC, McCarthy G, Spencer DD. Cortical somatosensory evoked potentials. II. Effects of excision of somatosensory or motor cortex in humans and monkeys. J Neurophysiol 66: 64–82, 1991b [DOI] [PubMed] [Google Scholar]
- Arezzo et al., 1979. Arezzo J, Legatt AD, Vaughan HG., Jr Topography and intracranial sources of somatosensory evoked potentials in the monkey. I. Early components. Electroencephalogr Clin Neurophysiol 46: 155–172, 1979 [DOI] [PubMed] [Google Scholar]
- Balzamo et al., 2004. Balzamo E, Marquis P, Chauvel P, Régis J. Short-latency components of evoked potentials to median nerve stimulation recorded by intracerebral electrodes in the human pre- and postcentral areas. Clin Neurophysiol 115: 1616–1623, 2004 [DOI] [PubMed] [Google Scholar]
- Barba et al., 2005. Barba C, Valeriani M, Colicchio G, Mauguière F. Short and middle-latency median nerve (MN) SEPs recorded by depth electrodes in human pre-SMA and SMA-proper. Clin Neurophysiol 116: 2664–2674, 2005 [DOI] [PubMed] [Google Scholar]
- Barba et al., 2008. Barba C, Valeriani M, Colicchio G, Mauguière F. New depth short-latency somatosensory evoked potential (SEP) component recorded in human SI area. Neurosci Lett 432: 179–183, 2008 [DOI] [PubMed] [Google Scholar]
- Barba et al., 2004. Barba C, Valeriani M, Colicchio G, Tonali P, Restuccia D. Parietal generators of low- and high-frequency MN (median nerve) SEPs: data from intracortical human recordings. Clin Neurophysiol 115: 647–657, 2004 [DOI] [PubMed] [Google Scholar]
- Bast et al., 2007. Bast T, Wright T, Boor R, Harting I, Feneberg R, Rupp A, Hoechstetter K, Rating D, Baumgärtner U. Combined EEG and MEG analysis of early somatosensory evoked activity in children and adolescents with focal epilepsies. Clin Neurophysiol 118: 1721–1735, 2007 [DOI] [PubMed] [Google Scholar]
- Baumgärtner et al., 1998. Baumgärtner U, Vogel H, Ellrich J, Gawehn J, Stoeter P, Treede RD. Brain electrical source analysis of primary cortical components of the tibial nerve somatosensory evoked potential using regional sources. Electroencephalogr Clin Neurophysiol 108: 588–599, 1998 [DOI] [PubMed] [Google Scholar]
- Bodegård et al., 2001. Bodegård A, Geyer S, Grefkes C, Zilles K, Roland PE. Hierarchical processing of tactile shape in the human brain. Neuron 31: 317–328, 2001 [DOI] [PubMed] [Google Scholar]
- Buchner et al., 1994. Buchner H, Fuchs M, Wischmann HA, Dossel O, Ludwig I, Knepper A, Berg P. Source analysis of median nerve and finger stimulated somatosensory evoked potentials: multichannel simultaneous recording of electric and magnetic fields combined with 3D-MR tomography. Brain Topogr 6: 299–310, 1994 [DOI] [PubMed] [Google Scholar]
- Buchner et al., 1996. Buchner H, Waberski TD, Fuchs M, Drenckhahn R, Wagner M, Wischmann HA. Postcentral origin of P22: evidence from source reconstruction in a realistically shaped head model and from a patient with a postcentral lesion. Electroencephalogr Clin Neurophysiol 100: 332–342, 1996 [DOI] [PubMed] [Google Scholar]
- Buchner et al., 1995. Buchner H, Waberski TD, Fuchs M, Wischmann HA, Wagner M, Drenckhahn R. Comparison of realistically shaped boundary-element and spherical head models in source localization of early somatosensory evoked potentials. Brain Topogr 8: 137–143, 1995 [DOI] [PubMed] [Google Scholar]
- Burke et al., 1982. Burke D, Gandevia SC, McKeon B, Skuse NF. Interactions between cutaneous and muscle afferent projections to cerebral cortex in man. Electroenceph Clin Neurophysiol 53: 349–360, 1982 [DOI] [PubMed] [Google Scholar]
- Cheney and Fetz, 1984. Cheney PD, Fetz EE. Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J Physiol 349: 249–272, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiber et al., 1986. Deiber MP, Giard MH, Mauguière F. Separate generators with distinct orientations for N20 and P22 somatosensory evoked potentials to finger stimulation? Electroencephalogr Clin Neurophysiol 65: 321–334, 1986 [DOI] [PubMed] [Google Scholar]
- Desmedt, 1978. Desmedt JE. Progress in Clinical Neurophysiology. Cerebral Motor Control in Man: Long Loop Mechanisms Basel: Karger, 1978 [Google Scholar]
- Desmedt et al., 1987. Desmedt JE, Nguyen TH, Bourguet M. Bit-mapped color imaging of human evoked potentials with reference to the N20, P22, P27 and N30 somatosensory responses. Electroencephalogr Clin Neurophysiol 68: 1–19, 1987 [DOI] [PubMed] [Google Scholar]
- Desmedt and Ozaki, 1991. Desmedt JE, Ozaki I. SEPs to finger joint input lack the N20-P20 response that is evoked by tactile inputs: contrast between cortical generators in area 3b and 2 in humans. Electroencephalogr Clin Neurophysiol 80: 513–521, 1991 [DOI] [PubMed] [Google Scholar]
- Frot and Mauguière, 2003. Frot M, Mauguière F. Dual representation of pain in the operculo-insular cortex in humans. Brain 126: 438–450, 2003 [DOI] [PubMed] [Google Scholar]
- Fuchs et al., 2002. Fuchs M, Kastner J, Wagner M, Hawes S, Ebersole JS. A standardized boundary element method volume conductor model. Clin Neurophysiol 113: 702–712, 2002 [DOI] [PubMed] [Google Scholar]
- Fuchs et al., 2007. Fuchs M, Wagner M, Kastner J. Development of volume conductor and source models to localize epileptic foci. J Clin Neurophysiol 24: 101–119, 2007 [DOI] [PubMed] [Google Scholar]
- Gardner, 1988. Gardner EP. Somatosensory cortical mechanisms of feature detection in tactile and kinesthetic discrimination. Can J Physiol Pharmacol 66: 439–454, 1988 [DOI] [PubMed] [Google Scholar]
- Gardner and Costanzo, 1980. Gardner EP, Costanzo RM. Neuronal mechanisms underlying direction sensitivity of somatosensory cortical neurons in awake monkeys. J Neurophysiol 43: 1342–1354, 1980 [DOI] [PubMed] [Google Scholar]
- Ghez and Shinoda, 1978. Ghez C, Shinoda Y. Spinal mechanisms of the functional stretch reflex. Exp Brain Res 32: 55–68, 1978 [DOI] [PubMed] [Google Scholar]
- Geyer et al., 1999. Geyer S, Schleicher A, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. I. Microstructural organization and interindividual variability. Neuroimage 10: 63–83, 1999 [DOI] [PubMed] [Google Scholar]
- Geyer et al., 2000. Geyer S, Schleicher A, Zilles K. Areas 3a, 3b, and 1 of human primary somatosensory cortex. II Spatial normalization to standard anatomical space. Neuroimage 11: 684–696, 2000 [DOI] [PubMed] [Google Scholar]
- Güllmar et al., 2010. Güllmar D, Haueisen J, Reichenbach JR. Influence of anisotropic electrical conductivity in white matter tissue on the EEG/MEG forward and inverse solution. A high-resolution whole head simulation study. Neuroimage 51: 145–163, 2010 [DOI] [PubMed] [Google Scholar]
- Hallez et al., 2009. Hallez H, Staelens S, Lemahieu I. Dipole estimation errors due to not incorporating anisotropic conductivities in realistic head models for EEG source analysis. Phys Med Biol 54: 6079–6093, 2009 [DOI] [PubMed] [Google Scholar]
- Hayashi et al., 1995. Hayashi N, Nishijo H, Ono T, Endo S, Tabushi E. Generators of somatosensory evoked potentials investigated by dipole tracing in the monkey. Neuroscience 68: 323–38, 1995 [DOI] [PubMed] [Google Scholar]
- Hsiao et al., 1993. Hsiao SS, O'Shaughnessy DM, Johnson KO. Effects of selective attention on spatial form processing in monkey primary and secondary somatosensory cortex. J Neurophysiol 70: 444–447, 1993 [DOI] [PubMed] [Google Scholar]
- Hyvärinen and Poranen, 1978. Hyvärinen J, Poranen A. Movement-sensitive and direction and orientation-selective cutaneous receptive fields in the hand area of the post-central gyrus in monkeys. J Physiol 283: 523–537, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones and Porter, 1980. Jones EG, Porter R. What is area 3A? Brain Res Rev 203: 1–45, 1980 [DOI] [PubMed] [Google Scholar]
- Jung et al., 2003. Jung P, Baumgärtner U, Bauermann T, Magerl W, Gawehn J, Stoeter P, Treede RD. Asymmetry in the human primary somatosensory cortex and handedness. Neuroimage 19: 913–923, 2003 [DOI] [PubMed] [Google Scholar]
- Jung et al., 2008. Jung P, Baumgärtner U, Magerl W, Treede RD. Hemispheric asymmetry of hand representation in human primary somatosensory cortex and handedness. Clin Neurophysiol 119: 2579–86, 2008 [DOI] [PubMed] [Google Scholar]
- Junghöfer et al., 1999. Junghöfer M, Elbert T, Tucker DM, Braun C. The polar average reference effect: a bias in estimating the head surface integral in EEG recording. Clin Neurophysiol 110: 1149–1155, 1999 [DOI] [PubMed] [Google Scholar]
- Kaas, 1983. Kaas JH. What, if anything, is SI? Organization of first somatosensory area of cortex. Physiol Rev 63: 206–231, 1983 [DOI] [PubMed] [Google Scholar]
- Kakigi, 1994. Kakigi R. Somatosensory evoked magnetic fields following median nerve stimulation. Neurosci Res 20: 165–174, 1994 [DOI] [PubMed] [Google Scholar]
- Kanovsky et al., 2003. Kanovsky P, Bares M, Rektor I. The selective gating of the N30 cortical component of the somatosensory evoked potentials of median nerve is different in the mesial and dorsolateral frontal cortex: evidence from intracerebral recordings. Clin Neurophysiol 114: 981–991, 2003 [DOI] [PubMed] [Google Scholar]
- Kristeva-Feige et al., 1997. Kristeva-Feige R, Grimm C, Huppertz HJ, Otte M, Schreiber A, Jäger D, Feige B, Büchert M, Hennig J, Mergner T, Lücking CH. Reproducibility and validity of electric source localization with high-resolution electroencephalography. Electroencephalogr Clin Neurophysiol 103: 652–660, 1997 [DOI] [PubMed] [Google Scholar]
- Lee and Seyal, 1998. Lee EK, Seyal M. Generators of short latency human somatosensory-evoked potentials recorded over the spine and scalp. J Clin Neurophysiol 15: 227–234, 1998 [DOI] [PubMed] [Google Scholar]
- Lee et al., 2009. Lee WH, Liu Z, Mueller BA, Lim K, He B. Influence of white matter anisotropy on EEG source localization: an experimental study. Conf Proc IEEE Eng Med Biol Soc 2009: 2923–2925, 2009 [DOI] [PubMed] [Google Scholar]
- Lenz et al., 1993. Lenz FA, Seike M, Lin YC, Baker FH, Rowland LH, Gracely RH, Richardson RT. Neurons in the area of human thalamic nucleus ventralis caudalis respond to painful heat stimuli. Brain Res 623: 235–240, 1993 [DOI] [PubMed] [Google Scholar]
- Lenz et al., 1983a. Lenz FA, Tatton WG, Tasker RR. Electromyographic response to displacement of different forelimb joints in the squirrel monkey. J Neurosci 3: 783–794, 1983a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz et al., 1983b. Lenz FA, Tatton WG, Tasker RR. The effect of cortical lesions on the electromyographic response to joint displacement in the squirrel monkey forelimb. J Neurosci 3: 795–805, 1983b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauguière and Desmedt, 1991. Mauguière F, Desmedt JE. Focal capsular vascular lesions can selectively deafferent the prerolandic or the parietal cortex: somatosensory evoked potentials evidence. Ann Neurol 30: 71–75, 1991 [DOI] [PubMed] [Google Scholar]
- Mauguière et al., 1983. Mauguière F, Desmedt JE, Courjon J. Astereognosis and dissociated loss of frontal or parietal components of somatosensory evoked potentials in hemispheric lesions. Detailed correlations with clinical signs and computerized tomographic scanning. Brain 106: 271–311, 1983 [DOI] [PubMed] [Google Scholar]
- McCarthy et al., 1991. McCarthy G, Wood CC, Allison T. Cortical somatosensory evoked potentials. I. Recordings in monkey Macaca fascicularis. J Neurophysiol 66: 53–63, 1991 [DOI] [PubMed] [Google Scholar]
- Michel et al., 2004. Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R. EEG source imaging. Clin Neurophysiol 115: 2195–2222, 2004 [DOI] [PubMed] [Google Scholar]
- Ossenblok et al., 2003. Ossenblok P, Leijten FS, de Munck JC, Huiskamp GJ, Barkhof F, Boon P. Magnetic source imaging contributes to the presurgical identification of sensorimotor cortex in patients with frontal lobe epilepsy. Clin Neurophysiol 114: 221–232, 2003 [DOI] [PubMed] [Google Scholar]
- Phillips, 1969. Phillips CG. The Ferrier lecture, 1968. Motor apparatus of the baboon's hand. Proc R Soc Lond B Biol Sci 173: 141–174, 1969 [DOI] [PubMed] [Google Scholar]
- Phillips et al., 1988. Phillips JR, Johnson KO, Hsiao SS. Spatial pattern representation and transformation in monkey somatosensory cortex. Proc Natl Acad Sci USA 85: 1317–1321, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini et al., 1989. Rossini PM, Babiloni F, Bernardi G, Cecchi L, Johnson PB, Malentacca A, Stanzione P, Urbano A. Abnormalities of short-latency somatosensory evoked potentials in parkinsonian patients. Electroencephalogr Clin Neurophysiol 74: 277–289, 1989 [DOI] [PubMed] [Google Scholar]
- Scherg, 1990. Scherg M. Fundamentals of dipole source potentials analysis. In: Auditory Evoked Magnetic Fields and Electronic Potentials, edited by Grandoni F, Hoke M, Romani GL. Basel: Karger, 1990, p. 40–69 [Google Scholar]
- Scherg, 1992. Scherg M. Functional imaging and localization of electromagnetic brain activity. Brain Topogr 5: 103–111, 1992 [DOI] [PubMed] [Google Scholar]
- Sonoo et al., 1991. Sonoo M, Shimpo T, Takeda K, Genba K, Nakano I, Mannen T. SEPs in two patients with localized lesions of the postcentral gyrus. Electroencephalogr Clin Neurophysiol 80: 536–546, 1991 [DOI] [PubMed] [Google Scholar]
- SöröS et al., 1999. SöröS P, Knecht S, Imai T, Gurtler S, Lutkenhöner B, Ringelstein EB, Henningsen H. Cortical asymmetries of the human somatosensory hand representation in right- and left-handers. Neurosci Lett 271: 89–92, 1999 [DOI] [PubMed] [Google Scholar]
- Strik and Lehmann, 1993. Strik WK, Lehmann D. Data-determined window size and space-oriented segmentation of spontaneous EEG map series. Electroenceph Clin Neurophysiol 87: 169–174, 1993 [DOI] [PubMed] [Google Scholar]
- Talairach and Tournoux, 1988. Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme, 1988 [Google Scholar]
- Theuvenet et al., 2005. Theuvenet PJ, van Dijk BW, Peters MJ, van Ree JM, Lopes da Silva FL, Chen AC. Whole-head MEG analysis of cortical spatial organization from unilateral stimulation of median nerve in both hands: no complete hemispheric homology. Neuroimage 28: 314–325, 2005 [DOI] [PubMed] [Google Scholar]
- Tiihonen et al., 1989. Tiihonen J, Hari R, Hämäläinen M. Early deflections of cerebral magnetic responses to median nerve stimulation. Electroencephalogr Clin Neurophysiol 74: 290–296, 1989 [DOI] [PubMed] [Google Scholar]
- Töpper et al., 1993. Töpper R, Schwarz M, Podoll K, Dömges F, Noth J. Absence of frontal somatosensory evoked potentials in Huntington's disease. Brain 116: 87–101, 1993 [DOI] [PubMed] [Google Scholar]
- Tracey et al., 1980. Tracey DJ, Asanuma C, Jones EG, Porter R. Thalamic relay to motor cortex: afferent pathways from brain stem, cerebellum, and spinal cord in monkeys. J Neurophysiol 44: 532–554, 1980 [DOI] [PubMed] [Google Scholar]
- Valeriani et al., 1998. Valeriani M, Restuccia D, Di Lazzaro V, Le Pera D, Barba C, Tonali P, Mauguiere F. Dipolar sources of the early scalp somatosensory evoked potentials to upper limb stimulation. Effect of increasing stimulus rates. Exp Brain Res 120: 306–315, 1998 [DOI] [PubMed] [Google Scholar]
- Valeriani et al., 1999. Valeriani M, Restuccia D, Di Lazzaro V, Le Pera D, Tonali P. Effect of movement on dipolar source activities of somatosensory evoked potentials. Muscle Nerve 22: 1510–1519, 1999 [DOI] [PubMed] [Google Scholar]
- van't Ent et al., 2001. van't Ent D, de Munck JC, Kaas AL. A fast method to derive realistic BEM models for E/MEG source reconstruction. IEEE Trans Biomed Eng 48: 1434–1443, 2001 [DOI] [PubMed] [Google Scholar]
- Vatta et al., 2002. Vatta F, Bruno P, Inchingolo P. Accuracy of EEG source reconstruction in the presence of brain lesions: modelling errors and surface electrodes' placement. Biomed Sci Instrum 38: 423–428, 2002 [PubMed] [Google Scholar]
- Vogel et al., 2003. Vogel H, Port JD, Lenz FA, Solaiyappan M, Krauss G, Treede RD. Dipole source analysis of laser-evoked subdural potentials recorded from parasylvian cortex in humans. J Neurophysiol 89: 3051–3060, 2003 [DOI] [PubMed] [Google Scholar]
- Wiesendanger and Miles, 1982. Wiesendanger M, Miles TS. Ascending pathway of low-threshold muscle afferents to the cerebral cortex and its possible role in motor control. Physiol Rev 62: 1234–1270, 1982 [DOI] [PubMed] [Google Scholar]
- Wood et al., 1985. Wood CC, Cohen D, Cuffin BN, Yarita M, Allison T. Electrical sources in human somatosensory cortex: identification by combined magnetic and potential recordings. Science 227: 1051–1053, 1985 [DOI] [PubMed] [Google Scholar]
- Wolters et al., 2006. Wolters CH, Anwander A, Tricoche X, Weinstein D, Koch MA, MacLeod RS. Influence of tissue conductivity anisotropy on EEG/MEG field and return current computation in a realistic head model: a simulation and visualization study using high-resolution finite element modeling. Neuroimage 30: 813–826, 2006 [DOI] [PubMed] [Google Scholar]
- Yamada, 2000. Yamada T. Neuroanatomic substrates of lower extremity somatosensory evoked potentials. J Clin Neurophysiol 17: 269–279, 2000 [DOI] [PubMed] [Google Scholar]
- Yousry et al., 1995. Yousry T, Schmid UD, Jassoy AG, Schmidt D, Eisner WE, Reulen H-J, Reiser MF, Lissner J. Topography of the cortical hand area: prospective study with functional MR imaging and direct motor mapping at surgery. Radiology 195: 23–29, 1995 [DOI] [PubMed] [Google Scholar]
- Yvert et al., 1997. Yvert B, Bertrand O, Thévenet M, Echallier JF, Pernier J. A systematic evaluation of the spherical model accuracy in EEG dipole localization. Electroencephalogr Clin Neurophysiol 102: 452–459, 1997 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2008. Zhang Y, van Drongelen W, Kohrman M, He B. Three-dimensional brain current source reconstruction from intra-cranial ECoG recordings. Neuroimage 42: 683–695, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]