Abstract
People can find objects in a visual scene fast and effortlessly. It is thought that this may be accomplished by creating a map of the outside world that incorporates bottom-up sensory and top-down cognitive inputs—a priority map. Eye movements are made toward the location represented by the highest activity on the priority map. We hypothesized that the lateral intraparietal area (LIP) of posterior parietal cortex acts as such a map. To test this, we performed low current microstimulation on animals trained to perform a foraging task and asked whether we could bias the animals to make a saccade to a particular stimulus, by creating an artificial peak of activity at the location representing that stimulus on the map. We found that microstimulation slightly biased the animals to make saccades to visual stimuli at the stimulated location, without actively generating saccades. The magnitude of this effect was small, but it appeared to be similar for all visual stimuli. We interpret these results to mean that microstimulation slightly biased saccade goal selection to the object represented at the stimulated location in LIP.
INTRODUCTION
When we scan a scene for an object, we subconsciously make no more than three eye movements per second, yet the search is fast and efficient. Based on behavioral findings, theoreticians have proposed biologically plausible models that explain this efficiency, using bottom-up salience and top-down cognitive inputs (Itti and Koch 2000; Koch and Ullman 1985). We (Ipata et al. 2009; Mirpour et al. 2009), among others (Gottlieb et al. 2009), have suggested that the lateral intraparietal area (LIP) of posterior parietal cortex acts similarly to one of these models—as a priority map (Fecteau and Munoz 2006; Serences and Yantis 2006). In this model, eye movements are made toward the highest activity, representing the most behaviorally important location of the scene (Ipata et al. 2006; Itti and Koch 2001).
Evidence that LIP may act as a priority map comes from a host of recording studies. They show that LIP neurons have activity related to eye movements; many of which respond before saccade initiation (Andersen et al. 1987; Barash et al. 1991; Gnadt and Andersen 1988). They also show that activity in LIP reflects cognitive information, such as reward likelihood (Dorris and Glimcher 2004; Sugrue et al. 2004), the selection of saccade targets during active visual search (Buschman and Miller 2007; Ipata et al. 2006; Thomas and Pare 2007), and the accumulation of evidence for decision making in direction discrimination tasks (Churchland et al. 2008; Roitman and Shadlen 2002). Finally, LIP activity is also affected by exogenous inputs (Balan and Gottlieb 2006). Thus activity within LIP contains all the information necessary for it to act as a priority map.
In addition to the appropriate activity needed for a priority map, LIP also has the connections with oculomotor areas, such as the frontal eye fields (FEFs) and superior colliculus (SC) (Andersen et al. 1985, 1990), which allow it to influence the selection of saccade goal targets. However, causal evidence that activity from LIP is actually used in this manner is limited (Balan and Gottlieb 2009; Hanks et al. 2006; Wardak et al. 2002). In this study, we asked whether low current microstimulation of LIP could bias animals to select a particular stimulus by creating an artificial peak on the priority map.
METHODS
All experiments were approved by the Chancellor's Animal Research Committee at UCLA as complying with the guidelines established in the Public Health Service Guide for the Care and Use of Laboratory Animals. Two monkeys (8–10 kg) were implanted with head posts, scleral coils, and recording cylinders during sterile surgery under general anesthesia as described previously (Bisley and Goldberg 2006; Mirpour et al. 2009). The animals were trained on the standard memory-guided saccade task (MGS) and on the foraging task (Fig. 1). Experiments were run using the REX system (Hays et al. 1982), and visual stimuli were presented on a CRT using the associated VEX software.
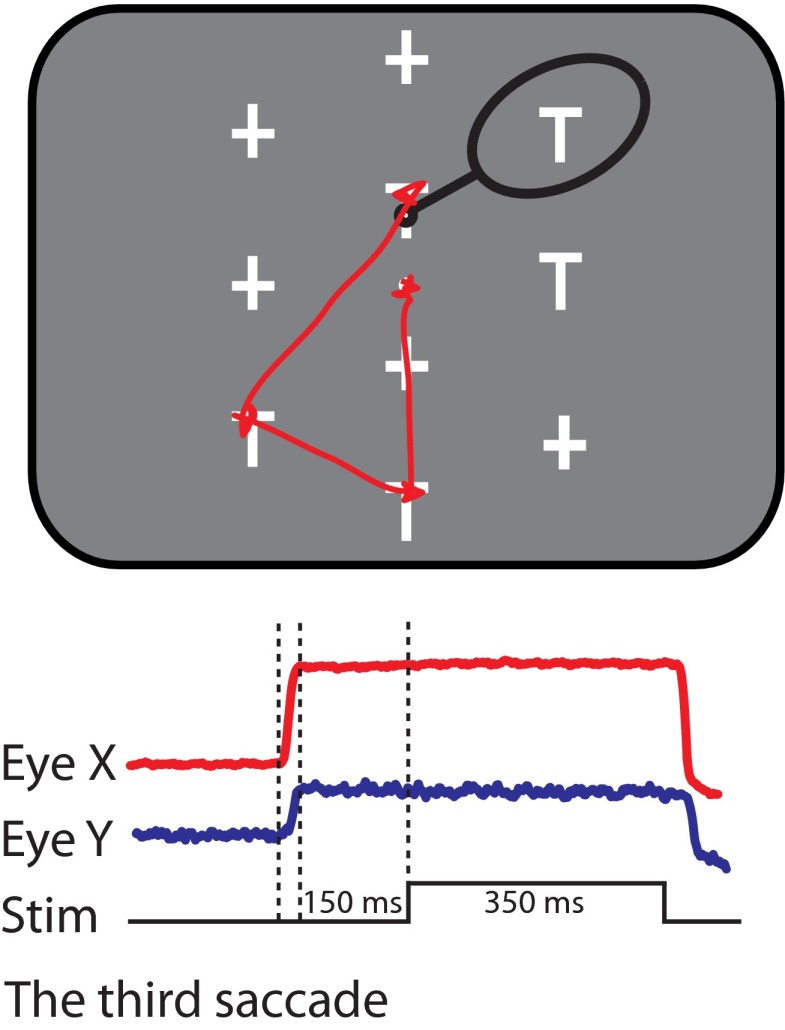
Fig. 1.
Task and behavior. In each trial, 5 distractors (+) and 5 potential targets (T) were presented. One T, the target, had a fluid reward linked to it, such that when the monkey looked at it for 500 ms within 8 s, he obtained the reward. The stimuli were arranged so that when looking at 1 stimulus (small black circle), another stimulus was usually centered in the receptive field of the lateral intraparietal area (LIP) multiunit activity (black oval). A 350-ms burst of biphasic pulses was injected into LIP 150 ms after the third saccade on ∼29% of trials.
To begin a trial of the foraging task, the monkeys had to fixate a spot placed on the center of the screen. After a delay of 450–700 ms, an array of five potential targets (T) and five distractors (+) were presented. One of the Ts had a juice reward associated with it (the target), such that if the monkey looked at it for 500 ms within 8 s after the start of trial, he would get the reward. The stimuli were arranged in such a fashion that, when the monkey looked at one stimulus, the receptive field of the multiunit LIP activity could encompass a single other stimulus. On each trial, the spatial arrangement of the stimulus array was identical, but the positions of the potential targets and distractors were randomly assigned (Mirpour et al. 2009). Thus from session to session, the locations of the objects were different, and within a session, there were 252 possible stimulus configurations.
We recorded extracellular multiunit activity from LIP using tungsten microelectrodes guided by coordinates from MRI images. The activity was considered to be in LIP if it showed a visual burst, sustained delay activity, and/or a perisaccadic burst in the MGS task (Barash et al. 1991). To be included, sites had to have at least perisaccadic activity or sustained delay activity. The size and position of the receptive field of the multiunit response was initially mapped by hand and then with a more detailed MGS mapping paradigm. For this procedure, the receptive fields were probed with nine points arranged in a 3 × 3 matrix within the contralateral visual field to estimate the coarse range of location and size of the receptive field. The output of these data was used to set up a more tightly constrained 25-point array in a 5 × 5 matrix. At the end of this procedure, the boundaries of the receptive field were defined with an accuracy of ∼0.5°. Receptive field eccentricities ranged from 5 to 15°. The diameter of the receptive fields ranged from 2.5 to 7.5°. We arranged the stimuli in the foraging task array in a way such that we had only one object inside the receptive field. If the size or eccentricity of the receptive field did not let us arrange 10 objects on the monitor without receptive field overlap, we excluded that site and looked for a new recording site. Therefore very large and/or eccentric receptive fields that did not pass the criteria were not included in the study.
If the location and size of the receptive field remained similar along a 100-μm path of the electrode, the foraging task was run, and microstimulation was performed in the center of the 100-μm cluster. Two thirds of trials were slated as microstimulation trials, but stimulation was only performed if the animal made ≥3 saccades, and the third saccade was not to the target. This resulted in stimulation on ∼29% of all trials. We performed microstimulation after the third saccade to balance the probability of having a fixated or unfixated T within the receptive field at the time of stimulation. Microstimulation was presented as a 350-ms burst of 20-μA peak-to-peak biphasic pulses with a frequency of 200 Hz (Bisley et al. 2001; Moore and Armstrong 2003; Salzman et al. 1990), occurring 150 ms after the third saccade. This window was chosen because it corresponded with the period during which a stabilized response was seen in the neural activity, which differentiated between targets, distractors, and previously fixated targets (Mirpour et al. 2009). Microstimulation was terminated on initiation of a saccade if it happened before the end of the 350-ms window of stimulation. After stimulation, the trials were continued like any other trial; if the monkeys found and maintained fixation on the target, they were rewarded.
Because microstimulation was only performed on trials with more than three saccades, we needed to create a set of control data that would have at least as many saccades per trial as the stimulation data. This is important because performance and saccade goal choices in one, two, and three saccade trials are quite different from those in trials with more than three saccades. To create these control data, we used a resampling method that matched the stimulation data as best as possible, while using as many different control trials as possible. For each iteration, a number of trials (equal to the number of stimulation trials) were randomly chosen from the nonstimulation data in such a way that the number of fixations in each nonstimulation trial had to be equal to or more than the matched stimulation trial. The probability of making a saccade to the receptive field and the percent correct in that set of control trials were calculated for each iteration. This process was repeated 10,000 times. The mean and SE for the probabilities were calculated from the resultant distribution and were used for plotting the data. Statistical comparisons were made between the control data and the stimulation data by testing whether the stimulation data lay above the top 97.5th percentile or below the 2.5th percentile of the distribution. This is labeled as a bootstrap test of significance in the text.
RESULTS
To study eye movements in unconstrained viewing, two rhesus macaques were trained on a visual foraging task in which they searched through 10 stimuli (5 potential targets, Ts, and 5 distractors, +s) to find the target, which was loaded with reward (Fig. 1). Once the stimuli appeared, the monkeys had 8 s to find the target and were free to move their eyes to any location; no eye movements were “errors” that would cancel the trial. To get the reward, the monkeys had to fixate the target for 500 ms; this lead to a strategy in which they usually looked from T to T, waiting at each for ∼600 ms. The details of the animals' behavior in this task have been examined in depth elsewhere (Mirpour et al. 2009). In brief, the animals are excellent at differentiating between targets and distractors and have a good memory of what objects they have seen before. We have described this as efficient search, using the term efficient to describe the ability to find the target using the smallest number of saccades and not to describe how optimal the scan path is. Importantly, we showed that the animals do not follow a single stereotyped scan path from trial to trial, meaning that they must use their memory to perform the task efficiently. We also showed that the activity in LIP during this task is related to the behavioral importance of the stimulus within the receptive field. Greatest activity is seen in response to potential targets, significantly less activity is seen in response to Ts that have been fixated and found not to contain the reward, and the weakest activity is seen in response to distractors, which never contain a reward (Mirpour et al. 2009). We predicted that if LIP is used to guide eye movements, low current microstimulation should bias the animals to make saccades toward the object in the receptive field of the stimulated neurons.
In each microstimulation session, we first identified the eccentricity and size of the receptive field by recording the LIP multiunit activity while the animals performed a delayed MGS. Once the receptive field was defined, we placed the stimuli within the search array such that when the animal looked at one stimulus, a single other stimulus was usually in the receptive field of the multiunit activity (Fig. 1). We microstimulated the center of a 100-μm cluster of LIP neurons on 29.2 ± 0.44% (SE from all sessions) of trials, after monkeys made the third saccade to an object that was not the target. Stimulation began 150 ms following the end of the third saccade and lasted for 350 ms or until the next saccade was initiated, whichever came first. We recorded the behavior in 30 microstimulation sessions (16 sessions for monkey E, 14 sessions for monkey D) and in 31 sham sessions, in which the same task was run but current injection was not performed. Performance was measured as the percentage of trials in which the animal found the target and received the reward out of the total number of trials in which the array appeared. The number of correct trials ranged from 405 to 1,905 (average, 964) per session. For all sessions, performance and behavior for stimulation (or sham stimulation) trials were compared with performance and behavior from a control set of data that was obtained using a resampling method to select nonstimulation trials with similar numbers of saccades (see methods for details). All analyses were done in each individual animal and, because all reported significant results were significant in each individual monkey, we only show the pooled data.
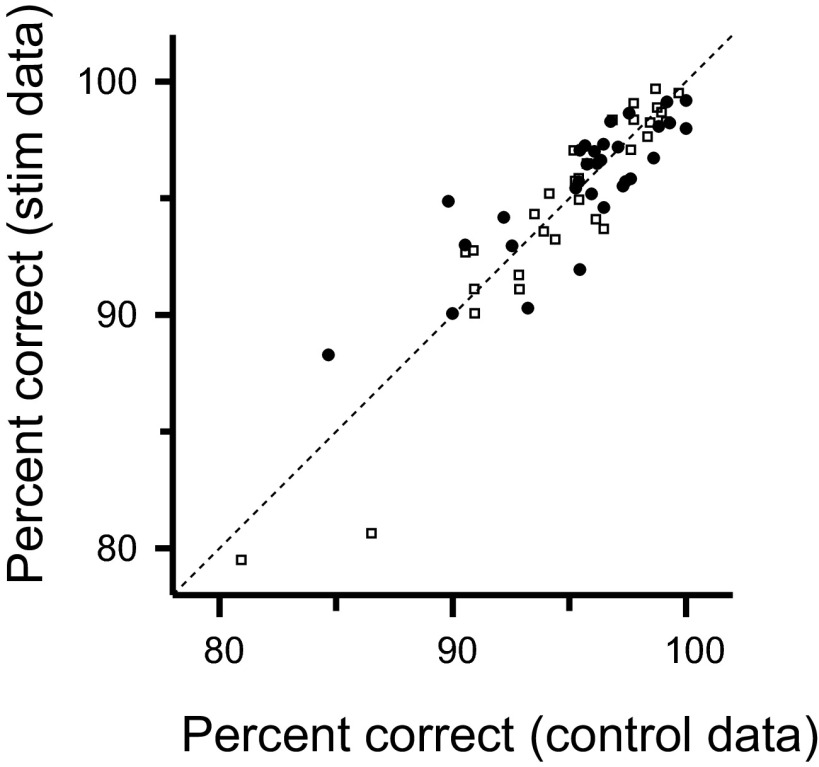
Monkeys were usually able to find the target within the 8-s time limit, and microstimulation did not change their overall performance. There was a significant difference in percent correct in only 1 of the 61 individual sessions (bootstrap test of significance, P < 0.05), and overall, performance was not significantly different in the microstimulation (P = 0.9, Wilcoxon signed-rank test) or sham (P = 0.7) sessions (Fig. 2).
Fig. 2.
Performance on the behavioral task. Percent correct of 30 microstimulation and 31 sham sessions. Each symbol represents the percent correct of stimulated trials compared with the percent correct of the control trials from a single session. Microstimulation and sham sessions are represented with filled circles and open squares, respectively. There was no significant difference between stimulated and control sessions in microstimulation (P = 0.9, Wilcoxon sign rank test) and sham (P = 0.7) sessions.
Microstimulation of LIP-biased eye movements toward items the monkeys did not usually look at. During normal behavior, the majority of saccades are made toward potential targets rather than distractors (Mirpour et al. 2009). Microstimulation of LIP during fixations in which there was a distractor inside the receptive field increased the average probability of making a saccade toward the receptive field compared with control trials, whereas there was no such difference in the sham sessions (Fig. 3A). Stimulation under these conditions occurred in 62.5 ± 4.9 trials per session. In 13 (43%) sessions, microstimulation significantly biased saccades toward distractors (red symbols in Fig. 3B; bootstrap test of significance), whereas it never significantly reduced the probability of going to the distractor. Thus this effect was highly significant across the population (P = 0.0002, Wilcoxon signed-rank test). In sham sessions, microstimulation significantly increased the probability of going to a distractor in four (13%) sessions and reduced the probability in one (3%) session. In addition, there was no overall bias toward distractors across the population (P = 0.63; Fig. 3C).
Fig. 3.
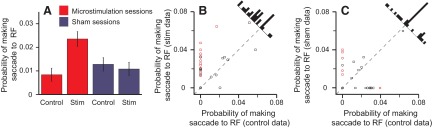
Probability of making a saccade to a distractor inside the receptive field (RF). A: the average (±SE) probability of making a saccade to the RF when there was a distractor inside it in stimulation (stim) and control trials across stimulation (red) and sham sessions (blue). B and C: each point represents the probability of making a saccade to the RF from a single session when there was a distractor inside the RF in stimulation trials (ordinate) compared with control trials (abscissa) in microstimulation (B) and sham (C) sessions. Histograms show the distribution of data points. Red points, single sessions with a significant difference between stimulation and control trials; circle symbol, monkey D; square, monkey E.
Microstimulation slightly increased the probability that the animals would look at Ts that they had already fixated. In normal behavior, monkeys rarely look at Ts that have been fixated and thus have been ruled out as containing the reward, and the activity of LIP neurons under these conditions is suppressed (Mirpour et al. 2009). In 33.2 ± 3.8 trials per session, a previously fixated T was in the receptive field when stimulation occurred. In this case, microstimulation significantly biased the probability of making a saccade to the receptive field in three (9%) sessions (red symbols in Fig. 4B). Such an effect was only seen in one sham session (Fig. 4C). In no case did microstimulation significantly decrease the probability that a saccade would be made to a fixated T. The average probability of making a saccade to a fixated T showed the same direction and magnitude of bias that we observed in the case of distractors inside the receptive field (cf. Figs. 4A and 3A); however, this difference was not significant for the data in Fig. 4 (P = 0.276). To see whether the bias was significant under more constrained conditions, we restricted our analyses to sessions in which monkeys did not make any saccades to fixated targets inside the receptive field in at least one condition (orange boxes in Fig. 4B). Our hypothesis was that, if the effect of microstimulation is real, but weak, it should be most obvious in sessions in which the animals best suppressed saccades to Ts that they had already fixated. Under these conditions, microstimulation significantly biased the eye movements toward the fixated Ts in the receptive field (P = 0.003; vertical orange box in Fig. 4B). To show that this shift is real, we performed three controls. First, we ran the same analysis on sham sessions and found that there was no significant difference in probability of looking at a fixated T in the receptive field, when only sessions in which the monkey never looked at the fixated T in control trials were included (vertical orange box in Fig. 4C; P = 0.25). The remaining two analyses tested the reverse assumption by only examining sessions in which microstimulation never biased the eyes toward the fixated T and showed that the probability of making a saccade to the fixated T did not increase in control trials, both in sham (P = 0.06) and microstimulation (P = 0.25) sessions (see horizontal orange boxes in Fig. 4, B and C).
Fig. 4.
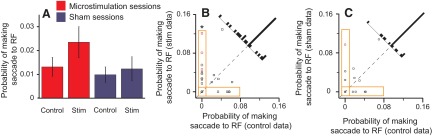
Probability of making a saccade to a previously fixated T inside the RF. A: the average (±SE) probability of making a saccade to the RF when there was a previously fixated T inside it in stimulation (stim) and control trials across stimulation (red) and sham sessions (blue). B and C: each point represents the probability of making a saccade to the RF from a single session when there was a previously fixated T inside the RF in stimulation trials (ordinate) compared with control trials (abscissa) in microstimulation (B) and sham (C) sessions. Histograms show the distribution of data points. Red points, single sessions with a significant difference between stimulation and control trials; circle symbol, monkey D; square, monkey E.
Microstimulation of LIP did not obviously bias the animals to look at a potential target, which they were likely to look at anyway. Because microstimulation was performed after the third saccade, there were often only two potential targets remaining, depending on how many of the previous three saccades were made to Ts. As such, trials in which a potential target was in the receptive field during stimulation only occurred 25.1 ± 2.1 times per session. In these trials, microstimulation did not change the average probability of the animals making a saccade toward the receptive field (Fig. 5, A and C; P = 0.64). Interestingly, when the data were examined closely, there was an increase in probability that was in the same direction and magnitude as the other effects and was absent in the sham data (cf. Figs. 5B and 3A and 4A, which are plotted on the same scale). We suggest that microstimulation is probably causing a similar small bias in behavior, but that given the size and variance of the probabilities in this condition, the bias is hidden in the noise and cannot be confirmed statistically.
Fig. 5.
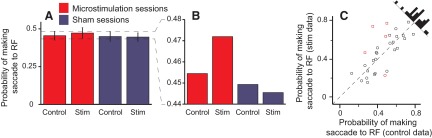
Probability of making a saccade to a potential target inside the RF. A: the average (±SE) probability of making a saccade to the RF when there was a potential target inside it in stimulation (stim) and control trials across stimulation (red) and sham sessions (blue). B: magnified version of A without the error bars, highlighting the trend of the effect of microstimulation. C: each point represents the probability of making a saccade to the RF from a single session when there was a potential target inside the RF in stimulation trials (ordinate) compared with control trials (abscissa) in microstimulation sessions. Histograms show the distribution of data points. Red points, single sessions with a significant difference between stimulation and control trials; circle symbol, monkey D; square, monkey E.
Microstimulation of LIP did not seem to bias saccade goal selection for subsequent saccades. It is possible that animals plan several saccades in advance and, by microstimulating LIP, we are more likely to bias subsequent saccades rather than the saccade being planned. To test this, we analyzed the probability that a subsequent saccade would be made to the visual stimulus that was inside the receptive field during stimulation. We found no evidence that microstimulation changed the probability of making a subsequent saccade to any of the stimuli; the average probability of making a saccade to a distractor, a fixated T, or a potential target was no different between stimulation and control trials (P = 0.8, 0.3, and 0.6, respectively; Wilcoxon signed rank tests). The same results were observed in sham sessions, with P values of 0.7, 0.6, and 0.5 for distractors, fixated Ts, and potential targets, respectively. We conclude that the effect of microstimulation is limited to the next saccade after stimulation. It should be mentioned that it is possible that we did not find any effect in subsequent saccades because the statistical power of the analysis is decreased as a result of the limited number of saccades and potential targets after the fourth saccade. However, if the lack of effect is true, it may be caused by a transient effect of stimulation or, if the induced activity persists, it may mean that activity within LIP is not remapped across saccades (Duhamel et al. 1992).
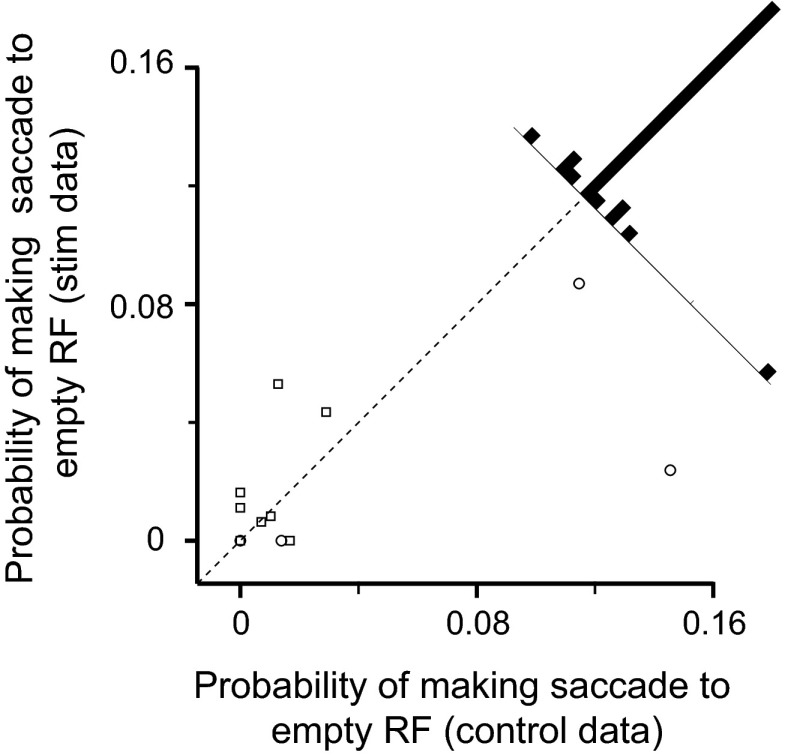
To test whether microstimulation of LIP was just eliciting saccades toward the receptive field, rather than biasing saccade goal selection, we calculated the probability of the animal making a saccade to the receptive field when there was no stimulus inside it. On average, empty receptive fields were stimulated in 131.6 ± 8.895 trials per session. Microstimulation of LIP did not increase the probability that a saccade would be made into the empty receptive field (P = 0.6; Fig. 6); in fact, the mean probability of going to the empty receptive field decreased from 0.0196 ± 0.008 to 0.0079 ± 0.004 when stimulation trials were compared with control trials.
Fig. 6.
Probability of making a saccade to an empty RF. Each point represents the probability of making a saccade to an empty RF from a single session in stimulation trials (ordinate) compared with control trials (abscissa). The histogram shows the distribution of data points. Circle symbol, monkey D; square, monkey E.
Saccadic metrics, such as saccade velocity and amplitude, were not significantly different between stimulation trials in stimulation sessions and sham sessions (Wilcoxon rank test, P = 0.6 and 0.9 for velocity and amplitude, respectively).
The effect of microstimulation was independent of the time that the saccade was made relative to the stimulation time. To test this, we pooled all of the stimulation data and looked at the probability that the next saccade would go to the stimulated receptive field as a function of the time that the saccade was initiated relative to the time that stimulation finished. The probability of going to the receptive field after stimulation was completed did not change as the delay between the end of stimulation and saccadic onset increased. There was a reduced probability that the saccade would go to the receptive field in trials in which the saccade was made before stimulation was due to be terminated, but this reduction was seen in the sham data as well. Furthermore, the mean latency of the saccade was 25 ms longer in stimulation sessions than in sham session (P = 0.003, T-test) but was independent of the class of the stimulus in the receptive field. This difference was solely caused by a significant increase in latency for saccades made away from the receptive field; there was no significant difference in latency for saccades made toward the receptive field. From these data, we conclude that microstimulation did not cause a premature motor movement and that the effect of microstimulation was not attenuated because of a gap between the end of microstimulation and the start of the next saccade.
DISCUSSION
Overall, we found that microstimulation of LIP biased saccade goal selection toward visual stimuli within the stimulated receptive field. These effects were small but consistent in magnitude, suggesting that activity in LIP is used to guide eye movements in a manner consistent with the priority map hypothesis.
Microstimulation appeared to bias saccades to all visual stimuli, from which we interpret that stimulation was only effective on a small proportion of trials. Different underlying mechanisms could have produced these results and, although determining the underlying mechanism is beyond reach of this study, we suggest some plausible mechanisms that could make such an effect. Overall, there are two possible ways that microstimulation of LIP can bias saccade goal selection. Either microstimulation adds a small amount of activity to the location on the priority map each time we stimulate or microstimulation has a strong effect, but only in a small percentage of stimulated trials. One way for the former to be true would be if the variance in response to the potential targets were substantially higher than the variance in responses to the fixated Ts or the distractors. If stimulation produces a very small bias, this may have a limited effect when a potential target is in the receptive field because of the large variance seen in this condition. On the other hand, stimulation would make the usually consistent response to a distractor slightly higher, and on trials in which the high variance results in low responses to the potential targets, the distractor would become the goal of the saccade. However, when we looked at the variance of the responses at the single neuron level, they were too consistent among categories for this explanation to be viable. Under these conditions, we would expect that microstimulation would have a much stronger effect when a potential target was in the receptive field than when a previously fixated T or a distractor was in the receptive field. We suggest that a more parsimonious explanation of our data is that microstimulation has a robust effect but only on a small number of trials. We can think of at least two plausible ways that this could occur. First, it is possible that the animals preplan a series of saccades and do not use the activity on the priority map on a saccade-by-saccade basis, although it is updated so that it can be used when needed. In this case, stimulation of LIP always modulates the activity on LIP neurons but may be ineffective on a large number of trials because the animal has already preplanned the next few saccades and ignores the activity in LIP. On the few trials in which the animal works on a saccade-by-saccade basis, stimulation biases saccades toward the object in the receptive field. We tend to think that this is unlikely given that Hanks et al. (2006) found similarly weak effects in a task in which only one eye movement needed to be made. Another way in which microstimulation may only be effective on a minority of trials relates to the state of the network involved in saccade target selection. It is possible that the artificial activity created by subthreshold stimulation is not effectively integrated into the network unless the state of the network is just right. For example, this could be in terms of when artificially induced spikes occur relative to the spikes driven by the endogenous activity. Although our data do not allow us to identify a specific mechanism, our bias is that a mechanism like this is more likely.
Microstimulation did not just trigger eye movements on a small number of trials. To test whether this was the case, we examined the probability that an eye movement would be made to an empty receptive field in stimulation and control trials and found no significant difference. It is possible that the reason we did not see a significant difference was because the expected difference was so small. We do not think this is the case for three reasons. First, we have more trials of this class than of any other class. This gives us the best chances to see a difference within a session. Second, because the effect of stimulation was small, it was generally easiest to see the effects when there were no saccades to the receptive field in the control condition. Because the monkeys rarely made eye movements to the empty receptive field, an effect of stimulation should be easy to see in this condition. Finally, in all the other conditions, the mean probability of going to the receptive field increased by a similar amount in stimulation trials, even when this difference was not significant. However, in this case, the probability of going to the RF in stimulation trials was actually less than in the control trials. Thus we are certain that we are not inducing saccades by microstimulation; a stimulus must be in the receptive field for stimulation to be effective.
Although the increase in probability of making a saccade to an object in the receptive field was very small, it confirms the causal relationship between LIP activity and the selection of the eye movement goal. It is not clear why microstimulation appeared to be so ineffective; however, there are a number of confounding factors that could contribute to this. The first is that the current we used was ≥10 times less than that which has been shown to elicit saccades in LIP (Constantin et al. 2007; Shibutani et al. 1984; Thier and Andersen 1998). By keeping the current at such low levels, we aimed to bias behavior by stimulating a minimal number of neurons with similar or identical receptive field properties (Salzman et al. 1990) and to minimize the possibility that we were stimulating fibers of passage, thus producing effects caused by stimulating areas unrelated to LIP. Second, unlike earlier visual areas or later oculomotor areas, LIP has a relatively poor topographic organization of neurons based on receptive field location (Ben Hamed et al. 2001), so while we stimulated neurons with consistent receptive field locations, there were probably many more neurons with similar receptive fields that were not stimulated. In line with this, higher currents were needed to measure behavioral changes in an attentional task with LIP microstimulation (Cutrell and Marrocco 2002), and behavioral effects on saccade goal selection were only found with wide spread inactivation of LIP (Wardak et al. 2002). Finally, in this task, the animals were free to move their eyes to any location in space; no eye movement was an “error.” Thus unlike microstimulation in a two-alternative forced choice paradigm (Hanks et al. 2006) or a reaction time task with only two potential target locations (Cutrell and Marrocco 2002), the induced activity was competing with the physiological activity elicited by the task for all 10 objects, increasing the chance that it would not create a competent or competitive physiological response, which could be used to guide saccade target selection.
Hanks et al. (2006) showed a similar magnitude of bias as the result of LIP microstimulation in the context of a two-alternative forced choice direction discrimination task. Based on the data they presented, it is difficult for us to interpret whether the small effect could be caused by a small bias on all trials or a strong effect on a small number of trials. In either case, the bias of saccade direction in that study was interpreted as an increase in the accumulation of sensory evidence in favor of one of the choices. In this study, there is no clear correct choice, and evidence does not need to be accumulated over time in the way it does for noisy motion stimuli—the Ts and distractors are distinct shapes that can be differentiated early in the ventral visual pathway (Kobatake and Tanaka 1994). However, the end result is similar; in both tasks, saccades are made to the peak activity in LIP. In the two-alternative forced choice task, a threshold must first be passed (Kiani et al. 2008; Roitman and Shadlen 2002), but in tasks not requiring the accumulation of temporal evidence, a threshold may not be necessary. Thus our hypothesis covers both studies, suggesting that LIP acts to integrate sensory (bottom-up) and cognitive (top-down) information for prioritizing the guidance of eye movements (Gnadt and Andersen 1988; Ipata et al. 2006; Roitman and Shadlen 2002; Thomas and Pare 2007; Wardak et al. 2002) and covert attention (Bisley and Goldberg 2006; Herrington and Assad 2009; Shepherd et al. 2009; Wardak et al. 2004).
A similarly small effect was reported when the FEF was stimulated with the same current but with a shorter duration. Moore and Fallah (2001) improved monkeys' performance by ∼3.9% by injecting a low current into FEF in a spatial attention task. This same stimulation has been shown to drive the modulation of V4 activity, similar to what is seen because of attention (Moore and Armstrong 2003). These results have been interpreted as meaning that FEF microstimulation acts to focus attention on objects represented by the receptive field of the stimulated neurons. Given that we have suggested that LIP acts to guide attention as well as eye movements (Bisley and Goldberg 2010), it may be predicted that LIP stimulation should enhance discriminability and further reduce the number of eye movements made to a distractor, which is the opposite of what we found. However, it is important to remember that this enhanced information would be fed into the oculomotor system via LIP, which we are stimulating. Therefore, although there would be a reduction of priority for the distractor coming out of V4, there is still a strong bias being created by the artificial stimulation, which would override this signal.
LIP is interconnected with FEF and the superior colliculus (Andersen et al. 1990), which have also been implied in the allocation and control of attention and eye movements (Cavanaugh and Wurtz 2004; Ignashchenkova et al. 2004; Moore et al. 2003; Noudoost et al. 2010; Schall 2004) and, in particular, in the selection of visual targets for saccades (Bichot and Schall 1999; Thompson and Schall 1999). It has also been shown that subthreshold microstimulation of these areas biases the direction of saccades during target selection (Opris et al. 2005; Schafer and Moore 2007). Although the microstimulation of FEF and SC has an effect on the selection of the saccade goal, it seems that it has a more crucial role in preparing an appropriate movement to foveate the selected target. We suggest that LIP activity is driven by visual input (Fanini and Assad 2009; Sereno and Maunsell 1998), including the accumulation of sensory evidence in time-dependent tasks (Kiani et al. 2008; Roitman and Shadlen 2002), and by top-down influences, including reward information (Dorris and Glimcher 2004; Platt and Glimcher 1999; Sugrue et al. 2004), stimulus relevance (Gottlieb et al. 1998; Ipata et al. 2009; Mirpour et al. 2009), and motor preparation (Gnadt and Andersen 1988). This activity is summed to create a priority map (Ipata et al. 2009), which is fed forward to FEF and SC and used to guide saccades. Thus the peak in activity created by stimulation of LIP is passed to these structures, influencing the goal of the next saccade.
GRANTS
This work was supported by the Gerald Oppenheimer Family Foundation, the Kirchgessner Foundation, a Klingenstein Fellowship Award in the Neurosciences, an Alfred P. Sloan Foundation Research Fellowship, a McKnight Scholar Award, and National Eye Institute Grant R01 EY-019273-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank the members of the University of California–Los Angeles, The Divison of Laboratory Animal Medicine, for superb animal care.
REFERENCES
- Andersen et al., 1985. Andersen RA, Asanuma C, Cowan WM. Callosal and prefrontal associational projecting cell populations in area 7A of the macaque monkey: a study using retrogradely transported fluorescent dyes. J Comp Neurol 232: 443–455, 1985 [DOI] [PubMed] [Google Scholar]
- Andersen et al., 1990. Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol 296: 65–113, 1990 [DOI] [PubMed] [Google Scholar]
- Andersen et al., 1987. Andersen RA, Essick GK, Siegel RM. Neurons of area 7 activated by both visual stimuli and oculomotor behavior. Exp Brain Res 67: 316–322, 1987 [DOI] [PubMed] [Google Scholar]
- Balan and Gottlieb, 2006. Balan PF, Gottlieb J. Integration of exogenous input into a dynamic salience map revealed by perturbing attention. J Neurosci 26: 9239–9249, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan and Gottlieb, 2009. Balan PF, Gottlieb J. Functional significance of nonspatial information in monkey lateral intraparietal area. J Neurosci 29: 8166–8176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash et al., 1991. Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. Saccade-related activity in the lateral intraparietal area. I. Temporal properties; comparison with area 7a. J Neurophysiol 66: 1095–1108, 1991 [DOI] [PubMed] [Google Scholar]
- Ben Hamed et al., 2001. Ben Hamed S, Duhamel JR, Bremmer F, Graf W. Representation of the visual field in the lateral intraparietal area of macaque monkeys: a quantitative receptive field analysis. Exp Brain Res 140: 127–144, 2001 [DOI] [PubMed] [Google Scholar]
- Bichot and Schall, 1999. Bichot NP, Schall JD. Effects of similarity and history on neural mechanisms of visual selection. Nat Neurosci 2: 549–554, 1999 [DOI] [PubMed] [Google Scholar]
- Bisley and Goldberg, 2006. Bisley JW, Goldberg ME. Neural correlates of attention and distractibility in the lateral intraparietal area. J Neurophysiol 95: 1696–1717, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley and Goldberg, 2010. Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci 33: 1–21, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisley et al., 2001. Bisley JW, Zaksas D, Pasternak T. Microstimulation of cortical area MT affects performance on a visual working memory task. J Neurophysiol 85: 187–196, 2001 [DOI] [PubMed] [Google Scholar]
- Buschman and Miller, 2007. Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 315: 1860–1862, 2007 [DOI] [PubMed] [Google Scholar]
- Cavanaugh and Wurtz, 2004. Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci 24: 11236–11243, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland et al., 2008. Churchland AK, Kiani R, Shadlen MN. Decision-making with multiple alternatives. Nat Neurosci 11: 693–702, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin et al., 2007. Constantin AG, Wang H, Martinez-Trujillo JC, Crawford JD. Frames of reference for gaze saccades evoked during stimulation of lateral intraparietal cortex. J Neurophysiol 98: 696–709, 2007 [DOI] [PubMed] [Google Scholar]
- Cutrell and Marrocco, 2002. Cutrell EB, Marrocco RT. Electrical microstimulation of primate posterior parietal cortex initiates orienting and alerting components of covert attention. Exp Brain Res 144: 103–113, 2002 [DOI] [PubMed] [Google Scholar]
- Dorris and Glimcher, 2004. Dorris MC, Glimcher PW. Activity in posterior parietal cortex is correlated with the relative subjective desirability of action. Neuron 44: 365–378, 2004 [DOI] [PubMed] [Google Scholar]
- Duhamel et al., 1992. Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science 255: 90–92, 1992 [DOI] [PubMed] [Google Scholar]
- Fanini and Assad, 2009. Fanini A, Assad JA. Direction selectivity of neurons in the macaque lateral intraparietal area. J Neurophysiol 101: 289–305, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau and Munoz, 2006. Fecteau JH, Munoz DP. Salience, relevance, and firing: a priority map for target selection. Trends Cogn Sci 10: 382–390, 2006 [DOI] [PubMed] [Google Scholar]
- Gnadt and Andersen, 1988. Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res 70: 216–220, 1988 [DOI] [PubMed] [Google Scholar]
- Gottlieb et al., 2009. Gottlieb J, Balan P, Oristaglio J, Suzuki M. Parietal control of attentional guidance: the significance of sensory, motivational and motor factors. Neurobiol Learn Mem 91: 121–128, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb et al., 1998. Gottlieb JP, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature 391: 481–484, 1998 [DOI] [PubMed] [Google Scholar]
- Hanks et al., 2006. Hanks TD, Ditterich J, Shadlen MN. Microstimulation of macaque area LIP affects decision-making in a motion discrimination task. Nat Neurosci 9: 682–689, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays et al., 1982. Hays AV, Richmond BJ, Optican LM. Unix-based multiple-process system, for real-time data acquisition and control. WESCON Conf Proc 2: 1–10, 1982 [Google Scholar]
- Herrington and Assad, 2009. Herrington TM, Assad JA. Neural activity in the middle temporal area and lateral intraparietal area during endogenously cued shifts of attention. J Neurosci 29: 14160–14176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignashchenkova et al., 2004. Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat Neurosci 7: 56–64, 2004 [DOI] [PubMed] [Google Scholar]
- Ipata et al., 2009. Ipata AE, Gee AL, Bisley JW, Goldberg ME. Neurons in the lateral intraparietal area create a priority map by the combination of disparate signals. Exp Brain Res 192: 479–488, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipata et al., 2006. Ipata AE, Gee AL, Goldberg ME, Bisley JW. Activity in the lateral intraparietal area predicts the goal and latency of saccades in a free-viewing visual search task. J Neurosci 26: 3656–3661, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti and Koch, 2000. Itti L, Koch C. A saliency-based search mechanism for overt and covert shifts of visual attention. Vision Res 40: 1489–1506, 2000 [DOI] [PubMed] [Google Scholar]
- Itti and Koch, 2001. Itti L, Koch C. Computational modelling of visual attention. Nat Rev Neurosci 2: 194–203, 2001 [DOI] [PubMed] [Google Scholar]
- Kiani et al., 2008. Kiani R, Hanks TD, Shadlen MN. Bounded integration in parietal cortex underlies decisions even when viewing duration is dictated by the environment. J Neurosci 28: 3017–3029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobatake and Tanaka, 1994. Kobatake E, Tanaka K. Neuronal selectivities to complex object features in the ventral visual pathway of the macaque cerebral cortex. J Neurophysiol 71: 856–867, 1994 [DOI] [PubMed] [Google Scholar]
- Koch and Ullman, 1985. Koch C, Ullman S. Shifts in selective visual attention: towards the underlying neural circuitry. Hum Neurobiol 4: 219–227, 1985 [PubMed] [Google Scholar]
- Mirpour et al., 2009. Mirpour K, Arcizet F, Ong WS, Bisley JW. Been there, seen that: a neural mechanism for performing efficient visual search. J Neurophysiol 102: 3481–3491, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore and Armstrong, 2003. Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature 421: 370–373, 2003 [DOI] [PubMed] [Google Scholar]
- Moore et al., 2003. Moore T, Armstrong KM, Fallah M. Visuomotor origins of covert spatial attention. Neuron 40: 671–683, 2003 [DOI] [PubMed] [Google Scholar]
- Moore and Fallah, 2001. Moore T, Fallah M. Control of eye movements and spatial attention. Proc Natl Acad Sci USA 98: 1273–1276, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noudoost et al., 2010. Noudoost B, Chang MH, Steinmetz NA, Moore T. Top-down control of visual attention. Curr Opin Neurobiol 20: 183–190, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opris et al., 2005. Opris I, Barborica A, Ferrera VP. Microstimulation of the dorsolateral prefrontal cortex biases saccade target selection. J Cogn Neurosci 17: 893–904, 2005 [DOI] [PubMed] [Google Scholar]
- Platt and Glimcher, 1999. Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature 400: 233–238, 1999 [DOI] [PubMed] [Google Scholar]
- Roitman and Shadlen, 2002. Roitman JD, Shadlen MN. Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci 22: 9475–9489, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman et al., 1990. Salzman CD, Britten KH, Newsome WT. Cortical microstimulation influences perceptual judgements of motion direction. Nature 346: 174–177, 1990 [DOI] [PubMed] [Google Scholar]
- Schafer and Moore, 2007. Schafer RJ, Moore T. Attention governs action in the primate frontal eye field. Neuron 56: 541–551, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall, 2004. Schall JD. On the role of frontal eye field in guiding attention and saccades. Vision Res 44: 1453–1467, 2004 [DOI] [PubMed] [Google Scholar]
- Serences and Yantis, 2006. Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci 10: 38–45, 2006 [DOI] [PubMed] [Google Scholar]
- Sereno and Maunsell, 1998. Sereno AB, Maunsell JH. Shape selectivity in primate lateral intraparietal cortex. Nature 395: 500–503, 1998 [DOI] [PubMed] [Google Scholar]
- Shepherd et al., 2009. Shepherd SV, Klein JT, Deaner RO, Platt ML. Mirroring of attention by neurons in macaque parietal cortex. Proc Natl Acad Sci USA 106: 9489–9494, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibutani et al., 1984. Shibutani H, Sakata H, Hyvarinen J. Saccade and blinking evoked by microstimulation of the posterior parietal association cortex of the monkey. Exp Brain Res 55: 1–8, 1984 [DOI] [PubMed] [Google Scholar]
- Sugrue et al., 2004. Sugrue LP, Corrado GS, Newsome WT. Matching behavior and the representation of value in the parietal cortex. Science 304: 1782–1787, 2004 [DOI] [PubMed] [Google Scholar]
- Thier and Andersen, 1998. Thier P, Andersen RA. Electrical microstimulation distinguishes distinct saccade-related areas in the posterior parietal cortex. J Neurophysiol 80: 1713–1735, 1998 [DOI] [PubMed] [Google Scholar]
- Thomas and Pare, 2007. Thomas NW, Pare M. Temporal processing of saccade targets in parietal cortex area LIP during visual search. J Neurophysiol 97: 942–947, 2007 [DOI] [PubMed] [Google Scholar]
- Thompson and Schall, 1999. Thompson KG, Schall JD. The detection of visual signals by macaque frontal eye field during masking. Nat Neurosci 2: 283–288, 1999 [DOI] [PubMed] [Google Scholar]
- Wardak et al., 2002. Wardak C, Olivier E, Duhamel JR. Saccadic target selection deficits after lateral intraparietal area inactivation in monkeys. J Neurosci 22: 9877–9884, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardak et al., 2004. Wardak C, Olivier E, Duhamel JR. A deficit in covert attention after parietal cortex inactivation in the monkey. Neuron 42: 501–508, 2004 [DOI] [PubMed] [Google Scholar]