Abstract
The accessory olfactory bulb (AOB), the first relay of chemosensory information in the Vomeronasal system, receives extensive cholinergic innervation from the basal forebrain. Cholinergic modulation of neuronal activity in the olfactory bulb has been hypothesized to play an important role in olfactory processing; however, little is known about the cellular actions of acetylcholine (ACh) within the AOB. Here using in vitro slice preparation, we show that muscarinic acetylcholine receptor (mAChR) activation increases neuronal excitability of granule and mitral/tufted cells (GCs and MCs) in the AOB. Activation of mAChRs increased excitability of GCs by three distinct mechanisms: induction of a long-lasting depolarization, activation of a slow afterdepolarization (sADP), and an increase in excitatory glutamatergic input due to MC depolarization. The depolarization and sADP were elicited by the selective agonist 4-[[[(3-chlorophenyl)amino]carbonyl]oxy]-N,N,N-trimethyl-2-butyn-1-aminium chloride (100 μM) and blocked by low concentrations of pirenzepine (300 nM), indicating that they result from activation of M1-like mAChRs. In contrast, cholinergic stimulation increased the excitability of MCs via recruitment of nicotinic AChRs (nAChRs) and M1-like mAChRs. Submaximal activation of these receptors, however, decreased the excitability of MCs. Surprisingly, we found that unlike GCs in the main olfactory bulb, GCs in the AOB are excited by mAChR activation in young postnatal neurons, suggesting marked differences in cholinergic regulation of development between these two regions of the olfactory bulb.
INTRODUCTION
The olfactory bulb (OB) is the site of initial information processing in the olfactory pathway. The most abundant neurons within the main and accessory OB (MOB and AOB, respectively) are the inhibitory granule cells (GCs). The GCs regulate the excitability of the principal projection neurons, the mitral and tufted cells (MCs) through GABAergic inhibition at reciprocal dendrodendritic synapses (Shepherd and Greer 1998). The processing of sensory information in the OB, and the relay of this information to higher centers by the MCs is crucial for the survival of most mammals (e.g., feeding and mating). The inhibitory synapses from GCs to MCs play an important role in olfactory processing and are the target of several afferent neuromodulatory systems to the OB (Ennis et al. 2007; Schoppa and Urban 2003).
The OB receives a rich cholinergic innervation from the nucleus of the horizontal limb of the diagonal band of Broca (HDB), located in the basal forebrain, which has divergent projections that innervate all layers of the OB (Kasa et al. 1995; Le Jeune and Jourdan 1991; Le Jeune et al. 1995; Nickell and Shipley 1988; Ojima et al. 1988; Zaborszky et al. 1986). Two types of cholinergic receptors, nicotinic and muscarinic receptors, mediate the actions of acetylcholine (ACh) throughout the brain (nAChRs and mAChRs, respectively). ACh receptors are further divided into subtypes that elicit distinct cellular effects on activation, thereby providing a diverse array of mechanisms to regulate neuronal activity (Gotti et al. 2006; Lanzafame et al. 2003). Both nAChRs and mAChRs are found in the OB, albeit with a differential pattern of distribution, suggesting that selective activation of these receptors could modulate different aspects of olfactory processing (Hill et al. 1993; Keiger and Walker 2000; Le Jeune et al. 1996; Whiteaker et al. 2009).
In the MOB, the cellular and behavioral consequences of cholinergic neuromodulation have been extensively studied. For example, blocking the cholinergic input to the MOB has a profoundly deleterious effect on odor discrimination, while enhancing cholinergic activity improves discrimination between chemically similar odorants (Linster et al. 2001; Mandairon et al. 2006; Ravel et al. 1994). In addition, in vitro studies have indicated that MCs and GCs are differentially modulated by the cholinergic system. Both inhibitory and excitatory muscarinic effects have been described in GCs while excitatory nicotinic and muscarinic effects have been described in MCs (Castillo et al. 1999; Pignatelli and Belluzzi 2008; Pressler et al. 2007). Furthermore, recent studies in the MOB have suggested that neuromodulation by the cholinergic system is developmentally regulated (Ghatpande and Gelperin 2009; Ghatpande et al. 2006). Thus in early postnatal development (<10 days), cholinergic neuromodulation exists only in MCs, while later in development, GCs begin to exhibit a cholinergic excitatory effect (Ghatpande and Gelperin 2009). This developmental shift in cholinergic neuromodulation may have an important implication for the functioning of developing GCs and/or their role in perinatal olfactory mediated behaviors (Brennan and Keverne 1997).
Despite the crucial role of the AOB in the processing of pheromonal information by the Vomeronasal system (Halpern and Martinez-Marcos 2003), the targets and cellular effects of the cholinergic system in this region remain insufficiently understood. Neuromodulation of the AOB circuitry by afferent input is thought to promote the structural and functional synaptic plasticity underlying AOB mediated behaviors that require learning (Brennan and Keverne 1997). Specifically, extensive studies have shown that modulation of AOB circuitry by the noradrenergic system underlies the formation of memory in the Bruce effect in mice (Brennan and Peele 2003). Here we characterize the cellular actions of cholinergic modulation in the AOB using whole cell recordings of GCs and MCs. Using selective pharmacological agents, we show that activation of an M1-like mAChR produces a long-lasting excitation in both GCs and MCs. However, the mAChR-mediated excitation differed between these cells. In GCs but not MCs, M1-like mAChR activation also elicited the appearance of a stimulus-driven slow afterdepolarization. In addition, cholinergic stimulation in MCs also involves the recruitment of ionotropic nAChRs. Surprisingly, we find that unlike the developmental shift in excitatory muscarinic response observed in the MOB, the M1-like excitatory action in GCs is present in the AOB from early postnatal ages throughout adulthood in the AOB. Together, these results indicate that GCs are directly excited by muscarinic receptor activation, resulting in an increase in the inhibitory input onto MCs, thereby decreasing MC activity. Concomitantly, stimulation of either nicotinic or muscarinic receptors would increase MC activity, resulting in an opposite effect on bulbar output. However, under submaximal cholinergic stimulation, only the inhibitory effect onto MCs prevails. Further, our study also provides evidence for developmental differences in the function of cholinergic modulation controlling neuronal components of the AOB compared with the MOB.
METHODS
Slice preparation
Experiments were performed in OB slices obtained from postnatal days 5–60 (P5 to P60) C57/BL6 mice using methods previously described (Smith et al. 2009). Male and female mice were used for our experiments as we did not find any gender differences in cholinergic effects. Briefly, animals were deeply anesthetized with isoflurane and decapitated, and brain slices were quickly prepared using a modified sucrose artificial cerebrospinal fluid (ACSF). A block of tissue, containing part of the frontal lobes and the olfactory bulbs, was glued with cyanoacrylate to a microslicer stage and bathed in chilled sucrose ACSF. Sagittal sections (250 μm) of the OB containing the AOB were sliced using a Leica vibrating microslicer (Redding, CA). Slices were then transferred to an incubation chamber containing normal ACSF (see following text) and left to recuperate first at 35°C for 30 min and then at room temperature until use. In all experiments, unless otherwise stated, the extracellular solution is ACSF with the following composition (in mM): 125 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 3 KCl, 2 CaCl2, 1 MgCl2, 3 myo-inositol, 0.3 ascorbic acid, 2 Na-pyruvate, and 15 glucose, continuously oxygenated (95% O2-5% CO2) to give a pH 7.4 and an osmolarity of ∼305 mosM.
Slices were placed in a submerged recording chamber mounted on the stage of an Olympus BX51, upright microscope, fitted with differential infrared interference contrast (IR-DIC) optics. Slices were observed with a ×40 water immersion objective and visualized using a CoolSNAP EZ camera (Photometrix, Tucson, AZ). Granule cells (GCs) were recognized by their morphology and position within the slices; in the AOB the lateral olfactory tract separates the GC layer from the mitral/tufted cell (MC) layer. Likewise, MCs were recognized by their spatial location and morphology. Most experiments, unless indicated, were carried out in the current-clamp mode using standard patch pipettes (3–7 MΩ resistance) pulled on a horizontal puller (Sutter, CA). All experiments were performed at room temperature.
Data acquisition and analysis
Recordings were performed using a dual EPC10 amplifier in current-and voltage-clamp mode (HEKA, Union City, NY). Data analysis was performed using macros written for the Igor Pro software (Wavemetrics, Wosego, OR). To elicit the slow afterdepolarization (sADP), GCs were stimulated every 30 s with a depolarizing current stimulus (500 ms) of variable intensity adjusted to elicit 3–12 action potentials. The afterhyperpolarization (AHP) was measured as the most negative value of membrane potential following the depolarizing stimulus, and its peak usually occurred within 100 ms after the end of the pulse. The sADP was measured as the most positive value of membrane potential after the end of the pulse and its peak generally occurred within 5–10 s of the end of stimulus. The baseline value of membrane potential prestimulus was subtracted from each of these values; therefore the reported values of sADP correspond to the ΔV. The size of the sADP reported here corresponds to averages of the largest sADP recorded in different cells in the presence of agonist or agonist plus antagonist. To quantify the increase in synaptic activity induced by mAChR activation, we calculated the frequency of spontaneous excitatory potentials before and after oxotremorine (Oxo) addition. The average dose-response curve (DRC) for nicotine (Nic) was obtained from cells where at least three different concentrations of nicotine were applied, including 30 μM, which was used to normalize the responses. The DRC for Oxo was obtained in each cell using a concentration range of 0.3–10 μM, and the responses were normalized to 10 μM. For Nic and Oxo, the DRC for each cell was fitted to the Hill equation using the IgorPro software. The current-voltage relation for Nic in MCs was obtained using a ramp protocol from –120 to +60 mV (300 mV/ms) and in the presence of Ni 100 μM, Cd 100 μM, d-2-amino-5-phosphonopentanoic acid (APV) 100 μM, 2,3-dihydroxy-6-nitro-7-sulfamoybenzo-(f)-quinoxaline (NBQX) 10 μM, BMI 10 μM, and TTX 1 μM. All values reported correspond to results from at least three experiments and error bars indicate the SE. Statistical differences were assessed by the paired t-test.
Solutions and pharmacological agents
In current-clamp experiments, the internal solution had the following composition (in mM): 120 K-gluconate, 10 Na-Gluconate, 4 NaCl, 10 HEPES-K, 10 Na phosphocreatine, 2 Na-ATP, 4 Mg-ATP, and 0.3 GTP adjusted to pH 7.3 with KOH. In voltage-clamp, the internal solution had the following composition (in mM): 125 Cs-gluconate, 4 NaCl, 2 MgCl2, 2 CaCl2, 2 EGTA, 10 HEPES, 2 Na-ATP, 4 Mg-ATP, and 0.3 GTP adjusted to pH 7.3 with CsOH. The osmolarity of the internal solutions was adjusted to 290–305 mosM. The following drugs were bath applied: N,N,N-trimethyl-4-(2-oxo-1-pyrolidinyl)-2-butyn-1-ammonium iodide (Oxo), 5,11-dihydro-11-[(4-methyl-1-piperazinyl) acetyl]-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one dihydrochloride (pirenzepine), 4-[[[(3-chlorophenyl)amino]carbonyl]oxy]-N,N,N-trimethyl-2-butyn-1-aminium chloride (MCN-A-343), 1,1-dimethyl-4-diphenylacetoxypiperidinium iodide (4-DAMP), 6-imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide (GABAzine), 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX), APV, NBQX, (−)-cytisine (Cys), mecamylamine hydrochloride (MM), dihydro-β-erythroidine hydrobromide (DHBE), (−)-nicotine ditartrate (Nic), methyllycaconitine citrate (MLA), LY367385, tetrodotoxin (TTX), and N-methyl-d-glucamine (NMDG). The speed of perfusion allowed for full solution exchange in <1 min. Antagonists were applied for ≥10 min, and drug washouts lasted for ≥10 min. All drugs were purchased from Tocris Cookson (UK).
RESULTS
M1-like muscarinic acetylcholine receptor activation excites granule cells
At least five different muscarinic receptor types have been identified all of which can be activated by the nonselective agonist Oxo. Application of Oxo (30 μM, 2–3 min) produced a long-lasting depolarization of GCs (14.8 ± 1.0 mV; n = 45, Fig. 1A, left trace). The depolarization had a slow onset (>45 s) and typically persisted several minutes (>10 min) after washout. In addition, Oxo induced the appearance of a sADP following a stimulus-evoked train of action potentials (5–50 pA; 500 ms, Fig. 1A, right). In control, a depolarizing current stimulus elicited several nonaccommodating spikes, which were followed by a small AHP at the end of the stimulus pulse (Fig. 1A, middle, ↓, –1.7 ± 0.3 mV, n = 4). Following this AHP (∼2 s), the membrane potential was not significantly different from baseline (baseline, −66.4 ± 2.0 mV; after stimulus, −66.5 ± 2.0 mV, n = 4. In the presence of Oxo, the stimulus pulse produced an increase in the number of evoked action potentials and the AHP was now overridden by a sADP (5.0 ± 0.3 mV, n = 20, Fig. 1A, right). The depolarization and the sADP were significantly reduced by pirenzepine (Pir, 300 nM), which at low concentrations selectively blocks M1 muscarinic acetylcholine receptors (M1-mAChR; Fig. 1B, left trace). In the presence of pirenzepine (300 nM), the depolarization was reduced by ∼88% (control, 14.0 ± 0.9 mV; in Pir, 1.6 ± 0.7 mV; P < 0.0005; n = 7, Fig. 1D, top) while the sADP was reduced by ∼92% (control, 5.7 ± 0.4 mV; in Pir, 0.5 ± 0.3 mV; P < 0.0002; n = 6, Fig. 1D, bottom). Furthermore, application of the selective M1-mAChR agonist MCN-A-343 (100 μM) mimicked the depolarization and sADP produced by Oxo (depolarization, 10.9 ± 1.6 mV; sADP, 4.4 ± 0.5 mV; n = 11, Fig. 1C, right and middle traces, and D). Activation of M1 and M3 mAChRs produce similar effects in various neuronal types (Lanzafame et al. 2003); however, the selective M3-mAChR antagonist 4-DAMP (300 nM) did not affect the depolarization induced by Oxo (depolarization; 15.3 ± 1.3 mV; sADP 4.5 ± 0.3 mV, n = 4; data not shown). Like Oxo, application of a high concentration of nicotine (300 μM) depolarized and increased excitatory synaptic activity in GCs (21.2 ± 0.6 mV, n = 5; Fig. 2A). However, these excitatory responses were drastically reduced in the presence of blockers of glutamatergic synaptic transmission (2.0 ± 0.6 mV; in 10 μM CNQX, 100 μM APV, and 100 μM LY367385; P < 0.002; Fig. 2C), indicating that this depolarization does not result from a direct nicotinic effect on GCs (see also following text).
Fig. 1.

Muscarinic acetylcholine receptor (mAChR) agonists excite granule cells (GCs). A, left: bath application of the nonselective muscarinic agonist oxotremorine (Oxo, 30 μM, 2 min) produced a robust membrane depolarization and sustained firing of action potentials. Right: in addition to membrane depolarization, Oxo (30 μM, 3 min) induced the appearance of a slow afterdepolarization (sADP) following a stimulus-induced train of action potentials (20 pA, 500 ms, right trace). In control the action potentials are followed by a small AHP (↓, see text). B, left trace: low concentration of the M1 mAChR antagonist pirenzepine (Pir, 300 nM) greatly reduced the Oxo-induced depolarization and sADP (right traces). Responses in A and B are from the same cell, the calibration bar is 20 mV and 3 min (traces on the left) and 10 mV and 2 s (traces on the right). The resting membrane potential (RMP) is −65 mV. C: the M1 mAChR agonist 4-[[[(3-chlorophenyl)amino]carbonyl]oxy]-N,N,N-trimethyl-2-butyn-1-aminium chloride (MCN-A-343; 100 μM, 3 min) mimics the Oxo-induced depolarization and sADP. Right: superimposed traces showing the stimulus-induced sADP obtained in control and MCN-A-343 from the cell in the middle panel. The calibration bar is 20 mV and 3 min (left traces) and 10 mV and 2 s (right traces); the RMP is −61 mV (left) and −64 mV (right). D: summary of the pharmacological profile of the excitatory muscarinic response in GCs. Pir (□) significantly reduced the Oxo-induced depolarization (■) and sADP (depolarization, top, *P < 0.0005; sADP, bottom, *P < 0.0002). Both excitatory effects were mimicked by MCN-A-343 (▨).
Fig. 2.
Muscarinic but not nicotinic acetylcholine receptor activation directly excites GCs. A, top: bath application of nicotine (Nic, 300 μM, 1 min) produced a robust depolarization that elicited firing of action potentials and increase in excitatory synaptic activity. Application of a mixture of glutamate receptor (GluR) blockers including 100 μM LY367385, 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX), and 100 μM d-2-amino-5-phosphonopentanoic acid (APV) produced a decrease in the excitatory synaptic activity and greatly reduced the response to nicotine. The calibration bar is 20 mV and 1 min. Bottom: in the presence of the same mixture of GluR blockers, application of carbachol (30 μM, 3 min) produced a robust depolarization and the appearance of a sADP following a stimulus-induced train of action potentials (20 pA, 500 ms, right trace). The calibration bar is 20 mV and 1 min for the left trace and 10 mV and 2 s for the right-hand traces. The RMP is −60 mV (top) and −61 mV (bottom). B: graph bar summarizing the effects of GluR blockers on the excitatory responses to carbachol (30 μM) and nicotine (300 μM). The nicotinic excitatory response (■) is significantly reduced in the presence of the blockers (□, * P < 0.002), while the response to carbachol is not affected (▨; see text).
Application of Oxo (30 μM) also produced an increase in synaptic activity in GCs most likely due to an increase in glutamatergic excitation at dendrodendritic synapses. This excitatory response results from activation of muscarinic receptors in MCs (see following text); accordingly, application of Oxo increased the frequency of excitatory postsynaptic potentials (EPSPs) by fourfold (baseline, 2.4 ± 0.6 Hz; Oxo, 9.4 ± 2 Hz; n = 4), and this effect was greatly reduced in the presence of ionotropic glutamate receptor blockers (NBQX and APV, 10 and 100 μM, respectively; Oxo plus blockers; 3.0 ± 0.8 Hz, P < 0.02; n = 4). Nevertheless, in the presence of the fast synaptic transmission blockers, the M1-like mAChR-induced depolarization of GCs was reduced only slightly (Oxo control, 16.0 ± 1.2 mV; Oxo plus blockers, 14.2 ± 1.7 mV; P < 0.3; n = 5). These results indicate the increase in the excitatory synaptic drive onto GCs only partially contributes to the M1-like mAChR-induced depolarization in GCs. Furthermore, we have previously shown that activation of metabotropic glutamate receptor type 1 (mGluR1) and α1 adrenergic receptors produce an excitatory response and activation of a sADP in GCs. In these studies, the α1 excitatory response was reduced by blockers of mGluR1 receptors, suggesting that α1 adrenergic receptor activation potentiates a basal mGluR1 activity (Smith et al. 2009). We wondered if activation of muscarinic receptors in GCs could act through a similar mechanism. Therefore we recorded GC responses to carbachol (30 μM) in the presence of a mixture of glutamate receptors blockers that included 100 μM LY367385; 10 μM CNQX, and 100 μM APV. As shown in Fig. 2A, in the presence of these blockers carbachol still produced a robust depolarization and the stimulus-induced sADP (depolarization: 15.3 ± 2.0 mV; sADP, 4.8 ± 0.5 mV; n = 4, Fig. 2C). Similarly, the depolarization and sADP produced by Oxo were not affected by 100 μM LY367385 (depolarization, 17.3 ± 1.2 mV; sADP, 5.8 ± 0.1 mV; n = 9, not shown). These results further indicate that the muscarinic response results from a direct action in GCs and that this excitatory response is not dependent on activation of mGluR1.
The muscarinic-induced depolarization and sADP are qualitatively similar to those previously described in the GCs of the MOB as well as in olfactory cortex (Constanti et al. 1993; Libri et al. 1994; Pressler et al. 2007). Interestingly, we have shown that activation of α1-adrenergic receptors also produces a similar excitatory effect in the GCs of the AOB (Smith et al. 2009). Further, the physiological and pharmacological properties of the excitation produced by Oxo and α1-adrenergic receptor agonists on GCs have indicated that activation of these receptors results in the recruitment of a nonselective cationic current, ICAN (Pressler et al. 2007; Smith et al. 2009). Accordingly, reducing the driving force for sodium ions by lowering the extracellular concentration to 10 mM by replacing the external Na ions with N-methyl-glucamine (NMGM), and in the presence of TTX (0.5 μM), greatly reduced the depolarization (control, 14.0 ± 3.5 mV; NMGM, 3.3 ± 0.3 mV; P < 0.05; n = 3, Fig. 3B) and sADP (control, 4.7 ± 0.6 mV; NMGM, 0.7 ± 0.1 mV; P < 0.02; n = 3, B) induced by M1-like mAChR activation. We note that extracellular Na substitution did not significantly reduce the AHP that follows the stimulus-induced action potentials in GCs (control, −1.8 ± 0.1 mV; NMGM, −1.5 ± 0.5 mV; n = 4, Fig. 3A, AHP indicated by ↓), indicating that the equilibrium potential for K ions was not perturbed under these conditions. A recent study in the AOB indicated that the increase in GABA mIPSC frequency recorded in MCs is sensitive to blockers of the M-current (Takahashi and Kaba 2010). However, we found that the selective blocker of the KCNQ K-channel, XE-991 (50 μM), failed to reduce the depolarization produced by Oxo (10 μM) (Oxo plus XE-991, 16.5 ± 1.5 mV; n = 3, data not shown). Together this suggests that the excitatory action produced by M1-like mAChR activation in GCs in the AOB is due to the activation of ICAN.
Fig. 3.
The sADP and depolarization is dependent on extracellular Na. A, top: in the presence of fast synaptic transmission blockers and TTX (1 μM, NBQX 10 μM, and APV 100 μM, see text), Oxo (30 μM) depolarized GCs (not shown) and induced the appearance of sADP (right trace; 5.1 mV in this cell) following a current stimulus (25 pA, 500 ms). Bottom traces: the extracellular Na concentration was reduced to 10 mM with iso-osmolar replacement with NMDG. In low Na, the Oxo-induced sADP following current stimulus (50 pA, 500 ms) and a depolarization (not shown) were almost completely abolished. The dotted line indicates the membrane potential before the depolarizing stimulus, control −64 mV, low Na −67 mV. ↓, the AHP following the current stimulus is not reduced in the low-Na solution (bottom right trace, see text). The calibration bar is 2 s and 10 mV, and 200 ms and 10 mV for the inset. B: graph bar summarizing the effects of low extracellular Na concentration (□) on the depolarization and sADP elicited by Oxo (30 μM); both the depolarization and sADP are significantly reduced in low Na (* P < 0.05, see text).
M1-like induced depolarization has a young age onset in granule cells
Recent studies have indicated that the muscarinic-induced excitation of GCs is developmentally regulated in the MOB (Ghatpande and Gelperin 2009; Ghatpande et al. 2006). Thus GCs at postnatal day 10 (P10) or younger do not exhibit a direct M1-mAChR excitatory response in the MOB (Ghatpande and Gelperin 2009). Surprisingly, in the AOB the M1-like mAChR mediated excitation of GCs was present at postnatal age younger than P10. As shown in Fig. 4A, the effect of Oxo (30 μM) was qualitatively similar at early postnatal days (P6, Fig. 4A, top left) to that of the adult (P60, 4A, top right), and both the depolarization and sADP were present (depolarization 16 ± 1 mV; sADP, 5.1 ± 0.3 mV; n = 9, Fig. 4A, inset). Additionally, Oxo increased the frequency of spontaneous EPSPs by about fourfold in these young postnatal GCs, consistent with activation of mAChRs in MCs (see following text, baseline, 0.5 ± 0.1 Hz; Oxo, 2.2 ± 0.5 Hz; n = 4). Furthermore, when we grouped the responses by age, we found no significant differences between cells at P < 10 days, onward (Fig. 4B). Additionally, the excitatory effect of Oxo at P6 was abolished by Pir (300 nM), which at nanomolar concentrations blocks M1 mAChRs (control: 13.3 ± 1.2 mV; in Pir, 0.9 ± 0.3 mV; P < 0.01, n = 3, Fig. 4C). More importantly, at P6, blockers of ionotropic glutamatergic receptors (10 μM NBQX and 100 μM APV) did not significantly reduce the Oxo induced excitatory response, suggesting a direct effect of Oxo on GCs (control, 16.6 ± 2.2 mV; Oxo plus blockers, 11.5 ± 1.3 mV, n = 5, P < 0.06, Fig. 4C). Thus GCs in the AOB do not exhibit a developmentally triggered switch on the site of action of M1-mAChR-mediated excitation as it has been shown in GCs of the MOB (Ghatpande and Gelperin 2009).
Fig. 4.
Excitatory muscarinic responses in GCs are present from early postnatal days. A: GCs recorded in slices from postnatal day 6 (P-6, left trace) exhibit a robust depolarization and stimulus-induced sADP (inset) in the presence of Oxo (30 μM). This response is qualitatively similar to the excitatory muscarinic response in adult mice (P-60, right trace). The RMP of both cells is –67 mV; calibration bar is 20 mV and 1 min and 10 mV and 1 s for the inset. B: bar graph showing the average depolarization in postnatal, age-grouped, cells. No significant difference is observed in the degree of depolarization induced by Oxo (30 μM) in these different groups. C: the muscarinic depolarization response in the young mice is insensitive to blockers of excitatory fast synaptic transmission (10 μM NBQX, and 100 μM APV, P < 0.06), but it was greatly reduced by the selective M1 mAChR antagonist Pir (300 nM, P < 0.01).
Nicotinic and M1 muscarinic acetylcholine receptor activation excites mitral cells
Cholinergic projections are found throughout the layers of the OB suggesting the potential regulation of different neuronal populations by this neuromodulatory system (Le Jeune and Jourdan 1991; Le Jeune et al. 1995; Ojima et al. 1988). To this end, application of the nonselective muscarinic agonist Oxo (30 μM, 2–3 min) also depolarized MCs in the AOB (12.8 ± 1.0 mV; n = 25, Fig. 5A). This depolarization was greatly reduced in the presence of Pir (300 nM; control, 13.0 ± 0.8 mV; in Pir, 1.3 ± 0.7 mV; P < 0.0001; n = 6, Fig. 5, B, right, and D) and mimicked by MCN-A-343 (100 μM; 9.5 ± 0.6 mV; n = 4, Fig. 5B, left). These results suggest that the muscarinic-induced depolarization in MCs is due to the activation of M1-like mAChRs. Surprisingly, activation of mAChRs was not accompanied by a stimulus-induced sADP in MCs. Thus at 5 s poststimulus (see methods), the membrane potential was similar in the absence and presence of Oxo (Fig. 5A, right). On the other hand, we observed an increase in the size of the AHP triggered by the stimulus, consistent with the depolarization of the membrane potential by Oxo (control, −2.1 ± 0.2 mV; Oxo, −4.9 ± 0.5 mV; P < 0.02; n = 3, data not shown). One possibility is that the sADP in MCs is relatively small and was therefore masked by recurrent inhibition from GCs triggered by our stimulus protocol. To test this possibility, we applied Oxo in the presence of blockers of glutamate (100 μM APV and 10 μM NBQX) and GABA ionotropic receptors (5 μM, GABAzine, Fig. 5C). In the presence of these blockers, the current-stimulus still failed to induce a sADP (baseline before stimulus, −56.0 ± 1 mV; after stimulus, −55.9 ± 0.9 mV; n = 3, Fig. 5C, bottom), while the depolarization produced by Oxo was not significantly different (control, 15.0 ± 2.7 mV; plus blockers, 16.4 ± 3.5 mV; n = 7, data not shown). These results suggest that the M1-mAChR depolarization in MCs is mechanistically different in MCs versus GCs.
Fig. 5.
M1-mAChR activation produces an excitatory response in mitral and tufted cells (MCs). A: MCs are depolarized by Oxo (30 μM; 3 min, left), but a train of stimulus-induced action potentials is not followed by a sADP (stimulus: 75 pA, 500 ms, right traces). Compared with GCs, the depolarization elicited by Oxo in MCs has a faster onset (<45 s), but it similarly lasted several minutes (>10 min, see Fig. 1). The RMP in this cell is –62 mV; the calibration bar is 20 mV and 1 min (left) and 10 mV and 1 s (right). B: the excitatory effect of Oxo is mimicked by the selective M1 mAChR agonist MCN-A-343 (100 μM, 2 min, left) and greatly reduced by Pir (300 nM, right). The RMP in these cells is –62 and –66 mV, respectively. C: Oxo (30 μM) still produced a robust depolarization in the presence of blockers of fast excitatory and inhibitory synaptic transmission (100 μM APV, 10 μM CNQX, 5 μM GABAzine). In the presence of blockers, the sADP was still present. D: bar graph summarizing the effects of selective mAChR agonist and antagonists in MCs. The depolarizing response of Oxo (30 μM, ■) was significantly decreased in the presence of Pir (300 nM, □, P < 0.02) and mimicked by MCN-A-343 (100 μM, ▨).
The cholinomimetic carbachol (CCh, 30 μM; 2–3 min) also depolarized MCs; however, under our recording conditions, the onset of this response was faster than in the Oxo response (CCh, 37.5 ± 3.2 s; Oxo, 104.1 ± 9.0 s; P < 0.005; n = 4, data not shown). In addition, application of Pir (300 nM) only partially reduced the depolarization produced by CCh (control, 12.0 ± 1.3 mV; in Pir, 8.75 ± 1.1 mV; P < 0.004; n = 4) while the onset of the response was unchanged (CCh control, 37.5 ± 3.2 s; in Pir, 37.2 ± 3.0 s; n = 4). The faster onset and partial sensitivity to Pir suggests that the depolarizing response produced by CCh is due to activation of mAChR and nicotinic AChRs (nAChR). Accordingly, application of the selective nAChR agonist Nic (30 μM) resulted in depolarization of MCs that in most cells resulted in robust firing (Fig. 6A, n = 7).
Fig. 6.
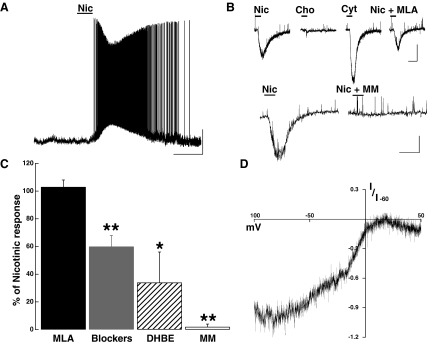
Nicotinic AChR activation excites MCs. A: bath application of Nic (30 μM, 1 min) produced a fast-onset depolarization in MCs (<20 s). The membrane potential in this cell is −66 mV. Calibration bar is 20 mV and 2 min. B, top: voltage-clamp recordings showing nAChR activated inward currents in presence of selective agonists and antagonists in the same cell. Nic (10 μM) produced a fast onset inward current (−153 pA), Cho (100 μM) failed to produce an inward current, while Cyt (10 μM) produced a larger response than Nic (−292 pA). The response to Nic was not significantly reduced in the presence of the a7-containing nAChR antagonist MLA (10 nM, −108 pA). Bottom trace: in a different cell, the response to Nic (30 μM, −231 pA) was completely abolished in the presence of MM (30 μM). For both cells, the calibration bar is 100 pA and 1 min. C: sensitivity of the nicotinic response to selective antagonists; the response to Nic was 103 ± 5% in MLA 10 nM, in Blockers (NBQX, APV, BMI and TTX) 60 ± 8%, 34 ± 22% in dihydro-β-erythroidine hydrobromide (DHBE) and 2 ± 2% in mecamylamine hydrochloride (MM, see text, *P < 0.05, ** P < 0.02). D: the inward current produced by Nic (30 μM) showed a strong inward rectification; I-V graph shows the average normalized current at −60 mV in 3 cells. In all cells, the holding potential is −60 mV.
Several receptor subunit composition and properties distinguish neuronal nAChR, including sensitivity to agonists and propensity to desensitization (Hogg et al. 2003). To further characterize the nicotinic response in MCs, we conducted voltage-clamp experiments. At −60 mV, bath application of Nic (1–300 μM; 30 s) produced a fast onset (<20 s) inward current (Fig. 6B, Nic 30 μM; −347 ± 27 pA; n = 36). In the presence of inhibitors of fast synaptic transmission (20 μM BMI, 10 μM NBQX, 100 μM APV) and TTX (1 μM), the response to Nic (30 μM) was only partially reduced (−267 ± 76 pA control; Nic + Blockers, −143 ± 20 pA, P < 0.01, n = 4, Fig. 6C), suggesting that a direct action of Nic on MCs contributed to the depolarization. The nicotinic response was nondesensitizing, as consecutive applications of Nic (within 10 min) resulted in responses that were similar in amplitude (1st application, −312 ± 53 vs. 2nd −337 ± 74 pA; n = 7) and exhibited dose dependency, with an EC50 of 42 ± 2 μM (n = 5, not shown). Additionally, the voltage dependency of the inward current produced by Nic (30 μM) exhibited the characteristically strong inward rectification of neuronal nAChRs. Figure 6D shows the voltage-dependency of the normalized inward current induced by Nic at −60 mV. The current at −40 mV was −84 ± 34 pA while at +30 mV was −18 ± 7 pA (n = 3).
We further characterized the properties of the nAChR in the AOB by using various pharmacological agents that distinguish between receptors with distinct subunit compositions. The nonselective neuronal nAChR agonist cytisine (Cyt) produced a greater effect than nicotine at the same concentrations (10 μM, Nic, −152 ± 54 pA vs. Cyt −295 ± 63 pA, P < 0.02, n = 3). Application of choline (100–1,000 μM) failed to depolarize MCs (Fig. 6B) while acetylcholine, like nicotine, produced a fast inward current (not shown). The nonselective nicotinic antagonist MM (30 μM; n = 4) completely blocked the response to Nic (30 μM; Fig. 6B, bottom; control, −275 ± 45 pA; in MM, −5 ± 5 pA, P < 0.02), while in the presence of the α4-containing nAChR antagonist DHBE (3 μM) the response to Nic was reduced to 39 ± 27% of control (P < 0.02, n = 3). The selective α7-containing nAChR antagonist MLA (10–30 nM) had no effect on the nicotinic response (Fig. 6B, top; control, −123 ± 47 pA; in MLA 10 nM, −130 ± 47 pA, n = 4). These results suggest that the nicotinic responses in MC are due to activation of α4β2* -like nAChRs. In addition, we found that like the M1-like mAChR response in GCs, MCs exhibited both nicotinic and M1-like muscarinic responses early in postnatal development (control, 12.6 ± 1.6 mV; in 300 nM Pir, 1.0 ± 0.97 mV; P < 0.02; n = 3, data not shown), suggesting that the receptor subtypes and their distribution among neuronal components of the AOB is established early in postnatal development.
Submaximal activation of nicotinic and muscarinic receptors decreases the output from MCs
Our results demonstrate that the main cholinergic effect on GCs is excitation mediated by M1-like mAChRs, which would result in an increase in inhibitory GABAergic input onto MCs. Concomitantly, the main cholinergic effect on MCs is excitation by both nicotinic and muscarinic receptors, which would result in lowering the threshold for excitatory sensory input and increasing the output from MCs. To ask which neuromodulatory action predominates on MCs (i.e., excitation or inhibition), we selectively activated nicotinic or muscarinic receptors with low agonist concentrations to elicit a submaximal excitatory effect on MCs while driving action potentials with current injection (3–8 Hz). To determine the submaximal dose for these experiments, we constructed dose-response curves for Oxo in the 0.3–10 μM concentration range. The EC50 was 3.11 ± 0.22 μM in GCs (n = 7) and 0.79 ± 0.02 μM in MCs (n = 4; not shown). In these experiments, the membrane potential was maintained at a steady value by manually injecting current. Surprisingly, we found that in the presence of either Nic (3 μM) or Oxo (3 μM), the frequency of stimulus-elicited action potentials in MCs was significantly depressed (Fig. 7A). In the presence of Nic, the firing frequency was decreased by 53 ± 12% (P < 0.002, n = 7, Fig. 7B), while in the presence of Oxo, it was reduced by 45 ± 11% (P < 0.002, n = 7, Fig. 7B), suggesting that under these conditions, the influence of increased inhibitory input from GCs overrides the excitation of MC. Accordingly, when the actions of these agonists were tested in the presence of GABAzine (5 μM) to block the inhibitory input from interneurons, only the excitatory effect prevailed and there was a slight increase in the frequency of firing (Nic, 10 ± 7%; Oxo, 15 ± 15%, Fig. 7B). Lowering the concentration of Oxo to 1 μM resulted in a smaller yet significant reduction in the frequency of MC firing (18 ± 3%; P < 0.003; n = 13, not shown), while in the presence of GABAzine the increase in frequency was also observed (15 ± 3%; P < 0.02; n = 10, not shown). Thus submaximal concentrations of Oxo decrease the firing rate in MCs.
Fig. 7.
Submaximal activation of nicotinic and muscarinic receptors decreases the output from MCs. A, top: a low concentration of Nic (3 μM) reduced the firing rate in this MC. In the same cell, the GABA receptor antagonist GABAzine (5 μM) blocked the inhibitory response produced by Nic and only a slight excitatory remained. Bottom traces: low concentrations of Oxo (3 μM) decreased the firing rate in this MC and GABAzine (5 μM) also reduced this inhibitory effect. Cells were manually clamped at −60 mV; the calibration bar in is 0.5 s and 20 mV. B: graph bar summarizing the effects of submaximal concentrations of Oxo (3 μM) and Nic (3 μM) in the firing frequency of MCs (see text, ■, *P < 0.002). Both Nic and Oxo significantly reduced the frequency of firing. In the presence of GABAzine (5 μM, □), the inhibitory effects of these agonists were greatly diminished leaving a slight excitatory effect.
DISCUSSION
Modulation of neuronal circuits in the OB by cholinergic and noradrenergic afferent systems plays a crucial role in the proper execution of several survival-dependent behaviors (Brennan 2004; Brennan and Keverne 1997; Wilson et al. 2004). Yet the mechanisms by which these afferent systems regulate neuronal excitability in the OB remain poorly understood. Here we provide evidence that the excitability of both GCs and MCs is enhanced by AChR activation in the AOB, a region involved in control of mating and aggressive behaviors, suggesting that the cholinergic system may play a role in regulating the neuronal processing required for these behaviors. Activation of M1-like mAChRs depolarized GCs and induced the appearance of a sADP following a stimulus-induced train of action potentials. In addition, MCs were also excited through activation of M1-like mAChRs and nAChRs, suggesting that cholinergic modulation may enhance excitability in the AOB and increase sensitivity of MCs to sensory input. However, our results demonstrate that under submaximal activation of these receptors, the main effect is inhibition of MC excitability. These results suggest that under physiological conditions, the cholinergic system may act to increase the overall inhibitory tone of MCs instead. Intriguingly, in the AOB the cholinergic excitatory action on GCs and MCs is present from early postnatal days, suggesting that unlike in the MOB, excitatory muscarinic responses do not exhibit a developmental switch, suggesting that neuromodulation of GCs in the AOB may play an important physiological role in early postnatal ages.
Modulation of neuronal excitability by acetylcholine, as in other sensory systems, plays an important role in olfactory processing. Cholinergic projections to the OB from the basal nuclei of the forebrain, in particular the HDB, are found throughout the different cellular layers of the OB, suggesting this system can modulate several neuronal components in the OB (Kasa et al. 1995; Le Jeune et al. 1996; Nickell and Shipley 1988; Ojima et al. 1988; Zaborszky et al. 1986). Studies in vivo have indicated that in the MOB acetylcholine enhances discrimination of similar odors and promotes odor learning (Chaudhury et al. 2009; Levy et al. 1997; Linster and Cleland 2002; Mandairon et al. 2006; Ravel et al. 1994; Roman et al. 1993). Field potential recordings in the olfactory bulb have reported conflicting results in response to cholinergic agents or stimulation in the HDB with some reporting decreased GC-MC inhibition (Elaagouby and Gervais 1992; Elaagouby et al. 1991; Kunze et al. 1991, 1992; Tsuno et al. 2008) and others, in agreement with our findings, reporting inhibition of MC (Nickell and Shipley 1988). It should be noted that the HDB projections include both GABAergic and cholinergic neurons that may underlie these discrepancies (Zaborszky et al. 1986). In contrast, only a few studies have addressed the cellular effects of the cholinergic system in the OB. Noticeably, most of these studies have been confined to the MOB where both inhibitory and excitatory cholinergic effects have been described (Castillo et al. 1999; Ghatpande and Gelperin 2009; Ghatpande et al. 2006; Pignatelli and Belluzzi 2008; Pressler et al. 2007). For example, cholinergic stimulation inhibited GC firing in cell-attached recordings and increased the frequency of GABA inhibitory postsynaptic currents (IPSCs) in whole cell recordings from MCs (Castillo et al. 1999). The increase in IPSC frequency was attributed to activation of presynaptic mAChRs at dendrodendritic synapses (Castillo et al. 1999; Ghatpande et al. 2006). These finding are consistent with the abundant expression of M1 receptor in the external plexiform layer of the OB, where most dendrodendritic synapses occur (Buckley et al. 1988; Spencer et al. 1986). We now provide evidence that activation of M1-like muscarinic receptors in GCs of the AOB produces a depolarization and a sADP following a stimulus-induced train of action potentials that increases the release of GABA onto MCs. Our results are in agreement with previous studies in the MOB (Pressler et al. 2007), showing that GCs exhibit an M1 muscarinic receptor excitation and an ADP. Characterization of the ionic mechanisms underlying the mAChR induced ADP indicated that this is due to the activation of a nonselective cationic current (ICAN), which occur through activation of transient receptor potential (TRP) channels (Yan et al. 2009). These data suggest that GCs in the AOB and MOB exhibit similar cellular mechanism of modulation by the cholinergic system and are in agreement with M1 excitatory effects found in other brain regions (Egorov et al. 2006; Haj-Dahmane and Andrade 1999). Only one other study to date has examined cholinergic neuromodulation in the AOB (Takahashi and Kaba 2010). In agreement with the robust M1-like mAChR-induced depolarization of GCs described here, this study reported that M1 receptor activation increased the frequency of GABA IPSCs in MCs. However, our data are consistent with previous work in the MOB indicating that muscarinic depolarization in GCs results from recruitment of a nonselective cationic current (ICAN) rather than the closure of K channels (M-current) as proposed by Takahashi et al. (2010). Further studies are necessary to determine the reason for this discrepancy; however, the use of different mice strains may be a contributing factor.
Muscarinic activation of nonselective cationic currents have been described in various regions of the brain where they promote long-lasting depolarization, providing an interesting mechanism for cholinergic-induced neuronal plasticity (Constanti et al. 1993; Egorov et al. 2006; Haj-Dahmane and Andrade 1996, 1999; Krnjevic et al. 1971; Schwindt et al. 1988). In general, GCs exhibit a hyperpolarized resting membrane potential, so coincident excitatory input can enhance the cholinergic excitatory effect. Glutamatergic inputs onto GCs occur mainly through dendrodendritic synapses and synapses from afferent fibers originating in the olfactory cortex. Basal dendrites and the soma of GCs receive synapses from centrifugal fibers and axon collaterals from MCs (Mouret et al. 2009). It has been suggested that this segregated pattern of connectivity is likely to have an important physiological role in GC function (Whitman and Greer 2007). Thus coincident excitatory activity at any of these sites could selectively potentiate the cholinergic depolarization of GCs, leading to an increased release of GABA to induce inhibition of MCs. In this regard, we hypothesize that the dual muscarinic and nicotinic excitation of MCs that leads to increased glutamatergic input at dendrodendritic synapses can also significantly contribute to the excitation of GCs. Interestingly, we recently showed that α-1 adrenergic and metabotropic glutamate receptor activation also depolarizes GCs and induces the appearance of an ADP (Smith et al. 2009). These results suggest that neuromodulation by these distinct afferent systems could use a convergent mechanism to increase the excitability of GCs. In addition, increased inhibition at dendrodendritic synapses may play an important role in the discrimination of sexual cues by the AOB, including those involved in the Bruce effect, emphasizing the important neuromodulatory role of these systems at dendrodendritic synapses (Hendrickson et al. 2008).
Cholinergic agonists also excited MCs; however, unlike GCs, this excitation recruited both muscarinic and nicotinic receptors, similar to the responses of MCs in the MOB. Diversity in subunit composition gives rise to a great number of homomeric and heteromeric nAChRs subtypes, each with unique physiological and pharmacological properties (Gotti et al. 2007; Luetje and Patrick 1991). The pharmacological profile of the nicotinic excitation we describe here suggests that the response in MCs is mediated by nAChRs of the α4β2* type (Albuquerque et al. 2009). Accordingly, we found that the nicotinic response was sensitive to DHBE but not to MLA. The α4β2* type nAChR exhibits a lesser degree of desensitization, as reported here, suggesting that nicotinic activation could tonically excite MCs. These results are in agreement with other studies that show that in the OB the most abundant nAChRs are the α7-type and α4β2*type (Hogg et al. 2003). In addition, we found that M1-like mAChR activation excited MCs; however, unlike GCs, the sADP was not present. One possibility is that M1 activation couples to different targets in MCs and/or that the ADP does not contribute substantially to the depolarization in MCs; we are currently addressing this question.
Intriguingly, in sharp contrast with the MOB, the excitatory cholinergic responses in MCs and GCs were present from early postnatal days (Ghatpande and Gelperin 2009; Ghatpande et al. 2006). Recordings from MCs in the MOB indicated that early postnatal M1 mAChR activation occurs on MCs, which then indirectly excite GCs through glutamate receptors, and only at around P10 do GCs become sensitive to direct mAChR activation. It's possible that this difference is due to the heterogeneity of GCs within the OB or differences in the species used (rat vs. mice). Nonetheless, the presence of M1 responses in GCs at early postnatal days suggests that cholinergic modulation could play an important role in the maturation of the AOB circuitry, which happens during the first week of postnatal development (Mouret et al. 2009; Salazar et al. 2006). Afferent neuromodulatory systems play an important role in olfactory learning both in the AOB and MOB (Brennan and Keverne 1997), thus it is tempting to speculate that neuromodulation by cholinergic system in the AOB may also play an important role in perinatal behaviors.
Although several studies have shown the presence of cholinergic fibers in the vicinity of MCs, the precise cellular distribution of muscarinic and nicotinic receptors in MCs is not known. We postulate that activation of somatic excitatory receptors could produce a more pronounced effect on MC output, while activation of receptors located on lateral dendrites could have a stronger effect on recurrent and lateral inhibition (i.e., local processing). Further studies are necessary to determine the contribution of either receptor type to the output and local processing of MCs. Nevertheless, we find that activation of either receptor with low concentrations of cholinergic agonists tends to promote overall inhibition in MCs; that is, the inhibitory drive from GCs, and to a lesser extent from PGs, dominates. Under these conditions, lateral and recurrent inhibition of MCs could be enhanced by cholinergic neuromodulation. Interestingly, ACh can also increase the inhibitory drive in other brain regions where it modulates the balance between excitation and inhibition (Lucas-Meunier et al. 2009). On the other hand, under decreased inhibitory activity from the interneurons (GC and PGs), the excitatory effect of muscarinic and nicotinic receptors on MCs predominates. Thus different levels of activity of afferent cholinergic fibers or stimulation of selective compartments within the MC could lead to different neuromodulatory effects on OB output. It is possible that in vivo several other factors may influence the neuromodulatory action of ACh at the network level. For example, ACh could produce differential activation of metabotropic and ionotropic cholinergic receptors or spatial and temporal constraints could bias these responses to distinct neuronal components (i.e., GCs vs. MCs). Nevertheless, our studies provide further insight on the cellular mechanism by which the cholinergic system modulates excitability in the bulb. Further in vivo studies are necessary to determine how these cellular mechanisms convene to functionally modify odor processing and output of the bulb.
GRANTS
This work was partially funded by National Institute of Deafness and Other Communicational Disorders Grant DC RO1-DC-009817 to R. C. Araneda.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank the members of the Araneda lab for their helpful comments on the manuscript.
REFERENCES
- Albuquerque et al., 2009. Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89: 73–120, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan, 2004. Brennan PA. The nose knows who's who: chemosensory individuality and mate recognition in mice. Horm Behav 46: 231–240, 2004 [DOI] [PubMed] [Google Scholar]
- Brennan and Keverne, 1997. Brennan PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Prog Neurobiol 51: 457–481, 1997 [DOI] [PubMed] [Google Scholar]
- Brennan and Peele, 2003. Brennan PA, Peele PF, Towards an understanding of the pregnancy-blocking urinary chemosignals of mice. Biochem Soc Trans 31: 152–155, 2003 [DOI] [PubMed] [Google Scholar]
- Buckley et al., 1988. Buckley NJ, Bonner TI, Brann MR. Localization of a family of muscarinic receptor mRNAs in rat brain. J Neurosci 8: 4646–4652, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo et al., 1999. Castillo PE, Carleton A, Vincent JD, Lledo PM. Multiple and opposing roles of cholinergic transmission in the main olfactory bulb. J Neurosci 19: 9180–9191, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury et al., 2009. Chaudhury D, Escanilla O, Linster C. Bulbar acetylcholine enhances neural and perceptual odor discrimination. J Neurosci 29: 52–60, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti et al., 1993. Constanti A, Bagetta G, Libri V. Persistent muscarinic excitation in guinea-pig olfactory cortex neurons: involvement of a slow post-stimulus afterdepolarizing current. Neuroscience 56: 887–904, 1993 [DOI] [PubMed] [Google Scholar]
- Egorov et al., 2006. Egorov AV, Unsicker K, von Bohlen und Halbach O. Muscarinic control of graded persistent activity in lateral amygdala neurons. Eur J Neurosci 24: 3183–3194, 2006 [DOI] [PubMed] [Google Scholar]
- Elaagouby and Gervais, 1992. Elaagouby A, Gervais R. ACh-induced long-lasting enhancement in excitability of the olfactory bulb. Neuroreport 3: 10–12, 1992 [DOI] [PubMed] [Google Scholar]
- Elaagouby et al., 1991. Elaagouby A, Ravel N, Gervais R. Cholinergic modulation of excitability in the rat olfactory bulb: effect of local application of cholinergic agents on evoked field potentials. Neuroscience 45: 653–662, 1991 [DOI] [PubMed] [Google Scholar]
- Ennis et al., 2007. Ennis M, Hamilton KA, Hayar A. Neurochemistry of the Main Olfactory System. New York: Springer, 2007 [Google Scholar]
- Ghatpande and Gelperin, 2009. Ghatpande AS, Gelperin A. Presynaptic muscarinic receptors enhance glutamate release at the mitral/tufted to granule cell dendrodendritic synapse in the rat main olfactory bulb. J Neurophysiol 101: 2052–2061, 2009 [DOI] [PubMed] [Google Scholar]
- Ghatpande et al., 2006. Ghatpande AS, Sivaraaman K, Vijayaraghavan S. Store calcium mediates cholinergic effects on mIPSCs in the rat main olfactory bulb. J Neurophysiol 95: 1345–1355, 2006 [DOI] [PubMed] [Google Scholar]
- Gotti et al., 2007. Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli ME. Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol 74: 1102–1111, 2007 [DOI] [PubMed] [Google Scholar]
- Gotti et al., 2006. Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci 27: 482–491, 2006 [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane and Andrade, 1996. Haj-Dahmane S, Andrade R. Muscarinic activation of a voltage-dependent cation nonselective current in rat association cortex. J Neurosci 16: 3848–3861, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Dahmane and Andrade, 1999. Haj-Dahmane S, Andrade R. Muscarinic receptors regulate two different calcium-dependent non-selective cation currents in rat prefrontal cortex. Eur J Neurosci 11: 1973–1980, 1999 [DOI] [PubMed] [Google Scholar]
- Halpern and Martinez-Marcos, 2003. Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: an update. Prog Neurobiol 70: 245–318, 2003 [DOI] [PubMed] [Google Scholar]
- Hendrickson et al., 2008. Hendrickson RC, Krauthamer S, Essenberg JM, Holy TE. Inhibition shapes sex selectivity in the mouse accessory olfactory bulb. J Neurosci 28: 12523–12534, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill et al., 1993. Hill JA, Jr, Zoli M, Bourgeois JP, Changeux JP. Immunocytochemical localization of a neuronal nicotinic receptor: the beta 2-subunit. J Neurosci 13: 1551–1568, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg et al., 2003. Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol 147: 1–46, 2003 [DOI] [PubMed] [Google Scholar]
- Kasa et al., 1995. Kasa P, Hlavati I, Dobo E, Wolff A, Joo F, Wolff JR. Synaptic and non-synaptic cholinergic innervation of the various types of neurons in the main olfactory bulb of adult rat: immunocytochemistry of choline acetyltransferase. Neuroscience 67: 667–677, 1995 [DOI] [PubMed] [Google Scholar]
- Keiger and Walker, 2000. Keiger CJ, Walker JC. Individual variation in the expression profiles of nicotinic receptors in the olfactory bulb and trigeminal ganglion and identification of alpha2, alpha6, alpha9, and beta3 transcripts. Biochem Pharmacol 59: 233–240, 2000 [DOI] [PubMed] [Google Scholar]
- Krnjevic et al., 1971. Krnjevic K, Pumain R, Renaud L. The mechanism of excitation by acetylcholine in the cerebral cortex. J Physiol 215: 247–268, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze et al., 1991. Kunze WA, Shafton AD, Kemm RE, McKenzie JS. Effect of stimulating the nucleus of the horizontal limb of the diagonal band on single unit activity in the olfactory bulb. Neuroscience 40: 21–27, 1991 [DOI] [PubMed] [Google Scholar]
- Kunze et al., 1992. Kunze WA, Shafton AD, Kemm RE, McKenzie JS. Intracellular responses of olfactory bulb granule cells to stimulating the horizontal diagonal band nucleus. Neuroscience 48: 363–369, 1992 [DOI] [PubMed] [Google Scholar]
- Lanzafame et al., 2003. Lanzafame AA, Christopoulos A, Mitchelson F. Cellular signaling mechanisms for muscarinic acetylcholine receptors. Receptors Channels 9: 241–260, 2003 [PubMed] [Google Scholar]
- Le Jeune et al., 1995. Le Jeune H, Aubert I, Jourdan F, Quirion R. Comparative laminar distribution of various autoradiographic cholinergic markers in adult rat main olfactory bulb. J Chem Neuroanat 9: 99–112, 1995 [DOI] [PubMed] [Google Scholar]
- Le Jeune et al., 1996. Le Jeune H, Aubert I, Jourdan F, Quirion R. Developmental profiles of various cholinergic markers in the rat main olfactory bulb using quantitative autoradiography. J Comp Neurol 373: 433–450, 1996 [DOI] [PubMed] [Google Scholar]
- Le Jeune and Jourdan, 1991. Le Jeune H, Jourdan F. Postnatal development of cholinergic markers in the rat olfactory bulb: a histochemical and immunocytochemical study. J Comp Neurol 314: 383–395, 1991 [DOI] [PubMed] [Google Scholar]
- Levy et al., 1997. Levy F, Richard P, Meurisse M, Ravel N. Scopolamine impairs the ability of parturient ewes to learn to recognize their lambs. Psychopharmacology 129: 85–90, 1997 [DOI] [PubMed] [Google Scholar]
- Libri et al., 1994. Libri V, Constanti A, Calaminici M, Nistico G. A comparison of the muscarinic response and morphological properties of identified cells in the guinea-pig olfactory cortex in vitro. Neuroscience 59: 331–347, 1994 [DOI] [PubMed] [Google Scholar]
- Linster and Cleland, 2002. Linster C, Cleland TA. Cholinergic modulation of sensory representations in the olfactory bulb. Neural Netw 15: 709–717, 2002 [DOI] [PubMed] [Google Scholar]
- Linster et al., 2001. Linster C, Garcia PA, Hasselmo ME, Baxter MG. Selective loss of cholinergic neurons projecting to the olfactory system increases perceptual generalization between similar, but not dissimilar, odorants. Behav Neurosci 115: 826–833, 2001 [DOI] [PubMed] [Google Scholar]
- Lucas-Meunier et al., 2009. Lucas-Meunier E, Monier C, Amar M, Baux G, Fregnac Y, Fossier P. Involvement of nicotinic and muscarinic receptors in the endogenous cholinergic modulation of the balance between excitation and inhibition in the young rat visual cortex. Cereb Cortex 19: 2411–2427, 2009 [DOI] [PubMed] [Google Scholar]
- Luetje and Patrick, 1991. Luetje CW, Patrick J. Both alpha- and beta-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J Neurosci 11: 837–845, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandairon et al., 2006. Mandairon N, Ferretti CJ, Stack CM, Rubin DB, Cleland TA, Linster C. Cholinergic modulation in the olfactory bulb influences spontaneous olfactory discrimination in adult rats. Eur J Neurosci 24: 3234–3244, 2006 [DOI] [PubMed] [Google Scholar]
- Mouret et al., 2009. Mouret A, Murray K, Lledo PM. Centrifugal drive onto local inhibitory interneurons of the olfactory bulb. Ann NY Acad Sci 1170: 239–254, 2009 [DOI] [PubMed] [Google Scholar]
- Nickell and Shipley, 1988. Nickell WT, Shipley MT. Two anatomically specific classes of candidate cholinoceptive neurons in the rat olfactory bulb. J Neurosci 8: 4482–4491, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojima et al., 1988. Ojima H, Yamasaki T, Kojima H, Akashi A. Cholinergic innervation of the main and the accessory olfactory bulbs of the rat as revealed by a monoclonal antibody against choline acetyltransferase. Anat Embryol 178: 481–488, 1988 [DOI] [PubMed] [Google Scholar]
- Pignatelli and Belluzzi, 2008. Pignatelli A, Belluzzi O. Cholinergic modulation of dopaminergic neurons in the mouse olfactory bulb. Chem Senses 33: 331–338, 2008 [DOI] [PubMed] [Google Scholar]
- Pressler et al., 2007. Pressler RT, Inoue T, Strowbridge BW. Muscarinic receptor activation modulates granule cell excitability and potentiates inhibition onto mitral cells in the rat olfactory bulb. J Neurosci 27: 10969–10981, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel et al., 1994. Ravel N, Elaagouby A, Gervais R. Scopolamine injection into the olfactory bulb impairs short-term olfactory memory in rats. Behav Neurosci 108: 317–324, 1994 [DOI] [PubMed] [Google Scholar]
- Roman et al., 1993. Roman FS, Simonetto I, Soumireu-Mourat B. Learning and memory of odor-reward association: selective impairment following horizontal diagonal band lesions. Behav Neurosci 107: 72–81, 1993 [DOI] [PubMed] [Google Scholar]
- Salazar et al., 2006. Salazar I, Sanchez-Quinteiro P, Cifuentes JM, Fernandez De Troconiz P. General organization of the perinatal and adult accessory olfactory bulb in mice. Anat Rec A Discov Mol Cell Evol Biol 288: 1009–1025, 2006 [DOI] [PubMed] [Google Scholar]
- Schoppa and Urban, 2003. Schoppa NE, Urban NN. Dendritic processing within olfactory bulb circuits. Trends Neurosci 26: 501–506, 2003 [DOI] [PubMed] [Google Scholar]
- Schwindt et al., 1988. Schwindt PC, Spain WJ, Foehring RC, Chubb MC, Crill WE. Slow conductances in neurons from cat sensorimotor cortex in vitro and their role in slow excitability changes. J Neurophysiol 59: 450–467, 1988 [DOI] [PubMed] [Google Scholar]
- Shepherd and Greer, 1998. Shepherd GM, Greer CA. Olfactory bulb. In: The Synaptic Organization of the Brain (4 ed.), edited by Shepherd GM. Oxford, UK: Oxford Univ. Press, 1998, p. 159450–204 [Google Scholar]
- Smith et al., 2009. Smith RS, Weitz CJ, Araneda RC. Excitatory actions of noradrenaline and metabotropic glutamate receptor activation in granule cells of the accessory olfactory bulb. J Neurophysiol 102: 1103–1114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer et al., 1986. Spencer DG, Jr, Horvath E, Traber J. Direct autoradiographic determination of M1 and M2 muscarinic acetylcholine receptor distribution in the rat brain: relation to cholinergic nuclei and projections. Brain Res 380: 59–68, 1986 [DOI] [PubMed] [Google Scholar]
- Takahashi and Kaba, 2010. Takahashi Y, Kaba H. Muscarinic receptor type 1 (M1) stimulation, probably through KCNQ/Kv7 channel closure, increases spontaneous GABA release at the dendrodendritic synapse in the mouse accessory olfactory bulb. Brain Res 1339: 26–40, 2010 [DOI] [PubMed] [Google Scholar]
- Tsuno et al., 2008. Tsuno Y, Kashiwadani H, Mori K. Behavioral state regulation of dendrodendritic synaptic inhibition in the olfactory bulb. J Neurosci 28: 9227–9238, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteaker et al., 2009. Whiteaker P, Wilking JA, Brown RW, Brennan RJ, Collins AC, Lindstrom JM, Boulter J. Pharmacological and immunochemical characterization of alpha2* nicotinic acetylcholine receptors (nAChRs) in mouse brain. Acta Pharmacol Sin 30: 795–804, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman and Greer, 2007. Whitman MC, Greer CA. Adult-generated neurons exhibit diverse developmental fates. Dev Neurobiol 67: 1079–1093, 2007 [DOI] [PubMed] [Google Scholar]
- Wilson et al., 2004. Wilson DA, Fletcher ML, Sullivan RM. Acetylcholine and olfactory perceptual learning. Learn Mem 11: 28–34, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan et al., 2009. Yan HD, Villalobos C, Andrade R. TRPC channels mediate a muscarinic receptor-induced afterdepolarization in cerebral cortex. J Neurosci 29: 10038–10046, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky et al., 1986. Zaborszky L, Carlsen J, Brashear HR, Heimer L. Cholinergic and GABAergic afferents to the olfactory bulb in the rat with special emphasis on the projection neurons in the nucleus of the horizontal limb of the diagonal band. J Comp Neurol 243: 488–509, 1986 [DOI] [PubMed] [Google Scholar]







