Abstract
Salivary nitrate from dietary or endogenous sources is reduced to nitrite by oral bacteria. In the acidic stomach, nitrite is further reduced to NO and related compounds, which have potential biological activity. We used an in vivo rat model as a bioassay to test effects of human saliva on gastric mucosal blood flow and mucus thickness. Gastric mucosal blood flow and mucus thickness were measured after topical administration of human saliva in HCl. The saliva was collected either after fasting (low in nitrite) or after ingestion of sodium nitrate (high in nitrite). In additional experiments, saliva was exchanged for sodium nitrite at different doses. Mucosal blood flow was increased after luminal application of nitrite-rich saliva, whereas fasting saliva had no effects. Also, mucus thickness increased in response to nitrite-rich saliva. The effects of nitrite-rich saliva were similar to those of topically applied sodium nitrite. Nitrite-mediated effects were associated with generation of NO and S-nitrosothiols. In addition, pretreatment with an inhibitor of guanylyl cyclase markedly inhibited nitrite-mediated effects on blood flow. We conclude that nitrite-containing human saliva given luminally increases gastric mucosal blood flow and mucus thickness in the rat. These effects are likely mediated through nonenzymatic generation of NO via activation of guanylyl cyclase. This supports a gastroprotective role of salivary nitrate/nitrite.
Introduction
The gastric mucosa is subjected to both endogenous (e.g., HCl, pepsin) and exogenous (e.g., ethanol, drugs, bacteria) caustic, toxic, and digestive substances. To withstand this constantly changing challenge it is of paramount importance that the mucosal defense systems can adapt instantly. Among these defense mechanisms, mucosal blood flow and mucus secretion play central roles (1–7). A rapid increase in mucosal blood flow following exposure to a luminal irritant allows for buffering of acid and removal of toxic compounds. An adequate blood flow is also important for the production of mucus and bicarbonate (HCO3–). Mucus contributes to mucosal defense by providing a barrier to luminal contents (8, 9). It represents a physical protection against bacteria and a chemical barrier to luminal acid. Bicarbonate is secreted by the epithelial cells into the mucus layer, thereby forming a pH gradient with almost neutral pH near the epithelium despite a more acidic gastric lumen (9–11).
PGs and NO are mediators of multiple aspects of mucosal barrier function (12, 13). They mediate increases in mucosal blood flow (6), stimulate mucus secretion (14, 15), and inhibit neutrophil adherence and activation (16). Luminal irritants can activate afferent sensory nerves, leading to a rapid increase in mucosal blood flow that is ultimately mediated by NO (17, 18). Suppression of PG or NO synthesis increases mucosal susceptibility to damage (19). A classic example of this is the gastric toxicity of NSAIDs, which is linked to the ability of these agents to suppress local PG synthesis in the gastric mucosa (20). Inhibition of NO synthesis is also detrimental to gastric mucosal integrity (21).
NO is typically produced from L-arginine by NO synthases (NOSs), and often acts on the target cell by activating guanylyl cyclase. A second fundamentally different pathway for NO production in biological systems has been described (22, 23). This production is nonenzymatic and results from reduction of inorganic nitrite to NO and other nitrogen oxides. The reaction is accelerated in acidic and reducing environments. Nonenzymatic NO production is especially high in the stomach because of the low pH. Intragastric NO is greatly increased following intake of inorganic nitrate (24). Ingested nitrate is rapidly absorbed proximally in the gastrointestinal tract, and about 25% of this nitrate is taken up from plasma by the salivary glands and secreted in saliva (25, 26). In the oral cavity, bacteria convert some of the nitrate to nitrite by the action of nitrate reductases (27). By this procedure, more nitrite enters the acidic stomach where it can be reduced to NO. Several in vitro studies indicate that nitrite-derived NO and related compounds play an important role in gastric host defense by enhancing the acid-dependent killing of swallowed pathogens (23, 28, 29). In this study, an in vivo rat model has been used as a bioassay to test effects of human saliva on gastric mucosal blood flow and mucus thickness. The same model was also used to study dose-dependent effects of acidified sodium nitrite.
Methods
Animal and tissue preparation.
All experiments were approved by the Uppsala University Ethical Committee for Animal Experiments and the Local Ethics Committee for Human Research at the Karolinska Institute. Male Sprague-Dawley rats (B&K Universal AB, Sollentuna, Sweden) weighing 165–280 g were kept in standard conditions of temperature (21–22°C) and illumination (12 h light/12 h darkness). The rats were kept in wide, mesh-bottomed cages with free access to pelleted food and tap water. Before the experiment they were fasted for 18–20 hours with free access to water. Anesthesia was conducted with 120 mg/kg thiobutabarbital sodium (Inactin; Sigma-Aldrich, St. Louis, Missouri, USA) given intraperitoneally and a PE-200 cannula was inserted into the trachea to facilitate spontaneous breathing. Core temperature was kept at 37–38°C by a heating pad regulated with a rectal thermistor. The right femoral artery was catheterized with a PE-50 cannula containing heparin (12.5 IU/ml) dissolved in 0.9% saline, and connected to a pressure transducer for continuous measurement of systemic blood pressure. The right femoral vein was cannulated for drug administration and continuous infusion of a modified Ringer solution (25 mM NaHCO3, 120 mM NaCl, 2.5 mM KCl, and 0.75 mM CaCl2) at a rate of 1 ml/h. In one group of animals, the left femoral vein was also cannulated for administration of N-nitro-L-arginine (L-NNA).
The gastric preparation for blood flow and mucus thickness measurements has been described previously in detail (15, 30). In brief, the abdomen was opened through a midline incision and the stomach was gently exteriorized. The forestomach was opened along the greater curvature and the rat was placed on its left side on a Lucite microscope stage. The corpus of the stomach was everted through the incision and loosely draped over a truncated cone with the luminal side up. A mucosal chamber with a hole in the bottom was placed over the stomach, exposing approximately 1.2 cm2 of the mucosal surface, and the junction was sealed with silicone grease. The mucosal chamber was filled with unbuffered 0.9% saline (5 ml) and kept at 37–38°C by warm water perfusing the bottom of the chamber. The saline was changed every 10 minutes and pH was measured. The animals were allowed to recover for at least 1 hour after surgical preparation until systemic blood pressure and gastric mucosal blood flow were stabilized.
Since basal acid secretion is very low or absent in fasted, anesthetized animals, pentagastrin was given to stimulate secretion. Pentagastrin (40 μg/kg/h intravenously) was given as a continuous infusion throughout the experiments, starting at least 30 minutes before the experiments. The time needed to achieve the desired acidity in the chamber (pH 2) was very long and highly variable (70–180 minutes). This is mainly due to the relatively large chamber volume (5 ml) in relation to the small acid-producing area exposed (approximately 10%) and the variability in acid secretion rate between the animals. Therefore, to make it possible to perform dose-response experiments and comparative studies in the same rat, chamber pH was set with exogenous acid in most experiments.
Blood flow.
Laser-Doppler flowmetry (PeriFlux 4001 Master and Periflux PF3; Perimed AB, Stockholm, Sweden) was used for mucosal blood flow measurements. The laser light (wavelength 635 nm, helium neon laser) is guided to the tissue by an optical fiber, and back-scattered light is detected by a pair of fibers with a separation of 0.5 mm. The nature of the Doppler shift from an illuminated tissue depends on the velocity and number of moving red blood cells (31). The accuracy of the Laser-Doppler flowmetry technique for gastrointestinal applications was described earlier (32, 33). The laser probe was fixed to a micromanipulator and kept at a distance of 0.5–1 mm from the gastric mucosa in the chamber solution. The recorded blood flow was considered to be mainly mucosal, since the amount of back-scattered light decreases exponentially with the distance from the probe and since a majority of the total blood flow in the gastric wall is mucosal. Blood flow was determined as a voltage signal and expressed as perfusion units, and was continuously recorded throughout the experiment. Blood flow was then expressed as percent of baseline values. Mean blood flow response was calculated from the area under the curve during a 10-minute period.
Mucus thickness.
Mucus thickness was measured using micropipettes connected to a micromanipulator (Leitz GmbH & Co., Oberkochen, Germany) (15, 34). The micropipettes were pulled to a tip diameter of 1–3 μm with a pipette puller (pp-83; Narishige Scientific Instrument Laboratories, Tokyo, Japan). To prevent mucus from adhering to the glass, the tip was dipped into a silicone solution (Wacker Silicone, Wacker-Chemie GmbH, Munich, Germany) and dried at 100°C for 30 minutes. The luminal surface of the mucus gel was visualized with carbon particles (extra pure activated charcoal; Merck Inc., Darmstadt, Germany). The epithelial cell surface was visible through the microscope. The micropipette was pushed into the mucus gel at an angle (a) of 25–35° to the epithelial cell surface, and the distance (D) traveled by the micropipette from the luminal surface of the mucus gel to the epithelial cell surface was measured with a digimatic indicator (IDC Series 543; Mitutoyo Corp., Tokyo, Japan) connected to the micromanipulator. Mucus gel thickness (T) was then calculated from the formula T = D(sin a). A mean value from four to five measurements at different locations was used as one observation. Removal of the outer loosely adherent mucus layer was performed by gentle suction with a thin catheter coupled to a syringe. The inner firmly adherent mucus layer remained and the thickness of this layer was immediately measured.
Collection of human saliva.
To obtain saliva with different nitrite content, saliva was collected from overnight fasting volunteers before and 1 hour after ingestion of sodium nitrate (0.1 mmol/kg). Previous studies have shown that salivary nitrite/nitrate is greatly increased 1 hour after a nitrate load (35). The saliva was mixed, centrifuged at 400 g for 10 minutes, and stored at –20°C until immediately before use. Nitrite and nitrate content of saliva was measured with the chemiluminescence method described below.
Headspace NO.
To determine the relative rate of NO formation from saliva and sodium nitrite we used an in vitro model. A plastic cup (100 ml) was placed over the mucosal chamber to collect headspace NO. The same concentrations and pH as in the in vivo experiments were used for sodium nitrite and saliva. For the sodium nitrite experiments, the chamber was filled with 5 ml isotonic HCl (pH 2), and nitrite (100 mM) was added through a hole in the base of the cup to a final concentration of 0.1, 0.5, 1, and 5 mM. For the saliva experiments, the chamber was filled with 2.5 ml saliva, after which 2.5 ml HCl (32 mM) was added to reach pH 2. In addition, we studied NO formation from saliva at pH 5.5. Headspace NO was measured through a sample tube connected to a chemiluminescence analyzing system (Aerocrine AB, Stockholm, Sweden). Calibration with cylinder gas (10 ppm NO; AGA AB, Lidingö, Sweden) was performed before each experiment. The sample flow rate was 100 ml/min, and peak NO concentration was measured after adding the compounds. The 8-mm hole in the base of the cup secured circulation of air during measurements.
Nitrite, nitrate, and S-nitrosothiols.
For measurements of nitrite, nitrate, and S-nitrosothiols, we used a chemiluminescence method as described in detail by Feelisch et al. (36). Human saliva was collected before and after ingestion of sodium nitrate as described above. After centrifugation at 400 g for 10 minutes, saliva was mixed (1:1) with isotonic HCl to reach pH 2 or pH 5.5. The solutions were kept at room temperature for 15 minutes before analysis.
In vivo experimental protocol.
The effects of topical administration of a variety of substances were tested in several groups of rats as described below. When the substances were coadministered with acid, an isotonic HCl (pH 2) was used. The total volume in the mucosal chamber was 5 ml in all experiments. All rats were given pentagastrin to secure a standardized endogenous acid secretion. Before each intervention a baseline mucosal blood flow was obtained during a 10-minute period to which each response was compared. The effects of each intervention were followed for 10 minutes. Mucosal blood flow and mean arterial blood pressure (MAP) were continuously registered during the experiments, and mucosal vascular resistance was calculated as the ratio of MAP to mucosal blood flow.
Effects of saliva on mucosal blood flow.
Rat stomachs were treated topically with human saliva collected after a nitrate load (n = 6) or with fasting human saliva (n = 5). Saliva (2.5 ml) and HCl were mixed in the mucosal chamber. In all animals, the response to sodium nitrite (1 mM) in pH 2 HCl was also tested. Between each intervention the mucosal chamber was emptied and replaced with saline.
Dose-dependent effects of sodium nitrite on mucosal blood flow.
Mucosal blood flow was measured in 7–13 animals after increasing doses of sodium nitrite. The mucosal chamber was filled with HCl (pH 2), and sodium nitrite (100 mM stock solution) was immediately added to a final concentration of 0.1, 0.5, 1, and 5 mM, respectively. Nitrite (1 mM) was also administered to six separate rats in an experiment in which chamber pH was allowed to drop to pH 2 spontaneously without addition of exogenous acid. Eight separate animals were exposed to HCl without addition of nitrite. Six additional animals were pretreated with an intravenous infusion of L-NNA (10 mg/kg bolus followed by 3 mg/kg/h throughout the experiments). After 30 minutes, when the blood flow was stable, the effects of 1 mM of sodium nitrite in HCl were studied.
Effects of guanylyl cyclase inhibition.
In six rats, mucosal blood flow responses to acidified sodium nitrite (1 mM or 5 mM) and the NO donor S-nitroso-N-acetyl-penicillamine (SNAP; 0.3 mM, pH 5.5) were studied before and after 30 minutes’ exposure in the mucosal chamber to the guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3,-a]quinoxalin-1-one (ODQ, 1 mM) dissolved in 5% DMSO. In three additional rats, acidified nitrite and SNAP were tested before and after 5% DMSO alone.
Effects of a COX inhibitor.
In four rats, the effects of sodium nitrite (1 mM, pH 2) on gastric mucosal blood flow were studied after pretreatment with the COX inhibitor indomethacin (3 mg/kg given intravenously).
Effects of an NO donor.
In four rats, the gastric mucosal blood flow response to the NO donor diethylenetriamine NONOate (DETA/NO) (1 mM, pH 5.5) was studied.
After all experiments intravital microscopy was performed to detect any mucosal injury.
Effects of saliva and sodium nitrite on mucus thickness.
Rats (n = 13) were divided into three groups in which nitrite-rich saliva (pH 2, n = 3), sodium nitrite (1 mM, pH 2, n = 5), or pH 2 HCl alone (n = 5) was applied for a total of 60 minutes. During this period the solutions in the chamber were replaced every 15 minutes to maintain nitrite concentration throughout the experiment. To standardize diffusion distance to the mucosa and to allow measurements of total mucus increase, the outer loosely adherent mucus layer was removed before intervention and the remaining inner firmly adherent mucus layer was measured. After the 60-minute study period, total mucus thickness and the inner firmly adherent mucus layer were measured again.
Intragastric NO generation.
In 14 additional rats we measured intragastric NO levels after pretreatment with sodium nitrate. Fasting rats were given either sodium nitrate (0.1 mmol/kg, n = 7) or the same amount of NaCl (control, n = 7) in 1 ml distilled water intragastrically. After 2 hours, the rats were anesthetized and a laparotomy was performed. A thin needle was inserted intragastrically via the stomach wall and the stomach was inflated with 4 ml of NO-free air. External clamps prevented passage of air into the esophagus or duodenum. The dilution of gastric gas was necessary to achieve a satisfactory gas volume for further analysis. After 15 seconds the air was aspirated and immediately injected into a rapid-response chemiluminescence NO analyzer (Aerocrine AB).
Chemicals.
The following chemicals and drugs were used: thiobutabarbital sodium (Inactin, Sigma-Aldrich), pentagastrin (Cambridge Laboratories Ltd., Wallsend, United Kingdom), 1 M HCl (Titrisol; Merck Inc.), ODQ (Tocris Cookson Inc., Ballwin, Missouri, USA), heparin (LEO Pharma, Ballerup, Denmark), SNAP, DETA/NO, L-NNA, DMSO, sodium nitrite, and sodium nitrate (Sigma-Aldrich).
Statistics.
Differences between groups of animals were evaluated by one-way ANOVA, followed by the Fisher protected least significant difference test. For comparison within groups, we used ANOVA for repeated measures followed by the Fisher protected least significant difference test. All statistical calculations were performed with a data analysis software system (STATISTICA version 6; StatSoft Inc., Tulsa, Oklahoma, USA). All data are presented as mean ± SEM. A P value of less than 0.05 was considered significant.
Results
MAP for all animals was 96 ± 2 mmHg and remained stable throughout the experiments. In the group of animals treated with L-NNA, MAP increased by 27% ± 9% 30 minutes after administration, with a parallel increase in gastric mucosal vascular resistance of 31% ± 16%. The baseline gastric mucosal blood flow for all animals was 97 ± 7 perfusion units. Intravital microscopy at the end of the experiments did not reveal any signs of mucosal damage.
Blood flow
Effects of saliva.
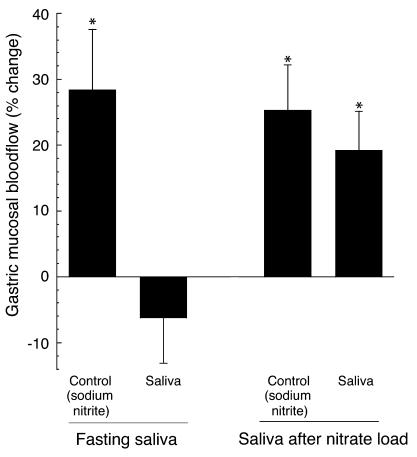
The mean nitrite content of saliva was 43 ± 14 μM in fasting saliva and 2.1 ± 0.3 mM after the nitrate load. Corresponding salivary nitrate levels were 0.33 ± 0.08 mM and 8.1 ± 0.9 mM, respectively. After mixing with HCl in the mucosal chamber, nitrite levels were 50% lower. Within a minute after administration of nitrite-rich saliva, mucosal blood flow increased, reaching a maximum at 2–3 minutes (Figure 1). Mean and maximum blood flow increases were 19% ± 6% and 35% ± 4% respectively, and did not differ significantly from those observed when using 1 mM sodium nitrite. No effects on blood flow were observed with fasting saliva (Figure 2).
Figure 1.
Dynamic changes in gastric mucosal vascular resistance (resistance), MAP, and gastric mucosal blood flow following topical application of human nitrite-rich saliva or sodium nitrite (1 mM) at pH 2 to the rat gastric mucosa. Values are expressed as mean ± SEM (n = 6).
Figure 2.
Changes in mean gastric mucosal blood flow during 10 minutes after topical application of human saliva or sodium nitrite to the rat gastric mucosa at pH 2. Nitrite levels were 23 μM in the experiments with fasting saliva (n = 5) and 1.1 mM after nitrate load (n = 6). In both groups the response to 1 mM sodium nitrite (NaNO2, pH 2) was also studied. *P < 0.05 compared with baseline.
Effects of sodium nitrite.
Gastric mucosal blood flow increased dose-dependently with luminal sodium nitrite exposure at pH 2. Mean blood flow increased by 14–50% in response to sodium nitrite (0.1–5 mM) (Figure 3), while maximum blood flow increased by 29–88% (Figure 4). In experiments without exogenous HCl, the time needed to reach pH 2 in the mucosal chamber was 70–180 minutes. Mean blood flow increased by 29% ± 18% (P < 0.05) in response to sodium nitrite (1 mM). Sodium nitrite (1 mM) at pH 5.5 or HCl alone at pH 2 had no effect on blood flow (data not shown). The blood flow response to sodium nitrite (1 mM, pH 2) was unaltered after treatment with L-NNA (23% ± 4% vs. 20% ± 11% increase).
Figure 3.
Effects of sodium nitrite (0.1–5 mM in HCl, pH 2) on gastric mucosal vascular resistance, MAP, and gastric mucosal blood flow. Values are expressed as mean ± SEM (n = 7–13).
Figure 4.
Maximum mucosal blood flow responses (upper panel, n = 7–13 rats) after topical application of human saliva or sodium nitrite (0.1–5 mM) to the gastric mucosa in relation to generation of NO (lower graphs, n = 5). Saliva or sodium nitrite solutions were mixed with HCl to pH 2. Final nitrite levels were 0.23 μM in experiments with fasting saliva and 1.1 mM when using saliva collected after a nitrate load. Values are expressed as mean ± SEM. *P < 0.01 compared with baseline.
Effects of ODQ.
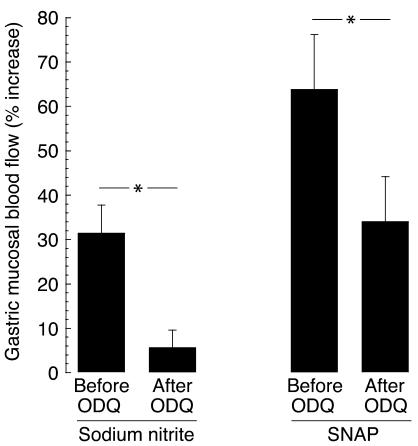
After pretreatment with ODQ, the gastric blood flow response to sodium nitrite (1 mM, pH 2) decreased from 32% ± 6% to 6% ± 4% (P = 0.02). Treatment with SNAP (0.3 mM, pH 5.5) resulted in a change in magnitude of the blood flow increase from 64% ± 12% to 34% ± 10% (P < 0.05, Figure 5). There was no change in blood flow response to sodium nitrite after pretreatment with vehicle (26% ± 5% vs. 38% ± 9%, P = 0.08).
Figure 5.
Effects of topical administration of sodium nitrite (1 mM, pH 2) and SNAP (0.3 mM) on rat gastric mucosal blood flow before and after topical pretreatment with the guanylyl cyclase inhibitor ODQ (1 mM, n = 6). Data are presented as mean ± SEM. *P < 0.05.
Effects of a COX inhibitor.
In rats pretreated with indomethacin, the mean increase in gastric mucosal blood flow in response to sodium nitrite (1 mM, pH 2) was 26% ± 8% (P < 0.05).
Effects of an NO donor.
Gastric mucosal blood flow increased by 31% ± 8% (P < 0.05) in response to the NO donor DETA/NO (1 mM, pH 5.5).
Mucus thickness
During the 60-minute experimental period, the total mucus layer increased by 39% ± 10% (from 75± 5 μm to 103 ± 5 μm, P < 0.01) in the saliva group, 33% ± 8% (from 78 ± 8 μm to 101 ± 7 μm, P < 0.01) in the sodium nitrite group, and 16% ± 8% (from 69 ± 1 μm to 80 ± 6 μm, not significant) in the control group. The inner firmly adherent mucus layer was thicker in the saliva group (81 ± 9 μm) and sodium nitrite group (89 ± 6 μm) than in the control group (55 ± 1 μm, P < 0.05, Figure 6).
Figure 6.
Thickness of the firmly adherent gastric mucus layer following 60 minutes of exposure to human saliva or sodium nitrite (1 mM). All experiments were performed at pH 2; in the control group the mucosa was exposed to acid alone. *P < 0.05 compared with control.
Generation of NO and S-nitrosothiols
When mixing human saliva with HCl to a final pH of 2, NO gas immediately appeared in the headspace above the solution. Peak NO levels generated from fasting saliva were 55 ± 4 ppb, while those from nitrite-rich saliva were 6,500 ± 450 ppb (Figure 4). Dose-dependent NO formation was noted when saliva was exchanged for sodium nitrite. Sodium nitrite at 1 mM resulted in NO levels similar to those of nitrite-rich saliva (5,550 ± 450 ppb, Figure 4). When changing the acidity from pH 2 to pH 5.5, lesser amounts of NO (175 ± 11 ppb) were generated from nitrite-rich saliva (Figure 7). Mixing saliva (nitrite 0.9 mM) with HCl at two different pH values resulted in formation of S-nitrosothiols (Figure 7). At pH 2 the amounts of S-nitrosothiols formed were tenfold higher (650 ± 125 nM) than at pH 5.5 (65 ± 9 nM).
Figure 7.
Generation of NO (filled circles) and S-nitrosothiols (open circles) from saliva mixed with HCl to pH 5.5 or pH 2. Saliva was collected from healthy volunteers after ingestion of nitrate (0.1 mmol/kg); nitrite content was 0.9 mM. Data are expressed as mean ± SEM of three experiments.
Intragastric NO generation in vivo
Mean intragastric NO levels were 73 ± 47 ppb in control animals (treated with NaCl) and 8,161 ± 3,070 ppb in rats given sodium nitrate (P < 0.01).
Discussion
We show here that nitrite-containing saliva increases gastric mucosal blood flow and thickness of the firmly adherent mucus layer when it comes in contact with the acidic lumen solution. Similar dose-dependent effects were seen when using sodium nitrite in the same experimental model. In contrast, fasting saliva had no effect on mucosal blood flow. All saliva used in this study was collected from the same persons before and after the nitrate load. Prior to the experiment, the subjects had been fasting overnight and measured nitrite levels were very low (<50 μM). After the nitrate load, salivary nitrite rose to about 2 mM. Taken together, these observations strongly suggest that the salivary component responsible for the increases in mucosal blood flow and mucus thickness is nitrite. However, nitrite itself is unlikely to be mediating these effects directly and independently since this anion was completely without effect when lumen pH was increased to 5.5 (the pH of saline). Also, replacing the saline with HCl at pH 2 without addition of nitrite did not cause any changes in mucosal blood flow, implying that protons were not mediating the effects. At a low pH, nitrite is rapidly converted to nitrous acid (pKa = 3.4), which in turn decomposes to a variety of biologically active nitrogen oxides including NO (23, 37). It is likely that the effects observed here are cGMP dependent and caused by NO, although effects of other closely related compounds cannot be excluded. The effects on blood flow and mucus formation were paralleled by formation of NO gas measured in the headspace above the solutions, and the nitrite-mediated effects were almost abolished by local pretreatment with ODQ, an inhibitor of guanylyl cyclase (38).
It is possible that acidified nitrite is acting indirectly via activation of NOSs in mucosal blood vessels and mucus-producing cells. This is however less likely since an NOS inhibitor (L-NNA) did not influence the effects caused by nitrite in this study. An effective inhibition of NOS was apparent by the rise in systemic blood pressure and mucosal vascular resistance seen after intravenous administration of the drug. NO has been shown to stimulate PG synthesis in the gastric mucosa (39), which could suggest that the nitrite-mediated effects are ultimately mediated by PGs. This also seems unlikely since in the experiments with indomethacin the mucosal blood flow response to nitrite was unaffected. The evidence here supports the interpretation that luminal nitrite–derived NO penetrates the mucus gel to the effector cells in the superficial mucosa (mucus-producing cells) and through the mucosa to the submucosal arterioles.
We also observed increases in gastric mucosal blood flow when the S-nitrosothiol SNAP was applied from the luminal side. The transport of nitrite-derived NO through the mucus gel and mucosa could partly be in the form of S-nitrosothiols. These compounds can function as stable carriers of NO (for example in blood), thereby increasing the half-life of NO and allowing for more distal effects (40). The stomach appears to be an ideal milieu for generation of S-nitrosothiols since the powerful nitrosylating agent N2O3 is generated from acidified nitrite (41). Indeed, we did observe formation of S-nitrosothiols, thereby confirming the results from a recent study by Richardson et al., who found S-nitrosothiols in human gastric aspirates following nitrate ingestion (42). In addition, we have here shown that saliva contains both substrates (nitrite and thiol groups) necessary for S-nitrosothiol formation in the acidic stomach. It is also likely that gastric thiols, e.g., from sulfur-containing glycoproteins in mucus or from glutathione produced by gastric epithelial cells (43), are S-nitrosylated by acidified nitrite.
The exact nature of the thiols in saliva remains to be determined. It will also be of interest to study whether the biological activity of salivary and gastric proteins (e.g., digestive enzymes or enzymes in the gastric mucosa) are affected by the S-nitrosylation occurring in the stomach. Indeed, S-nitrosylation of the critical sulfur-containing amino acid cysteine at the active site of enzymes has emerged as a fundamental mechanism for regulating enzyme activity (40, 44). Taken together, from the present data it is not possible to finally confirm whether the effects of acidified nitrite are caused by NO directly or via generation of S-nitrosothiols, or by a combination of the two. In the experiments using a non–thiol-containing pure NO donor (DETA/NO), a similar increase in blood flow was noted, which suggests that direct effects of NO indeed are possible.
When comparing the effects of nitrite-rich saliva with those of exogenous sodium nitrite, it was noted that the peak in gastric blood flow was somewhat higher with sodium nitrite treatment, while the effect was more prolonged with saliva (Figure 1). The reason for this difference is not clear. One possible explanation is that saliva favors the formation of S-nitrosothiols, which release NO more slowly over a longer period of time. A more simplistic explanation could be that the saliva dissolves more slowly than sodium nitrite in the saline solution of the chamber, thereby delaying the release of NO close to the mucosal surface.
NO and PG’s are the two major terminal mediators of increases in gastric mucosal blood flow in response to various agents (13, 45). A study by Takeuchi et al. showed a 25–30% increase in blood flow when using a PGI2 analogue topically (46). Conversely, inhibition of PG production with indomethacin decreases basal gastric mucosal blood flow by about 30% in our model (47). In the present study the response to NO donators and to nitrite-rich saliva were of the same magnitude, underscoring the potential physiological importance of this system.
This study also examined the effects of nitrite and saliva on gastric mucus generation in rats stimulated to produce acid. The mucus layer covering the gastric mucosa has been shown to consist of two layers, with an outer layer that can easily be removed by gentle suction, leaving an inner firmly adherent mucus layer that is apparently not affected by this maneuver (34). The firmly adherent inner mucus layer is an important part of gastric mucosal defense, providing protection against acid back-diffusion (11), whereas the outer loosely adherent mucus is probably not as important in this function. Nitrite-rich saliva or sodium nitrite significantly increased the thickness of the inner firmly adherent mucus compared with the control situation with acid alone. The exact mechanism behind these effects remains to be determined.
Protective gastric mechanisms automatically improve in a situation of high acid output, since bicarbonate production increases in parallel (48). Thus, the mucosa is less sensitive to luminal irritants (e.g., exogenous acid challenge) if endogenous acid output is stimulated (9). Notably, NO production in the lumen is also autoregulated, with more NO being generated when pH is low (37). Since NO is uncharged, it can probably diffuse more easily over the mucus membrane than protons can, thereby aiding feedback mechanisms and signaling to the underlying mucosa.
There is today substantial evidence that both endogenous and exogenously delivered NO serve important gastroprotective functions (13). Various NO-donating drugs afford strong protection against ulcerogenic agents both in animals and in humans (49–51). For example, Fiorucci et al. showed in a clinical trial that the gastric lesions caused by aspirin are almost completely avoided if an NO-donating moiety is incorporated into the drug (49). Very recent animal studies have indicated that similar protective effects are afforded by dietary nitrate (52–54).
The amount of NO produced nonenzymatically in the stomach in vivo is dependent on several parameters, including gastric acidity, salivary nitrite levels, and local redox conditions (37). The acidity used here (pH 2) and the amounts of nitrate ingested were chosen to resemble what is physiologically achievable. The acidity of the human stomach varies over the day and is often below pH 1. The amount of nitrate ingested in this study (0.1 mmol/kg) corresponds to what is found in about 150–300 g of spinach (55). This intake resulted in salivary nitrite levels above 1 mM. When using sodium nitrite in the dose-response experiments, we observed effects on blood flow already at 0.1–0.5 mM, suggesting that even a substantially lower nitrate intake would generate enough nitrite in saliva to have biological effects in the stomach.
There has been widespread discussion about health risks related to the amount of nitrate in our diet. When dietary nitrate enters saliva it is rapidly reduced to nitrite in the mouth by mechanisms discussed above. Saliva containing large amounts of nitrite is acidified in the normal stomach to enhance generation of N-nitrosamines (56), which are powerful carcinogens in the experimental setting. More recently, it has been suggested that NO in the stomach could also be carcinogenic (57). A great number of studies have been performed examining the relationship between nitrate intake and gastric cancer in humans and animals. In general it has been found that there is either no relationship or an inverse relationship, such that a high nitrate intake is associated with a lower rate of cancer (58–60). Recently, studies have been performed suggesting that not only is nitrate harmless but in fact it may even be beneficial (27, 28, 37, 54). Indeed, acidified nitrite may be an important part of gastric host defense against swallowed pathogens. The results presented here further support the interpretation that dietary nitrate is gastroprotective. They also suggest that the oral microflora, instead of being potentially harmful, is living in a true symbiotic relationship with its host. The host provides nitrate, which is an important nutrient for many anaerobic bacteria. In return, the bacteria help the host by generating the substrate (nitrite) necessary for generation of NO in the stomach.
In conclusion, ingestion of inorganic nitrate results in rapid accumulation of nitrite in saliva. In the acidic stomach, nitrite-containing saliva generates NO with a concomitant cGMP-dependent increase in mucosal blood flow. Furthermore, the firmly adherent mucus layer increases in thickness. These results indicate that dietary nitrate may serve important gastroprotective functions.
Acknowledgments
This study was supported by grants from the Ekhaga Foundation, the Swedish Research Council (08646, 12585, and 12586), Karolinska Institute, the Swedish Heart-Lung Foundation, the Jeanssen Foundation, and the European Union Sixth Framework Porgramme. We thank Mirco Govoni for expert help with the nitrite/S-nitrosothiol measurements and Annika Jägare for expert technical assistance.
Footnotes
See the related Commentary beginning on page 19.
Håkan Björne and Joel Petersson contributed equally to this work.
Conflict of interest: The authors have declared that no conflict of interest exists.
Nonstandard abbreviations used: N-nitro-L-arginine (L-NNA); mean arterial blood pressure (MAP); S-nitroso-N-acetyl-penicillamine (SNAP); 1H-[1,2,4]oxadiazolo[4,3,-a]quinoxalin-1-one (ODQ); diethylenetriamine NONOate (DETA/NO).
References
- 1.Cheung LY, Chang N. The role of gastric mucosal blood flow and H+ back-diffusion in the pathogenesis of acute gastric erosions. J. Surg. Res. 1977;22:357–361. doi: 10.1016/0022-4804(77)90157-3. [DOI] [PubMed] [Google Scholar]
- 2.McGreevy JM, Moody FG. Protection of gastric mucosa against aspirin-induced erosions by enhanced blood flow. Surg. Forum. 1977;28:357–359. [PubMed] [Google Scholar]
- 3.Leung FW, Itoh M, Hirabayashi K, Guth PH. Role of blood flow in gastric and duodenal mucosal injury in the rat. Gastroenterology. 1985;88:281–289. doi: 10.1016/s0016-5085(85)80181-5. [DOI] [PubMed] [Google Scholar]
- 4.Stein HJ, Bauerfeind P, Hinder RA, Koerfer J, Blum AL. Luminal acid reduces gastric mucosal blood flow in the ischemic stomach. J. Surg. Res. 1989;46:616–619. doi: 10.1016/0022-4804(89)90031-0. [DOI] [PubMed] [Google Scholar]
- 5.Allen A, Garner A. Mucus and bicarbonate secretion in the stomach and their possible role in mucosal protection. Gut. 1980;21:249–262. doi: 10.1136/gut.21.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pique JM, Whittle BJ, Esplugues JV. The vasodilator role of endogenous nitric oxide in the rat gastric microcirculation. Eur. J. Pharmacol. 1989;174:293–296. doi: 10.1016/0014-2999(89)90324-5. [DOI] [PubMed] [Google Scholar]
- 7.Wallace JL, Granger DN. The cellular and molecular basis of gastric mucosal defense. FASEB J. 1996;10:731–740. doi: 10.1096/fasebj.10.7.8635690. [DOI] [PubMed] [Google Scholar]
- 8.Allen A, Flemstrom G, Garner A, Kivilaakso E. Gastroduodenal mucosal protection. Physiol. Rev. 1993;73:823–857. doi: 10.1152/physrev.1993.73.4.823. [DOI] [PubMed] [Google Scholar]
- 9.Synnerstad I, Johansson M, Nylander O, Holm L. Intraluminal acid and gastric mucosal integrity: the importance of blood-borne bicarbonate. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G121–G129. doi: 10.1152/ajpgi.2001.280.1.G121. [DOI] [PubMed] [Google Scholar]
- 10.Schade C, Flemstrom G, Holm L. Hydrogen ion concentration in the mucus layer on top of acid-stimulated and -inhibited rat gastric mucosa. Gastroenterology. 1994;107:180–188. doi: 10.1016/0016-5085(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 11.Phillipson M, Atuma C, Henriksnas J, Holm L. The importance of mucus layers and bicarbonate transport in preservation of gastric juxtamucosal pH. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G211–G219. doi: 10.1152/ajpgi.00223.2001. [DOI] [PubMed] [Google Scholar]
- 12.Wallace JL, Tigley AW. Review article: new insights into prostaglandins and mucosal defence. Aliment. Pharmacol. Ther. 1995;9:227–235. doi: 10.1111/j.1365-2036.1995.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 13.Wallace JL, Miller MJ. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512–520. doi: 10.1053/gast.2000.9304. [DOI] [PubMed] [Google Scholar]
- 14.Brown JF, Hanson PJ, Whittle BJ. Nitric oxide donors increase mucus gel thickness in rat stomach. Eur. J. Pharmacol. 1992;223:103–104. doi: 10.1016/0014-2999(92)90824-n. [DOI] [PubMed] [Google Scholar]
- 15.Sababi M, Nilsson E, Holm L. Mucus and alkali secretion in the rat duodenum: effects of indomethacin, N omega-nitro-L-arginine, and luminal acid. Gastroenterology. 1995;109:1526–1534. doi: 10.1016/0016-5085(95)90640-1. [DOI] [PubMed] [Google Scholar]
- 16.Wallace JL, Ma L. Inflammatory mediators in gastrointestinal defense and injury. Exp. Biol. Med. 2001;226:1003–1015. doi: 10.1177/153537020122601107. [DOI] [PubMed] [Google Scholar]
- 17.Holzer P. Neural emergency system in the stomach. Gastroenterology. 1998;114:823–839. doi: 10.1016/s0016-5085(98)70597-9. [DOI] [PubMed] [Google Scholar]
- 18.Holzer P. Chemosensitive afferent nerves in the regulation of gastric blood flow and protection. Adv. Exp. Med. Biol. 1995;371B:891–895. [PubMed] [Google Scholar]
- 19.MacNaughton WK, Cirino G, Wallace JL. Endothelium-derived relaxing factor (nitric oxide) has protective actions in the stomach. Life Sci. 1989;45:1869–1876. doi: 10.1016/0024-3205(89)90540-7. [DOI] [PubMed] [Google Scholar]
- 20.Wallace JL. Nonsteroidal anti-inflammatory drugs and the gastrointestinal tract. Mechanisms of protection and healing: current knowledge and future research. Am. J. Med. 2001;110:19S–23S. doi: 10.1016/s0002-9343(00)00631-8. [DOI] [PubMed] [Google Scholar]
- 21.Sugata H, Ueno T, Shimosegawa T, Yoshimura T. Direct detection of nitric oxide and its roles in maintaining gastric mucosal integrity following ethanol-induced injury in rats. Free Radic. Res. 2003;37:159–169. doi: 10.1080/1071576021000036461. [DOI] [PubMed] [Google Scholar]
- 22.Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamin N, et al. Stomach NO synthesis. Nature. 1994;368:502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 24.McKnight GM, et al. Chemical synthesis of nitric oxide in the stomach from dietary nitrate in humans. Gut. 1997;40:211–214. doi: 10.1136/gut.40.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tannenbaum SR, Weisman M, Fett D. The effect of nitrate intake on nitrite formation in human saliva. Food Cosmet. Toxicol. 1976;14:549–552. doi: 10.1016/s0015-6264(76)80006-5. [DOI] [PubMed] [Google Scholar]
- 26.Spiegelhalder B, Eisenbrand G, Preussman R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet. Toxicol. 1976;14:545–548. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- 27.Duncan C, et al. Protection against oral and gastrointestinal diseases: importance of dietary nitrate intake, oral nitrate reduction and enterosalivary nitrate circulation. Comp. Biochem. Physiol. A Physiol. 1997;118:939–948. doi: 10.1016/s0300-9629(97)00023-6. [DOI] [PubMed] [Google Scholar]
- 28.Dykhuizen R, et al. Antimicrobial effect of acidified nitrite on gut pathogens: importance of dietary nitrate in host defence. Antimicrob. Agents Chemother. 1996;40:1422–1425. doi: 10.1128/aac.40.6.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Xu X, Verstraete W. The bactericidal effect and chemical reactions of acidified nitrite under conditions simulating the stomach. J. Appl. Microbiol. 2001;90:523–529. doi: 10.1046/j.1365-2672.2001.01278.x. [DOI] [PubMed] [Google Scholar]
- 30.Holm-Rutili L, Obrink KJ. Rat gastric mucosal microcirculation in vivo. Am. J. Physiol. 1985;248:G741–G746. doi: 10.1152/ajpgi.1985.248.6.G741. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson GE, Tenland T, Oberg PA. Evaluation of a laser Doppler flowmeter for measurement of tissue blood flow. IEEE Trans. Biomed. Eng. 1980;27:597–604. doi: 10.1109/TBME.1980.326582. [DOI] [PubMed] [Google Scholar]
- 32.Holm-Rutili L, Berglindh T. Pentagastrin and gastric mucosal blood flow. Am. J. Physiol. 1986;250:G575–G580. doi: 10.1152/ajpgi.1986.250.5.G575. [DOI] [PubMed] [Google Scholar]
- 33.Kvietys PR, Shepherd AP, Granger DN. Laser-Doppler, H2 clearance, and microsphere estimates of mucosal blood flow. Am. J. Physiol. 1985;249:G221–G227. doi: 10.1152/ajpgi.1985.249.2.G221. [DOI] [PubMed] [Google Scholar]
- 34.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 35.Zetterquist W, Pedroletti C, Lundberg JO, Alving K. Salivary contribution to exhaled nitric oxide. Eur. Respir. J. 1999;13:327–333. doi: 10.1034/j.1399-3003.1999.13b18.x. [DOI] [PubMed] [Google Scholar]
- 36.Feelisch M, et al. Concomitant S-, N-, and heme-nitros(yl)ation in biological tissues and fluids: implications for the fate of NO in vivo. FASEB J. 2002;16:1775–1785. doi: 10.1096/fj.02-0363com. [DOI] [PubMed] [Google Scholar]
- 37.Weitzberg E, Lundberg JO. Nonenzymatic nitric oxide production in humans. Nitric Oxide. 1998;2:1–7. doi: 10.1006/niox.1997.0162. [DOI] [PubMed] [Google Scholar]
- 38.Garthwaite J, et al. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol. Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- 39.Uno H, et al. Nitric oxide stimulates prostaglandin synthesis in cultured rabbit gastric cells. Prostaglandins. 1997;53:153–162. doi: 10.1016/s0090-6980(97)00013-0. [DOI] [PubMed] [Google Scholar]
- 40.Foster MW, McMahon TJ, Stamler JS. S-nitrosylation in health and disease. Trends Mol. Med. 2003;9:160–168. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 41.Keshive M, Singh S, Wishnok JS, Tannenbaum SR, Deen WM. Kinetics of S-nitrosation of thiols in nitric oxide solutions. Chem. Res. Toxicol. 1996;9:988–993. doi: 10.1021/tx960036y. [DOI] [PubMed] [Google Scholar]
- 42.Richardson G, et al. The ingestion of inorganic nitrate increases gastric S-nitrosothiol levels and inhibits platelet function in humans. Nitric Oxide. 2002;7:24–29. doi: 10.1016/s1089-8603(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 43.Body SC, Sasame HA, Body MR. High concentrations of glutathione in glandular stomach: possible implications for carcinogenesis. Science. 1979;205:1010–1012. doi: 10.1126/science.572989. [DOI] [PubMed] [Google Scholar]
- 44.Stamler JS, Lamas S, Fang FC. Nitrosylation. The prototypic redox-based signaling mechanism. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 45.Miller TA. Protective effects of prostaglandins against gastric mucosal damage: current knowledge and proposed mechanisms. Am. J. Physiol. 1983;245:G601–G623. doi: 10.1152/ajpgi.1983.245.5.G601. [DOI] [PubMed] [Google Scholar]
- 46.Takeuchi K, et al. Facilitation by endogenous prostaglandins of capsaicin-induced gastric protection in rodents through EP2 and IP receptors. J. Pharmacol. Exp. Ther. 2003;304:1055–1062. doi: 10.1124/jpet.102.044156. [DOI] [PubMed] [Google Scholar]
- 47.Holm L, Jagare A. Influence of tactile stimulation of the rat gastric mucosa on blood flow and acid output. Am. J. Physiol. 1993;265:G303–G309. doi: 10.1152/ajpgi.1993.265.2.G303. [DOI] [PubMed] [Google Scholar]
- 48.Teorell T. The acid-base balance of the secreting isolated gastric mucosa. J. Physiol. (Lond.) 1951;114:267–276. doi: 10.1113/jphysiol.1951.sp004618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiorucci S, et al. Gastrointestinal safety of NO-aspirin (NCX-4016) in healthy human volunteers: a proof of concept endoscopic study. Gastroenterology. 2003;124:600–607. doi: 10.1053/gast.2003.50096. [DOI] [PubMed] [Google Scholar]
- 50.Andrews FJ, Malcontenti-Wilson C, O’Brien PE. Protection against gastric ischemia-reperfusion injury by nitric oxide generators. Dig. Dis. Sci. 1994;39:366–373. doi: 10.1007/BF02090210. [DOI] [PubMed] [Google Scholar]
- 51.Holm L, Phillipson M, Perry MA. NO-flurbiprofen maintains duodenal blood flow, enhances mucus secretion contributing to lower mucosal injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G1090–G1097. doi: 10.1152/ajpgi.00480.2001. [DOI] [PubMed] [Google Scholar]
- 52.Larauche M, et al. Protective effect of dietary nitrate on experimental gastritis in rats. Br. J. Nutr. 2003;89:777–786. doi: 10.1079/BJN2003845. [DOI] [PubMed] [Google Scholar]
- 53.Larauche M, Bueno L, Fioramonti J. Effect of dietary nitric oxide on gastric mucosal mast cells in absence or presence of an experimental gastritis in rats. Life Sci. 2003;73:1505–1516. doi: 10.1016/s0024-3205(03)00480-6. [DOI] [PubMed] [Google Scholar]
- 54.Miyoshi M, et al. Dietary nitrate inhibits stress-induced gastric mucosal injury in the rat. Free Radic. Res. 2003;37:85–90. doi: 10.1080/1071576021000086632. [DOI] [PubMed] [Google Scholar]
- 55.Chung SY, et al. Survey of nitrate and nitrite contents of vegetables grown in Korea. Food Addit. Contam. 2003;20:621–628. doi: 10.1080/0265203031000124146. [DOI] [PubMed] [Google Scholar]
- 56.Tannenbaum SR, Sisnkey AJ, Weisman M, Bishop W. Nitrite in human saliva. Its possible relationship to nitrosamine formation. J. Natl. Cancer Inst. 1974;53:79–84. [PubMed] [Google Scholar]
- 57.Iijima K, et al. Dietary nitrate generates potentially mutagenic concentrations of nitric oxide at the gastroesophageal junction. Gastroenterology. 2002;122:1248–1257. doi: 10.1053/gast.2002.32963. [DOI] [PubMed] [Google Scholar]
- 58.Crampton RF. Carcinogenic dose-related response to nitrosamines. Oncology. 1980;37:251–254. doi: 10.1159/000225446. [DOI] [PubMed] [Google Scholar]
- 59.Knight TM, et al. Nitrate and nitrite exposure in Italian populations with different gastric cancer rates. Int. J. Epidemiol. 1990;19:510–515. doi: 10.1093/ije/19.3.510. [DOI] [PubMed] [Google Scholar]
- 60.Al-Dabbagh S, Forman D, Bryson D, Stratton I, Doll R. Mortality of nitrate fertiliser workers. Br. J. Ind. Med. 1986;43:507–515. doi: 10.1136/oem.43.8.507. [DOI] [PMC free article] [PubMed] [Google Scholar]