Abstract
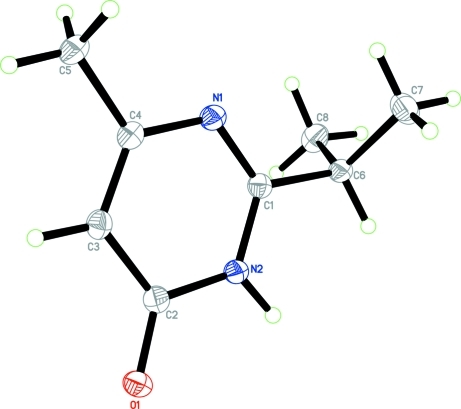
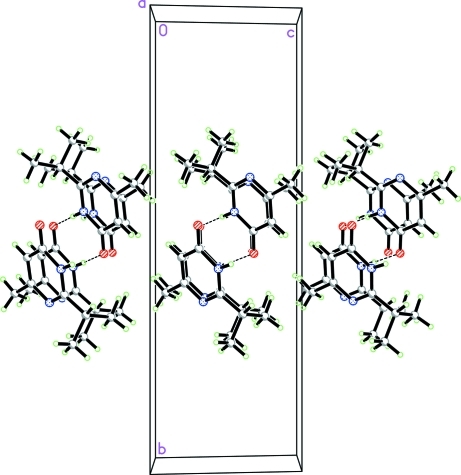
The molecular structure of the title compound, C8H12N2O, indicates that 2-isopropyl-6-methylpyrimidin-4-ol (the enol–form) undergoes an enol-to-keto tautomerism during the crystallization process. The pyrimidin-4(3H)-one group is essentially planar, with a maximum deviation of 0.081 (1) Å for the O atom. In the crystal structure, symmetry-related molecules are linked into centrosymmetic dimers via pairs of intermolecular N—H⋯O hydrogen bonds, generating R 2 2(8) rings. These dimers are stacked along the a axis.
Related literature
For applications of pyridinium derivatives, see: Condon et al. (1993 ▶); Maeno et al. (1990 ▶); Gilchrist (1997 ▶); Selby et al. (2002 ▶). For hydrogen-bond motifs, see: Bernstein et al. (1995 ▶). For the stability of the temperature controller used in the data collection, see: Cosier & Glazer (1986 ▶).
Experimental
Crystal data
C8H12N2O
M r = 152.20
Monoclinic,
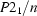
a = 4.8627 (2) Å
b = 22.6320 (8) Å
c = 7.4228 (3) Å
β = 96.495 (2)°
V = 811.66 (5) Å3
Z = 4
Mo Kα radiation
μ = 0.08 mm−1
T = 100 K
0.74 × 0.14 × 0.07 mm
Data collection
Bruker SMART APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2009 ▶) T min = 0.940, T max = 0.994
7806 measured reflections
2371 independent reflections
1958 reflections with I > 2σ(I)
R int = 0.026
Refinement
R[F 2 > 2σ(F 2)] = 0.039
wR(F 2) = 0.103
S = 1.06
2371 reflections
148 parameters
All H-atom parameters refined
Δρmax = 0.32 e Å−3
Δρmin = −0.20 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810034276/lh5121sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810034276/lh5121Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H1N2⋯O1i | 0.937 (15) | 1.844 (14) | 2.7809 (11) | 178.7 (10) |
Symmetry code: (i) 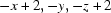 .
.
Acknowledgments
MH and HKF thank the Malaysian Government and Universiti Sains Malaysia for the Research University Golden Goose grant No. 1001/PFIZIK/811012. MH also thanks Universiti Sains Malaysia for a post-doctoral research fellowship.
supplementary crystallographic information
Comment
Pyrimidine derivatives are very important molecules in biology and have many application in the areas of pesticide and pharmaceutical agents (Condon et al., 1993). For example, imazosulfuron, ethirmol and mepanipyrim have been commercialized as agrochemicals (Maeno et al., 1990). Pyrimidine derivatives have also been developed as antiviral agents, such as AZT, which is the most widely used anti-AIDS drug (Gilchrist, 1997). Recently, a new series of highly active herbicides of substituted azolylpyrimidines were reported (Selby et al., 2002). Keeping in view of the importance of the pyrimidine derivatives, the title compound (I) was presented.
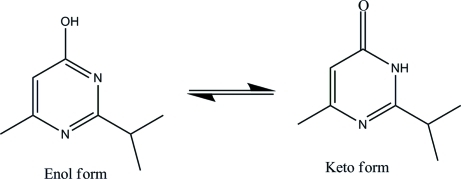
The title molecule, (Fig. 1), exists in the keto-form although 2-isopropyl-4-hydroxy-6-methylpyrimidine (the enol-form) was used for crystallization. This indicates the compound undergoes an enol-to-keto tautomerism during the crystallization process (Fig. 3). The C2═O1 bond length is 1.2497 (11) Å. The pyrimidin-4(3H)-one group is essentially planar with a maximum deviation of 0.081 (1) Å for atom O1. In the crystal structure (Fig. 2), adjacent molecules are linked via pairs of intermolecular N—H···O hydrogen bonds to form dimers, generating R22(8) rings (Bernstein et al., 1995). These dimers are stacked along the a-axis.
Experimental
Hot methanol solution (20 ml) of 2-isopropyl-4-hydroxy-6-methylpyrimidine (46 mg, Aldrich) was warmed over a heating magnetic stirrer for 5 minutes. The resulting solution was allowed to cool slowly at room temperature. Crystals of the title compound appeared from the mother liquor after a few days.
Refinement
All H atoms were located in a difference Fourier map and refined freely.
Figures
Fig. 1.
The molecular structure of the title compound. Displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.
The crystal packing of the title compound, viewed approximately along the a-axis. Hydrogen bonds are shown as dashed lines.
Fig. 3.
The title compound and the tautomeric form.
Crystal data
| C8H12N2O | F(000) = 328 |
| Mr = 152.20 | Dx = 1.245 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2yn | Cell parameters from 2995 reflections |
| a = 4.8627 (2) Å | θ = 2.9–30.0° |
| b = 22.6320 (8) Å | µ = 0.08 mm−1 |
| c = 7.4228 (3) Å | T = 100 K |
| β = 96.495 (2)° | Needle, colourless |
| V = 811.66 (5) Å3 | 0.74 × 0.14 × 0.07 mm |
| Z = 4 |
Data collection
| Bruker SMART APEXII CCD area-detector diffractometer | 2371 independent reflections |
| Radiation source: fine-focus sealed tube | 1958 reflections with I > 2σ(I) |
| graphite | Rint = 0.026 |
| φ and ω scans | θmax = 30.1°, θmin = 1.8° |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | h = −5→6 |
| Tmin = 0.940, Tmax = 0.994 | k = −26→31 |
| 7806 measured reflections | l = −10→10 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.039 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.103 | All H-atom parameters refined |
| S = 1.06 | w = 1/[σ2(Fo2) + (0.049P)2 + 0.163P] where P = (Fo2 + 2Fc2)/3 |
| 2371 reflections | (Δ/σ)max < 0.001 |
| 148 parameters | Δρmax = 0.32 e Å−3 |
| 0 restraints | Δρmin = −0.20 e Å−3 |
Special details
| Experimental. The crystal was placed in the cold stream of an Oxford Cryosystems Cobra open-flow nitrogen cryostat (Cosier & Glazer, 1986) operating at 100.0 (1) K. |
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.77782 (15) | 0.03007 (3) | 0.81600 (9) | 0.02023 (18) | |
| N1 | 0.43265 (17) | −0.13343 (3) | 0.84671 (10) | 0.01613 (18) | |
| N2 | 0.75959 (17) | −0.06091 (3) | 0.94759 (10) | 0.01473 (17) | |
| C1 | 0.64511 (19) | −0.11546 (4) | 0.95755 (12) | 0.01455 (19) | |
| C2 | 0.6596 (2) | −0.01885 (4) | 0.82081 (12) | 0.0158 (2) | |
| C3 | 0.4232 (2) | −0.03768 (4) | 0.70385 (12) | 0.0169 (2) | |
| C4 | 0.32239 (19) | −0.09370 (4) | 0.71759 (12) | 0.0156 (2) | |
| C5 | 0.0868 (2) | −0.11656 (5) | 0.58916 (13) | 0.0190 (2) | |
| C6 | 0.7737 (2) | −0.15603 (4) | 1.10544 (13) | 0.01614 (19) | |
| C7 | 0.8468 (3) | −0.21559 (5) | 1.02578 (15) | 0.0249 (2) | |
| C8 | 0.5752 (2) | −0.16347 (5) | 1.25028 (14) | 0.0222 (2) | |
| H1N2 | 0.915 (3) | −0.0510 (7) | 1.0283 (19) | 0.034 (4)* | |
| H3A | 0.338 (3) | −0.0101 (6) | 0.6146 (18) | 0.025 (3)* | |
| H5A | −0.064 (3) | −0.1299 (6) | 0.655 (2) | 0.036 (4)* | |
| H5B | 0.011 (3) | −0.0864 (7) | 0.503 (2) | 0.044 (4)* | |
| H5C | 0.151 (3) | −0.1505 (6) | 0.5237 (19) | 0.034 (4)* | |
| H6A | 0.946 (3) | −0.1369 (5) | 1.1613 (16) | 0.018 (3)* | |
| H7A | 0.922 (3) | −0.2424 (6) | 1.124 (2) | 0.031 (3)* | |
| H7B | 0.984 (3) | −0.2117 (6) | 0.9349 (19) | 0.031 (4)* | |
| H7C | 0.681 (3) | −0.2348 (6) | 0.9648 (19) | 0.036 (4)* | |
| H8A | 0.661 (3) | −0.1874 (6) | 1.3518 (19) | 0.029 (3)* | |
| H8B | 0.408 (3) | −0.1849 (6) | 1.1978 (18) | 0.031 (4)* | |
| H8C | 0.519 (3) | −0.1249 (6) | 1.2967 (19) | 0.032 (4)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0210 (4) | 0.0159 (3) | 0.0229 (4) | −0.0031 (3) | −0.0012 (3) | 0.0032 (3) |
| N1 | 0.0159 (4) | 0.0163 (4) | 0.0157 (4) | −0.0010 (3) | 0.0002 (3) | −0.0005 (3) |
| N2 | 0.0146 (4) | 0.0143 (4) | 0.0150 (4) | −0.0008 (3) | 0.0005 (3) | 0.0004 (3) |
| C1 | 0.0146 (4) | 0.0146 (4) | 0.0146 (4) | 0.0003 (3) | 0.0026 (3) | −0.0007 (3) |
| C2 | 0.0159 (4) | 0.0161 (4) | 0.0156 (4) | 0.0005 (3) | 0.0028 (3) | 0.0007 (3) |
| C3 | 0.0165 (5) | 0.0185 (4) | 0.0153 (4) | 0.0014 (3) | 0.0006 (3) | 0.0019 (3) |
| C4 | 0.0143 (4) | 0.0185 (5) | 0.0142 (4) | 0.0005 (3) | 0.0019 (3) | −0.0018 (3) |
| C5 | 0.0164 (5) | 0.0227 (5) | 0.0172 (4) | −0.0011 (4) | −0.0013 (3) | −0.0022 (4) |
| C6 | 0.0157 (4) | 0.0151 (4) | 0.0168 (4) | −0.0011 (3) | −0.0015 (3) | 0.0009 (3) |
| C7 | 0.0301 (6) | 0.0172 (5) | 0.0260 (5) | 0.0036 (4) | −0.0026 (4) | −0.0004 (4) |
| C8 | 0.0202 (5) | 0.0268 (5) | 0.0193 (5) | −0.0016 (4) | 0.0012 (4) | 0.0061 (4) |
Geometric parameters (Å, °)
| O1—C2 | 1.2497 (11) | C5—H5B | 0.978 (16) |
| N1—C1 | 1.3105 (12) | C5—H5C | 0.978 (15) |
| N1—C4 | 1.3777 (12) | C6—C7 | 1.5297 (14) |
| N2—C1 | 1.3595 (12) | C6—C8 | 1.5332 (14) |
| N2—C2 | 1.3874 (12) | C6—H6A | 0.990 (12) |
| N2—H1N2 | 0.937 (15) | C7—H7A | 0.985 (14) |
| C1—C6 | 1.5114 (13) | C7—H7B | 1.004 (14) |
| C2—C3 | 1.4254 (13) | C7—H7C | 0.980 (15) |
| C3—C4 | 1.3672 (13) | C8—H8A | 0.982 (14) |
| C3—H3A | 0.968 (13) | C8—H8B | 0.987 (14) |
| C4—C5 | 1.4972 (13) | C8—H8C | 0.988 (15) |
| C5—H5A | 0.973 (16) | ||
| C1—N1—C4 | 116.83 (8) | H5A—C5—H5C | 107.8 (12) |
| C1—N2—C2 | 123.08 (8) | H5B—C5—H5C | 109.9 (12) |
| C1—N2—H1N2 | 119.2 (9) | C1—C6—C7 | 110.47 (8) |
| C2—N2—H1N2 | 117.7 (9) | C1—C6—C8 | 109.54 (8) |
| N1—C1—N2 | 123.11 (9) | C7—C6—C8 | 111.50 (8) |
| N1—C1—C6 | 119.97 (8) | C1—C6—H6A | 107.5 (7) |
| N2—C1—C6 | 116.92 (8) | C7—C6—H6A | 108.9 (7) |
| O1—C2—N2 | 120.02 (9) | C8—C6—H6A | 108.9 (7) |
| O1—C2—C3 | 126.12 (9) | C6—C7—H7A | 109.9 (8) |
| N2—C2—C3 | 113.86 (8) | C6—C7—H7B | 112.5 (8) |
| C4—C3—C2 | 120.21 (9) | H7A—C7—H7B | 109.4 (11) |
| C4—C3—H3A | 121.2 (8) | C6—C7—H7C | 110.8 (9) |
| C2—C3—H3A | 118.6 (8) | H7A—C7—H7C | 106.5 (12) |
| C3—C4—N1 | 122.85 (9) | H7B—C7—H7C | 107.6 (11) |
| C3—C4—C5 | 121.86 (9) | C6—C8—H8A | 110.7 (8) |
| N1—C4—C5 | 115.27 (8) | C6—C8—H8B | 109.4 (8) |
| C4—C5—H5A | 110.6 (9) | H8A—C8—H8B | 106.8 (12) |
| C4—C5—H5B | 112.3 (9) | C6—C8—H8C | 111.6 (8) |
| H5A—C5—H5B | 107.2 (12) | H8A—C8—H8C | 109.3 (11) |
| C4—C5—H5C | 108.9 (8) | H8B—C8—H8C | 109.0 (11) |
| C4—N1—C1—N2 | −1.11 (13) | C2—C3—C4—N1 | 2.79 (14) |
| C4—N1—C1—C6 | 178.69 (8) | C2—C3—C4—C5 | −175.72 (8) |
| C2—N2—C1—N1 | 0.98 (14) | C1—N1—C4—C3 | −0.79 (13) |
| C2—N2—C1—C6 | −178.83 (8) | C1—N1—C4—C5 | 177.82 (8) |
| C1—N2—C2—O1 | −178.41 (8) | N1—C1—C6—C7 | 52.88 (12) |
| C1—N2—C2—C3 | 0.98 (12) | N2—C1—C6—C7 | −127.31 (9) |
| O1—C2—C3—C4 | 176.61 (9) | N1—C1—C6—C8 | −70.32 (11) |
| N2—C2—C3—C4 | −2.73 (13) | N2—C1—C6—C8 | 109.50 (9) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H1N2···O1i | 0.937 (15) | 1.844 (14) | 2.7809 (11) | 178.7 (10) |
Symmetry codes: (i) −x+2, −y, −z+2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LH5121).
References
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
- Bruker (2009). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Condon, M. E., Brady, T. E., Feist, D., Malefyt, T., Marc, P., Quakenbush, L. S., Rodaway, S. J., Shaner, D. L. & Tecle, B. (1993). Brighton Crop Protection Conference on Weeds, pp. 41–46. Alton, Hampshire, England: BCPC Publications.
- Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst.19, 105–107.
- Gilchrist, T. L. (1997). Heterocyclic Chemistry, 3rd ed., pp. 261–276. Singapore: Addison Wesley Longman.
- Maeno, S., Miura, I., Masuda, K. & Nagata, T. (1990). Brighton Crop Protection Conference on Pests and Diseases, pp. 415–422. Alton, Hampshire, England: BCPC Publications.
- Selby, T. P., Drumm, J. E., Coats, R. A., Coppo, F. T., Gee, S. K., Hay, J. V., Pasteris, R. J. & Stevenson, T. M. (2002). ACS Symposium Series, Vol. 800, Synthesis and Chemistry of Agrochemicals VI, pp. 74–84. Washington DC: American Chemical Society.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810034276/lh5121sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810034276/lh5121Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report