Abstract
The title compound, C14H10Cl4O4S, is a 2,2,2-trichloroethyl-protected precursor of 4′-chlorobiphenyl-3-yl sulfate, a sulfuric acid ester of 4′-chlorobiphenyl-3-ol. The Caromatic—O and O—S bond lengths of the Caromatic—O—S unit are comparable to those in structurally analogous biphenyl-4-yl 2,2,2-trichloroethyl sulfates with no electronegative chlorine substituent in the benzene ring with the sulfate ester group. The dihedral angle between the aromatic rings is 27.47 (6)°.
Related literature
For similar structures of sulfuric acid biphenyl-4-yl ester 2,2,2-trichloro-ethyl esters, see: Li et al. (2008 ▶, 2010a
▶,b
▶,c
▶). For a review of structures of sulfuric acid aryl mono esters, see: Brandao et al. (2005 ▶). For further discussion of dihedral angles in chlorinated biphenyl derivatives, see: Lehmler et al. (2002 ▶); Shaikh et al. (2008 ▶); Vyas et al. (2006 ▶). For additional background to hydroxylated polychlorinated biphenyls, see: Bergman et al. (1994 ▶); Buckman et al. (2006 ▶); Dirtu et al. (2010 ▶); Liu et al. (2006 ▶, 2009 ▶); Nomiyama et al. (2010 ▶); Wang et al. (2006 ▶).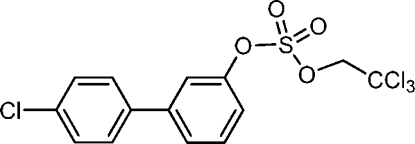
Experimental
Crystal data
C14H10Cl4O4S
M r = 416.08
Monoclinic,
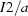
a = 21.1900 (3) Å
b = 5.8543 (1) Å
c = 26.6803 (5) Å
β = 98.304 (1)°
V = 3275.06 (10) Å3
Z = 8
Mo Kα radiation
μ = 0.87 mm−1
T = 90 K
0.41 × 0.22 × 0.06 mm
Data collection
Nonius KappaCCD diffractometer
Absorption correction: multi-scan (SCALEPACK; Otwinowski & Minor, 1997 ▶) T min = 0.718, T max = 0.950
30021 measured reflections
3759 independent reflections
3242 reflections with I > 2σ(I)
R int = 0.041
Refinement
R[F 2 > 2σ(F 2)] = 0.026
wR(F 2) = 0.067
S = 1.05
3759 reflections
208 parameters
H-atom parameters constrained
Δρmax = 0.39 e Å−3
Δρmin = −0.38 e Å−3
Data collection: COLLECT (Nonius, 1998 ▶); cell refinement: SCALEPACK (Otwinowski & Minor, 1997 ▶); data reduction: DENZO-SMN (Otwinowski & Minor, 1997 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XP in SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXL97 and local procedures.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810031338/om2350sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810031338/om2350Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
This research was supported by grants ES05605, ES013661 and ES017425 from the National Institute of Environmental Health Sciences, NIH.
supplementary crystallographic information
Comment
Hydroxylated metabolites of polychlorinated biphenyls (OHPCBs) are present in the serum of humans (Bergman et al., 1994; Dirtu et al., 2010) and other animals (Buckman et al., 2006; Nomiyama et al., 2010). Recent studies also indicate that the mammalian cytosolic sulfotransferases catalyze the formation of sulfuric acid esters from OHPCBs (Liu et al., 2006; Liu et al., 2009; Wang et al., 2006). However, our knowledge of the metabolic and toxicologic significance of these sulfation reactions has been hindered by a lack of information on the structural and chemical properties of the sulfuric acid ester products. As one component of our continuing studies on the properties of the sulfuric acid esters of OHPCBs, we report here the structure of 4'-chloro-biphenyl-3-yl 2,2,2-trichloroethyl sulfate.
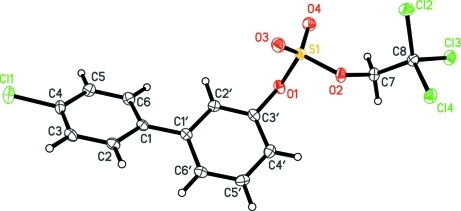
Several authors have proposed that the Caromatic—O and the corresponding O—S bond lengths are correlated with the stability of sulfuric acid conjugates (Brandao et al., 2005; Li et al., 2010a-c; Li et al., 2008). The Caromatic—O (i.e., C3'-O1) and O—S (i.e., O1—S1) bond lengths of the title compound are 1.4338 (17) Å and 1.5685 (11) Å, respectively. These values are comparable to the corresponding bond lengths reported for analogous biphenyl-4-yl 2,2,2-trichloroethyl sulfates with no electronegative chlorine substituent in the sulfated benzene ring (Li et al., 2010a-c; Li et al., 2008), which suggest that, analogous to biphenyl-4-yl sulfates, the 4'-chloro-biphenyl-3-yl sulfate corresponding to the title compound may be stable under physiological conditions (Brandao et al., 2005; Li et al., 2010c).
The dihedral angle of the biphenyl moiety of OHPCBs and, consequently, their sulfuric acid conjugates is associated with their affinity for cellular target molecules and, therefore, may correlate with their toxicity. The title compound adopts a solid state dihedral angle of 27.47 (6)°. The solid state dihedral angles of structurally related biphenyl-4-yl 2,2,2-trichloroethyl sulfates without ortho chlorine substituents ranges from 4.9 (2)° to 41.84 (16)° (Li et al., 2010a,b; Li et al., 2008). This large range of dihedral angles suggests, similar to the parent PCBs (Lehmler et al., 2002; Shaikh et al., 2008; Vyas et al., 2006), a conformational flexibility of the biphenyl moiety that allows the title compound and analogous biphenyl-4-yl sulfates to adopt an energetically less favorable conformation in the solid state due to crystal packing effects.
Experimental
The title compound was synthesized from 3',4'-dichlorobiphenyl-4-ol by sulfation with 2,2,2-trichloroethyl sulfonyl chloride using 4-dimethylaminopyridine as catalyst (Li et al., 2010c). Crystals suitable for crystal structure analysis were obtained by slowly evaporating a methanolic solution of the title compound.
Refinement
H atoms were placed in idealized positions and were constrained with distances of 0.99 Å for CH2 and 0.95 Å for CarH, and with Uiso(H) = 1.2Ueq of their attached C atom.
Figures
Fig. 1.
View of the title compound showing the atom-labeling scheme. Displacement ellipsoids are drawn at the 50% probability level.
Crystal data
| C14H10Cl4O4S | F(000) = 1680 |
| Mr = 416.08 | Dx = 1.688 Mg m−3 |
| Monoclinic, I2/a | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -I 2ya | Cell parameters from 4127 reflections |
| a = 21.1900 (3) Å | θ = 1.0–27.5° |
| b = 5.8543 (1) Å | µ = 0.87 mm−1 |
| c = 26.6803 (5) Å | T = 90 K |
| β = 98.304 (1)° | Plate, colourless |
| V = 3275.06 (10) Å3 | 0.41 × 0.22 × 0.06 mm |
| Z = 8 |
Data collection
| Nonius KappaCCD diffractometer | 3759 independent reflections |
| Radiation source: fine-focus sealed tube | 3242 reflections with I > 2σ(I) |
| graphite | Rint = 0.041 |
| Detector resolution: 18 pixels mm-1 | θmax = 27.5°, θmin = 1.5° |
| ω scans at fixed χ = 55° | h = −27→27 |
| Absorption correction: multi-scan (SCALEPACK; Otwinowski & Minor, 1997) | k = −7→7 |
| Tmin = 0.718, Tmax = 0.950 | l = −34→34 |
| 30021 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.026 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.067 | H-atom parameters constrained |
| S = 1.05 | w = 1/[σ2(Fo2) + (0.0294P)2 + 3.9224P] where P = (Fo2 + 2Fc2)/3 |
| 3759 reflections | (Δ/σ)max = 0.001 |
| 208 parameters | Δρmax = 0.39 e Å−3 |
| 0 restraints | Δρmin = −0.38 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.205499 (17) | 0.54226 (7) | 0.300571 (14) | 0.01597 (9) | |
| O1 | 0.18726 (5) | 0.4208 (2) | 0.24808 (4) | 0.0172 (2) | |
| O2 | 0.19895 (5) | 0.3440 (2) | 0.33919 (4) | 0.0183 (2) | |
| O3 | 0.15849 (5) | 0.7045 (2) | 0.30865 (4) | 0.0221 (2) | |
| O4 | 0.27060 (5) | 0.6040 (2) | 0.30274 (4) | 0.0248 (3) | |
| Cl1 | −0.064775 (19) | 1.32997 (7) | 0.026719 (15) | 0.02388 (10) | |
| Cl2 | 0.279773 (19) | 0.45519 (7) | 0.437358 (15) | 0.02180 (10) | |
| Cl3 | 0.333188 (17) | 0.00690 (7) | 0.427046 (15) | 0.02125 (10) | |
| Cl4 | 0.199083 (17) | 0.05436 (7) | 0.434813 (14) | 0.01980 (10) | |
| C1 | 0.01131 (7) | 0.7573 (3) | 0.13260 (5) | 0.0141 (3) | |
| C2 | −0.05371 (7) | 0.8102 (3) | 0.12421 (6) | 0.0169 (3) | |
| H2 | −0.0823 | 0.7255 | 0.1414 | 0.020* | |
| C3 | −0.07744 (7) | 0.9834 (3) | 0.09145 (6) | 0.0182 (3) | |
| H3 | −0.1217 | 1.0176 | 0.0863 | 0.022* | |
| C4 | −0.03566 (7) | 1.1058 (3) | 0.06635 (6) | 0.0173 (3) | |
| C5 | 0.02901 (7) | 1.0572 (3) | 0.07324 (6) | 0.0179 (3) | |
| H5 | 0.0571 | 1.1412 | 0.0555 | 0.021* | |
| C6 | 0.05201 (7) | 0.8843 (3) | 0.10634 (6) | 0.0169 (3) | |
| H6 | 0.0964 | 0.8512 | 0.1114 | 0.020* | |
| C1' | 0.03627 (7) | 0.5740 (3) | 0.16854 (5) | 0.0139 (3) | |
| C2' | 0.09909 (7) | 0.5839 (3) | 0.19354 (5) | 0.0147 (3) | |
| H2' | 0.1259 | 0.7090 | 0.1881 | 0.018* | |
| C3' | 0.12148 (7) | 0.4097 (3) | 0.22605 (5) | 0.0150 (3) | |
| C4' | 0.08533 (7) | 0.2238 (3) | 0.23630 (6) | 0.0169 (3) | |
| H4' | 0.1025 | 0.1062 | 0.2588 | 0.020* | |
| C5' | 0.02240 (7) | 0.2170 (3) | 0.21209 (6) | 0.0175 (3) | |
| H5' | −0.0044 | 0.0935 | 0.2186 | 0.021* | |
| C6' | −0.00152 (7) | 0.3877 (3) | 0.17880 (5) | 0.0155 (3) | |
| H6' | −0.0444 | 0.3785 | 0.1625 | 0.019* | |
| C7 | 0.25272 (7) | 0.1927 (3) | 0.35354 (6) | 0.0166 (3) | |
| H7A | 0.2910 | 0.2522 | 0.3406 | 0.020* | |
| H7B | 0.2431 | 0.0387 | 0.3391 | 0.020* | |
| C8 | 0.26470 (7) | 0.1800 (3) | 0.41106 (6) | 0.0153 (3) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.01324 (17) | 0.0176 (2) | 0.01656 (19) | −0.00114 (14) | 0.00035 (13) | 0.00386 (14) |
| O1 | 0.0119 (5) | 0.0242 (6) | 0.0157 (5) | 0.0030 (4) | 0.0023 (4) | 0.0016 (5) |
| O2 | 0.0130 (5) | 0.0241 (6) | 0.0179 (5) | 0.0007 (4) | 0.0025 (4) | 0.0087 (5) |
| O3 | 0.0221 (6) | 0.0204 (6) | 0.0228 (6) | 0.0036 (5) | 0.0000 (4) | −0.0015 (5) |
| O4 | 0.0158 (5) | 0.0291 (7) | 0.0282 (6) | −0.0073 (5) | −0.0011 (4) | 0.0095 (5) |
| Cl1 | 0.0251 (2) | 0.0248 (2) | 0.0220 (2) | 0.00861 (17) | 0.00412 (15) | 0.00689 (16) |
| Cl2 | 0.0263 (2) | 0.0171 (2) | 0.0219 (2) | −0.00104 (15) | 0.00333 (15) | −0.00356 (15) |
| Cl3 | 0.01544 (17) | 0.0210 (2) | 0.0267 (2) | 0.00532 (15) | 0.00114 (14) | 0.00543 (16) |
| Cl4 | 0.01582 (17) | 0.0237 (2) | 0.02059 (19) | −0.00115 (15) | 0.00512 (14) | 0.00656 (15) |
| C1 | 0.0139 (7) | 0.0161 (7) | 0.0124 (7) | −0.0002 (6) | 0.0017 (5) | −0.0024 (6) |
| C2 | 0.0122 (7) | 0.0210 (8) | 0.0178 (7) | −0.0024 (6) | 0.0032 (5) | −0.0008 (6) |
| C3 | 0.0115 (7) | 0.0231 (8) | 0.0196 (8) | 0.0011 (6) | 0.0003 (6) | −0.0021 (6) |
| C4 | 0.0196 (7) | 0.0177 (8) | 0.0137 (7) | 0.0047 (6) | 0.0000 (6) | 0.0007 (6) |
| C5 | 0.0179 (7) | 0.0195 (8) | 0.0175 (8) | −0.0004 (6) | 0.0066 (6) | 0.0017 (6) |
| C6 | 0.0120 (7) | 0.0203 (8) | 0.0190 (7) | 0.0020 (6) | 0.0048 (5) | −0.0003 (6) |
| C1' | 0.0127 (6) | 0.0173 (8) | 0.0123 (7) | 0.0005 (6) | 0.0034 (5) | −0.0017 (6) |
| C2' | 0.0132 (7) | 0.0170 (8) | 0.0149 (7) | −0.0011 (6) | 0.0050 (5) | −0.0005 (6) |
| C3' | 0.0109 (6) | 0.0199 (8) | 0.0144 (7) | 0.0023 (6) | 0.0027 (5) | −0.0021 (6) |
| C4' | 0.0213 (7) | 0.0158 (8) | 0.0143 (7) | 0.0018 (6) | 0.0051 (6) | 0.0006 (6) |
| C5' | 0.0215 (8) | 0.0166 (8) | 0.0155 (7) | −0.0053 (6) | 0.0067 (6) | −0.0028 (6) |
| C6' | 0.0145 (7) | 0.0186 (8) | 0.0138 (7) | −0.0019 (6) | 0.0034 (5) | −0.0033 (6) |
| C7 | 0.0168 (7) | 0.0180 (8) | 0.0152 (7) | 0.0029 (6) | 0.0030 (5) | 0.0024 (6) |
| C8 | 0.0136 (7) | 0.0145 (7) | 0.0180 (7) | 0.0016 (6) | 0.0031 (5) | 0.0009 (6) |
Geometric parameters (Å, °)
| S1—O3 | 1.4153 (12) | C4—C5 | 1.386 (2) |
| S1—O4 | 1.4191 (11) | C5—C6 | 1.385 (2) |
| S1—O1 | 1.5685 (11) | C5—H5 | 0.9500 |
| S1—O2 | 1.5714 (11) | C6—H6 | 0.9500 |
| O1—C3' | 1.4338 (17) | C1'—C2' | 1.401 (2) |
| O2—C7 | 1.4503 (18) | C1'—C6' | 1.403 (2) |
| Cl1—C4 | 1.7412 (16) | C2'—C3' | 1.378 (2) |
| Cl2—C8 | 1.7677 (16) | C2'—H2' | 0.9500 |
| Cl3—C8 | 1.7710 (15) | C3'—C4' | 1.381 (2) |
| Cl4—C8 | 1.7693 (15) | C4'—C5' | 1.396 (2) |
| C1—C2 | 1.398 (2) | C4'—H4' | 0.9500 |
| C1—C6 | 1.401 (2) | C5'—C6' | 1.384 (2) |
| C1—C1' | 1.485 (2) | C5'—H5' | 0.9500 |
| C2—C3 | 1.385 (2) | C6'—H6' | 0.9500 |
| C2—H2 | 0.9500 | C7—C8 | 1.521 (2) |
| C3—C4 | 1.385 (2) | C7—H7A | 0.9900 |
| C3—H3 | 0.9500 | C7—H7B | 0.9900 |
| O3—S1—O4 | 121.62 (8) | C6'—C1'—C1 | 121.85 (13) |
| O3—S1—O1 | 110.62 (6) | C3'—C2'—C1' | 119.08 (14) |
| O4—S1—O1 | 105.34 (7) | C3'—C2'—H2' | 120.5 |
| O3—S1—O2 | 105.39 (7) | C1'—C2'—H2' | 120.5 |
| O4—S1—O2 | 109.79 (6) | C2'—C3'—C4' | 123.88 (14) |
| O1—S1—O2 | 102.53 (6) | C2'—C3'—O1 | 116.77 (13) |
| C3'—O1—S1 | 119.08 (9) | C4'—C3'—O1 | 119.27 (14) |
| C7—O2—S1 | 118.95 (9) | C3'—C4'—C5' | 116.82 (14) |
| C2—C1—C6 | 117.78 (14) | C3'—C4'—H4' | 121.6 |
| C2—C1—C1' | 120.96 (13) | C5'—C4'—H4' | 121.6 |
| C6—C1—C1' | 121.26 (13) | C6'—C5'—C4' | 120.90 (14) |
| C3—C2—C1 | 121.53 (14) | C6'—C5'—H5' | 119.6 |
| C3—C2—H2 | 119.2 | C4'—C5'—H5' | 119.6 |
| C1—C2—H2 | 119.2 | C5'—C6'—C1' | 121.33 (14) |
| C2—C3—C4 | 119.01 (14) | C5'—C6'—H6' | 119.3 |
| C2—C3—H3 | 120.5 | C1'—C6'—H6' | 119.3 |
| C4—C3—H3 | 120.5 | O2—C7—C8 | 107.85 (12) |
| C3—C4—C5 | 121.24 (15) | O2—C7—H7A | 110.1 |
| C3—C4—Cl1 | 119.25 (12) | C8—C7—H7A | 110.1 |
| C5—C4—Cl1 | 119.48 (12) | O2—C7—H7B | 110.1 |
| C6—C5—C4 | 119.01 (14) | C8—C7—H7B | 110.1 |
| C6—C5—H5 | 120.5 | H7A—C7—H7B | 108.5 |
| C4—C5—H5 | 120.5 | C7—C8—Cl2 | 110.51 (11) |
| C5—C6—C1 | 121.43 (14) | C7—C8—Cl4 | 110.88 (11) |
| C5—C6—H6 | 119.3 | Cl2—C8—Cl4 | 110.07 (8) |
| C1—C6—H6 | 119.3 | C7—C8—Cl3 | 106.40 (10) |
| C2'—C1'—C6' | 117.97 (14) | Cl2—C8—Cl3 | 109.34 (8) |
| C2'—C1'—C1 | 120.18 (13) | Cl4—C8—Cl3 | 109.56 (8) |
| O3—S1—O1—C3' | −24.84 (13) | C2—C1—C1'—C6' | 27.7 (2) |
| O4—S1—O1—C3' | −158.01 (11) | C6—C1—C1'—C6' | −152.88 (15) |
| O2—S1—O1—C3' | 87.12 (11) | C6'—C1'—C2'—C3' | 1.4 (2) |
| O3—S1—O2—C7 | −158.44 (11) | C1—C1'—C2'—C3' | −178.90 (13) |
| O4—S1—O2—C7 | −25.84 (13) | C1'—C2'—C3'—C4' | −0.8 (2) |
| O1—S1—O2—C7 | 85.75 (11) | C1'—C2'—C3'—O1 | 176.06 (13) |
| C6—C1—C2—C3 | −0.5 (2) | S1—O1—C3'—C2' | 91.88 (14) |
| C1'—C1—C2—C3 | 178.90 (14) | S1—O1—C3'—C4' | −91.13 (15) |
| C1—C2—C3—C4 | 0.2 (2) | C2'—C3'—C4'—C5' | −0.5 (2) |
| C2—C3—C4—C5 | 0.4 (2) | O1—C3'—C4'—C5' | −177.25 (13) |
| C2—C3—C4—Cl1 | −177.88 (12) | C3'—C4'—C5'—C6' | 1.2 (2) |
| C3—C4—C5—C6 | −0.7 (2) | C4'—C5'—C6'—C1' | −0.6 (2) |
| Cl1—C4—C5—C6 | 177.54 (12) | C2'—C1'—C6'—C5' | −0.7 (2) |
| C4—C5—C6—C1 | 0.4 (2) | C1—C1'—C6'—C5' | 179.55 (14) |
| C2—C1—C6—C5 | 0.2 (2) | S1—O2—C7—C8 | 129.66 (11) |
| C1'—C1—C6—C5 | −179.25 (14) | O2—C7—C8—Cl2 | −58.24 (14) |
| C2—C1—C1'—C2' | −152.01 (15) | O2—C7—C8—Cl4 | 64.09 (14) |
| C6—C1—C1'—C2' | 27.4 (2) | O2—C7—C8—Cl3 | −176.84 (10) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: OM2350).
References
- Bergman, Å., Klasson-Wehler, E. & Kuroki, H. (1994). Environ. Health Perspect.102, 464–469. [DOI] [PMC free article] [PubMed]
- Brandao, T. A. S., Priebe, J. P., Damasceno, A. S., Bortoluzzi, A. J., Kirby, A. J. & Nome, F. (2005). J. Mol. Struct.734, 205–209.
- Buckman, A. H., Wong, C. S., Chow, E. A., Brown, S. B., Solomon, K. R. & Fisk, A. T. (2006). Aquat. Toxicol.78, 176–185. [DOI] [PubMed]
- Dirtu, A. C., Jaspers, V. L. B., Cernat, R., Neels, H. & Covaci, A. (2010). Environ. Sci. Technol.44, 2876–2883. [DOI] [PubMed]
- Lehmler, H.-J., Parkin, S. & Robertson, L. W. (2002). Chemosphere, 46, 485–488. [DOI] [PubMed]
- Li, X., Parkin, S., Duffel, M. W., Robertson, L. W. & Lehmler, H.-J. (2010a). Acta Cryst. E66, o1615–o1616. [DOI] [PMC free article] [PubMed]
- Li, X., Parkin, S., Duffel, M. W., Robertson, L. W. & Lehmler, H.-J. (2010b). Acta Cryst. E66, o1073. [DOI] [PMC free article] [PubMed]
- Li, X., Parkin, S., Duffel, M. W., Robertson, L. W. & Lehmler, H.-J. (2010c). Environ. Int. doi:10.1016/j.envint.2009.1002.1005. [DOI] [PMC free article] [PubMed]
- Li, X., Parkin, S., Robertson, L. W. & Lehmler, H.-J. (2008). Acta Cryst. E64, o2464. [DOI] [PMC free article] [PubMed]
- Liu, Y., Apak, T. I., Lehmler, H.-J., Robertson, L. W. & Duffel, M. W. (2006). Chem. Res. Toxicol.19, 1420–1425. [DOI] [PubMed]
- Liu, Y., Smart, J. T., Song, Y., Lehmler, H.-J., Robertson, L. W. & Duffel, M. W. (2009). Drug Metab. Dispos.37, 1065–1072. [DOI] [PMC free article] [PubMed]
- Nomiyama, K., Murata, S., Kunisue, T., Yamada, T. K., Mizukawa, H., Takahashi, S. & Tanabe, S. (2010). Environ. Sci. Technol.44, 3732–3738. [DOI] [PubMed]
- Nonius (1998). COLLECT Nonius BV, Delft, The Netherlands.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr. & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Shaikh, N. S., Parkin, S., Luthe, G. & Lehmler, H. J. (2008). Chemosphere, 70, 1694–1698. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Vyas, S. M., Parkin, S. & Lehmler, H.-J. (2006). Acta Cryst. E62, o2905–o2906.
- Wang, L.-Q., Lehmler, H.-J., Robertson, L. W. & James, M. O. (2006). Chem. Biol. Interact.159, 235–246. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536810031338/om2350sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810031338/om2350Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report