SUMMARY
The incidence of Bell’s palsy has been estimated in a health district of a major Italian city, taking also into consideration the potential risk factors that might influence the occurrence of Bell’s palsy. A matched case-control was therefore designed, by collecting data from the Emergency Departments of four Hospitals belonging to the same Health District in Rome (Italy), coordinated by a tertiary referral centre University Hospital. All patients affected by Bell’s palsy within the health district and four controls for each case were included. Controls were selected from other ENT patients, and were matched for hospital admission, week of disease onset, and climate conditions. Information regarding possible risk factors was collected using standardized telephone interviews. The resulting dataset was analyzed using multiple conditional logistic regression. The study group comprised 381 patients with acute, unilateral, peripheral facial palsy, clinically diagnosed as Bell’s palsy observed between 1st January 2006 and 31st December 2008. The cumulative incidence of Bell’s palsy was found to be 53.3/100.000/year. Among the risk factors, age was found to influence onset of Bell’s palsy, with an odds ratio of 2% for each one-year increase in age, with a linear trend (95% CI = 1-3%; p = 0.005). Bell’s palsy was found to occur with an annual incidence close to previous reports. Among the possible known risk factors (diabetes, pregnancy, etc.), only aging was found to play a significant role.
KEY WORDS: Bell's palsy, Epidemiology, Risk factors
RIASSUNTO
L’incidenza di paralisi di Bell è stata calcolata in un Distretto Sanitario di una grande città italiana, analizzando anche i potenziali fattori di rischio che potrebbero influenzarla. è stato disegnato uno studio caso-controllo, raccogliendo i dati dai Dipartimenti di Emergenza di quattro Ospedali romani, coordinato dall’Ospedale Universitario di riferimento. Sono stati esaminati tutti i pazienti affetti da paralisi di Bell nell’ambito del medesimo Distretto Sanitario e quattro controlli per ciascun caso. I controlli sono stati selezionati da pazienti giunti nello stesso giorno presso lo stesso Ospedale del paziente/caso, affetti da altra patologia di ambito otorinolaringoiatrico. Sono state anche prese in considerazione sia la settimana dell’anno in esame che le relative condizioni meteorologiche. Le informazioni sui possibili fattori di rischio sono state ottenute mediante intervista telefonica. I risultati sono stati analizzati mediante regressione logistica multipla. Il gruppo di studio è risultato composto da 381 pazienti con paralisi facciale periferica acuta ed unilaterale, diagnosticati come paralisi di Bell tra il 1° gennaio 2006 ed il 31 dicembre 2008. L’incidenza è risultata pari a 53,3 casi per 100.000 abitanti per anno, dunque non discostante da precedenti rilievi della letteratura. Tra i fattori di rischio, l’invecchiamento è risultato l’unica variabile in grado di influenzare l’insorgenza di paralisi di Bell, con in media la possibilità di un incremento del 2% per anno, con tendenza lineare (95% CI = 1-3%; p = 0,005).
Introduction
Bell’s palsy (BP) accounts for almost 75% of all cases of acute facial palsy (FP), the incidence varying in the different countries around the world. Different rates of incidence have been reported, in the medical literature, depending on the geographical regions under study.
The annual incidence has been reported to range between 11 and 40 or 8 and 240 cases per 100,000 subjects 1 2.
The aetiology of BP is still unclear: viral infections, vascular disease, hypertension and diabetes have been indicated as possible causal agents 3-6. Adour, in 1977, suggested that reactivation of Herpes simplex type 1 virus may play a major role in the pathogenesis of BP 7 and, more recently, increasing evidence of reactivation of latent HSV-1 from cranial nerve ganglia has been reported 8 9.
BP would appear to be more frequent in the Japanese population and in the Mediterranean Countries. It is still unclear whether these differences are due to racial susceptibility, or rather to environmental, geographic or climatic factors 10. Experimental findings would support a significant role played by low temperatures, which might be related to a higher incidence of BP in the colder period of the year 1. The age peak incidence occurs between 15 and 45 years 11. Females and males are equally affected, although the incidence in the former is higher during pregnancy 12.
Although a satisfactory recovery for patients with BP is thought to depend on the combined treatment with steroids and anti-viral drugs during the first week after onset, the role of this therapeutic association has recently been rediscussed 13 14. Some Authors are also suggesting that about 20-31% of patients with BP not receiving an appropriate treatment, run a higher risk of residual facial muscle weakness with complications, such as synkinesis or hypercontractures that would cause secondary physiological and psychological sequelae 15-17. The most important prognostic factors for an unsatisfactory prognosis are: complete palsy, absence of recovery in the first three weeks and age over 60 years.
Despite the many studies carried out on the incidence and risk factors of BP, studies investigating all the aspects of this pathological condition are indeed very few. More studies are, instead, essential in order to be able to associate diagnosis and treatment with suitable prevention, especially for patients at higher risk of recurrences and complications.
The aim of the present study was to estimate the incidence of BP in a large health district of a South-European Capital city, and to study potential risk factors that may influence BP occurrence, applying a case-control design.
Methods
The area under study refers to the health district 'ASL RM/E' of Rome, Italy (Fig. 1). This area has a population of about 500,000 inhabitants. Acute health conditions, in this area, are managed by four Emergency Departments (ED) belonging to different Hospitals where, according to the National Health System, all patients are systematically referred by general practitioners. All patients admitted to those four EDs, between 1st January 2006 and 31st December 2008, with a diagnosis of BP, were included in the study. Diagnosis was always made by the Otolaryngologist, as consultant for the ED physician. Diagnosis was based on: a negative otoscopic examination and lack of other possible coexisting signs/ symptoms, as well as on results of imaging techniques (computed tomography [CT], magnetic resonance imaging [MRI]) when considered useful. Audiological tests, including stapedial reflex elicitation, were only occasionally carried out and were not taken into account in this study.
Fig. 1. Rome (Italy) and its Municipals. The four Emergency Departments included in the study belong to the XVIII, XIX and XX Municipals (circled).
An estimate of the total general population referring to each hospital, in that period of time, was carried out, obtaining population data from the authorities of each municipal area forming the health district. The cumulative incidence has been calculated by dividing the number of BP patients in each ED (numerator) by the total general population referring to each ED (denominator).
All patients included in the estimation of BP incidence could potentially constitute the cases of the case-control study. However, of the 381 cases detected and used to calculate the cumulative incidence of the disease, 177 (46.5%) were lost to follow-up. The case-control study was carried out only on the cohort collected at the ED of the Authors’ Hospital, i.e., on 82 patients. For each of these four controls were selected as follows: Each time a new case was selected, four patients that arrived at the outpatient ENT ward, during the same week as the case and that met the inclusion criteria outlined below have been recruited. Therefore, controls were matched for hospital admission, week of disease onset, and climate conditions. In order to meet the inclusion criteria, control patients should have had an ENT-related pathological condition or symptom. All cases and all controls underwent a standardized telephone interview (Table I) performed by a trained physician, including questions concerning:
Table I. Questionnaire for the subjects included in the incidence and the case/control study.
| Incidence Study Group |
| Did you perform a CT scan? |
| Did you perform an MRI scan? |
| Which medical therapy has been prescribed? |
| Did you perform any physical rehabilitation? |
| For how long have you suffered from facial palsy (FP)? |
| Did the FP fully recover? |
| Did you suffer from any FP sequela? |
| How many episodes of FP did you have? |
| Case-Control Study |
| Did anybody in your family ever suffer from FP? |
| Do you suffer from diabetes? |
| Are you affected by hypertension? |
| Have you ever had measles or Herpes zoster? |
| Have you ever had Herpes symplex? |
| Did you have FP during pregnancy? |
| Did you have FP after anti-flu vaccination? |
diagnosis, treatment, duration of illness, time of recovery and outcome;
constitutional factors (age, sex, pregnancy) and coexisting pathological conditions (diabetes, hypertension, past herpetic infections).
The resulting dataset was analyzed using multiple conditional logistic regression.
The present study was approved by the Ethics Committee of the University Hospital which acted as coordinator. All the patients interviewed were correctly informed about the aim of the study.
Results
The cumulative incidence of BP for each of the four EDs ranged from 48.1 to 57.3 per 100,000 per year. The cumulative incidence for the global area was 53.3 per 100,000 per year (Table II).
Table II. Incidence of BP distribution in the four Hospitals belonging to the Health District 'ASL RM/E" in Rome, Italy.
| Hospital | Total number of cases of BP, in 3 years | Estimated population at risk | Cumulative incidence (per 100,000 per year) |
|---|---|---|---|
| S. Andrea | 125 | 218,357 | 57.25 |
| S. Filippo | 97 | 180,557 | 53.72 |
| Gemelli | 94 | 180,557 | 52.06 |
| S. Carlo | 65 | 135,038 | 48.13 |
| Total | 381 | 714,509 | 53.32 |
Age was normally distributed (mean value 50.3 years [SD 19.5]). Males were slightly more affected (53.7%) than females and 2.4% of females were pregnant at the time of BP onset. BP duration had a log-normal distribution and a geometric mean of 45.9 days (SD 142.5; min 1; max 1.825). The comparison between cases and controls is outlined in Table III, while the ENT diseases in the Control group are listed in Table IV.
Table III. Comparison between cases and controls.
| Variable and Category | Cases | Controls | Total |
|---|---|---|---|
| Mean age (SD) | 50.3 (19.5) | 44.1 (22.6) | 45.4 (22.1) |
| Male sex | 44 (53.7%) | 168 (51.2%) | 212 (51.7%) |
| Diabetes mellitus | 11 (13.4%) | 57 (17.4%) | 68 (19.6%) |
| Varicella | 67 (81.7%) | 273 (83.2%) | 340 (82.9%) |
| Herpes simplex | 32 (39.0%) | 130 (39.6%) | 162 (39.5%) |
| Herpes zoster | 8 (9.8%) | 46 (14.0%) | 54 (13.2.%) |
| Hypertension | 32 (39.0%) | 125 (38.1%) | 157 (38.3%) |
| Pregnancy | 2 (2.4%) | 2 (0.6%) | 4 (1.0%) |
| Anti-flu vaccination | 5 (6.1%) | 16 (4.9%) | 21 (5.1%) |
| Total | 82 (100%) | 328 (100%) | 410 (100%) |
Table IV. Types of diagnosis in the control group.
| Diagnosis in the control group | n. (%) |
|---|---|
| Acute dyspnoea | 10 (3.04) |
| Acute pharyngitis | 11 (3.35) |
| Acute mastoiditis | 3 (0.91) |
| Acute external otitis | 48 (14.63) |
| Acute otitis media | 31 (9.45) |
| Acute scialoadenitis | 2 (0.60) |
| Acute sinusitis | 13 (3.96) |
| Acute tonsillitis | 8 (2.43) |
| Anaphylactic shock | 2 (0.60) |
| Ear perichondritis | 3 (0.91) |
| Earwax plug | 12 (3.65) |
| External auditory canal foreign body | 4 (1.21) |
| External ear pimple | 3 (0.91) |
| Facial trauma | 20 (6.09) |
| Foreign body ingestion | 8 (2.43) |
| GERD | 12 (3.65) |
| Haemophtysis | 2 (0.60) |
| Laterocervical lymphoadenopathy | 2 (0.60) |
| Mononucleosis | 2 (0.60) |
| Nasal foreign body | 3 (0.91) |
| Nasal fractures | 27 (8.23) |
| Nose pimple | 2 (0.60) |
| Nosebleeding | 84 (25.60) |
| Peritonsillar abscess | 2 (0.60) |
| Submandibular lymphadenopathy | 1 (0.30) |
| Sudden deafness | 12 (3.65) |
| Vocal chord oedema | 1 (0.30) |
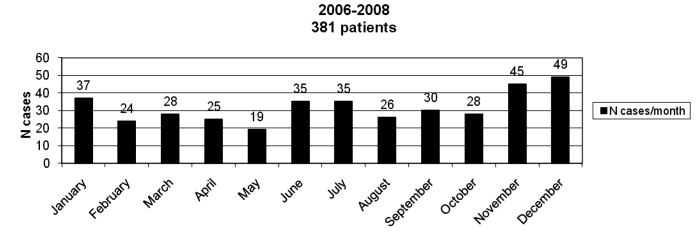
After the case-mix adjustment, all risk factors considered in the case-control study had a similar distribution both in the case and control groups, except for age (Table V); the odds of PB increased on average by 2% for each one-year increase in age with a linear trend (95% CI 1-3%; p = 0.005). Pregnancy was of borderline significance. The effect of climate conditions (Fig. 2) could not be assessed since the study was matched for those variables.
Table V. Multiple conditional logistic regression output, modelling the odds of BP occurrence.
| Factor and Category | Mutually adjusted OR | P | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|
| Male vs. Female | 1.37 | 0.241 | 0.81 | 2.32 |
| 1 year increase in age | 1.02 | 0.005 | 1.01 | 1.03 |
| Diabetes | 0.59 | 0.185 | 0.27 | 1.29 |
| Varicella | 0.74 | 0.397 | 0.37 | 1.48 |
| Herpes simplex | 0.99 | 0.959 | 0.58 | 1.68 |
| Herpes zoster | 0.60 | 0.241 | 0.26 | 1.41 |
| Hypertension | 0.74 | 0.295 | 0.42 | 1.30 |
| Pregnancy | 5.40 | 0.099 | 0.73 | 40.20 |
| Vaccination | 1.26 | 0.711 | 0.37 | 4.27 |
Fig. 2. Monthly distribution of BP in the study group.
Discussion
This study consisted of two parts: estimate of the cumulative incidence of BP and assessment of some potential risk factors for BP onset. There are two main limitations potentially affecting the estimation of incidence. On the one hand, the diagnosis of BP can be made by different medical expertise, in which case, the results cannot be generalized. However, in all our cases, the diagnosis was made by emergency doctors at the Hospital Emergency Department, always supported both by ENT or Neurological specialists and negative imaging results (MRI, CT). Our denominators could also be unreliable. By using population data, it has been assumed that all BP patients living in the study areas were admitted to the Hospitals belonging to the same areas, and that no patients affected by BP and living in other areas were admitted to the hospitals belonging to the study areas. However, although the exact incidence of BP is not yet known, BP can be considered a rare disease. Therefore, it would appear that our denominators may be considered significant. In fact, if one carries out a sensitivity analysis and changes the denominators by a 10% factor, the resulting cumulative incidence would change by about 5 points only. In this regard, the fact that there is no heterogeneity among the four Hospitals, which all show similar incidence rates (Table I), is reassuring.
In contrast to most viral infections, BP can be considered endemic rather than epidemic in nature. This would mainly be related to post-primary infection symbiosis between the virus and human host as well as to its selfinoculated pathogenesis. Although endemic, BP has been shown to be fairly frequent worldwide, however with a different incidence 1. This latter can be in relation to territorial (demographic, climatic, seasonal and probably racial), as well as socio-economic (Health Care System, methodology of study) factors. In fact, methodology may be considered the weakest point of most of the previous epidemiologic studies. Such studies should, in fact, be based on three main principles: correct diagnosis, adequate sample number and case-control statistical analysis.
The present study has been following these principles with a diagnosis achieved, in 50% of the cases, by a multidisciplinary approach, and imaging-supported in the other 50%, as exclusion criterion. Although the sample has been limited to 16% of the entire City population, it has been shown to be adequate for the analysis of the annual incidence since relative values for each Emergency Department overlapped, with 50 cases of BP per year, hence slightly higher than the maximum value reported in the literature 1.
As in previous investigations 18 19, this study also included a case-control statistical analysis, having as its major target the risk index assessment of the main factors involved in BP onset and prognostic recovery between the affected and unaffected population. These factors have been selected among those suggested in the literature, i.e., hypertension, diabetes, primary Herpes Simplex infection, anti-influenza vaccination in the three months prior to BP, age, pregnancy and climatic factors 18-21.
There is one main limitation potentially affecting the evaluation of BP risk factors, in terms of selection bias. In fact, 46.5% of the cases have not been interviewed. However, this was not due to patients’ self-selection but to logistic problems and, therefore, losses to follow-up occurred in a fairly random fashion.
The fact that elderly people are more likely to be affected by BP than young people, with a linear trend, represents an interesting finding. It is tempting to suggest that a more thorough investigation focused on the aetiology of this disease should take into account also different attitudes or metabolic assets linked to aging, which may represent the real risk factors for BP.
The present study has revealed a mean age value of BP patients higher than that reported in the literature, with a lower inferior value (8 vs. 15), higher superior value (87 vs. 60), minimal percentage in children under 8 years old (2.4%) and a high percentage in adults over 60 years (40%). These latter data could be in relation to the longer life expectancy that during the last 30 years has been recorded in the Country of reference, contrary to the rest of the world in which this trend has been noticed only since 2007, long after the major previous epidemiological studies were carried out.
Contrary to previous studies that have highlighted the possibility of BP occurrence in patients over 60 years of age when diabetes and hypertension are also present 2 5 7 18 19, these pathological conditions have not represented a significant risk factor for BP. Furthermore, no role could be attributed to primary herpetic infection for differentiating normal from the affected population. A larger sample size would be helpful in clarifying this point.
The effect of climate on BP onset has been, in the majority of studies, investigated through the effect of minimum and maximum temperatures in the hottest and coldest seasons. In the present study, it has been considered important to assess not only the seasonal variations but also other specific characteristics typical of the different planet areas, such as relative humidity, precipitations and wind speed in the different seasons 20. Since climate factors have already been investigated, their effect (and their potential confounding effect) were nullified by designing a matched study.
The prognostic target of the present study led us to develop, within the questionnaire, a section dedicated to the BP evolution, based on the frequency of sequels (observational analysis) and on their correlation to the main risk factors (statistical analysis). In our study group, the incomplete recovery as flaccid sequel has rarely been reported. This favourable prognosis could be in agreement with the duration of the disease, i.e., on average slightly more than one month, although it could have been biased by its underestimate by the affected patients. In this regard, the major cause of underestimation is represented by synkineses that, instead of being perceived as unpleasant complications, often gave a false perception of facial recovery by temporarily improving both static and dynamic facial symmetry 21. Duration of the disease has been shown to be highly correlated with BP sequels: the longer the duration, the greater the likelihood of incomplete recovery.
Since hypercontractions and synkineses may develop also after the complete recovery of flaccid symptoms, it is logical to assume that, in order to adequately formulate an appropriate prognostic evaluation, a long, up to three-four years, follow-up is required.
The present epidemiological study, carried out by applying an appropriate protocol on incidence and risk factors for BP onset and evolution, has been shown to be in line with data in the literature. Being retrospective, less prognostic value could be drawn for other aspects of BP, since it has taken into account, as risk factors, only the duration, but not the severity of the disease. Further prospective studies are, therefore, needed with a longer observation time and an increased sample size.
Acknowledgements
Authors are grateful to the Emergency and ENT Departments of 'Gemelli', 'San Carlo di Nancy', 'San Filippo' Hospitals in Rome for contributing to the collection of data for the present study.
References
- 1.Diego-Sastre JI, Prim-Espada MP, Fernández-García F. The epidemiology of Bell’s palsy. Rev Neurol. 2005;41:287–290. [PubMed] [Google Scholar]
- 2.Finsterer J. Management of peripheral facial nerve palsy. Eur Arch Otorhinolaryngol. 2008;265:743–752. doi: 10.1007/s00405-008-0646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole J. About face. Cambridge: Mit Press; 2003. [Google Scholar]
- 4.Linder T, Bossart W, Bodmer D. Bell’s palsy and herpes simplex virus: fact or mystery? Otol Neurotol. 2005;26:109–113. doi: 10.1097/00129492-200501000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Morris AM, Deeks SL, Hill MD, et al. Annualized incidence and spectrum of illness from an outbreak investigation of Bell’s palsy. Neuroepidemiol. 2002;21:255–261. doi: 10.1159/000065645. [DOI] [PubMed] [Google Scholar]
- 6.Shmorgun D, Chan WS, Ray JG. Association between Bell’s palsy in pregnancy and pre-eclampsia. Q J Med. 2002;95:359–362. doi: 10.1093/qjmed/95.6.359. [DOI] [PubMed] [Google Scholar]
- 7.Adour KK. Incidence and management of Bell’s palsy. In: Fisch U, editor. Facial nerve surgery. 1st ed. Amstelveen, The Netherlands: Kugler Medical Publications; 1977. pp. 319–328. [Google Scholar]
- 8.Baringer JR. Herpes simplex virus and Bell’s palsy. Ann Intern Med. 1996;124:63–65. doi: 10.7326/0003-4819-124-1_part_1-199601010-00010. [DOI] [PubMed] [Google Scholar]
- 9.Ljostad U, Okstad S, Topstad T, et al. Acute peripheral facial palsy in adults. J Neurol. 2005;252:672–676. doi: 10.1007/s00415-005-0715-1. [DOI] [PubMed] [Google Scholar]
- 10.Gregg G. Some observations on Bell’s palsy in Belfast during the period 1949 to 1958. Arch Phys Med. 1961;42:602–608. [PubMed] [Google Scholar]
- 11.Peitersen E. The spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngol Suppl. 2002;549:4–30. [PubMed] [Google Scholar]
- 12.Gillman GS, Schaitkin BM, May M, et al. Bell’s palsy in pregnancy: a study of recovery outcomes. Otolaryngol Head Neck Surg. 2002;126:26–30. doi: 10.1067/mhn.2002.121321. [DOI] [PubMed] [Google Scholar]
- 13.Lockhart P, Daly F, Pitkethly M, et al. Antiviral treatment for Bell’s palsy (idiopathic facial paralysis) Cochrane Database Syst Rev. 2009;4:CD001869–CD001869. doi: 10.1002/14651858.CD001869.pub4. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan FM, Swan IR, Donnan PT, et al. A randomised controlled trial of the use of aciclovir and/or prednisolone for the early treatment of Bell’s palsy: the BELLS study. Health Technol Assess. 2009;13:1–130. doi: 10.3310/hta13470. [DOI] [PubMed] [Google Scholar]
- 15.Ross B, Nedzelski JM, McLean JA. Efficacy of feedback training in long-standing facial nerve paresis. Laryngoscope. 1991;101:744–750. doi: 10.1288/00005537-199107000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Satoh Y, Kanzaki J, Yoshihara S. A comparison and conversion table of ‘the House-Brackmann facial nerve grading system’ and ‘the Yanagihara grading system’. Auris Nasus Larynx. 2000;27:207–212. doi: 10.1016/s0385-8146(99)00049-8. [DOI] [PubMed] [Google Scholar]
- 17.Shafshak TS. The treatment of facial palsy from the point of view of physical and rehabilitation medicine. Eur Medicophys. 2006;42:41–47. [PubMed] [Google Scholar]
- 18.Pecket P, Schattner A. Concurrent Bell’s palsy and diabetes mellitus: a diabetic mononeuropathy? J Neurol Neurosurg Psychiatry. 1982;45:652–655. doi: 10.1136/jnnp.45.7.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savadi-Oskouei D, Abedi A, Sadeghi-Bazargani H. Independent role of hypertension in Bell’s palsy: a case-control study. Eur Neurol. 2008;60:253–257. doi: 10.1159/000151701. [DOI] [PubMed] [Google Scholar]
- 20.Danielides V, Patrikakos G, Nousia CS, et al. Weather conditions and Bell’s palsy: five-year study and review of the literature. BMC Neurol. 2001;1:7–7. doi: 10.1186/1471-2377-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanaya K, Ushio M, Kondo K, et al. Recovery of facial movement and facial synkinesis in Bell’s palsy patients. Otol Neurotol. 2009;30:640–644. doi: 10.1097/MAO.0b013e3181ab31af. [DOI] [PubMed] [Google Scholar]