SUMMARY
Recently, a new indicator of vestibular otolithic function has been reported: it is a series of negative-positive myogenic potentials recorded by surface electrodes on the skin beneath the eyes in response to bone-conducted vibration (BCV) delivered to the forehead at the hairline in the midline (Fz). The potential is called the ocular vestibular-evoked myogenic potential (oVEMP) and the first component of this (n10) is a small (~ 8 μV), short latency (~ 10 ms), negative potential. In healthy subjects, who are looking up, the n10 responses to Fz bone-conducted vibration are symmetrical beneath the two eyes. In the present investigation, in 17 patients with unilateral surgical vestibular loss, marked asymmetries were observed between the n10 beneath the two eyes: n10 is small or absent beneath the eye on the side opposite the operated ear, confirming previous evidence that n10 is a crossed vestibulo-ocular response unlike p13 of bone-conducted vibration cervical VEMPs (cVEMPs) is a ipsilateral vestibular response and also it is absent in this type of subjects. These results, together with evidence from patients with superior vestibular neuritis allow us to conclude: the asymmetry of the n10 response to Fz bone-conducted vibration is an indicator of utricular macula/superior vestibular nerve dysfunction on the operated side in patients with unilateral vestibular loss.
KEY WORDS: Vestibular function, Otolith, Bone conduction, VEMPs, oVEMPs, Unilateral vestibular loss
RIASSUNTO
Di recente in letteratura è stato presentato e descritto un nuovo indicatore della funzione otolitica. Il nuovo elemento elettrofisiologico è rappresentato da una serie, negativa-positiva, di potenziali vestibolari miogenici registrati mediante elettrodi di superficie posti sulla cute proprio sotto gli occhi del soggetto sottoposto ad esame. Tali potenziali sono evocati e documentabili in risposta ad una vibrazione ossea portata dall’esaminatore al cosiddetto punto Fz, il punto mediano della linea di inserzione del cuoio capelluto con la fronte. Il potenziale è denominato Potenziale Evocato Vestibolare Miogenico Oculare (oVEMP). è costituito da una "piccola" componente (onda n10) in termini di ampiezza (~ 8 μV), a breve latenza (~ 10 ms). La caratteristica fondamentale della onda n10 è che il grafo elemento è negativo. Dunque in soggetti sani che guardano verso l’alto, l’onda n10 in risposta allo stimolo vibratorio portato al punto Fz è simmetrica in termini di ampiezza. Noi abbiamo dimostrato che in 17 pazienti sottoposti in precedenza a chirurgia del basicranio con susseguente perdita totale, o pressoché totale, della funzione vestibolare, si possono evidenziare marcate asimmetrie tra le due onde n10 registrate sotto i due occhi. La n10 infatti è piccola o assente sotto l’occhio controlaterale al lato operato. Tale risultato conferma e valida precedenti evidenze. L’onda n10 infatti è una risposta vestibolo oculare crociata a differenza dell’onda p13 del Potenziale Evocato Vestibolare Miogenico Cervicale (cVEMPs), risposta vestibolare ipsilaterale, anch’essa similmente assente nella coorte di pazienti presa in esame. Tali risultati, insieme con l’evidenza che deriva dai soggetti affetti da nevrite della componente del Nervo Vestibolare Superiore, ci portano a concludere che la ridotta o assente onda n10, in risposta alla stimolazione condotta per via ossea, è un indicatore dell’assente funzione della macula utricolare, ovvero della disfunzione del nervo vestibolare superiore del lato operato in pazienti con perdita totale, o pressoché totale, della funzione vestibolare unilaterale.
Introduction
Air-conducted sounds (ACS) and bone-conducted vibration (BCV) have been proposed as two effective methods to evoke vestibular myogenic potentials originating from selective activation of the otolithic end organs, respectively saccular and utricular macula. Since the vestibular system has projections to many muscle systems, there are many vestibular-evoked myogenic potentials (VEMPs). Historically, the first VEMPs were inhibitory potentials recorded over contracted sternocleidomastoid muscles 1-3 and these cervical VEMPs (cVEMPs) are caused by otolithic saccular receptors and afferents primarily in the inferior vestibular nerve 4. Saccular receptors and irregular afferents are activated at short latency by ACS 5-7. For this reason, cVEMPs have become a widely used clinical test of human saccular, inferior vestibular nerve and vestibulo-collic function 3 8-10 and recent studies in the monkey 11 have further confirmed the above interpretation. More recently, evidence has appeared that BCV selectively activates one group of vestibular afferents, in guinea pig, at very low stimulus intensities 12, otolith irregular afferents, and that has allowed a new version of the VEMP – the ocular VEMP (oVEMP) – to BCV stimulation to be developed 13.
Moderate BCV stimuli applied to the midline of the forehead at the hairline (a location called Fz) cause waves to travel around and through the head, analogous to seismic waves produced by an earthquake or a tsunami. These waves result in linear accelerations which have been measured by two tri-axial linear accelerometers on the mastoids. BCV at Fz causes symmetrical linear acceleration at both mastoids with the largest component being in the inter-aural direction and both mastoids are stimulated almost equally 13 14. These linear accelerations are effective stimuli for otolith receptors and, in guinea pigs, such BCV selectively activates one class of otolith afferents at low intensities – otolith irregular afferents which originate from Type I receptors primarily at the striola of the maculae 12. Suzuki et al. 15 had found, in cats, that selective unilateral utricular nerve stimulation by high frequency electrical stimuli caused excitatory activity in the contralateral inferior oblique (IO) and inferior rectus (IR) muscles and in the ipsilateral superior oblique (SO) and superior rectus (SR) muscles, resulting in conjugate, mainly torsional, eye movements by both eyes. BCV then generates linear accelerations which is the appropriate stimulus for otolithic receptors, including utricular receptors, in human subjects and patients. It follows that such activation of the otolithic receptors would be expected to result in activation of the human contralateral IO and IR muscles. A finding consistent with that expectation is that as the subject or patient looks up during Fz BCV stimulation, so their eye position in the orbit is elevated and the belly of the IO is brought closer to the recording electrodes 13 16 the size of the n10 potential of the oVEMP in turn increases 13.
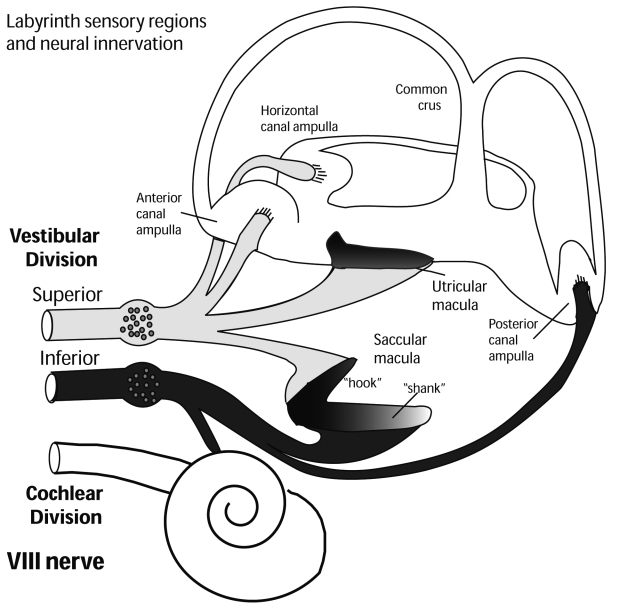
Therefore these small myogenic oVEMP potentials, in human subjects, to Fz BCV are probably caused by otolithic utricular receptors and afferents primarily in the superior vestibular nerve 4 (Fig. 1). Patients without vestibular function due to systemic gentamicin do not have oVEMPs but deaf subjects with residual vestibular function have normal n10s. The n10 is distinct from the R1 component of a blink 16. oVEMPs can be recorded by surface electrodes below the eyes (Fig. 2) in healthy subjects and patients evoked by a hand-held, Bruel and Kjaer (Naerum, Denmark), Mini-shaker 4810, fitted with a short bolt (2 cm long, M4) terminated in a bakelite cap 1.5 cm in diameter using short tone burst. In this way, it is possible to record a small negative wave, n10, with at a latency of around 10 ms to peak.
Fig. 1. Neural innervation of vestibular sense organs of labyrinth (using information reported in de Burlet 4).
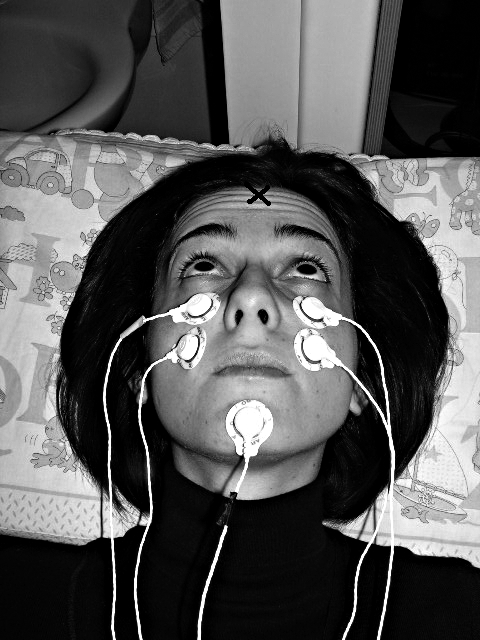
Fig. 2. Electrode configuration for optimum recording of oVEMPs. The person shown is not a patient but one of the co-workers and one of the healthy subjects. NB subject is looking upwards in her median plane. The point marked X indicates location of Fz.
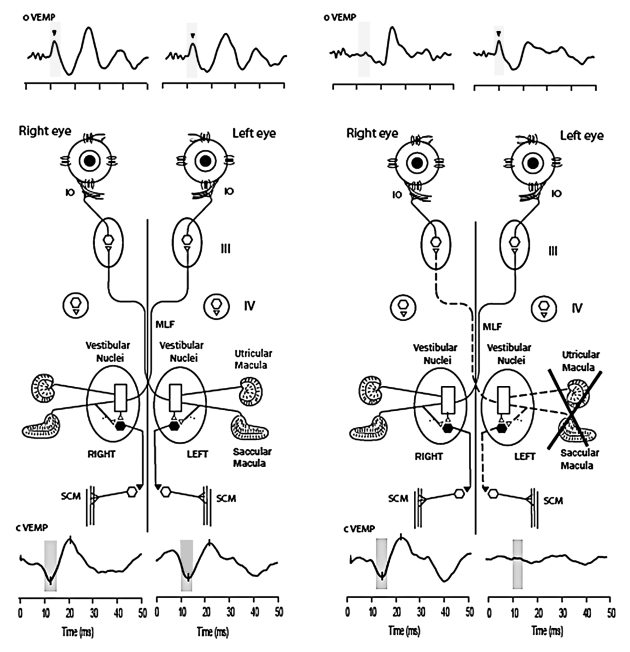
Since the n10 is a negative potential, it indicates excitation directly in the activated extra-ocular muscles, unlike the p13 of the cVEMP, which is a positive (inhibitory) potential indicating the inhibition in the activated sternocleidomastoid (SCM) muscles.
cVEMP and oVEMP are valuable for testing vestibulospinal and vestibulo-ocular pathways and both are complementary for identifying the affected side in patients with unilateral vestibular loss (uVL) and even the status of the saccular and utricular receptors 3 9 17-19. It has been argued that if the oVEMP depends on utricular, as opposed to saccular, function, then patients with complete uVL should show loss of oVEMPs and cVEMPs: they should present with an absent or reduced n10 beneath the eye opposite their affected ear (the contra-lesional eye) and an absent or reduced p13 of the cVEMPs on their ipsilesional SCM muscles, indicating loss of the utricular and saccular function, respectively. This result was reported in a small study on 11 patients 18.
In the present investigation, attempts were made to confirm this previous report by testing an entirely new group of 17 uVL patients tested in an independent clinic. This group of patients represents an adequate sample size to assess the characteristics of the new indicator, n10, of the utricular function and the asymmetry ratio (AR) in cases of known unilateral vestibular loss (uVL).
Materials and methods
A total of 17 patients (6 male; 11 female, aged between 32 and 73 years, mean age 57) all of whom gave informed consent to the study, were tested. Of these, 7 had uVL due to removal of the eighth cranial nerve for treatment of vestibular schwannoma and 10 had uVL due to removal of the vestibular nerve (neurectomy) for treatment of Ménière’s disease. This was the criterion for inclusion in this study. All these patients had good compensation. These subjects were enrolled in this prospective study between October 2008 and March 2009.
The results obtained in these 17 patients were combined with those from 50 healthy subjects without any vestibular disturbances, age range 16-86 years, mean age 38, all tested with informed consent. None of the healthy subjects reported any auditory, vestibular, neurological or visual problems (apart from standard refractive errors). All procedures were performed in accordance with the Helsinki declaration, and were approved by the Institutional Review Board and all subjects and patients gave informed consent to the investigation. The 17 uVL patients enrolled in this study had no evidence of vestibular function, on their operated side, with other tests (Fitzgerald-Hallpike and ice-water calorics, angular acceleration tests, head impulses, ACS and BCV cVEMPs). On the basis of this evidence, it was concluded these patients had probably lost all peripheral vestibular function, on their operated side, following the surgical procedure. All 17 uVL patients had no, or reduced, oVEMP and cVEMP responses following stimulation of their affected ear by BCV, indicating that the utricular and saccular otolithic receptors and their afferents, were not functional either in the superior or inferior vestibular nerve (Fig. 1 and Fig. 3).
Fig. 3. Recordings of cVEMPs (lower traces) and oVEMPs (upper traces) from a healthy subject and a uVL patient. In each recording stimulus onset occurred at time labelled 0. In response to BCV stimuli there are clear oVEMP from IO and IR muscles and cVEMPs from SCM on both sides in the healthy subject. The uVL patient, in contrast, shows a crossed pattern: reduction or absence of the n10 wave recorded under the contra-lateral eye and reduction or absence of p13 of cVEMP potentials recorded from ipsilateral SCM on operated side. Yet, in the healthy subject 500 Hz BCV at Fz by a short tone burst causes a symmetric oVEMP recorded by electrodes under each eye with equal amplitude n10 components (arrowheads). In contrast, the same Fz stimulus causes an asymmetric n10 component of oVEMP response in patient: there is a clear n10 recorded from beneath the ipsilesional eye, whereas the amplitude of n10 from beneath the contra-lesional eye is reduced. NB crossed-dissociation in patient: cVEMP is normal, whereas oVEMP is not in one side in the other side oVEMP is normal whereas cVEMP is not.
Method of stimulation and recording Fz BCV ocular- VEMPs
Fz bone-conducted vibration (BCV) was delivered using a hand-held, Bruel and Kjaer (Naerum, Denmark), Mini-shaker 4810, fitted with a short bolt (2 cm long, M5) which terminated in a bakelite cap 1.5 cm in diameter. The "at end of this cap was the contact point for the stimulator on the subject’s forehead in the midline at the hairline (the point known as Fz). There was excellent electrical isolation between the 4810 and the subject to avoid artifacts from the Mini-shaker contaminating the recordings of the small ocular myogenic potentials, and the use of a bakelite cap cemented on the head of the M5 bolt in the 4810 ensured electrical isolation. The 4810 was driven by computer generated signals, usually consisting of 50 repetitions (at a repetition rate of 3/s) of a 500 Hz tone burst lasting for a total of 7 ms (including a 2 ms rise and a 2 ms fall with a zero crossing start and 5 ms duration) or a square wave of 1 ms duration, amplified using a 300 W amplifier. This tone burst was referred to as a mini tone burst (MTB). Stimulus intensity after amplification was 130 dB SPL measured by an artificial mastoid (Model 4930) Bruel and Kjaer (Naerum, Denmark). This intensity is comparable to that of a light tap on the forehead by a tendon hammer. Unrectified EMG was sampled at 20 kHz and band-pass filtered between 3 and 500 Hz, and averaged with a Medelec Amplaid MK12 (Amplifon, Milan, Italy) averager. To minimize artifacts, 2 m long leads were used. Each lead was shielded individually and the shielding connected to the ground electrode attached to the chin or the sternum of the subject.
The patients and healthy subjects were instructed to maintain visual fixation on a target placed 25 degrees above their visual straight ahead (Fig. 2), which brought the IO and the inferior rectus (IR) of both eyes close to the recording electrode.
Subjects and patients were asked to lie in a supine position on a bed with their head supported by a pillow, but positioned so that the head was horizontal or pitched slightly nose down, with the chin close to the chest (Fig. 2). The skin beneath the eyes was cleaned very carefully with alcohol wipes (with the patient’s eyes closed), and surface EMG electrodes were applied to record the surface potentials from beneath both eyes as shown in Fig. 2. The self-adhesive pads around each electrode were cut to allow the active (+) electrode placement close to the lower eyelid, with the reference electrode (-) placed 2 cm directly below the active electrode (as illustrated), taking care that, no electrical bridge was formed between the conductive gel of the two closely juxtaposed electrodes 13 16 17. The electrodes were positioned to be aligned with the centre of the pupil as the subject looked up at a distant target exactly in the midline (i.e., it is very important that the eye position in the orbit be elevated during n10 measures). The ground electrode was on the chin or sternum.
At this point, a systems check was carried out; the size and polarity of the surface potentials were checked requesting the subject to execute vertical saccades between two dots: one 5° above and the other 5° below the central fixation dot. These 10° saccades generated raw EMG steps of approximately 50 μV and it is necessary that these EMG steps be of approximately equal amplitude (within about 20%) for both eyes, otherwise asymmetrical n10s can be due to electrode or ocular artifacts rather than to vestibular deficits. The oVEMP to Fz BCV stimulation is a series of negative-positive potentials and it is the first negative potential (n10) which has been shown to be due to utricular function 13 16-18 and it is the n10 component which was measured in this study. The amplitude of n10 was measured from baseline to peak.
Method of stimulation and recording Fz BCV cervical VEMPs
Subjects lay supine on a bed. The skin over the SCM muscles was thoroughly cleaned with alcohol wipes and surface EMG electrodes were used to record the responses from both SCMs. The subject or patient was requested to lift his/her head from the pillow while the operator stimulated at Fz with the 500 Hz BCV stimulus. The cVEMP to 500 Hz Fz BCV stimulation is a series of positivenegative potentials, and it is the peak-to-peak difference between the first positive and first negative potential (p13- n23) which was measured here 2.
An asymmetry ratio (AR) was calculated for n10, for uVL patients and healthy subjects, using a version of the standard Jongkees formula for asymmetry calculations in vestibular testing:
Results
oVEMPs and cVEMPs, in response to Fz BCV stimulation by a 4810 Mini-shaker, were found in all 50 successive unselected healthy subjects tested. Averaging the EMG response to the 50 stimulus presentations elicits negative/ positive EMG responses from beneath both eyes with a latency of around 6-8 ms to the foot of the first negativegoing EMG response (n10) and a latency of around 10 ms to peak of n10. In healthy subjects, n10 responses were of approximately equal amplitude and similar in shape under each eye, although the amplitudes varied very considerably from person to person.
The averages from one patient and one healthy subject (Fig. 3) show the main features of the oVEMP and cVEMP responses to 500 Hz Fz BCV. In healthy subjects the 500 Hz brief tone burst of BCV at Fz produced a small (about 5-10 µV) negative potential at a latency of about 10 ms (n10) of approximately equal amplitude beneath both eyes There were considerable individual differences between subjects in the amplitude of that n10 potential as reported in previous studies 13 17.
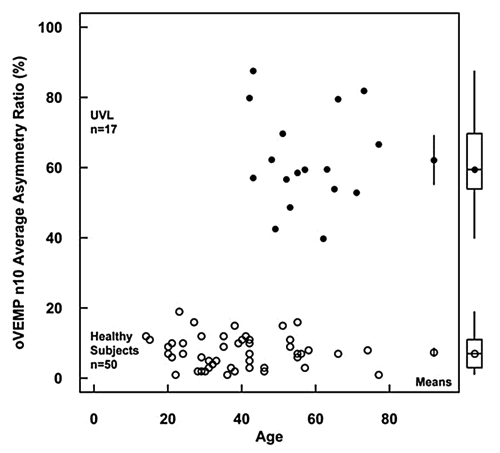
The n10 AR values for healthy subjects and uVL patients are shown in Figure 4. The average AR for BCV oVEMPs for all the uVL patients was 62.16 ± 13.76, n = 17, (95% CIs 69.24-55.08) which was significantly greater (p > 0.001) than the AR value of unselected normal subjects, 7.38% ± 4.54 SD, n = 50 (95% CIs 8.67-6.09). The value of AR for uVL patients here is close to the mean AR of the 11 patients in the previous study 18 (78.79 ± 13.01) and, likewise, the mean AR for healthy subjects is similar to the value for 67 healthy subjects reported elsewhere 17 (11.73 ± 8.26).
Fig. 4. Asymmetry ratios for BCV Fz oVEMPs of all 17 patients with unilateral vestibular loss (filled circles) plotted as a function of age. Also shown are ARs for BCV Fz oVEMPs of 50 healthy subjects (empty circles). Means and two-tailed 95% CI for mean are shown within the square and the boxplots for the medians, quartiles and ranges are shown outside the square. All patients have an asymmetry ratio greater than any normal subject tested.
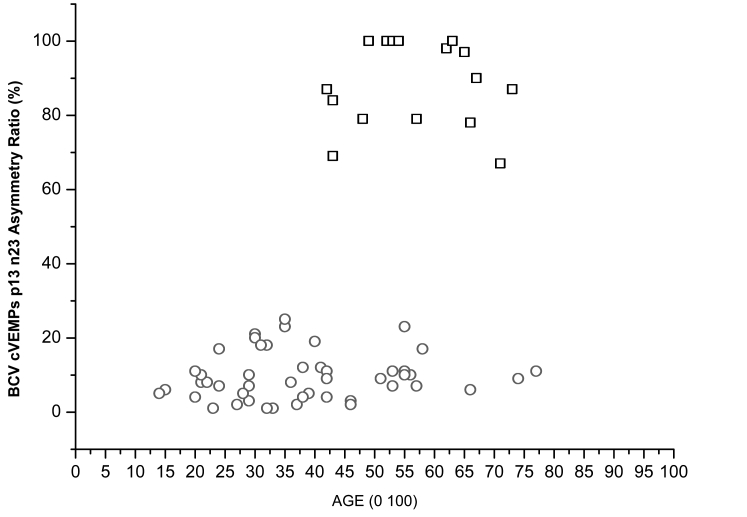
The p13 n23 AR values for healthy subjects and uVL patients are shown in Figure 5.
Fig. 5. Asymmetry ratios for BCV Fz cVEMPs of all 17 patients with unilateral vestibular loss (squares) plotted as function of age. Also shown are ARs for BCV Fz cVEMPs of 50 healthy subjects (circles).
The reduction of the n10 under the contra-lesional eye, in uVL patients, shows that the n10 component of the oVEMP is a crossed vestibulo-ocular response whereas the p13 n-23 components of the cVEMP is an uncrossed vestibulo-collic response (Fig. 3) 13 17.
Discussion
Calorics test and the head impulse test (HIT) 19 were used to define semicircular canal function, but testing separately otolith function (utricular and saccular macula) in a clinical environment has been difficult until now. Recently, however, two simple, safe and important tests of otolith function have been reported – first the cVEMP from contracted SCM muscles to an ACS 1 or BCV 8 and secondly the oVEMP to BCV 13.
The VEMP arises from modulation of background EMG activity and differs from neural potentials in that it requires tonic contraction of the muscle 20. Therefore, when the IO and IR muscles are used as the target muscle, BCV to the midline of the forehead at the hairline causes small short-latency negative (n10) myogenic potentials, recorded from surface electrodes beneath the eyes, with the individual looking up which brings the IO and the IR close to the recording electrodes.
Linear accelerations from a vibration, or a tap, or a tilt or a translation, all cause a change in firing of otolithic afferents because all these stimuli are linear accelerations and all result in otolithic hair cell receptors being deflected and primary otolithic afferents being activated. Acceleration measures, at the mastoids, show that 500 Hz BCV is a series of brief, rapid changes in linear acceleration (a series of jerks), and, therefore, might be expected to activate preferentially the jerk-sensitive otolith afferents, irregular otolith afferents 21. That is the case: irregular otolith afferents, originating from the Type 1 receptors at the striola of the maculae, are sensitive to changes in linear acceleration and are vigorously activated by 500 Hz bone-conducted vibration 12. Other otolith neurons, regular otolith afferents which are activated by low frequencies, are not significantly activated by 500 Hz BCV 12. Semicircular canal neurons do not respond to such 500 Hz linear acceleration stimuli at comparable intensities 12. This type of selective otolith activation will result in otolith-ocular and otolith-spinal responses, as Suzuki et al. have shown in cats 15.
However, one question emerges from these results: if the cohort of patients comprises all uVL, why is the value of their AR% not exactly 100%? In most uVL patients, there was a very small n10 wave present under the contralateral operated side, so the AR is less than 100%. Three possibilities can be evoked: the first is that not all the fibres of the vestibular nerve had been cut by the surgeon. The second, there is the possibility that some otolith fibres travel in the cochlear division (and are, therefore, spared by the surgeon) and may, therefore, produce the n10 ocular potential. Finally, it is not known, at the present time, whether the otolith utricular pathway is anatomically completely crossed.
As we have shown, Fz BCV stimuli are able to cause evoked potentials – oVEMPs and cVEMPs – which complement each other very well. cVEMPs, in response to BCV, are of otolith origin and are useful in the testing of ipsilateral sacculo-collic vestibulo-spinal pathways whereas oVEMPs to bone-conducted vibrations are also of otolith origin and most probably of utricular origin but are useful to test crossed vestibulo-ocular pathways 13 22. In a clinical setting oVEMPs present one great advantage over cVEMPs. Recording ocular vestibular potentials requires only that the subject lies on a bed with two pillows under his/her head and gazes upwards, for short trials lasting only about 20 sec. In contrast, recording cVEMPs requires sustained contraction of the neck muscles which is physically demanding, especially in more senior patients. From these considerations, it is important to emphasize that the oVEMP n10 is a negative potential whereas p13 of the cVEMPs is a positive potential reflecting, not the activation, but the inhibition, of the contracted neck muscle. For this reason, it can be affected both by the extent of the inhibitory drive as well as the extent of the activation of the sternocleido muscles.
Finally, our results and recordings of BCV Fz oVEMPs, in uVL patients, confirm previous results 23 and show that this stimulus is valid in exploring otolithic utricular receptors and afferents in the superior vestibular nerve function.
Conclusions
Healthy subjects have symmetrical n10 potentials to 500 Hz Fz BCV. uVL patients have reduced or absent n10 on the side opposite the operated ear, thus confirming a previous report and supporting the interpretation that n10 to 500 Hz Fz BCV is valid in the testing of utricular function. This is a new technique for assessing utricular function which is easy to perform and is well tolerated by patients. It should be part of the series of otoneurological tests with a solid scientific basis like, for example, the Head Impulse Test and Caloric Test. The results discussed in this report have shown that asymmetries in amplitude of the small (about 5-10 µV) negative potential, at a latency of about 10 ms (n10), recorded beneath the eyes, in response to Fz BCV stimulation, are useful in defining the localization of the utricular macula/superior vestibular nerve damage or dysfunction.
Acknowledgements
Authors are grateful for support of NH&MRC of Australia and the Garnett Passe and Rodney Williams Memorial Foundation.
List of abbreviations
AR: asymmetry ratio for the relative size of the n10 of the oVEMPs for the two eyes; ACS: air-conducted sound; BCV: bone-conducted vibration; EMG: electromyogram; Fz: the location on the forehead in the midline at the hairline; Fz BCV: bone-conducted vibration delivered to Fz; B-71: the standard clinical bone conduction oscillator (Radioear B71); 4810: the Bruel and Kjaer Mini-shaker; cVEMPs: cervical vestibular-evoked myogenic potentials; oVEMPs: ocular vestibular-evoked myogenic potentials; n10: the initial negative potential in the oVEMP response at latency of around 10 ms; TH: tendon hammer (or reflex hammer); UVD: unilateral vestibular deafferentation.
References
- 1.Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry. 1994;57:190–197. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halmagyi GM, Yavor RA, Colebatch JG. Tapping the head activates the vestibular system: a new use for the clinical reflex hammer. Neurology. 1995;45:1927–1929. doi: 10.1212/wnl.45.10.1927. [DOI] [PubMed] [Google Scholar]
- 3.Welgampola MS. Evoked potential testing in neuro-otology. Curr Opin Neurol. 2008;21:29–35. doi: 10.1097/WCO.0b013e3282f39184. [DOI] [PubMed] [Google Scholar]
- 4.Burlet HM. Zur Innervation der Macula sacculi bei Säugetieren. Anat Anzeig. 1924;58:26–32. [Google Scholar]
- 5.McCue MP, Guinan JJ. Acoustically responsive fibers in the vestibular nerve of the cat. J Neurosci. 1994;14:6058–6070. doi: 10.1523/JNEUROSCI.14-10-06058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murofushi T, Curthoys IS. Physiological and anatomical study of click-sensitive primary vestibular afferents in the guinea pig. Acta Otolaryngol. 1997;117:66–72. doi: 10.3109/00016489709117994. [DOI] [PubMed] [Google Scholar]
- 7.Murofushi T, Curthoys IS, Topple AN, et al. Responses of guinea pig primary vestibular neurons to clicks. Exp Brain Res. 1995;103:174–178. doi: 10.1007/BF00241975. [DOI] [PubMed] [Google Scholar]
- 8.Welgampola MS, Rosengren SM, Halmagyi GM, et al. Vestibular activation by bone-conducted sound. J Neurol Neurosurg Psychiatry. 2003;74:771–778. doi: 10.1136/jnnp.74.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welgampola MS, Colebatch JG. Characteristics and clinical application of vestibular-evoked myogenic potentials. Neurology. 2005;64:1682–1688. doi: 10.1212/01.WNL.0000161876.20552.AA. [DOI] [PubMed] [Google Scholar]
- 10.Halmagyi GM, Curthoys IS, Colebatch JG, et al. Vestibular responses to sound. Ann NY Acad Sci. 2005;1039:54–67. doi: 10.1196/annals.1325.006. [DOI] [PubMed] [Google Scholar]
- 11.Tsubota M, Shojaku H, Hori E, et al. Effects of the vestibular nerve section on sound-evoked myogenic potentials in the sternocleidomastoid muscle of monkey. Clin Neurophysiol. 2007;118:1488–1493. doi: 10.1016/j.clinph.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Curthoys IS, Kim J, McPhedran SK, et al. Bone-conducted vibration selectively activates irregular primary otolithic vestibular neurons in the guinea pig. Exp Brain Res. 2006;175:256–267. doi: 10.1007/s00221-006-0544-1. [DOI] [PubMed] [Google Scholar]
- 13.Iwasaki S, McGarvie LA, Halmagyi,, et al. Head taps evoke a crossed vestibulo-ocular reflex. Neurology. 2007;68:1227–1229. doi: 10.1212/01.wnl.0000259064.80564.21. [DOI] [PubMed] [Google Scholar]
- 14.Bekesy G. Experiments in Hearing. New York: McGraw Hill; 1960. [Google Scholar]
- 15.Suzuki JI, Tokumasu K, Goto K. Eye movements from single utricular nerve stimulation in the cat. Acta Otolaryngol. 1969;68:350–362. doi: 10.3109/00016486909121573. [DOI] [PubMed] [Google Scholar]
- 16.Smulders YE, Welgampola M, Burgess AM, et al. The n10 component of the ocular vestibular-evoked myogenic potential (oVEMP) is distinct from the R1 component of the blink reflex. Clin Neurophysiol. 2009;120:1567–1576. doi: 10.1016/j.clinph.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Iwasaki S, Smulders YE, Burgess AM, et al. Ocular vestibular-evoked myogenic potentials to bone-conducted vibration of the midline forehead at Fz in healthy subjects. Clin Neurophysiol. 2008;119:2135–2147. doi: 10.1016/j.clinph.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki S, Smulders YE, Burgess AM, et al. Ocular vestibular-evoked myogenic potentials in response to boneconducted vibration of the midline forehead at Fz. A new indicator of unilateral otolithic loss. Audiol Neurotol. 2008;13:396–404. doi: 10.1159/000148203. [DOI] [PubMed] [Google Scholar]
- 19.Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–739. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- 20.Colebatch JC, Rothwell JC. Motor unit excitability changes mediating vestibulocollic reflexes in sternocleidomastoid muscle. Clin Neurophysiol. 2004;115:2567–2573. doi: 10.1016/j.clinph.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg JM. Afferent diversity and the organization of central vestibular pathways. Exp Brain Res. 2000;130:277–297. doi: 10.1007/s002210050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosengren SM, Todd NPM, Colebatch JG. Vestibular-evoked extraocular potentials produced by stimulation with boneconducted sound. Clin Neurophysiol. 2005;116:1938–1948. doi: 10.1016/j.clinph.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki S, Chihara Y, Smulders Y, et al. The role of the utricular macula and the superior vestibular nerve in the generation of ocular vestibular-evoked myogenic potentials to bone-conducted vibration at Fz. Clin Neurophysiol. 2009;120:588–593. doi: 10.1016/j.clinph.2008.12.036. [DOI] [PubMed] [Google Scholar]