Abstract
Background
Few randomized trials comparing antiretroviral therapy (ART) regimens have been conducted in resource-limited settings.
Methods
In the Republic of South Africa, antiretroviral-naive HIV-infected individuals >14 years old, with <200 CD4+ cells/mm3 or prior AIDS diagnosis were randomized to: EFV or LPV/r with either ZDV+ddI or d4T+3TC in an open-label 2×2 factorial study and followed for: the primary outcome of AIDS or death; pre-specified secondary outcomes including CD4+ and viral load changes, treatment discontinuations and grade 4 events.
Results
1771 persons were randomized and followed for a median of 24.7 months. AIDS or death occurred among 163 participants assigned EFV and 157 LPV/r (HR=1.04; 95% CI 0.84–1.30) and among 170 assigned ZDV+ddI and 150 assigned d4T+3TC (HR=1.15; 95% CI: 0.93–1.44). HIV RNA was lower (p<0.001) and CD4+ count greater (p<.01) over follow-up for d4T+3TC versus ZDV+ddI. Rates of potentially life-threatening adverse events and overall treatment discontinuation were similar for d4T+3TC and ZDV+ddI; however, more participants discontinued d4T due to toxicity (12.6%) than other treatments (<5%).
Conclusion
EFV or LPV/r are effective components of first-line ART. The poorer viral and immune responses with ZDV+ddI and the greater toxicity-associated discontinuation rate with d4T+3TC suggest these treatments be used cautiously as initial therapy.
Trial Registration
clinicaltrials.gov Identifier: NCT00342355
Keywords: antiretroviral therapy, efavirenz, lopinavir/ritonavir, randomized controlled trial, treatment naïve, stavudine, didanosine, zidovudine, lamivudine
Introduction
HIV is the leading cause of death in sub-Saharan Africa with 1.7 million adult deaths in 2006 [1]. While developed nations have access to more than twenty-five antiretroviral agents for the treatment of HIV infection [2] access to potent ART is markedly constrained in large parts of Africa. In the Republic of South Africa (RSA) in 2004 when the national rollout of ART began [3], only seven drugs were available [4]. In 2006, only 21% of persons with AIDS in RSA were treated with ART [5].
Few randomized trials have been conducted in resource-limited settings to compare different ART regimens. The initial treatment for HIV is important in these settings since individuals present for treatment with advanced disease and the available regimens are limited.
The Phidisa II trial was designed to compare the effect of different initial ART treatments on AIDS or death among individuals with advanced HIV infection.
Methods
Study population
Eligible participants were uniformed RSA military personnel and family who were eligible to receive services from the military health system[6]. HIV infected adult patients (>14 years of age) were enrolled at one of six study sites; were antiretroviral naïve (defined as ART for <7 days) and had CD4+ T lymphocyte cell counts <200 cells/mm3 (or ≤14% for patients post-splenectomy) and/or had any history of or current AIDS-defining illness [7]. Patients with pulmonary tuberculosis had CD4+ counts <200 cells/mm3 to be eligible. Laboratory criteria for eligibility included; haemoglobin ≥9 g/dL (≥8 g/dL for women), neutrophil count >500 cells/μL, platelet count >25000/μL and serum liver transaminases <5 times upper limit of normal range. The study was approved by SANDF and NIAID institutional review boards and written informed consent was obtained from all participants.
Study design and outcome measures
PHIDISA II was a randomized, open-label comparison of four different ART regimens in a 2×2 factorial design. Eligible participants were randomly allocated in equal proportions to receive: 1) efavirenz (EFV, Stocrin®, Merck) 600 mg daily + zidovudine (ZDV, Retrovir®, GlaxoSmithKline) 300 mg twice daily + didanosine (ddI, Videx® or Videx EC®, Bristol-Myers Squibb) 125 mg twice daily for persons <60 kg, 200 mg twice daily for persons >60 kg or 250 mg and 400 mg daily for the enteric-coated formulation (available beginning December 2005); 2) EFV + stavudine (d4T, Zerit®, Bristol-Myers Squibb) 30 mg twice daily for persons <60 kg, 40 mg twice daily for persons >60 kg + lamivudine (3TC®, GlaxoSmithKline) 150 mg twice daily; 3) lopinavir/ritonavir 400 mg/100 mg twice daily (LPV/r, Kaletra, Abbott) + ZDV+ddI; or 4) LPV/r+d4T+3TC (Figure 1).
Figure 1.
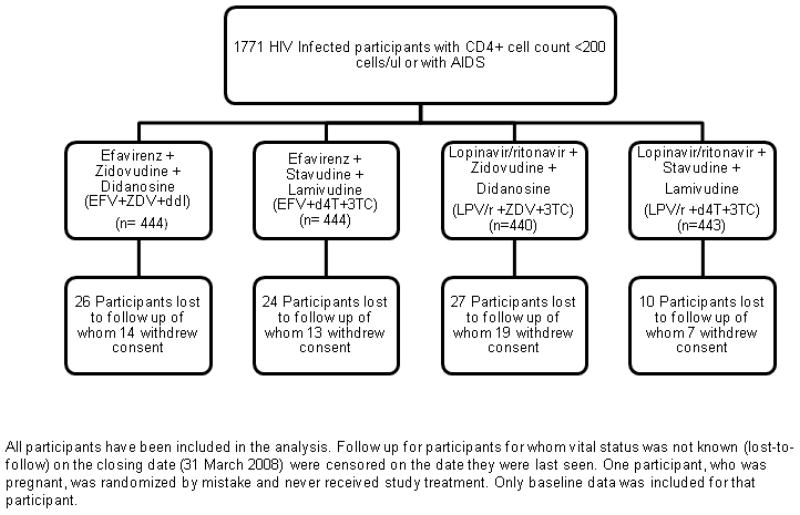
Randomization and follow-up of subjects on study
Phidisa II study design.
Women who were taking EFV and became pregnant on study were switched to nevirapine (Viramune®, Boehringer-Ingleheim) 200 mg daily for 14 days, then 200 mg twice daily during pregnancy. Persons with active tuberculosis were ineligible for study until induction therapy was completed. Persons with treatment-limiting side effects to: EFV were switched to nevirapine, LPV/r were switched to EFV, ddI or d4T were switched to ZDV+3TC, ZDV were switched to d4T+3TC. Those with an AIDS-related illness after 24 weeks of therapy and persons with virologic failure (failure to achieve a plasma HIV viral load <400 RNA copies/ml by week 36 or a rebound in viral load to >20,000 copies/ml, in the absence of any intercurrent illness, after having been suppressed to <400 copies/mL) were switched to the 3 drugs they were not receiving. Resistance testing was not routinely used. For a second virologic failure or drug toxicities, other ART was used as available. All patients received prophylaxis for Pneumocystis jiroveci pneumonia and toxoplasmosis until CD4+ count increased, per current guidelines [8].
The protocol specified primary outcome for the study was AIDS or death; pre-specified secondary outcomes included CD4+ and viral load changes, treatment discontinuations and grade 4 events.
An Endpoint Review Committee (ERC) reviewed site reported AIDS defining illnesses, blinded to treatment assignment. Events classified as confirmed or probable by the ERC, using pre-established criteria, were considered endpoints, as were all deaths irrespective of cause.
The planned sample size of 2,800 participants provided 80% power to detect a 20% difference in the hazard for progression to AIDS or death for two primary comparisons: 1) EFV versus LPV/r; and 2) d4T+3TC versus ZDV+ddI. Follow-up was to continue until 635 primary events had accrued (estimated at 5 years) assuming 30% of people with CD4+ counts <200 cells/μL or symptomatic HIV disease would develop disease progression or die (the primary event) and the primary event rate in year one was twice as great as in subsequent years. We assumed that 2.5% of participants would be lost to follow-up per year.
Randomization was stratified by site using randomly mixed permuted blocks of different sizes. Assignments were obtained by calling a central toll-free number.
Data collection and follow-up of participants
Medical histories, complete physical examination, and laboratory studies, including plasma HIV RNA, CD4+ count, haematology and clinical chemistry, were obtained prior to randomization. Follow-up visits for data collection occurred monthly for 3 months and every 3 months thereafter. Interim histories were taken, selected symptoms assessed and graded on a 4-point scale (mild to potentially life-threatening)[9], adherence with study medications assessed and reinforced by pharmacists, and CD4+ counts, HIV-RNA levels, and haematology and clinical chemistry tests performed. Physicians completed a questionnaire annually assessing participants’ clinical features of lipodystrophy. All changes to ART were recorded. All grade 4 events that occurred throughout follow-up were reported and coded according to the Medical Dictionary for Regulatory Activities (version 12.0). A final visit was conducted to confirm endpoint and vital status as of the study closing date, 31 March 2008.
Statistical analysis
The randomized treatment groups were compared by intention-to-treat; subjects were not censored for drug switches or discontinuation. Kaplan-Meier survival curves and Cox’s proportional hazards models were used to compare the main effects (EFV versus LPV/r and d4T+3TC versus AZT+ddI) for progression to AIDS or death, death, grade 4 events, and HIV RNA < 400 copies/mL [10]. Follow-up was censored when participants were lost to follow-up; otherwise, on 31 March 2008.
The hazard ratio (HR) for the main effects was estimated from a Cox model stratified by site (6 strata) and with two binary indicators, one for each main effect. An interaction term was added to assess whether the two factors studied were additive. The proportional hazards assumption was tested by including an interaction term between the randomized treatment indicators and log-transformed follow-up time. Treatment comparisons were summarized with HRs and 95% confidence intervals (CIs) from the Cox models. Longitudinal regression (mixed effects) models were used to compare CD4+ counts and other laboratory measures over follow-up between groups. The percentage of participants with lipodystrophy and with targeted symptoms at 12-months were compared with chi-square tests.
Statistical analyses were performed using SAS (Version 8.2). All reported p-values are 2-sided.
Interim monitoring of safety and efficacy
An independent Data and Safety Monitoring Board (DSMB) reviewed interim analyses from Phidisa II 5 times. In June 2007, the DSMB requested a plan to increase enrolment rate; only 1,600 of 2,800 patients had been enrolled after 28 months for a planned 2 year enrollment period. Coincident with slow enrollment were reports outside of the trial detailing toxicities related to d4T. The investigators decided after considering the DSMB request, without knowledge of the treatment comparisons, to end the study early to inform use of d4T in RSA. Enrolment ceased December 31st 2007; follow-up ended March 31, 2008.
Role of the funding source
The study was supported by SANDF, NIAID, and the US Department of Defense. None of these entities nor their employees or contractors had any financial interest in the drugs used. Employees and contractors of these agencies participated in study design, data collection, data analysis, data interpretation, and writing the report. The corresponding author had access to all data and final responsibility for the decision to submit for publication.
Results
Accrual and patient characteristics
Between February 2004 and December 2007, 1771 persons were randomized: 444 participants to EFV+ZDV+ddI; 444 participants to EFV+d4T+3TC; 440 participants to LPV/r+ZDV+ddI; and 443 participants to LPV/r+d4T+3TC (Figure 1).
One participant was mistakenly randomized and did not receive study medication. All other participants received their assigned treatment following randomization. The treatment groups were well balanced at entry (Table 1). Median CD4+ count at screening was 106 cells/μL. Thirty-seven percent of participants had no history of tuberculosis, AIDS, or AIDS-related symptoms; 31% had a history of AIDS-related symptoms but no history of tuberculosis or other AIDS-defining illness; and 32% had a history of tuberculosis or AIDS.
Table 1.
Baseline Characteristics of the Participants by Treatment Group
| EFV+ZDV+ddI |
EFV+d4T+3TC |
LPV/r+ZDV+ddI |
LPV/r+d4T+3TC |
|
|---|---|---|---|---|
| No. | 444 | 444 | 440 | 443 |
| Average age (years) (SD) | 35.3 (5.4) | 35.5 (5.5) | 35.3 (5.5) | 35.6 (5.5) |
| Female sex (%) | 32.9 | 30.6 | 32.5 | 32.1 |
| Median CD4+ cell count (cells/mm3) (25th, 75th percentiles) | 107 (48, 156) | 102 (38, 155) | 104 (43, 151) | 112 (51, 154) |
| Median plasma HIV-RNA level (copies/mL) (25th, 75th percentiles) | 131,000 (50200, 228000) | 136,100 (54200, 302000) | 157,000 (49200, 325000) | 152,000 (57400, 307000) |
| Mean Haemoglobin (g/dl) (SD) | 12.7 (2.0) | 12.6 (2.0) | 12.6 (1.9) | 12.5 (2.1) |
| Mean Body Mass Index (BMI) (kg/m2) (SD) | 23.9 (4.9) | 23.6 (4.8) | 23.6 (4.7) | 23.7 (4.6) |
Follow-up and Treatment Changes
Median follow-up was 24.7 months. For 87 (4.9%) participants the primary endpoint status was unknown at study closing date (range among treatments: 2.3% to 6.1%) (Figure 1).
Overall, 37% of participants discontinued or switched one or more drugs in their regimen. This percent did not differ by treatment group (p=0.51). The percent of follow-up time on the assigned regimen was 79.8% for EFV+ZDV+ddI, 85.4% for EFV+d4T+3TC, 80.5% for LPV/r+ZDV+ddI, and 81.8% for LPV/r+d4T+3TC. 27.3% of participants assigned to EFV+ZDV+ddI discontinued/changed all three drugs in the regimen, this percentage was 21.4% for those assigned to EFV+d4T+3TC, 23.2% to LPV/r+ZDV+ddI, and 16.5% to LPV/r+d4T+3TC (p=0.001). The percent of follow-up time off of all antiretroviral therapy ranged from 2.2% (LPV/r+d4T+3TC) to 3.6% (EFV+ZDV+ddI). Self-reported adherence (taking all or most of prescribed pills) ranged from 95.3% for LPV/r to 97.1% for EFV.
HIV RNA and CD4+ Cell Count Responses
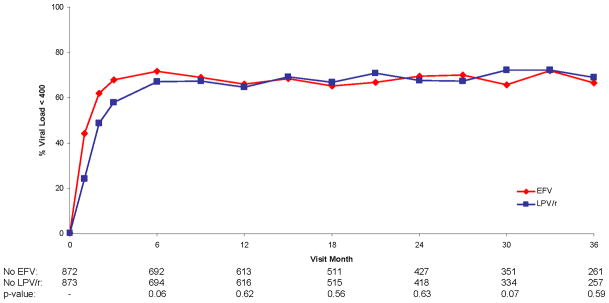
For participants assigned EFV, the percent achieving an HIV RNA level < 400 copies/mL was more rapid than for those assigned LPV/r, as reflected in a HR (EFV versus LPV/r) for time to viral suppression significantly greater than 1.0 [HR=1.48 (95% CI: 1.30 to 1.67; p<0.001)]. After 6 months the percent with HIV RNA <400 copies/mL was similar in the two groups (Figure 2a). The more rapid decline of HIV RNA level on EFV than LPV/r did not differ by the NRTI combination used (p=0.79 for interaction).
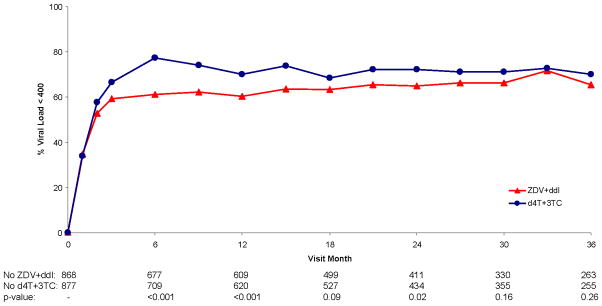
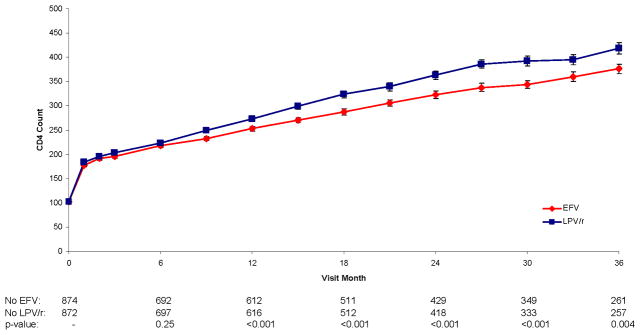
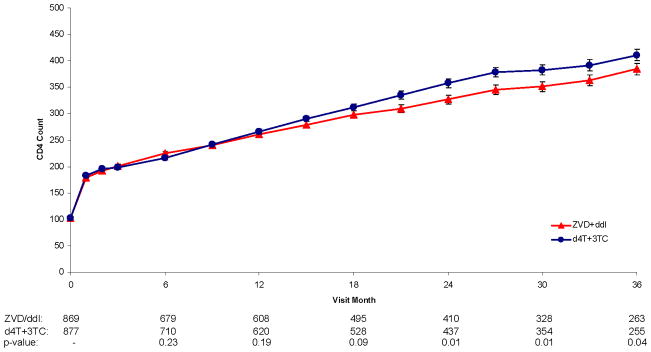
Figure 2.
Cumulative percent of subjects with HIV viral loads <400 copies/mL for EFV vs. LPV/r (2a) and for ZDV + ddI vs. d4T + 3TC (2b). CD4+ lymphocyte cell counts/mm3 for EFV vs. LPV/r (2c) and for ZDV + ddI vs. d4T + 3TC (2d) The numbers below the x-axis represent the numbers of subjects in each group at each time point.
Figure 2a. Percent Viral Load < 400 Over Follow-up for Efavirenz vs. Lopinavir/Ritonavir
Figure 2b. Percent Viral Load < 400 Over Follow-up for Zidovudine + Didanosine vs. Stavudine + Lamivudine
Figure 2c. CD4 Count Over Follow-up for Efavirenz vs. Lopinavir/Ritonavir
Figure 2d. CD4 Count Over Follow-up for Zidovudine + Didanosine vs. Stavudine + Lamivudine
This pronounced effect on HIV RNA level in the first 6 months did not result in greater increase in CD4+ counts for EFV versus LPV/r (Figure 2c); averaged over all of follow-up, CD4+ count was approximately 18 cells lower on EFV than LPV/r (p<0.001); treatment differences were greater in the later, compared to earlier, follow-up period (the p-value for the treatment by visit interaction was <0.001). The greater increases in CD4+ count for LPV/r than EFV did not vary by the NRTI combination used (p=0.21 for interaction).
For participants assigned d4T+3TC achievement of an HIV RNA level < 400 copies/mL was more rapid than for ZDV+ddI. The HR for ZDV+ddI versus d4T+3TC for time to HIV RNA <400 copies/mL was 0.79 (95% CI: 0.70 to 0.90; p<0.001). Differences between the NRTI combinations diminished with longer follow-up (Figure 2b).
CD4+ counts were similar for the first 12 months; afterwards, there were greater increases in CD4+ counts for d4T+3TC compared to ZDV+ddI (Figure 2d) (p-value for the treatment by visit interaction was <0.001). After 12 months, the greater effects of LPV/r and d4T+3TC on CD4+ count are additive, resulting in the best CD4+ cell count response. For example, at 24 months, the CD4+ cell count increase was 274 cells/mm3 for those assigned LPV/r+d4T+3TC versus 249 cells for LPV/r+ZDV+ddI, 238 cells for EFV+d4T+3TC, and 200 cells/mm3 for those assigned EFV+ZDV+ddI (p<0.001 for differences among the four groups).
Clinical Endpoints
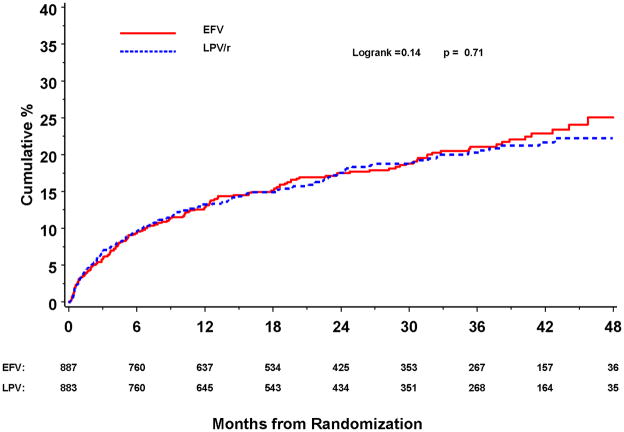
163 participants in the EFV group (rate = 9.1/100 person-years) and 157 in the LPV/r group (8.7/100 person-years) had an AIDS event or died from any cause (primary endpoint) (Table 2). Cumulative event rates after 12, 24, and 36 months were 12.9%, 17.5%, and 21.1% for those assigned EFV and 13.2%, 17.6% and 20.3% for those assigned LPV/r (Figure 3a). The HR for AIDS or death was 1.04 (95% CI: 0.84–1.30; p=0.71) (p=0.48 for proportional hazards assumption). This HR (EFV versus LPV/r) was 1.23 (95% CI: 0.91–1.66) for those also assigned ZDV+ddI and 0.87 (95% CI: 0.63–1.20) for those assigned d4T+3TC (p=0.13 for interaction).
Table 2.
Summary of Primary and Major Secondary Endpoints
| Endpointi | PI/NNRTI Comparison No. of Patients with Endpoint (Rate per 100 person years) |
NRTI Comparison No. Of Patients with Endpoint (Rate per 100 person years) |
||||||
|---|---|---|---|---|---|---|---|---|
| EFV (N=888) | LPV/r (N=883) | HR (95% CI) | P-value | ZDV+ddI (N=884) | d4T+3T C (N=887) | HR (95% CI) | P-value | |
| AIDS or death | 163 (9.1) | 157 (8.7) | 1.04 (0.84–1.30) | 0.71 | 170 (9.6) | 150 (8.2) | 1.15 (0.93–1.44) | 0.20 |
| Death | 106 (5.6) | 102 (5.4) | 1.05 (0.80–1.37) | 0.75 | 111 (5.9) | 97 (5.1) | 1.15 (0.88–1.51) | 0.31 |
| AIDS | 80 (4.4) | 69 (3.7) | 1.18 (0.85–1.62) | 0.32 | 83 (4.6) | 66 (3.5) | 1.29 (0.94–1.79) | 0.12 |
| Grade 4 event | 137 (7.9) | 129 (7.2) | 1.07 (0.84–1.36) | 0.57 | 142 (8.1) | 124 (7.0) | 1.16 (0.91–1.48) | 0.23 |
Figure 3.
Kaplan-Meier curves for death or progression of disease for EFV vs. LPV/r (3a), death for EFV vs. LPV/r (3b), death or progression of disease for ZDV + ddI vs. d4T + 3TC (3c), and death for ZDV + ddI vs. d4T + 3TC (3d).
Figure 3a. KM Curve for Death or Progression of Disease for Efavirenz Versus Lopinavir/Ritonavir
Figure 3b. KM Curve for Death for Efavirenz Versus Lopinavir/Ritonavir
Figure 3c. KM Curve for Death or Progression of Disease for Zidovudine + Didanosine Versus Stavudine +Lamivudine
Figure 3d. KM Curve for Death for Zidovudine + Didanosine Versus Stavudine +Lamivudine
170 participants in the ZDV+ddI group (9.6/100 person-years) and 150 in the d4T+3TC group (8.2/100 person-years) had an AIDS event or died from any cause. Cumulative event rates after 12, 24, and 36 months were 13.9%, 18.7%, and 22.2% for those assigned ZDV+ddI and 12.2%, 16.4% and 19.2% for those assigned d4T+3TC (Figure 3c). The HR for AIDS or death was 1.15 (95% CI: 0.93–1.44, p=0.20) (p=0.86 for proportional hazards assumption).
The majority (171) of the 320 primary events were death (53%), most occurring in the first 6 months of follow-up. There were 106 deaths among those assigned EFV (5.6/100 person-years) and 102 among those assigned LPV/r (5.4/100 person-years) (Table 2). Thirty-seven of the 208 deaths followed a confirmed or probable AIDS event. The HR (EFV versus LPV/r) for death from any cause was 1.05 (95% CI: 0.80–1.37) (Figure 3b). The HR for death was similar for those assigned ZDV+ddI and d4T+3TC (p=0.23 for interaction).
There were 111 deaths in the ZDV+ddI groups and 97 in the d4T+3TC groups. The HR for death from any cause was 1.15 (95% CI: 0.88–1.51) (Table 2, Figure 3d).
HRs for AIDS were 1.18 (95% CI: 0.85–1.62) for EFV versus LPV/r and 1.29 (95% CI: 0.94–1.79) for ZDV+ddI versus d4T+3TC (Table 2). The most common AIDS events were tuberculosis (113 participants); cryptococcal disease (15); Candida esophagitis (8), Kaposi sarcoma (7), and Mycobacterium avium complex (2).
Adverse Events and Reasons for Treatment Discontinuation
Grade 4 event rates were similar for different treatment groups. The HR for grade 4 events for EFV versus LPV/r groups was 1.07 (95% CI: 0.84–1.36); for ZDV+ddI versus d4T+3TC it was 1.16 (95% CI: 0.91–1.48) (Table 2)[11]. Of grade 4 adverse events, only blood and lymphatic disorders differed significantly between treatment groups (21 participants on ZDV+ddI, 7 on d4T+3TC; p=.01). Most of these events were anemia. Grade 4 lactic acidosis occurred among 6 persons taking d4T+3TC and 2 persons taking ZDV+ddI. Grade 4 pancreatitis occurred in 3 participants assigned d4T+3TC and 8 persons assigned ZDV+ddI.
D4T was discontinued for toxicity (n=112, 12.6% of participants) more frequently than other drugs; discontinuation rates ranged from 2.6% for 3TC to 4.3% for LPV/r. Thirty-nine (35%) stopped d4T for peripheral neuropathy, 30 (27%) for lipodystrophy, 25 (22%) for hyperlactatemia, 5 (5%) for lactic acidosis, 5 (5%) for lipoatrophy, and 8 (7%) for other reasons.
Compared to ZDV and ddI, d4T and 3TC had lower rates of discontinuation due to virologic failure – 2.5% for 3TC and 2.6% for those assigned d4T versus 6.1% for both ddI and ZDV.
At 12 months, based on the physician’s assessment of lipodystropy, significantly higher rates of fat wasting in the face, arms, buttocks, and legs were found in the d4T+3TC group versus the ZDV+ddI group (Appendix Table 1). There were no significant differences between EFV and LPV/r groups in any lipodystrophy measure at 12-months.
Selected symptoms were assessed at each follow-up visit; the percent with grade 1 or higher conditions through 12-months is summarized in Appendix Table 2. Compared to EFV, the LPV/r group had higher rates of nausea (23% versus 16%), diarrhea (25% versus 12%) and vomiting (11% versus 7%) (p<.01 for each) through 12-months; ZDV+ddI had higher rates of nausea (22% versus 17%) constipation (11% versus 7%) and fatigue (24% versus 19%) (p<0.05 for each), and lower rates of peripheral neuropathy compared to the 3TC+d4T group (17% versus 26%) (p<0.001).
Weight and Laboratory Changes
In all groups, weight increased following randomization and continued throughout follow-up. Overall, weight increases were similar for the EFV versus LPV/r groups (average difference = 0.16 kg; p=0.62); the average weight increase was 0.60 kg greater for those assigned d4T+3TC versus those assigned ZDV+ddI (p=0.06).
SGPT (average difference = 0.24 log IU/L), SGOT (0.13 log IU/L), albumin (0.70 g/L), hemoglobin (0.19 g/dL), and hematocrit (0.51%) were significantly greater and creatinine (−4.1 μmol/L) was significantly lower for those assigned EFV compared to LPV/r during follow-up (p=0.003 for hematocrit and p<0.001 for other laboratory measures). These measures also differed between the NRTI groups: SGPT (0.10 log IU/L), albumin (0.43 g/L), hemoglobin (0.35 g/dL), and hematocrit (0.96%) were significantly greater and platelet count (−9.0 109/L) was lower for those assigned d4T+3TC compared to ZDV+ddI (p=0.01 for albumin and p<0.001 for other laboratory measures).
Discussion
The Phidisa II trial was initiated to provide randomized evidence on the relative risks and benefits of available antiretroviral drugs in the RSA in 2006. A factorial design was employed to assess combined and separate effects of a protease inhibitor (LPV/r) and a NNRTI (EFV) and two NRTI combinations (d4T+3TC and ZDV+ddI). Consequent to stopping the trial early, the number of primary events reported and adjudicated (320) was about one-half of that planned and confidence intervals are wide for the clinical outcome comparisons. EFV had a more rapid effect in suppressing HIV RNA level than LPV/r and LPV/r had a small, but significantly greater, effect on raising CD4+ counts. The clinical relevance of this difference is uncertain. The HR for the primary endpoint was 1.04, but, due to the smaller than planned number of endpoints, we cannot rule out the possibility of a 30% higher rate of AIDS or death and a 37% higher death rate on EFV compared to LPV/r.
Grade 4 event rates were similar for EFV and LPV/r and discontinuation rates did not differ.
ZDV+ddI was not as effective as d4T+3TC in suppressing HIV RNA level or in raising CD4+ count, but the clinical significance of this difference is uncertain. There was an excess risk of AIDS or death, and of death alone for ZDV+ddI compared to d4T+3TC of 15% but the confidence around this estimate was wide with non-significant differences. Grade 4 event rates did not differ between the NRTI combinations, but more participants discontinued d4T due to toxicity than other NRTIs. This was offset by a lower rate of treatment discontinuation attributed to virologic failure.
The greater early effect of EFV compared to LPV/r on suppression of HIV RNA levels has been reported in another randomized trial (N=503) [12], but not in an observational trial (N=1550) [13]. In our study, the differential effect on HIV RNA disappeared after 6 months. The superiority of d4T+3TC compared to ZDV+ddI for HIV RNA and CD4+ count was also observed in a Botswana study [14]. A specific ARV-associated mutational pattern was identified peculiar to the HIV-1C subtype found in sub-Saharan Africa when treated with the ZDV+ddI that may lead to early virological failure [15]. Effects of EFV versus LPV/r and ZDV+ddI versus d4T+3TC on HIV RNA and CD4+ count changes appear to be additive. Taken together, a greater drop in HIV RNA is evident for those assigned a d4T+3TC-containing regimen in the first 24 months, with the greatest effect in the first 6 months for those also taking EFV. CD4+ increases after the first year are greater for participants taking d4T+3TC and LPV/r. The group with poorest CD4+ response (EFV+ZDV+ddI) also had the highest rate of AIDS or death (10.6/100 person-years).
More than one-half of the deaths on study, 108 of 208 (51.9%), occurred within the first 6 months of beginning antiretroviral therapy. For all clinical outcomes, there were no differences between groups in the first 6 months, consistent with other studies initiating antiretroviral therapy in similar settings [16–21]. The marked decrease in the mortality rate over time for all regimens suggests that these regimens are potent and introducing ART into an ARV-naïve population with advanced disease is the most critical issue in subsequent successful control of HIV infection. The marked decrease in deaths after six months on therapy argues strongly for initiating ART earlier than the CD4+ count <200 cells/mm3 criterion for entry onto this study [22–24].
Preceding the early termination of our study, there were reports of hyperlactataemia, lipoatrophy, and pancreatitis related to d4T from other African cohorts [25–29]. The frequency of severe cases in our cohort was small. Though recent data and guidelines recommend limiting d4T use due to toxicity [11, 30, 31], d4T use in this trial was well tolerated by many participants for up to 36 months. A reasonable approach would be to consider the use of d4T when no other options exist and closely monitor its use.
A major limitation of the study was our inability to precisely characterize the treatment differences with respect the primary endpoint of AIDS or death. The open-label study design could have impacted assessments of symptoms and other subjective conditions. A strength of our study is the excellent follow-up and adherence to the assigned regimens and the well-characterized virologic and immunologic responses to the regimens studied.
In summary, our findings show that either EFV or LPV/r can be supported as components of first line ART. Regimens containing ZDV + ddI should be monitored for slower virologic response and more modest immunologic enhancement and d4T+3TC monitored for higher rates of toxicities in this setting. The results of this study indicate that randomized trials can be successfully conducted in this setting to obtain evidence to support policy development for ART roll-out programs and the provision of care to people with advanced HIV disease in Southern Africa.
Acknowledgments
The authors wish to thank the entire Phidisa staff, as well as the participants who devoted their time and effort to this research project. The authors wish to acknowledge the following Specialist consultants: Clindev who provided regulatory monitoring, Science Applications International Corporation (SAIC) and BARC (Bio Analytical Research Corporation) who provided laboratory and personnel support, and the Henry Jackson Foundation for project and personnel support.
Funding was provided by the United States Department of Defense (US DoD) and the United States, National Institutes of Health (NIH) through the National Institute of Allergy and Infectious Diseases (NIAID). The South African Military Health Service (SAMHS) and the National Institute of Allergy and Infectious Diseases, NIH, USA signed a cooperative agreement to conduct this research project.
The Phidisa II Writing Team for Project Phidisa
Contributors
Collaborators contributed to the study from the South Africa Medical Health Services (SAMHS), South African Defence Forces, Pretoria, South Africa; National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, Bethesda, Maryland, United States of America; Critical Care Medicine Department, Clinical Center, National Institutes of Health, Bethesda, Maryland, United States of America; Charisma Health, Pretoria, South Africa; University of Minnesota (UM), Minneapolis, Minnesota, United States of America; National Centre in HIV Epidemiology and Clinical Research, University of New South Wales. Sydney, Australia; Science Applications International Corporation (SAIC), Frederick, Maryland, United States of America; Palladian Partners Incorporated, Silver Spring, Maryland, United States of America; KwaZulu-Natal Department of Health, Pietermaritzburg, South Africa; United States Department of Defense (US DoD), Washington, DC, United States of America.
Senior Protocol Writing team- A. Ratsela (SAMHS National Principal Investigator), M. Polis (NIAID co-Principal Investigator), S. Dhlomo (KwaZulu-Natal Department of Health), S. Emery (University of New South Wales), G. Grandits (UM), P. Khabo (Charisma), T. Khanyile (SAMHS), S. Komati (SAMHS), J.D. Neaton (UM), C. Naidoo (SAMHS), D. Magongoa (SAMHS), D. Qolohle (SAMHS)
Executive Committee Present – S. Brodine (DOD), H.C. Lane (NIAID), N. Motumi (SAMHS), M. Radebe (SAMHS), K. Mokoena (SAMHS)
Past - X. Currie (SAMHS), A. Jamuna (SAMHS), P. J Oelofse (SAMHS), S. Ngqakayi (SAMHS), L. Siwisa (SAMHS), S. Swanapoel (SAMHS)
Data and Safety Monitoring Board-J. Levin (Chairperson-Pretoria, South Africa), W. N. Rida (Bethesda, Maryland, USA), T. Morodi (Pretoria, South Africa), Y. Leeuw (Pretoria, South Africa)
SAMHS South Africa Clinical sites Principal Investigator/Study Coordinator-1-Military Site, Pretoria- S. Hassim, L. Malan; 3-Military Site, Cape Town- H. Somarro, T. Mokhathi, Bloemfontein- N. Mokwena, N. Coangae; Mtubatuba- T. Khanyile, Z. Yokwana; Mthata- B. Mabindla, G. Manqola; Phalaborwa- M. Maluleke, T. Tseka
Phidisa Headquarter staff- J. Dlamini (Charisma), L. Ledwaba (Charisma), M. Marumo (SAMHS), U. Matchaba (Charisma), J. Mthethwa (SAMHS), P. Sangweni (Charisma)
US Phidisa team- B. Baseler (SAIC), R. Eckes (NIAID), H. Masur (NIH Clinical Center), B. Grace (SAIC), G. Morgan (Palladian), L. McNay(NIAID), J. Metcalf (NIAID), S. Orsega (NIAID), A. Pau (NIAID), J. Tavel (NIAID), J. Zuckerman (NIAID), M. Simpson (SAIC), H, Highbarger (SAIC), R. Dewar (SAIC),
Statistical and Data Management support- University of Minnesota- A. DuChene, M. Harrison, A. Lifson
Footnotes
Sean Emery has received grant support, travel awards and honoriums from Abbott, Boehnringer-Ingleheim, Bristol Myers Squibb, Gilead Sciences, GlaxoSmithKline, Merck, and Tibotec. All other authors do not have a commercial or other association that may pose a conflict of interest.
This work was presented as poster abstract #594 during session 107 at the Conference on Retroviruses and Opportunistic Infections in February 8–11, 2009 at Montreal, Canada.
References
- 1.Joint United Nations Programme on HIV/AIDS., NetLibrary Inc. 2006 report on the global AIDS epidemic. Geneva: UNAIDS; 2006. A UNAIDS 10th anniversary special ed. [Google Scholar]
- 2.United States. Dept. of Health and Human Services., National Institutes of Health (U.S.). Office of AIDS Research. Advisory Council. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents: November 3, 2008. Washington, D.C: Dept. of Health and Human Services; 2008. [Google Scholar]
- 3.Karim SS, Churchyard GJ, Karim QA, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet. 2009;374:921–933. doi: 10.1016/S0140-6736(09)60916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Health SANDo. National Antiretroviral Treatment Guidelines. Johannesburg: Jacana; 2004. [Google Scholar]
- 5.Joint United Nations Programme on HIV/AIDS., World Health Organization. Progress on global access to HIV antiretroviral therapy a report on “3 by 5”, June 2005 and beyond. Geneva: World Health Organization: UNAIDS; 2006. [Google Scholar]
- 6.Motumi N, Emery S, Lane HC. Project Phidisa: development of clinical research capacity within the South African National Defence Force. Curr Opin HIV AIDS. 2007;2:69–76. doi: 10.1097/COH.0b013e328011e9c0. [DOI] [PubMed] [Google Scholar]
- 7.CDC. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR. 1992:41. [PubMed] [Google Scholar]
- 8.CDC. Centers for Disease Control and Prevention. Guidelines for preventing opportunistic infections among HIV-infected persons - 2002 recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. MMWR. 2002;51:1–52. [PubMed] [Google Scholar]
- 9.DAIDS. Division of AIDS (DAIDS) Table for Grading Severity of Adult Adverse Experiences for the HIV Prevention Trials Network. Regulatory Compliance Center, Office for Policy in Clinical Research Operations of the Division of AIDS; 1994. [Google Scholar]
- 10.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 2. Hoboken, N.J: J. Wiley; 2002. Wiley series in probability and statistics. [Google Scholar]
- 11.Dlamini J, Ledwaba L, Mokwena N, et al. Lactic Acidosis in a Randomized Trial of d4T-vs ddI-based First-line Therapy in HIV-infected Adults in South Africa. Vol. 283. Infectious Disease Society of America; Philadelphia, Pennsylvania: 2009. [Google Scholar]
- 12.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Domingo P, Suarez-Lozano I, Torres F, et al. First-line antiretroviral therapy with efavirenz or lopinavir/ritonavir plus two nucleoside analogues: the SUSKA study, a non-randomized comparison from the VACH cohort. J Antimicrob Chemother. 2008;61:1348–58. doi: 10.1093/jac/dkn121. [DOI] [PubMed] [Google Scholar]
- 14.Bussmann H, Wester CW, Thomas A, et al. Response to Zidovudine/Didanosine-Containing Combination Antiretroviral Therapy Among HIV-1 Subtype C-Infected Adults in Botswana: Two-Year Outcomes from a Randomized Clinical Trial. J Acquir Immune Defic Syndr. 2009;51:37–46. doi: 10.1097/QAI.0b013e31819ff102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novitsky V, Wester CW, DeGruttola V, et al. The reverse transcriptase 67N 70R 215Y genotype is the predominant TAM pathway associated with virologic failure among HIV type 1C-infected adults treated with ZDV/ddI-containing HAART in southern Africa. AIDS Res Hum Retroviruses. 2007;23:868–78. doi: 10.1089/aid.2006.0298. [DOI] [PubMed] [Google Scholar]
- 16.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–8. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 17.Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet. 2006;368:1254–9. doi: 10.1016/S0140-6736(06)69117-4. [DOI] [PubMed] [Google Scholar]
- 18.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 19.Marazzi MC, Liotta G, Germano P, et al. Excessive early mortality in the first year of treatment in HIV type 1-infected patients initiating antiretroviral therapy in resource-limited settings. AIDS Res Hum Retroviruses. 2008;24:555–60. doi: 10.1089/aid.2007.0217. [DOI] [PubMed] [Google Scholar]
- 20.Etard JF, Ndiaye I, Thierry-Mieg M, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS. 2006;20:1181–9. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 21.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emery S, Neuhaus JA, Phillips AN, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–44. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 23.Lawn SD, Little F, Bekker LG, et al. Changing mortality risk associated with CD4 cell response to antiretroviral therapy in South Africa. AIDS. 2009;23:335–42. doi: 10.1097/QAD.0b013e328321823f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–63. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stead D, Osler M, Boulle A, Rebe K, Meintjes G. Severe hyperlactataemia complicating stavudine first-line antiretroviral therapy in South Africa. Antivir Ther. 2008;13:937–43. [PubMed] [Google Scholar]
- 26.Makinson A, Moing VL, Kouanfack C, Laurent C, Delaporte E. Safety of stavudine in the treatment of HIV infection with a special focus on resource-limited settings. Expert Opin Drug Saf. 2008;7:283–93. doi: 10.1517/14740338.7.3.283. [DOI] [PubMed] [Google Scholar]
- 27.Group LAIS. Risk factors for lactic acidosis and severe hyperlactataemia in HIV-1-infected adults exposed to antiretroviral therapy. AIDS. 2007;21:2455–64. doi: 10.1097/QAD.0b013e3282f08cdc. [DOI] [PubMed] [Google Scholar]
- 28.Wester CW, Okezie OA, Thomas AM, et al. Higher-than-expected rates of lactic acidosis among highly active antiretroviral therapy-treated women in Botswana: preliminary results from a large randomized clinical trial. J Acquir Immune Defic Syndr. 2007;46:318–22. doi: 10.1097/QAI.0b013e3181568e3f. [DOI] [PubMed] [Google Scholar]
- 29.Bolhaar MG, Karstaedt AS. A high incidence of lactic acidosis and symptomatic hyperlactatemia in women receiving highly active antiretroviral therapy in Soweto, South Africa. Clin Infect Dis. 2007;45:254–60. doi: 10.1086/518976. [DOI] [PubMed] [Google Scholar]
- 30.Brinkman K. Stavudine in antiretroviral therapy: is this the end? AIDS. 2009;23:1727–9. doi: 10.1097/QAD.0b013e32832d3c5e. [DOI] [PubMed] [Google Scholar]
- 31.Health RoSADo. Clinical Guidelines for the Management of HIV and AIDS in Adults and Adolescents. National Department of Health; 2010. [Google Scholar]