Abstract
Background
The purpose of this study was to conduct a preliminary, post-market, home study of the Flexitouch® system to examine the potential efficacy of the device as a component of self-care in breast cancer survivors with truncal lymphedema.
Methods and Results
A quasi-experimental, pre-treatment, post-treatment design was used. Twelve participants received a total of ten self-administered, consecutive, one hour per day treatments. Treatments one and two were observed by study staff and the remaining eight were unobserved. Assessments were conducted at baseline, after the first two treatments, mid-way through therapy, and at the end-of-study. Logs revealed 100% compliance with the eight prescribed unobserved home treatments. Symptoms were assessed by self-report symptom surveys. Signs, objectively observed physical phenomenon, were assessed by staff-initiated skin examination and circumferential truncal measurements. Statistically significant improvement in truncal symptoms and sleep were found. Changes in function and girth were not statistically significant in this initial study.
Conclusions
Breast cancer survivors with truncal lymphedema may benefit from using an advanced pneumatic compression devices with truncal treatment as part of their self-care program. Participants were highly compliant in device use. Further research of this intervention is warranted. To facilitate future research, clinically meaningful reductions in truncal girth should be defined.
Introduction
Despite improved cancer treatments designed to decrease the incidence of secondary lymphedema in breast cancer survivors, new lymphedema cases continue to occur. Incidence of upper extremity (arm) lymphedema in women after breast cancer treatment is estimated to be 20%–36% up to 2 years post-treatment, increasing to 30%–45% at 15 years or more post-treatment.1 Rates in low-income breast cancer survivors may actually be higher.2 Some breast cancer survivors experience problematic swelling in the truncal areas (chest, axilla, shoulder, breast, and/or back). One retrospective review of 234 women reported a 21% incidence of acute breast edema immediately after radiotherapy treatment.3 A second study conducted one year after surgery, upon physical examination, found breast edema in 48% of patients who had axillary clearance with positive nodes, 35% of patients who had axillary dissection with negative nodes, and 23% of patients following sentinel lymph node biopsy.4 However, when using ultrasound as the assessment tool in lieu of physical examination, the same study found that 69%–70% of participants undergoing axillary dissection had breast swelling. Reports on the incidence of swelling elsewhere in the trunk have been more limited. However, one study reported that 10% of patients experienced swelling in their back, and 22% reported edema in their armpit area 3 to 4 years after cancer treatment.5
Although the impact of arm lymphedema has been well studied and documented, very little attention has been focused on the impact of truncal edema. Practitioners have observed the distress experienced by their patients related to trunk and/or breast edema, including difficulties involving clothing and undergarments, difficulty sleeping, pain/discomfort associated with increased breast weight, erythema, and tissue swelling in the axilla.6 Swelling in the breast, shoulder, and posterior axilla causes local discomfort, heat, increased pressure in the tissues, and may exacerbate postoperative neuropathic symptoms and reduce function.7 A recent study identified axilla edema as the problem that most limited activities 12 months post-breast cancer treatment.8 While there was improvement over the 6-month reports, the author noted that some patients had abandoned leisure activities altogether, along with experiencing a reduction in their ability to work.8 Despite the limited data available regarding the symptom profile associated with truncal lymphedema, it is clear that some degree of physical and psychological sequelae is associated with this disorder.
The current gold standard of treatment for all forms (primary and secondary), stages (initial onset and chronic), and locations (arm or trunk) of lymphedema is two-phase complete decongestive therapy (CDT). Phase-one includes professionally administered Manual Lymphatic Drainage (MLD), multilayer short stretch compressive bandaging, exercise, and meticulous skin care.9 Phase-two involves self-care components such as self-MLD or use of a pneumatic compression device (PCD), skin care, exercise, and the wearing of compression garments.
PCDs have been developed during the last 25 years as both alternative and complementary treatments to MLD. More recently developed lower-pressure devices, when used with appropriate training and education, are believed to be safer than their older counterparts.10,11 The Flexitouch® system (Fig. 1) is an advanced, programmable PCD that is cleared by the Food and Drug Administration for home use. This device is the only PCD designed to emulate the therapeutic techniques of MLD. The Flexitouch® system for upper extremities includes three compressive garments: 1) trunk; 2) chest; and 3) arm (see Fig. 1). Software programming allows for variation of compression patterns to meet individualized needs. The system applies light, dynamic, variable pressure to the affected arm, and beyond the upper arm and limb junction to the trunk and chest, using multi-chambered, inflatable, and stretchable fabric garments. Published studies and case reports suggest that breast cancer-associated limb and truncal edema may be effectively treated with the Flexitouch® system.12–15 Phase 2 self-care for breast cancer survivors with lymphedema is difficult and it is especially onerous for those with truncal swelling to perform self-MLD effectively in often hard-to-reach swollen areas. The Flexitouch® system's design, which includes garments to treat truncal swelling, may offer much needed self-care assistance to these patients.
FIG. 1.
Flexitouch® System for upper extremity.
To-date, no studies have tested the potential benefit of the application of the Flexitouch® system truncal and chest garments on the self-management of truncal lymphedema in breast cancer survivors. The purpose of this study was to conduct a preliminary, post-market, home study of the Flexitouch® system to examine the potential efficacy of the device as a component of self-care in breast cancer survivors with truncal lymphedema.
Materials and Methods
Participants
Approval was obtained from the Vanderbilt University Institutional Review Board and the Vanderbilt-Ingram Cancer Center Scientific Review Committee. Participants in this study had a history of breast cancer treated with surgery (±radiation) with resulting lymphedema in one arm and coexisting truncal swelling. Additional inclusion criteria included: completion of all therapy at least 6 months prior to study entry, 21 years of age or older, willing and able to drive to the study site as needed, and currently not using a compression pump or undergoing MLD by a therapist. Individuals were excluded if they were pregnant, or had: congestive heart failure, chronic/acute renal disease, cor pulmonale, nephrotic syndrome, nephrosis, liver failure or cirrhosis, pulmonary edema, thrombophlebitis, deep vein thrombosis, infection, inflammation in the trunk or arms, a history of bilateral breast cancer, active cancer, had metal implants that would interfere with bioimpedance measurement equipment, or were unable to stand upright. Those with pacemakers and internally implanted defibrillators, and those <3 months post-chest or arm surgery (affected arm only) were also excluded.
Participants were identified and recruited from an existing breast cancer survivor registry of patients known to have lymphedema and from the community at-large. Thirteen were screened using a three-phase screening process. Phase 1: initial telephone screening to ascertain self-reported truncal swelling; Phase 2: further medical information was obtained with consent and reviewed by the principal investigator and study physician; and Phase 3: on-site physical screening for truncal swelling (asymmetrical and palpable swelling). Twelve were enrolled and all completed the study. Participants were primarily well-educated, female Caucasians residing in rural and urban metropolitan areas in the southern and mid-western regions of the United States (Table 1).
Table 1.
Sample Characteristics
| Demographic Characteristics | ||
| Marital status | ||
| Married/partnered | 9 (75%) | |
| Single | 3 (25%) | |
| Work status | ||
| Employed full time | 6 (50%) | |
| Employed part time | 2 (17%) | |
| Not working | 4 (33%) | |
| Mean (Median) | SD (Min, Max) | |
| Age (years) | 55.3 (55) | 10.2 (43, 79) |
| Years of education (years) | 14.9 (15) | 2.1 (12, 18) |
| Medical Characteristics | ||
| Type of cancer treatment | ||
| Surgery | 2 (17%) | |
| Surgery and radiation | 3 (25%) | |
| Surgery and chemotherapy | 2 (17%) | |
| Surgery radiation and chemotherapy | 5 (42%) | |
| Type of Surgery | ||
| Lumpectomy | 6 (50%) | |
| Mastectomy | 6 (50%) | |
| Time since breast cancer diagnosis (years) | 5.4 (4) | 3.7 (1, 13) |
| Months until lymphedema onset (months) | 16.9 (11) | 23.1 (1, 85) |
| Lymphedema duration (months) | 52.6 (38) | 42.6 (3, 134) |
| Age at lymphedema onset (years) | 50.8 (47) | 11.7 (39, 78) |
Protocol
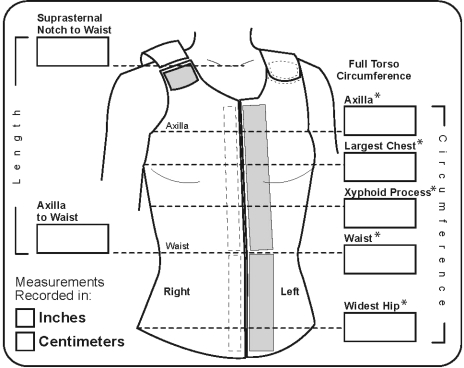
This was a quasi-experimental (single group), pre-test, post-test design.16 Participants were assessed upon enrollment in the study. They completed ten 1-hour per day treatments over 10 consecutive days. Participants were reassessed between treatments five and six, and after treatment ten. Symptoms, physical (truncal), psychological, or situational, and symptom burden were assessed by self-report with the Lymphedema Symptom Intensity and Distress Survey-Arm and Trunk (LSIDS-AT).17 The LSIDS-AT requires participants to indicate the presence of a symptom (“yes” or “no”). Participants then rate all “yes” symptoms for intensity and distress on separate 10 point numeric scales, with 1 representing “slight” and 10 representing “severe.” A symptom burden score is then derived by multiplying the intensity and distress values for each symptom to arrive at a weighted value, that may range from ‘0’ (symptom not reported) to ‘100’ (maximum intensity and distress). The Functional Assessment Screening Questionnaire (FASQ) was used to assess function.18 Signs, objectively observed physical phenomenon, were assessed as follows: skin condition by physical examination using a standardized checklist and trunk circumference was measured in five locations (Fig. 2) using a no-stretch, Gulick II Tape. Study staff were trained to within 0.2 cm of variation during measurement.
FIG. 2.
Truncal measurement locations. *Five areas measured in this study. Reprinted with permission from JoViPack Corporation.
Intervention
Participants were seen in a laboratory setting for baseline assessments, nurse administered training on use of the PCD, and an initial supervised self-treatment. Participants completed the self-report surveys. Study staff completed physical measurements and examinations. Prior to treatment one, participants viewed a training video demonstrating the appropriate Flexitouch® system garments and controller operation. Participants were then fitted for the compression garments and instructed on the use of the Flexitouch® system. Participants then gave a return demonstration on garments and device application and usage, and were reeducated if needed. To ensure proper PCD technique was followed and to observe for any participant problems, treatment one was conducted under staff supervision in our laboratory. Therefore, immediately following the training session, participants were asked to void, to remove any constrictive clothing and jewelry, and to change into scrub suits. An arm stockinette was placed over the affected arm and the compression garments were donned. During the treatment, participants rested supine on a massage table with their head and affected arm on pillows. Study staff remained present during the treatment to observe the participant for tolerance and comfort.
Following initial treatment, participants were given instructions for home treatments two through ten and a diary in which to record their home use. Study staff observed the second self-administered treatment in the participant's home to ensure that consistency in techniques taught in our laboratory were maintained in the home environment. Treatments three through ten were unobserved. Study staff was available by phone to answer any questions that arose during the ten days on the study.
Analyses
SPSS (17.0) was used to generate descriptive statistical summaries of patient characteristics and values of the study outcome variables at each time of assessment. As this was a single group pre-post design, all major analyses were within the group. Frequency distributions were used for summarizing the nominal and ordinal variables. Due to the highly skewed nature of the continuous variables and small sample size, median, minimum, and maximum values were used to summarize central tendency and variability of the continuous data. However, because means are so commonly reported, those values are also included in the textual descriptive summaries. Friedman Tests were used to test for statistically significant changes in each of the outcome measures (e.g., overall symptom scores, specific symptom burden values, function, number of signs) within the group of subjects between any of the observed assessment periods in the study. If an overall statistically significant finding was observed, post-hoc pairwise tests were conducted using Wilcoxon Signed-Ranks tests. Using a Bonferroni-adjusted alpha of 0.017 for concluding statistical significance for those measures with three times of assessment (0.05/3) and 0.008 for those measures with four times of assessment (0.05/6). The summary truncal change values was normally distributed, therefore a one-sample t-test was used for testing whether those values were statistically significantly greater than zero.
Results
Use of phone, available staff, and diary records
Few participants telephoned the available staff during the days they were not seen in-person. Calls primarily concerned how much to tighten the garment, and one patient called to make sure the “air noise” she heard during use was appropriate. Diary recordings revealed 100% compliance with the prescribed number of treatments.
Adverse events
No adverse events related to study participation were reported. No participants asked to stop the treatment.
Symptoms
While the level of symptom burden was not heavy in this sample, those with substantial levels of burden demonstrated improvements that were manifested in statistically significant decreases in both the number of truncal lymphedema symptoms (Friedman: Χ2(df=2) = 13.74, p = 0.001) and overall symptom burden (Friedman: Χ2(df=2) = 13.74, p = 0.001) throughout the course of the study. Of the 17 truncal symptoms assessed, the average number of truncal symptoms reported at baseline was 6.6 (Median = 7.0). The minimum number reported was two, and the maximum number was 11. A statistically significant change in the group's report over the course of the study was demonstrated (Friedman: Χ2(df=2) = 13.74, p = 0.001), with pairwise post-hoc tests revealing that most of that change occurring as a reduction in number of symptoms between baseline and study mid-point (M = 3.3, Median = 3.0, Min = 0, Max = 10, p = 0.003). That reduction remained steady through the end of the study (M = 3.6, Median = 3.5, Min = 0, Max = 10, p = 0.004 from baseline). The difference between the number of symptoms reported at mid-point and end-of study was not statistically significant (p > than adjusted alpha of 0.017).
An identical pattern was seen in the symptom burden values for the study group. Of a maximum score of 100, the average symptom burden score reported at baseline was 6.9 (Median = 4.3, Min = 0, Max = 27). A statistically significant change in the group's report over the course of the study was demonstrated (Friedman: Χ2(df=2) = 13.74, p = 0.001), with pairwise post-hoc tests again revealing that most of that change occurring as a reduction in symptom burden between baseline and mid-point of the study (M = 2.9, Median = 1.0, Min = 0, Max = 15, p = 0.010). That reduction remained statistically significant at the end of the study (M = 1.9, Median = 1.3, Min = 0, Max = 7, p = 0.009 from baseline). The difference between the overall symptom burden scores reported at mid-point and end-of study was not statistically significant (p > than adjusted alpha of 0.017).
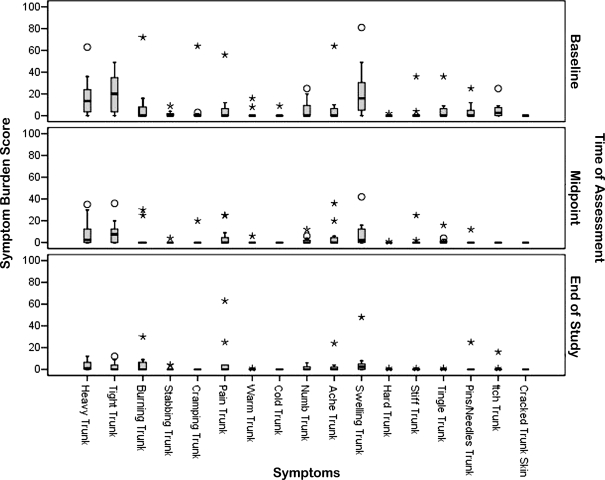
Analysis of the 17 individual truncal symptoms revealed that the overall symptom burden score improvement was due to a reduction in the burden of a subset of symptoms which were severe at baseline. The individual truncal symptom burden scores at each time of assessment are shown in Figure 3. Statistically significant reductions in perceptions of truncal heaviness (Friedman: Χ2(df=2) = 15.07, p = 0.001), swelling (Friedman: Χ2(df=2) = 14.37, p = 0.001), and tightness (Friedman: Χ2(df=2) = 12.63, p = 0.002) were reported, as well as changes in itchiness (Friedman: Χ2(df=2) = 12.00, p = 0.002). Post-hoc pairwise analysis of differences between each of the times of assessment in the study revealed that there were statistically significant reductions in each of these symptoms from baseline to the mid-point (p < 0.017). Those reductions appeared to be stable so that the level of burden for perceived heaviness, swelling, and tightness remained statistically significantly less at the end of the study than they were at baseline (p < 0.017). None of the comparisons between the assessments at mid-point and end-of-study were statistically significant for these individual symptom scores (p > than adjusted alpha of 0.017).
FIG. 3.
Individual symptom burden scores at time of assessment.
Finally, among the nontruncal symptoms included in the LSIDS-AT, difficulty sleeping, showed statistically significant reduction in symptom burden from baseline (M = 19.7, Median = 12.5, Min = 0, Max = 56) to the mid-point of the study (M = 4.2, Median = 0.0, Min = 0, Max = 36) and remained at the end of the study (M = 5.7, Median = 0.0, Min = 0, Max = 36) (both, p = 0.008). Again, any difference between the values for difficulty sleeping at mid-point and at end-of-study were not statistically significant (p > than adjusted alpha of 0.017).
Function
This was a high functioning sample of breast cancer survivors with lymphedema. Baseline assessment identified very few functional problems (M = 22, median = 21, out of a possible 60, Min = 14, Max = 34). No statistically significant change in function was demonstrated (Friedman: Χ2(df=2) = 1.22, p = 0.543).
Signs
Skin
Analysis of the number of skin conditions (maximum possible = 18) at each nursing assessment revealed a statistically significant difference in those values (Friedman: Χ2(df=3) = 9.00, p = 0.029). There was a general trend of increasing reports of skin conditions throughout the course of the study. Post-hoc pairwise tests of differences between each time of assessment revealed that number reported at the end of the study (M = 3.3, Median = 3.0, Min = 2, Max = 5) was statistically significantly higher than that number reported at baseline (M = 2.5, Median = 2.5, Min = 0, Max = 5, p = 0.007). None of the other pairwise comparisons were statistically significant (adjusted alpha >0.008).
Truncal circumferential changes
Overall summary, as well as specific truncal area changes in circumferential measures from baseline to end-of-study, were not statistically significant; however, given patients' self-reports of symptom improvement some possible clinically significant changes were noted. For the group as a whole, average summary change was −2.51 cm (SD 5.77), with a maximum decrease of −10.97 cm and a maximum increase of 4.75 cm. Table 2 shows truncal circumferential changes scores for each of the five locations measured for each participant.
Table 2.
Truncal Circumferential Changes in Centimeters (Change Scores)
| Participant | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | ||||||||||||
| Axilla | 3.00 | −2.28 | 0.50 | 0.65 | −0.30 | −0.35 | −3.15 | 0.35 | −2.85 | −0.65 | 1.60 | 1.40 |
| Largest chest | −0.15 | −0.10 | −1.43 | 0.47 | −4.55 | −0.95 | −3.13 | −6.95 | 0.30 | 1.35 | 0.70 | 0.35 |
| Xyphoid | −0.80 | −0.30 | −1.38 | −0.35 | −0.15 | −1.95 | −2.35 | −0.30 | −0.55 | 0.83 | 0.30 | −0.15 |
| Waist | −0.80 | 0.68 | −2.78 | −0.35 | −3.95 | −2.20 | −1.7 | −1.78 | −5.15 | −3.88 | −0.15 | 1.50 |
| Hip | −0.75 | 1.50 | 2.00 | −0.63 | −1.90 | 0.25 | −0.65 | −0.25 | 0.00 | 3.03 | 1.25 | −0.05 |
| Summary | .50 | −.50 | −3.08 | −.20 | −10.85 | 4.75 | −10.97 | −8.92 | −8.25 | .68 | 3.70 | 3.05 |
Discussion
All participants were receptive to home use of the device as a component of at-home self-care. The intervention had a high acceptance rate and no adverse events attributable to the device. The small number of phone calls to the staff suggests that the initial on-site education, coupled with a home observation/re-education visit, was an effective educational approach, supportive of Phase 2 self-care.
Symptoms with statistically significant improvement included those related to tightness and heaviness, specifically in the swollen truncal areas, and in sleep. This suggests the Flexitouch® system may be an effective treatment device to relieve or decrease symptom burden. These findings over a short treatment period are compelling and warrant further investigation. These results also suggest that there is a need to examine more formally what degree of circumferential reduction is clinically meaningful to patients, as the degree of reduction needed for symptomatic relief may fall below the amount required to obtain true statistical significance or that believed by clinicians to be significantly relevant, particularly in Phase 2 self-care.
Although there was an improvement in symptom burden, there was no improvement in overall function. The participants in this study demonstrated a high level of baseline functioning. Therefore, it may not be reasonable to expect significant functional improvement in this already high functioning group. The baseline functional level in our study is in contrast to that reported by Karki8 where axillary edema was reported to significantly limit activities related to arm movement post-breast cancer treatment. Despite the high functional level, our participants still experienced significant symptom relief. This suggests that symptom burden may be a more clinically meaningful targeted outcome than function in some populations with lymphedema. Alternatively, perhaps the FASQ may not address the type of functionality impacted by breast cancer subjects with truncal lymphedema.
This study demonstrated that most participants experienced some circumferential reduction in their most swollen areas. Thus, the Flexitouch® system was clearly able to move fluid from this region of the body. These findings, when considered in light of a recent case study that reported a range of 1–4 cm reduction after 2 months of home therapy with the Flexitouch® system in five of five cases, suggest this may be an efficacious way to manage truncal lymphedema and that further study in an adequately powered trial is warranted.19
Significant symptomatic relief in the truncal area was obtained after five treatments and maintained throughout the duration of therapy. While statistical significance was not achieved in reduction of truncal girth, some degree of a clinically meaningful reduction in swelling was achieved as reflected by the improvement of self-reported symptoms. This was a nonrandomized trial without a control group. Thus, improvement in self-reported symptoms could reflect a placebo effect. Future studies with a larger cohort of patients, should be designed to control for placebo effect. Additionally, findings from this study must be considered in light of the small sample size. To elaborate on these findings, it would be appropriate for future research to ensure inclusion of a study population stratified by baseline functional impairment to discern if the treatment impact varies in a study population that is not as high functioning as the one studied here. In addition, a larger, longer term study of lymphedema self-care incorporating the Flexitouch® system for breast cancer survivors with arm and/or truncal lymphedema is indicated.
Conclusions
Breast cancer survivors with treatment associated truncal lymphedema may benefit from using an advanced PCD with truncal treatment as part of their self-care program. These preliminary findings suggest the Flexitouch® system as a component of self-care may improve patient outcomes and warrants further exploration in a larger study.
Acknowledgments
The authors wish to thank Joey O'Dell for her assistance with the manuscript and the breast cancer survivors who participated in this study.
Author Disclosure Statement
All authors acknowledge that the study funding was provided by Tactile Systems Technology, Incorporated, 1331 Tyler Street NE, Suite 200, Minneapolis, MN 55413.
References
- 1.Erickson VS. Pearson ML. Ganz PA. Adams J. Kahn KL. Arm edema in breast cancer patients. J Natl Cancer Inst. 2001;93:96–111. doi: 10.1093/jnci/93.2.96. [DOI] [PubMed] [Google Scholar]
- 2.Eversley R. Estrin D. Dibble S. Wardlaw L. Pedrosa M. Favila–Penney W. Post-treatment symptoms among ethnic minority breast cancer survivors. Oncol Nurs Forum. 2005;32:250–256. doi: 10.1188/05.ONF.250-256. [DOI] [PubMed] [Google Scholar]
- 3.Back M. Guerrieri M. Wratten C. Steigler A. Impact of radiation therapy on acute toxicity in breast convervation therapy for early breast cancer. Clin Oncol. 2004;16:12–16. doi: 10.1016/j.clon.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Ronka RH. Pamilo MS. von Smitten KA. Leidenius MH. Breast lymphedema after breast conserving treatment. Acta Oncol. 2004;43:551–557. doi: 10.1080/02841860410014867. [DOI] [PubMed] [Google Scholar]
- 5.Bosompra K. Ashikaga T. O'Brien PJ. Nelson L. Skelly J. Swelling, numbness, pain, and their relationship to arm function among breast cancer survivors: A disablement process model perspective. Breast J. 2002;8:338–348. doi: 10.1046/j.1524-4741.2002.08603.x. [DOI] [PubMed] [Google Scholar]
- 6.American Cancer Society. Lymphedema: Understanding and managing lymphedema after cancer treatment: From the experts at the American Cancer Society. Atlanta: American Cancer Society; 2006. [Google Scholar]
- 7.Williams A. Moffatt C. Franks P. A phenomenological study of the lived experiences of people with lymphoedema. Intl J Pall Nurs. 2004;10:279–286. doi: 10.12968/ijpn.2004.10.6.13270. [DOI] [PubMed] [Google Scholar]
- 8.Karki A. Simonen R. Malkia E. Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med. 2005;37:180–188. doi: 10.1080/16501970410024181. [DOI] [PubMed] [Google Scholar]
- 9.Petrek JA. Pressman PI. Smith RA. Lymphedema: Current issues in research and management. CA Cancer J Clin. 2000;50:292–307. doi: 10.3322/canjclin.50.5.292. [DOI] [PubMed] [Google Scholar]
- 10.Boris M. Weindorf S. Lasinski B. The risk of genital edema after external pump compression for lower limb lymphedema. Lymphology. 1998;31:15–20. [PubMed] [Google Scholar]
- 11.Cheville AL. McGarvey CL. Petrek JA. Russo SA. Taylor ME. Thiadens SRJ. Lymphedema management. Semin Radiat Oncol. 2003;13:290–301. doi: 10.1016/S1053-4296(03)00035-3. [DOI] [PubMed] [Google Scholar]
- 12.Cannon S. Pneumatic compression devices for in-home management of lymphedema: Two case reports. Cases J. 2009;23:6625. doi: 10.1186/1757-1626-2-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayrovitz HN. Brown–Cross D. Mayrovitz BL. Golla AH. Lymphedema: Role of truncal clearance as a therapy component. Home Health Care Manag Pract. 2009;21:325–337. [Google Scholar]
- 14.Rockson SG. Lymphedema. Am J Med. 2001;110:288–295. doi: 10.1016/s0002-9343(00)00727-0. [DOI] [PubMed] [Google Scholar]
- 15.Wilburn O. Wilburn P. Rockson S. A pilot, prospective evaluation of a novel alternative for maintenance therapy of breast cancer-associated. BMC Cancer. 2006;6:84–94. doi: 10.1186/1471-2407-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook T. Campbell D. Peracchio L. Quasi-experimentation. Rand McNally; Chicago: 1979. [Google Scholar]
- 17.Ridner SH. Dietrich MS. Development of the lymphedema symptoms intensity and distress survey arm. J Clin Oncol (Meeting Abstracts) 2010;28:9125. [Google Scholar]
- 18.Millard R. The functional assessment screening questionnaire: Application for evaluating pain-related disability. Arch Phys Med Rehabil. 1989;70:303–307. [PubMed] [Google Scholar]
- 19.Hammond Tina M. Mayrovitz Harvey N. Programmable intermittent pneumatic compression as a component of therapy for breast cancer treatment-related truncal and arm lymphedema. Home Health Care Management Practice. 2010;22:397–402. [Google Scholar]