Abstract
Mucin 1 (MUC1), a tumor-associated antigen, is a transmembrane glycoprotein expressed by normal epithelial cells and overexpressed by carcinomas of epithelial origin. Autoantibodies against MUC1 are often found in circulation, either free or bound to immune complexes, which might contribute to limit tumor outgrowth and dissemination by antibody-dependent cell-mediated cytotoxicity, and were found favorably predictive of survival in early breast cancer patients. There is no commercial enzyme-linked immunosorbent assay (ELISA) kit for detecting the anti-MUC1 antibodies in human serum thus far. To detect circulating anti-MUC1 antibodies, we established an indirect ELISA (I-ELISA) using a recombinant MUC1 protein containing six tandem repeat sequences of MUC1 after the antigenicity and specificity of the protein were confirmed. The I-ELISA had a sensitivity of 91.3% and a specificity of 94.1% when a competitive I-ELISA was used as a reference test. The results showed that more patients with benign breast tumors (P = 0.001) and breast cancer patients before primary treatment (P = 0.010) were found to have anti-MUC1 IgG than healthy women; anti-MUC1 IgG before primary treatment was found more than after primary treatment (P = 0.016) in breast cancer patients. Interestingly, the anti-MUC1 IgG serum level was reversely correlated to that of CA15-3 antigen in advanced-stage patients (r = −0.4294, P = 0.046). Our study has demonstrated the suitability of the established I-ELISA for detecting circulating anti-MUC1 antibodies in human serum. Furthermore, we found that circulating anti-MUC1 antibodies may still bind MUC1 shed into blood in stage IV breast cancer, which can support the use of MUC1-target immune therapy strategies.
Mucin 1 (MUC1), also called cancer antigen 15-3 (CA15-3) or polymorphic epithelial mucin, is a transmembrane glycoprotein with variable number tandem repeats (VNTR) of a 20-amino-acid motif as its large extracellular fragment. The repeat units contain potential O glycosylation sites represented by serine and threonine residues, which act as a scaffold for the attachment of O-glycans, resulting in the formation of a highly glycosylated extended repetitive structure (22). CA15-3 is defined as the glycoprotein that binds with two monoclonal antibodies (MAbs): DF3 and 115D8. The DF3 antibody recognizes the VNTR of MUC1 (sequence DTRPAPGS), which corresponds to amino acids Asp-Thr-Arg-Pro-Ala-Pro-Gly-Ser. The 115D8 MAb is the solid-phase capture antibody, which binds to a peptide-carbohydrate epitope on the same repeat (11). As a tumor-associated antigen, MUC1 is overexpressed on various carcinomas of epithelial origin, including breast cancer, pancreatic cancer, ovarian cancer, and multiple myeloma, etc. Because of its deficient glycosylation with exposed VNTR in cancer cells, MUC1 can behave as a self-antigen to stimulate an immune response, which provides evidence for vaccine immunotherapy of targeting MUC1 (6, 19, 29).
Free and compound autoantibodies against MUC1 can be detected both in patients with malignant tumors and in healthy people (2, 17, 24). Studies have demonstrated that circulating anti-MUC1 antibodies may be used as a favorable prognostic factor for patients with early breast cancer and pancreatic cancer (7, 25). In addition, previous studies have shown that the antibodies might contribute to limit tumor outgrowth and dissemination by antibody-dependent cellular cytotoxicity (1, 8, 28). It is believed that free anti-MUC1 antibodies can bind MUC1 and form MUC1 circulating immune complexes (MUC1-CIC) in blood circulation (3); however, patients with stage IV of breast cancer present low MUC1-CIC, although more common anti-MUC1 antibodies and MUC1 exist in their sera (4, 26). A contradictory result indicated that anti-MUC1 antibodies in stage IV of breast cancer could not bind or neutralize MUC1 antigen, and they were of low affinity (4).
Thus far, there is no commercial enzyme-linked immunosorbent assay (ELISA) kit for detecting the anti-MUC1 antibodies in human serum. Mostly, synthetic MUC1 VNTR peptides were used as coating antigens in ELISA for detecting anti-MUC1 antibodies in human sera (13, 27). Alternatively, recombinant MUC1 VNTR containing peptide was also used as antigen for detecting circulating anti-MUC1 antibodies by Western blotting (9). Although the recombinant MUC1 VNTR containing peptide expressed in Escherichia coli cannot be glycosylated as in eukaryotic cells, it has been demonstrated to be efficient in detecting anti-MUC1 antibody because MUC1 is less or not glycosylated when expressed in tumor cells.
In the present study, we constructed a recombinant MUC1 protein, 8R-MUCPT, which contained six MUC1 VNTRs. After the antigenicity and specificity of the 8R-MUCPT were verified, we established an indirect ELISA (I-ELISA) using 8R-MUCPT as a coating antigen to detect anti-MUC1 antibodies in the sera of patients with benign breast tumors and breast cancer. The results have demonstrated the potential of this recombinant MUC1 protein as detecting antigen and the suitability of the established I-ELISA for detecting circulating anti-MUC1 antibodies. In addition, the results suggested that anti-MUC1 antibodies in serum may play a role in neutralizing MUC1 VNTR core peptides and forming MUC1-CIC. By analyzing the relationship between circulating MUC1 and anti-MUC1 antibodies in advanced-stage patients, we were able to deduce the same neutralizing role for the antibodies in stage IV breast cancer.
MATERIALS AND METHODS
Specimens.
A total of 200 serum samples were obtained from 56 healthy women (median age, 58.5 years; range, 25 to 80 years), 22 patients with benign breast tumors (median age, 55 years; range, 22 to 70 years), and 122 breast cancer patients (median age, 57 years; range, 29 to 77 years) before or after primary treatment including surgery, postoperative radiotherapy, and/or chemotherapy in the Second Hospital of Jilin University and Tumor Hospital of Jilin Province, Changchun, China. Serum samples were collected and stored at −70°C until analyzed. Eleven cases of CA15-3 antigen-positive sera and eleven cases of CA15-3 antigen-negative sera in advanced stage were screened by using a Protein Chip kit for Multi-Tumor Marker Detection (Shanghai Health Digit Co., Ltd., Shanghai, China).
The patients with benign breast tumors included seven with fibroadenoma, three with fibroadenosis, five with nomenclature adenosis, four with hyperplasia, and three with intraductal papillary neoplasms. The breast cancer patients included 69 with infiltrating duct carcinoma, 26 with adenocarcinoma, 17 with infiltrating lobular carcinoma, 3 with intraductal carcinoma, 4 with mucinous adenocarcinoma, 2 with atypical medullary carcinoma, and 1 with medullary carcinoma. The group of healthy women included 2 pregnant and 54 nonpregnant women, 26 lactating and 30 nonlactating women, and 4 nulliparous women.
Preparation of recombinant 8R-MUCPT protein.
Human MUC1 VNTR encoding sequence containing six tandem repeats was directly obtained by PCR using pET28a-HSP65-MUC1 plasmid, which was previously constructed in our lab (14), as a template. The MUC1-encoded gene was then subcloned into prokaryotic expression vector pET26b+ plasmid, in which there was an eight-arginine encoding sequence at the N-terminal insertion end, to construct a recombinant pET26b+-8R-MUCPT plasmid (21). The insertion in the plasmid was then analyzed by DNA sequencing. In the present study, the pET26b+-8R-MUCPT plasmid was directly transformed into bacterial strain E. coli BL21(DE3). The recombinant 8R-MUCPT protein was expressed in transformed E. coli induced by IPTG (isopropyl-β-d-thiogalactopyranoside), purified by Ni-chromate affinity chromatography, and then verified by Western blotting with MAb against MUC1 VNTR (anti-MUC1 VNTR MAb; BD Pharmingen). Endotoxin in the purified protein was determined by Limulus amebocyte lysate assay.
Preparation of anti-8R-MUCPT serum and immunohistochemical analysis.
One female rabbit was immunized with 8R-MUCPT (100 μg/each) plus complete Freund adjuvant for the first two immunizations and incomplete Freund adjuvant for the third immunization on days 0, 14, and 28. Endotoxin in the purified protein was <0.1 EU/μg. The rabbit serum was collected after checking the antibody against 8R-MUCPT protein by indirect ELISA and stored at −70°C until use. The animal disposal was overseen by Animal Research Ethics Committees of Jilin University. Immunohistochemical analysis was performed according to standard procedures; paraffin-embedded specimens were treated with 10 mM sodium citrate buffer at 98°C for 5 min and incubated overnight at 4°C with the prepared anti-8R-MUCPT mouse serum and anti-MUC1 VNTR MAb; negative controls were incubated with saline phosphate buffer (PBS; 0.005 M NaPO4H2, 0.005 M Na2PO4H, 0.15 M NaCl) instead of the antibodies described above. Visualization was performed with diaminobenzidine, and tissues were counterstained with hematoxylin.
Dot blot and inhibition test.
8R-MUCPT protein was dotted onto nitrocellulose membranes in four different doses and blocked by 5% milk-phosphate-buffered saline (PBS) at 4°C overnight. The next day, the membranes were preincubated with either anti-MUC1 VNTR MAb at a 1:50 dilution or anti-HBsAg MAb (Bioson Corp., China) at a 1:20 dilution at room temperature for 3 h. After washing, the membranes were incubated with a positive serum of anti-MUC1 antibody derived from a MUC1-positive breast cancer patient in 1:10 dilution at 4°C overnight and then washed and incubated with peroxidase-conjugated goat anti-human IgG (Dingguo Corp., China) diluted to 1:200 in 5% milk-PBS for 1 h. After washing, 3.3′-diaminobenzidine (DAB) was added to the membranes for coloration reaction.
Blocking experiment.
Four serum samples positive for anti-MUC1 antibodies were incubated with either 8R-MUCPT or synthetic peptides of polyarginine (poly-R) or polyhistidine (poly-H) at the same molar concentration, followed by detection of their anti-MUC1 antibodies by I-ELISA with 8R-MUCPT as a coating antigen.
Competitive indirect ELISA (CI-ELISA).
Capture antibody anti-MUC1 VNTR MAb diluted to 1:1,000 in 0.05 mol/liter of carbonate buffer (pH 9.6) was coated onto a 96-well plat-bottom plate at 4°C overnight. The plate was blocked by using 5% milk-PBS at 37°C for 2 h. After washing, the plate was incubated with 8R-MUCPT (30 μg/ml) at 42°C for 0.5 h and then washed. As a competitive antibody, anti-MUC1 VNTR MAb was added into triplicate wells of the plate at dilutions of 1:150, 1:300, or 1:450 (1, 1.5, or 3 μg/ml, respectively) in PBS, followed by incubation at 42°C for 0.5 h. After a washing step, serum samples were added at a dilution of 1:2 with 10% milk-PBS and cultured at 42°C for 0.5 h, followed by incubation with a secondary antibody, peroxidase-conjugated goat anti-human IgG, at dilution of 1:400 in 5% milk-PBS at 42°C for 0.5 h. o-Phenylenediamine dihydrochloride (OPD) was added to stop the reaction. A curve chart of the relationship between the optical density (OD) and the dilution of the blocking antibody was made by scientific graphics processing of Microsoft Excel 2003 data for each sample. The positive or negative results were confirmed in at least two repeated experiments.
I-ELISA.
8R-MUCPT protein (5 μg/ml) in 0.05 mol/liter of carbonate buffer (pH 9.6) was coated onto 96-well plat-bottom plates at 4°C for 17 h. The plates were washed with PBS for three times, blocked with 5% milk-PBS at 37°C for 2 h, and then incubated with sera from patients at a 1:40 dilution with 10% milk-PBS at 37°C for 2 h. After being washed with 0.05% Tween 20-PBS three times, the plates were incubated with peroxidase-conjugated goat anti-human IgG or IgM at a 1:1,000 dilution with 5% milk-PBS at 37°C for 1.25 h. The stop reaction was performed by adding OPD after three washes with 0.05% Tween 20-PBS. The OD was determined at A492 in an ELISA autoreader (Labsystems, Helsinki, Finland). All samples were measured in duplicate. Cutoff values were defined as the mean OD value plus three standard deviations (SD) for anti-MUC1 IgG and the mean OD value plus two SD for anti-MUC1 IgM. Determination of the mean OD value and the SD were based on the serum anti-MUC1 antibodies levels from 36 healthy women whose OD values were all less than the mean OD value from the serum samples of 56 healthy women.
Statistical analysis.
Statistical analysis was performed using SPSS software (version 10.0; SPSS, Inc.) and data analysis software (Microsoft Excel 2003). Comparisons among percentages of different groups were tested by using the chi-square test, and differences among groups in clinical stages were analyzed by using a Kruskal-Wallis Test. The correlation between two experimental groups was evaluated by linear regression analysis. For all of these tests, a P value of <0.05 as determined in a two-tailed test was considered significant.
RESULTS
Establishment of an indirect ELISA with 8R-MUCPT for detecting circulating anti-MUC1 antibodies in human serum.
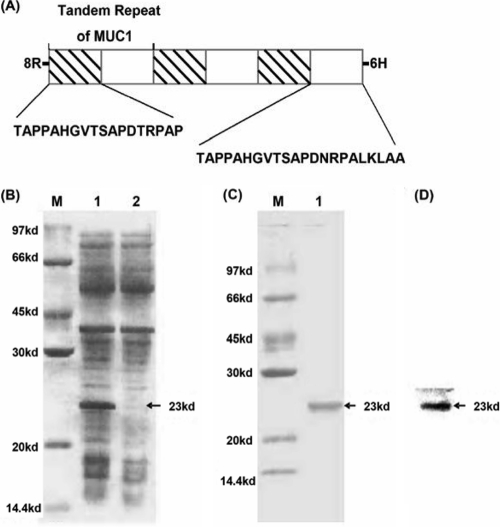
To develop an ELISA for detecting circulating anti-MUC1 antibodies, we first constructed 8R-MUCPT, which included six MUC1 VNTRs (Fig. 1A). The expressed 8R-MUCPT was analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 1B and C), and the purified 8R-MUCPT was identified by Western blotting (Fig. 1D). The results showed that 8R-MUCPT was highly expressed in E. coli and able to be specifically recognized by anti-MUC1 VNTR MAb.
FIG. 1.
Construction, expression, and identification of recombinant 8R-MUCPT. (A) Structure of 8R-MUCPT protein. (B) Analysis of recombinant 8R-MUCPT protein expressed in E. coli on an SDS-10% PAGE gel. Lane M, marker; lane 1, lysate of E. coli induced by IPTG; lane 2, lysate of E. coli without induction. (C) Analysis of purified 8R-MUCPT protein on an SDS-10% PAGE gel. Lane M, marker; lane 1, purified 8R-MUCPT protein. (D) Identification of purified 8R-MUCPT protein by Western blotting with anti-MUC1 VNTR MAb. The arrows indicate 8R-MUCPT protein.
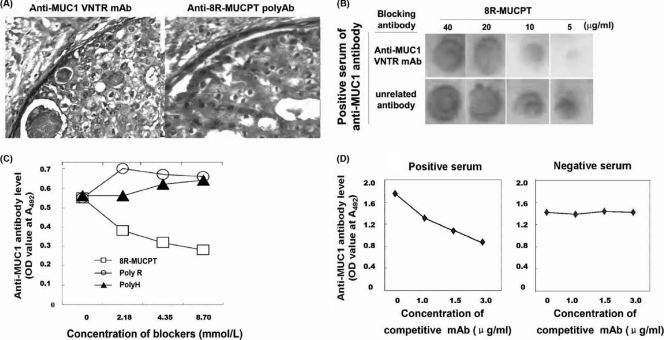
To confirm the antigenicity and specificity of 8R-MUCPT for measuring circulating anti-MUC1 antibodies, three tests were performed. Rabbit anti-8R-MUCPT serum was used as anti-MUC1 polyclonal antibody (PAb; anti-MUC1 PAb) for immunohistochemical staining of breast cancer tissue. The anti-MUC1 PAb could recognize MUC1 expressed on breast cancer tissue, as well as anti-MUC1 MAb (Fig. 2A), which revealed that 8R-MUCPT possesses high antigenic capacity and natural MUC1-mimic antigenicity. The dot blot and inhibition test showed that 8R-MUCPT could be recognized by the sera of a natural anti-MUC1 antibody that could be inhibited by anti-MUC1 VNTR MAb when its amount was high enough to neutralize 8R-MUCPT on the dot, such as 5 μg/ml (Fig. 2B), which demonstrates that natural anti-MUC1 antibody could recognize an epitope on 8R-MUCPT very similar to that of anti-MUC1 VNTR MAb. A blocking experiment was set up which showed that 8R-MUCPT could efficiently neutralize natural anti-MUC1 antibody in the sera. but poly-R or poly-H could not (Fig. 2C), and fused partners of poly-R or poly-H in 8R-MUCPT did not dramatically affect 8R-MUCPT as a detecting antigen. The result suggests that 8R-MUCPT possesses high specificity.
FIG. 2.
Analysis of the antigenicity and specificity of 8R-MUCPT as detecting antigen. (A) Immunohistochemical staining of breast cancer sections by anti-MUC1 VNTR MAb (left) or anti-8R-MUCPT serum (right). (B) Dot blot and inhibition test. 8R-MUCPT was spotted onto a nitrocellulose filter in four dosages. The serum reactivity to 8R-MUCPT could be abolished by preincubation with anti-MUC1VNTR MAb but not by preincubation with anti-HBSAg MAb. (C) Blocking experiment. The sera of four patients with breast cancer were mixed with 8R-MUCPT, poly-R, or poly-H in equal molar rations, and then their anti-MUC1 antibody levels were detected by the I-ELISA. The serum reactivity to 8R-MUCPT in an I-ELISA could be blocked by being mixed with 8R-MUCPT, but poly-R or poly-H could not block this reactivity. (D) CI-ELISA. 8R-MUCPT was captured by anti-MUC1 VNTR MAb as a coating antigen. The serum samples were directly added into the wells or mixed with three different concentrations of anti-MUC1 VNTR MAb (as a competitive antibody), respectively, before addition to the well. Each point represents the average OD of triplet wells. There was growing inhibition with increasing concentrations of competitive antibody, and the inhibition rate induced by the competitive antibody at 3 μg/ml was at least 30% for the positive serum. There was no inhibition for the negative serum.
For verifying whether the established I-ELISA could be used to detect anti-MUC1 antibodies in the sera of patients with breast tumors, 40 serum samples were examined. Detection was performed by I-ELISA using 8R-MUCPT as a coating antigen, and a competitive I-ELISA with commercial anti-MUC1 VNTR MAb as a reference test for the CI-ELISA (Fig. 2D) could be used as a qualitative method for detecting MUC1. In the CI-ELISA, anti-MUC1 VNTR MAb at different dilutions was used to bind 8R-MUCPT first for competing with natural anti-MUC1 antibody in human sera. The anti-MUC1 antibody level detected by I-ELISA and CI-ELISA was consistent, except for one sample that was determined to be positive in the I-ELISA but negative in the CI-ELISA and two samples that were determined to be negative in the I-ELISA but positive in the CI-ELISA. Accordingly, I-ELISA had a calculated sensitivity of 91.3% and a specificity of 94.1%.
Detection of circulating antibodies against MUC1 VNTR in the sera of the patients with breast tumors and health women by the I-ELISA.
The established I-ELISA method was used to detect anti-MUC1 IgG and IgM in the sera of patients with breast tumors or healthy women. The results are presented in Table 1. Anti-MUC1 IgG was detected in 32.8% of the breast cancer patients, while IgM was detected in 15.8% of the breast cancer patients; a statistically significant correlation was found between the two antibodies (r = 0.5943). IgG antibodies to MUC1 were found in 23.2% of the healthy women, 63.6% of the patients with benign breast tumors (P = 0.001 versus healthy women), and 50.0% of the patients with breast cancer before primary treatment (P = 0.010 versus healthy women). Comparatively, the positive rate of anti-MUC1 IgG antibodies in breast cancer patients before primary treatment was significantly was higher than that (26.7%) in breast cancer patients after primary treatment (P = 0.016). It was also noted that there were no significant differences in anti-MUC1 IgM levels among healthy women, benign breast tumor patients, and breast cancer patients. The levels of circulating anti-MUC1 antibodies were also similar in breast cancer patients in different stages, or with or without lymph node metastasis.
TABLE 1.
Detection of circulating anti-MUC1 antibodies in healthy women and in patients with benign and malignant breast tumors
| Subject group | No. of positive sera/total no. of sera tested (%)a |
|
|---|---|---|
| IgG | IgM | |
| Healthy women | 13/56 (23.2) | 12/47 (25.5) |
| Women with benign breast tumors | 14/22 (63.6)* | 3/20 (15.0) |
| Women with breast cancer | 40/122 (32.8) | 18/114 (15.8) |
| Before primary treatment | 16/32 (50.0)# | 8/32 (25.0) |
| After primary treatment | 24/90 (26.7)† | 10/82 (12.2) |
| Clinical stageb | ||
| I | 2/9 (22.2) | 0/9 (0) |
| II | 14/51 (27.5) | 5/49 (10.2) |
| III | 1/7 (14.3) | 1/6 (16.7) |
| IV | 5/17 (29.4) | 4/16 (25.0) |
| Lymph nodec | ||
| + | 11/31 (35.5) | 3/28 (10.7) |
| − | 6/35 (17.1) | 2/32 (6.3) |
*, P = 0.001 (compared to healthy women); #, P = 0.010 (compared to healthy women); †, P = 0.016 (compared to the breast cancer patients prior to primary treatment).
According to AUCC1988 and only referring to patients after primary treatment.
Only referring to patients after primary treatment and clinical stages I and II.
Relationship between circulating MUC1 antigen and anti-MUC1 IgG in patients with advanced carcinomas.
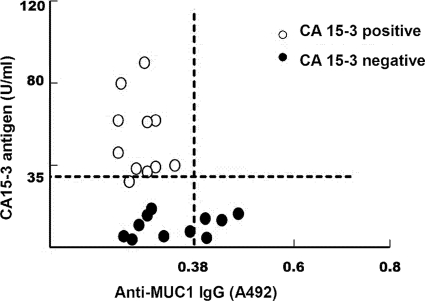
To analyze the relationship between serum MUC1 antigen and anti-MUC1 antibodies in patients with advanced-stage cancer, we selected 11 cases of CA15-3 antigen-positive sera and 11 cases of CA15-3 antigen-negative sera that were screened out by using a Protein Chip kit for Multi-Tumor Marker Detection. By the established I-ELISA, we evaluated the circulating anti-MUC1 antibody levels of the selected 22 serum samples. Circulating anti-MUC1 antibodies showed a negative correlation with serum CA15-3 expression, and it was statistically significant only between the serum level of CA15-3 antigen and the serum level of anti-MUC1 IgG (r = −0.4294, P = 0.046). As shown in Fig. 3, the serum samples were divided into three groups. In one group, positive anti-MUC1 IgG was detected together with negative CA15-3 antigen. In the second group, negative anti-MUC1 IgG was shown with negative CA15-3 antigen. In the third group, both negative anti-MUC1 IgG and positive CA15-3 antigen were found. These results suggest that circulating levels of anti-MUC1 antibodies are negatively correlated with the level of MUC1 antigen in the advanced-stage patients.
FIG. 3.
Relationship between circulating CA15-3 antigen and anti-MUC1 IgG in patients with the advanced stage. The levels of anti-MUC1 IgG in CA15-3 antigen-positive and -negative sera from the advanced-stage patients were measured by I-ELISA using 8R-MUCPT as a coating antigen. The horizontal line represents the cutoff value of CA15-3 antigen. The vertical line represents the cutoff value of anti-MUC1 IgG (r = 0.4294, P = 0.046 [serum CA15-3 antigen versus anti-MUC1 IgG]).
DISCUSSION
In this study, we analyzed circulating anti-MUC1 antibodies in patients with benign or malignant breast tumors by using an I-ELISA with E. coli-expressed recombinant MUC1 VNTR-containing peptide. To establish a credible ELISA method for detecting serum antibodies against MUC1 VNTR, we expressed recombinant MUC1 VNTR-containing peptide consisting of six tandem repeats of MUC1 VNTR (8R-MUCPT) in E. coli. The 8R-MUCPT, with no glycosylation, is be able to mimic the naturally expressed MUC1 in tumor cells. Compared to synthetic peptides, 8R-MUCPT contains more epitopes and is more efficient for coating the plates. In addition, 8R-MUCPT possesses antigenicity of naturally expressed MUC1 and high specificity, having the crucial property as a coating antigen of detecting the anti-MUC1 antibodies in clinical samples.
With high specificity, the CI-ELISA has been used to qualify anti-MUC1 antibody and to determine the sensitivity and/or specificity of the established I-ELISA. A capture-antibody anti-MUC1 VNTR MAb was used for firm adhesion in the experiment, and its validity has been verified by the inhibition results. The comparison results indicated that the detection capacities of the two ELISAs were nearly equivalent. The differences in epitopes that we observed were due to antigen captured by an antibody versus antigen bound to a plate when comparing the CI-ELISA to the indirect ELISA, but the difference is less because the anti-MUC1 MAb only binds one tandem repeat in the recombinant MUC1 protein with six tandem repeats. In addition, the serum samples were diluted 1:40 for the I-ELISA and diluted 1:2 for the CI-ELISA, which suggests that the I-ELISA developed here is more sensitive.
In agreement with the findings of Croce et al. (4), the anti-MUC1 IgG and IgM serum levels of breast cancer patients in our study were 32.8 and 15.8% positive, respectively, whereas in the study of Croce et al. the levels were elevated in 32 and 14%, respectively, although different ELISA method cutoff values might have led to differences in the positive rates. In addition, we also found a positive correlation between the two antibodies.
Consistent with the results of von Mensdorff-Pouilly et al. (25), we found that positive rates of circulating anti-MUC1 IgG in patients with benign breast tumors and breast cancer before primary treatment were higher than those in healthy women. After statistical analysis, we found that our data demonstrated statistical significance, whereas the earlier findings (25) did not, possibly due to the relatively few samples and sample sources. However, similar results were reported in colorectal cancer patients; Nakamura et al. (16) reported that the positive rate of anti-MUC1 IgG showed a significant increase in colorectal cancer and a higher frequency in benign disease than in healthy subjects. According to Croce et al. (4), frequent detection of anti-MUC1 IgG may be related to an early stimulation, such as pregnancy and lactation, in which a second challenge may induce immunoglobulin switching. That is, the second challenge or the presence of tumor may lead to an increase in anti-MUC1 IgG. Furthermore, we infer that the circulating level of the natural anti-MUC1 antibody may correlate with the frequency and the amount of exposed MUC1 core peptide.
Our study showed that primary treatment was correlated with decreasing positive rates of circulating anti-MUC1 antibodies in breast cancer patients, although similar findings were also reported in other cancer patients (10). Compared to the level before primary treatment, the circulating anti-MUC1 IgG level in breast cancer patients after primary treatment was low (P = 0.016). Moreover, for anti-MUC1 IgM, the circulating level was lower after primary treatment than before primary treatment, although it was not statistically significant. We postulate a possible reason for this: in the course of primary treatment, including surgery, chemotherapy, or radiotherapy, membrane-bound MUC1 antigens following cell necrosis are shed into the blood to neutralize circulating anti-MUC1 antibodies (24), leading to the reduced levels of circulating anti-MUC1 antibodies. In addition, a longer course of disease and latent micrometastasis after primary treatment may also cause the increase of MUC1 antigen, and thus the numbers of circulating anti-MUC1 antibodies are reduced for greater neutralization (5, 12, 23).
According to the results of other groups before primary treatment (4, 25), we also found slightly elevated serum levels of anti-MUC1 IgG in patients with stage II compared to those detected in stage I patients, and slightly decreased serum levels in stage III patients, whereas there were again elevated levels in patients with stage IV breast cancer after primary treatment. As for circulating anti-MUC1 IgM, stage II, III, and IV patients had slightly increased levels compared to stage I patients, an observation also noted in an earlier study (4). Therefore, there are many similarities in the changes of circulating anti-MUC1 antibodies at different stages of breast cancer before and after primary treatment, which implies these changes are related to the stages of the disease but not to the primary treatment. We presume that the slightly elevated serum levels of anti-MUC1 antibodies in patients with stage II may be caused by more exposed circulating MUC1 as direct immunogen, and the slightly decreased levels of anti-MUC1 IgG in stage III may be due to more neutralization from MUC1 antigen.
A negative correlation between circulating anti-MUC1 antibodies and MUC1 antigen has been found in patients with some malignant tumors (4, 20, 23). In the present study, we found a significantly negative correlation between the CA15-3 (MUC1 antigen) serum level and that of the anti-MUC1 IgG antibody in patients with an advanced stage of breast cancer.
Stage IV breast cancer patients presented more circulating MUC1 and anti-MUC1 antibodies, but the MUC1-CIC was low, which seemed to indicate that anti-MUC1 antibodies were of low affinity in stage IV patients (4, 26). However, in the present study, we found that circulating MUC1 and anti-MUC1 antibodies in stage IV breast cancer patients showed a significantly negative correlation. That meant high levels of circulating MUC1 should correspond to a low level of anti-MUC1 antibody or that a low level of circulating MUC1 should correspond to a high level of anti-MUC1 antibody in an individual patient. The simultaneous increase in serum MUC1 and anti-MUC1 antibodies should thus be a characteristic in later-stage breast cancers. Accordingly, we deduced that anti-MUC1 may still have the ability to bind MUC1 in stage IV breast cancer patients. This deduction may be in agreement with a recent report in which the overexpression of MUC1 antigen was associated with the absence of both regional recurrence and distance metastasis (18).
In summary, we examined serum samples from patients with benign and malignant breast tumors using the established I-ELISA. Our findings indicate that anti-MUC1 antibodies in serum play a role in binding MUC1 VNTR in breast cancer, including in stage IV.
Acknowledgments
Ethical support was provided by Litian Sun of Jilin University.
All authors read and approved the final manuscript. The authors report no conflicts of interest.
Footnotes
Published ahead of print on 28 September 2010.
REFERENCES
- 1.Braun, D. P., K. A. Crist, F. Shaheen, E. D. Staren, S. Andrews, and J. Parker. 2005. Aromatase inhibitors increase the sensitivity of human tumor cells to monocyte-mediated, antibody-dependent cellular cytotoxicity. Am. J. Surg. 190:570-571. [DOI] [PubMed] [Google Scholar]
- 2.Croce, M. V., M. Isla-Larrain, A. Capafons, M. R. Price, and A. Segal-Eiras. 2001. Humoral immune response induced by the protein core of MUC1 mucin in pregnant and healthy women. Breast Cancer Res. Tr. 69:1-11. [DOI] [PubMed] [Google Scholar]
- 3.Croce, M. V., M. T. Isla-Larrain, M. R. Price, and A. Segal-Eiras. 2001. Detection of circulating mammary mucin (Muc1) and MUC1 immune complexes (Muc1-CIC) in healthy women. Int. J. Biol. Markers 16:112-120. [DOI] [PubMed] [Google Scholar]
- 4.Croce, M. V., M. T. Isla-Larrain, S. O. Demichelis, J. R. Gori, M. R. Price, and A. Segal-Eiras. 2003. Tissue and serum MUC1 mucin detection in breast cancer patients. Breast Cancer Res. Treat. 81:195-207. [DOI] [PubMed] [Google Scholar]
- 5.Ebeling, F. G., P. Stieber, M. Untch, D. Nagel, G. E. Konecny, U. M. Schmitt, A. Fateh-Moghadam, and D. Seidel. 2002. Serum CEA and CA 15-3 as prognostic factors in primary breast cancer. Br. J. Cancer 86:1217-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulley, J. L., P. M. Arlen, K. Y. Tsang, J. Yokokawa, C. Palena, D. J. Poole, C. Remondo, V. Cereda, J. L. Jones, M. P. Pazdur, J. P. Higgins, J. W. Hodge, S. M. Steinberg, H. Kotz, W. L. Dahut, and J. Schlom. 2008. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin. Cancer Res. 14:3060-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamanaka, Y., Y. Suehiro, M. Fukui, K. Shikichi, K. Imai, and Y. Hinoda. 2003. Circulating anti-MUC1 IgG antibodies as a favorable prognostic factor for pancreatic cancer. Int. J. Cancer 103:97-100. [DOI] [PubMed] [Google Scholar]
- 8.Heuser, C., M. Ganser, A. Hombach, H. Brand, G. Denton, F. G. Hanisch, and H. Abken. 2003. An anti-MUC1-antibody-interleukin-2 fusion protein that activates resting NK cells to lysis of MUC1-positive tumour cells. Br. J. Cancer 89:1130-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinoda, Y., N. Nakagawa, H. Nakamura, Y. Makiguchi, F. Itoh, M. Adachi, T. Yabana, K. Imai, and A. Yachi. 1993. Detection of a circulating antibody against a peptide epitope on a mucin core protein, MUC1, in ulcerative colitis. Immunol. Lett. 35:163-168. [DOI] [PubMed] [Google Scholar]
- 10.Hirasawa, Y., N. Kohno, A. Yokoyama, K. Kondo, K. Hiwada, and M. Miyake. 2000. Natural autoantibody to MUC1 is a prognostic indicator for non-small cell lung cancer. Am. J. Respir. Crit. Care. Med. 161:589-594. [DOI] [PubMed] [Google Scholar]
- 11.Klee, G. G., and W. E. Schreiber. 2004. MUC1 gene-derived glycoprotein assays for monitoring breast cancer (CA 15-3, CA 27.29, BR): are they measuring the same antigen? Arch. Pathol. Lab. Med. 128:1131-1135. [DOI] [PubMed] [Google Scholar]
- 12.Kokko, R., K. Holli, and M. Hakama. 2002. CA 15-3 in the follow-up of localized breast cancer: a prospective study. Eur. J. Cancer 38:1189-1193. [DOI] [PubMed] [Google Scholar]
- 13.Kotera, Y., J. Darrell Fontenot, G. Pecher, R. S. Metzgar, and O. J. Finn. 1994. Humoral immunity against a tandem repeat epitope of human mucin MUC1 in sera from breast pancreatic and colon cancer patients. Cancer Res. 54:2856-2860. [PubMed] [Google Scholar]
- 14.Li, D. P., H. Li, P. Y. Zhang, X. L. Wu, H. F. Wei, L. Wang, M. Wan, P. Deng, Y. Zhang, and L. J. Wang. 2006. Heat shock fusion protein induces both specific and nonspecific anti-tumor immunity. Eur. J. Immunol. 36:1324-1336. [DOI] [PubMed] [Google Scholar]
- 15.Mann, K. 1990. Tumor markers in testicular cancer. Urologe A 29:77-86. (In German.) [PubMed] [Google Scholar]
- 16.Nakamura, H., Y. Hinoda, N. Nakagawa, Y. Makiguchi, F. Itoh, T. Endo, and K. Imai. 1998. Detection of circulating anti-MUC1 mucin core protein antibodies in patients with colorectal cancer. J. Gastroenterol. 33:354-361. [DOI] [PubMed] [Google Scholar]
- 17.Rabassa, M. E., M. V. Croce, A. Pereyra, and A. Segal-Eiras. 2006. MUC1 expression and anti-MUC1 serum immune response in head and neck squamous cell carcinoma (HNSCC): a multivariate analysis. BMC. Cancer 6:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rakha, E. A., R. W. Boyce, D. Abd El-Rehim, T. Kurien, A. R. Green, E. C. Paish, J. F. Robertson, and I. O. Ellis. 2005. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC, and MUC6) and their prognostic significance in human breast cancer. Mod. Pathol. 18:1295-1304. [DOI] [PubMed] [Google Scholar]
- 19.Ramlau, R., E. Quoix, J. Rolski, M. Pless, H. Lena, E. Lévy, M. Krzakowski, D. Hess, E. Tartour, M. P. Chenard, J. M. Limacher, N. Bizouarne, B. Acres, C. Halluard, and T. Velu. 2008. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV non-small cell lung cancer. J. Thorac. Oncol. 3:735-744. [DOI] [PubMed] [Google Scholar]
- 20.Richards, E.R., P. L. Devine, R. J. Quin, J. D. Fontenot, B. G. Ward, and M. A. McGuckin. 1998. Antibodies reactive with the protein core of MUC1 mucin are present in ovarian cancer patients and healthy women. Cancer Immunol. Immunother. 46:245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang, Y., H. Li, P. Y. Zhang, D. P. Li, Y. M. Wang, H. F. Wei, A. L. Wang, Y. L. Yu, and L. Y. Wang. 2005. Prokaryotic expression and purification of 8R-MUC1 core peptides fusion protein. J. Jilin Univ. 31:21-24. [Google Scholar]
- 22.Taylor-Papadimitriou, J., J. M. Burchell, T. Plunkett, R. Graham, I. Correa, D. Miles, and M. Smith. 2002. MUC1 and the immunobiology of cancer. J. Mamm. Gland Biol. Neoplasia 7:209-221. [DOI] [PubMed] [Google Scholar]
- 23.Treon, S. P., P. Maimonis, D. Bua, G. Young, N. Raje, J. Mollick, D. Chauhan, Y. T. Tai, T. Hideshima, Y. Shima, J. Hilgers, S. von Mensdorff-Pouilly, A. R. Belch, L. M. Pilarski, and K. C. Anderson. 2000. Elevated soluble MUC1 levels and decreased anti-MUC1 antibody levels in patients with multiple myeloma. Blood 96:3147-3153. [PubMed] [Google Scholar]
- 24.Varela, J. C., C. Atkinson, R. Woolson, T. E. Keane, and S. Tomlinson. 2008. Upregulated expression of complement inhibitory proteins on bladder cancer cells and anti-MUC1 antibody immune selection. Int. J. Cancer 123:1357-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Mensdorff-Pouilly, S., A. A. Verstraeten, P. Kenemans, F. G. Snijdewint, A. Kok, G. J. Van Kamp, M. A. Paul, P. J. Van Diest, S. Meijer, and J. Hilgers. 2000. Survival in early breast cancer patients is favorably influenced by a humoral immune response to polymorphic epithelial mucin. J. Clin. Oncol. 18:574-583. [DOI] [PubMed] [Google Scholar]
- 26.von Mensdorff-Pouilly, S., M. M. Gouretvich, P. Kenemans, A. A. Verstraeten, S. V. Litvinov, G. J. van Kamp, S. Meijer, J. Vermorken, and J. Hilgers. 1996. Humoral immune response to polymorphic epithelial mucin (MUC1) in patients with benign and malignant breast tumors. Eur. J. Cancer 32:1325-1331. [DOI] [PubMed] [Google Scholar]
- 27.von Mensdorff-Pouilly, S., M. M. Gourevitch, P. Kenemans, A. A. Verstraeten, G. J. van Kamp, A. Kok, K. van Uffelen, F. G. Snijdewint, M. A. Paul, and S. Meijer. 1998. An enzyme-linked immunosorbent assay for the measurement of circulating antibodies to polymorphic epithelial mucin (MUC1). Tumour Biol. 19:186-195. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto, M., A. Bharti, Y. Li, and D. Kufe. 1997. Interaction of the DF3/MUC1 breast carcinoma associated antigen and β-catenin in cell adhesion. J. Biol. Chem. 272:12492-12494. [DOI] [PubMed] [Google Scholar]
- 29.Yang, E., X. F. Hu, and P. X. Xing. 2007. Advances of MUC1 as a target for breast cancer immunotherapy. Histol. Histopathol. 22:905-922. [DOI] [PubMed] [Google Scholar]