Abstract
Acute otitis media (AOM) is an inflammatory reaction in the middle ear, most often occurring in young children. Streptococcus pneumoniae, nontypeable Haemophilus influenzae, and Moraxella catarrhalis are the most common bacteria isolated. Intercellular adhesion molecule 1 (ICAM-1) is involved in the innate immune response to infection by microorganisms, in effective antigen presentation, and in subsequent T-cell activation. Here we prospectively studied levels of serum soluble ICAM-1 (sICAM-1) before, at the time of, and after antimicrobial treatment of AOM in a group of 138 children ages 6 to 30 months. Middle ear fluids were collected by tympanocentesis to identify otopathogens. We found that (i) serum levels of sICAM-1 were significantly higher in S. pneumoniae-, nontypeable H. influenzae-, and M. catarrhalis-infected children than in well children (P < 0.001), confirming that a systemic inflammatory response occurs during AOM; (ii) sICAM-1 levels varied from no elevation (110 ng/ml) to elevation to high levels (maximum, 1,470 ng/ml) among children with AOM; (iii) in paired samples, sICAM-1 levels increased 4- to 20-fold when children developed AOM compared to their sICAM-1 levels before infection; and (iv) the level of sICAM-1 returned to the pre-AOM level at the convalescent stage of AOM after successful antimicrobial therapy. We conclude that AOM often causes a systemic inflammatory reaction, as measured by elevation of the serum sICAM-1 level, and that a high variability in sICAM-1 responses occurs with the presence of otopathogens during AOM.
Acute otitis media (AOM) is defined by the presence of middle ear effusion (MEE) with acute onset of symptoms of inflammation of the middle ear. Although more than half of patients who develop AOM have fever (14), the condition is considered a localized, mucosal infection. Currently, AOM is regarded as relatively benign due to spontaneous resolution of the infection in a majority of patients (42). The complications and sequelae of bacterial systemic invasion from the middle ear, including mastoiditis, brain abscess, and meningitis, are sufficiently rare that they have recently been considered less consequential in comparison to the consequences from the costs of antimicrobial treatment and overtreatment (2).
The cause and pathogenesis of otitis media are multifactorial, involving viral and bacterial infections. The most frequently isolated bacteria in AOM are Streptococcus pneumoniae (20 to 55% of cases), nontypeable Haemophilus influenzae (15 to 40%), and Moraxella catarrhalis (10 to 25%) (12, 25, 29). When bacteria gain entry into the middle ear space, they damage the middle ear mucosa directly by releasing toxins and indirectly by provoking both specific immunological and general inflammatory responses in the host. A prominent feature of the host response is an influx of inflammatory cells into the middle ear.
The migration of leukocytes into sites of inflammation is mediated by numerous factors, including intercellular adhesion molecule 1 (ICAM-1; CD54). ICAM-1 is a member of the immunoglobulin (Ig)-like superfamily (43); it is expressed on endothelial cells, monocytes, fibroblasts, leukocytes, epithelial cells, macrophages, mitogen-stimulated T lymphoblasts, germinal center B cells, and dendritic cells (48). Soluble isoforms of ICAM-1 (sICAM-1) shed from the surface of activated cells and can be quantified in biological fluids, allowing insights into early events of leukocyte recruitment (47). sICAM-1 levels have been reported to be the initial marker of inflammatory reactions in various diseases, such as allergic rhinitis, tuberculosis, sarcoidosis, rheumatoid arthritis, and meningitis (3, 7, 27, 33). However, to date, there is no information on sICAM-1 expression in children with AOM.
In this study, the first to ever evaluate the concentrations of systemic (serum) sICAM-1 from children with AOM, we sought to determine if (i) the levels of sICAM-1 increased during S. pneumoniae, nontypeable H. influenzae, or M. catarrhalis infections; (ii) sICAM-1 levels, as a marker of the innate immune response, varied among children with AOM; (iii) sICAM-1 levels increased when children developed AOM compared to their sICAM-1 levels before infection; and (iv) the sICAM-1 level returned to the pre-AOM level at the convalescent stage of AOM after successful antimicrobial therapy.
MATERIALS AND METHODS
Subjects.
The experimental human samples evaluated in this study were collected in the first 3 years to 5 years as part of a prospective study funded by the National Institute for Deafness and Communication Disorders that commenced in June 2006. All the samples were collected from the children at 6, 9, 12, 15, 18, 24, and 30 months of age. Informed consent was obtained at enrollment from the parents or guardians. The diagnosis of AOM was based on symptoms of fever, irritability, or earache; signs of inflammation (red or yellow color or bulging) of the tympanic membrane; and the presence of middle year fluid (MEF), as documented by tympanocentesis. After being diagnosed with AOM, patients received various antibiotic treatments and returned for a follow-up visit 3 weeks later, in addition to scheduled visits. Children with a history of chronic or recurrent AOM, other infections, chronic diseases, and other diseases were excluded. A virus infection was diagnosed on the basis of the observation and examination of clinical symptoms and signs, such as fever, rhinorrhea, and cough, along with a decreased white blood cell count and a predominance of lymphocytes, and verified via multiplex PCR using a Seeplex RV12 detection kit (Seegene, MD), following the manufacturer's instruction.
Serum and PBMCs.
Blood specimens were collected from the study patients at each visit. Four milliliters of heparinized peripheral venous blood was drawn from each patient and control donor. The specimens were centrifuged at 2,000 rpm (model AccuSpin-1; Fisher Scientific) at room temperature for 10 min. Peripheral blood mononuclear cells (PBMCs) were isolated on Ficoll gradients and stored at −80°C. Serum was immediately stored as aliquots at −80°C until it was assayed.
MEF.
Tympanocentesis was performed for all patients with the use of a 20-gauge spinal needle attached to a 3.0-ml sterile syringe; the anteroinferior portion of the intact tympanic membrane was punctured. The fluid (0.1 to 0.2 ml) was immediately aspirated into the sterile syringe and sent in transport medium for processing for bacteriologic culture within 3 h.
Bacteriology.
Swabs of the middle ear aspirate were plated on Trypticase agar medium containing 5% sheep blood and chocolate agar. The plates were incubated aerobically at 37°C in a 5% CO2 atmosphere for 48 h. Presumptive identification of S. pneumoniae was based on the presence of alpha hemolysis and inhibition of optochin, and the identity was confirmed by a positive slide agglutination test, according to established CLSI procedures. Identification of Haemophilus influenzae was based on Gram's stain, growth on chocolate agar medium, failure to grow on Trypticase agar with added sheep blood, and a nutritional requirement for both hemin and NAD. Organisms that failed to agglutinate with polyvalent antisera to H. influenzae groups a, c to f, and b (Phadebact; Pharmacia) were considered untypeable. Identification of M. catarrhalis was based on Gram's stain, oxidase reaction, and the catarrhalis disk reaction (Remel, KS). Whenever the pathogen was questionable, verification of the identity of the pathogen was performed by multiplex PCR, as described previously (28).
sICAM-1 assay.
sICAM-1 levels in the serum samples were measured using specific sandwich immunoassays (enzyme-linked immunosorbent assay [ELISA] kits from Bender MedSystems Europe, Vienna, Austria) based on recombinant soluble adhesion molecules supplied by the manufacturer's standards. Ninety-six-well ELISA plates were coated with capture anti-human sICAM-1 monoclonal antibody (MAb). All serum samples were diluted in diluent buffer provided with the kits. The known standards and duplicate test samples were added, and the mixtures were incubated for 2 h at room temperature. After the binding of sICAM-1 to the immobilized MAb, a second peroxidase (horseradish peroxidase)-conjugated streptavidin detecting anti-human sICAM-1 monoclonal antibody was added for 1 h. For color development, substrate solution [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] with 0.03% H2O2 was added. The optical densities (ODs) of the plates at 450 nm were read with a microplate reader. Standard curves were generated using known concentrations of human sICAM-1 in a series of dilutions ranging from 10 ng/ml to 0.156 ng/ml. Because the concentration in each sample corresponds to the OD readout of the sample, its value was derived from standard curves by regression analysis. Final concentrations were calculated by multiplying the given values by the dilution factor, the results are reported as the mean concentration (ng/ml) ± standard deviation (SD). Each experiment was repeated at least twice.
Microarray.
Total RNA was extracted from PBMCs using a QIAamp RNA blood minikit (Qiagen, MD), according to the manufacturer's instructions. Double-stranded cDNA generated from total RNA was labeled with cyanine 5 and subsequently hybridized to 30,968 human genome probes and 1,082 experimental control probes in a Human OneArray array system, according to the manufacturer's standard protocols (PhalanxBio Inc., CA). Microarrays were scanned at 5-μm resolution using an Agilent scanner. Raw intensity signals for each microarray were captured using a Molecular Dynamics Axon 4100A scanner and were measured using GenePixPro software. The data from all microarrays in each experimental set were then analyzed using Omicsoft Array Studio software; control and missing features were removed, and the remaining signals were quantile normalized. Student's t test was performed after technical replicates were combined to calculate P values.
qRT-PCR.
One hundred nanograms of total RNA was reverse transcribed to cDNA using an RT2 first-strand kit (SABiosciences, MD). Quantitative real-time reverse transcriptase PCR (qRT-PCR) was performed using an RT2 profiler PCR array system kit (SABiosciences) with a CFX 96 thermocycler (Bio-Rad). The threshold and baseline were set automatically using the PCR/array analysis method, according to the manufacturer's instructions (SABiosciences). Threshold cycle (CT) data were uploaded into the data analysis template on the manufacturer's website (SABiosciences). The relative expression of genes compared with the expression in control samples was calculated on the website using the ΔΔCT method and five housekeeping genes as controls.
Statistical analysis.
Analysis of variance was used for analyzing multiple-group data. Two-tailed analysis was used throughout, with significance defined as a P value of <0.05. Power analysis was done using paired and unpaired t test calculations. The statistical analysis included both paired analysis for each patient (AOM at the acute-phase visit versus AOM at the convalescent-phase visit) and comparison of the magnitude of the changes in the mean values. Comparisons between patients and healthy controls included means and standard deviations.
RESULTS
Serum levels of sICAM-1 in children with AOM.
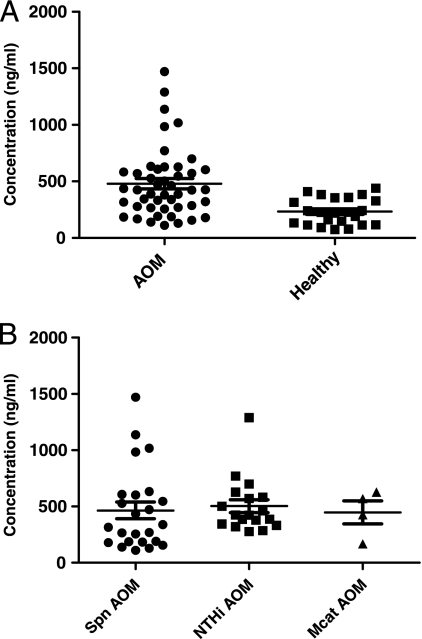
A total of 46 children with AOM, including 23 males and 23 females, were analyzed. The mean age of the children was 13 months. The children were infected with S. pneumoniae (n = 24), nontypeable H. influenzae (n = 18), or M. catarrhalis (n = 4). Twenty-three age-matched healthy children without any AOM symptoms or signs were used as controls for comparison. It was found that sera from patients with bacterial AOM had significantly elevated sICAM-1 levels (479 ± 305 ng/ml) compared to those for the controls (232 ± 117 ng/ml) (P = 0.0004) (Fig. 1A). There was no significant difference in serum sICAM-1 levels among children infected by S. pneumoniae, nontypeable H. influenzae, or M. catarrhalis (Fig. 1B).
FIG. 1.
(A) Comparison of serum sICAM-1 levels between 23 healthy children and 46 children with AOM. The samples were collected from the children at 6 months to 30 months of age. The MEF of all children with AOM (n = 46) was positive for otopathogens (S. pneumoniae [Spn], nontypeable H. influenzae [NT Hi], or M. catarrhalis [Mcat]). Healthy children (n = 23) did not have any symptoms or signs of AOM. The experiments were repeated twice, with duplicate wells being used for each test. The concentration (ng/ml) was derived from the absorbance (OD at 450 nm) based on a standard curve. (B) Comparison of the serum sICAM-1 levels among children with AOM caused by S. pneumoniae, nontypeable H. influenzae, and M. catarrhalis. The sICAM-1 levels in serum from 46 children with AOM were analyzed (as described for panel A) with different otopathogens in the middle ears: S. pneumoniae, n = 24; nontypeable H. influenzae, n = 18; M. catarrhalis, n = 4. No significant change existed among the groups.
sICAM-1 levels vary among children with AOM.
The systemic adaptive immune response to AOM is known to vary among children by age at the time of infection, a key predictor of the response to treatment. Therefore, we evaluated the variations in sICAM-1 levels among the 46 children with AOM described above according to the age of the child at the time of infection (Fig. 2). It was found that there were no changes in sICAM-1 levels among children who experienced AOM at age ≤18 months. However, the levels of sICAM-1 were significantly increased when the children experienced AOM at the age of 18 to 24 months compared to the levels in younger children (P < 0.05). For 24- to 30-month-old children with AOM, the levels of sICAM-1 were not statistically different from those in the younger group of 6 to 18 months of age, but only 4 children were in the oldest age group.
FIG. 2.
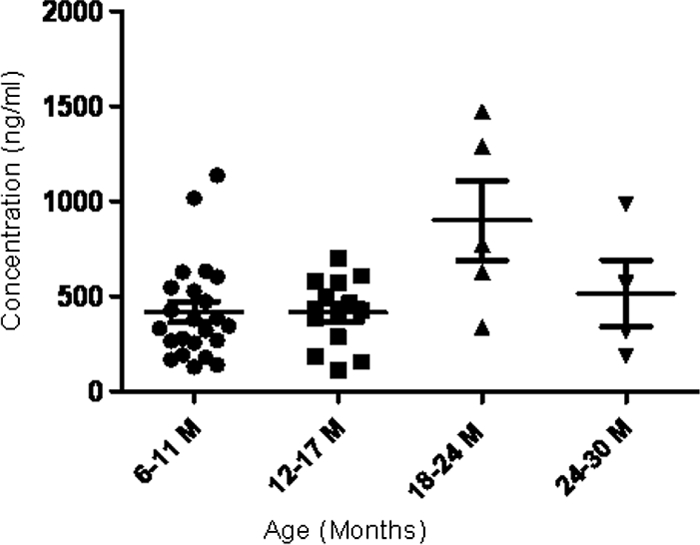
Serum sICAM-1 level change in 46 children with AOM of different ages. P < 0.05 for the group aged 18 to 24 months (M) versus the group aged 6 to 11 months (or 12 to 17 months); P > 0.05 for the group aged 18 to 24 months versus the group aged 24 to 30 months.
Serum sICAM-1 dynamic change during AOM progression.
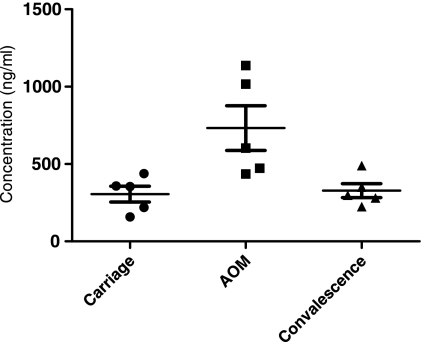
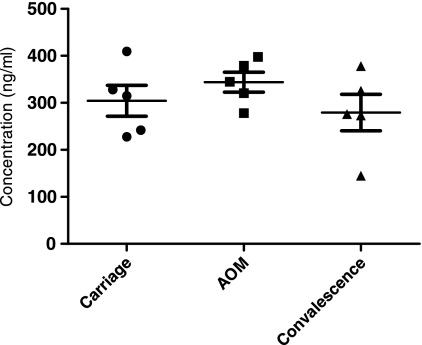
AOM development occurs as a dynamic progression, including the asymptomatic carriage, AOM, and convalescent stages. To study the status of serum sICAM-1 during the progression of AOM, we tested a total of 10 children at a time when they were experiencing asymptomatic carriage of an ototpathogen (during the healthy stage without AOM symptoms and signs), when the children developed AOM (when bacteria were present in MEF and clinical AOM), and in the convalescent stage (when bacteria were presumed to be eradicated from MEF after pathogen-directed antibiotic treatment). We found that the patterns of change in sICAM-1 levels were different for S. pneumoniae and nontypeable H. influenzae. For 5 children studied who developed AOM due to S. pneumoniae, it was found that serum levels of sICAM-1 were low (306 ± 134 ng/ml) when children were in the healthy stage and S. pneumoniae was carried in the nasopharynx (NP), that ICAM-1 levels significantly increased (733 ± 323 ng/ml) when the children experienced AOM compared to the levels during their carriage stage (P < 0.05), and that sICAM-1 levels dropped back to the levels of the asymptomatic carriage stage after successful antibiotic treatment (Fig. 3). The serum sICAM-1 levels for 5 children who carried nontypeable H. influenzae in their NPs, then developed AOM from nontypeable H. influenzae, and then recovered after antibiotic therapy are shown in Fig. 4. The pattern of sICAM-1 levels for children infected with H. influenzae appears to be different from that for children infected with S. pneumoniae. Asymptomatic carriage in the NPs was associated with a modest elevation in the level of sICAM-1 (304 ± 73 ng/ml); increased during AOM, but not significantly (P = 0.34); and fell toward normal during convalescence.
FIG. 3.
Serum sICAM-1 levels in 5 children during asymptomatic (carriage) stage, at onset of AOM, and during convalescence from infection due to S. pneumoniae, as follows: 306 ± 134 ng/ml (mean ± SD) for carriage stage, 733 ± 323 ng/ml at onset of AOM, and 329 ± 101 ng/ml during convalescence. P = 0.02 for AOM versus carriage; P = 0.03 for AOM versus convalescence.
FIG. 4.
Serum sICAM-1 levels in 5 children during asymptomatic (carriage) stage, at onset of AOM, and during convalescence from infection due to nontypeable H. influenzae, as follows: 304 ± 73 ng/ml (mean ± SD) for carriage, 344 ± 47 ng/ml at onset of AOM, and 280 ± 87 ng/ml during convalescence. P = 0.34 for AOM versus carriage; P = 0.18 for AOM versus convalescence.
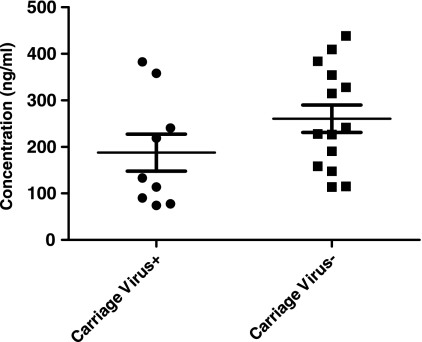
Serum levels of sICAM-1 in healthy children with and without nasopharyngeal carriage of otopathogens.
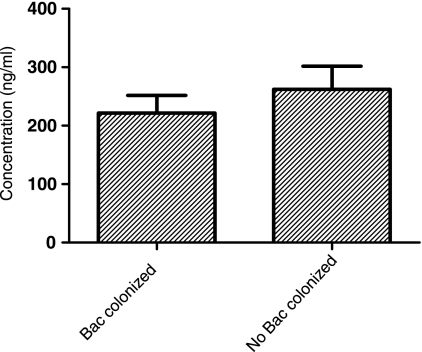
The bacterial carriage of otopathogens in the NPs of healthy children is quite common (20 to 50%), and carriage rates are 100% during AOM episodes (19, 46). To investigate whether or not NP colonization by otopathogens in healthy children influences the expression of sICAM-1, sera were collected from 17 healthy children with bacterial colonization of the NPs and 6 children without bacterial colonization of the NPs. No significant difference in the levels of sICAM-1 in the NPs of children colonized with otopathogens (222 ± 124 ng/ml) and children not colonized with bacteria (262 ± 97 ng/ml) was found (Fig. 5).
FIG. 5.
Comparison of serum sICAM-1 levels in children with otopathogen colonization (n = 17) and without otopathogen colonization (n = 6). The results showed that the levels of sICAM-1 were not different in the two groups (P > 0.05). Bac, bacteria.
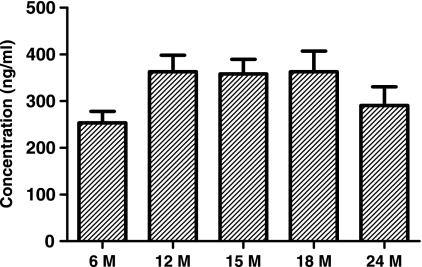
Serum levels of sICAM-1 in healthy children of various ages.
We also evaluated the sICAM-1 levels in 36 additional children when they were healthy at different ages. The tests were performed when children were 6 months (n = 20), 12 months (n = 3), 15 months (n = 4), 18 months (n = 4), and 24 months (n = 5) old. Serum sICAM-1 levels in 6-month-olds were lower than those in the other groups, but the difference was not statistically significant (Fig. 6).
FIG. 6.
Comparison of serum sICAM-1 levels in 36 healthy children without NP carriage of any AOM pathogen by age (M, months). There is no difference among the groups.
Serum levels of sICAM-1 during viral URI and nasopharyngeal carriage of otopathogens.
It is known that AOM occurs concurrently with viral upper respiratory tract infections in >90% of cases of AOM in children (41). Viral upper respiratory infection (URI) impairs host defenses, thereby contributing to subsequent bacterial superinfection. To study if the presence of a respiratory virus in the NPs would change the expression of sICAM-1, the sera from the 9 children clinically diagnosed to be infected by a respiratory virus and 14 children without an apparent respiratory virus infection were tested; no difference in sICAM-1 levels was found (Fig. 7). Among the 9 samples from children with clinically diagnosed virus infection, 2 samples with medium serum sICAM-1 levels were selected to check the virus species by multiplex PCR, and it was found that both of the samples were parainfluenza virus positive.
FIG. 7.
Serum sICAM-1 levels in 9 virus-positive and 14 virus-negative children when otopathogens were also present in the nasopharynx. P > 0.05 for virus-positive versus virus-negative groups.
Transcription regulation of ICAM-1 in S. pneumoniae AOM.
ICAM-1 exists in two forms: a membrane form (mICAM-1) and a soluble form. mICAM-1 produces the soluble form of ICAM-1 by undergoing proteolysis (10). The level of the soluble form of ICAM-1 is increased during inflammation in proportion to the level of mICAM-1. To study transcriptome regulation of ICAM-1, we randomly selected one child from whom PBMCs were obtained at a time of health, during AOM caused by S. pneumoniae, and during convalescence. We found that the ICAM-1 gene from PBMCs was upregulated 2.52 times during AOM compared with the level of regulation at the preinfection carriage stage. In the convalescent stage after treatment, the ICAM-1 gene was downregulated 10.21 times compared with the level of regulation during AOM (Table 1).
TABLE 1.
Expression of ICAM-1 at transcriptional levela
| Method | No. of patients | Fold change |
|
|---|---|---|---|
| AOM/carri. | AOM/conval. | ||
| Microarray | 1 | 2.52 | 10.21 |
| qRT-PCR | 6 | 2.76 | 1.13 |
Total RNA was extracted from peripheral blood mononuclear cells from the same child at three time points: at the time of AOM caused by S. pneumoniae, before the S. pneumoniae infection (carriage [carri.]), and after the infection during the convalescent (conval.) stage. Microarray analysis and qRT-PCR were performed as described in Materials and Methods. Data were analyzed after normalization (see Materials and Methods). The data for qRT-PCR are the mean of 6 children.
To verify the results of microarray analysis, the total RNAs were extracted from PBMCs derived from 6 children and qRT-PCR was performed with ICAM-1-specific primers. Similar to the microarray analysis, the mean levels of ICAM-1 in 6 children with AOM caused by S. pneumoniae were upregulated 2.8-fold compared to the levels during the healthy stage. After successful treatment of AOM, the expression of the ICAM-1 gene was reduced to 1.1-fold compared to that during AOM caused by the S. pneumoniae (Table 1). Thus, the transcriptome pattern was similar to that observed in the serum sICAM-1 obtained via ELISA.
DISCUSSION
The present study shows that serum levels of sICAM-1 are significantly higher in S. pneumoniae-, nontypeable H. influenzae-, and M. catarrhalis AOM-infected children than in well children, confirming that a systemic inflammatory response occurs during AOM; that sICAM-1 levels vary from no elevation to high elevations among children with AOM; that in paired samples the sICAM-1 levels increase when children develop AOM due to S. pneumoniae compared to their sICAM-1 levels before infection; and that the level of sICAM-1 returns to pre-AOM levels during the convalescent stage of AOM after successful antimicrobial therapy. When children develop AOM due to nontypeable H. influenzae, however, significant increases in sICAM-1 levels are not detected. We also evaluated the levels of sICAM-1 in healthy children at ages 6, 12, 15, 18, 24, and 30 months, during NP carriage of otopathogens with and without concurrent viral upper respiratory infections, and found that neither the age of the child nor the presence of a viral URI impacted serum sICAM-1 levels in our study population. In addition, we evaluated transcription regulation of ICAM-1 in AOM caused by S. pneumoniae. To our knowledge, this is the first report on the detailed changes of sICAM-1 levels in the sera of children with AOM.
Similar to the erythrocyte sedimentation and C-reactive protein, ICAM-1 is inducible by proinflammatory mediators and by bacterial products in association with bacterial infection. It is well documented that ICAM-1 is endogenously expressed on various cell types and makes possible reversible adhesion and signal transduction between cells, processes critical to T-cell development. Increased levels of ICAM-1 promote cell-cell interactions, playing a critical role in leukocyte recruitment and leading to prolonged and, sometimes, excessive inflammation (52).
Our study focused on children ages 6 to 30 months with AOM, and we compared the sICAM-1 levels in the sera of children with AOM and the sera of healthy children; in the sera of children whose NPs were colonized and in the sera of children whose NPs were not colonized with potential AOM otopathogens; as well as in the sera of individual children obtained pre-AOM, at the time of AOM, and during convalescence from AOM. We found that the mean levels of serum sICAM-1 in healthy children whose NPs were colonized and not colonized with otopathogens were the same, measured as a mean of 232 ng/ml. This quantity is similar to the serum sICAM-1 levels in healthy adults ages 20 to 50 years (203 ng/ml) (8). At the time of onset of AOM in the children, we studied the serum levels of sICAM-1 and found that they increased 2.1 times compared with the level in the healthy controls to a mean level of about 500 ng/ml, a level similar to those obtained during other infectious diseases (15, 49). With effective antibiotic treatment and clinical resolution of AOM, we found that serum sICAM-1 returned to preinfection levels. We interpret these results to suggest that the inflammatory reaction in the middle ear during bacterial AOM is associated with a systemic inflammatory response and that after recovery from AOM the decrease in the levels of sICAM-1 in the sera reflect a resolution of inflammation.
Serum sICAM-1 levels varied widely among children with AOM in our study. The subjects whom we studied were selected on the basis of the bacterial species causing AOM; they were otherwise healthy, except for concurrent clinically diagnosed viral upper respiratory infections, and had no other infections or diseases. Two possibilities contributing to this variation that might be considered were the severity of the disease and the age of the child. All the children in the current study had bulging tympanic membranes, suggestive of a clearly established AOM. However, there is no recognized system to score the severity of AOM. Methods used to classify AOM, such as symptoms (questionnaire from parents), body temperature, and signs (on otoscopy examination) are subjective. For example, the severity of ear pain depends on an individual's pain sensitivity threshold. Therefore, we did not pursue an analysis of the association between symptoms and signs and sICAM-1 levels. Recently, we have found that the adaptive immune response to NP carriage of otopathogens increases with age in our study cohort (13), and others have found an association of younger age and susceptibility to AOM (38), so we evaluated variations in sICAM-1 levels according to the age of the child at the time of AOM. We found that when children experienced AOM when they were under the age of 18 months, they had lower sICAM-1 levels than 18- to 23-month old children. Witkowska et al. (51) reported that there was no difference in serum sICAM-1 levels in adults ages 42 to 81 years with abdominal aortic aneurysms. El-Sawy et al. (17) did not find any difference in serum sICAM-1 levels in children ages 6 to 12 years with bronchial asthma exacerbations.
Serum sICAM-1 levels showed a dynamic change during AOM progression, and that the change in S. pneumoniae infection appeared to be different from that in nontypeable H. influenzae infection. Although AOM is usually treated as a single entity, both studies with humans and experimental animal studies suggest that there are differences in host responses, depending on the organism involved (23, 29, 36). There are indications that nontypeable H. influenzae antigens evoke a greater local inflammatory response than pneumococcal antigens (35). However, there is no report on the serum level change of sICAM-1 in nontypeable H. influenzae-infected children with AOM. S. pneumoniae infection, on the other hand, is clinically more severe and involves a higher risk of serious disease and intracranial complications (6, 39, 44). S. pneumoniae induces better systemic protection against reinfections than do nontypeable H. influenzae and M. catarrhalis (9, 29, 36). However, our study shows that the levels of sICAM-1 in children with AOM infected by S. pneumoniae, nontypeable H. influenzae, or M. catarrhalis are similar. In addition, we found the levels of sICAM-1 in the sera of children infected by nontypeable H. influenzae were higher than those in the sera of healthy children (P < 0.05); however, there was no significant change during the progression of AOM in the same child at the three stages. Animal experiments showed that AOM appears 1 day after nontypeable H. influenzae inoculation and 3 days after S. pneumoniae inoculation and that lower transcript levels of cytokines such as interleukin-6 (IL-6), IL-1alpha, tumor necrosis factor alpha, and IL-10 were detected in S. pneumoniae-infected animals than in nontypeable H. influenzae-infected animals (34). Genetic analysis showed that variation in innate immunoresponse genes, such as the TNFA-863A, TNFA-376G, TNFA-238G, IL-10-1082A, and IL-6-174G alleles, might result in altered cytokine production that leads to altered inflammatory responses (18) and, hence, may contribute to altered ICAM-1 levels as well. The relationship between ICAM-1 alleles and disease susceptibility in other diseases has been reported. For example, a mutation of the coding region of ICAM-1, ICAM-1Kilifi, causing a change from Lys to Met in the loop region, increased the susceptibility of Kenyan children to severe malaria (31). Matsuzawa et al. found that the allelic frequency of K469E was significantly higher both in patients with Crohn's disease and in patients with ulcerative colitis than in controls (33a). ICAM-1 genotype G/G (corresponding to Lys469Glu) exhibited a higher frequency in patients with grade II astrocytomas (11). Therefore, the finding of no significant difference in sICAM-1 levels in nontypeable H. influenzae-infected children during AOM and during their preinfection carrier stage might be influenced by genetic factors such as ICAM-1 gene polymorphisms. This is an area of interest for further study with children with AOM.
Serum levels of sICAM-1 did not appear to vary in magnitude in healthy children of various ages (between 6 and 30 months). El-Sawy (17) and Abdelrazik et al. (1) reported that no significant correlation was found between age and serum sICAM-1 levels in healthy children at the ages of 6 to 12 years. Our results are consistent with their observations. Serum levels of sICAM-1 in healthy children did not appear to vary during NP colonization with otopathogens. This is consistent with the clinical observation of an absence of signs of inflammation in the nasal mucosa when otopathogen colonization occurs. In addition, since the subjects studied in the present study were selected on the basis of the presence of AOM caused by different otopathogens and subjects with other infections, chronic diseases, and other diseases were excluded, the main clinical context where AOM must be differentiated from a second infection is the circumstance where a viral URI is occurring simultaneously. Therefore, we studied the sICAM-1 levels of children with viral URIs but without AOM. We found that the serum levels of sICAM-1 did not vary during viral URIs in our study population. There are previous reports on the upregulation of sICAM-1 after infection by respiratory viruses in vitro (16, 22). However, observations in vivo were different from the results obtained in vitro. Lai et al. (32) found that mean sICAM-1 concentrations were similar between respiratory syncytial virus (RSV)-positive and RSV-negative patients with acute bronchiolitis. Kosai et al. (30) tested plasma levels of sICAM-1 in patients with bacterial pneumonia coinfected with influenza virus and those not coinfected. They found similar levels of sICAM-1 in the two groups.
The elevation of ICAM-1 levels caused by infection with otopathogens has been previously reported in vitro. A recent study showed that M. catarrhalis lipooligosaccharide (LOS) stimulates proinflammatory cytokine production and selectively induces ICAM-1 expression on human monocytes via Toll-like receptor 4 (TLR4)-dependent and CD14-dependent pathways (52). Avadhanula et al. found that nontypeable H. influenzae infection increased the level of ICAM-1 expression on carcinomic human alveolar basal epithelial (A549) cells in vitro (5). Limited information on sICAM-1 expression in humans with chronic serous and mucoid otitis media has been previously described. Himi et al. (24) measured the levels of sICAM-1 in MEEs of children with chronic serous and mucoid otitis media and found that MEEs contained significantly higher levels of sICAM-1 than the sera of healthy children, but they did not comparatively study serum sICAM-1 levels in children with chronic serous otitis media and children with mucoid otitis media (24). Russo et al. studied the ICAM-1 levels in middle ear serous and mucoid effusions in children with otitis media with effusion (a clinical condition distinctly different from AOM) and did not find elevated levels (45). Ganbo et al. (21) also studied the levels of sICAM-1 in MEEs of subjects ages 3 to 79 years with mucoid otitis media and found that the mean level of sICAM-1 was 1,440 ng/ml, whereas the mean level in the MEEs of subjects with serous otitis media was 430 ng/ml. In this study we focused on the serum sICAM-1 expression levels in children with AOM and the relationship between sICAM-1 levels and the infecting organism as well as the dynamic change in ICAM-1 levels during the process of AOM development. Because chronic serous and mucoid otitis media are pathological conditions very different from AOM, no direct comparison of the significance of sICAM-1 levels in MEEs or sera from those populations and ours is biologically relevant.
Our results lead to new, interesting questions regarding the role of ICAM-1 in polymicrobial infections of the upper respiratory tract, an area of ongoing research by several groups. Passariello et al. have demonstrated that the significant enhancement of S. aureus infections following human rhinovirus (HRV) infections in vitro is mediated by the enhanced levels of inflammatory cytokines released from HRV-infected cells and the subsequent overexpression of ICAM-1 (38). The phenomenon could be prevented by blocking ICAM-1 or IL-6 and IL-8 activities with neutralizing antibodies (38). In vitro, by upregulation of expression of ICAM-1, RSV and influenza virus promote nontypeable H. influenzae and S. pneumoniae colonization of the NPs and adherence of these bacteria to respiratory epithelial cells (4). Moreover, ICAM-1 can promote the uptake of bacterial pathogens by macrophages and increase neutrophil recruitment (26, 37). Frick et al. (20) and Humlicek et al. (26) found that adherence of nontypeable H. influenzae to respiratory epithelial cells rapidly induced ICAM-1 expression, a process that they hypothesized would facilitate the recruitment of neutrophils to sites of nontypeable H. influenzae infection. Xie and Gu demonstrated that leukocyte recruitment mediated by enhanced ICAM-1 levels after M. catarrhalis infection may also result in increased bacterial adhesion to the respiratory tract (52). In the current study, although there is no direct evidence to illustrate that the enhanced ICAM-1 level has promoted neutrophil recruitment to the middle ear, the literature and the fact that the elevation of serum sICAM-1 levels in children with AOM positive for bacteria in MEF suggest that the intercellular adhesion molecules are upregulated during middle ear inflammation and that the increased ICAM-1 levels may contribute to innate immune responses through increasing leukocyte recruitment to the middle ear.
In conclusion, our study demonstrates that the elevation of serum sICAM-1 levels in children with AOM is correlated to pathogen presence and an inflammatory reaction in the middle ear. Moreover, our study raises new questions about the role of the sICAM-1 level during otopathogen infection, and answers to those questions may help us develop and introduce early interventions to moderate the acute inflammatory process and abort disease progression from colonization in the respiratory system (NPs) to AOM.
Acknowledgments
This work was supported by grant R01DC08671 from the National Institutes of Health and the Thrasher Research Fund (to M.P.).
This study would not have been possible without the help and dedication of our study coordinator, Sally Thomas; our nurses and staff; collaborating pediatricians from Long Pond Pediatrics, Genesis Pediatrics, Rainbow Pediatrics, and Lewis Pediatrics; and the parents who consented to this long and challenging study.
Footnotes
Published ahead of print on 6 October 2010.
REFERENCES
- 1.Abdelrazik, N., M. Fouda, M. H. Zaghloul, and D. Abbas. 2008. Serum level of intercellular adhesion molecule-1 in children with malignant lymphoma. Med. Princ. Pract. 17:233-238. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics. 2004. Subcommittee on Management of Acute Otitis Media. Diagnosis and management of acute otitis media. Pediatrics 113:1451-1465. [DOI] [PubMed] [Google Scholar]
- 3.Amiri, M. V., S. D. Mansoori, M. Shekar-Abi, S. M. Mirsaeidi, S. Zahirifard, M. K. Dizaji, P. Tabarsi, A. Halvani, S. D. Tabatabaee, and M. R. Masjedi. 2004. SICAM-1 as a serum marker for follow-up of pulmonary tuberculosis therapy. Tanaffos 3:55-63. [Google Scholar]
- 4.Avadhanula, V., Y. Wang, A. Portner, and E. Adderson. 2007. Nontypeable Haemophilus influenzae and Streptococcus pneumoniae bind respiratory syncytial virus glycoprotein, J. Med. Microbiol. 56:1133-1137. [DOI] [PubMed] [Google Scholar]
- 5.Avadhanula, V., C. A. Rodriguez, G. C. Ulett, L. O. Bakaletz, and E. E. Adderson. 2006. Nontypeable Haemophilus influenzae adheres to intercellular adhesion molecule 1 (ICAM-1) on respiratory epithelial cells and upregulates ICAM-1 expression. Infect. Immun. 74:830-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barry, B., J. Delattre, F. Vie, J. P. Bedos, and P. Gehanno. 1999. Otogenic intracranial infections in adults. Laryngoscope 109:483-487. [DOI] [PubMed] [Google Scholar]
- 7.Baumer, I., G. Zissel, M. Schlaak, and J. Muller-Quernheim. 1998. Soluble intercellular adhesion molecule 1 (sICAM-1) in bronchoalveolar lavage (BAL) cell cultures and in the circulation of patients with tuberculosis, hypersensitivity pneumonitis and sarcoidosis. Eur. J. Med. Res. 3:288-294. [PubMed] [Google Scholar]
- 8.Biesiada, G., J. Czepiel, I. Sobczyk-Krupiarz, D. Salamon, A. Garlicki, and T. Mach. 2009. Levels of sVCAM-1 and sICAM-1 in patients with Lyme disease. Pol. Arch. Med. Wewn. 119:200-204. [PubMed] [Google Scholar]
- 9.Branefors-Helander, P., T. Dahlberg, and O. Nylén. 1975. Acute otitis media. A clinical, bacteriological and serological study of children with frequent episodes of acute otitis media. Acta Otolaryngol. (Stockholm) 80:399-409. [DOI] [PubMed] [Google Scholar]
- 10.Budnik, A., M. Grewe, K. Gyufko, and J. Krutmann. 1996. Analysis of the production of soluble ICAM-1 molecules by human cells. Exp. Hematol. 24:352-359. [PubMed] [Google Scholar]
- 11.Burim, R. V., S. A. Teixeira, B. O. Colli, F. M. Peria, L. F. Tirapelli, S. K. Marie, S. M. Malheiros, S. M. Oba-Shinjo, A. A. Gabbai, P. A. Lotufo, and C. G. Carlotti-Júnior. 2009. ICAM-1 (Lys469Glu) and PECAM-1 (Leu125Val) polymorphisms in diffuse astrocytomas. Clin. Exp. Med. 9:157-163. [DOI] [PubMed] [Google Scholar]
- 12.Casey, J. R., D. G. Adlowitz, and M. E. Pichichero. 2010. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr. Infect. Dis. J. 29:304-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casey, J. R., and M. E. Pichichero. 2004. Changes in frequency and pathogens causing acute otitis media in 1995-2003. Pediatr. Infect. Dis. J. 23:824-828. [DOI] [PubMed] [Google Scholar]
- 14.Chandler, S. M., S. M. Garcia, and D. P. McCormick. 2007. Consistency of diagnostic criteria for acute otitis media: a review of the recent literature. Clin. Pediatr. (Phila.) 46:99-108. [DOI] [PubMed] [Google Scholar]
- 15.Chihara, J., T. Yamamoto, D. Kurachi, and S. Nakajima. 1994. Soluble ICAM-1 in sputum of patients with bronchial asthma. Lancet 343:1108. [DOI] [PubMed] [Google Scholar]
- 16.Chini, B. A., M. A. Fiedler, L. Milligan, T. Hopkins, and J. M. Stark. 1998. Essential roles of NF-kB and C/EBP in the regulation of intercellular adhesion molecule-1 after respiratory syncytial virus infection of human respiratory epithelial cell cultures. J. Virol. 72:1623-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Sawy, I. H., O. M. Badr-El-Din, O. E. El-Azzouni, and H. A. Motawae. 1999. Soluble intercellular adhesion molecule-1 in sera of children with bronchial asthma exacerbation. Int. Arch. Allergy Immunol. 119:126-132. [DOI] [PubMed] [Google Scholar]
- 18.Emonts, M., R. H. Veenhoven, S. P. Wiertsema, J. J. Houwing-Duistermaat, V. Walraven, R. de Groot, P. W. M. Hermans, and E. A. Sanders. 2007. Genetic polymorphisms in immunoresponse genes TNFA, IL6, IL10, and TLR4 are associated with recurrent acute otitis media. Pediatrics 120:814-823. [DOI] [PubMed] [Google Scholar]
- 19.Faden, H., L. Brodsky, J. Bernstein, J. Stanievich, D. Krystofik, et al. 1989. Otitis media in children: local immune response to nontypeable Haemophilus influenzae. Infect. Immun. 57:3555-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frick, A. G., T. D. Joseph, L. Pang, A. M. Rabe, J. W. St Geme III, and D. C. Look. 2000. Haemophilus influenzae stimulates ICAM-1 expression on respiratory epithelial cells. J. Immunol. 164:4185-4196. [DOI] [PubMed] [Google Scholar]
- 21.Ganbo, T., K. Hisamatsu, S. Shimomura, T. Nakajima, H. Inoue, and Y. Murakami. 1995. Inhibition of mucociliary clearance of the eustachian tube by leukotriene C4 and D4. Ann. Otol. Rhinol. Laryngo1. 104:231-236. [DOI] [PubMed] [Google Scholar]
- 22.Gao, J., S. Choudhary, A. K. Banerjee, and B. P. De. 2000. Human parainfluenza virus type 3 upregulates ICAM-1 (CD54) expression in a cytokine-independent manner. Gene Expr. 9:115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heikkinen, T., F. Ghaffar, A. O. Okorodudu, and T. Chonmaitree. 1998. Serum interleukin-6 in bacterial and nonbacterial acute otitis media. Pediatrics 102:296-299. [DOI] [PubMed] [Google Scholar]
- 24.Himi, T., M. Kamimura, A. Kataura, and K. Imai. 1994. Quantitative analysis of soluble cell adhesion molecules in otitis media with effusion. Acta Otolaryngol. (Stockholm) 114:285-288. [DOI] [PubMed] [Google Scholar]
- 25.Howie, V. M., R. Dillard, and B. Lawrence. 1985. In vivo sensitivity test in otitis media: efficacy of antibiotics. Pediatrics 75:8-13. [PubMed] [Google Scholar]
- 26.Humlicek, A. L., L. Pang, and D. C. Look. 2004. Modulation of airway inflammation and bacterial clearance by epithelial cell ICAM-1. Am. J. Physiol. Lung Cell. Mol. Physiol. 287:L598-L607. [DOI] [PubMed] [Google Scholar]
- 27.Jaber, S. M., E. A. Hamed, and S. A Hamed. 2009. Adhesion molecule levels in serum and cerebrospinal fluid in children with bacterial meningitis and sepsis. J. Pediatr. Neurosci. 4:76-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaur, R., D. G. Adlowitz, J. R. Casey, M. Zeng, and M. E. Pichichero. 23 March 2010. Simultaneous assay for four bacterial species including Alloiococcus otitidis using multiplex-PCR in children with culture negative acute otitis media. Pediatr. Infect. Dis. J. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 29.Klein, J. O. 1994. Otitis media. Clin. Infect. Dis. 19:823-833. [DOI] [PubMed] [Google Scholar]
- 30.Kosai, K., M. Seki, K. Yanagihara, S. Nakamura, S. Kurihara, K. Izumikawa, H. Kakeya, Y. Yamamoto, T. Tashiro, and S. Kohno. 2008. Elevated levels of high mobility group box chromosomal protein-1 (HMGB-1) in sera from patients with severe bacterial pneumonia coinfected with influenza virus. Scand. J. Infect. Dis. 40:338-342. [DOI] [PubMed] [Google Scholar]
- 31.Kun, J. F., J. Klabunde, B. Lell, D. Luckner, M. Alpers, J. May, C. Meyer, and P. G. Kremsner. 1999. Association of the ICAM-1Kilifi mutation with protection against severe malaria in Lambarene, Gabon. Am. J. Trop. Med. Hyg. 61:776-779. [DOI] [PubMed] [Google Scholar]
- 32.Lai, C.-C., H.-Y. Tai, H.-D. Shen, W.-T. Chung, R.-L. Chung, and R.-B. Tang. 2004. Elevated levels of soluble adhesion molecules in sera of patients with acute bronchiolitis J. Microbiol. Immunol. Infect. 37:153-156. [PubMed] [Google Scholar]
- 33.Mégarbane, B., P. Marchal, A. Marfaing-Koka, O. Belliard, F. Jacobs, I. Chary, et al. 2004. Increased diffusion of soluble adhesion molecules in meningitis, severe sepsis and systemic inflammatory response without neurological infection is associated with intrathecal shedding in cases of meningitis. Intensive Care Med. 30:867-874. [DOI] [PubMed] [Google Scholar]
- 33a.Matsuzawa, J., K. Sugimura, Y. Matsuda, M. Takazoe, K. Ishizuka, T. Mochizuki, S. S. Seki, O. Yoneyama, H. Bannnai, K. Suzuki, T. Honma, and H. Asakura. 2003. Association between K469E allele of intercellular adhesion molecule 1 gene and inflammatory bowel disease in a Japanese population. Gut 52:75-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melhus, A., and A. F. Ryan. 2000. Expression of cytokine genes during pneumococcal and nontypeable Haemophilus influenzae acute otitis media in the rat. Infect. Immun. 68:4024-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller, M. B., P. J. Koltai, and S. V. Hetherington. 1990. Bacterial antigens and neutrophil granule proteins in middle ear effusions. Arch. Otolaryngol. Head Neck Surg. 116:335-337. [DOI] [PubMed] [Google Scholar]
- 36.Murphy, T. F. 1996. Branhamella catarrhalis: epidemiology, surface antigenic structure, and immune response. Microbiol. Rev. 60:267-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Brien, A. D., T. J. Standiford, K. A. Bucknell, S. E. Wilcoxen, and R. Paine III. 1999. Role of alveolar epithelial cell intercellular adhesion molecule-1 in host defense against Klebsiella pneumoniae. Am. J. Physiol. 276:L961-L970. [DOI] [PubMed] [Google Scholar]
- 38.Passariello, C., S. Schippa, C. Conti, P. Russo, F. Poggiali, E. Garaci, and A. T. Palamara. 2006. Rhinoviruses promote internalisation of Staphylococcus aureus into non-fully permissive cultured pneumocytes. Microbes Infect. 8:758-766. [DOI] [PubMed] [Google Scholar]
- 39.Petersen, C. G., T. Ovesen, and C. B. Pedersen. 1998. Acute mastoidectomy in a Danish county from 1977-1996 with focus on the bacteriology. Int. J. Pediatr. Otorhinolaryngol. 45:21-29. [DOI] [PubMed] [Google Scholar]
- 40.Reference deleted.
- 41.Revai, K., L. A. Dobbs, S. Nair, J. A. Patel, J. J. Grady, and T. Chonmaitree. 2007. Incidence of acute otitis media and sinusitis complicating upper respiratory tract infection: the effect of age. Pediatrics 119:e1408-e1412. [DOI] [PubMed] [Google Scholar]
- 42.Rosenfeld, R. M., and D. Kay. 2003. Natural history of untreated otitis media. Laryngoscope 113:1645-1657. [DOI] [PubMed] [Google Scholar]
- 43.Rothlein, R., M. L. Dustin, S. D. Marlin, and T. A. Springer. 1986. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J. Immunol. 137:1270-1274. [PubMed] [Google Scholar]
- 44.Rudberg, R. D. 1954. Acute otitis media: comparative therapeutic results of sulphonamide and penicillin administered in various forms. Acta Otolaryngol. (Stockholm) 113(Suppl.):9-79. [PubMed] [Google Scholar]
- 45.Russo, E., C. W. Smith, E. M. Friedman, E. O. Smith, and S. L. Kaplan. 2004. Cell adhesion molecules and cytokines in middle ear effusions in children with or without recent acute otitis media. Otolaryngol. Head Neck Surg. 130:242-248. [DOI] [PubMed] [Google Scholar]
- 46.Samuelson, A., S. Borrelli, R. Gustafson, L. Hammarstrom, C. I. Smith, et al. 1995. Characterization of Haemophilus influenzae isolates from the respiratory tract of patients with primary antibody deficiencies: evidence for persistent colonizations. Scand. J. Infect. Dis. 27:303-313. [DOI] [PubMed] [Google Scholar]
- 47.Springer, T. A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76:301-314. [DOI] [PubMed] [Google Scholar]
- 48.Sulik, A., M. Wojtkowska, D. Rozkiewicz, and E. Oldak. 2006. Increase in adhesion molecules in cerebrospinal fluid of children with mumps and mumps meningitis. Scand. J. Immunol. 64:420-424. [DOI] [PubMed] [Google Scholar]
- 49.Terada, N., A. Konno, T. Yamashita, et al. 1993. Serum level of soluble ICAM-1 in subjects with nasal allergy and ICAM-1 mRNA expression in nasal mucosa. Jpn. J. Allergol. 42:87-93. [PubMed] [Google Scholar]
- 50.Reference deleted.
- 51.Witkowska, A. M., M. H. Borawska, and M. Gacko. 2006. Relationship among TNF-α, sICAM-1, and selenium in presurgical patients with abdominal aortic aneurysms. Biol. Trace Element Res. 114:31-40. [DOI] [PubMed] [Google Scholar]
- 52.Xie, H., and X. X. Gu. 2008. Moraxella catarrhalis lipooligosaccharide selectively upregulates ICAM-1 expression on human monocytes and stimulates adjacent naïve monocytes to produce TNF-alpha through cellular cross-talk. Cell. Microbiol. 10:1453-1467. [DOI] [PubMed] [Google Scholar]