Abstract
A previously observed rise in the plasma viral load postpartum in both treated and untreated HIV-positive women remains unexplained. Virological and immunological markers were evaluated in HIV-negative controls and HIV-positive pregnant women with and without antiretroviral treatment. Plasma HIV RNA, CD4/CD8 T cells, and serum activation markers were sequentially measured during the third trimester, at delivery, and 2 to 8 weeks postpartum in a cohort of HIV-positive pregnant women (n = 96) enrolled in a maternal-fetal HIV transmission study and a control group of HIV-negative pregnant women (n = 28). Mean plasma HIV RNA (P = 0.003) increased from delivery to postpartum, and mean CD4 T cells (P = 0.002) and serum β2-microglobulin (P < 0.0001) increased from the third trimester through postpartum among the HIV-positive women. Mean CD8 T cells increased from the third trimester through postpartum in women receiving zidovudine (ZDV) and in those not treated (P < 0.05) but remained stable in those on highly active antiretroviral therapy (HAART) and the HIV-negative controls. Increases in serum β2-microglobulin were correlated with increases in HIV RNA (P = 0.01). HIV-positive pregnant women showed postpartum increases in plasma HIV RNA, CD4 T cells, and serum β2-microglobulin regardless of the treatment regimen. The rise in CD4 T cells and β2-microglobulin was also observed in HIV-negative pregnant women, suggesting hormonal changes and/or labor-induced cytokines may contribute to immune activation. Immune activation correlated with increased plasma HIV RNA in postpartum women despite treatment, although HAART appeared to blunt the effect. The observed rise in plasma HIV RNA postpartum, which correlated with markers of immune activation, may have implications for enhanced transmission to infants through early breast-feeding and to sexual partners.
Antiretrovirals (ARV) given to mothers during pregnancy and delivery and to infants have been shown to significantly reduce HIV perinatal transmission. The AIDS Clinical Trials Group Protocol (ACTG) 076 Study Group demonstrated that zidovudine (ZDV) treatment decreased perinatal transmission rates from 25.5% to less than 8% (7). Highly active antiretroviral therapy (HAART) given during pregnancy further reduced transmission rates to less than 1.5% (8).
In previous studies, an unexplained rise in plasma HIV RNA was observed postpartum for both treated and untreated HIV-positive women (6, 9). Immune activation may play a role, since proinflammatory and immunoregulatory cytokines are known to influence induction of HIV-1 transcription (5, 10, 12-13, 16). Elevated tumor necrosis factor alpha (TNF-α) and β2-microglobulin levels have been shown to be part of overall immune activation in HIV-positive individuals compared to findings for HIV-negative ones (14). Serum activation markers correlate well with the HIV plasma viral load and have been proposed as prognostic markers for HIV progression (11).
In normal pregnancies, serum neopterin and inflammatory cytokine levels in amniotic fluid increase toward term (4). The onset of labor has been associated with elevated levels of interleukin 1β (IL-1β), IL-6, and TNF-α in amniotic fluid and cervical secretions and increases in leukocyte density, particularly neutrophils and macrophages (21-22).
We evaluated virological and immunological markers in HIV-positive pregnant women on several different treatment regimens to assess whether elevated levels of immune activation markers correlated with increases in the plasma viral load. To explore the potential effects of pregnancy and labor per se on immune activation, we also measured markers of immune activation in HIV-negative pregnant women.
MATERIALS AND METHODS
Study population.
A secondary data analysis was performed using patient medical records and laboratory results from HIV-positive pregnant women (n = 96) prospectively enrolled in a maternal-fetal HIV transmission study through the Maternal Child Immunology Clinic of the Mattel Children's Hospital at the University of California, Los Angeles (UCLA), and the Los Angeles Pediatric AIDS Consortium from 1989 to 2003. Pregnant women diagnosed with HIV were referred to the transmission study by their medical care providers. HIV-negative pregnant women (n = 28) were enrolled as immunologic controls. These women had normal uncomplicated pregnancies and were recruited from an ethnic and socioeconomic clinic population similar to that of the HIV-positive cohort. The study received approval from the UCLA Medical Institutional Review Board. Participants were included in the analysis based on availability of specimens from the third trimester (>24 weeks gestation), delivery, and 2 to 8 weeks postpartum periods and complete clinical data.
Stratification of study population by ARV treatment regimen.
For the analysis, HIV-positive women were stratified into four groups based on their maternal ARV treatment regimen. Treatment regimens reflected established standards of care at the time of study enrollment. Group I (n = 11) was untreated with ARV, having enrolled when treatment was unavailable (1989 to 1992). Groups II and III were all initially treatment naive. The majority of Group IV was treatment naive, having been diagnosed with HIV during pregnancy. Group II (n = 12) received prenatal ZDV only. Group III (n = 37) received both prenatal and postpartum ZDV, having enrolled when ZDV treatment was given routinely during the prenatal period and continued treatment postpartum was contingent on having a CD4 count of less than 500 cells/ml (1993 to 1997). Group IV (n = 36) enrolled during 1997 to 2002 and received both prenatal and postpartum HAART regimens comprised of two nucleoside reverse transcriptase inhibitors ZDV and lamivudine (3TC) given in combination with a protease inhibitor, either nelfinavir or ritonavir. Groups II, III, and IV all received ZDV at delivery in addition to their respective prenatal/postpartum treatment regimens. Women remained on the HAART regimen though the postpartum sample collection.
Laboratory assays.
Plasma HIV RNA, CD4/CD8 T cells, and serum activation markers were sequentially measured at three time points: third trimester (week 25 to delivery), delivery, and early postpartum (weeks 2 to 8). Viral load and T-cell assays were performed in real time; serum activation marker assays were performed using frozen specimens. The postpartum time point depended on each patient's scheduled follow-up visit and availability of laboratory results (2 to 8 weeks).
Plasma HIV RNA was quantified using the Amplicor HIV-1 Monitor standard and ultrasensitive assays (Roche Molecular Systems, Somerville, NJ), with detection thresholds of ≥400 copies per milliliter and ≥50 copies per milliliter, respectively.
The lymphocyte phenotype was analyzed with a FACScan flow cytometer (Becton Dickinson, Mountain View, CA), using fluorochrome-labeled monoclonal antibodies and fluorescence detection by laser flow cytometry (24).
Serum activation marker levels were quantified using commercial assays. β2-Microglobulin was measured using the Imx β2 M kit (Abbott Laboratories, North Chicago, IL), neopterin with the ELI test (BRAHMS Diagnostica GmbH, Berlin, Germany), and TNF-α with the Innotest hTNF-α (Innogenetics, Gent, Belgium) and TNF-α EASIA (BioSource Europe SA, Nivelles, Belgium) kits. The assays were performed by the UCLA Immunology Core Laboratory, which has established median values using these assays for HIV-positive and HIV-negative individuals (1).
Statistical analysis.
Longitudinal data analysis of maternal plasma HIV RNA, CD4/CD8 T cells, and serum activation markers used the Proc Mixed method (SAS 8.02). Correlations were analyzed by the Pearson correlation coefficients method. Proc GLM methods were used to compare treatment effects on the different variables at each time point.
RESULTS
Demographic characteristics.
The study population of HIV-positive pregnant women was comprised of 51% Latina, 24% Caucasian, and 20% African-American women, as well as 6% other ethnicities, including Asian and Native American. The mean age at delivery was 26.5 years, with no significant difference across treatment groups or in the HIV negative-control group.
Mother-to-child transmission of HIV.
Among group I mothers who received no ARV treatment, HIV transmission to infants occurred in 36% of the pregnancies (n = 4). In contrast, among group III mothers who received prenatal and postpartum ZDV, HIV transmission occurred in 13.5% of pregnancies (n = 5). HIV-positive infants were diagnosed based upon positive HIV-DNA PCR results at two different time points. There were no HIV-positive infants born to mothers in groups II and IV. None of the infants were breast-fed in any of the four maternal treatment groups.
Plasma viral load.
As shown in Table 1, mean maternal plasma HIV RNA decreased from the third trimester to delivery within all three groups receiving prenatal ARV treatment. However, viral levels increased from delivery to 2 to 8 weeks postpartum within all four groups (P = 0.003). Among untreated women (group I), mean HIV RNA levels increased from the third trimester to delivery and continued to increase postpartum. Women who received prenatal ZDV only (group II) experienced a transient drop from the third trimester to delivery, followed by an increase postpartum. A similar pattern of change was observed in those who remained on either ZDV (group III) or HAART (group IV) postpartum, characterized by a decrease from the third trimester to delivery followed by an increase postpartum. Postpartum increases in HIV RNA (P < 0.05) occurred in women both on ZDV and on HAART despite continued ARV treatment.
TABLE 1.
Mean levels of plasma HIV RNA, CD4, CD8, and serum β2-microglobulin in HIV-positive pregnant women by treatment group and in HIV-negative pregnant women at the third trimester, delivery, and postpartum period
| Factor measured and treatment groupa | Mean level (± SE) |
||
|---|---|---|---|
| Third trimester | Delivery | Postpartum | |
| HIV RNA (copies/ml) | |||
| Group I* | 7,248 (±1,529) | 37,970 (±11,027) | 51,517 (±7,396) |
| Group II* | 8,399 (±2,802) | 4,539 (±2,026) | 16,854 (±5,020) |
| Group III* | 42,004 (±10,512) | 15,519 (±3,103) | 34,742 (±6,692) |
| Group IV* | 5,740 (±2,089) | 1,321 (±392) | 3,901 (±1,258) |
| CD4 (cells/ml) | |||
| Group I | 622 (±60) | 729 (±47) | 744 (±44) |
| Group II | 692 (±68) | 759 (±80) | 900 (±82) |
| Group III | 333 (±27) | 360 (±31) | 426 (±32) |
| Group IV | 444 (±34) | 500 (±36) | 565 (±45) |
| HIV-negative* | 1,011 (±63) | 985 (±92) | 1,081 (±62) |
| CD8 (cells/ml) | |||
| Group I* | 799 (±52) | 919 (±79) | 1,057 (±81) |
| Group II* | 850 (±71) | 998 (±144) | 1,218 (±147) |
| Group III* | 809 (±55) | 880 (±68) | 1,038 (±71) |
| Group IV* | 904 (±67) | 906 (±68) | 1,013 (±70) |
| HIV-negative* | 667 (±47) | 719 (±53) | 718 (±44) |
| β2-Microglobulin (mg/liter) | |||
| Group I* | 1.68 (±0.16) | 2.26 (±0.27) | 2.36 (±0.23) |
| Group II* | 1.39 (±0.07) | 1.56 (±0.10) | 1.73 (±0.11) |
| Group III* | 1.78 (±0.08) | 1.81 (±0.09) | 2.33 (±0.10) |
| Group IV* | 1.58 (±0.09) | 1.79 (±0.07) | 1.74 (±0.07) |
| HIV-negative* | 1.15 (±0.05) | 1.39 (±0.10) | 1.38 (±0.04) |
Group I, untreated with ARV; group II, ZDV prenatal only; group III, ZDV prenatal and postpartum; group IV, HAART prenatal and postpartum. *, P < 0.05 for time trend.
CD4 T cells.
Mean numbers of CD4 T cells increased within each of the four HIV-positive groups from the third trimester through 2 to 8 weeks postpartum (P = 0.002), as shown in Table 1. Untreated women had increases from the third trimester to delivery, which continued to the postpartum period. A similar pattern of increases from the third trimester to delivery to the postpartum period was observed in the three treatment groups. HIV-negative pregnant women showed a transient drop from the third trimester to delivery but then had an increase postpartum as well. A postpartum increases in numbers of CD4 T cells were observed in all four of the HIV-positive groups (P = 0.01) despite the concurrent rise in HIV RNA.
CD8 T cells.
Mean CD8 T cells increased from the third trimester through 2 to 8 weeks postpartum in the two ZDV treatment groups and in the untreated group (P < 0.05) but remained stable in the HAART group and the HIV-negative controls. As shown in Table 1, untreated women had increases in mean CD8 T cells from the third trimester to delivery and continued to show an increase postpartum. A similar pattern of increase from the third trimester to delivery to postpartum was observed in both ZDV treatment groups. Increases in CD8 T cells occurred from delivery to postpartum among women on the HAART regimen and from the third trimester to delivery among HIV-negative women.
Serum activation markers.
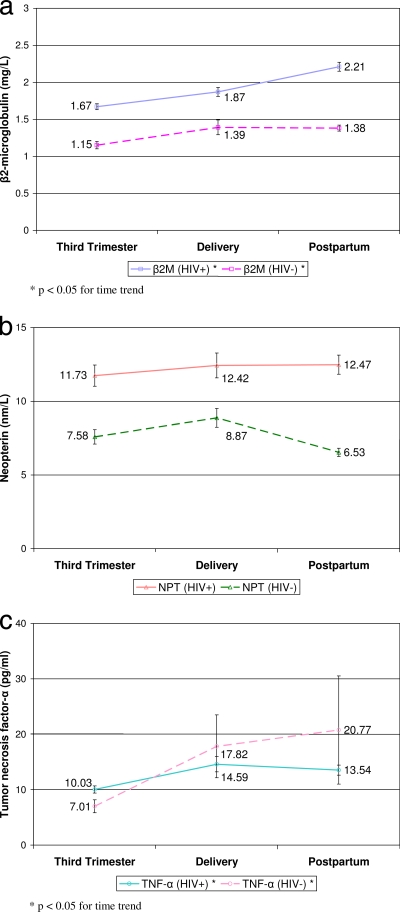
β2-Microglobulin and neopterin levels were higher for HIV-positive women than for HIV-negative controls (P < 0.01) at all time points, as shown in Fig. 1. TNF-α levels were higher for HIV-positive women than for HIV-negative controls only at the third trimester (P = 0.01).
FIG. 1.
Mean levels of serum activation markers in HIV-positive and HIV-negative pregnant women at the third trimester (pretreatment), at delivery, and postpartum (2 to 8 weeks).
Among HIV-positive women, β2-microglobulin levels correlated with the levels of neopterin and TNF-α at all three time points. β2-Microglobulin levels correlated with neopterin levels at the third trimester (r = 0.36; P = 0.024), delivery (r = 0.64; P = 0.001), and postpartum (r = 0.63; P < 0.001). β2-Microglobulin levels correlated with TNF-α levels at the third trimester (r = 0.49; P < 0.001), delivery (r = 0.54; P = 0.009) and postpartum (r = 0.57; P < 0.001).
When HIV-positive women were stratified by treatment groups, mean β2-microglobulin levels increased from the third trimester through 2 to 8 weeks postpartum within each group (P < 0.0001), but there was no difference across groups, as shown in Table 1. Mean β2-microglobulin levels increased from the third trimester to delivery and continued to increase postpartum among untreated women and in both ZDV treatment groups. Women on the HAART regimen also had mean β2-microglobulin levels that increased from the third trimester to delivery but stabilized postpartum, a pattern similar to that seen among HIV-negative controls. Observed increases in β2-microglobulin correlated within individuals with increases in HIV RNA (P = 0.01).
DISCUSSION
HIV-positive pregnant women in our study showed postpartum increases in mean plasma HIV RNA, CD4 T cells, and serum β2-microglobulin regardless of ARV treatment. Since the rise in CD4 T cells and β2-microglobulin was also seen in HIV-negative pregnant women, this suggests hormonal changes and/or labor-induced cytokines may contribute to the observed immune activation. The immune activation correlated with increased plasma HIV RNA in postpartum women despite treatment. Increases in the plasma viral load were also observed for women who continued on ZDV and HAART postpartum, although HAART appeared to blunt the effect.
Our observation that CD4 and CD8 T cells increased from the third trimester through the early postpartum period in HIV-positive women is consistent with findings of previous studies (27). Among HIV-negative women, the decrease observed from the third trimester to delivery reflects immunosuppression seen in normal pregnancies (3). CD8 T cell levels remained stable from delivery to the early postpartum period among women receiving HAART and more closely resembled the pattern of change observed among HIV-negative women. This result suggests a blunting of immune activation in HAART recipients compared with findings for women not on treatment or receiving ZDV monotherapy.
Our finding of higher β2-microglobulin levels at all time points among HIV-positive women than for HIV-negative controls concurs with previous results (23). β2-Microglobulin, neopterin, and TNF-α levels increased from the third trimester to delivery for both HIV-positive and HIV-negative women, suggesting the labor process may be involved in the observed immune activation. There were not enough samples available for neopterin (NPT) and TNF-α testing for the results from HIV-positive women to be stratified by treatment group. We were unable to evaluate the potential effect of labor on CD4/CD8 T cells and serum activation markers because more than 90% of the women in our study had vaginal deliveries with labor. Further studies with women who have elective Cesarean delivery, therefore allowing evaluation of the effect of labor on immune activation, would be of interest.
Immune activation that results in a systemic increase in the viral load may further enhance transmission risk. We observed a rise in postpartum plasma HIV RNA accompanied by a concurrent increase in CD4 T cells and serum β2-microglobulin. Increases in serum β2-microglobulin correlated best with increases in HIV RNA. Within the genital tract, localized immune activation may upregulate viral replication and increase the transmission risk (25). Elevated concentrations of proinflammatory cytokines and immune activation markers have been shown to correlate with significant viral load increases in female genital secretions upon development of genital ulceration (17). This immune activation may contribute to the increased HIV transmission risk observed with sexually transmitted disease (STD) coinfections, especially when ulcerative sores are present (28). A higher plasma maternal viral load and mastitis have been associated with elevated levels of HIV RNA in breast milk, which increases the risk of HIV transmission through breast-feeding (15, 20). Immune activation may exacerbate the mastitis by recruiting inflammatory cells to enter the breast milk and by upregulating viral replication in the infected cells of the inflamed breast tissue, resulting in higher levels of virus in the breast milk.
In this analysis, we were unable to evaluate the potential impact of treatment adherence levels and HIV-1 drug resistance prevalence. Lack of adherence may lead to an increase in the viral load, a decrease in CD4 T cells, and development of drug resistance (18). HIV-1 drug resistance may have contributed to the observed increase in the viral load. However, postpartum increases in viral load, CD4 T cell numbers, and β2-microglobulin also occurred among untreated women, suggesting the observed increases cannot be explained solely by low treatment adherence or HIV-1 drug resistance.
Current treatment recommendations from the U.S. Public Health Service call for postpartum women with nadir CD4 counts of less than 500 to continue their antiretroviral regimens (19). Considering our findings, it would be of interest to further evaluate proinflammatory cytokines in women who continue or interrupt International Maternal Pediatric Adolescent AIDS Clinical Trials (HAART) postpartum in both breast-feeding and non-breast-feeding populations, as planned in upcoming studies, such as the PROMISE study (Promoting Maternal-Infant Survival Everywhere), a large, multicountry clinical trial by the NIH IMPAACT network. Studies in resource-limited sectors have shown reduced rates of postnatal transmission among HIV-exposed infants of breast-feeding mothers when the infants are given ARV treatment through the first 6 to 14 weeks of life (2, 26; A. Bedri and the Six-Week Extended Dose Nevirapine [SWEN] Study Team, presented at the 17th Conference on Retroviruses and Opportunistic Infections, San Francisco, CA, 2010). The SWEN studies extended the infants' nevirapine (NVP) regimen through 6 weeks of age, while the Postexposure Prophylaxis of Infants (PEPI) study extended regimens of either NVP or NVP in combination with ZDV through 14 weeks of age (2). The observed correlation between a postpartum rise in plasma HIV RNA and immune activation may have implications for enhanced transmission both to infants by early breast-feeding and also to sexual partners.
Acknowledgments
We are grateful for the contributions of Audra Deveikis of Long Beach Memorial Hospital, Long Beach, CA, and Margaret Keller of Harbor-UCLA Medical Center for their participation in the recruitment and clinical care of the mothers, Jaime Deville and Karin Nielsen of the UCLA Care-4-Families Clinic for their assistance with the postpartum follow-up of the mothers, and Najib Aziz of the UCLA Immunology Core Laboratory for his assistance with the serum activation markers assays.
Funding for this study was provided by the National Institutes of Health (R0132440, R01-HD30629, and UCLA CFAR AI028697), Pediatric AIDS Clinical Trials Group (U01-AI27550), Clinical Research Center (M01-RR00865), and Universitywide AIDS Research Program of the University of California Research (Postdoctoral Research Fellowship Award to Hong-Ha M. Truong [F01-LA-0930]).
Footnotes
Published ahead of print on 27 October 2010.
REFERENCES
- 1.Aziz, N., P. Nishanian, J. M. Taylor, R. T. Mitsuyasu, J. M. Jacobson, B. J. Dezube, M. M. Lederman, R. Detels, and J. L. Fahey. 1999. Stability of plasma levels of cytokines and soluble activation markers in patients with human immunodeficiency virus infection. J. Infect. Dis. 179:843-848. [DOI] [PubMed] [Google Scholar]
- 2.Bedri, A., B. Gudetta, A. Isehak, S. Kumbi, S. Lulseged, Y. Mengistu, A. V. Bhore, R. Bhosale, V. Varadhrajan, N. Gupte, J. Sastry, N. Suryavanshi, S. Tripathy, F. Mmiro, M. Mubiru, C. Onyango, A. Taylor, P. Musoke, C. Nakabiito, A. Abashawl, R. Adamu, G. Antelman, R. C. Bollinger, P. Bright, M. A. Chaudhary, J. Coberly, L. Guay, M. G. Fowler, A. Gupta, E. Hassen, J. B. Jackson, L. H. Moulton, U. Nayak, S. B. Omer, L. Propper, M. Ram, V. Rexroad, A. J. Ruff, A. Shankar, and S. Zwerski. 2008. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet 372:300-313. [DOI] [PubMed] [Google Scholar]
- 3.Burns, D. N., P. Nourjah, H. Minkoff, J. Korelitz, R. J. Biggar, S. Landesman, A. Rubinstein, D. Wright, and R. P. Nugent. 1996. Changes in CD4+ and CD8+ cell levels during pregnancy and post partum in women seropositive and seronegative for human immunodeficiency virus-1. Am. J. Obstet. Gynecol. 174:1461-1468. [DOI] [PubMed] [Google Scholar]
- 4.Burns, D. N., P. Nourjah, D. J. Wright, H. Minkoff, S. Landesman, A. Rubinstein, J. J. Goedert, and R. P. Nugent. 1999. Changes in immune activation markers during pregnancy and postpartum. J. Reprod. Immunol. 42:147-165. [DOI] [PubMed] [Google Scholar]
- 5.Butera, S. T., B. D. Roberts, and T. M. Folks. 1993. Regulation of HIV-1 expression by cytokine networks in a CD4+ model of chronic infection. J. Immunol. 150:625-634. [PubMed] [Google Scholar]
- 6.Cao, Y., P. Krogstad, B. T. Korber, R. A. Koup, M. Muldoon, C. Macken, J. L. Song, Z. Jin, J. Q. Zhao, S. Clapp, I. S. Chen, D. D. Ho, and A. J. Ammann. 1997. Maternal HIV-1 viral load and vertical transmission of infection: the Ariel Project for the prevention of HIV transmission from mother to infant. Nat. Med. 3:549-552. [DOI] [PubMed] [Google Scholar]
- 7.Connor, E. M., R. S. Sperling, R. Gelber, P. Kiselev, G. Scott, M. J. O'Sullivan, R. VanDyke, M. Bey, W. Shearer, R. L. Jacobson, et al. 1994. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N. Engl. J. Med. 331:1173-1180. [DOI] [PubMed] [Google Scholar]
- 8.Cooper, E. R., M. Charurat, L. Mofenson, I. C. Hanson, J. Pitt, C. Diaz, K. Hayani, E. Handelsman, V. Smeriglio, R. Hoff, and W. Blattner. 2002. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J. Acquir. Immune Defic. Syndr. 29:484-494. [DOI] [PubMed] [Google Scholar]
- 9.Ekpini, R. A., J. N. Nkengasong, T. Sibailly, C. Maurice, C. Adje, B. B. Monga, T. H. Roels, A. E. Greenberg, and S. Z. Wiktor. 2002. Changes in plasma HIV-1-RNA viral load and CD4 cell counts, and lack of zidovudine resistance among pregnant women receiving short-course zidovudine. AIDS 16:625-630. [DOI] [PubMed] [Google Scholar]
- 10.Fahey, J. L., J. M. Taylor, R. Detels, B. Hofmann, R. Melmed, P. Nishanian, and J. V. Giorgi. 1990. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N. Engl. J. Med. 322:166-172. [DOI] [PubMed] [Google Scholar]
- 11.Fahey, J. L., J. M. Taylor, B. Manna, P. Nishanian, N. Aziz, J. V. Giorgi, and R. Detels. 1998. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T-cell measurements. AIDS 12:1581-1590. [DOI] [PubMed] [Google Scholar]
- 12.Folks, T. M., K. A. Clouse, J. Justement, A. Rabson, E. Duh, J. H. Kehrl, and A. S. Fauci. 1989. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc. Natl. Acad. Sci. U. S. A. 86:2365-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folks, T. M., J. Justement, A. Kinter, C. A. Dinarello, and A. S. Fauci. 1987. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science 238:800-802. [DOI] [PubMed] [Google Scholar]
- 14.Giron-Gonzalez, J. A., A. Lopez-Sanchez, J. Elvira, E. Perez, and C. Fernandez-Gutierrez. 2000. Effect of patient adherence to antiretroviral therapy on CD4+ cell count, HIV-1 RNA, and serum concentrations of tumor necrosis factor and its soluble receptors. Eur. J. Clin. Microbiol. Infect. Dis. 19:852-858. [DOI] [PubMed] [Google Scholar]
- 15.John, G. C., R. W. Nduati, D. A. Mbori-Ngacha, B. A. Richardson, D. Panteleeff, A. Mwatha, J. Overbaugh, J. Bwayo, J. O. Ndinya-Achola, and J. K. Kreiss. 2001. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J. Infect. Dis. 183:206-212. [DOI] [PubMed] [Google Scholar]
- 16.Kottilil, S., J. Gamberg, I. Bowmer, J. Trahey, C. Howley, M. Gallant, and M. Grant. 2000. Human immunodeficiency virus type 1 replication, immune activation, and circulating cytotoxic T cells against uninfected CD4+ T cells. J. Clin. Immunol. 20:175-186. [DOI] [PubMed] [Google Scholar]
- 17.Lawn, S. D., S. T. Butera, and T. M. Folks. 2001. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin. Microbiol. Rev. 14:753-777, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mannheimer, S., G. Friedland, J. Matts, C. Child, and M. Chesney. 2002. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin. Infect. Dis. 34:1115-1121. [DOI] [PubMed] [Google Scholar]
- 19.National Institutes of Health. 24 May 2010. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States, p. 1-117. National Institutes of Health, Bethesda, MD.
- 20.Nduati, R., D. Mbori-Ngacha, G. John, B. Richardson, and J. Kreiss. 2000. Breastfeeding in women with HIV. JAMA 284:956-957. [PubMed] [Google Scholar]
- 21.Olah, K. S., G. S. Vince, J. P. Neilson, G. Deniz, and P. M. Johnson. 1996. Interleukin-6, interferon-gamma, interleukin-8, and granulocyte-macrophage colony stimulating factor levels in human amniotic fluid at term. J. Reprod. Immunol. 32:89-98. [DOI] [PubMed] [Google Scholar]
- 22.Osman, I., A. Young, M. A. Ledingham, A. J. Thomson, F. Jordan, I. A. Greer, and J. E. Norman. 2003. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol. Hum. Reprod. 9:41-45. [DOI] [PubMed] [Google Scholar]
- 23.Piwowar, E. M., S. B. Tugume, R. M. Grant, T. Lutalo, K. Pattishall, and E. Katongole-Mbidde. 1995. Beta-2 microglobulin values among human immunodeficiency virus (HIV)-negative, HIV-positive asymptomatic, and HIV-positive symptomatic Ugandans. Clin. Diagn. Lab. Immunol. 2:236-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plaeger-Marshall, S., V. Isacescu, S. O'Rourke, J. Bertolli, Y. J. Bryson, and E. R. Stiehm. 1994. T cell activation in pediatric AIDS pathogenesis: three-color immunophenotyping. Clin. Immunol. Immunopathol. 71:19-26. [DOI] [PubMed] [Google Scholar]
- 25.Quinn, T. C., M. J. Wawer, N. Sewankambo, D. Serwadda, C. Li, F. Wabwire-Mangen, M. O. Meehan, T. Lutalo, and R. H. Gray. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N. Engl. J. Med. 342:921-929. [DOI] [PubMed] [Google Scholar]
- 26.Taha, T. E., J. Kumwenda, S. R. Cole, D. R. Hoover, G. Kafulafula, M. G. Fowler, M. C. Thigpen, Q. Li, N. I. Kumwenda, and L. Mofenson. 2009. Postnatal HIV-1 transmission after cessation of infant extended antiretroviral prophylaxis and effect of maternal highly active antiretroviral therapy. J. Infect. Dis. 200:1490-1497. [DOI] [PubMed] [Google Scholar]
- 27.Tuomala, R. E., L. A. Kalish, C. Zorilla, H. Fox, W. Shearer, A. Landay, S. H. Vermund, S. Landesman, and D. Burns. 1997. Changes in total, CD4+, and CD8+ lymphocytes during pregnancy and 1 year postpartum in human immunodeficiency virus-infected women. The Women and Infants Transmission Study. Obstet. Gynecol. 89:967-974. [DOI] [PubMed] [Google Scholar]
- 28.Wawer, M. J., R. H. Gray, N. K. Sewankambo, D. Serwadda, X. Li, O. Laeyendecker, N. Kiwanuka, G. Kigozi, M. Kiddugavu, T. Lutalo, F. Nalugoda, F. Wabwire-Mangen, M. P. Meehan, and T. C. Quinn. 2005. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J. Infect. Dis. 191:1403-1409. [DOI] [PubMed] [Google Scholar]