Abstract
The objectives of this study were to compare relative vaccine-specific serum immunoglobulin concentrations, vaccine-specific lymphoproliferative responses, and cytokine profiles of proliferating lymphocytes between 3-day-old foals, 3-month-old foals, and adult horses after vaccination with a killed adjuvanted vaccine. Horses were vaccinated intramuscularly twice at 3-week intervals with a vaccine containing antigens from bovine viral respiratory pathogens to avoid interference from maternal antibody. Both groups of foals and adult horses responded to the vaccine with a significant increase in vaccine-specific IgGa and IgG(T) concentrations. In contrast, only adult horses and 3-month-old foals mounted significant vaccine-specific total IgG, IgGb, and IgM responses. Vaccine-specific concentrations of IgM and IgG(T) were significantly different between all groups, with the highest concentrations occurring in adult horses, followed by 3-month-old foals and, finally, 3-day-old foals. Only the adult horses mounted significant vaccine-specific lymphoproliferative responses. Baseline gamma interferon (IFN-γ) and interleukin-4 (IL-4) concentrations were significantly lower in 3-day-old foals than in adult horses. Vaccination resulted in a significant decrease in IFN-γ concentrations in adult horses and a significant decrease in IL-4 concentrations in 3-day-old foals. After vaccination, the ratio of IFN-γ/IL-4 in both groups of foals was significantly higher than that in adult horses. The results of this study indicate that the humoral and lymphoproliferative immune responses to this killed adjuvanted vaccine are modest in newborn foals. Although immune responses improve with age, 3-month-old foals do not respond with the same magnitude as adult horses.
Development of the equine immune system occurs relatively early during fetal life. Lymphocytes are present in the peripheral blood of the equine fetus by day 120 of gestation, and they proliferate in response to mitogens by day 140 (21). Specific antibody responses to in utero vaccination with coliphage T2 have been detected in equine fetuses as early as day 200 of gestation (16). In other studies, administration of a Venezuelan equine encephalomyelitis antigen to equine fetuses between 232 and 283 days of gestational age resulted in serum neutralization titers higher than those elicited by the same preparation in adult horses (18, 19). Recent work supports these findings, showing that active B-cell development and immunoglobulin isotype switching occur during equine gestation and the neonatal period (27). Proliferation of peripheral blood lymphocytes in response to mitogens is slightly reduced at birth but rapidly increases to adult levels (6, 25). Foals also have normal lymphokine-activated killing (LAK) cell activity of peripheral blood lymphocytes at birth and during early life (6).
Although these findings suggest that newborn foals should be able to mount adequate immune responses at birth, maternal antibodies acquired through ingestion of colostrum have been shown to exert a considerable suppressive effect on antibody production (13, 29). In addition, the recognized type 2 bias in the immune responses of murine and human neonates, along with the recent finding that young foals are deficient in their ability to produce gamma interferon (IFN-γ) in response to stimulation with mitogens, has led to the widespread hypothesis that foals are born with an inherent inability to mount a strong cell-mediated immune response (3, 4). Foals may not develop cytotoxic T-lymphocyte responses until 6 to 8 weeks of age (20). Recently, we have demonstrated that newborn foals can produce robust IFN-γ responses and high concentrations of all IgG subclasses when they are challenged intrabronchially with virulent Rhodococcus equi (10-12). However, there are no studies comparing the primary humoral and cell-mediated immune responses of newborn foals to those of older foals and adult horses following vaccination in the absence of vaccine-specific maternal antibody interference. A thorough understanding of immune responses of newborn foals following vaccination would be essential for the future development of rational strategies for vaccination against pathogens likely to infect foals early in life.
The objectives of this study were to compare serum IgM and IgG subclass concentrations, antigen-specific lymphoproliferative responses, and cytokine profiles of proliferating lymphocytes of newborn foals, older foals, and adult horses following vaccination with a killed adjuvanted vaccine. A killed adjuvanted vaccine was selected because most vaccines commercially available for use in horses currently are of this type. The central hypothesis for the present study was that newborn foals mount inferior immune responses to a killed vaccine compared to adult horses.
MATERIALS AND METHODS
Animals and experimental design.
Thirty-two healthy Thoroughbred or Thoroughbred-cross foals were used. The foals were considered healthy on the basis of adequate transfer of passive immunity at 24 h of age, findings of complete blood cell counts and physical examination at the time of vaccination, and daily monitoring until completion of the study. Healthy adult horses (n = 6) were also used. Foals were randomly assigned to one of two age groups indicating age at the time of the first vaccination: 3 days old (n = 11) and 3 months old (n = 15). Each animal received a series of two intramuscular injections of a killed adjuvanted cattle vaccine (Triangle4; Fort Dodge Animal Health, Fort Dodge, IA) at 3-week intervals. This vaccine included antigens from type II bovine viral diarrhea virus, infectious bovine rhinotracheitis virus, parainfluenza virus type 3, and bovine respiratory syncytial virus. The vaccine was selected on the basis of the lack of detectable serum antibody to these antigens in horses as well as demonstrated safety and immunogenicity in horses (26). Blood was collected from each animal prior to vaccination (baseline), 3 weeks after administration of the first dose and prior to administration of the second dose (dose 1), and 3 weeks after administration of the second dose (dose 2).
Blood collection and cell separation.
One hundred milliters of blood was collected by jugular venipuncture using heparin as the anticoagulant. In addition, blood (10 ml) was collected without anticoagulant for separation of serum. Peripheral blood mononuclear cells (PBMCs) were harvested from blood samples by density gradient centrifugation (Ficoll-Paque; Amersham Biosciences, Pittsburgh, PA), washed 3 times with phosphate-buffered saline (PBS), and counted using a hemacytometer. Aliquots of 3 × 107 PBMCs were cryogenically preserved in 90% fetal bovine serum and 10% dimethyl sulfoxide (DMSO) in liquid nitrogen until they were used for lymphocyte proliferation and cytokine assays (see below). Serum was stored at −80°C until it was used for measurement of vaccine-specific immunoglobulin concentrations.
Vaccine-specific serum immunoglobulin concentrations.
Vaccine-specific relative IgM, total IgG, IgGa, IgGb, and IgG(T) concentrations in serum were determined by enzyme-linked immunosorbent assay (ELISA) as previously described (11). Optimal dilutions of reagents were determined by checkerboard titration. Briefly, wells in 96-well microtiter plates (Immulon II; Thermo Fisher Scientific, Waltham, MA) were coated at 4°C overnight with whole vaccine (Triangle4; Fort Dodge Animal Health) diluted 1:250 in carbonate-bicarbonate buffer (pH 9.6; total volume, 100 μl/well). Plates were washed 4 times with PBS-0.05% Tween 20 between each of the following incubations. Plates were blocked with PBS-1% bovine serum albumin for 1 h at room temperature. Serum from each experimental animal was diluted 1:100, and 100 μl was added to each well for 1 h of incubation at room temperature. To determine isotype-specific responses, 100 μl of peroxidase-conjugated goat anti-equine IgGa (1:5,000), IgGb (1:5,000), IgG(T) (1:1,000), and IgM (1:2,500) (Serotec, Raleigh, NC) was added to the wells for 1 h of incubation at room temperature. After addition of substrate [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid); Roche Diagnostics, Indianapolis, IN], the plates were incubated for 45 min in the dark at room temperature and the optical density at 405 nm (OD405) was measured. For each immunoglobulin subisotype measured, serum from a high responder was serially diluted to generate a standard curve for relative quantification of immunoglobulin concentrations in the experimental animals. The sample dilutions used to generate the standard curve were run on each plate to correct for interplate variability. Wells incubated without serum were used as blanks to subtract out the background absorbance. Each sample was run in triplicate, and the mean OD was used.
Vaccine-specific lymphocyte proliferation.
Immediately after they were thawed, PBMCs were washed twice and placed in minimal essential medium alpha (MEMα) supplemented with 10% horse serum, 2 mM glutamine, and penicillin-streptomycin (100 U and 100 μg per ml, respectively). More than 80% of the cells were viable after they were thawed, as assessed by trypan blue (Mediatech, Herndon, VA) exclusion. Lymphoproliferative responses were assessed using a nonradioactive colorimetric assay. The results of this assay have been shown to correlate closely with those of conventional radioactive [3H]thymidine incorporation in many species, including the horse (2, 30). In preliminary experiments, the adjuvant of the killed vaccine was found to exert mitogenic effects on equine lymphocytes, thereby preventing our ability to detect vaccine-specific lymphoproliferative responses. A modified live vaccine (Pyramid5; Fort Dodge Animal Health) containing the same viral agents as the killed adjuvanted vaccine was used as a source of antigen for the lymphoproliferative assay. The advantage of the modified live vaccine was that antigen and adjuvants were provided in separate vials. Vaccine antigen was diluted in MEMα and inactivated by heating at 60°C for 1 h. The optimal concentration of antigen (1:7,000) was determined in preliminary experiments. Aliquots (100 μl) of cells (1 × 106 cells/ml) were placed in triplicate wells of 96-well black plates with flat, clear-bottom wells (Corning Inc., Corning, NY). Cells were separately incubated either without antigen (blank), with 5 μg/ml of pokeweed mitogen (positive control), or with vaccine antigen. The cells were stimulated at 37°C for 72 h in 6% CO2. Eighteen hours before the end of the assay, 20 μl of alamar blue (Accumed International Inc., Westlake, OH) was added to each well and fluorescence was determined with a fluorometer (Synergy HT; BioTek Instruments Inc., Winooski, VT) using an excitation wavelength of 530 nm and measurement of emission at 590 nm. Change in fluorescence was calculated as the mean of the stimulated cells minus the mean of the cells without antigen or mitogen (blank).
mRNA expression of interleukin-2 (IL-2) and IL-10.
PBMCs were cultured in triplicate wells for 24 h in the presence of the vaccine antigen as described above. Time of stimulation (24 h) was selected on the basis of peak mRNA expression in preliminary experiments with adult horse PBMCs. Isolation of total RNA was performed with an RNeasy kit (Qiagen Inc., Valencia, CA), according to the manufacturer's instructions. The RNA concentration was measured by determination of the OD260. All RNA samples were treated with amplification-grade DNase I (Gibco BRL, Rockville, MD) to remove any trace of genomic DNA contamination. Briefly, 1 U of DNase I and 1 μl of 10× DNase I reaction buffer were mixed with 1 μg of total RNA for a total volume of 10 μl. The mixture was incubated for 10 min at room temperature and then inactivated by the addition of 1 μl of 25 mM EDTA and incubation at 65°C for 10 min.
A commercial kit was used to synthesize cDNA (Advantage RT-for-PCR kit; Clontech, Palo Alto, CA), according to the protocol of the manufacturer. Briefly, 1 μg of DNase-treated total RNA was mixed with 1 μl of oligo(dT)18 primer (20 μM), and the mixture was heated at 70°C for 2 min. After the mixture was cooled to room temperature, the following reagents were added: 4 μl of 5× reaction buffer, 1 μl of deoxynucleoside triphosphates (10 mM each), 0.5 μl of RNase inhibitor, (40 U/μl), and 1 μl of Moloney murine leukemia virus reverse transcriptase (200 U/μl). The mixture was incubated at 42°C for 1 h, heated at 94°C for 5 min, diluted to a final volume of 100 μl, and stored at −70°C until being used for PCR analysis.
Gene-specific primers and internal oligonucleotide probes for equine glyceraldehyde-3-phosphate dehydrogenase (G3PDH), IL-2, and IL-10 have been previously reported (8). The internal probes were labeled at the 5′ end with the reporter dye 6-carboxyfluorescein and at the 3′ end with the quencher dye 6-carboxytetramethylrhodamine. Amplification of 2 μl of cDNA was performed in a 25-μl PCR mixture containing 900 nM concentrations of each primer, 250 nM TaqMan probe, and 12 μl of TaqMan universal PCR master mix (Applied Biosystems). Amplification and detection were performed with an ABI Prism 7900 sequence detection system (Applied Biosystems) with initial incubation steps at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. cDNA from equine blood mononuclear cells stimulated for 24 h with concanavalin A was used as a positive control and was run on each plate as a calibrator sample. Each sample was assayed in triplicate, and the mean value was used for comparison. Samples without cDNA were included in the amplification reactions to determine background fluorescence and to check for contamination. DNase-treated RNA samples were subjected to PCR using the G3PDH primers to confirm the absence of genomic DNA contamination. Relative gene expression was calculated using the method described by Pfaffl (22).
IFN-γ and IL-4 concentrations.
Supernatants of 2 × 107 PBMCs stimulated as described above for 72 h were collected and stored at −80°C until use. Supernatants were concentrated using a centrifugal filter device (Amicon Ultra; Millipore, Billerica, MA), according to the manufacturer's instruction. The concentrations of IL-4 and IFN-γ proteins in the concentrated supernatant were measured using commercially available equine cytokine ELISA kits (R&D Systems, Minneapolis, MN), according to the manufacturer's instructions. For each assay, a seven-point standard curve obtained using a 2-fold serial dilution of recombinant equine IFN-γ or IL-4 (starting at 1,000 pg/ml) was used for quantification and as a positive control. The lower detection limits were 30 and 16 pg/ml for IFN-γ and IL-4, respectively.
Statistical analysis.
Normality and equality of variance of the data were assessed using the Kolmogorov-Smirnov and Levene's tests, respectively. Vaccine-specific immunoglobulin concentrations and cytokine mRNA expression data were not normally distributed and were rank transformed prior to analysis. A two-way analysis of variance for repeated measurements was used to determine the effects of vaccination (baseline, dose 1, and dose 2) and experimental group (3-day-old, 3-month-old, and adult horses) and the interactions between vaccination and experimental group on antibody concentrations, lymphoproliferative responses, and cytokine induction. When appropriate, multiple pairwise comparisons were done using the Holm-Sidak test. A P value of <0.05 was considered significant. Normally distributed data are presented as means ± standard deviations (SDs) or mean and individual data points. Rank-transformed data are presented as medians and individual data points.
RESULTS
Vaccine-specific immunoglobulin concentrations.
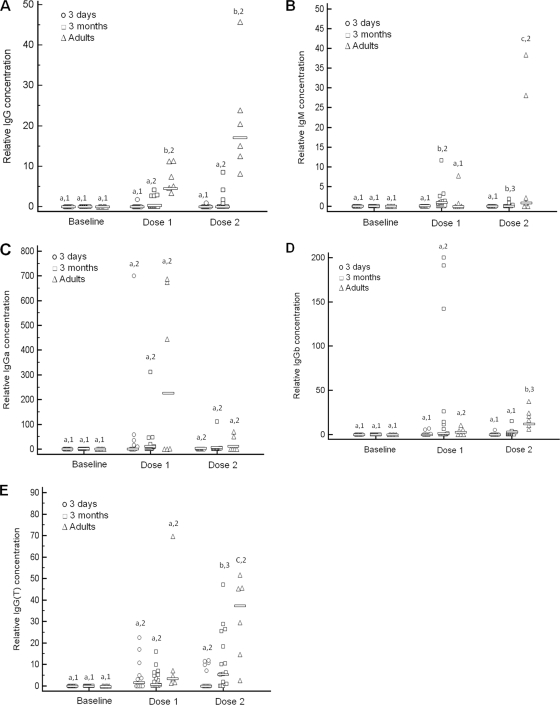
Both groups of foals and adult horses responded to the vaccine with a significant increase in relative vaccine-specific IgGa and IgG(T) concentrations (Fig. 1). In contrast, only adult horses and 3-month-old foals mounted significant vaccine-specific total IgG, IgGb, and IgM responses. Relative concentrations of vaccine-specific total IgG and IgGb after administration of two doses were significantly higher in adult horses than in both groups of foals. Relative concentrations of vaccine-specific IgM and IgG(T) after administration of two doses were significantly different among all groups, with the highest relative concentrations occurring in adult horses, followed by 3-month-old foals and, finally, 3-day-old foals (Fig. 1). Relative concentrations of IgGa were not significantly different between groups.
FIG. 1.
Relative vaccine-specific serum total IgG (A), IgM (B), IgGa (C), IgGb (D), and IgG(T) (E) concentrations determined by capture ELISA. Adult horses, 3-day-old foals, and 3-month-old foals were vaccinated with a killed adjuvanted vaccine twice with 3 weeks between administrations. Serum was collected from each animal prior to vaccination (baseline), 3 weeks after administration of the first dose and prior to administration of the second dose (dose 1), and 3 weeks after administration of the second dose (dose 2). Symbols represent individual data points. Horizontal bars indicate median values. Different numbers (1, 2, and 3) within an age group indicate significant differences between sample time points. Different letters (a, b, and c) within a time point indicate significant differences between age groups. Significance was set at a P value of <0.05.
Vaccine-specific lymphoproliferative responses and cytokine induction.
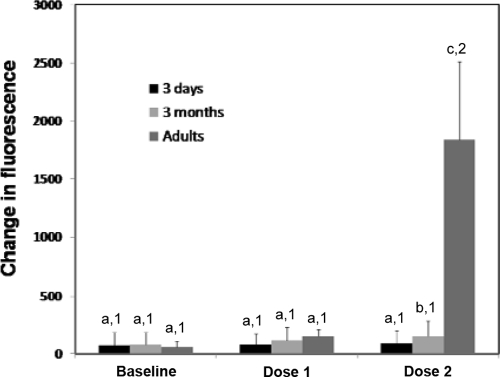
Adult horses had significantly greater lymphoproliferative responses to the vaccine antigen after the second dose of vaccine compared to those measured at the baseline or after a single dose (Fig. 2). In both groups of foals, vaccine-induced lymphoproliferative responses after vaccination were not significantly different from baseline values (Fig. 2). Lymphoproliferative responses of adult horses after administration of two doses of vaccine were significantly higher than that of either group of foals (Fig. 2). Three-month-old foals had significantly greater lymphoproliferative responses after two doses of vaccine than 3-day-old foals. There was no significant effect of vaccination on lymphoproliferative responses to pokeweed mitogen, but there was a significant effect of group (P = 0.016). Lymphoproliferative responses to pokeweed mitogen in adult horses (1,470 ± 1,058) were significantly higher than those of 3-day-old foals (387 ± 533) and 3-month-old foals (806 ± 1,608). Lymphoproliferative responses to pokeweed mitogen were not significantly different between the two groups of foals.
FIG. 2.
Mean (±SD) vaccine-specific lymphoproliferative responses determined by a colorimetric lymphocyte proliferation assay. Adult horses, 3-day-old foals, and 3-month-old foals were vaccinated with a killed adjuvanted vaccine twice with 3 weeks between administrations. PBMCs were collected from each animal prior to vaccination (baseline), 3 weeks after administration of the first dose and prior to administration of the second dose (dose 1), and 3 weeks after administration of the second dose (dose 2). Different numbers (1, 2, and 3) within an age group indicate significant differences between sample time points. Different letters (a, b, and c) within a time point indicate significant differences between age groups. Significance was set at a P value of <0.05.
Cytokine induction in vaccine-stimulated PBMCs.
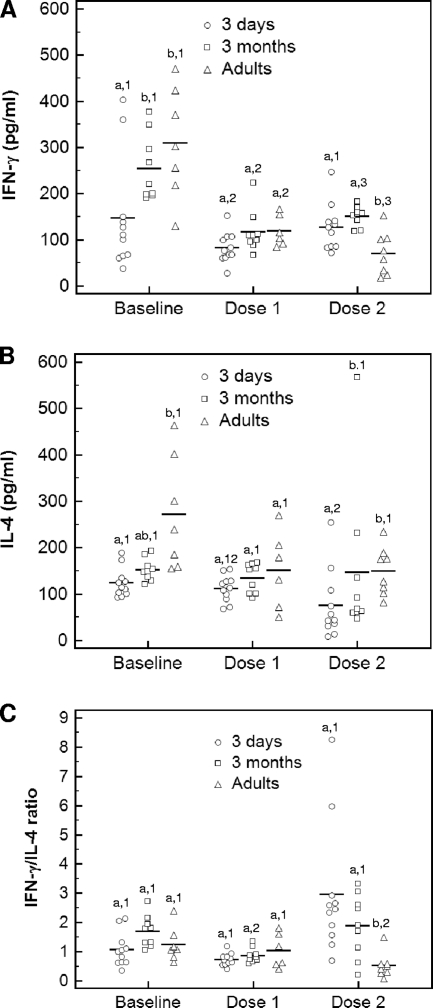
Baseline IFN-γ concentrations were significantly lower in 3-day-old foals than in 3-month-old foals and adult horses (Fig. 3A). Vaccination resulted in a significant decrease in IFN-γ concentrations after the first dose of the vaccine in 3-day-old foals and after both doses in 3-month-old foals and adult horses. After two doses of the vaccine, IFN-γ concentrations were significantly lower in adult horses than in both groups of foals (Fig. 3A). Baseline IL-4 concentrations were significantly lower in 3-day-old foals than in adult horses (Fig. 3B). Concentrations of IL-4 significantly decreased after the second vaccination compared to those at the baseline in 3-day-old foals. Concentrations of IL-4 after the second vaccination were significantly lower in 3-day-old foals than in 3-month-old foals and adult horses (Fig. 3B). There was a significant increase in the IFN-γ/IL-4 ratio after the second dose of vaccine in 3-day-old foals and a significant decrease in the same ratio in adult horses. The IFN-γ/IL-4 ratio of both groups of foals was significantly higher than that of adult horses after the second dose of the vaccine (Fig. 3C).
FIG. 3.
Mean concentrations of IFN-γ (A) and IL-4 (B) and IFN-γ/IL-4 ratio (C) in the supernatants of PBMCs stimulated with vaccine antigens determined by ELISA. Adult horses, 3-day-old foals, and 3-month-old foals were vaccinated with a killed adjuvanted vaccine twice with 3 weeks between administrations. PBMCs were collected from each animal prior to vaccination (baseline), 3 weeks after administration of the first dose and prior to administration of the second dose (dose 1), and 3 weeks after administration of the second dose (dose 2). Different numbers (1, 2, and 3) within an age group indicate significant differences between sample time points. Different letters (a, b, and c) within a time point indicate significant differences between age groups. Significance was set at a P value of <0.05.
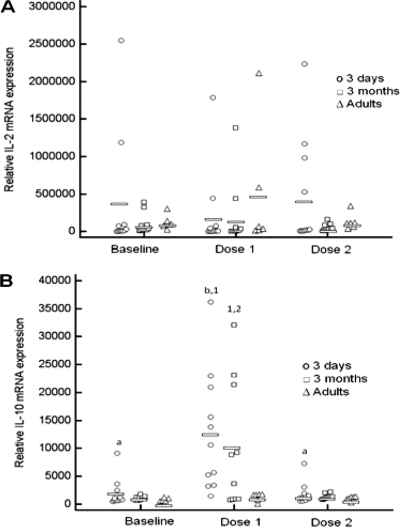
There was a significant effect of group (P = 0.033) on relative IL-2 mRNA expression, but the effect of vaccination (P = 0.17) and the interactions between group and vaccination (P = 0.97) were not statistically significant. Relative IL-2 mRNA expression in adult horses was significantly higher than that in both groups of foals (Fig. 4A). Baseline relative IL-10 mRNA expression was not significantly different between groups. There was a significant increase in relative IL-10 mRNA expression after the first dose of the vaccine in 3-day-old foals only, but expression returned to the baseline after the second dose (Fig. 4B). Relative IL-10 mRNA expression after the first dose of the vaccine was significantly higher in 3-day-old foals than in adult horses (Fig. 4B). Relative IL-10 mRNA expression did not change significantly with vaccination in 3-month-old foals or in adult horses.
FIG. 4.
Relative levels of IL-10 (A) and IL-2 (B) mRNA expression in PBMCs stimulated with vaccine antigens. Adult horses, 3-day-old foals, and 3-month-old foals were vaccinated with a killed adjuvanted vaccine twice with 3 weeks between administrations. PBMCs were collected from each animal prior to vaccination (baseline), 3 weeks after administration of the first dose and prior to administration of the second dose (dose 1), and 3 weeks after administration of the second dose (dose 2). Symbols represent individual data points. Horizontal bars indicate median values. Different numbers (1, 2, and 3) within an age group indicate significant differences between sample time points. Different letters (a, b, and c) within a time point indicate significant differences between age groups. Significance was set at a P value of <0.05.
DISCUSSION
Although vaccination of horses is widely practiced and forms an important part of infectious disease control programs, very little is known regarding development of immune responses following vaccination in newborn foals. Response to vaccination is generally assessed in a population of healthy adult horses. However, age has a profound effect on immune responses, as evidenced by the fact that old horses have decreased antibody production and lymphoproliferative responses to some vaccines (9). Current equine vaccination guidelines state that vaccination of foals should begin at between 3 and 6 months of age, depending on the specific vaccine. This is because maternal antibody acquired through ingestion of colostrum has been shown to exert a considerable suppressive effect on antibody production. This is substantiated by the fact that the onset of antibody production is advanced in colostrum-deprived foals compared to foals with adequate transfer of passive immunity (13). The rate of decline of maternal antibodies varies depending on the nature of the antigen. For many pathogens, the concentration of maternal antibody in foals falls to nonprotective levels by 2 to 3 months of age. For equine influenza and tetanus, maternal antibodies in foals born from mares vaccinated in the last 2 months of pregnancy can persist until approximately 6 months of age and prevent adequate immune responses in foals vaccinated prior to reaching that age (29). There are no studies comparing primary humoral and cell-mediated immune responses of newborn foals to those of older foals and adult horses following vaccination in the absence of antigen-specific maternal antibody interference. A thorough understanding of the default immune response of newborn foals following vaccination is essential for the future development of rational vaccination strategies for pathogens expected to cause disease early in life.
The killed adjuvanted vaccine selected for use in the present study was well tolerated and invoked both humoral and lymphoproliferative responses in adult horses. Relative vaccine-specific serum concentrations of IgM, IgG(T), IgGa, IgGb, and total IgG all increased following vaccination. Vaccine-specific total IgG, IgM, IgG(T), and IgGb concentrations in adult horses were the highest after the second vaccination. In contrast, vaccine-specific IgGa concentrations increased after the first vaccination, but a considerable decrease in IgGa concentrations was observed after the second vaccination in most horses. These results are in accordance with those of Slack et al. (26), who reported a peak in IgGa concentrations 2 weeks following administration of the second dose of the same vaccine to adult horses and a substantial decrease in IgGa concentrations at 3 weeks. Although newborn foals mounted statistically significant vaccine-specific IgGa and IgG(T) responses, the magnitude of these responses was modest compared to that of older foals and adult horses. In one study, vaccination of 8- to 15-day-old foals with two doses of a DNA vaccine expressing the vapA gene of Rhodococcus equi failed to elicit a measurable antibody response, while the same vaccine elicited robust antigen-specific IgG responses in adults (15). In contrast, infection of 7-day-old foals with live virulent R. equi resulted in a significant increase in IgGa, IgGb, IgGc, and IgM concentrations compared to preinfection values (11). In the same study, postinfection IgGa and IgGb concentrations in infected foals were significantly higher than those achieved following administration of the same inoculum to adult horses (11). Administration of a higher inoculum of the same virulent R. equi strain resulted in significantly higher IgG(T) and IgM responses (10). Collectively, these findings indicate that newborn foals can mount adequate humoral immune responses if they are provided with the right stimulus. However, the nature and dose of antigen and possibly the type of adjuvant have a profound effect on the magnitude and IgG subclass of the response in newborn foals.
The present study showed that the antigen-specific lymphoproliferative responses of adult horses are substantially greater than those of foals. Similarly, 7-day-old foals infected with virulent R. equi had decreased lymphoproliferative responses to R. equi antigens compared to adult horses (10). Although the proportion of B lymphocytes in the peripheral blood of foals (20 to 30%) is significantly greater than that of adult horses (5 to 10%) (7), the resulting small difference in the proportion of T lymphocytes between foals and adults is unlikely to be responsible for the profound difference in antigen-specific lymphocyte proliferation observed in the present study. Limited antigen-specific lymphoproliferative responses in foals are unlikely to be a result of an impaired proliferative ability of neonatal lymphocytes because previous studies have indicated that foals and adult horses have similar lymphoproliferative responses in response to stimulation with concanavalin A (6, 11). In the present study, lymphoproliferative responses to pokeweed mitogen were significantly higher in adult horses than in both groups of foals. The decreased antigen-specific lymphoproliferative responses in neonatal foals may be the result of the cell type that functions as the antigen-presenting cell during the initial immune response (23). Alternatively, decreased antigen-specific lymphoproliferative responses in neonatal foals may be the result of impaired or immature function of antigen-presenting cells. Recent studies have shown that monocyte-derived dendritic cells from foals are phenotypically different from those from adult horses, having decreased major histocompatibility complex class II and CD86 expression (5, 17).
Cell-mediated immune responses of murine and human neonates are generally thought to be biased toward a Th2 response (1). Several studies have documented that newborn foals are deficient in their ability to induce IFN-γ in response to stimulation with mitogens (3, 4). These findings, along with the peculiar susceptibility of foals to infection with R. equi, a facultative intracellular pathogen known to cause disease in immunocompetent mice only when a Th2 response is experimentally induced (14), have led to the hypothesis that T-cell responses from newborn foals may be biased toward a Th2 cytokine profile. However, experimental infection of neonatal foals with virulent R. equi triggers induction of IFN-γ mRNA transcription in a manner that is similar to that in adult horses, indicating that foals can mount adequate IFN-γ responses if they are provided the proper stimulus (11, 12). Thorough assessment of the Th1/Th2 polarization of the foals' immune responses also necessitates measurement of Th2 cytokines, such as IL-4. Recent data demonstrate that foals are also deficient in their ability to produce IL-4 in response to stimulations with mitogens, suggesting that a clear-cut polarization toward a Th2 response is unlikely in neonatal foals (24, 28). The relative Th1/Th2 polarization of equine neonatal immune responses would be better assessed by measuring antigen-specific responses after vaccination rather than after stimulation with mitogens. To the authors' knowledge, the present study is the first to measure Th1 and Th2 cytokines in response to vaccination of newborn foals with a killed adjuvanted vaccine. Consistent with studies using mitogens, baseline (prior to vaccination) IFN-γ and IL-4 concentrations in the present study were significantly lower in 3-day-old foals than in adult horses. However, the IFN-γ/IL-4 ratio after vaccination was significantly higher in both groups of foals than in adult horses. These results indicate that although basal cytokine secretion in neonatal foals may be considerably dampened, there is not a clear bias toward a Th2 response to the vaccine used in the present study.
In conclusion, the present study demonstrated considerably decreased humoral and lymphoproliferative responses in newborn foals after vaccination with a killed vaccine compared to that in adult horses even in the absence of vaccine-specific maternal antibody interference. Although immune responses to the vaccine improved with age, 3-month-old foals did not respond with the same magnitude as adult horses. However, newborn foals do not have a bias toward a Th2 response in response to vaccination. Additional studies are needed to determine the effects of type of antigen, dose of antigen, and form of adjuvant on induction of robust humoral and cell-mediated immune responses in foals.
Acknowledgments
This study was funded by the Morris Animal Foundation.
Footnotes
Published ahead of print on 13 October 2010.
REFERENCES
- 1.Adkins, B. 2000. Development of neonatal Th1/Th2 function. Int. Rev. Immunol. 19:157-171. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, S. A., R. M. Gogal, Jr., and J. E. Walsh. 1994. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 170:211-224. [DOI] [PubMed] [Google Scholar]
- 3.Boyd, N. K., N. D. Cohen, W. S. Lim, R. J. Martens, M. K. Chaffin, and J. M. Ball. 2003. Temporal changes in cytokine expression of foals during the first month of life. Vet. Immunol. Immunopathol. 92:75-85. [DOI] [PubMed] [Google Scholar]
- 4.Breathnach, C. C., T. Sturgill-Wright, J. L. Stiltner, A. A. Adams, D. P. Lunn, and D. W. Horohov. 2006. Foals are interferon gamma-deficient at birth. Vet. Immunol. Immunopathol. 112:199-209. [DOI] [PubMed] [Google Scholar]
- 5.Flaminio, M. J., D. V. Nydam, H. Marquis, M. B. Matychak, and S. Giguère. 2009. Foal monocyte-derived dendritic cells become activated upon Rhodococcus equi infection. Clin. Vaccine Immunol. 16:176-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flaminio, M. J., B. R. Rush, E. G. Davis, K. Hennessy, W. Shuman, and M. J. Wilkerson. 2000. Characterization of peripheral blood and pulmonary leukocyte function in healthy foals. Vet. Immunol. Immunopathol. 73:267-285. [DOI] [PubMed] [Google Scholar]
- 7.Flaminio, M. J., B. R. Rush, and W. Shuman. 1999. Peripheral blood lymphocyte subpopulations and immunoglobulin concentrations in healthy foals and foals with Rhodococcus equi pneumonia. J. Vet. Intern. Med. 13:206-212. [DOI] [PubMed] [Google Scholar]
- 8.Garton, N. J., M. Gilleron, T. Brando, H. H. Dan, S. Giguère, G. Puzo, J. F. Prescott, and I. C. Sutcliffe. 2002. A novel lipoarabinomannan from the equine pathogen Rhodococcus equi. Structure and effect on macrophage cytokine production. J. Biol. Chem. 277:31722-31733. [DOI] [PubMed] [Google Scholar]
- 9.Horohov, D. W., A. A. Adams, and T. M. Chambers. 2010. Immunosenescence of the equine immune system. J. Comp. Pathol. 142(Suppl. 1):S78-S84. [DOI] [PubMed] [Google Scholar]
- 10.Jacks, S., and S. Giguère. 2010. Effects of inoculum size on cell-mediated and humoral immune responses of foals experimentally infected with Rhodococcus equi: a pilot study. Vet. Immunol. Immunopathol. 133:282-286. [DOI] [PubMed] [Google Scholar]
- 11.Jacks, S., S. Giguère, P. C. Crawford, and W. L. Castleman. 2007. Experimental infection of neonatal foals with Rhodococcus equi triggers adult-like gamma interferon induction. Clin. Vaccine Immunol. 14:669-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacks, S., S. Giguère, and J. F. Prescott. 2007. In vivo expression of and cell-mediated immune responses to the plasmid-encoded virulence-associated proteins of Rhodococcus equi in foals. Clin. Vaccine Immunol. 14:369-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeffcott, L. B. 1974. Studies on passive immunity in the foal. 1. Gamma-globulin and antibody variations associated with the maternal transfer of immunity and the onset of active immunity. J. Comp. Pathol. 84:93-101. [DOI] [PubMed] [Google Scholar]
- 14.Kanaly, S. T., S. A. Hines, and G. H. Palmer. 1995. Cytokine modulation alters pulmonary clearance of Rhodococcus equi and development of granulomatous pneumonia. Infect. Immun. 63:3037-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez, A. M., M. T. Hines, G. H. Palmer, D. P. Knowles, D. C. Alperin, and S. A. Hines. 2003. Analysis of anamnestic immune responses in adult horses and priming in neonates induced by a DNA vaccine expressing the vapA gene of Rhodococcus equi. Vaccine 21:3815-3825. [DOI] [PubMed] [Google Scholar]
- 16.Martin, B. R., and K. A. Larson. 1973. Immune response of equine fetus to coliphage T2. Am. J. Vet. Res. 34:1363-1364. [PubMed] [Google Scholar]
- 17.Merant, C., C. C. Breathnach, K. Kohler, C. Rashid, P. Van Meter, and D. W. Horohov. 2009. Young foal and adult horse monocyte-derived dendritic cells differ by their degree of phenotypic maturity. Vet. Immunol. Immunopathol. 131:1-8. [DOI] [PubMed] [Google Scholar]
- 18.Mock, R. E., D. O. Morgan, M. M. Jochim, and T. F. Lock. 1978. Antibody response of the fetus and adult equine to Venezuelan equine encephalomyelitis virus (VEE-TC-84): immunoglobulins G (a&b), M, and T. Equine Infect. Dis. IV:209-219. [Google Scholar]
- 19.Morgan, D. O., J. T. Bryans, and R. E. Mock. 1975. Immunoglobulins produced by the antigenized equine fetus. J. Reprod. Fertil. Suppl., p. 735-738. [PubMed]
- 20.Patton, K. M., T. C. McGuire, M. T. Hines, R. H. Mealey, and S. A. Hines. 2005. Rhodococcus equi-specific cytotoxic T lymphocytes in immune horses and development in asymptomatic foals. Infect. Immun. 73:2083-2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perryman, L. E., T. C. McGuire, and R. L. Torbeck. 1980. Ontogeny of lymphocyte function in the equine fetus. Am. J. Vet. Res. 41:1197-1200. [PubMed] [Google Scholar]
- 22.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ridge, J. P., E. J. Fuchs, and P. Matzinger. 1996. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science 271:1723-1726. [DOI] [PubMed] [Google Scholar]
- 24.Ryan, C., S. Giguère, J. Hagen, C. Hartnett, and A. E. Kalyuzhny. 2010. Effect of age and mitogen on the frequency of interleukin-4 and interferon gamma secreting cells in foals and adult horses as assessed by an equine-specific ELISPOT assay. Vet. Immunol. Immunopathol. 133:66-71. [DOI] [PubMed] [Google Scholar]
- 25.Sanada, Y., H. Noda, and H. Nagahata. 1992. Development of lymphocyte blastogenic response in the neonatal period of foals. Zentralbl. Veterinarmed. A 39:69-75. [DOI] [PubMed] [Google Scholar]
- 26.Slack, J., J. M. Risdahl, S. J. Valberg, M. J. Murphy, B. R. Schram, and D. P. Lunn. 2000. Effects of dexamethasone on development of immunoglobulin G subclass responses following vaccination of horses. Am. J. Vet. Res. 61:1530-1533. [DOI] [PubMed] [Google Scholar]
- 27.Tallmadge, R. L., K. McLaughlin, E. Secor, D. Ruano, M. B. Matychak, and M. J. Flaminio. 2009. Expression of essential B cell genes and immunoglobulin isotypes suggests active development and gene recombination during equine gestation. Dev. Comp. Immunol. 33:1027-1038. [DOI] [PubMed] [Google Scholar]
- 28.Wagner, B., A. Burton, and D. Ainsworth. 2010. Interferon-gamma, interleukin-4 and interleukin-10 production by T helper cells reveals intact Th1 and regulatory T(R)1 cell activation and a delay of the Th2 cell response in equine neonates and foals. Vet. Res. 41:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson, W. D., J. E. Mihalyi, S. Hussey, and D. P. Lunn. 2001. Passive transfer of maternal immunoglobulin isotype antibodies against tetanus and influenza and their effect on the response of foals to vaccination. Equine Vet. J. 33:644-650. [DOI] [PubMed] [Google Scholar]
- 30.Witonsky, S., R. M. Gogal, Jr., V. Buechner-Maxwell, and S. A. Ahmed. 2003. Immunologic analysis of blood samples obtained from horses and stored for twenty-four hours. Am. J. Vet. Res. 64:1003-1009. [DOI] [PubMed] [Google Scholar]