Abstract
The objective of this study was to evaluate the efficacy of influenza and meningococcal vaccines in healthy subjects exposed to the anti-interleukin-1β (anti-IL-1β) monoclonal antibody canakinumab. This was an open-label, parallel group, randomized, single-center study of healthy subjects (aged 18 to 45 years). At baseline, antibody (Ab) titers were measured and subjects were randomized (1:1) to a single 300-mg canakinumab dose administered subcutaneously (s.c.) or received no treatment (control group). After 2 weeks, subjects were treated with inactivated, unadjuvanted influenza and conjugated group C meningococcal (MenC) vaccines, administered intramuscularly (i.m.). The primary efficacy variable was the response (≥2-fold increase in Ab titer in ≥2 of 3 influenza virus strains) after 4 weeks in subjects treated with canakinumab compared to the control group. Secondary efficacy variables were the antibody response to vaccines at different thresholds and time points. Fifty-one of 112 subjects screened were randomized to canakinumab (n = 25) or the control group (n = 26). Antibody responses to vaccinations measured against different influenza virus strains and one MenC strain at 4 weeks were comparable in the canakinumab and control groups. The primary efficacy variable, the response to influenza vaccination (≥2-fold increase in Ab titer in ≥2 of 3 serotypes) at 4 weeks, was shown in 24/25 subjects in the canakinumab group compared to 25/25 subjects in the control group. Antibody responses remained comparable in the two groups at the different time points assessed. Headache was the most frequently reported adverse event. No deaths or serious adverse events were reported during the study. We concluded that a single dose of 300 mg canakinumab s.c. does not affect the induction or persistence of antibody responses after vaccination with unadjuvanted influenza or alum-adjuvanted MenC vaccines in healthy subjects.
Patients with autoinflammatory diseases have an increased risk of mortality due to infections (7a). This may be due to immunomodulatory effects of the disease itself or due to the immunosuppressive effects of the agents used for the treatment of the disease conditions (6, 12). Increased risk of serious infections in patients with autoinflammatory diseases like Muckle-Wells syndrome (MWS) or systemic juvenile idiopathic arthritis (sJIA) who are treated with immunosuppressive agents such as anti-tumor necrosis factor (TNF) antibody therapy, corticosteroids, or other agents has been previously reported (2, 7). Among biologics used for these indications, high doses of a biological agent, anakinra, increased the risk of serious infections in patients with such autoimmune conditions, especially in the presence of comorbidity factors (22). Patients with autoinflammatory diseases are therefore potential candidates for vaccinations, for example, against influenza virus.
Vaccination against influenza is currently recommended for patients with chronic autoinflammatory diseases (1, 10). Previous trials have shown that vaccination against influenza virus is safe and that it induces a satisfactory antibody response, although one that is possibly lower than in healthy controls (4, 9, 19). The antibody response of rheumatoid arthritis (RA) patients to vaccination against influenza does not seem to be affected by the use of prednisone, disease-modifying antirheumatic drugs (DMARDs), or TNF-α blockers (4, 9). The extensive use of biologics in the treatment of autoimmune diseases has increased the incidence of infections in such populations (12) and has shown the importance for innate immunity to be still responsive in cases of concomitant use of TNF antagonists or other cytokine inhibitors (22).
Vaccination against meningococcal infection is recommended in populations at risk (17, 25). Some of these vaccines are adjuvanted with aluminum salts. The adjuvanticity of aluminum salts has recently been shown to involve caspase-1 activation and interleukin-1β (IL-1β) secretion (5, 8, 16). As a consequence, the effectiveness of alum-adjuvanted vaccines might be affected by IL-1β inhibitors, such as canakinumab.
Canakinumab (ACZ885) is a high-affinity, fully human anti-IL-1β monoclonal antibody (an IgG1/κ isotype) with a long half-life of 28 to 30 days (15). Canakinumab binds to human IL-1β, blocking the interaction of this cytokine with its receptors, thus functionally neutralizing the bioactivity of IL-1β without preventing the binding of the natural inhibitor, IL-1Ra, or the binding of IL-1α to the IL-1 receptors. IL-1β is recognized as one of the principal proinflammatory cytokines in a variety of inflammatory conditions. Canakinumab is under clinical development for the treatment of autoinflammatory diseases such as cryopyrin-associated periodic fever syndrome (CAPS), for which it has been recently approved by the European Medicines Agency and FDA, sJIA, gout, chronic obstructive pulmonary disease (COPD), and diabetes. Although IL-1β inhibition by canakinumab is well tolerated and provides complete and sustained clinical remission in patients with autoinflammatory diseases such as CAPS (14, 15), its effect on vaccine effectiveness in such patients has not been studied. Therefore, it is of high importance to evaluate whether canakinumab might interfere with vaccinations, as inflammatory diseases like gout, sJIA, CAPS, etc., require life-long treatment which may start early in childhood.
Preclinical evidence with canakinumab suggests that intraperitoneal administration of a surrogate antibody (01BSUR; an analogous antibody that recognizes the intended antigen in different species but does not cross-react with the human antigen) has no inhibitory effects on IgM or IgG antibody titers (unpublished data). However, there is no direct clinical evidence for the lack of interference of canakinumab with the effectiveness of vaccination with or without alum adjuvant at therapeutic doses. The present study, therefore, was conducted to evaluate the efficacies of influenza and meningococcal vaccines in healthy subjects exposed to canakinumab.
MATERIALS AND METHODS
Subjects.
A total of 51 adults (aged 18 to 65 years), out of 112 screened, were enrolled in the study. Inclusion criteria of the study included healthy male and female subjects (oral body temperature, 35.0 to 37.5°C; systolic/diastolic blood pressure, 90 to 140/50 to 90 mm Hg; pulse rate, 40 to 90 beats/min) who were between 18 and 65 years old (body weight, ≥50 kg; body mass index, 18 to 29 kg/m2), and negative tuberculin skin test reaction (purified protein derivative [PPD] at 5 tuberculin units [TU, producing <5-mm induration, 48 to 72 h after administration at the screening visit or within 2 months prior to the screening visit). Subjects who had a positive PPD skin test as defined by existing guidelines (3) with documentation of a Mycobacterium bovis BCG vaccination who were at low environmental risk for tuberculosis infection or reactivation and had a negative chest X-ray were also included. Female subjects were required to have a negative pregnancy test at screening and prior to dosing and to be using an effective method of contraception (e.g., birth control pills, double-barrier contraception, etc.) during the study (from the date of screening) and for at least 3 months after the last dose.
The key exclusion criteria were the following: vaccination of any kind during the preceding 1 year; meningococcal vaccination at any time during the subject's life; influenza vaccination in the 2 years prior to screening; allergy to vaccination, investigational compound/compound class, or egg products; active infection; autoimmune or other significant systemic disease; liver or respiratory disease; impaired renal function; use of any prescription drug or herbal supplement within 4 weeks prior to initial dosing; use of an over-the-counter (OTC) medication or dietary supplement (vitamins included) within 2 weeks prior to initial dosing; history of drug or alcohol abuse within 12 months prior to dosing; pregnancy; any surgical or medical condition that might significantly alter the absorption, distribution, metabolism, or excretion of drugs or which may jeopardize study participation.
All subjects provided written informed consent, and the study was conducted in accordance with the International Committee on Harmonisation (ICH) guidelines for good clinical practice (GCP). The study received approval from the institutional review board or ethical review committee of the University Hospital Centre, Rennes, France.
Study design.
This was a phase I, single-center, open-label, randomized, parallel group, single-dose study to evaluate the effectiveness of influenza vaccination and conjugated group C meningococcal (MenC) vaccination following concomitant (≤2 weeks) exposure to a single subcutaneous (s.c.) dose of canakinumab at 300 mg. The study included a 14-day screening period, and after measurement of antibody (Ab) titers at baseline, eligible subjects were randomized to a single canakinumab 300-mg s.c. dose or no treatment (control group), followed by inactivated unadjuvanted influenza (Agrippal) and conjugated alum-adjuvanted MenC (Menjugate) vaccines given separately intramuscularly (i.m.) in both arms after 2 weeks. The influenza vaccine contained the following virus strains: A/Brisbane/59/2007 (A/H1N1); A/Uruguay/716/2007 (A/H3N2); B/Florida/4/2006 (B). The vaccines are licensed in several countries worldwide and were sourced locally for the trial.
Efficacy and safety assessments.
The primary efficacy variable was the antibody response to influenza vaccination (≥2-fold increase in Ab titer in ≥2 of 3 virus strains) at 4 weeks postvaccination in subjects treated with canakinumab compared to the control group. Secondary efficacy variables were the response (≥2-fold increase in Ab titer in ≥2 of 3 virus strains) to influenza vaccine at 6 weeks postvaccination, response (≥4-fold increase in Ab titer in ≥2 of 3 virus strains) to influenza vaccine at 4 weeks postvaccination, and response (≥4-fold increase in Ab titer) to meningococcal vaccine at 4 and 6 weeks postvaccination. In addition, the safety and tolerability of canakinumab in healthy subjects during vaccination were evaluated.
Vaccine-specific serum Ab responses were assessed at 2, 4, and 6 weeks postvaccination. The antibody response to the three influenza vaccine virus strains was evaluated by hemagglutination inhibition test (HI test; limit of quantification [LOQ], titer of 10) according to standard procedures (20). The antibody response to MenC was evaluated by serum bactericidal assay (SBA; LOQ, titer of 4) using the MenC C11 strain and prescreened human plasma as the source of complement (23). Antibody responses are reported as either a ≥2- or ≥4-fold increase in titer for influenza vaccine and as a ≥4-fold increase in titer for MenC vaccine, or a rise from a nonprotective baseline level of <1:40 to ≥1:40 for influenza and of <1:8 to >1:8 for MenC in antibodies at 4 weeks after vaccination compared to baseline. Geometric mean antibody titers were also calculated for the three influenza antigens and for MenC.
The safety assessment of treatment with canakinumab consisted of monitoring and recording all adverse events (AEs) and serious adverse events (SAEs), hematology, biochemistry parameters, pregnancy tests, vital signs, and physical examinations.
Statistical analyses.
The sample size determination was based on the primary end point, i.e., the proportion of subjects with a ≥2-fold increase in Ab titer in ≥2 of 3 virus strains at 4 weeks post-influenza virus vaccination. A sample size of 50 subjects (25 subjects in each group) provided 68% power with a one-sided α-level of 0.05 to establish noninferiority, assuming a control response rate of 75% and a noninferiority margin of 30%. The same calculation applied to MenC, for which the control response rate was again expected to be 75%. The differences in proportions responding in the two groups together with 90% confidence intervals (CI) were calculated, and noninferiority was concluded if the lower 90% CI excluded a difference of 30% or more. Efficacy and safety variables were summarized using descriptive statistics.
RESULTS
Characteristics of the enrolled subjects.
Of the 51 enrolled subjects, 25 and 26 subjects were randomized into the canakinumab and control groups, respectively. One subject in the control group had missing assessment values for the 2- and 4-week postvaccination visits and was therefore not included in the efficacy analysis for those visits. The baseline demographics of the enrolled subjects are presented in Table 1.
TABLE 1.
Demographic characteristics of the enrolled subjects
| Characteristic | Value for group |
||
|---|---|---|---|
| Canakinumab (n = 25) | Control (n = 26) | Total (n = 51) | |
| Mean age, in yrs (SD) | 25.2 (5.7) | 26.7 (6.3) | 26.0 (6.0) |
| Mean ht, in cm (SD) | 167.7 (7.9) | 170.1 (8.8) | 169.0 (8.3) |
| Mean wt, in kg (SD) | 63.0 (9.9) | 64.0 (11.0) | 63.5 (10.4) |
| Gender, n (%) | |||
| Male | 7 (28) | 11 (42) | 18 (35) |
| Female | 18 (72) | 15 (58) | 33 (65) |
| Race, n (%) | |||
| Caucasian | 24 (96) | 26 (100) | 50 (98) |
| Other | 1 (4) | 0 (0) | 1 (2) |
Efficacy of influenza and meningococcal vaccinations.
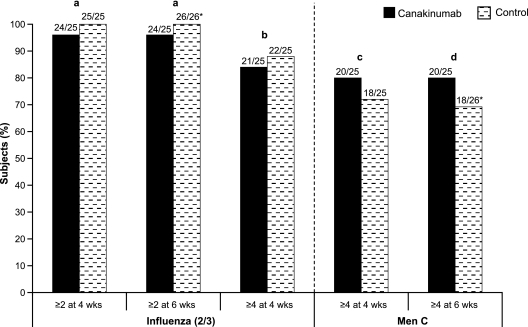
Response to influenza vaccination (≥2-fold change in Ab titer in ≥2 of 3 virus strains) at 4 and 6 weeks postvaccination was seen in 24/25 (96%) subjects in the canakinumab group and all (100%) subjects in the control group, respectively. A response of a ≥4-fold increase at 4 weeks postvaccination was shown in 21/25 (84%) and 22/25 (88%) subjects in the canakinumab and control groups (Fig. 1).
FIG. 1.
Proportion of subjects with ≥2- and ≥4-fold increases in antibody titers from baseline following vaccination. Numbers above each vertical bar indicate the number of subjects with the indicated increase in Ab titer from baseline and the total number of subjects analyzed. *, one subject in the control group was missing assessment values at 4 weeks postvaccination and was therefore not included in the analysis for that time point. a, the difference in proportion was −0.04 (95% CI, −0.12 to 0.04); b, the difference in proportion was −0.04 (95% CI, −0.23 to 0.15); c, the difference in proportion was 0.08 (95% CI, −0.16 to 0.32); d, the difference in proportion was 0.11 (95% CI, −0.13 to 0.34).
In the canakinumab group, 20/25 (80%) of subjects showed a ≥4-fold increase in titer in response to MenC vaccination both at 4 and 6 weeks postvaccination, compared to 18/25 (72%) and 18/26 (69.2%) subjects after 4 and 6 weeks in the control group (Fig. 1) The proportions of subjects who had a ≥2- and ≥4-fold increase in Ab titer in response to influenza vaccination and a ≥4-fold increase in Ab titer in response to MenC antigen at 2, 4, and 6 weeks after vaccination are presented in Table 2.
TABLE 2.
Proportion of subjects showing ≥2- or ≥4-fold increase in Ab titer from baseline for each serotype and time point
| Vaccine and serotype | Treatment group | Fold increase in titer from baseline | % of subjects achieving predefined postvaccination titer at: |
||
|---|---|---|---|---|---|
| 2 wks | 4 wks | 6 wks | |||
| Influenza | |||||
| A/Brisbane/59/2007 | Canakinumab | ≥2 | 100.0 | 100.0 | 100.0 |
| ≥4 | 100.0 | 100.0 | 96.0 | ||
| Control | ≥2 | 100.0 | 100.0 | 100.0 | |
| ≥4 | 100.0 | 100.0 | 100.0 | ||
| A/Uruguay/716/2007 | Canakinumab | ≥2 | 80.0 | 80.0 | 80.0 |
| ≥4 | 72.0 | 68.0 | 68.0 | ||
| Control | ≥2 | 88.0 | 88.0 | 88.5 | |
| ≥4 | 80.0 | 68.0 | 65.4 | ||
| B/Florida/4/2006 | Canakinumab | ≥2 | 92.0 | 88.0 | 92.0 |
| ≥4 | 68.0 | 64.0 | 64.0 | ||
| Control | ≥2 | 88.0 | 96.0 | 92.3 | |
| ≥4 | 76.0 | 72.0 | 69.2 | ||
| Meningitis | |||||
| MenC (strain C11) | Canakinumab | ≥4 | 84.0 | 80.0 | 80.0 |
| Control | ≥4 | 76.0 | 72.0 | 69.2 | |
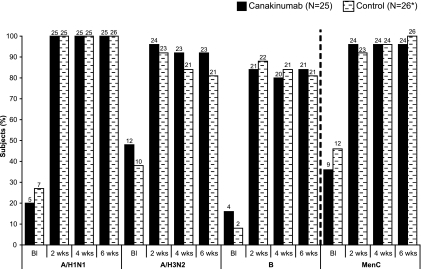
The proportion of all subjects who showed an increase in protective antibodies (from nonprotective baseline titer of <1/40 for influenza and <1/8 for meningococcus) at different time points was comparable between the canakinumab and control groups (Fig. 2).
FIG. 2.
Proportions of subjects with a protective level of Ab titer at the indicated time points postvaccination (protective levels were defined as ≥1/40 for influenza and ≥1/8 for meningitis). *, one subject in the control group had missing assessment values at 2 and 4 weeks postvaccination and was not included in those visit analyses. Values on top of each vertical bar indicate the number of subjects who had a protective Ab titer at the indicated time point. BI, baseline; A/H1N1, influenza virus A/Brisbane/59/2007; A/H3N2, influenza virus A/Uruguay/716/2007; B, influenza virus B/Florida/2006.
Levels of antibodies to influenza and MenC vaccines.
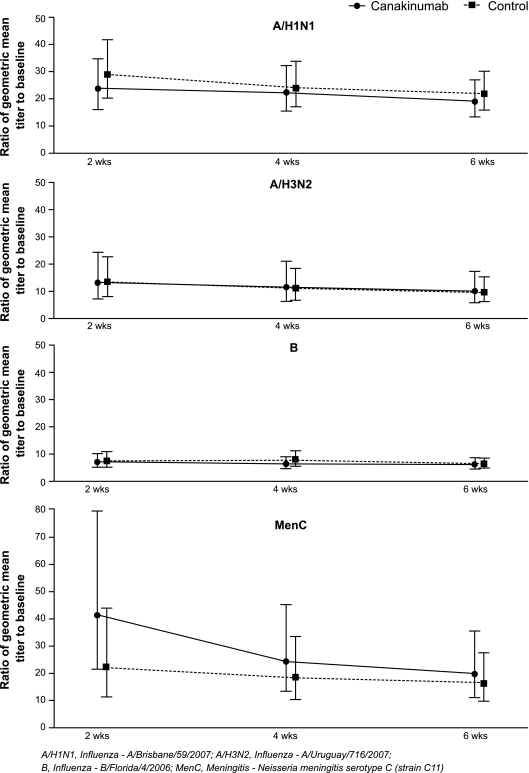
All the subjects in the canakinumab and control groups had clinically significant increases in their geometric mean titers of HI antibody against each of the influenza antigens tested and of antibody titers against MenC at 2, 4, and 6 weeks postvaccination. The antibody titers that were reached and the antibody titer persistence over time were comparable between the canakinumab and the control groups (Fig. 3). The distribution of peak titer values was similar between the two groups, as were the geometric mean titers (Florida, 1:72 for canakinumab, 1:82 for control; Brisbane, 1:368 for canakinumab, 1:376 for control; Uruguay, 1:378 for canakinumab, 1:312 for control; MenC, 1:199 for canakinumab, 1:186 for control).
FIG. 3.
Ratios (90% CI) to baseline of geometric mean titers at different time points for the canakinumab and control group serotypes. A/H1N1, influenza virus A/Brisbane/59/2007; A/H3N2, influenza virus A/Uruguay/716/2007; B, influenza virus B/Florida/2006.
Safety and tolerability.
No deaths or serious adverse events were reported during the study. The most commonly reported AEs were headache, influenza-like syndrome, and pharyngitis (Table 3). There were no discontinuations due to AEs in this study.
TABLE 3.
Most frequent AEs observed during the study
| AE | No. (%) with AE |
|
|---|---|---|
| Canakinumab (n = 25) | Control (n = 26) | |
| Headache | 3 (12.0) | 5 (19.2) |
| Influenza-like syndrome/aching | 0 (0.0) | 2 (7.7) |
| Pharyngitis | 0 (0.0) | 2 (7.7) |
DISCUSSION
This study evaluated the efficacy of influenza and meningococcal vaccines in healthy subjects exposed to canakinumab, which was measured as the rise in antibody titers over time after administration of unadjuvanted influenza and alum-adjuvanted MenC vaccines. Taken together, the data presented here show that treatment with canakinumab does not affect the development of a protective response against influenza or against MenC. Indeed, the response to both vaccines was comparable between the canakinumab and control groups, who in this study were healthy volunteers. Overall, the response to influenza vaccination showed a marked consistency across the three virus strains in the healthy subjects in this study, whereas in patients with autoimmune conditions the response can be uneven (10). Almost all subjects in both the canakinumab and control groups showed a ≥2- and ≥4-fold Ab titer rise in response to the A/H1N1 influenza virus strain at 2, 4, and 6 weeks postvaccination. Variations in the percentages of subjects responding to other virus strains were observed at all time points postvaccination in both groups. The level of response measured at 6 weeks postvaccination was clinically reassuring, given the fact that we considered seroconversion based on responses to two out of three virus strains.
The results of this study should be considered in the wider context of different mechanisms of actions for biologics, which target diverse pathways in autoimmune diseases. Previous reports have shown that patients with RA and psoriatic arthritis treated with adalimumab and etanercept were still able to develop an effective response to the tested influenza and pneumococcal vaccines (13, 18), while other agents, like rituximab, did affect vaccine effectiveness (21). The percentage of healthy subjects responding to influenza vaccination observed in the present study was higher than the percentage of subjects responding to pneumococcal vaccination (11) or to tetanus toxoid with Abatacept 750 (24).
The potential influence of canakinumab on nonadjuvant and adjuvant-based influenza and MenC vaccines was explored in our study. The seroconversion rate (defined as an antibody titer increase of ≥4-fold) was comparable in the canakinumab and control groups, while the rate of protection (based on a threshold antibody titer of ≥1:8) in both the groups was at least 96% at 4 and 6 weeks postvaccination. The data are in agreement with our preclinical findings and show that in the presence of canakinumab, healthy subjects achieve protective levels of antibodies after vaccination, independent of adjuvant use.
To further validate these findings in healthy subjects, a similar study of patients with autoimmune conditions such as CAPS should be considered. Nevertheless, this is the first clinical evidence that suggests that in the presence of an anti-IL-1β antibody, vaccination with two of the most common vaccines in clinical practice is well tolerated and is most likely to be highly effective.
Conclusion.
Blockade of IL-1β by canakinumab with a 300-mg s.c. single dose in healthy volunteers does not interfere with the efficacy of influenza or meningococcal vaccines, as measured by protective antibody titers, despite the fact that the adjuvant properties of aluminum salts are mediated by IL-1β. Further studies are planned to confirm the results in patients with autoinflammatory conditions who are treated with canakinumab.
Acknowledgments
We thank Nicolaos Gaitatzis, who was responsible for clinical serology for flu testing. We also thank the medical writer Vikrant Pallapotu (MSCDIndia, Novartis) for his assistance with drafting the manuscript and incorporating subsequent revisions and Kirstin Stricker for critical review of the manuscript.
Footnotes
Published ahead of print on 20 October 2010.
REFERENCES
- 1.Abu-Shakra, M., J. Press, D. Buskila, and S. Sukenik. 2007. Influenza vaccination of patients with systemic lupus erythematosus: safety and immunogenecity issues. Autoimmun. Rev. 6:543-546. [DOI] [PubMed] [Google Scholar]
- 2.Bongartz, T., A. J. Sutton, M. J. Sweeting, I. Buchan, E. L. Matteson, and V. Montori. 2006. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 295:2275-2285. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2000. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm. Rep. 49(RR06):1-54. [PubMed] [Google Scholar]
- 4.Chalmers, A., D. Scheifele, C. Patterson, D. Williams, J. Weber, R. Shuckett, and A. Teufel. 1994. Immunization of patients with rheumatoid arthritis against influenza: a study of vaccine safety and immunogenicity. J. Rheumatol. 21:1203-1206. [PubMed] [Google Scholar]
- 5.De Gregorio, E., E. Tritto, and R. Rappuoli. 2008. Alum adjuvanticity: unraveling a century old mystery. Eur. J. Immunol. 38:2068-2071. [DOI] [PubMed] [Google Scholar]
- 6.Doran, M. F., C. S. Crowson, G. R. Pond, W. M. O'Fallon, and S. E. Gabriel. 2002. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 46:2287-2293. [DOI] [PubMed] [Google Scholar]
- 7.Doran, M. F., C. S. Crowson, G. R. Pond, W. M. O'Fallon, and S. E. Gabriel. 2002. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 46:2294-2300. [DOI] [PubMed] [Google Scholar]
- 7a.Efthimiou, R. A., A. Furlan, G. Gasbarrini, A. Gava, I. Koné-Paut, R. Manna, L. Punzi, F. S. Sutterwala, I. Touitou, and A. Doria. 2006. Autoinflammatory syndromes and infections: pathogenetic and clinical implications. Clin. Exp. Rheumatol. 26(Suppl. 48):S53-S61. [PubMed] [Google Scholar]
- 8.Eisenbarth, S. C., O. R. Colegio, W. O'Connor, F. S. Sutterwala, and R. A. Flavell. 2008. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453:1122-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fomin, I., D. Caspi, V. Levy, N. Varsano, Y. Shalev, D. Paran, D. Levartovsky, I. Litinsky, I. Kaufman, I. Wigler, E. Mendelson, and O. Elkayam. 2006. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann. Rheum. Dis. 65:191-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gluck, T., and U. Muller-Ladner. 2008. Vaccination in patients with chronic rheumatic or autoimmune diseases. Clin. Infect. Dis. 46:1459-1465. [DOI] [PubMed] [Google Scholar]
- 11.Go, E. S., and Z. K. Ballas. 1996. Anti-pneumococcal antibody response in normal subjects: a meta-analysis. J. Allergy Clin. Immunol. 98:205-215. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez, C., A. S. Cetner, J. E. Jordan, S. N. Puangsuvan, and J. K. Robinson. 2008. Tuberculosis in the age of biologic therapy. J. Am. Acad. Dermatol. 59:363-380. [DOI] [PubMed] [Google Scholar]
- 13.Kaine, J. L., A. J. Kivitz, C. Birbara, and A. Y. Luo. 2007. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J. Rheumatol. 34:272-279. [PubMed] [Google Scholar]
- 14.Lachmann, H. J., I. Kone-Paut, J. B. Kuemmerle-Deschner, K. S. Leslie, E. Hachulla, P. Quartier, X. Gitton, A. Widmer, N. Patel, and P. N. Hawkins. 2009. Use of canakinumab in the cryopyrin-associated periodic syndrome. N. Engl. J. Med. 360:2416-2425. [DOI] [PubMed] [Google Scholar]
- 15.Lachmann, H. J., P. Lowe, S. D. Felix, C. Rordorf, K. Leslie, S. Madhoo, H. Wittkowski, S. Bek, N. Hartmann, S. Bosset, P. N. Hawkins, and T. Jung. 2009. In vivo regulation of interleukin 1β in patients with cryopyrin-associated periodic syndromes. J. Exp. Med. 206:1029-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, H., S. B. Willingham, J. P. Ting, and F. Re. 2008. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J. Immunol. 181:17-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makwana, N., and F. A. Riordan. 2007. Bacterial meningitis: the impact of vaccination. CNS Drugs 21:355-366. [DOI] [PubMed] [Google Scholar]
- 18.Mease, P. J., C. T. Ritchlin, R. W. Martin, A. B. Gottlieb, S. W. Baumgartner, D. J. Burge, and J. B. Whitmore. 2004. Pneumococcal vaccine response in psoriatic arthritis patients during treatment with etanercept. J. Rheumatol. 31:1356-1361. [PubMed] [Google Scholar]
- 19.Oren, S., M. Mandelboim, Y. Braun-Moscovici, D. Paran, J. Ablin, I. Litinsky, D. Comaneshter, D. Levartovsky, E. Mendelson, R. Azar, I. Wigler, A. Balbir-Gurman, D. Caspi, and O. Elkayam. 2008. Vaccination against influenza in patients with rheumatoid arthritis: the effect of rituximab on the humoral response. Ann. Rheum. Dis. 67:937-941. [DOI] [PubMed] [Google Scholar]
- 20.Palmer, D. F., W. R. Dowle, M. T. Coleman, and G. C. Schild. 1975. Haemagglutination inhibition test, p. 25-62. Advanced laboratory techniques for influenza diagnosis. Procedural guide, pt. 2. U.S. Department of Health, Education and Welfare, Public Health Service, Atlanta, GA.
- 21.Salemi, S., A. Picchianti-Diamanti, V. Germano, I. Donatelli, A. Di Martino, M. Facchini, R. Nisini, R. Biselli, C. Ferlito, E. Podesta, A. Cappella, F. Milanetti, F. Rossi, R. Amodeo, F. Tabacco, R. Di Rosa, B. Lagana, and R. D'Amelio. 2010. Influenza vaccine administration in rheumatoid arthritis patients under treatment with TNFα blockers: safety and immunogenicity. Clin. Immunol. 134:113-120. [DOI] [PubMed] [Google Scholar]
- 22.Salliot, C., M. Dougados, and L. Gossec. 2009. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann. Rheum. Dis. 68:25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snape, M. D., K. P. Perrett, K. J. Ford, T. M. John, D. Pace, L. M. Yu, J. M. Langley, S. McNeil, P. M. Dull, F. Ceddia, A. Anemona, S. A. Halperin, S. Dobson, and A. J. Pollard. 2008. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. JAMA 299:173-184. [DOI] [PubMed] [Google Scholar]
- 24.Tay, L., F. Leon, G. Vratsanos, R. Raymond, and M. Corbo. 2007. Vaccination response to tetanus toxoid and 23-valent pneumococcal vaccines following administration of a single dose of abatacept: a randomized, open-label, parallel group study in healthy subjects. Arthritis Res. Ther. 9:R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodard, J. L., and D. M. Berman. 2006. Prevention of meningococcal disease. Fetal Pediatr. Pathol. 25:311-319. [DOI] [PubMed] [Google Scholar]