Abstract
Norwalk virus (NV) is an enteric pathogen from the genus Norovirus and a major cause of nonbacterial gastroenteritis in humans. NV virus-like particles (VLPs) are known to elicit systemic and mucosal immune responses when delivered nasally; however, the correlates of immune protection are unknown, and codelivery with a safe and immunogenic mucosal adjuvant may enhance protective anti-NV immune responses. Resiquimod (R848), an imidazoquinoline-based Toll-like receptor 7 and/or 8 (TLR7/8) agonist, is being evaluated as an adjuvant in FDA-approved clinical vaccine trials. As such, we evaluated the adjuvant activity of two imidazoquinoline-based TLR7 and TLR7/8 agonists when codelivered intranasally with plant-derived NV VLPs. We also compared the activity of these agonists to the gold standard mucosal adjuvant, cholera toxin (CT). Our results indicate that codelivery with the TLR7 agonist, gardiquimod (GARD), induces NV VLP-specific serum IgG and IgG isotype responses and mucosal IgA responses in the gastrointestinal, respiratory, and reproductive tracts that are superior to those induced by R848 and comparable to those induced by the mucosal adjuvant CT. This study supports the continued investigation of GARD as a mucosal adjuvant for NV VLPs and possible use for other VLP-based vaccines for which immune responses at distal mucosal sites (e.g., respiratory and reproductive tracts) are desired.
Norwalk virus (NV), the prototype virus of the genus Norovirus, is a major cause of nonbacterial gastroenteritis in humans (12). Annually, noroviruses cause an estimated 21 million infections in the United States and 200 thousand deaths among children in developing countries (40). This disease burden strongly indicates the need for a robust vaccine; however, currently there is no FDA-approved norovirus vaccine available (22). Extensive research has focused on the development of NV virus-like particles (VLPs) as vaccine antigens that can be delivered mucosally (6, 9, 12, 16, 19, 20, 22, 26, 31, 33, 45). VLPs are self-assembled empty shells that mimic authentic viral antigenicity but lack a nucleic acid core. The success of VLPs as vaccine antigens has been shown by the FDA approval of VLP-based hepatitis B virus (HBV) and human papillomavirus (HPV) vaccines (13, 34, 39).
The use of mucosal vaccine adjuvants may increase norovirus VLP immunogenicity and potentially broaden the nature of the resulting immune responses. Mucosal adjuvants with the ability to skew adaptive immune responses toward a CD4+ T-helper type 1 (Th1) phenotype are highly attractive. Th1 responses characterized by gamma interferon (IFN-γ) secretion, macrophage activation, and CD8+ cytotoxic T-cell action provide effective protection against intracellular pathogens, including viruses (35, 36, 42). Cholera toxin (CT), a powerful, gold standard mucosal adjuvant, was previously shown to enhance systemic IgG immune responses when codelivered with NV VLPs administered intranasally or orally; however, due to toxicity in human clinical trials, CT is not safe for use in a clinical NV vaccine (23, 37). Synthetic molecules that mimic pathogen-associated molecular patterns (PAMPs) are a potential class of mucosal adjuvants because they are particularly powerful at stimulating pattern recognition receptors (PRRs) of the innate immune system (28, 42, 44). Activation through one family of PRRs, the Toll-like receptors (TLRs), leads to the maturation of antigen-presenting cells and the stimulation of antigen-specific Th1 cells, thereby inducing both innate and acquired immunity as well as immunological memory (55).
Numerous TLR agonists are currently being developed as adjuvants. One such agonist, monophosphoryl lipid A (MPL; Toll-like receptor 4 [TLR4] agonist), has been approved for clinical use in a HPV VLP-based vaccine in the United States as well in HBV vaccines in Europe and Argentina (14, 28). MPL has also been shown to induce higher antibody titers than aluminum salts which are the most commonly used adjuvant in FDA-approved vaccines (14, 24, 42). The TLR9 agonist, CpG oligonucleotide, has also been used in clinical trials as a vaccine adjuvant and was shown to effectively enhance antibody responses against HBV and anthrax (28, 29, 52).
Imidazoquinoline-based TLR7 and/or TLR8 (TLR7/8) agonists are attractive to pursue as mucosal adjuvants because they have a high safety profile in humans, are most effective when delivered topically, and have robust adjuvant qualities (5, 11, 30, 38, 46, 51). Resiquimod (R848), an imidazoquinoline-based TLR7/8 agonist, is currently being investigated as an adjuvant in at least three FDA-approved clinical trials (www.clinicaltrials.gov). Both R848 and another imidazoquinoline-based TLR7 agonist, gardiquimod (GARD), have been shown to induce the secretion of Th1 cytokines, including IFN-γ, alpha interferon (IFN-α), interleukin 12 (IL-12), and tumor necrosis factor alpha (TNF-α) both in vitro and in vivo (5, 21, 32, 38, 47). When evaluated as adjuvants in murine models, R848 and GARD were shown to promote adaptive immune responses to codelivered antigens and provide protection against live infection challenges (5, 48, 51, 56). These studies are the basis of our investigation of these imidazoquinoline-based TLR agonists as mucosal adjuvants for VLP antigens.
Nasal epithelial cells have not been extensively studied for TLR expression. In this study, we defined the immunomodulatory specificity of intranasally delivered TLR agonists R848 and GARD for the induction of NV VLP-specific antibody production. We also compared the immunomodulatory activity of the imidazoquinoline-based adjuvants to the mucosal adjuvant cholera toxin. The immune response was measured in serum and at other sites known to be part of the common mucosal immune system (CMIS) (23). Our results indicate that codelivery with GARD produces a superior antigen-specific immune response systemically and at CMIS sites, including sites in the enteric tract (salivary and intestinal), than codelivery with R848 and that the response with GARD is comparable to that induced by CT.
MATERIALS AND METHODS
NV VLP preparation.
Norwalk virus (NV) VLPs were purified from an extract of Nicotiana benthamiana after inoculation of the plants using viral vectors derived from a tobacco mosaic virus (TMV)-based system as previously described (45). Briefly, three TMV-derived viral vector constructs (5′ cytosolic module, integrase, and 3′ NV capsid protein module) were grown in Agrobacterium tumefaciens (optical density at 600 nm [OD600] of 0.6) and then centrifuged at 6,000 × g for 10 min. Equal amounts of the three bacterial pellets were combined and suspended (OD600 of 0.1) in infiltration buffer [10 mM 2-(N-morpholino)ethanesulfonic acid, 10 mM MgSO4 [pH 5.5]). N. benthamiana plants, 5 to 6 weeks old, were inverted in the bacterial suspension within a sealed chamber and then infiltrated with Agrobacterium by two rounds of vacuum pump-induced air extraction and vacuum release for 1 min each. At 13 days postinfection, fresh leaf material (0.2 to 0.8 g/ml) was homogenized in an ice-cold, fresh acid extraction buffer (25 mM sodium phosphate, 100 mM NaCl, 50 mM sodium ascorbate, 1 mM EDTA [EMD Chemicals, Gibbstown, NJ], 2 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin [pH 5.75]) by blending for 1 to 2 min. The reagents for infiltration and extraction buffers were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise noted. Homogenates were immediately filtered through four layers of cheesecloth into 50-ml conical tubes, incubated on ice for 1 h, and then centrifuged at 2,590 × g for 20 min at 4°C. The supernatant was transferred to a new 50-ml conical tube, incubated at 4°C for 24 h, and then centrifuged as described above. This procedure was repeated again at 48 h postextraction to remove acid-precipitated plant cell endogenous proteins, the majority of which was ribulose bisphosphate carboxylase-oxygenase (Rubisco). The NV VLP extract was adjusted to pH 7.3 using dibasic sodium phosphate (EMD Chemicals), filtered (cold) through a 0.22-μm bottle-top filter (Corning Life Sciences, Lowell, MA), and then concentrated 10-fold using a stirred-cell apparatus (Millipore, Bedford, MA) with a 30,000-kDa cutoff membrane, from which the retentate (containing NV VLPs) was stored at 4°C. The NV VLPs were further purified by ion-exchange chromatography with DEAE Sepharose FF resin (GE Healthcare, Piscataway, NJ) to remove small molecules, including endotoxin. Purified NV VLPs were collected in the DEAE cellulose flowthrough fraction and stored at 4°C. The endotoxin level was less than 75 endotoxin units (EU)/dose as measured by the chromogenic Limulus amebocyte lysate assay per the manufacturer's instructions (Cambrex Corporation, East Rutherford, NJ).
Intranasal immunization.
All animals were housed in American Association for Laboratory Animal Care (AALAC)-approved quarters, provided unlimited access to food and water, and handled in accordance with the Animal Welfare Act and Arizona State University (ASU) Institutional Animal Care and Use Committee (IACUC). Prior to initiation of any treatment, inbred female BALB/c mice (Charles River Laboratories International, Inc., Wilmington, MA), 5 weeks old, were randomly distributed into 7 groups and allowed to acclimate for at least 1 week prior to immunization. Mice were immunized intranasally with 5 to 25 μg tobacco-derived NV VLPs on days 0 and 21. NV VLPs were codelivered without or with candidate adjuvants which include the following: 1 μg cholera toxin (CT) (List Biological Laboratories, Inc., Campbell, CA) (1.0 mg/ml in phosphate-buffered saline [PBS]); 10 μg gardiquimod (GARD) (InvivoGen, San Diego, CA) (2.5 mg/ml in PBS); or 10 to 25 μg resiquimod (R848) (generously provided by Mark Tomai of 3M Drug Delivery Systems, St. Paul, MN) (5.9 mg/ml in 1.65% [wt/wt] l-tartaric acid in double-distilled water [ddH2O] [pH 5.0]). Sixteen mice were immunized with 25 μg NV VLP plus 25 μg R848 or 25 μg NV VLP plus 10 μg R848; half of these animals were euthanized on day 56, and half were euthanized on day 119. Thirteen mice were mock immunized with PBS, of which five were euthanized on day 56 and eight on day 119. Eight mice were immunized with NV VLP alone (25 μg), NV VLP (25 μg) plus CT (1 μg), NV VLP (25 μg) plus GARD (10 μg), or NV VLP (5 μg) plus R848 (25 μg). Intranasal immunizations were administered to conscious mice by gently distributing 5 to 10 μl of the antigen with or without adjuvant dropwise in each naris using a P20 pipette. Negative-control mice received 10 μl PBS alone. Mice were not anesthetized for intranasal immunizations.
Sample collection.
Serum samples, fecal pellets, and vaginal lavage samples were collected prior to the first immunization on day 0 (preimmune) and on days 12, 21, 42, 56, 84, and 119. Serum was isolated by centrifugation of blood samples collected using Goldenrod animal bleeding lancets (MEDIpoint Inc., Mineola, NY) to bleed the submandibular vein and following euthanization on day 56 or 119 by cardiac venipuncture. Fecal pellets (44 ± 2 mg) were diluted in 500 μl PBS containing 0.05% sodium azide, incubated on ice for 10 min, sonicated using the Fast Prep FP120 homogenizer (Qbiogene Inc., Carlsbad, CA) on a speed setting of 4.0 for 25 s, and then clarified by centrifugation as previously described (45). Vaginal lavage samples were collected using oral feeding needles (Braintree Scientific Inc., Braintree, MA) to lavage 100 μl PBS intravaginally. Vaginal lavage samples were further diluted in an additional 100 μl PBS and clarified by centrifugation. Mice were euthanized on day 56 (25 μg NV VLP plus 25 μg R848, n = 8; 25 μg NV VLP plus 10 μg R848, n = 8; PBS, n = 5) or day 119 (25 μg NV VLP plus 1 μg CT, n = 8; 25 μg NV VLP plus 10 μg GARD, n = 8; 25 μg NV VLP plus 25 μg R848, n = 7; 25 μg NV VLP plus 10 μg R848, n = 8; 5 μg NV VLP plus 25 μg R848, n = 8; 25 μg NV VLP, n = 8; PBS, n = 8) in accordance with the Animal Welfare Act and ASU IACUC. Salivary samples were collected by inserting a small cellulose swab (Aspen Surgical, Calendonia, MI) into the oral cavity and allowing sufficient time (∼10 to 15 min) for saliva absorption. The swab was then placed into a tube containing 200 μl PBS. After absorption, the bottom of the tube was cut off and placed into a new tube, and salivary samples were collected by centrifugation. Nasal lavage samples were collected by flushing each naris with 50 μl PBS. Intestinal lavage samples were collected by removing 6 mm of the small intestine, 2 mm beyond the stomach, and flushing the length of the intestine with 1 ml PBS. Bronchoalveolar lavage samples were collected by making a small incision transecting the ventral region of trachea and slowly lavaging the lungs with 300 μl PBS using a 22-gauge 1-in. metal gavage needle (Braintree Scientific Inc.), being careful to avoid overfill/rupture of the lungs; Vascu-Statt bulldog clamps (Scanlan International, St. Paul, MN) were used to clamp the trachea and close the incision to ensure the lungs were a closed system. Four of the eight mice were randomly selected from each immunization group, and bronchoalveolar lavage samples were collected and assayed from the animals euthanized on day 119. All samples were clarified by centrifugation and stored at −80°C prior to analysis. Each mucosal sample was collected in a systematic and consistent manner to minimize potential variability in sample collection.
NV-specific enzyme-linked immunosorbent assays (ELISAs).
Enzyme immunoassay (EIA)/radioimmunoassay (RIA) 96-well polystyrene high-binding plates (Corning Life Sciences) were coated for 4 h at room temperature with 100 μl per well of 0.5 μg/ml insect cell-derived NV VLPs. Insect cell-derived NV VLPs were manufactured using a recombinant baculovirus expression system in Sf9 cells by Invitrogen (Carlsbad, CA) and purified as previously described for plant-derived VLPs (45). The plates were blocked overnight at 4°C with 10% (wt/vol) (fecal and intestinal samples) or 5% (all other samples) dry milk in PBS. Prior to loading samples, the plates were washed three times with PBS containing 0.05% Tween 20 (PBS-T). The samples were prepared in 2.5% (serum samples) or 5% (mucosal samples) dry milk in PBS-T, serially diluted 2-fold down the microtiter plate, and incubated for 2 h at 37°C to permit antibody binding as previously described (20, 45). To determine background binding, 2.5% or 5% dry milk in PBS-T was analyzed in wells containing NV antigen. The plates were washed four times with PBS-T and incubated for 1 h at 37°C with horseradish peroxidase (HRP)-conjugated goat anti-mouse antibodies diluted in PBS-T containing 2.5% dry milk diluted as follows: IgG (1:5,000) (Southern Biotech, Birmingham, AL), IgG1 (1:2,500) (Santa Cruz Biotechnology Inc., Santa Cruz, CA), IgG2a (1:2,500) (Santa Cruz Biotechnology Inc.), or IgA (1:5,000) (Sigma-Aldrich). After the plates were washed four times with PBS-T, they were developed with 100 μl per well of 4% 3,3′,5,5′-tetramethylbenzidine (TMB) peroxidase liquid substrate system (KPL Inc., Gaithersburg, MD) for 7 min (IgG1, IgG2a, and IgA), 12 min (serum IgG), or 15 min (vaginal IgG). Color development was stopped by the addition of an equal volume of 1 M phosphoric acid. Absorbance measurements were made at 450 nm using an MRX automatic plate reader (Dynex Technologies, Chantilly, VA). The lowest dilution for each of the samples was 1:2 for mucosal samples or 1:100 for serum samples. Endpoint titers are reported as the reciprocal of the highest dilution that had an absorbance value greater than or equal to 0.065 to 0.1 above the background (0.065 for serum and 0.1 for all mucosal samples).
Splenocyte isolation and NV-specific enzyme-linked immunospot (ELISPOT) assay.
Spleens were harvested following euthanization on day 56 or 119. Single-cell suspensions were prepared in RPMI 1640 medium (Invitrogen) supplemented with 2% fetal bovine serum (FBS) (Invitrogen) by homogenization using metal mesh sieves (Sigma-Aldrich). The cells from 2 or 3 spleens were pooled for a total of 3 pools per immunization group with the exception of the day 56 mock-immunized (PBS-immunized) group (2 pools). Splenocytes were washed twice with PBS by centrifugation at 450 × g for 5 min, and the pelleted cells were suspended in RPMI 1640 medium supplemented with 2% FBS, filtered through a 70-μm nylon cell strainer (BD Biosciences, Franklin Lakes, NJ), and enumerated with a hemocytometer. Cell suspensions were seeded into Millititer HA 96-well plates (Millipore) precoated with 2 μg/ml insect cell-derived NV VLPs (Invitrogen). Millititer HA 96-well plates were prepared by incubating with 100 μl insect cell-derived NV VLPs at 37°C for 3 h, washing four times with PBS, and blocking with 200 μl RPMI 1640 medium supplemented with 2% FBS at 37°C for 3 h. After the plates were washed three times with PBS, isolated splenocytes were added in duplicate at 2.0 × 105, 1.0 × 106, and 5.0 × 106 cells/well (day 56) or 1.0 × 106, 5.0 × 106, and 2.5 × 107 (day 119) and incubated at 37°C for 4 h. The plates were then washed three times with PBS and three times with PBS-T and then incubated with 100 μl of HRP-conjugated goat anti-mouse IgG diluted 1:5,000 in PBS-T at 4°C overnight. After three washes with PBS, the plates were developed with 100 μl of 3-amino-9-ethyl-carbazole (AEC) substrate solution (BD Biosciences) for 25 min, washed with ddH2O, and air dried. Spots were counted with the ImmunoSpot S3B ELISPOT analyzer (Cellular Technology Ltd., Shaker Heights, OH) and expressed as the average number of NV-specific IgG-secreting cells per 1 × 107 splenocytes.
Statistical analysis.
Prism software (GraphPad Inc., San Diego, CA) was used to graph and make statistical comparisons of all data. NV VLP-specific IgA and IgG antibody titers are expressed as geometric mean titers (GMT) for each immunization group at each time point. All responders and nonresponders were included in the computation of the GMT. Negative samples were assigned a value of 1.0 for the purpose of calculating the GMT. Values for groups were compared using the Kruskal-Wallis one-way analysis of variance (ANOVA) followed by a Dunn's posttest at each time point. ELISPOT data were analyzed by ANOVA followed by Bonferroni's multiple-comparison posttest. All statistical comparisons displayed graphically were made between individual treatment groups versus the group given PBS alone. Statistical significance was considered to be a P value of <0.05.
RESULTS
Intranasal immunization with NV VLPs codelivered with CT or GARD is superior to R848 in the induction of a robust NV-specific systemic immune response.
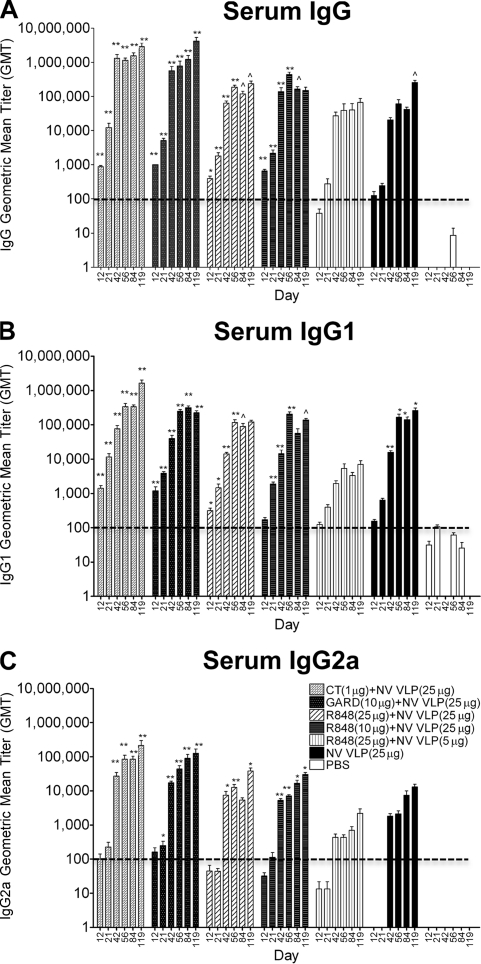
Previous studies (6, 9, 20, 22, 31, 33, 45) have shown that NV VLPs elicited humoral and mucosal immune responses after immunization via either the enteric or intranasal route. In our study, NV VLPs (5 or 25 μg) were administered intranasally twice over a 3-week interval on days 0 and 21, with or without CT (1 μg), GARD (25 μg), or R848 (10 or 25 μg). Serum was assayed for NV VLP-specific IgG and IgG isotype levels by ELISAs. NV VLP boosting on day 21 dramatically increased serum IgG, IgG1, and IgG2a titers (Fig. 1). Serum IgG and IgG isotype levels remained elevated throughout the study (to day 119), and IgG2a levels were consistently lower than IgG and IgG1 levels in all immunization groups (Fig. 1). Minimal levels of antigen-specific antibodies were detected in some of the PBS-immunized (mock-immunized) samples, and all GMT values were included in the graphs and statistical comparisons.
FIG. 1.
Serum Norwalk virus (NV)-specific IgG and isotype production following intranasal immunization with NV virus-like particles (VLPs) codelivered with or without cholera toxin (CT) or Toll-like receptor (TLR) ligands. Female BALB/c mice were immunized intranasally with NV VLPs (5 or 25 μg) on days 0 and 21 with or without CT (1 μg), gardiquimod (GARD) (10 μg), or resiquimod (R848) (10 or 25 μg). Serum samples were collected on days 0, 12, 21, 42, 56, 84, and 119 and analyzed for NV VLP-specific IgG (A), IgG1 (B), and IgG2a (C) by ELISA. By day 42, all mice that received NV VLPs codelivered with or without adjuvant responded with a positive antibody titer (geometric mean titer [GMT] of ≥100) for IgG and IgG1. In addition, all mice responded with a positive anti-NV IgG2a titer except for those receiving R848 (25 μg) plus NV VLP (5 μg) on days 42 (5/8 mice), 56 (6/8 mice), 84 (6/8 mice), and 119 (7/8 mice) or those receiving NV VLP alone on day 42 (7/8 mice). Antigen-specific IgG and IgG2a were not detected (GMT < 100) in all preimmune samples; however, background levels of IgG1 were detected in preimmune samples (data not shown). Error bars represent the standard errors of the means. Values that were significantly different from the values for the PBS control group are shown as follows: ∧, P < 0.05; *, P < 0.01; **, P < 0.001. The horizontal thick broken line indicates the limit of detection for the assay.
Following intranasal immunization with NV VLPs codelivered with CT or GARD, all mice produced serum NV VLP-specific IgG and IgG1 responses by day 12, as well as IgG2a responses by day 42, that were significantly higher than mock-immunized mice (P < 0.001) and remained significantly higher throughout the study (P < 0.001) (Fig. 1). From days 21 to 119, mice immunized with CT or GARD produced on average 36- or 21-fold-higher serum IgG levels, 7- or 3-fold-higher IgG1 levels, and 62- or 61-fold-higher IgG2a levels, respectively, relative to mice immunized with antigen alone (Fig. 1). The IgG (CT, days 42 and 56; GARD, days 12 to 42) and IgG2a (CT and GARD, days 42 and 56) responses were significantly higher than the responses in mice immunized with antigen alone (P < 0.05).
The antigen-to-adjuvant dose relationship of the TLR7/8 agonist R848 was evaluated by immunizing mice with either a 5-μg or 25-μg dose of NV VLP antigen codelivered with either 10 or 25 μg R848. One mouse immunized with 25 μg NV VLPs codelivered with 25 μg R848 died on day 56; however, no indications of immunotoxicity associated with the adjuvant or antigen were observed (i.e., no systemic lymphoproliferation, hepatomegaly, or splenomegaly). Immunization with a low dose of antigen (5 μg) codelivered with the highest dose of R848 (25 μg) did not elicit significant IgG, IgG1, or IgG2a responses compared to mock-immunized mice (P > 0.05) (Fig. 1).
In contrast, immunization with a 25-μg dose of antigen codelivered with either 10 or 25 μg R848 did elicit significant IgG, IgG1, and IgG2a production on most days compared to mock-immunized mice (P < 0.05) (Fig. 1). These responses, however, were not significantly different compared to mice immunized with antigen alone (P > 0.05) (Fig. 1). On average, after day 21, mice immunized with a standard dose of antigen codelivered with R848 induced IgG levels that were only 5-fold (10 μg R848) or 2-fold (25 μg R848) higher, respectively, than the levels in mice immunized with antigen alone (Fig. 1A). In addition, IgG1 and IgG2a levels in both groups immunized with R848 were comparable to or lower than those in mice immunized with NV VLP alone throughout the study (Fig. 1B and C). In comparison to CT-immunized mice, both R848-immunized groups induced IgG, IgG1, and IgG2a antibodies at levels that were 6-, 5-, and 4-fold lower, respectively (P > 0.05). Similarly, in comparison to GARD-immunized mice, both R848-immunized groups produced IgG, IgG1, and IgG2a antibodies at levels that were 6-, 2-, and 3-fold lower, respectively (P > 0.05) (Fig. 1).
Intranasal immunization with NV VLPs codelivered with CT or GARD is superior to R848 in the induction of a robust NV-specific mucosal immune response in the enteric pathway (gastrointestinal tract [fecal, salivary, and intestinal]).
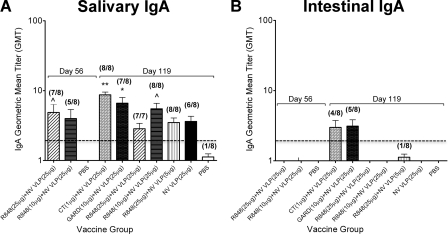
Noroviruses are enteric pathogens. We therefore evaluated the ability of TLR agonists to induce strong antigen-specific IgA production throughout the gastrointestinal tract by analyzing NV VLP-specific IgA content in the saliva, small intestine, and fecal extracts of mice immunized with NV VLPs codelivered with CT or TLR ligands.
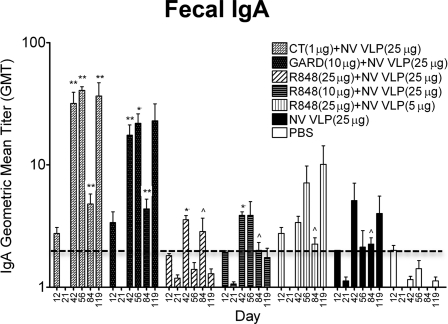
As observed with systemic IgG and IgG isotype responses (Fig. 1), intranasal codelivery with CT or GARD induced the highest and most consistent IgA immune responses in the gastrointestinal tract (Fig. 2 and 3). Fecal IgA titers increased by 4-fold following NV VLP boosting on day 21, and the addition of CT or GARD to NV VLPs increased fecal IgA levels by 32- and 18-fold, respectively. After day 42, fecal IgA levels fluctuated; however, mice immunized with CT (days 42 to 119) or GARD (days 42 to 84) had significantly elevated fecal IgA responses compared to mock-immunized mice (P < 0.01). On day 56, fecal IgA titers were also significantly higher than those in mice immunized with antigen alone (CT, P < 0.001; GARD, P < 0.01) (Fig. 2). Similarly, mice immunized with CT or GARD produced intestinal IgA titers that were 3-fold higher and salivary IgA titers that were 2-fold higher than mice immunized with antigen alone (Fig. 3). The salivary IgA levels were significantly higher than those in mock-immunized mice (CT, P < 0.001; GARD, P < 0.01) (Fig. 3).
FIG. 2.
Fecal NV-specific IgA production following intranasal immunization with NV VLPs codelivered with or without CT or TLR ligands. Fecal pellets were collected on days 0, 12, 21, 42, 56, 84, and 119 and analyzed for NV VLP-specific IgA by ELISAs. By day 42, all mice that received NV VLPs codelivered with or without adjuvant responded with a positive fecal IgA titer (GMT ≥ 2) except for those receiving R848 (25 μg) plus NV VLP (25 μg) (14/16 mice), R848 (25 μg) plus NV VLP (5 μg) (7/8 mice), or NV VLP alone (7/8 mice). Fecal antigen-specific IgA was not detected (GMT < 2) in all preimmune samples (data not shown). Error bars represent the standard errors of the means. Values that were significantly different from the values for the PBS control group are shown as follows: ∧, P < 0.05; *, P < 0.01; **, P < 0.001. The horizontal thick broken line indicates the limit of detection for the assay.
FIG. 3.
NV-specific IgA production in the gastrointestinal tract following intranasal immunization with NV VLPs codelivered with or without CT or TLR ligands. Mice were euthanized on days 56 and 119. Salivary swabs (A) and intestinal lavage samples (B) were collected and analyzed for NV VLP-specific IgA by ELISAs. The number of mice responding with a positive antibody titer (GMT ≥ 2) to the total number of mice is indicated in parentheses above each bar. Error bars represent the standard errors of the mean. Values that were significantly different from the values for the PBS control group are shown as follows: ∧, P < 0.05; *, P < 0.01; **, P < 0.001. There were no significant differences between the groups in panel A. The horizontal thick broken line indicates the limit of detection for the assay.
In contrast to immunization with CT or GARD, immunization with R848 induced fecal and intestinal IgA titers that were lower than or comparable to those in mice immunized with antigen alone (Fig. 2 and 3). While immunization with a standard dose of antigen codelivered with R848 elicited significant fecal IgA production on days 42 (P < 0.01) and 84 (P < 0.05) as well as salivary IgA production on days 56 (25 μg R848, P < 0.05) and 119 (10 μg R848, P < 0.05) compared to mock-immunized mice, fecal IgA titers were not consistently high, and fecal and salivary IgA titers were lower than those in mice immunized with CT or GARD (Fig. 2 and 3). Compared to mice immunized with CT or GARD, IgA titers in mice immunized with R848 were 11- or 7-fold lower, respectively, in fecal samples and 3- or 2-fold lower, respectively, in salivary and intestinal samples. The difference in IgA titers reached statistical significance in fecal extracts on days 56 (CT, P < 0.01; GARD, P < 0.05) and 119 (CT, P < 0.05) compared to mice immunized with R848 and a standard dose of antigen (Fig. 2).
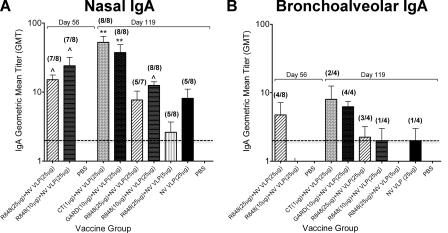
Intranasal immunization with NV VLPs codelivered with CT or GARD is superior to R848 in the induction of a robust NV-specific mucosal immune response at distal sites in the common mucosal immune system. (i) Respiratory tract (nasal and bronchoalveolar).
Although NV primarily infects the gastrointestinal tract, NV VLPs may be useful for displaying heterologous epitopes as delivery systems for mucosal immunizations (22); therefore, antigen-specific IgA and IgG production was evaluated at other mucosal sites, including the respiratory and reproductive tracts. To examine the mucosal immune response in the respiratory tract, nasal and bronchoalveolar lavage samples were collected following euthanization on days 56 and 119 and analyzed for antigen-specific IgA content. Similar to results observed for systemic (Fig. 1) and gastrointestinal (Fig. 2 and 3) immune responses, immunization with CT or GARD induced the highest nasal and bronchoalveolar NV VLP-specific IgA titers (Fig. 4). Nasal IgA titers were significantly higher than mock-immunized mice (P < 0.001) (Fig. 4A). Compared to immunization with antigen alone, codelivery with CT or GARD enhanced nasal IgA titers by 8- and 6-fold, as well as bronchoalveolar IgA titers by 4- and 3-fold, respectively (Fig. 4). Although mice immunized with a standard dose of antigen codelivered with R848 produced significantly higher nasal IgA levels at day 56 than mock-immunized mice (P < 0.05), no such response was observed in bronchoalveolar samples at day 56 (Fig. 4). Furthermore, in comparison to CT- and GARD-immunized mice, nasal IgA titers in all R848 immunization groups were on average 10- and 7-fold lower, respectively; likewise, bronchoalveolar IgA titers were 5- and 4-fold lower, respectively (Fig. 4).
FIG. 4.
NV-specific IgA production in the respiratory tract following intranasal immunization with NV VLPs codelivered with or without CT or TLR ligands. Following euthanization on days 56 and 119, nasal (A) and bronchoalveolar (B) lavage samples were collected and analyzed for NV VLP-specific IgA by ELISAs. The number of mice responding with a positive antibody titer (GMT ≥ 2) to the total number of mice is indicated in parentheses above each respective bar. Error bars represent the standard errors of the means. Values that were significantly different from the values for the PBS control group are shown as follows: ∧, P < 0.05; *, P < 0.01; **, P < 0.001.There were no significant differences between the groups in panel B. The horizontal thick broken line indicates the limit of detection for the assay.
(ii) Reproductive tract (vaginal).
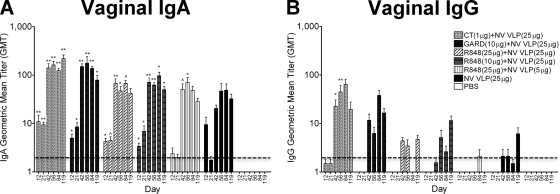
To examine the mucosal immune response in the female reproductive tract, vaginal lavage samples were collected and analyzed for NV VLP-specific IgA and IgG content. Vaginal IgA and IgG levels increased rapidly after NV VLP boosting on day 21. IgA levels remained consistently high, while IgG levels fluctuated from days 42 to 119. In addition, IgA levels were consistently higher than IgG levels in all immunization groups (Fig. 5). Mice immunized with NV VLPs codelivered with CT or GARD produced significant levels of vaginal IgA by day 12 that remained significantly elevated up to day 119 compared to mock-immunized mice (CT, P < 0.001; GARD, P < 0.01) (Fig. 5A). IgA levels in mice immunized with CT or GARD were also significantly higher than mice immunized with antigen alone on days 21 (CT, P < 0.01) and 42 (GARD, P < 0.05) (Fig. 5A). Significant IgG production was observed in mice immunized with CT from days 42 to 56 compared to mock-immunized mice (P < 0.01) and mice immunized with antigen alone (P < 0.05) (Fig. 5B). All R848-immunized groups did not produce significant IgG responses (Fig. 5B). While mice immunized with a standard dose of antigen codelivered with R848 produced significantly higher levels of vaginal IgA than mock-immunized mice on most days (10 μg R848, P < 0.01; 25 μg R848, P < 0.05), these levels were not significantly higher than mice immunized with antigen alone (P > 0.05) and were lower than those in mice immunized with CT or GARD (Fig. 5A). Moreover, mice immunized with a standard dose of antigen codelivered with R848 produced significantly lower IgG levels on days 42 (10 μg R848) and 56 (10 and 25 μg R848) than mice immunized with CT (P < 0.01).
FIG. 5.
Vaginal NV-specific IgA and IgG production following intranasal immunization with NV VLPs with or without CT or TLR ligands. Vaginal lavage samples were collected on days 0, 12, 21, 42, 56, 84, and 119 and analyzed for NV VLP-specific IgA (A) and IgG (B) by ELISAs. By day 42, all mice that received NV VLPs codelivered with or without adjuvant responded with a positive vaginal IgA antibody titer (GMT ≥ 2). None of the mice responded with a positive vaginal IgG titer (GMT ≥ 2) following a single immunization (days 12 and 21), except for the CT-treated group (1/8 mice). By day 119, 6/8 mice responded with a positive IgG titer in groups receiving NV VLP (25 μg) codelivered with CT, GARD, or R848 (10 μg), whereas groups immunized with NV VLP (25 μg) plus R848 (25 μg), NV VLP (5 μg) plus R848 (25 μg), or NV VLP alone responded with a positive titer in 4/7, 0/8, or 4/8 mice, respectively. Vaginal antigen-specific IgA and IgG were not detected (GMT < 2) in all preimmune samples (data not shown). Error bars represent the standard errors of the means. Values that were significantly different from the values for the PBS control group are shown as follows: ∧, P < 0.05; *, P < 0.01; **, P < 0.001. The horizontal thick broken line indicates the limit of detection for the assay.
Intranasal immunization with NV VLPs codelivered with CT or TLR ligands increases the frequency of NV-specific IgG-secreting splenocytes.
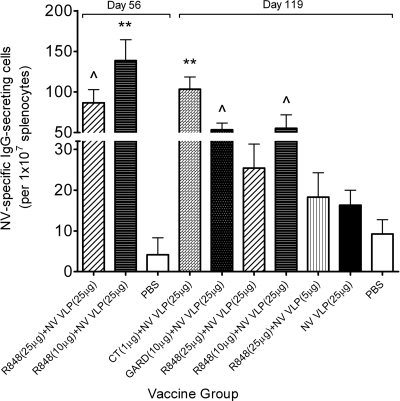
The frequency of NV VLP-specific IgG-secreting splenocytes was analyzed in mice following immunization with NV VLPs codelivered with or without CT or TLR ligands. Following euthanization on day 56 or 119, splenocytes were isolated, and 3 pools per group were prepared and analyzed in duplicate to determine the frequency of antigen-specific IgG-secreting cells by an ELISPOT assay. Mice immunized with NV VLPs codelivered with CT or GARD had significantly more IgG-secreting cells than mock-immunized mice (P < 0.001 and P < 0.05, respectively) (Fig. 6). In addition, mice immunized with a 25-μg dose of antigen codelivered with 10 or 25 μg R848 had significantly more IgG-secreting cells at day 56 than mock-immunized mice (P < 0.05 or P < 0.001, respectively). By day 119, however, the group immunized with 25 μg NV VLPs codelivered with 25 μg R848 was the only resiquimod group to have a significantly higher frequency of IgG-secreting cells than mock-immunized mice (P < 0.05), and the frequency in all mice immunized with R848 was significantly lower than mice immunized with CT (P < 0.05) (Fig. 6).
FIG. 6.
Frequency of NV-specific IgG-secreting cells in the spleen following intranasal immunization with NV VLPs with or without CT or TLR ligands. Following euthanization on day 56 or 119, splenocytes were isolated and pooled into 3 groups to determine the frequency of NV VLP-specific IgG-secreting cells by an ELISPOT assay. Results are expressed as the average number of antigen-specific IgG-secreting cells per 1 × 107 splenocytes. Error bars represent the standard errors of the means. Values that were significantly different from the values for the PBS control group are shown as follows: ∧, P < 0.05; **, P < 0.001.
DISCUSSION
Plant-derived NV VLPs have been shown to enhance antigen-specific systemic and mucosal immune responses when delivered orally in mice and humans. It is not known, however, whether the immune responses induced by NV VLPs is sufficient to provide protection against a live virus challenge (16, 22). Codelivery of NV VLPs with a safe and immunogenic mucosal adjuvant may enhance antigen-specific immune responses and potentially confer protection against a NV infection. CT, which is the gold standard in experimental studies of mucosal adjuvants for subunit vaccines, has a safety profile that makes it inappropriate for consideration in commercial nasal vaccines (37). It has been used, however, to show greatly enhanced systemic NV VLP-specific IgG responses when administered orally or intranasally in mice.
PRR agonists are currently widely studied as potential vaccine adjuvants because they induce immune responses that mimic the natural response during a live viral infection (28). This has led to one commercial success; MPL (a TLR4 agonist) was recently approved for use in an HPV VLP-based vaccine by the FDA (14). In the current study, we report the adjuvant activity of two imidazoquinoline-based TLR agonists, R848 and GARD, when intranasally codelivered with plant-derived NV VLPs. Our results indicate that the TLR7 agonist GARD induces antigen-specific systemic and mucosal immune responses superior to those induced by the TLR7/8 agonist R848 and comparable to those induced by CT.
Previous studies have demonstrated the role of R848 in the induction of a Th1 immune response through the activation of transcription factor nuclear factor κ B (NF-κB) and the secretion of proinflammatory cytokines like IFN-γ, IFN-α, TNF-α, and IL-12 (1, 2, 21, 53). R848 has also been evaluated as a vaccine adjuvant in murine models using ovalbumin (OVA) (48, 49, 51). Intranasal administration of OVA codelivered with R848 enhanced antigen-specific nasal IgA production, and subcutaneous and oral administration enhanced IFN-γ-dependent Th1 immune responses as measured by increased IgG2a production (43, 48, 49, 51). As reviewed by Tomai et al. (49) and Wu et al. (54), it is well established in the literature that R848 induces a primary Th1 immune response when delivered as an adjuvant.
In the present study, the dose-ranging adjuvant activity of R848 was evaluated for a NV VLP-based vaccine. In contrast to previous vaccine studies using OVA, intranasal codelivery with R848 did not consistently induce Th1 or robust mucosal immune responses against NV VLPs. NV-specific antibody ELISAs and ELISPOT assays indicate that immunization with 5 μg NV VLPs codelivered with 25 μg R848 was not sufficient to elicit significant anti-NV VLP immune response. While immunization with 25 μg NV VLPs codelivered with 10 or 25 μg R848 did elicit significant serum IgG and IgG isotype responses as well as fecal, salivary, nasal, and vaginal IgA responses compared to mock-immunized mice, in all cases the responses were not significantly higher than those elicited by mice immunized with antigen alone (Fig. 1 to 5). Similar IgG1 results were observed with subcutaneous OVA codelivery with R848 (51). In most cases, IgG2a titers were lower than IgG1 titers, suggesting the presence of a predominant Th2 response to NV VLP. In comparison to mice immunized with CT or GARD, systemic and mucosal NV-specific antibody levels in mice immunized with R848, regardless of dose, were consistently lower. Therefore, R848 did not induce sufficient mucosal IgA or systemic Th1 responses when codelivered with plant-derived NV VLPs.
GARD, the other imidazoquinoline-based TLR agonist used as an NV VLP vaccine adjuvant in this study, has been previously shown to enhance the maturation of antigen-presenting cells and the secretion of proinflammatory Th1-like cytokines, including IFN-γ, IFN-α, TNF-α, and IL-12 (5, 32). To date, only one study has reported the role of GARD as an adjuvant in a candidate vaccine formulation. This report was for a novel tuberculosis (TB) vaccine (5). GARD codelivered with the TB antigen intradermally induced IFN-γ production and skewed serum IgG antibody subclass responses to IgG1, indicative of a Th2 immune response. Codelivery with GARD also conferred protection against a live TB challenge infection (5). In our study, the adjuvant activity of GARD was evaluated by immunizing mice intranasally with GARD codelivered with NV VLPs. Codelivery with GARD consistently and significantly enhanced serum IgG, IgG1, and IgG2a levels relative to mock-immunized mice. Similar to the TB study, GARD induced higher IgG1 levels than IgG2a, suggesting the induction of a predominant Th2 response. In addition to enhancing systemic responses, codelivery with GARD also consistently and significantly enhanced mucosal IgA responses in gastrointestinal, respiratory, and reproductive tract samples (Fig. 2 to 5). Furthermore, in most cases, the total IgG, IgG isotypes, and IgA antibody titers induced by GARD were higher than those induced by R848 and comparable to those induced by the mucosal adjuvant CT. Therefore, the current study shows that GARD is an effective alternative to CT because it enhances a mixed Th1/Th2 systemic immune response as well as mucosal immune responses when delivered intranasally.
The differential immunogenic responses elicited by the two imidazoquinoline-based TLR agonists R848 and GARD may be due, in part, to the inherent nature of murine TLRs. Currently, TLR1 to TLR10 have been identified in humans, and TLR1 to TLR9 and TLR11 have been identified in mice (3, 15, 50). TLR7 and TLR8 are distinct classes of TLRs present in the endosomal compartments of innate immune cells that detect viral nucleic acids in the form of single-stranded RNA (ssRNA) (7, 8). In humans, TLR7 is predominantly expressed by B cells and plasmacytoid dendritic cells with some reports of expression in monocytes and macrophages (25, 27). In contrast, in mice, TLR7 is more broadly expressed on monocytes, macrophages, and the mature dendritic cell population found in mice, CD8α+ dendritic cells (4, 10). TLR8 is predominantly expressed on human myeloid dendritic cells, monocyte-derived dendritic cells, monocytes, macrophages, and neutrophils with some reports on regulatory T cells (17, 27, 41), whereas murine TLR8 is believed to be nonfunctional based on stimulation experiments with ssRNA and/or imadazoquinoline-based TLR8 agonists (18, 49). Therefore, in mice, TLR7/8 agonists such as R848 act solely through TLR7 (49). In our study, the reduced immunogenic effects of R848 compared to GARD, a strict TLR7 agonist, may be partially due to the fact that in mice, TLR8 is nonfunctional and R848 acts primarily through murine TLR7. It remains to be determined whether R848 could be used successfully as an adjuvant in humans for NV VLP vaccine.
In this study, we demonstrate that immunization with GARD significantly enhances NV VLP-specific systemic and mucosal responses in mice at a level that is statistically similar to that induced by CT, whereas immunization with R848 enhances immune responses only slightly, not to levels comparable to those elicited by GARD or CT. Therefore, our studies support the continued investigation of GARD as a potential adjuvant candidate for our intranasally delivered plant-derived NV VLPs. Future studies may determine the effectiveness of codelivering multiple TLR agonists to enhance systemic and mucosal IgA responses in mice and compare intranasal and parenteral routes of NV VLPs and PRR agonists, including GARD.
Acknowledgments
We thank Jacki Kilbourne and Daaimah LaVigne for their technical assistance with animal work.
The R848 used in this study was generously supplied by Mark Tomai at 3M Drug Delivery Systems. This work was supported by a grant from the Sexually Transmitted Infections and Topical Microbicides Cooperative Research Center (1 U19 AI062150-01).
Footnotes
Published ahead of print on 20 October 2010.
REFERENCES
- 1.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 2.Ahonen, C. L., S. J. Gibson, R. M. Smith, L. K. Pederson, J. M. Lindh, M. A. Tomai, and J. P. Vasilakos. 1999. Dendritic cell maturation and subsequent enhanced T-cell stimulation induced with the novel synthetic immune response modifier R-848. Cell. Immunol. 197:62-72. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S., and H. Hemmi. 2003. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 85:85-95. [DOI] [PubMed] [Google Scholar]
- 4.Asselin-Paturel, C., G. Brizard, K. Chemin, A. Boonstra, A. O'Garra, A. Vicari, and G. Trinchieri. 2005. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J. Exp. Med. 201:1157-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldwin, S. L., S. Bertholet, M. Kahn, I. Zharkikh, G. C. Ireton, T. S. Vedvick, S. G. Reed, and R. N. Coler. 2009. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine 27:3063-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ball, J. M., M. E. Hardy, R. L. Atmar, M. E. Conner, and M. K. Estes. 1998. Oral immunization with recombinant Norwalk virus-like particles induces a systemic and mucosal immune response in mice. J. Virol. 72:1345-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton, G. M. 2007. Viral recognition by Toll-like receptors. Semin. Immunol. 19:33-40. [DOI] [PubMed] [Google Scholar]
- 8.Boehme, K. W., and T. Compton. 2004. Innate sensing of viruses by Toll-like receptors. J. Virol. 78:7867-7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donaldson, E. F., L. C. Lindesmith, A. D. Lobue, and R. S. Baric. 2008. Norovirus pathogenesis: mechanisms of persistence and immune evasion in human populations. Immunol. Rev. 225:190-211. [DOI] [PubMed] [Google Scholar]
- 10.Doxsee, C. L., T. R. Riter, M. J. Reiter, S. J. Gibson, J. P. Vasilakos, and R. M. Kedl. 2003. The immune response modifier and Toll-like receptor 7 agonist S-27609 selectively induces IL-12 and TNF-alpha production in CD11c+CD11b+CD8- dendritic cells. J. Immunol. 171:1156-1163. [DOI] [PubMed] [Google Scholar]
- 11.Edwards, L., A. Ferenczy, L. Eron, D. Baker, M. L. Owens, T. L. Fox, A. J. Hougham, K. A. Schmitt, and the HPV Study Group. 1998. Self-administered topical 5% imiquimod cream for external anogenital warts. Arch. Dermatol. 134:25-30. [DOI] [PubMed] [Google Scholar]
- 12.Estes, M. K., J. M. Ball, R. A. Guerrero, A. R. Opekun, M. A. Gilger, S. S. Pacheco, and D. Y. Graham. 2000. Norwalk virus vaccines: challenges and progress. J. Infect. Dis. 181(Suppl. 2):S367-S373. [DOI] [PubMed] [Google Scholar]
- 13.Garland, S. M., M. Steben, H. L. Sings, M. James, S. Lu, R. Railkar, E. Barr, R. M. Haupt, and E. A. Joura. 2009. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J. Infect. Dis. 199:805-814. [DOI] [PubMed] [Google Scholar]
- 14.Giannini, S. L., E. Hanon, P. Moris, M. Van Mechelen, S. Morel, F. Dessy, M. A. Fourneau, B. Colau, J. Suzich, G. Losonksy, M. T. Martin, G. Dubin, and M. A. Wettendorff. 2006. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine 24:5937-5949. [DOI] [PubMed] [Google Scholar]
- 15.Gill, N., E. J. Davies, and A. A. Ashkar. 2008. The role of Toll-like receptor ligands/agonists in protection against genital HSV-2 infection. Am. J. Reprod. Immunol. 59:35-43. [DOI] [PubMed] [Google Scholar]
- 16.Glass, R. I., U. D. Parashar, and M. K. Estes. 2009. Norovirus gastroenteritis. N. Engl. J. Med. 361:1776-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorden, K. B., K. S. Gorski, S. J. Gibson, R. M. Kedl, W. C. Kieper, X. Qiu, M. A. Tomai, S. S. Alkan, and J. P. Vasilakos. 2005. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J. Immunol. 174:1259-1268. [DOI] [PubMed] [Google Scholar]
- 18.Gorden, K. K., X. X. Qiu, C. C. Binsfeld, J. P. Vasilakos, and S. S. Alkan. 2006. Activation of murine TLR8 by a combination of imidazoquinoline immune response modifiers and polyT oligodeoxynucleotides. J. Immunol. 177:6584-6587. [DOI] [PubMed] [Google Scholar]
- 19.Green, K. Y., J. F. Lew, X. Jiang, A. Z. Kapikian, and M. K. Estes. 1993. Comparison of the reactivities of baculovirus-expressed recombinant Norwalk virus capsid antigen with those of the native Norwalk virus antigen in serologic assays and some epidemiologic observations. J. Clin. Microbiol. 31:2185-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerrero, R. A., J. M. Ball, S. S. Krater, S. E. Pacheco, J. D. Clements, and M. K. Estes. 2001. Recombinant Norwalk virus-like particles administered intranasally to mice induce systemic and mucosal (fecal and vaginal) immune responses. J. Virol. 75:9713-9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196-200. [DOI] [PubMed] [Google Scholar]
- 22.Herbst-Kralovetz, M., H. S. Mason, and Q. Chen. 2010. Norwalk virus-like particles as vaccines. Expert Rev. Vaccines 9:299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmgren, J., J. Adamsson, F. Anjuere, J. Clemens, C. Czerkinsky, K. Eriksson, C. F. Flach, A. George-Chandy, A. M. Harandi, M. Lebens, T. Lehner, M. Lindblad, E. Nygren, S. Raghavan, J. Sanchez, M. Stanford, J. B. Sun, A. M. Svennerholm, and S. Tengvall. 2005. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunol. Lett. 97:181-188. [DOI] [PubMed] [Google Scholar]
- 24.Holmgren, J., A. M. Harandi, and C. Czerkinsky. 2003. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Expert Rev. Vaccines 2:205-217. [DOI] [PubMed] [Google Scholar]
- 25.Hornung, V., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2002. Quantitative expression of Toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531-4537. [DOI] [PubMed] [Google Scholar]
- 26.Huang, Z., G. Elkin, B. J. Maloney, N. Beuhner, C. J. Arntzen, Y. Thanavala, and H. S. Mason. 2005. Virus-like particle expression and assembly in plants: hepatitis B and Norwalk viruses. Vaccine 23:1851-1858. [DOI] [PubMed] [Google Scholar]
- 27.Jarrossay, D., G. Napolitani, M. Colonna, F. Sallusto, and A. Lanzavecchia. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31:3388-3393. [DOI] [PubMed] [Google Scholar]
- 28.Kanzler, H., F. J. Barrat, E. M. Hessel, and R. L. Coffman. 2007. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat. Med. 13:552-559. [DOI] [PubMed] [Google Scholar]
- 29.Klinman, D. M. 2006. CpG oligonucleotides accelerate and boost the immune response elicited by AVA, the licensed anthrax vaccine. Expert Rev. Vaccines 5:365-369. [DOI] [PubMed] [Google Scholar]
- 30.Lebwohl, M., S. Dinehart, D. Whiting, P. K. Lee, N. Tawfik, J. Jorizzo, J. H. Lee, and T. L. Fox. 2004. Imiquimod 5% cream for the treatment of actinic keratosis: results from two phase III, randomized, double-blind, parallel group, vehicle-controlled trials. J. Am. Acad. Dermatol. 50:714-721. [DOI] [PubMed] [Google Scholar]
- 31.LoBue, A. D., J. M. Thompson, L. Lindesmith, R. E. Johnston, and R. S. Baric. 2009. Alphavirus-adjuvanted norovirus-like particle vaccines: heterologous, humoral, and mucosal immune responses protect against murine norovirus challenge. J. Virol. 83:3212-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma, Y., L. Poisson, G. Sanchez-Schmitz, S. Pawar, C. Qu, G. J. Randolph, W. L. Warren, E. M. Mishkin, and R. G. Higbee. 2010. Assessing the immunopotency of Toll-like receptor agonists in an in vitro tissue-engineered immunological model. Immunology 130:374-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mason, H. S., J. M. Ball, J. J. Shi, X. Jiang, M. K. Estes, and C. J. Arntzen. 1996. Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc. Natl. Acad. Sci. U. S. A. 93:5335-5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McAleer, W. J., E. B. Buynak, R. Z. Maigetter, D. E. Wampler, W. J. Miller, and M. R. Hilleman. 1984. Human hepatitis B vaccine from recombinant yeast. Nature 307:178-180. [DOI] [PubMed] [Google Scholar]
- 35.Mosmann, T. R., H. Cherwinski, M. W. Bond, M. A. Giedlin, and R. L. Coffman. 2005. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 175:5-14. [PubMed] [Google Scholar]
- 36.Mosmann, T. R., and R. L. Coffman. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 37.Mutsch, M., W. Zhou, P. Rhodes, M. Bopp, R. T. Chen, T. Linder, C. Spyr, and R. Steffen. 2004. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N. Engl. J. Med. 350:896-903. [DOI] [PubMed] [Google Scholar]
- 38.Otero, M., S. A. Calarota, B. Felber, D. Laddy, G. Pavlakis, J. D. Boyer, and D. B. Weiner. 2004. Resiquimod is a modest adjuvant for HIV-1 gag-based genetic immunization in a mouse model. Vaccine 22:1782-1790. [DOI] [PubMed] [Google Scholar]
- 39.Paavonen, J., D. Jenkins, F. X. Bosch, P. Naud, J. Salmeron, C. M. Wheeler, S. N. Chow, D. L. Apter, H. C. Kitchener, X. Castellsague, N. S. de Carvalho, S. R. Skinner, D. M. Harper, J. A. Hedrick, U. Jaisamrarn, G. A. Limson, M. Dionne, W. Quint, B. Spiessens, P. Peeters, F. Struyf, S. L. Wieting, M. O. Lehtinen, and G. Dubin. 2007. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 369:2161-2170. [DOI] [PubMed] [Google Scholar]
- 40.Patel, M. M., M. A. Widdowson, R. I. Glass, K. Akazawa, J. Vinje, and U. D. Parashar. 2008. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg. Infect. Dis. 14:1224-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng, G., Z. Guo, Y. Kiniwa, K. S. Voo, W. Peng, T. Fu, D. Y. Wang, Y. Li, H. Y. Wang, and R. F. Wang. 2005. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science 309:1380-1384. [DOI] [PubMed] [Google Scholar]
- 42.Pulendran, B., and R. Ahmed. 2006. Translating innate immunity into immunological memory: implications for vaccine development. Cell 124:849-863. [DOI] [PubMed] [Google Scholar]
- 43.Quarcoo, D., S. Weixler, R. A. Joachim, P. Stock, T. Kallinich, B. Ahrens, and E. Hamelmann. 2004. Resiquimod, a new immune response modifier from the family of imidazoquinolinamines, inhibits allergen-induced Th2 responses, airway inflammation and airway hyper-reactivity in mice. Clin. Exp. Allergy 34:1314-1320. [DOI] [PubMed] [Google Scholar]
- 44.Rajagopal, D., C. Paturel, Y. Morel, S. Uematsu, S. Akira, and S. S. Diebold. 2010. Plasmacytoid dendritic cell-derived type I interferon is crucial for the adjuvant activity of Toll-like receptor 7 agonists. Blood 115:1949-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santi, L., L. Batchelor, Z. Huang, B. Hjelm, J. Kilbourne, C. J. Arntzen, Q. Chen, and H. S. Mason. 2008. An efficient plant viral expression system generating orally immunogenic Norwalk virus-like particles. Vaccine 26:1846-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slade, H. B., M. L. Owens, M. A. Tomai, and R. L. Miller. 1998. Imiquimod 5% cream (Aldara). Expert Opin. Invest. Drugs 7:437-449. [DOI] [PubMed] [Google Scholar]
- 47.Thomsen, L. L., P. Topley, M. G. Daly, S. J. Brett, and J. P. Tite. 2004. Imiquimod and resiquimod in a mouse model: adjuvants for DNA vaccination by particle-mediated immunotherapeutic delivery. Vaccine 22:1799-1809. [DOI] [PubMed] [Google Scholar]
- 48.Tomai, M. A., L. M. Imbertson, T. L. Stanczak, L. T. Tygrett, and T. J. Waldschmidt. 2000. The immune response modifiers imiquimod and R-848 are potent activators of B lymphocytes. Cell. Immunol. 203:55-65. [DOI] [PubMed] [Google Scholar]
- 49.Tomai, M. A., R. L. Miller, K. E. Lipson, W. C. Kieper, I. E. Zarraga, and J. P. Vasilakos. 2007. Resiquimod and other immune response modifiers as vaccine adjuvants. Expert Rev. Vaccines 6:835-847. [DOI] [PubMed] [Google Scholar]
- 50.Uematsu, S., and S. Akira. 2006. Toll-like receptors and innate immunity. J. Mol. Med. 84:712-725. [DOI] [PubMed] [Google Scholar]
- 51.Vasilakos, J. P., R. M. Smith, S. J. Gibson, J. M. Lindh, L. K. Pederson, M. J. Reiter, M. H. Smith, and M. A. Tomai. 2000. Adjuvant activities of immune response modifier R-848: comparison with CpG ODN. Cell. Immunol. 204:64-74. [DOI] [PubMed] [Google Scholar]
- 52.Verthelyi, D., and D. M. Klinman. 2003. Immunoregulatory activity of CpG oligonucleotides in humans and nonhuman primates. Clin. Immunol. 109:64-71. [DOI] [PubMed] [Google Scholar]
- 53.Wagner, T. L., C. L. Ahonen, A. M. Couture, S. J. Gibson, R. L. Miller, R. M. Smith, M. J. Reiter, J. P. Vasilakos, and M. A. Tomai. 1999. Modulation of TH1 and TH2 cytokine production with the immune response modifiers, R-848 and imiquimod. Cell. Immunol. 191:10-19. [DOI] [PubMed] [Google Scholar]
- 54.Wu, J. J., D. B. Huang, and S. K. Tyring. 2004. Resiquimod: a new immune response modifier with potential as a vaccine adjuvant for Th1 immune responses. Antiviral Res. 64:79-83. [DOI] [PubMed] [Google Scholar]
- 55.Xagorari, A., and K. Chlichlia. 2008. Toll-like receptors and viruses: induction of innate antiviral immune responses. Open Microbiol. J. 2:49-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang, W. W., and G. Matlashewski. 2008. Immunization with a Toll-like receptor 7 and/or 8 agonist vaccine adjuvant increases protective immunity against Leishmania major in BALB/c mice. Infect. Immun. 76:3777-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]