Abstract
Merck V710 is a novel vaccine containing the conserved Staphylococcus aureus iron surface determinant B shown to be protective in animal models. A phase I, multicenter, double-blind study of the dose range was conducted to assess the immunogenicity and safety of an adjuvanted liquid formulation of V710. A total of 124 adults (18 to 55 years of age) were randomized 1:1:1:1 to receive one 0.5-ml intramuscular injection of V710 (5 μg, 30 μg, or 90 μg) or saline placebo. A positive immune response was defined as at least a 2-fold increase in IsdB-specific IgG levels from baseline levels. Local and systemic adverse events were assessed for 5 and 14 days, respectively, following vaccination. Positive immune responses were detected in 12 (67%) of the 18 subjects in the groups receiving 30 and 90 μg V710 tested at day 10. At day 14, a significantly greater proportion of subjects manifested a positive immune response with higher geometric mean concentrations in the V710 30-μg (86%; geometric mean concentration of 116 μg/ml) and 90-μg (87%; geometric mean concentration of 131 μg/ml) dose groups than in the V710 5-μg (29%; geometric mean concentration of 51 μg/ml) or placebo (4%; geometric mean concentration of 23 μg/ml) groups. Immune responses were durable through day 84. Subjects <40 and ≥40 years of age had comparable immune responses. The most common adverse events were injection-site pain, nausea, fatigue, and headache, usually of mild intensity. No immediate reactions or serious adverse events were reported. In this first study of V710 in humans, a single 30-μg or 90-μg dose was more immunogenic than the 5-μg dose or placebo. Immune responses were evident by 10 to 14 days after vaccination in most responders.
Staphylococcus aureus causes a wide variety of infections ranging from superficial soft-tissue infections to sepsis and death (8, 15, 16, 19, 25, 30). The intact skin and mucous membranes of healthy individuals are usually effective barriers to staphylococci, but when these natural barriers are breached, the risk of serious staphylococcal infection grows. S. aureus infections appear to be increasing in both community and hospital settings. Antibiotic susceptibility does not guarantee successful outcomes in patients with serious S. aureus infections because of the intrinsic virulence of the organism and/or the frailty of the host. Furthermore, even in community-acquired infections, methicillin-resistant S. aureus (MRSA) is becoming commonplace (5, 16, 19, 30, 34, 37). Multidrug-resistant S. aureus has emerged as a real threat, especially when associated with decreased susceptibility to vancomycin (1, 2, 4, 6, 7, 9, 14, 20, 28, 38, 41). Resistance to newer antistaphylococcal antibiotics, such as linezolid and daptomycin, is starting to appear (31-33, 36). Widespread antimicrobial resistance has progressively limited safe and effective therapeutic options and has led to renewed efforts to develop prophylactic vaccines. A vaccine that protects against the large majority of nosocomial and community-acquired S. aureus strains could reduce the substantial morbidity and mortality associated with these common infections (13, 22, 23, 26). However, the antigenic diversity of pathogenic S. aureus strains has complicated and slowed vaccine development (10, 12, 17, 24, 27, 29, 40, 44).
To address an unmet need, a vaccine designated V710 that contains a highly conserved immunogenic surface protein called iron surface determinant B (IsdB) has been developed by Merck. Several properties of IsdB make it an attractive vaccine antigen (21, 42, 43). The protein is a member of the well-characterized LPXG family of S. aureus surface-exposed proteins, which ensures its accessibility by circulating antibodies. In addition, the protein is highly conserved, as evidenced by its expression in all of the >50 diverse S. aureus isolates (including community and hospital MRSA strains) screened to date. Preclinical studies in rats and rhesus monkeys have demonstrated rapid immune responses to vaccination with V710 (21). Vaccine efficacy has been shown in murine models of sepsis, deep-wound infection with dissemination, and catheter-associated bacteremia. Protection from lethal infection correlated with anti-IsdB antibody concentrations. This report describes the safety and immunogenicity results from the first dose escalation trial of V710 in humans.
MATERIALS AND METHODS
Primary objectives.
There were two primary objectives of this phase I study. The immunogenicity objective was to evaluate the frequency and magnitude of serological immune responses generated 14 days following the administration of V710 (for each of the three doses) relative to placebo. The safety objective was to evaluate the tolerability of ascending doses of V710 versus placebo when administered as a single intramuscular injection.
Study design.
Merck V710 protocol 001 was a randomized, multicenter, double-blind, placebo-controlled trial to evaluate increasing dosages (5 μg, 30 μg, and 90 μg) of V710 in healthy adults. For this study, V710 was prepared as a liquid formulation using Merck aluminum adjuvant in a single batch. Clinical vaccine supplies of V710 were provided as single-dose vials and stored at the sites at 2°C to 8°C. The placebo was sterile saline without adjuvant purchased from a commercial distributor in individual-dose vials. Since the vaccine and placebo had slightly different appearances, treatment-unblinded personnel at each study site were responsible for handling clinical supply shipments and administering vaccine or placebo to subjects by intramuscular injection into the deltoid muscle; these unblinded individuals were not involved in any postvaccination assessments.
Healthy adults between 18 and 55 years of age were eligible. Breast-feeding women or women who were or might become pregnant during the study period were excluded. Other exclusion criteria included any serious S. aureus infection during the previous year, fever (>100.4°F) in the prior 48 h, receipt of a live-virus vaccine during the last month, receipt of any vaccine in the preceding 2 weeks, an underlying immunocompromising disorder, recent use of corticosteroids or other immunosuppressive medications, chronic skin conditions, bleeding diathesis, or active recreational drug use. The study was conducted in accordance with principles of good clinical practice and was approved by the appropriate institutional review boards and regulatory agencies. Each participant signed an informed-consent form prior to undergoing any study-related procedures.
The trial was divided into two enrollment stages: sequential dose-escalating stage A followed by open-enrollment stage B. For each stage, subjects were randomly assigned to a vaccine or placebo group according to a computer-generated randomized allocation schedule. In each part of stage A, safety was successively assessed in 12 subjects randomized in a 3:1 ratio of V710 to placebo, starting with a 5-μg dose, then a 30-μg dose, and finally a 90-μg dose of vaccine for 14 days following the receipt of vaccine or placebo. Before enrollment could commence in the next higher-dose period in stage A, safety data at the lower dose had to be reviewed and approved by a Safety Evaluation Committee composed of representatives of the industry sponsor and independent experts. Following the completion of all three parts of stage A, all accumulated safety data were again evaluated by the Safety Evaluation Committee. Only after the safety results from stage A were deemed acceptable did enrollment in stage B commence. In stage B, subjects were randomized in a 1:1:1:1 ratio to one of the three dose strengths of V710 or placebo. A total of at least 84 patients (21 per group) were targeted for enrollment in stage B. Enrollment in stage B was stratified by age (18 to 39 years versus 40 to 55 years), with approximately equal numbers of subjects planned for the two age strata.
Immunogenicity evaluation.
Antibody concentrations were assayed by an IsdB enzyme-linked immunosorbent assay of blood collected at screening (prevaccination) and on days 7, 14, 28, 56, and 84 following vaccination (21). Blood was also collected at postvaccination days 3 and 10 from subjects in stage A. The primary immunogenicity time point was day 14. Sera were analyzed for IsdB-specific antibodies using a total IgG assay on a Luminex (Austin, TX) platform.
Safety evaluation.
Subjects were observed for 30 min after vaccination for immediate reactions. Local adverse events at the injection site and body temperature were monitored for 5 days following vaccination. Data for systemic adverse events were collected for 14 days following vaccination. Subjects were asked to record body temperatures and local (injection-site) reactions for days 1 to 5 and other (systemic) adverse events for days 1 to 14 on a standardized vaccination report card, which prompted subjects about four specific symptoms (nausea, headache, myalgia, and fatigue). Deaths from any cause and vaccine-related serious adverse events were to be reported for the entire 84-day duration of the study. Blood and urine were collected from subjects during stage A both at screening (prevaccination) and on postvaccination day 7 for blood counts, liver and renal function tests, and urinalysis.
Statistical analyses.
All vaccinated subjects without serious protocol violations were included in the per-protocol immunogenicity analyses. Serious protocol violations were defined as high baseline anti-IsdB IgG concentrations (>142 μg/ml), concomitant vaccination(s), or missing data. Immunogenicity measurements assessed at all time points for each treatment group included the proportion of subjects with a ≥2-fold rise in the antibody concentration from the baseline (defined as a “positive” immune response based on an estimate of assay variability derived from a validation study), geometric mean concentrations, and geometric mean fold rises in antibody concentrations from the baseline. The proportion of subjects with a ≥4-fold increase in antibody concentration from the baseline was also calculated at day 14 for each treatment group.
To evaluate the primary immunogenicity hypothesis that positive immune responses on day 14 would be higher in at least one vaccine group than in the placebo group, differences in the proportions of subjects in the per-protocol immunogenicity analyses with positive immune responses on day 14 postvaccination between the placebo group and each of the three V710 dose groups were assessed for statistical significance sequentially (starting with the highest potency compared with placebo) using the normal approximation for testing of two independent binomial proportions (one-tailed α = 0.025). V710 was to be deemed immunogenic relative to placebo if a dose was identified that had a response proportion statistically higher than that of placebo. Serious protocol violators were excluded from the per-protocol immunogenicity analysis, and no adjustment was made for missing data.
To compare the relative immunogenicities of the three V710 doses, differences in immune response rates and fold differences in geometric mean antibody concentrations (with corresponding 95% confidence intervals [CIs]) were calculated between the 5-μg and 30-μg groups, the 5-μg and 90-μg groups, and the 30-μg and 90-μg groups. To address possible differences in V710 immunogenicity between the two age strata, response rates and geometric mean concentrations were summarized by treatment group for subjects 18 to 39 years of age and for subjects 40 to 55 years of age. Analyses of fold differences in geometric mean concentrations after vaccination were adjusted for the baseline concentration using an analysis of covariance model.
All vaccinated subjects were included in the safety analysis. Any adverse event judged to be possibly, probably, or definitely related to the vaccine was tallied as a vaccine-related adverse event. The incidence of vaccine-related serious adverse events during the entire 84-day postvaccination study period with the corresponding 95% CI was summarized for each treatment group. No formal hypothesis testing was specified per protocol with regard to between-group comparisons of frequencies of adverse events. The intensity of injection-site reactions was graded by the investigator as being mild, moderate, or severe.
RESULTS
Subject accounting and demographics.
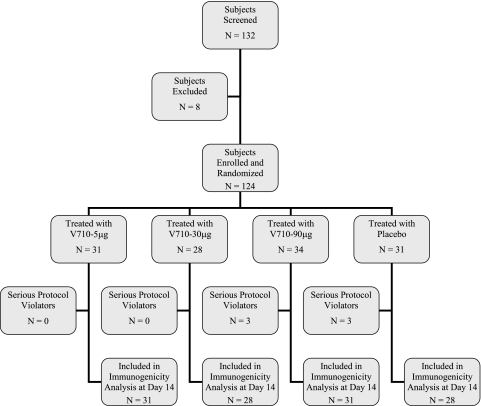
Subjects were enrolled at six sites across the United States. Overall, 36 subjects were randomized in the three sequential enrollment periods of stage A, followed by 88 subjects randomized in stage B (Fig. 1). Eight subjects were excluded from entry, including seven cases that involved a clinical or laboratory abnormality at screening.
FIG. 1.
Subject accounting and disposition. All enrolled patients were randomized and vaccinated. All vaccinated patients were included in the safety analysis. There were no deaths during the study period. A total of 119 (96%) of the 124 vaccinated subjects completed the trial through postvaccination day 84. Of the five subjects who discontinued the study, three were lost to follow-up (one V710 5-μg recipient and two placebo recipients), one moved (a V710 5-μg recipient), and one joined the Army Reserve (a V710 90-μg recipient). No subject discontinued the study because of an adverse event. Six subjects (three each in the V710 90-μg and placebo groups) were excluded from the per-protocol immunogenicity analysis at day 14 because of serious protocol violations (including high baseline antibody concentration in two subjects, concomitant vaccination[s] in one subject, and missing data for three subjects).
All 124 enrolled subjects were randomized and vaccinated. A total of 119 (96%) of the 124 vaccinated subjects completed the trial through postvaccination day 84. Five subjects discontinued the study: three were lost to follow-up (one V710 5-μg recipient and two placebo recipients), and two relocated (one each in the V710 5-μg and V710 90-μg groups). There were no deaths during the study. No subject discontinued the study because of an adverse event. Six subjects were excluded from the per-protocol immunogenicity analysis at day 14 because of serious protocol violations, including high baseline concentrations (n = 2), concomitant vaccinations (n = 1), or missing data (n = 3).
For the most part, demographic characteristics were comparably distributed across the four treatment arms (Table 1). Approximately equal numbers of subjects were entered into the prespecified age strata of 18 to 39 years (n = 65 [52%]) and 40 to 55 years (n = 59 [48%]). Eighteen (15%) subjects were ≥50 years old. A large majority of vaccinated subjects in each group were white (range, 58% to 77%), although a greater proportion of black subjects were randomized to the 5-μg (29%) and 30-μg (29%) V710 groups than to the 90-μg V710 (12%) and placebo (13%) groups.
TABLE 1.
Demographic characteristics of vaccinated subjectsa
| Characteristic | No. (%) for group |
|||
|---|---|---|---|---|
| V710 5 μg (n = 31) | V710 30 μg (n = 28) | V710 90 μg (n = 34) | Placebo (n = 31) | |
| Gender | ||||
| Male | 18 (58) | 7 (25) | 18 (53) | 13 (42) |
| Female | 13 (42) | 21 (75) | 16 (47) | 18 (58) |
| Age (yr)b | ||||
| 18-29 | 10 (32) | 5 (18) | 8 (24) | 10 (32) |
| 30-39 | 7 (23) | 8 (29) | 12 (35) | 5 (16) |
| 40-49 | 11 (36) | 8 (29) | 11 (32) | 11 (36) |
| 50-55 | 3 (10) | 7 (25) | 3 (9) | 5 (16) |
| Race or ethnicity | ||||
| White | 18 (58) | 19 (68) | 26 (77) | 21 (68) |
| Black | 9 (29) | 8 (29) | 4 (12) | 4 (13) |
| Asian | 3 (10) | 0 (0) | 1 (3) | 3 (10) |
| Hispanic | 1 (3) | 1 (4) | 2 (6) | 0 (0) |
| Native American | 0 (0) | 0 (0) | 0 (0) | 2 (7) |
| Multiracial | 0 (0) | 0 (0) | 1 (3) | 1 (3) |
All randomized subjects received vaccine or placebo.
Enrollment was stratified in stage B by ages of <40 and ≥40 years.
Immunogenicity results.
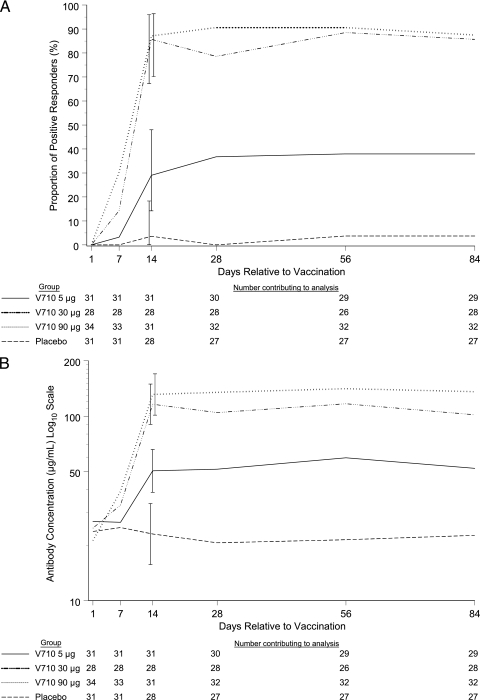
Positive immune responses were not detected in any of the 29 V710 recipients across the three vaccine dose groups or in the seven placebo recipients tested on postvaccination day 3 during stage A (Fig. 2A). Immune responses became evident as early as day 7 in a small minority of V710 recipients, including 4/28 (14%) and 10/33 (33%) subjects in the 30- and 90-μg V710 groups, respectively. Of the 18 subjects in the 30- and 90-μg V710 groups tested on postvaccination day 10, 12 (6 of 9 [67%] in each group) had positive immune responses at that time. At the primary immunogenicity time point (day 14 postvaccination), a similar proportion of subjects manifested positive immune responses in the V710 30- and 90-μg groups (86% and 87%, respectively), which were statistically superior to responses achieved in the V710 5-μg group and placebo groups (29% and 4%, respectively) (one-tailed P value of <0.025 for each pairwise comparison) (Table 2). Likewise, the absolute geometric mean concentration and its fold increase from baseline at postvaccination day 14 were higher for the V710 30- and 90-μg groups than those achieved for the V710 5-μg group and placebo groups. Antibody concentrations at day 14 were numerically higher than those at day 7 in all V710 dose groups. The magnitude of the immune responses persisted from day 14 through day 84 without much change (Fig. 2B). The immunogenicity profiles were comparable among the younger (18 to 39 years of age) and older (40 to 55 years of age) cohorts of subjects (Table 3).
FIG. 2.
Proportion of subjects with a positive immune response (A) and geometric mean concentrations of IsdB-specific IgG (B) over time. All vaccinated patients without serious protocol violations were included in these analyses. No missing data were imputed. Bars at day 14 represent 95% confidence intervals.
TABLE 2.
Immunogenicity results at the primary time point (day 14 postvaccination)
| Dose group | ≥2-fold increase in antibody concn from baseline (positive response)a |
≥4-fold increase in antibody concn from baselinea |
Geometric mean antibody concn (μg/ml) (95% CI) | Geometric mean fold rise in antibody concn from baseline (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| No. of subjects/no. of vaccinated subjects | % of subjects | 95% CI | No. of subjects/no. of vaccinated subjects | % of subjects | 95% CI | |||
| V710 (5 μg) | 9/31 | 29 | 14, 48 | 3/31 | 10 | 2, 26 | 51 (39, 66) | 1.8 (1.4, 2.2) |
| V710 (30 μg) | 24/28 | 86 | 67, 96 | 15/28 | 54 | 34, 72 | 116 (90, 149) | 4.4 (3.3, 5.7) |
| V710 (90 μg) | 27/31 | 87 | 70, 96 | 22/31 | 71 | 52, 86 | 131 (101, 170) | 5.5 (4.0, 7.5) |
| Placebo | 1/28 | 4 | 0, 18 | 1/28 | 4 | 0, 18 | 23 (16, 34) | 1.0 (0.9, 1.2) |
Shown are the numbers of subjects with the specified increase in antibody concentration and the numbers of vaccinated subjects in the corresponding treatment group.
TABLE 3.
Antibody responses by prespecified age groupings at day 14 postvaccination
| Age group (yr) and parameter | Value for group (95% CI) |
|||
|---|---|---|---|---|
| V710 5 μg (n = 31) | V710 30 μg (n = 28) | V710 90 μg (n = 31) | Placebo (n = 28) | |
| 18-39 | ||||
| % of subjects with ≥2-fold rise in antibody concn (positive response) | 29 (10, 56) | 85 (55, 98) | 78 (52, 94) | 8 (0, 36) |
| Geometric mean antibody concn (μg/ml) | 47 (33, 67) | 113 (74, 172) | 116 (79, 169) | 37 (24, 57) |
| Geometric mean fold rise in antibody concn from baseline | 1.7 (1.4, 2.0) | 3.4 (2.5, 4.6) | 4.8 (3.0, 7.9) | 1.1 (0.8, 1.6) |
| 40-55 | ||||
| % of subjects with ≥2-fold rise in antibody concn (positive response) | 29 (8, 58) | 87 (60, 98) | 100 (75, 100) | 0 (0, 22) |
| Geometric mean antibody concn (μg/ml) | 55 (35, 88) | 119 (84, 168) | 156 (110, 223) | 15 (9, 27) |
| Geometric mean fold rise in antibody concn from baseline | 2.0 (1.2, 3.1) | 5.4 (3.5, 8.5) | 6.6 (4.4, 9.7) | 0.9 (0.8, 1.0) |
Safety results.
No subject experienced an immediate postvaccination reaction, a documented temperature of ≥99.9°F (≥37.7°C) during the 5 days following vaccination, or a serious adverse event at any time during the study. The most common vaccine-related adverse events were injection-site pain and headache (Table 4), all of which were judged to be of mild or moderate intensity. Injection-site pain and headache were each more frequent in V710 recipients than placebo recipients. Of the 18 subjects reporting headache in the three V710 groups, 13 cases were mild and 5 cases were moderate in intensity. Of the four subjects reporting headache in the placebo group, one case was mild and three cases were moderate in intensity. Similarly, of the 46 subjects reporting injection-site pain in the three V710 groups, 44 cases were mild and 2 cases were moderate in intensity. Of the seven subjects with injection-site pain in the placebo group, all cases were mild in intensity. The most common systemic adverse events (fatigue, headache, myalgia, and nausea) were evenly distributed over the V710 dose and placebo groups. The frequencies of adverse events were comparable for the younger and older cohorts of subjects.
TABLE 4.
Frequencies of the most common vaccine-related adverse eventsa
| Adverse event | No. (%) of subjects |
|||
|---|---|---|---|---|
| V710 5 μg (n = 31) | V710 30 μg (n = 28) | V710 90 μg (n = 34) | Placebo (n = 31) | |
| Systemicb | ||||
| Fatigue | 4 (13) | 2 (7) | 3 (9) | 3 (10) |
| Headache | 6 (19) | 5 (18) | 3 (9) | 4 (13) |
| Myalgia | 3 (10) | 2 (7) | 2 (6) | 5 (16) |
| Nausea | 2 (7) | 3 (11) | 3 (9) | 1 (3) |
| Injection site | ||||
| Bruising | 0 (0) | 1 (4) | 0 (0) | 1 (3) |
| Erythema | 3 (1) | 0 (0) | 0 (0) | 1 (3) |
| Pain | 14 (45) | 16 (57) | 16 (47) | 7 (23) |
| Swelling | 2 (7) | 3 (11) | 1 (3) | 3 (10) |
All vaccinated patients were included in the safety assessment. Injection-site reactions and body temperature were monitored for 5 days following vaccination. Data for systemic adverse events were collected for 14 days following vaccination.
Includes all systemic adverse events occurring in ≥10% of any treatment group. Subjects were asked to record adverse events on a standardized vaccination report card, which explicitly asked about nausea, headache, myalgia, and fatigue.
None of the laboratory abnormalities detected in 7 (19%) of the 36 subjects tested during stage A on day 7 were graded as being more than mild toxicity on a predefined scale. Four (11%) subjects developed laboratory adverse events considered to be possibly vaccine related by the investigator: decreased hemoglobin concentration (one subject in the 5-μg V710 group), increased serum alkaline phosphatase level (two subjects in the 90-μg V710 group), and increased urinary protein level (one subject in the placebo group).
DISCUSSION
V710 protocol 001 represents the first trial for the Merck IsdB S. aureus vaccine in humans. Preclinical studies in rats and rhesus macaques indicated that V710 had the potential to provide relatively rapid protection against a broad spectrum of S. aureus isolates (21). In addition, no safety signals with the vaccine were identified in animal toxicology studies. This initial phase I study was conducted with the expressed purpose to assess the immunogenicity, safety, and tolerability of the adjuvanted liquid formulation of V710 in healthy adults between 18 and 55 years of age.
V710 was immunogenic following a single vaccination with all three dosages evaluated (5 μg, 30 μg, or 90 μg). In a small subset of 36 subjects tested on postvaccination day 3, none developed positive immune responses (as defined by a ≥2-fold increase in IsdB-specific IgG concentrations relative to baseline concentrations). Only a minority of subjects developed positive immune responses by day 7. For the 36 subjects tested on day 10, the majority of subjects given the two higher V710 doses exhibited positive immune responses. Most subjects receiving the higher two doses of V710 had positive immune responses at day 14. Significantly more subjects manifested a positive immune response and achieved higher geometric mean antibody concentrations with the 30- and 90-μg doses than with the 5-μg dose or placebo by postvaccination day 14. Antibody titers in subjects receiving the higher doses of vaccine were comparable to levels achieved in the preclinical studies with rhesus macaques (21). Subsequent to day 14, immune response rates and antibody concentrations remained relatively stable throughout the course of the study to day 84. V710 generated immune responses in the older cohort of subjects (40 to 55 years of age) that were at least comparable to those noted for the younger cohort of subjects (18 to 39 years of age). Serious adverse events were not encountered, although mild-to-moderate injection-site reactions and constitutional symptoms (primarily headache) were seen in vaccine recipients. Because the placebo did not contain an adjuvant, the observed increase in the frequency of pain at the injection site in the vaccine groups (which was comparable across doses) relative to the placebo group could be a consequence of the adjuvant and/or the protein antigen.
Vaccine strategies to prevent S. aureus infections may need to be tailored to the specific population at risk. High-risk groups include dialysis and cancer patients with chronic indwelling catheters, recipients of prosthetic valves or joints, and patients recovering from cardiothoracic or other major surgery (3, 15, 25, 45). The peak risk period for cardiac surgical patients is limited to the intra- and postoperative periods, whereas the risk is indefinitely ongoing for dialysis patients with end-stage renal disease. A vaccine containing S. aureus type 5 and 8 capsular polysaccharides conjugated to nontoxic recombinant Pseudomonas aeruginosa exotoxin A (StaphVAX; Nabi Biopharmaceuticals, Arlington, VA) conferred partial short-term protection for approximately 40 weeks against S. aureus bacteremia in patients undergoing hemodialysis in an initial study (39, 40); however, a larger subsequent study failed to demonstrate any protection at this same time point (S. Deresinski, presented at the 12th International Symposium on Staphylococci and Staphylococcal Infections, Cairns, Australia, 7 to 10 September 2008 [jeny.ipro.org/attachment.php?attachmentid=4692&d=1276005061]). An appropriately powered efficacy study is under way to confirm that V710 can induce relatively rapid immune responses and provide protection against serious S. aureus infections following single-dose vaccination in an at-risk population. The antibody concentrations against IsdB necessary for reliable protection from serious infections need to be better established (10, 27, 29, 44). The role and effectiveness of booster doses in patients at chronic risk for S. aureus infections remain to be elucidated (11). The possible impact of vaccination on mucocutaneous colonization has not been examined to date (3, 18, 35, 45). The contributions of the adjuvant to immunogenicity and tolerability cannot be established from these data.
Our findings imply that adjuvanted V710 could provide meaningful protection within 2 weeks of vaccination for patients anticipated to have a definable finite period of high risk for developing an S. aureus infection in the near term. Antibodies were detected by 7 to 14 days after vaccination in healthy subjects and persisted for at least 84 days. No differences in the safety profiles of the two higher-V710-dose groups were identified, and both the 30-μg and 90-μg dosages of V710 generated generally similar immune response rates and antibody concentrations, affording an opportunity to select a dose for future clinical trials that is well bracketed in terms of safety and immunogenicity.
Acknowledgments
We thank all the volunteers and investigators who participated in this study. The contributions of Larry Padget to an earlier poster presentation of these results and the instructive guidance of Tessie McNeely regarding the preclinical V710 studies are gratefully recognized. We also appreciate the technical assistance of Joann DiLullo and Karyn Davis in preparing the manuscript.
This study was funded by Merck & Co., Inc. Present and past employees of Merck may own stock or stock options in the company. All academic authors have been investigators for Merck. C.H. has received contract support from Merck related to its S. aureus, influenza virus, and HIV-1 vaccine programs and received fees for service on safety advisory committees for HIV-1 vaccine trials. The spouse of H.E.G. is employed by Merck.
Footnotes
Published ahead of print on 13 October 2010.
REFERENCES
- 1.Applebaum, P. C. 2007. Reduced glycopeptide susceptibility in methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Antimicrob. Agents 30:398-408. [DOI] [PubMed] [Google Scholar]
- 2.Bagga, B., A. K. Reddy, and P. Garg. 2010. Decreased susceptibility to quinolones in methicillin-resistant Staphylococcus aureus isolated from ocular infections at a tertiary eye care centre. Br. J. Ophthalmol. 94:1407-1408. [DOI] [PubMed] [Google Scholar]
- 3.Bode, L. G., J. A. Kluytmans, H. F. Wertheim, D. Bogaers, C. M. Vandenbroucke-Grauls, R. Roosendaal, A. Troelstra, A. T. Box, A. Voss, I. van der Tweel, A. van Belkum, H. A. Verbrugh, and M. C. Vos. 2010. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N. Engl. J. Med. 362:9-17. [DOI] [PubMed] [Google Scholar]
- 4.Bordon, J., R. N. Master, R. B. Clark, P. Duvvuri, J. A. Karlowsky, K. Ayesu, A. Klotchko, R. Kapoor, and J. Ramirez. 2010. Methicillin-resistant Staphylococcus aureus resistance to non-beta-lactam antimicrobials in the United States from 1996 to 2008. Diagn. Microbiol. Infect. Dis. 67:395-398. [DOI] [PubMed] [Google Scholar]
- 5.Boyce, J. M., B. Cookson, K. Christiansen, S. Hori, J. Vuopio-Varkila, S. Kocagoz, A. Y. Oztop, C. M. Vandenbroucke-Grauls, S. Harbarth, and D. Pittet. 2005. Methicillin-resistant Staphylococcus aureus. Lancet Infect. Dis. 5:653-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cafiso, V., T. Bertuccio, D. Spina, F. Campanile, D. Bongiorno, M. Santagati, A. Sciacca, C. Sciuto, and S. Stefani. 2010. Methicillin resistance and vancomycin heteroresistance in Staphylococcus aureus in cystic fibrosis patients. Eur. J. Clin. Microbiol. Infect. Dis. 29:1277-1285. [DOI] [PubMed] [Google Scholar]
- 7.Castanheira, M., A. A. Watters, J. M. Bell, J. D. Turnidge, and R. N. Jones. 2010. Fusidic acid resistance rates and prevalence of resistance mechanisms among Staphylococcus spp. isolated in North America and Australia (2007-2008). Antimicrob. Agents Chemother. 54:3614-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, F. Y., B. B. MacDonald, J. E. Peacock, Jr., D. M. Musher, P. Triplett, J. M. Mylotte, A. O'Donnell, M. M. Wagener, and V. L. Yu. 2003. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 82:322-332. [DOI] [PubMed] [Google Scholar]
- 9.Chang, S., D. M. Sievert, J. C. Hageman, M. L. Boulton, F. C. Tenover, F. P. Downes, S. Shah, J. T. Rudrik, G. R. Pupp, W. J. Brown, D. Cardo, and S. K. Fridkin. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342-1347. [DOI] [PubMed] [Google Scholar]
- 10.Ekstedt, R. D., and K. Yoshida. 1969. Immunity to staphylococcal infection in mice: effect of living versus killed vaccine, role of circulating antibody, and induction of protection-inducing antigen(s) in vitro. J. Bacteriol. 100:745-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fattom, A., S. Fuller, M. Propst, S. Winston, L. Muenz, D. He, R. Naso, and G. Horwith. 2004. Safety and immunogenicity of a booster dose of Staphylococcus aureus types 5 and 8 capsular polysaccharide conjugate vaccine (StaphVAX) in hemodialysis patients. Vaccine 23:656-663. [DOI] [PubMed] [Google Scholar]
- 12.Fattom, A. I., G. Horwith, S. Fuller, M. Propst, and R. Naso. 2004. Development of StaphVAX, a polysaccharide conjugate vaccine against S. aureus infection: from the lab bench to phase III clinical trials. Vaccine 22:880-887. [DOI] [PubMed] [Google Scholar]
- 13.Filice, G. A., J. A. Nyman, C. Lexau, C. H. Lees, L. A. Bockstedt, K. Como-Sabetti, L. J. Lesher, and R. Lynfield. 2010. Excess costs and utilization associated with methicillin resistance for patients with Staphylococcus aureus infection. Infect. Control Hosp. Epidemiol. 31:365-373. [DOI] [PubMed] [Google Scholar]
- 14.Foucault, M. L., P. Courvalin, and C. Grillot-Courvalin. 2009. Fitness cost of VanA-type vancomycin resistance in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:2354-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowler, V. G., Jr., M. K. Olsen, G. R. Corey, C. W. Woods, C. H. Cabell, L. B. Reller, A. C. Cheng, T. Dudley, and E. Z. Oddone. 2003. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch. Intern. Med. 163:2066-2072. [DOI] [PubMed] [Google Scholar]
- 16.Fridkin, S. K., J. C. Hageman, M. Morrison, L. T. Sanza, K. Como-Sabetti, J. A. Jernigan, K. Harriman, L. H. Harrison, R. Lynfield, and M. M. Farley. 2005. Methicillin-resistant Staphylococcus aureus disease in three communities. N. Engl. J. Med. 352:1436-1444. [DOI] [PubMed] [Google Scholar]
- 17.Harro, J. M., B. M. Peters, G. A. O'May, N. Archer, P. Kerns, R. Prabhakara, and M. E. Shirtliff. 2010. Vaccine development in Staphylococcus aureus: taking the biofilm phenotype into consideration. FEMS Immunol. Med. Microbiol. 59:306-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honda, H., M. J. Krauss, C. M. Coopersmith, M. H. Kollef, A. M. Richmond, V. J. Fraser, and D. K. Warren. 2010. Staphylococcus aureus nasal colonization and subsequent infection in intensive care unit patients: does methicillin resistance matter? Infect. Control Hosp. Epidemiol. 31:584-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, L. B., M. O. Almoujahed, K. Ilg, L. Maolood, and R. Khatib. 2003. Staphylococcus aureus bacteremia: compliance with standard treatment, long-term outcome and predictors of relapse. Scand. J. Infect. Dis. 35:782-789. [DOI] [PubMed] [Google Scholar]
- 20.Khosrovaneh, A., K. Riederer, S. Saeed, M. S. Tabriz, A. R. Shah, M. M. Hanna, M. Sharma, L. B. Johnson, M. G. Fakih, and R. Khatib. 2004. Frequency of reduced vancomycin susceptibility and heterogeneous subpopulation in persistent or recurrent methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 38:1328-1330. [DOI] [PubMed] [Google Scholar]
- 21.Kuklin, N. A., D. J. Clark, S. Secore, J. Cook, L. D. Cope, T. McNeely, L. Noble, M. J. Brown, J. K. Zorman, X. M. Wang, G. Pancari, H. Fan, K. Isett, B. Burgess, J. Bryan, M. Brownlow, H. George, M. Meinz, M. E. Liddell, R. Kelly, L. Schultz, D. Montgomery, J. Onishi, M. Losada, M. Martin, T. Ebert, C. Y. Tan, T. L. Schofield, E. Nagy, A. Meineke, J. G. Joyce, M. B. Kurtz, M. J. Caulfield, K. U. Jansen, W. McClements, and A. S. Anderson. 2006. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect. Immun. 74:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, B. Y., P. J. Ufberg, R. R. Bailey, A. E. Wiringa, K. J. Smith, A. J. Nowalk, C. Higgins, A. R. Wateska, and R. R. Muder. 2010. The potential economic value of a Staphylococcus aureus vaccine for neonates. Vaccine 28:4653-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, B. Y., A. E. Wiringa, R. R. Bailey, G. J. Lewis, J. Feura, and R. R. Muder. 2010. Staphylococcus aureus vaccine for orthopedic patients: an economic model and analysis. Vaccine 28:2465-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindsay, J. A. 2007. Prospects for a MRSA vaccine. Future Microbiol. 2:1-3. [DOI] [PubMed] [Google Scholar]
- 25.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 26.Lucero, C. A., J. Hageman, E. R. Zell, S. Bulens, J. Nadle, S. Petit, K. Gershman, S. Ray, L. H. Harrison, R. Lynfield, G. Dumyati, J. M. Townes, W. Schaffner, and S. K. Fridkin. 2009. Evaluating the potential public health impact of a Staphylococcus aureus vaccine through use of population-based surveillance for invasive methicillin-resistant S. aureus disease in the United States. Vaccine 27:5061-5068. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy, A. J., and J. A. Lindsay. 2010. Genetic variation in Staphylococcus aureus surface and immune evasion genes is lineage associated: implications for vaccine design and host-pathogen interactions. BMC Microbiol. 10:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDougal, L. K., G. E. Fosheim, A. Nicholson, S. N. Bulens, B. M. Limbago, J. E. Shearer, A. O. Summers, and J. B. Patel. 2010. Emergence of resistance among USA300 methicillin-resistant Staphylococcus aureus isolates causing invasive disease in the United States. Antimicrob. Agents Chemother. 54:3804-3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Middleton, J. R. 2008. Staphylococcus aureus antigens and challenges in vaccine development. Expert Rev. Vaccines 7:805-815. [DOI] [PubMed] [Google Scholar]
- 30.Miller, L. G., F. Perdreau-Remington, G. Rieg, S. Mehdi, J. Perlroth, A. S. Bayer, A. W. Tang, T. O. Phung, and B. Spellberg. 2005. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 352:1445-1453. [DOI] [PubMed] [Google Scholar]
- 31.Moise, P. A., D. North, J. N. Steenbergen, and G. Sakoulas. 2009. Susceptibility relationship between vancomycin and daptomycin in Staphylococcus aureus: facts and assumptions. Lancet Infect. Dis. 9:624. [DOI] [PubMed] [Google Scholar]
- 32.Morales, G., J. J. Picazo, E. Baos, F. J. Candel, A. Arribi, B. Pelaez, R. Andrade, M. A. de la Torre, J. Fereres, and M. Sanchez-Garcia. 2010. Resistance to linezolid is mediated by the cfr gene in the first report of an outbreak of linezolid-resistant Staphylococcus aureus. Clin. Infect. Dis. 50:821-825. [DOI] [PubMed] [Google Scholar]
- 33.Nannini, E., B. E. Murray, and C. A. Arias. 2010. Resistance or decreased susceptibility to glycopeptides, daptomycin, and linezolid in methicillin-resistant Staphylococcus aureus. Curr. Opin. Pharmacol. 10:516-521. [DOI] [PubMed] [Google Scholar]
- 34.Orendi, J. M., N. Coetzee, M. J. Ellington, E. Boakes, B. D. Cookson, K. J. Hardy, P. M. Hawkey, and A. M. Kearns. 2010. Community and nosocomial transmission of Panton-Valentine leucocidin-positive community-associated methicillin-resistant Staphylococcus aureus: implications for healthcare. J. Hosp. Infect. 74:258-264. [DOI] [PubMed] [Google Scholar]
- 35.Perl, T. M., J. J. Cullen, R. P. Wenzel, M. B. Zimmerman, M. A. Pfaller, D. Sheppard, J. Twombley, P. P. French, and L. A. Herwaldt. 2002. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N. Engl. J. Med. 346:1871-1877. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez-Garcia, M., M. A. de la Torre, G. Morales, B. Pelaez, M. J. Tolon, S. Domingo, F. J. Candel, R. Andrade, A. Arribi, N. Garcia, F. Martinez Sagasti, J. Fereres, and J. Picazo. 2010. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA 303:2260-2264. [DOI] [PubMed] [Google Scholar]
- 37.Scazzocchio, F., L. Aquilanti, C. Tabacchini, V. Iebba, and C. Passariello. 21 June 2010, posting date. Microbiological and molecular characterization of nosocomial and community Staphylococcus aureus isolates. Epidemiol. Infect. doi: 10.1017/S095026881000138X. [DOI] [PubMed]
- 38.Schwartz, B. S., C. J. Graber, B. A. Diep, L. Basuino, F. Perdreau-Remington, and H. F. Chambers. 2009. Doxycycline, not minocycline, induces its own resistance in multidrug-resistant, community-associated methicillin-resistant Staphylococcus aureus clone USA300. Clin. Infect. Dis. 48:1483-1484. [DOI] [PubMed] [Google Scholar]
- 39.Shinefield, H., S. Black, A. Fattom, G. Horwith, S. Rasgon, J. Ordonez, H. Yeoh, D. Law, J. B. Robbins, R. Schneerson, L. Muenz, S. Fuller, J. Johnson, B. Fireman, H. Alcorn, and R. Naso. 2002. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N. Engl. J. Med. 346:491-496. [DOI] [PubMed] [Google Scholar]
- 40.Shinefield, H. R. 2006. Use of a conjugate polysaccharide vaccine in the prevention of invasive staphylococcal disease: is an additional vaccine needed or possible? Vaccine 24(Suppl. 2):S2-S9. [DOI] [PubMed] [Google Scholar]
- 41.Sieradzki, K., R. B. Roberts, S. W. Haber, and A. Tomasz. 1999. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N. Engl. J. Med. 340:517-523. [DOI] [PubMed] [Google Scholar]
- 42.Ster, C., F. Beaudoin, M. S. Diarra, M. Jacques, F. Malouin, and P. Lacasse. 2010. Evaluation of some Staphylococcus aureus iron-regulated proteins as vaccine targets. Vet. Immunol. Immunopathol. 136:311-318. [DOI] [PubMed] [Google Scholar]
- 43.Stranger-Jones, Y. K., T. Bae, and O. Schneewind. 2006. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 103:16942-16947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Therrien, R., P. Lacasse, G. Grondin, and B. G. Talbot. 2007. Lack of protection of mice against Staphylococcus aureus despite a significant immune response to immunization with a DNA vaccine encoding collagen-binding protein. Vaccine 25:5053-5061. [DOI] [PubMed] [Google Scholar]
- 45.Yu, V. L., A. Goetz, M. Wagener, P. B. Smith, J. D. Rihs, J. Hanchett, and J. J. Zuravleff. 1986. Staphylococcus aureus nasal carriage and infection in patients on hemodialysis. Efficacy of antibiotic prophylaxis. N. Engl. J. Med. 315:91-96. [DOI] [PubMed] [Google Scholar]