Abstract
Background
We have accumulated multiple lines of evidence supporting the ability of HB-EGF to protect the intestines from injury and to augment the healing of partial-thickness scald burns of the skin. The aim of the current study was to investigate the role of heparin-binding EGF-like growth factor (HB-EGF) in intestinal anastomotic wound healing.
Materials and Methods
HB-EGF(−/−) knockout (KO) mice (n= 42) and their HB-EGF(+/+) wild type (WT) counterparts (n= 33), as well as HB-EGF transgenic (TG) mice (n=26) and their (WT) counterparts (n=27), underwent division and reanastomosis of the terminal ileum. In addition, WT mice (n=21) that received enteral HB-EGF (800µg/kg) underwent the same operative procedure. Anastomotic bursting pressure was measured at 3 and 6 days postoperatively. Tissue sections were stained with hematoxylin & eosin to assess anastomotic healing, and Picrosirus red to assess collagen deposition. Immunohistochemistry using anti-von Willebrand factor antibodies was performed to assess angiogenesis. Complications and mortality were also recorded.
Results
HB-EGF KO mice had significantly lower bursting pressures, lower healing scores, higher mortality and higher complication rates postoperatively compared to WT mice. Collagen deposition and angiogenesis were significantly decreased in KO mice compared to WT mice. Conversely, HB-EGF TG mice had increased anastomotic bursting pressure, higher healing scores, lower mortality, lower complication rates, increased collagen deposition and increased angiogenesis postoperatively compared to WT mice. WT mice that received HB-EGF had increased bursting pressures compared to non-HB-EGF treated mice.
Conclusion
Our results demonstrate that HB-EGF is an important factor involved in the healing of intestinal anastomoses.
Keywords: heparin-binding EGF-like growth factor, intestine, anastomosis
INTRODUCTION
Rapid and effective wound-healing is of paramount importance to the surgeon. Failure of wound-healing generally leads to potentially life-threatening complications, additional surgical procedures, increased length of hospital stay, increased cost, and long-term disability.[1] Inadequate healing and consequent leakage from bowel anastomosis are a significant cause of postoperative morbidity and mortality.[2]
During the past few years, a series of candidate key players in the wound-healing scenario have been identified. These include not only a variety of different growth factors and cytokines, but also molecules that are involved in cell–cell and cell–matrix interactions, and proteins responsible for cell stability and cell migration.[3] The effects of growth hormone and different growth factors on the healing of bowel anastomoses are currently being evaluated in an attempt to identify agents that may promote and improve the anastomotic healing process. This is especially important in seriously ill patients with altered metabolism and impaired tissue healing.[2]
Heparin-binding EGF-like growth factor (HB-EGF) was initially identified in the conditioned medium of cultured human macrophages[4] and later found to be a member of the epidermal growth factor (EGF) family.[5] Like other family members, HB-EGF binds to the EGF receptor (EGFR; ErbB-1), inducing its phosphorylation. Unlike most EGF family members, HB-EGF has the ability to bind strongly to heparin. Cell-surface heparan-sulfate proteoglycans (HSPG) can act as highly abundant, low affinity receptors for HB-EGF. HB-EGF mRNA expression has been demonstrated in multiple tissues including skin, lung, heart, intestine, kidney, skeletal muscle, brain, male reproductive system, lymph nodes, thymus and spleen.[6, 7] HB-EGF is produced by many different cell types including epithelial cells, and it is mitogenic and chemotactic for smooth muscle cells, keratinocytes, hepatocytes and fibroblasts. HB-EGF exerts its mitogenic effects by binding to and activation of EGF receptor subtypes ErbB-1 and ErbB-4.[8] In addition, HB-EGF exerts chemotactic effects when binding to the HB-EGF specific receptor N-arginine dibasic convertase (NrdC).[9] Importantly, endogenous HB-EGF is protective in various pathologic conditions and plays a pivotal role in mediating the earliest cellular responses to proliferative stimuli and cellular injury.[10]
We have previously demonstrated the presence of HB-EGF protein in the basal layer of normal human dermis.[11] In addition, we have identified HB-EGF in human burn wound fluid following thermal injury, and have localized it immunohistochemically to epithelial cells of the marginal epidermis and dermal appendages of excised human burn tissue.[12] Furthermore, after thermal injury of the skin there is increased expression of endogenous HB-EGF in marginal surface keratinocytes.[13] We have also shown that topical application of HB-EGF accelerates partial thickness burn wound healing by increasing keratinocyte proliferative activity, in part via enhanced production of endogenous transforming growth factor α mRNA.[14]
We have accumulated multiple lines of evidence supporting the role of HB-EF in protection of the intestines from a variety of insults including intestinal ischemia/reperfusion (I/R) injury[15], hemorrhagic shock and resuscitation(HS/R)[16] and necrotizing enterocolitis (NEC)[17, 18]. Several investigators have shown that significant metabolic differences exist between wound healing in the skin and in the gastrointestinal tract.[19] Our findings of the ability of HB-EGF to promote cutaneous burn wound healing and to protect the intestines from various types of injuries prompted us to investigate the ability of HB-EGF to affect intestinal anastomotic wound healing.
MATERIALS AND METHODS
Animals
To investigate the effects of HB-EGF loss-of-function on intestinal anastomotic wound healing, HB-EGF(−/−) knockout (KO) mice (n=42) and their HB-EGF(+/+) wild type (WT) counterparts (n=33) were randomly selected to undergo terminal ileal division and reanastomosis. HB-EGF KO mice on a C57BL/6J × 129 background and their HB-EGF WT C57BL/6J × 129 counterparts were kindly provided by Dr. David Lee (Chapel Hill, NC).[20] In HB-EGF KO mice, HB-EGF exons 1 and 2 were replaced with PGK-Neo, thus deleting the signal peptide and propeptide domains. The desired targeting events were verified by Southern blots of genomic DNA and exon-specific polymerase chain reaction, with Northern blots confirming the absence of the respective transcripts.[20]
To investigate the effects of overexpression of HB-EGF on intestinal anastomotic wound healing, HB-EGF transgenic (TG) mice (n=26) and their (WT) FVB counterparts (n=27) underwent the same procedure. HB-EGF TG mice were produced in our laboratory and were designed to specifically overexpress the human HB-EGF precursor (proHB-EGF) in the intestine using a 12.4 kb villin regulatory and promoter sequence to drive human proHB-EGF gene expression.[21] The promoter of the villin gene ensures the constant expression of HB-EGF throughout the entire intestine from the duodenum to the colon, from embryogenesis to adulthood. Furthermore, the villin promoter targets transgene expression throughout the entire crypt-villous axis. The pBSII-12.4kbVill plasmid containing the 12.4kb promoter fragment from the villin gene was a generous gift from Dr. Deborah Gumucio (University of Michigan, Ann Arbor, MI).[22]
To investigate the effects of exogenous administration of HB-EGF on intestinal anastomotic wound healing,C57Bl/6J × 129 WT mice (n=21) received HB-EGF (800 µg/kg) administered by gastric gavage once per day for 3 days prior to anastomosis surgery. Mice were gastric gavaged using a 20 gauge feeding needle (Braintree Scientific, Braintree, MA) with 1 ml 0.9% saline solution (Baxter Healthcare Corporation, Deerfield, IL), containing 800 µg/kg HB-EGF. The HB-EGF used was Good Manufacturing Process (GMP) grade human mature HB-EGF produced in Pichia pastoris yeast and supplied to us by Trillium Therapeutics, Inc, (Toronto, Canada).
The dose of HB-EGF administered was the most efficacious dose of enterally administered HB-EGF leading to protection of the intestines from injury based on our previous studies.[23]
Intestinal anastomosis
The experimental protocol was performed according to the guidelines for the ethical treatment of experimental animals and approved by the Institutional Animal Care and Use Committee of Nationwide Children’s Hospital, Columbus, Ohio (#AR06-00094). Mice were anesthetized with inhaled 2% Isoflurane (Butler Animal Health, Dublin, OH). The abdomen was clipped and prepared with a solution of 70% alcohol. Operations were performed with the aid of an operating microscope (10× magnification, Carl Zeiss Inc., Thornwood, NY). Through a midline incision, the ileocecal junction was identified. The intervention consisted of division of the terminal ileum approximately 5 cm proximal to the ileocecal junction, with reanastomosis using 8-0 nylon sutures (Ethicon, Somerville, NJ).The surgical technique for the intestinal anastomosis was standardized and we used eight interrupted sutures equally spaced in a single layer to reconstruct the continuity of the intestine in an end-to-end fashion. The abdomen was closed in a single layer using 4.0 nylon sutures (Ethicon, Somerville, NJ) and the animals were resuscitated with a subcutaneous injection of Lactated Ringers solution (1 ml) (Baxter Healthcare Corporation, Deerfield, IL). Animals were allowed to recover in a warmed incubator (37°C) for 4 h after surgery, and they re ceived 5% dextrose in water for 48 h postoperatively, after which they were provided with water and solid food ad libitum. Postoperatively, animals received analgesia every 12 h for 48 h using buprenorphine hydrochloride (0.1mg/ml, 0.15mg/kg body weight) (Reckitt Benckiser Healthcare, Hull, England).
Bursting pressure measurements
On postoperative days 3 and 6 (POD 3 and 6), animals were euthanized by cervical dislocation, the abdomen was opened, and any evidence of anastomotic dehiscence, abscess or intestinal obstruction was recorded. The site of the anastomosis was excised with a 3 cm margin proximally and distally. A 4.0 nylon ligature suture (Ethicon, Somerville, NJ) was placed across the bowel 2 cm distal to the anastomosis. After the introduction of a catheter into the intestinal lumen through the proximal end of the sample, a watertight seal was created using a second 4.0 nylon suture ligature. The catheter was simultaneously connected to a BS-8000 infusion pump (Braintree Scientific, Braintree, MA) and a pressure transducer (Grass, West Warwick, RI). The intestine was placed in a 37°C water bath and air was introduced in the intestine at 3 ml/min via the infusion pump. The pressure signal was transduced into voltage and displayed continuously on a monitor. An abrupt drop in pressure registered on the pressure curve, as well as the appearance of air bubbles in the water bath, was recorded as the bursting point. The intestine was inspected to confirm that the burst occurred at the site of the anastomosis.
Histologic analysis and grading of anastomotic healing
The section of terminal ileum containing the anastomotic site was opened longitudinally and fixed in 10% neutralized formaldehyde overnight. Paraffin embedded tissue sections (5 µm) were prepared and stained with hematoxylin and eosin (H&E) for histologic analysis. Tissue sections were analyzed using conventional light microscopy (Primo Star, Carl Zeiss Inc., Thornwood, NY) and photographed at 20× magnification. To assess healing at the anastomotic site, slides were graded using a modified healing scoring system that was initially described by Gϋven et al.[24] The accumulation of polymorphonuclear leukocytes (PMN), lymphocytes and macrophages (inflammation); presence of mucosal epithelium at the anastomotic site, and submucosal-muscular layer repair were recorded on histopathologic examination and scored from 1 to 4, as summarized in Table 1. The grading and quantification of intestinal healing scores, collagen deposition, and angiogenesis quantification were performed blindly by two independent individuals.
Table 1.
Intestinal anastomosis wound healing grading system
| Score | Inflammation | Mucosal Epithelium |
Submucosal Healing |
|---|---|---|---|
| 4 | Absent | Normal epithelium | Good |
| 3 | Slight | Mucosa mostly covered by epithelium |
Moderate bridge |
| 2 | Moderate | Mucosa partially covered by epithelium |
Weak bridge |
| 1 | Severe | Mucosa damaged completely, epithelium absent |
Absent |
Collagen quantification
Picrosirius red staining for collagen analysis was performed using paraffin sections that were deparaffinized, hydrated, and stained with 0.1% Sirius red F3B (Pfaltz & Bauer, Waterbury, CT) in a saturated solution of Picric Acid (Ricca Chemical Company, Arlington, TX) with a very small amount of solid Picric Acid (Electron Microscopy Sciences, Hatfield, PA) to ensure saturation for 1 hour at room temperature. The sections were then washed in two changes of acidified water, dehydrated in three changes of 100% ethanol, cleared with xylene and mounted. Tissue sections were analyzed using conventional light microscopy (Primo Star, Carl Zeiss Inc., Thornwood, NY) and photographed at 20× magnification. Collagen deposition at the site of the anastomosis was quantified using Image Pro-Plus software, version 4.1.0.0 for Windows (Media Cybernetics, Bethesda, MD). All images were taken under the same camera settings and exposure times, images were digitized, and the area positive for Picrosirius red staining was recognized, using fixed threshold values. The intensity of the positive area was calculated by Image Pro-Plus software, representing the total collagen deposited at the anastomotic area and expressed as optical density in pixels. Collagen deposition was quantified using the formula:
Collagen deposition (optical density) = total intensity of the positive area / total area of the anastomotic site.
Angiogenesis quantification
Angiogenesis in anastomotic sites was examined by von Willebrand Factor (vWF) immunostaining of endothelial cells. Immunofluorescent staining was carried out as follows. After deparaffinizing and rehydration, tissue sections were blocked with 10% donkey serum/PBS for 20 min at RT. Tissue sections were incubated in a rabbit primary polyclonal antibody to vWF (Abcam, Cambridge, MA) diluted to 10% in donkey serum/PBS at 4°C overnight, as recommended by the manufacturer. After three 10 min washes with PBS/0.1% Tween 20, tissue sections were incubated with a secondary anti-rabbit IgG antibody conjugated with Cy3 (Jackson ImmunoResearch Laboratories, Inc, West Grove, PA) diluted to 10% donkey serum/PBS for 1 h at RT. After three 10 min washes with PBS+0.1% Tween 20, sections were mounted with ProLong Gold antifade reagent with DAPI (Invitrogen, Carlsbad, CA, US). Quantification of vWF expression in anastomotic sites was performed using ImageJ software, version 1.39u for Windows (National Institutes of Health, Bethesda, MD) and was represented by the ratio of vWF fluorescent intensity per anastomotic area.
In addition, since tubular blood vessel formation signifies a later stage of angiogenesis and functional blood vessel maturation, tubular blood vessels per unit area were quantified on POD 6. The process of angiogenesis is defined in four stages with maturation as the final step.[25] One of the criteria for mature blood vessels is formation of an interconnecting lumen. In our quantification of mature blood vessels, only blood vessels with a lumen were considered to be mature.
Statistical analysis
The Student’s t test was used for comparing bursting pressure measurements, anastomosis healing scores, collagen deposition and angiogenesis between groups. Statistical analyses were performed using Microsoft Office Excel, version 2007 for Windows. The Fisher’s Exact test, SAS (version 9.1,SAS Institute, Cary, NC) was used for comparing the rate of complications between groups. p-values less then 0.05 were considered statistically significant. Results are expressed as mean ± SD.
RESULTS
Anastomotic Bursting Pressure
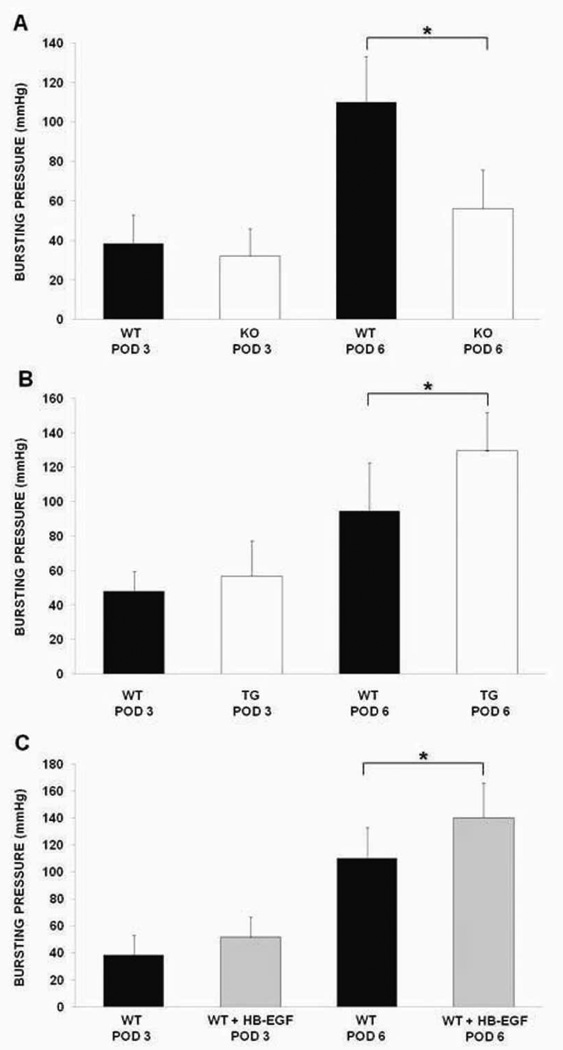
Anastomotic bursting pressure measurements on POD 3 revealed that WT mice had slightly higher bursting pressures compared to HB-EGF KO mice (38.34 ± 14.47 mmHg vs. 33.18 ± 15.63 mmHg; p=0.36) (Figure 1A). However, on POD 6, WT mice had significantly higher bursting pressures than KO mice (110.05 ± 22.93 mmHg vs. 55.95 ± 19.82 mmHg; p<0.01).
Figure 1.
Bursting pressure measurements. A) Terminal ileal bursting pressures in HB-EGF KO mice compared to their WT C57BL/6J × 129 counterparts on POD 3 and 6; B) Terminal ileal bursting pressures in HB-EGF TG mice compared to their WT FVB counterparts on POD 3 and 6; C) Terminal ileal bursting pressures on POD 3 and 6 in WT C57BL/6J × 129 mice compared to WT C57BL/6J × 129 mice that received HB-EGF (800µg/kg) via gastric gavage once a day for three days prior to surgery. WT, wild type mice; KO, HB-EGF knockout mice; TG, HB-EGF transgenic mice; POD, post-operative day; * p<0.05.
We next compared anastomotic bursting pressure in HB-EGF TG mice and their WT counterparts. We found that HB-EGF TG mice had slightly increased anastomotic bursting pressure on POD 3 (56.91 ± 20.47 mmHg vs. 47.86 ± 11.54 mmHg; p=0.26) (Figure 1B). On POD 6, HB-EGF TG mice had significantly increased anastomotic bursting pressures (129.57 ± 22.09 mmHg vs. 94.41 ± 28.12 mmHg; p=0.04).
When comparing anastomotic bursting pressures in WT mice and WT mice that received HB-EGF prior to surgery, we found that on POD3, WT mice that received HB-EGF had slightly higher bursting pressures compared to non-HB-EGF treated WT mice (51.39 ± 15.11 mmHg vs. 38.34 ± 14.47 mmHg; p=0.06). On POD 6, WT animals that received HB-EGF had significantly increased anastomotic bursting pressures compared to non-HB-EGF treated WT mice. (140.12 ± 25.79 mmHg vs. 110.05 ± 22.93 mmHg; p=0.01) (Figure 1C)
Anastomotic Histology and Healing Scores
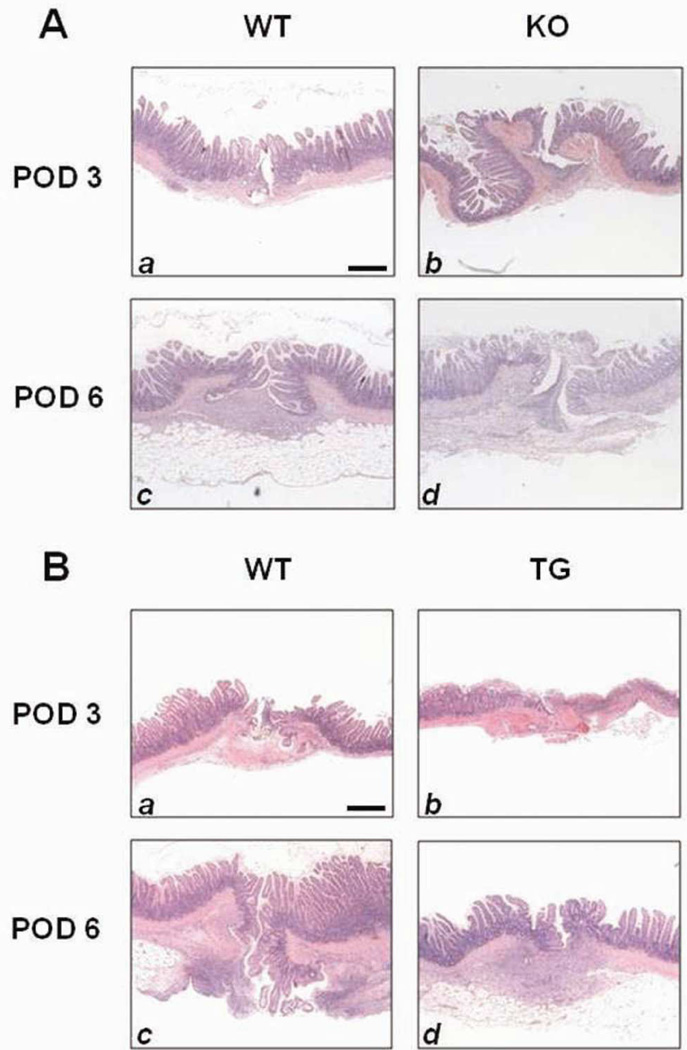
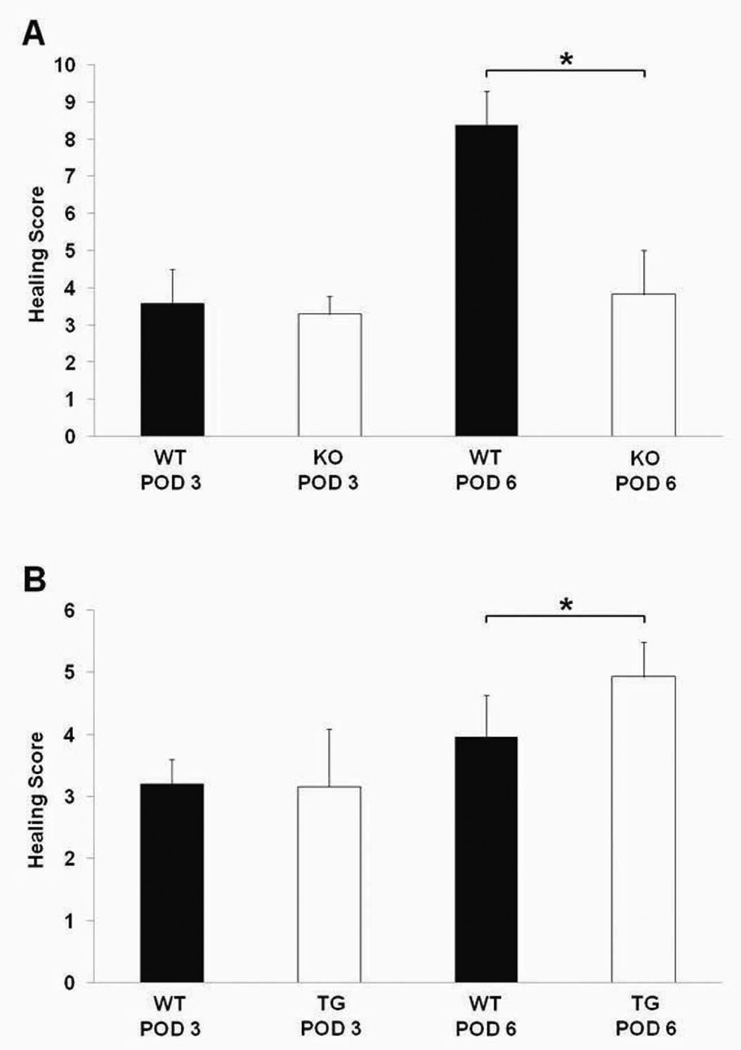
HB-EGF KO mice had significantly delayed anastomotic healing, with severe inflammation around the site of the sutures and in the submucosal space (Figure 2A). When healing scores were compared between WT and HB-EGF KO mice on POD 3, there were no significant differences present (3.58 ± 0.91 vs.3.28 ± 0.48; p=0.47). However, on POD 6, WT mice demonstrated significantly improved healing scores compared to HB-EGF KO mice (8.37 ± 0.91 vs. 3.83 ± 1.16; p<0.01) (Figure 3A).
Figure 2.
Histology of anastomoses. Shown are representative H&E stained histological images of ileal anastomoses on POD 3 and 6. A) Histology of HB-EGF KO mice and their WT C57BL/6J × 129 counterparts ; B) Histology of HB-EGF TG mice and their WT FVB counterparts. Magnification 20×. Scale bar = 100µm.
Figure 3.
Anastomotic healing scores. A) Healing scores in HB-EGF KO mice compared to their WT C57BL/6J × 129 counterparts on POD 3 and 6; B) Healing scores in HB-EGF TG mice compared to their WT FVB counterparts on POD 3 and 6. WT, wild type mice; KO, HB-EGF knockout mice; TG, HB-EGF transgenic mice; POD, post-operative day; * p<0.05.
HB-EGF TG mice displayed minimal signs of inflammation on POD 6, with partial coverage of the mucosa by epithelial cells and significant submucosal healing (Figure 2B). There were no significant difference in healing scores between WT and HB-EGF TG mice on POD 3 (3.21 ± 0.39 vs. 3.16 ± 0.93; p=0.90), however, on POD 6 HB-EGF TG mice had significantly increased healing scores (4.93 ± 0.56 vs. 3.96 ± 0.68; p<0.01) (Figure 3B).
Anastomotic Complications
HB-EGF KO mice had more anastomotic complications and increased mortality compared to their WT counterparts (Table 2A). On POD 3, 38.8% of KO mice displayed complications (bleeding 5.5%, anastomotic dehiscence 16.6%, abscess formation at the anastomotic site 16.6%), compared to a 25% complication rate in their WT counterparts (p=0.48), (anastomotic dehiscence 12.5%, abscess formation 12.5%). The mortality of KO mice on POD 3 was higher than the mortality of WT mice (16.6% vs. 12.5%; p=1.0). On POD 6, 45.8% of KO mice had complications (anastomotic dehiscence 8.3%, abscess formation 16.6%, intestinal obstruction 20.8%), whereas only 29.4% of WT mice had complications (p=0.34), (anastomotic dehiscence 5.8%, abscess formation 5.8%, intestinal obstruction 17.6%). The mortality of KO mice on POD 6 was higher than the mortality of WT mice (20.8% vs. 11.7%, p=0.68).
Table 2.
Mortality and anastomotic complications
| A | ||||
|---|---|---|---|---|
| Complications | WT (n=16) POD 3 |
KO (n=18) POD 3 |
WT (n=17) POD 6 |
KO (n=24) POD 6 |
| Mortality | 2 (12.5%) | 3 (16.6%) | 2 (11.7%) | 5 (20.8%) |
| Bleeding | - | 1 (5.5%) | - | - |
| Dehiscence | 2 (12.5%) | 3 (16.6%) | 1 (5.8%) | 2 (8.3%) |
| Abscess | 2 (12.5%) | 3 (16.6%) | 1 (5.8%) | 4 (16.6%) |
| Obstruction | - | - | 3 (17.6%) | 5 (20.8%) |
| All complications | 6 (37.5%) | 7 (55.3%) | 7 (40.9%) | 16 (66.5%) |
| B | ||||
|---|---|---|---|---|
| Complications | WT (n=16) POD 3 |
TG (n=16) POD 3 |
WT (n=11) POD 6 |
TG (n=10) POD 6 |
| Mortality | 2 (12.5%) | 1 (6.2%) | 1 (9%) | 1 (10%) |
| Bleeding | 1 (6.2%) | - | - | - |
| Dehiscence | - | 1 (6.2%) | 1 (9%) | - |
| Abscess | 2 (12.5%) | 3 (18.7%) | 1 (9%) | 1 (10%) |
| Obstruction | 1 (6.2%) | 2 (12.5%) | 2 (18.1%) | 1 (10%) |
| All complications | 6 (37.4%) | 7 (43.6%) | 5 (45.1%) | 3 (30%) |
| C | ||||
|---|---|---|---|---|
| Complications | WT (n=16) POD 3 |
WT + HB-EGF (n=10) POD 3 |
WT (n=11) POD 6 |
WT + HB-EGF (n=11) POD 6 |
| Mortality | 2 (12.5%)* | - | 2 (11.7%)* | - |
| Bleeding | - | - | - | - |
| Dehiscence | 2 (12.5%)* | - | 1 (5.8%)* | - |
| Abscess | 2 (12.5%)* | - | 1 (5.8%)* | - |
| Obstruction | - | - | 3 (17.6%)* | - |
| All complications | 6 (37.5%) | - | 7 (40.9%) | - |
WT, wild type mice; WT + HB-EGF, wild type mice that received 800µg/kg HB-EGF; POD, post-operative day;
p<0.05.
When comparing HB-EGF TG mice to their WT counterparts, on POD 3 37.5% of TG mice had local complications (anastomotic dehiscence 6.2%, abscess formation 18.7%, intestinal obstruction 12.5%), compared to a 25% complication rate in WT mice (p=0.70) (intraperitoneal bleeding with hematoma at the site of the anastomosis 6.2%, abscess formation 12.5%, intestinal obstruction 6.2%). (Table 2B). On POD 3, TG mice had a lower mortality then WT mice (6.2% vs. 12.5%, p=1.0). On POD 6, 20% of TG mice had complications (abscess formation 10%, intestinal obstruction 10%), whereas 36.4% of WT mice had complications (p=0.64), (anastomotic dehiscence 9%, abscess formation 9%, intestinal obstruction 18.1%). The mortality of TG and WT mice on POD 6 was similar (10% vs.9%).
On POD 3, WT mice had significantly increased local complications (anastomotic dehiscence 12.5% and abscess formation 12.5%) compared to no complications noted in WT animals that received enteral HB-EGF prior to surgery. The mortality on POD 3 was 12.5% in WT animals and 0% in WT animals that received HB-EGF. Similar results were noted on POD 6, when WT mice had significantly increased local complications (anastomotic dehiscence 5.8%, abscess formation 5.8% and intestinal obstruction 17.6%) compared to no complications noted in WT animals that received HB-EGF. The mortality on POD 6 was 11.7% in WT animals and 0% in WT animals that received HB-EGF.
Collagen quantification
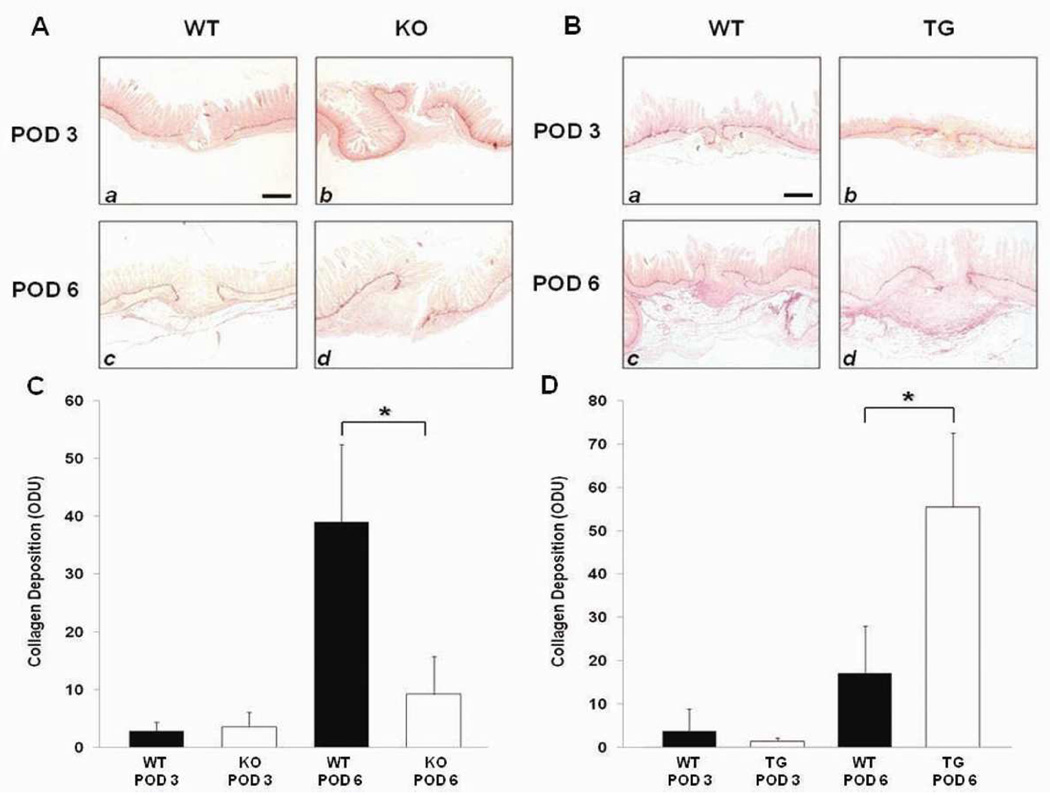
Picrosirius red staining of histologic sections was used to assess collagen deposition at the anastomotic sites (Figure 4 A,B). Image analysis quantification of Picrosirius red staining of the anastomotic sites revealed no significant differences in collagen deposition on POD 3 in either HB-EGF KO mice and their WT counterparts (Figure 4A), or in HB-EGF TG mice and their WT counterparts (Figure 4B). However, on POD 6 there was significantly greater collagen deposition in WT compared to KO mice (38.96 ± 13.41 vs. 9.18 ± 6.49; p<0.01) (Figure 4A) and less collagen deposition in WT mice compared to TG mice (17.2 ± 10.91 vs. 55.6 ± 17.08; p<0.01) (Figure 4B).
Figure 4.
Picrosirius red staining for collagen deposition. A) Histology of HB-EGF KO mice and their WT C57BL/6J × 129 counterparts, magnification 20×, scale bar = 100µm; B) Histology of HB-EGF TG mice and their WT FVB counterparts, magnification 20×, scale bar= 100µm; C) Quantification of collagen deposition in HB-EGF KO mice compared to their WT C57BL/6J × 129 counterparts on POD 3 and 6; D) Quantification of collagen deposition in HB-EGF TG mice compared to their WT FVB counterparts on POD 3 and 6. WT, wild type mice; KO, HB-EGF knockout mice; TG, HB-EGF transgenic mice; POD, post-operative day; * p<0.05.
Angiogenesis
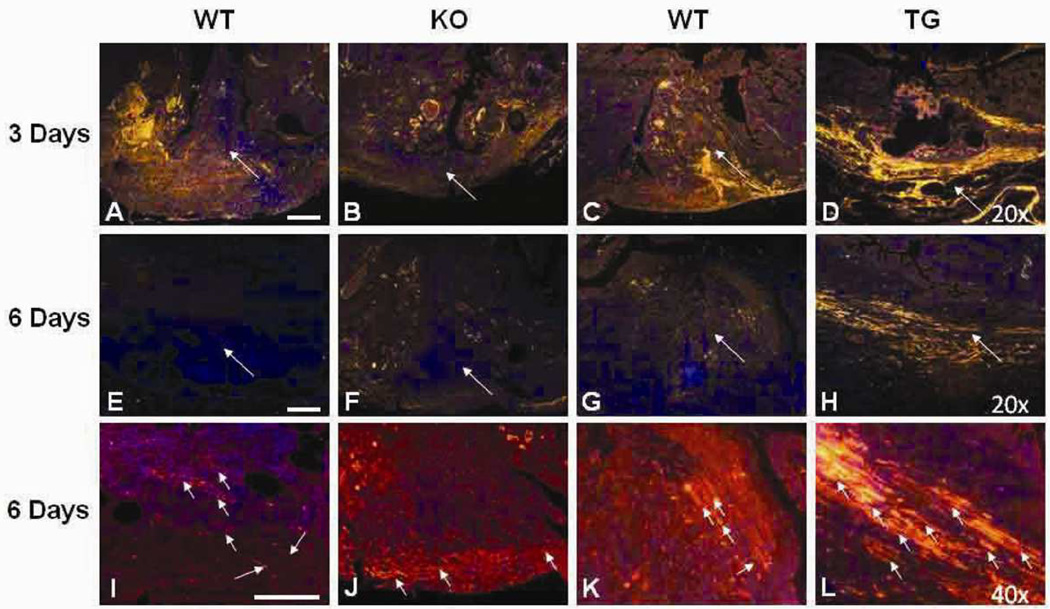
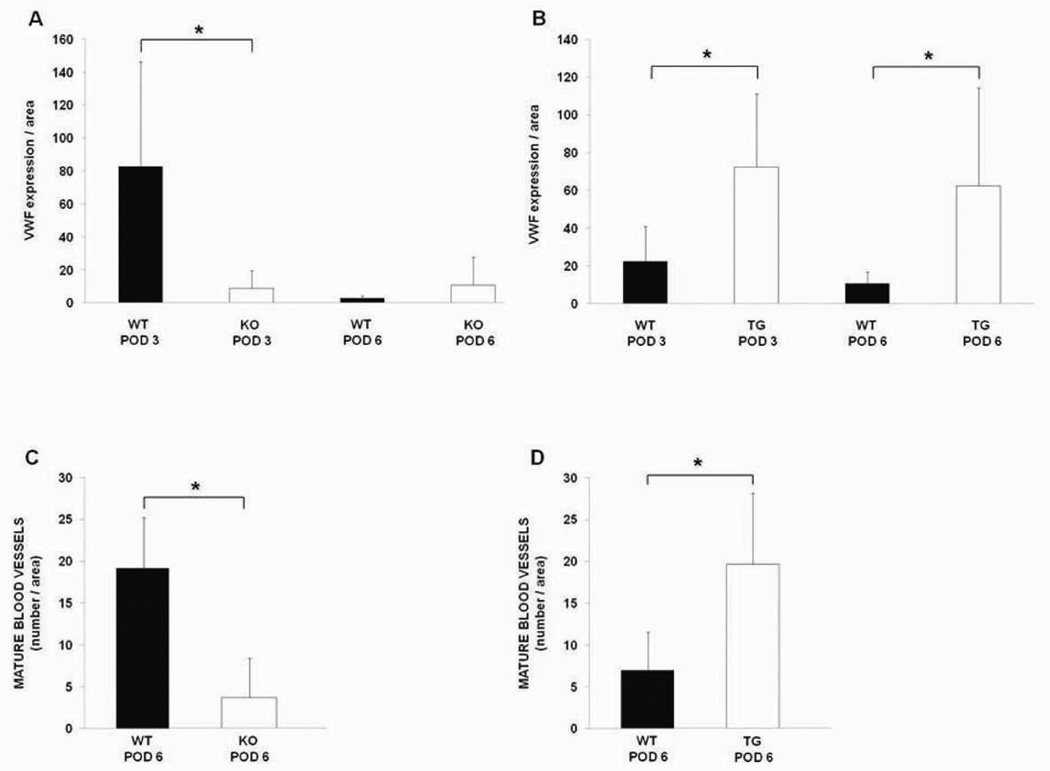
We initially used vWF immunostaining to identify endothelial cells at the anastomotic sites (Figures 5, 6A,B). HB-EGF KO mice had significantly decreased vWF expression at the anastomotic sites compared to WT mice on POD 3 (8.7 ± 10.9 vs. 82.9 ± 63.5, p<0.05) (Figure 6A). However, by POD 6 we noted a marked decrease in vWF staining at the anastomotic sites in the WT mice. Despite this decline in vWF expression in WT mice, we noted that the anastomotic sites of WT mice had maturation of the vasculature, with an increase in mature, longer blood vessels at the 6 day time point. HB-EGF KO mice had significantly fewer mature blood vessels per unit area compared to WT mice on POD 6 (3.6 ± 4.7 vs. 19.2 ± 6.1, p<0.01) (Figure 6C). On the other hand, HB-EGF TG mice had higher vWF expression at the anastomotic sites compared to WT mice on POD 3 and 6 (72.6 ± 38.6 vs. 22.6 ± 18.3 and 62.5 ± 51.8 vs. 10.8 ± 6.1, respectively, p<0.05 for both time points) (Figure 6B). HB-EGF TG mice also had increased numbers of mature blood vessels at the anastomotic sites compared to WT mice on POD 6 (19.6 ± 8.5 vs. 7 ± 4.5, p<0.05) (Figure 6D).
Figure 5.
von Willebrand factor (vWF) immunohistochemistry of anastomotic sites. Shown are representative photomicrographs of vWF immunostaining of the anastomotic sites. Magnification: A-H, 20×; I-L, 40×. Red, vWF (Cy3); blue: DAPI, nuclear staining; WT, wild type; KO, HB-EGF knock out mice; TG, HB-EGF transgenic mice; * p<0.05. Long white arrows indicate the anastomotic sites. The small white arrows in high power panels I-L indicate mature blood vessels containing lumens. Scale bar = 100µm.
Figure 6.
Quantification of angiogenesis at anastomotic sites. A, B) quantification of vWF staining in HB-EGF KO and TG mice compared to their WT counterparts on POD 3 and 6, as determined using ImageJ Software, version 1.39u for Windows, represented by the ratio of vWF fluorescent intensity per anastomotic area. C, D) quantification of mature tubular blood vessels per anastomotic area in HB-EGF KO and TG mice compared to their WT counterparts on POD 6. WT, wild type; KO, HB-EGF knock out mice; TG, HB-EGF transgenic mice; * p<0.05.
DISCUSSION
HB-EGF is an immediate early gene that plays a pivotal role in mediating the earliest cellular responses to proliferative stimuli and cellular injury.[26] Previous studies from our laboratory and from others have shown that expression of endogenous HB-EGF is significantly increased in response to tissue damage,[13, 27] hypoxia[28] and oxidative stress,[29] and during wound healing and regeneration.[12] Endogenous HB-EGF has been shown to play a role in the healing of various tissues, as demonstrated by its increased levels in the kidney in response to I/R injury,[30, 31] the brain in response to I/R injury[32] and the liver in response to partial hepatectomy or hepatotoxin injury.[33] HB-EGF production is increased in the skin in response to thermal injury in patients,[12] and promotes cutaneous burn wound healing when applied topically in mice.[14]
We have previously shown that adult HB-EGF KO mice have increased intestinal injury upon exposure to intestinal I/R[34], HS/R [35] and NEC[36]. Furthermore, we have shown that administration of exogenous HB-EGF under experimental conditions protects the intestines from diverse injuries including intestinal I/R,[15] HS/R,[16] and NEC.[18, 23] The current study now demonstrates that HB-EGF KO mice have delayed intestinal anastomotic wound healing and that HB-EGF TG mice have augmented anastomotic wound healing. Thus, HB-EGF not only protects the intestines from injury, but plays an important role in healing of intestinal anastomoses.
The strength of an intestinal anastomosis is mainly derived from collagen fibrils located in the submucosa. During the first few postoperative days, anastomotic strength is low, as collagen is degraded secondary to collagenase activity at the site of the injury. Early anastomotic strength is therefore dependent on the suture- or staple-holding capacity of existing collagen until large amounts of new collagen can be synthesized by both fibroblasts and smooth muscle cells.[1] Bursting pressure is therefore lowest during the first 3 days after surgery, after which anastomotic strength rapidly increases. Bursting pressure is 50% of normal in small bowel anastomoses, and 35–75% of normal in large-bowel anastomoses, at two to three days postoperatively.[1] Bursting pressure approaches 100% of normal at 7 days post-operatively, after which the intestine will generally burst outside of the suture line.[1] These findings are consistent with our results that showed that on POD 3 the bursting pressures were low in all groups of animals studied, with significant differences noted only on POD 6.
Healing of the intestinal astomosis and, in particular, the development of early postoperative anastomotic strength, may be compromised by numerous technical, local and systemic factors.[37] In our study, lack of endogenous HB-EGF expression resulted in decreased bursting pressure and lower collagen deposition at the anastomotic site, elements that strongly correlated with compromised anastomotic strength and delayed healing. In addition, the higher complication rate in HB-EGF KO mice, and their increased mortality on POD 3 and 6, demonstrate the importance of endogenous HB-EGF in the healing process. HB-EGF TG mice had no significant differences in mortality or overall complication rates on POD 3 or 6 compared to their WT counterparts, although it should be recognized that the numbers of animals studied are relatively low.
The effect of growth factors on the healing of bowel anastomoses is a topic of great interest, and a number of studies have investigated the expression of these factors in the context of anastomotic healing. Buckmire et al., using a rat model of colonic anastomosis, examined the temporal expression of the TGF-beta1 (TGF-β1), epidermal growth factor (EGF), and platelet derived growth factor B (PDGF-BB) genes, and their temporal relationship to the expression of the procollagen type 1 (PROC I) gene.[38] They found significantly increased TGF-β1 showed significantly increased gene expression in the healing colonic perianastomotic tissues, with expression that had a temporal correlation with the expression of the PROC I gene. Brasken et al. examined the expression of EGF and EGF receptor genes in the healing of colonic anastomoses in rats and found enhanced EGF receptor gene expression, suggesting that anastomotic healing was associated with the presence of EGF or an EGF-like substance, and that its activity increases during the first postoperative week.[39] Furthermore, Sakallioglu et al.[40], reported that local application of low dose EGF to the anastomotic site in the form of microspheres in gelatin increased wound collagen, bursting pressure and healing in colonic anastomoses even in the presence of steroid inhibition.[40] We have previously shown that exogenous HB-EGF administration has important clinical significance, protecting the intestines from a variety of insults including intestinal ischemia/reperfusion (I/R) injury[15], hemorrhagic shock and resuscitation(HS/R)[16] and necrotizing enterocolitis (NEC)[17, 18]. Data from our current study shows that exogenous administration of HB-EGF enhances the healing of intestinal anastomoses and reduces anastomotic complications and mortality.
Global deletion of the HB-EGF gene results in abnormal embryonic cardiac cushion development resulting in defective valvulogenesis, with stenosis of the semilunar and atrioventricular valves leading to cardiac hypertrophy.[20] Chalothorn et al. used 4 month old HB-EGF KO mice to study the effect of HB-EGF on angiogenesis in a hindlimb ischemia model.[41] As part of those studies, the authors demonstrated that HB-EGF KO mice had cardiac hypertrophy without evidence of heart failure, with normal hemodynamic parameters. Furthermore, 6 month old HB-EGF KO mice only had a 25% reduction in left ventricular fractional shortening.[20] This is less than the mildest level of human heart failure which is characterized by an ejection fraction that is reduced by at least 40%.[20] Jackson et al. also reported that HB-EGF KO mice have defects in the lungs, including fewer alveoli, abnormally thickened mesenchymal tissue and a high fraction of immature, glycogen-containing cells.[20] Despite these findings, HB-EGF KO mice did not display any clinical evidence of pulmonary distress in our studies. Furthermore, in parallel studies of HB-EGF KO compared to WT adult mice, we noted no differences in oxygen saturation of these mice either prior to or after surgical manipulation (unpublished observations). In addition, we used echocardiography to comparatively evaluate cardiac heart function in 1 month old WT and HB-EGF KO mice and found no differences in heart rate, stroke volume, fractional shortening of the ventricle or cardiac output between WT and HB-EGF KO mice (unpublished observations).
Angiogenesis is crucial to anastomotic wound healing. We have previously shown that HB-EGF stimulates capillary tube formation in vitro[42] and angiogenesis in vivo. [43] HB-EGF has also been shown to enhance neurogenesis and angiogenesis after focal cerebral ischemia in rats,[44] and has been implicated in tumor growth, angiogenesis and metastasis.[45, 46] Ishii et al. found that local administration of vascular endothelial growth factor (VEGF) to colonic anastomoses accelerates wound healing and strengthens the anastomosis by increasing angiogenesis.[47] The results of our current study show that HB-EGF KO mice have significantly decreased angiogenesis at the anastomotic site, and HB-EGF TG mice have significantly increased angiogenesis at the anastomotic site, compared with WT animals. Thus, HB-EGF regulates angiogenesis in healing intestinal anastomoses.
In conclusion, our results provide evidence for a role for HB-EGF in intestinal anastomotic wound healing. HB-EGF therapy may provide a potentially therapeutic approach to improve and promote healing of intestinal anastomoses, and to minimize morbidity and mortality after bowel surgery.
ACKNOWLEDGMENTS
The authors acknowledge the following financial support for this work: NIH R01 DK074611 and R01 GM061193 (GEB), The Samuel J. Roessler Memorial Fellowship (JKO) and The Firefighters Endowment Fund of Nationwide Children’s Hospital (XY). The authors would like to thank Dr. David Lee (Chapel Hill, NC) for supplying HB-EGF KO and WT mice, Dr. Deborah Gumucio (Ann Arbor, MI) for providing us with the pBSII-12.4kbVill plasmid containing the 12.4kb promoter fragment from the villin gene, Laurie Goodchild, DVM for her assistance with care of the mice, and William Gardner Ph.D. and Wei Wang, M.S., M.A.S. for their assistance with statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Thompson SK, Chang EY, Jobe BA. Clinical review: Healing in gastrointestinal anastomoses, part I. Microsurgery. 2006;26:131. doi: 10.1002/micr.20197. [DOI] [PubMed] [Google Scholar]
- 2.Egger B, Tolmos J, Procaccino F, et al. Keratinocyte growth factor promotes healing of left-sided colon anastomoses. American journal of surgery. 1998;176:18. doi: 10.1016/s0002-9610(98)00104-4. [DOI] [PubMed] [Google Scholar]
- 3.Grose R, Werner S. Wound-healing studies in transgenic and knockout mice. Molecular biotechnology. 2004;28:147. doi: 10.1385/MB:28:2:147. [DOI] [PubMed] [Google Scholar]
- 4.Besner G, Higashiyama S, Klagsbrun M. Isolation and characterization of a macrophage-derived heparin-binding growth factor. Cell Regul. 1990;1:811. doi: 10.1091/mbc.1.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higashiyama S, Abraham JA, Miller J, et al. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 6.Vaughan TJ, Pascall JC, Brown KD. Tissue distribution of mRNA for heparin-binding epidermal growth factor. Biochem J. 1992;287(Pt 3):681. doi: 10.1042/bj2870681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abraham JA, Damm D, Bajardi A, et al. Heparin-binding EGF-like growth factor: characterization of rat and mouse cDNA clones, protein domain conservation across species, and transcript expression in tissues. Biochem.Biophys.Res.Commun. 1993;190:125. doi: 10.1006/bbrc.1993.1020. [DOI] [PubMed] [Google Scholar]
- 8.Junttila TT, Sundvall M, Maatta JA, et al. Erbb4 and its isoforms: selective regulation of growth factor responses by naturally occurring receptor variants. Trends in cardiovascular medicine. 2000;10:304. doi: 10.1016/s1050-1738(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 9.Nishi E, Prat A, Hospital V, et al. N-arginine dibasic convertase is a specific receptor for heparin-binding EGF-like growth factor that mediates cell migration. The EMBO journal. 2001;20:3342. doi: 10.1093/emboj/20.13.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis PD, Hadfield KM, Pascall JC, et al. Heparin-binding epidermal-growth-factor-like growth factor gene expression is induced by scrape-wounding epithelial cell monolayers: involvement of mitogen-activated protein kinase cascades. Biochem J. 2001;354:99. doi: 10.1042/0264-6021:3540099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downing MT, Brigstock DR, Luquette M, et al. Immunohistochemical localization of heparin-binding epidermal growth factor-like growth factor in normal skin and skin cancers. Histochem.J. 1997;29:735. doi: 10.1023/a:1026417202351. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy DW, Downing MT, Brigstock DR, et al. Production of heparin-binding epidermal growth factor-like growth factor (HB-EGF) at sites of thermal injury in pediatric patients. J Invest Dermatol. 1996;106:49. doi: 10.1111/1523-1747.ep12327214. [DOI] [PubMed] [Google Scholar]
- 13.Cribbs RK, Harding PA, Luquette MH, et al. Endogenous production of heparin-binding EGF-like growth factor during murine partial-thickness burn wound healing. J Burn Care Rehabil. 2002;23:116. doi: 10.1097/00004630-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Cribbs R, Luquette M, Besner G. Acceleration of partial-thickness burn wound healing with topical application of heparin-binding EGF-like growth factor (HB-EGF) J.Burn Care Rehabil. 1998;19:95. doi: 10.1097/00004630-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 15.El-Assal ON, Besner GE. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology. 2005;129:609. doi: 10.1016/j.gastro.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 16.El-Assal ON, Radulescu A, Besner GE. Heparin-binding EGF-like growth factor preserves mesenteric microcirculatory blood flow and protects against intestinal injury in rats subjected to hemorrhagic shock and resuscitation. Surgery. 2007;142:234. doi: 10.1016/j.surg.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Feng J, El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor reduces intestinal apoptosis in neonatal rats with necrotizing enterocolitis. J Pediatr Surg. 2006;41:742. doi: 10.1016/j.jpedsurg.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Radulescu A, Zorko NA, Yu X, et al. Preclinical neonatal rat studies of heparin-binding EGF-like growth factor in protection of the intestines from necrotizing enterocolitis. Pediatr Res. 2009;65:437. doi: 10.1203/PDR.0b013e3181994fa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thornton FJ, Barbul A. Healing in the gastrointestinal tract. The Surgical clinics of North America. 1997;77:549. doi: 10.1016/s0039-6109(05)70568-5. [DOI] [PubMed] [Google Scholar]
- 20.Jackson LF, Qiu TH, Sunnarborg SW, et al. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. The EMBO journal. 2003;22:2704. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CL, Mehta VB, Zhang HY, et al. Intestinal phenotype in mice overexpressing a heparin-binding EGF-like growth factor transgene in enterocytes. Growth Factors. 2010;28:82. doi: 10.3109/08977190903407365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madison BB, Dunbar L, Qiao XT, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 23.Feng J, El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor decreases the incidence of necrotizing enterocolitis in neonatal rats. J Pediatr Surg. 2006;41:144. doi: 10.1016/j.jpedsurg.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Guven A, Pehlivan M, Gokpinar I, et al. Early glutamine-enriched enteral feeding facilitates colonic anastomosis healing: light microscopic and immunohistochemical evaluation. Acta histochemica. 2007;109:122. doi: 10.1016/j.acthis.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Daniel TO, Abrahamson D. Endothelial signal integration in vascular assembly. Annual review of physiology. 2000;62:649. doi: 10.1146/annurev.physiol.62.1.649. [DOI] [PubMed] [Google Scholar]
- 26.Bulus N, Barnard JA. Heparin binding epidermal growth factor-like growth factor is a transforming growth factor beta-regulated gene in intestinal epithelial cells. Biochem Biophys Res Commun. 1999;264:808. doi: 10.1006/bbrc.1999.1600. [DOI] [PubMed] [Google Scholar]
- 27.Xia G, Martin AE, Besner GE. Heparin-binding EGF-like growth factor downregulates expression of adhesion molecules and infiltration of inflammatory cells after intestinal ischemia/reperfusion injury. J Pediatr Surg. 2003;38:434. doi: 10.1053/jpsu.2003.50075. [DOI] [PubMed] [Google Scholar]
- 28.Jin K, Mao XO, Sun Y, et al. Heparin-binding epidermal growth factor-like growth factor: hypoxia-inducible expression in vitro and stimulation of neurogenesis in vitro and in vivo. J Neurosci. 2002;22:5365. doi: 10.1523/JNEUROSCI.22-13-05365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank GD, Mifune M, Inagami T, et al. Distinct mechanisms of receptor and nonreceptor tyrosine kinase activation by reactive oxygen species in vascular smooth muscle cells: role of metalloprotease and protein kinase C-delta. Mol Cell Biol. 2003;23:1581. doi: 10.1128/MCB.23.5.1581-1589.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Homma T, Sakai M, Cheng HF, et al. Induction of heparin-binding epidermal growth factor-like growth factor mRNA in rat kidney after acute injury. J Clin Invest. 1995;96:1018. doi: 10.1172/JCI118087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakai M, Zhang M, Homma T, et al. Production of heparin binding epidermal growth factor-like growth factor in the early phase of regeneration after acute renal injury. Isolation and localization of bioactive molecules. J Clin Invest. 1997;99:2128. doi: 10.1172/JCI119386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka N, Sasahara M, Ohno M, et al. Heparin-binding epidermal growth factor-like growth factor mRNA expression in neonatal rat brain with hypoxic/ischemic injury. Brain Res. 1999;827:130. doi: 10.1016/s0006-8993(99)01319-0. [DOI] [PubMed] [Google Scholar]
- 33.Kiso S, Kawata S, Tamura S, et al. Expression of heparin-binding EGF-like growth factor in rat liver injured by carbon tetrachloride or D-galactosamine. Biochem.Biophys.Res.Commun. 1996;220:285. doi: 10.1006/bbrc.1996.0397. [DOI] [PubMed] [Google Scholar]
- 34.El-Assal ON, Paddock H, Marquez A, et al. Heparin-binding epidermal growth factor-like growth factor gene disruption is associated with delayed intestinal restitution, impaired angiogenesis, and poor survival after intestinal ischemia in mice. J Pediatr Surg. 2008;43:1182. doi: 10.1016/j.jpedsurg.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang HY, Radulescu A, Besner GE, et al. Heparin-binding epidermal growth factor-like growth factor is essential for preservation of gut barrier function after hemorrhagic shock and resuscitation in mice. Surgery. 2009;146:334. doi: 10.1016/j.surg.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radulescu A, Yu X, Orvets ND, et al. Deletion of the heparin-binding epidermal growth factor-like growth factor gene increases susceptibility to necrotizing enterocolitis. J Pediatr Surg. 2010;45:729. doi: 10.1016/j.jpedsurg.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ortolan EV, Spadella CT, Caramori C, et al. Microscopic, morphometric and ultrastructural analysis of anastomotic healing in the intestine of normal and diabetic rats. Exp Clin Endocrinol Diabetes. 2008;116:198. doi: 10.1055/s-2007-993147. [DOI] [PubMed] [Google Scholar]
- 38.Buckmire MA, Parquet G, Greenway S, et al. Temporal expression of TGF-beta1, EGF, and PDGF-BB in a model of colonic wound healing. J Surg Res. 1998;80:52. doi: 10.1006/jsre.1998.5326. [DOI] [PubMed] [Google Scholar]
- 39.Brasken P, Renvall S, Sandberg M. Expression of epidermal growth factor and epidermal growth factor receptor genes in healing colonic anastomoses in rats. The European journal of surgery = Acta chirurgica. 1991;157:607. [PubMed] [Google Scholar]
- 40.Sakallioglu AE, Yagmurlu A, Dindar H, et al. Sustained local application of low-dose epidermal growth factor on steroid-inhibited colonic wound healing. J Pediatr Surg. 2004;39:591. doi: 10.1016/j.jpedsurg.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 41.Chalothorn D, Moore SM, Zhang H, et al. Heparin-binding epidermal growth factor-like growth factor, collateral vessel development, and angiogenesis in skeletal muscle ischemia. Arterioscler Thromb Vasc Biol. 2005;25:1884. doi: 10.1161/01.ATV.0000175761.59602.16. [DOI] [PubMed] [Google Scholar]
- 42.Mehta VB, Besner GE. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors. 2007;25:253. doi: 10.1080/08977190701773070. [DOI] [PubMed] [Google Scholar]
- 43.Mehta VB, Zhou Y, Radulescu A, et al. HB-EGF stimulates eNOS expression and nitric oxide production and promotes eNOS dependent angiogenesis. Growth Factors. 2008;26:301. doi: 10.1080/08977190802393596. [DOI] [PubMed] [Google Scholar]
- 44.Sugiura S, Kitagawa K, Tanaka S, et al. Adenovirus-mediated gene transfer of heparin-binding epidermal growth factor-like growth factor enhances neurogenesis and angiogenesis after focal cerebral ischemia in rats. Stroke. 2005;36:859. doi: 10.1161/01.STR.0000158905.22871.95. [DOI] [PubMed] [Google Scholar]
- 45.Adam RM, Kim J, Lin J, et al. Heparin-binding epidermal growth factor-like growth factor stimulates androgen-independent prostate tumor growth and antagonizes androgen receptor function. Endocrinology. 2002;143:4599. doi: 10.1210/en.2002-220561. [DOI] [PubMed] [Google Scholar]
- 46.Ongusaha PP, Kwak JC, Zwible AJ, et al. HB-EGF is a potent inducer of tumor growth and angiogenesis. Cancer research. 2004;64:5283. doi: 10.1158/0008-5472.CAN-04-0925. [DOI] [PubMed] [Google Scholar]
- 47.Ishii M, Tanaka E, Imaizumi T, et al. Local VEGF administration enhances healing of colonic anastomoses in a rabbit model. European surgical research. Europaische chirurgische Forschung. 2009;42:249. doi: 10.1159/000210671. [DOI] [PubMed] [Google Scholar]