Abstract
Glycation, or nonenzymatic glycosylation, is a chemical reaction between reactive carbonyl-containing compounds and biomolecules containing free amino groups. Carbonyl-containing compounds include reducing sugars such as glucose or fructose, carbohydrate-derived compounds such as methylglyoxal and glyoxal, and nonsugars such as polyunsaturated fatty acids. The latter group includes molecules such as proteins, DNA, and amino lipids. Glycation-induced damage to these biomolecules has been shown to be a contributing factor in human disorders such as Alzheimer's disease, atherosclerosis, and cataracts and in diabetic complications. Glycation also affects Escherichia coli under standard laboratory conditions, leading to a decline in bacterial population density and long-term survival. Here we have shown that as E. coli aged in batch culture, the amount of carboxymethyl lysine, an advanced glycation end product, accumulated over time and that this accumulation was affected by the addition of glucose to the culture medium. The addition of excess glucose or methylglyoxal to the culture medium resulted in a dose-dependent loss of cell viability. We have also demonstrated that glyoxylase enzyme GloA plays a role in cell survival during glycation stress. In addition, we have provided evidence that carnosine, folic acid, and aminoguanidine inhibit glycation in prokaryotes. These agents may also prove to be beneficial to eukaryotes since the chemical processes of glycation are similar in these two domains of life.
One factor that may affect the long-term survival of bacterial cells in a population is the level of damage incurred by macromolecules via the nonenzymatic process of glycation, first described by Louis-Camille Maillard (16). The Maillard reaction is responsible for the formation of several compounds identified as advanced glycation end products (AGEs) (9). In vivo this reaction appears to play a role in the aging process, as it leads to slow degradation of molecules. The principal mechanisms of glycation-related damage involve cross-links between proteins and/or DNA, modifying or destroying their functional properties (2, 8, 38). Most studies of glycation have been performed with eukaryotes because of its relationship to aging and disorders such as Alzheimer's disease and diabetes (6, 21, 30, 42). However, several studies (32, 33) have shown that glycation also takes place in Escherichia coli, affecting protein and DNA of this prokaryote.
Many biochemical pathways produce reactive dicarbonyl intermediates, such as glyoxal and methylglyoxal (MG), which can further react with DNA, proteins, or other biomolecules to form AGEs (8, 36). Reaction of glucose with amino groups of proteins and subsequent formation of reactive dicarbonyls via a series of reactions involving Schiff base and Amadori product intermediates have been well documented (40). Methylglyoxal can be formed by spontaneous decomposition of glycolytic triose phosphates such as dihydroxyacetone phosphate (DHAP) (1) or can be produced enzymatically from DHAP by the E. coli enzyme methylglyoxal synthase (MgsA) (12). MG synthesis usually requires an environment low in phosphate and high in DHAP, a situation that occurs most frequently under high-glucose conditions (25, 26). If MG is not degraded, MG accumulation will lead to cell death (12). E. coli maintains pathways for the detoxification of methylglyoxal, including glyoxalase enzymes I and II (encoded by gloA and gloB, respectively), which convert MG to S-lactoyl glutathione and then to d-lactate (12). This system has been proposed to be the predominant MG detoxification system in E. coli (12, 29).
Glyoxal is also a toxic dicarbonyl compound capable of damaging cells via AGE formation. One of the AGEs formed in the presence of glyoxal is carboxymethyl lysine (CML), which has been used extensively as a biomarker for aging (11, 20, 31, 39). CML can be formed by different pathways: glucose can be oxidized to glyoxal, which can react with protein to form CML (1, 17); glucose can also react with protein to form fructoselysine (an Amadori product), which can undergo oxidative cleavage to form CML (1). In this study, we investigated CML formation in E. coli growing under standard and glycation-prone laboratory conditions. Since AGE formation may negatively affect cell survival and reproduction during long-term batch culture (35), we hypothesized that CML would accumulate in these cultures as cells progress through stationary phase.
One product that may interfere with AGE formation is carnosine (β-alanyl-l-histidine), a naturally occurring dipeptide in many organisms. Although its mechanism of action has not been fully determined, there is evidence that both the free amino group derived from the β-alanine and the imidazole ring of histidine compete with amino groups of proteins in the presence of reactive dicarbonyl compounds (7, 24). In this study we designed assays to determine the effect of carnosine (and other compounds) on survival of cultures of E. coli under a variety of experimental conditions. Additionally, since strains lacking glyoxalase enzymes I and II have a reduced ability to detoxify methylglyoxal, we hypothesized that gloA and/or gloB mutants would require larger amounts of carnosine than would wild-type strains to survive in the presence of this toxic electrophile.
MATERIALS AND METHODS
Strains, media, and culture conditions.
All bacterial strains used were derived from ZK126 (W3110 ΔlacU169 tna-2), a descendant of E. coli K-12. Strains with mutations in gloA or gloB and the gloA gloB double mutant (Table 1) were constructed by Red recombinase-mediated homologous recombination, including gene replacement with a chloramphenicol resistance (Camr) or kanamycin resistance (Kanr) cassette flanked by FLP recombinase (FLP recombination target [FRT]) sites (10). These strains displayed the same growth, survival, and competition phenotypes as did the parent strain under standard culture conditions (data not shown). The Camr mutations were transduced by bacteriophage P1 into the genetically marked strain ZK1142 (Nalr) (15, 43). Removal of Camr cassettes was achieved by transformation of each strain with a temperature-sensitive plasmid expressing FLP recombinase (10). Experiments were performed at 37°C, with aeration, either in 18- by 150-mm test tubes in a TC-7 rolling drum (New Brunswick Scientific, Edison, NJ) or in 250-ml flasks on a shaking platform (250 rpm). Luria-Bertani (LB) medium was prepared according to the manufacturer's instructions (Difco), and broth was supplemented with glucose (0 to 0.8% [wt/vol]) where indicated. Carnosine, folic acid, aspirin (acetylsalicylic acid), and aminoguanidine (AG), as well as S-lactoyl glutathione and B vitamins, were obtained from Sigma-Aldrich (St. Louis, MO); grape seed extract was obtained from a local commercial wine manufacturer. The pH of the LB medium was set by adding HEPES (pH 7.0; Sigma-Aldrich) to 100 mM and monitored by removing aliquots and measuring pH on standard pH paper (EMD Chemicals, La Jolla, CA). For quantitative assays, either 5- or 50-ml cultures were inoculated 1:1,000 (vol/vol) from overnight cultures started from frozen LB-glycerol stocks, and viable counts were determined by serial dilution of cells removed periodically from the cultures, followed by plating on LB agar (28). The limit of detection in all experiments was 1,000 CFU/ml.
TABLE 1.
E. coli strains used in glycation studies
| Strain | Genotypea |
|---|---|
| Parental strains | |
| ZK126 | W3110 ΔlacU169tna-2 |
| ZK1142 | ZK126 Nalr |
| ZK1143 | ZK126 Strr |
| GloA− strains | |
| SF2671 | ZK126 gloA::Camr |
| SF2672 | ZK126 gloA::Kanr |
| SF2673 | SF2671 gloA::FRT |
| SF2674 | ZK1142 gloA::Camr |
| SF2675 | SF2674 gloA::FRT |
| SF2676 | ZK1143 gloA::Camr |
| SF2677 | SF2676 gloA::FRT |
| GloB− strains | |
| SF2678 | ZK126 gloB::Camr |
| SF2679 | SF2678 gloB::FRT |
| SF2680 | ZK1142 gloB::Camr |
| SF2681 | SF2680 gloB::FRT |
| SF2682 | ZK1143 gloB::Camr |
| SF2683 | SF2682 gloB::FRT |
| GloA− GloB− strains | |
| SF2684 | ZK126 gloA::FRT gloB::Kanr |
| SF2685 | SF2684 gloA::FRT gloB::FRT |
| SF2686 | ZK1142 gloA::FRT gloB::Kanr |
| SF2687 | SF2686 gloA::FRT gloB::FRT |
| SF2688 | ZK1143 gloA::FRT gloB::Kanr |
| SF2689 | SF2688 gloA::FRT gloB::FRT |
Abbreviations: Nalr, nalidixic acid resistant; Strr, streptomycin resistant; Camr, chloramphenicol resistant; Kanr, kanamycin resistant; FRT, chloramphenicol or kanamycin resistance cassette removed with FLP recombinase, leaving a single FRT site in its place (see reference 10).
Isolation of total E. coli protein.
Fifty-milliliter LB cultures were grown in 250-ml flasks. Ten milliliters was removed from each flask periodically for processing, over 5 days. On days 1 and 3, cultures contained ∼1010 CFU/ml in all flasks; on day 5, cell densities were ∼109 CFU/ml in the cultures containing only LB medium and ∼106 CFU/ml in the LB/glucose medium. Cells were pelleted by centrifugation at 4,800 rpm for 15 min, followed by resuspension of cells in 10 mM Tris-HCl (pH 7.5) and 0.15 M NaCl. Cells were then sonicated, on ice, twice at maximum setting at 1-min intervals (Biosonik III probe; Bronwill Scientific) followed by enzymatic digestion with DNase I (50 μg/ml), RNase A (50 μg/ml), and lysozyme (20 μg/ml) at 37°C for 1 h. Protein levels in the lysate, containing cellular debris, were quantified using the Quick Start Bradford dye reagent, following the manufacturer's instructions (Bio-Rad).
ELISA.
One hundred microliters of purified protein at concentrations ranging from 0.5 to 1.0 mg/ml was loaded into and incubated overnight in standard polystyrene 96-well plates (Corning, Inc.) at 4°C. The next day, wells were washed twice with phosphate-buffered saline (PBS)-Tween 20 (0.05%), blocked with 200 μl of blocking solution (PBS plus 5% bovine serum albumin; 1 h, 37°C), washed again three times in PBS-Tween 20, and incubated (1 h, 37°C) with 100 μl of horseradish peroxidase (HRP)-conjugated anti-CML antibody obtained from Cosmobio, Japan (diluted 1:1,000 in PBS-Tween 20). After an additional wash (PBS-Tween 20) to remove excess antibody, 200 μl of ortho-phenylenediamine-HCl (OPD) color reagent (Sigma-Aldrich) was added for 30 min (in the dark at room temperature) and plates were read in a Bio-Rad enzyme-linked immunosorbent assay (ELISA) plate reader at 450 nm, as specified by the manufacturer (Sigma-Aldrich). Three separate 100-ml cultures were assayed in quadruplicate on ELISA plates, for a total of 12 measurements for each sample type.
Antiglycation activity assays.
In order to identify potential toxic effects of antiglycation agents, initial tests were performed using LB cultures supplemented with potential protective agents but without “stressor” compounds that induce glycation (e.g., glucose or methylglyoxal). A maximum nontoxic concentration was determined for each potential protective compound (see Table 2) as follows: cells were inoculated at ∼106 CFU/ml in fresh LB medium containing various concentrations of the potential protective agent up to the maximum achievable solubility in LB medium. After ∼1 day of incubation, viable counts were determined. The maximum concentration of potential protective agent that did not affect cell viability was determined. This maximum concentration was then tested for protective ability against lethal concentrations of stressor compounds (determined to inhibit growth at ∼1 day after inoculation at ∼106 CFU/ml). If any effect was observed, the concentration of protective agent was then reduced to determine the lowest effective concentration that yielded protection against the stressor compound(s).
TABLE 2.
Concentrations of chemical agents required to protect against toxic levels of glucose or methylglyoxal
| Compound | Concn (mM) required for protection from: |
Toxicity (mM) | |
|---|---|---|---|
| Glucose (0.6%) | Methylglyoxal (2.1 mM) | ||
| Carnosine | 20 | 10 | —a |
| Folic acid | 0.5 | 0.4 | 100 |
| Aminoguanidine | 0.1 | 1 | — |
—, agent was not lethal to cells at the maximum solubility tested (see Materials and Methods).
RESULTS
Glucose and fructose toxicity.
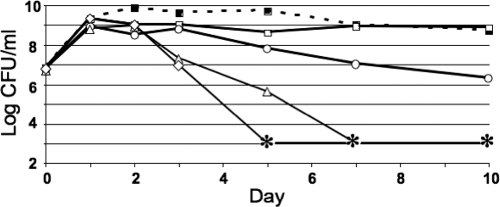
When buffered standard LB medium was supplemented with different concentrations of glucose, E. coli populations displayed different survival patterns depending on the amount of glucose added. In LB medium, E. coli tolerated glucose at concentrations up to 0.2% (wt/vol). At a concentration of 0.4%, final cell yields were reduced to 1% of untreated cultures; amounts at or above 0.6% glucose were lethal within 1 week of initial inoculation (Fig. 1). Similar results were observed with high concentrations of fructose, as cells lost viability after 5 days of treatment in the presence of 0.6% fructose (data not shown) but survived at high density at 0.2%. In all experiments, the pH of the medium did not appreciably change (data not shown).
FIG. 1.
Glucose toxicity profile. Cells were inoculated with the indicated amounts of glucose added: 0.2% (open squares), 0.4% (open circles), 0.6% (open triangles), and 0.8% (open diamonds). The dashed line with filled squares represents LB medium without glucose. Asterisks indicate titers below the limit of detection of <1,000 CFU/ml; titering error was ±2- to 3-fold (28). Representative data are shown (n = 3).
CML detection.
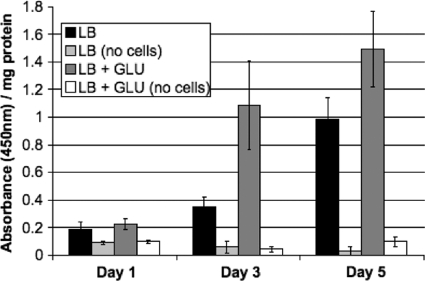
We investigated CML formation in E. coli growing under standard and glycation-prone laboratory conditions, in the presence or absence of additional glucose, respectively. An antibody specific for carboxymethyl lysine was used to determine the level of CML. As shown in Fig. 2, the amount of CML detected by ELISA increased significantly over time in cultures containing only LB medium, evidence that cells in batch culture are most likely subject to glycation-induced damage under standard laboratory conditions. CML formation in a mock-treated culture without cells was negligible (Fig. 2), indicating that glycation products do not form spontaneously in the medium in the absence of cellular activity. After 3 days of incubation, the level of CML increased at least 4-fold in cultures supplemented with 0.4% glucose over the level in those containing LB medium alone, and after day 5, levels doubled again (Fig. 2).
FIG. 2.
ELISA using anti-CML antibody in cultures containing either LB medium (black bars) or LB medium supplemented with 0.4% glucose (GLU) (dark gray bars). Cell-free controls are indicated in the absence (light gray bars) or presence (white bars) of glucose. Cultures were initially inoculated with approximately 106 CFU/ml and harvested after 1, 3, or 5 days of incubation. Data are the averages of 12 trials with standard deviation shown.
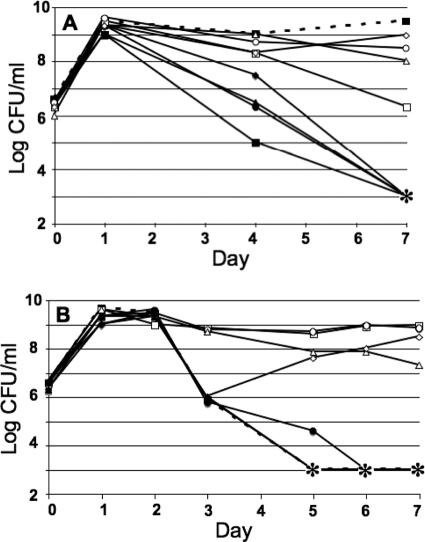
Protection against glucose toxicity by carnosine.
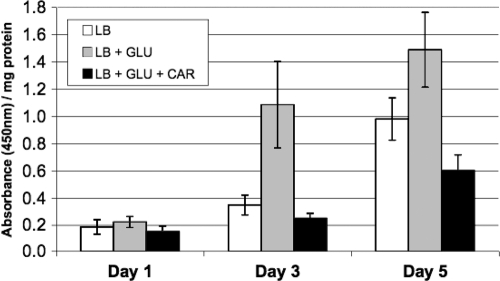
Cells were inoculated in LB medium with lethal concentrations of glucose (0.6%) and various concentrations of carnosine (Fig. 3). As shown in Fig. 3A, carnosine displayed protective ability at concentrations of 20 mM or higher (concentrations below 20 mM did not prevent a decrease in viability over 1 week of incubation). When carnosine was added at different times after inoculation of glucose-containing medium, carnosine was protective if added within 3 days of the initial inoculation; addition on day 4 or 5 had no protective effect (Fig. 3B). An anti-CML antibody was used to detect the level of CML in cells growing in the presence or absence of glucose and/or carnosine. As shown in Fig. 4, after 3 days of incubation the level of CML increased approximately 3-fold in cultures supplemented with glucose over the level in those containing LB medium alone. When carnosine was added to cultures at the start of the experiment, however, CML levels did not increase relative to LB cultures and were, in fact, slightly below baseline values (Fig. 4).
FIG. 3.
Carnosine protection against glucose toxicity. (A) Cells were incubated in LB medium with glucose (GLU; 0.6% [33.3 mM]) and the following concentrations of carnosine: 0 mM (filled squares), 1 mM (filled circles), 5 mM (filled triangles), 10 mM (filled diamonds), 20 mM (open squares), 50 mM (open circles), 100 mM (open triangles), and 200 mM (open diamonds). The dashed line with filled boxes represents an LB control culture with no additional glucose or carnosine. The asterisk indicates titers below the limit of detection. Representative data are shown (n = 3). (B) Addition of carnosine at different times after inoculation. Cells were inoculated simultaneously with glucose (0.6% [33.3 mM]), and then carnosine (50 mM) was added on different days for 5 days. Time of carnosine addition: day 0 (open circles), day 1 (open squares), day 2 (open triangles), day 3 (open diamonds), day 4 (filled circles), or day 5 (filled diamonds). Asterisks indicate titers below the limit of detection of <1,000 CFU/ml. Representative data are shown (n = 2).
FIG. 4.
ELISA using anti-CML antibody. Cells were harvested 1, 3, or 5 days after inoculation. Absorbance measurements were obtained to quantify the relative amount of CML per milligram of total protein in each culture (see Materials and Methods). Data are the averages of 12 measurements with standard deviation shown. GLU, glucose (0.4%); CAR, carnosine (50 mM).
Carnosine protection against methylglyoxal toxicity.
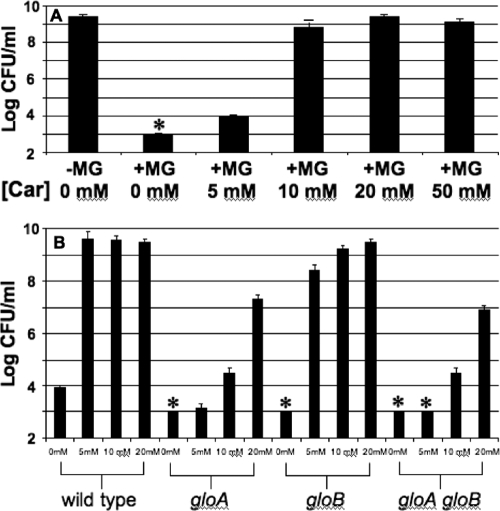
To investigate if carnosine can suppress MG-mediated toxicity in E. coli, cells were incubated in LB medium supplemented with MG and/or carnosine and cell survival was determined (Fig. 5). As shown in Fig. 5A, bacteria were able to grow to maximum density (109 CFU/ml) in cultures where at least 10 mM carnosine was added to the medium during outgrowth. However, in assays involving glyoxalase mutants with a decreased capacity to detoxify methylglyoxal (29), higher concentrations of carnosine were needed to protect gloA mutant strains or the gloA gloB double mutant than were needed to protect wild-type cells (Fig. 5B) (see Discussion).
FIG. 5.
Carnosine protection against methylglyoxal toxicity. (A) Cultures were inoculated at ∼106 CFU/ml and then grown overnight (18 to 22 h) in the presence or absence of methylglyoxal (MG, 2.1 mM) and/or carnosine (Car) at the given concentrations. (B) Comparison of wild-type strains with glyoxylase mutants. Growth yield data are shown with the concentration of carnosine indicated from 0 to 20 mM. Asterisks indicate cell titers that were below the limit of detection (103 CFU/ml).
Other potential protective agents.
Other compounds with reported antiglycating ability were investigated by using assays similar to those developed here to study carnosine. The results of these assays are summarized in Table 2. Specifically, we found that both folic acid and aminoguanidine each displayed some protective capacity against glucose and/or MG-related toxicity. Other compounds tested that did not show protection in these assays included green tea extract, S-lactoyl glutathione, and B vitamins B1, B6, and B12.
DISCUSSION
Taken together, the data suggest that bacteria suffer damage from glycation in a manner similar to that of eukaryotic cells. As shown here, some amount of glycation was detectable in cells growing under standard laboratory conditions in batch culture (Fig. 2), most likely due to the action of reactive electrophiles that are generated as part of normal metabolism. When the culture was supplemented with glucose, the amount of glycation increased significantly (Fig. 2). Although E. coli has evolved various protective mechanisms against toxic electrophiles (12), we speculate that glycation may nevertheless contribute to cell death and negatively affect the long-term survival of these and other bacterial populations during long-term stationary phase (14).
Carnosine is a naturally occurring dipeptide with the ability to act as an antioxidant and/or antiglycation agent in cells (19). Synthesized endogenously by carnosine-anserine synthetase in many eukaryotes, it is found in long-lived mammalian tissues (such as neurons and muscle tissue) at concentrations up to 20 mM (37, 41). It has been shown that protein cross-links induced in vitro by methyglyoxal are eliminated in the presence of carnosine (24). Carnosine may react directly with MG and sequester it; its amino group and imidazole ring may bind to reactive dicarbonyl groups (3, 7, 24), although the structure of MG-carnosine adducts has yet to be determined (37). Alternatively, Hipkiss and Brownson (22, 23) have proposed that proteins may become “carnosinylated” at carbonyl groups and that this may protect them from degradation and/or cross-linking. Our findings provide further evidence for carnosine's ability to suppress the formation of AGEs in vivo (Fig. 4). Carnosine protected cells from lethal concentrations of glucose (Fig. 3A) and methylglyoxal (Fig. 5A). Furthermore, it protected E. coli even when added after 3 days of exposure to toxic concentrations of glucose (Fig. 3B), and more carnosine was needed to protect gloA mutants, which are less capable of degrading intracellular methylglyoxal, than to protect wild-type cells (Fig. 5B). The slight increase in sensitivity of gloB single mutants was likely due to the fact that the functional gloA gene product can remove MG, resulting in accumulation of a less toxic intermediate (29). A proposed model of protection against AGE formation is shown in Fig. 6.
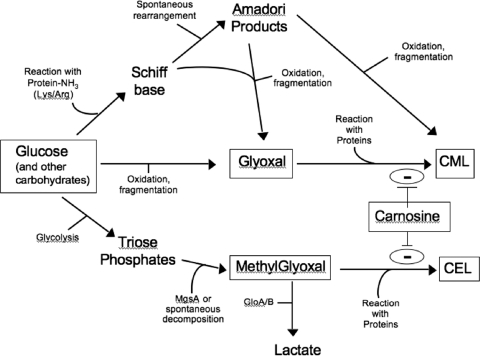
FIG. 6.
Proposed model of carnosine protection against glycation. Carnosine most likely interacts with reactive carbonyl intermediate compounds (e.g., glyoxal and methylglyoxal) to inhibit the formation of advanced glycated end products (e.g., CML and carboxyethyl lysine). Other mechanisms of protection are also probable (see text for details). Abbreviations: MgsA, methylglyoxal synthase; CML, carboxymethyl lysine; CEL, carboxyethyl lysine. GloA and GloB are glyoxylase enzymes I and II, respectively.
Other chemical agents with possible antiglycating ability were investigated using our bacterial model system. Folic acid (folate/vitamin B9) protected E. coli from toxic concentrations of both glucose and methylglyoxal (Table 2). Although the exact mechanism of action for this effect has not been determined, it has been shown that folate can modulate cellular glutathione levels, which may act as a defense against oxidant and alkylating agent damage, since glutathione is an essential component of the glyoxylase I/II system(s) used to detoxify methylglyoxal (13). In one study, rats fed a high-folate diet were found to have greater hepatic glutathione concentrations than were those on standard or folate-deficient diets (5). Other investigators found that methylglyoxal-resistant E. coli mutants have increased activity in their glutathione-forming enzyme system (34). Therefore, we postulate that the protective ability of folate under glycation-prone conditions may be due to its influence on glutathione. Further studies using glyoxylase mutants should help to clarify the role(s) of folate and/or glutathione in protection against glycating agents.
Aminoguanidine (AG) has previously been shown to reduce the severity of AGE-influenced pathologies in mammalian systems (27), probably by reaction with glycation intermediates and/or toxic dicarbonyl compounds via its free amino groups (4). We demonstrated that AG can also display protective effects in a bacterial system, allowing E. coli to survive when grown in medium containing lethal concentrations of glucose or methylglyoxal (Table 2). It may be useful to determine if AG acts synergistically with carnosine or any of the other antiglycation agents previously discussed.
We have developed a novel prokaryotic model system to study the effects of compounds that may protect against glycation. This study focused on carnosine, an agent that may be one of several compounds that Gallant and colleagues (18) refer to as “geroprotectors,” substances that can prevent some of the deterioration associated with the aging process. Additionally, folate and aminoguanidine displayed protective effects in the presence of agents known to induce glycation (Table 2). Other chemicals, such as grape seed extract and aspirin, may also have protective benefits (E. D. Pepper and S. E. Finkel, unpublished observation). Further studies using this bioassay may shed light on the biological relevance of these compounds in long-term survival of bacteria and clinically relevant eukaryotic systems.
Acknowledgments
We thank Vyacheslav Palchevskiy and Angela May Wong for helpful suggestions and Steven Goodman, Steven Bradforth, Zhenya Gershman, Alison Kraigsley, Christopher Corzett, and Kavita Chavan for comments on the manuscript.
This work was supported in part by NSF CAREER award MCB 0237975 to S.E.F. E.D.P. was supported in part by an NIH/NIA Neuroendocrinology of Aging training grant.
Footnotes
Published ahead of print on 15 October 2010.
REFERENCES
- 1.Ahmed, M. U., E. Brinkmann Frye, T. P. Degenhardt, S. R. Thorpe, and J. W. Baynes. 1997. N-epsilon-carboxyethyllysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem. J. 324:565-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, M. U., S. R. Thorpe, and J. W. Baynes. 1986. Identification of N-epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated proteins. J. Biol. Chem. 261:4889-4894. [PubMed] [Google Scholar]
- 3.Aldini, G., M. Carini, G. Beretta, S. Bradamante, and R. M. Facino. 2002. Carnosine is a quencher of 4-hydroxy-nonenal, through what mechanism of reaction? Biochem. Biophys. Res. Commun. 298:699-706. [DOI] [PubMed] [Google Scholar]
- 4.Booth, A. A., R. G. Khalifah, P. Todd, and B. G. Hudson. 1997. In vitro kinetic studies of formation of antigenic advanced glycation end products (AGEs), novel inhibition of post-Amadori glycation pathways. J. Biol. Chem. 272:5430-5437. [DOI] [PubMed] [Google Scholar]
- 5.Branda, R. F., Z. Chen, E. M. Brooks, S. J. Naud, T. D. Trainer, and J. J. McCormack. 2002. Diet modulates the toxicity of cancer chemotherapy in rats. J. Lab. Clin. Med. 140:358-368. [DOI] [PubMed] [Google Scholar]
- 6.Bras, I. D., C. M. Colitz, D. F. Kusewitt, H. Chandler, P. Lu, A. J. Gemensky-Metzler, and D. A. Wilkie. 2007. Evaluation of advanced glycation end-products in diabetic and inherited canine cataracts. Graefes Arch. Clin. Exp. Ophthalmol. 245:249-257. [DOI] [PubMed] [Google Scholar]
- 7.Brownson, C., and A. Hipkiss. 2000. Carnosine reacts with a glycated protein. Free Radic. Biol. Med. 28:1564-1570. [DOI] [PubMed] [Google Scholar]
- 8.Bucala, R., and A. Cerami. 1992. Advanced glycosylation, chemistry, biology, and implications for diabetes and aging. Adv. Pharmacol. 23:1-34. [DOI] [PubMed] [Google Scholar]
- 9.Chuyen, N. V. 2006. Toxicity of the AGEs generated from the Maillard reaction, on the relationship of food-AGEs and biological-AGEs. Mol. Nutr. Food Res. 50:1140-1149. [DOI] [PubMed] [Google Scholar]
- 10.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyer, D. G., J. A. Dunn, S. R. Thorpe, K. E. Bailie, T. J. Lyons, D. R. McCance, and J. W. Baynes. 1993. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J. Clin. Invest. 91:2463-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson, G. P. 1999. Protective mechanisms against toxic electrophiles in Escherichia coli. Trends Microbiol. 7:242-247. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson, G. P., and I. R. Booth. 1998. Importance of glutathione for growth and survival of Escherichia coli cells, detoxification of methylglyoxal and maintenance of intracellular K+. J. Bacteriol. 180:4314-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkel, S. E. 2006. Long-term survival during stationary phase: evolution and the GASP phenotype. Nat. Rev. Microbiol. 4:113-120. [DOI] [PubMed] [Google Scholar]
- 15.Finkel, S. E., and R. Kolter. 1999. Evolution of microbial diversity during prolonged starvation. Proc. Natl. Acad. Sci. U. S. A. 96:4023-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finot, P. A. 2005. Historical perspective of the Maillard reaction in food science. Ann. N. Y. Acad. Sci. 1043:1-8. [DOI] [PubMed] [Google Scholar]
- 17.Fu, M. X., J. R. Requena, A. J. Jenkins, T. J. Lyons, J. W. Baynes, and S. R. Thorpe. 1996. The advanced glycation end product, (N-epsilon-carboxymethyl)-lysine, is a product of both lipid peroxidation and glycoxidation reactions. J. Biol. Chem. 271:9982-9986. [DOI] [PubMed] [Google Scholar]
- 18.Gallant, S., M. Semyonova, and M. Yuneva. 2000. Carnosine as a potential anti-senescence drug. Biochemistry (Moscow) 65:866-868. [PubMed] [Google Scholar]
- 19.Gariballa, S., and A. Sinclair. 2000. Carnosine, physiological properties and therapeutic potential. Age Ageing 29:207-210. [DOI] [PubMed] [Google Scholar]
- 20.Genuth, S., W. Sun, P. Cleary, D. R. Sell, W. Dahms, J. Malone, W. Sivitz, and V. M. Monnier. 2005. DCCT Skin Collagen Ancillary Study Group. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes 54:3103-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geroldi, D., C. Falcone, and E. Emanuele. 2006. Soluble receptor for advanced glycation end products, from disease marker to potential therapeutic target. Curr. Med. Chem. 13:1971-1978. [DOI] [PubMed] [Google Scholar]
- 22.Hipkiss, A. R., and C. Brownson. 2000. Carnosine reacts with protein carbonyl groups, another possible role for the anti-ageing peptide? Biogerontology 1:217-223. [DOI] [PubMed] [Google Scholar]
- 23.Hipkiss, A. R. 1999. Carnosine and protein carbonyl groups, a possible relationship. Biochemistry (Moscow) 65:771-778. [PubMed] [Google Scholar]
- 24.Hobart, L. J., I. Seibel, G. S. Yeargans, and N. W. Seidler. 2004. Anti-crosslinking properties of carnosine, significance of histidine. Life Sci. 75:1379-1389. [DOI] [PubMed] [Google Scholar]
- 25.Hopper, D. J., and R. A. Cooper. 1972. The purification and properties of Escherichia coli methylglyoxal synthase. Biochem. J. 128:321-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hopper, D. J., and R. A. Cooper. 1971. The regulation of Escherichia coli methylglyoxal synthase; a new control site in glycolysis? FEBS Lett. 13:213-216. [DOI] [PubMed] [Google Scholar]
- 27.Kern, T., and R. Engerman. 2001. Pharmacological inhibition of diabetic retinopathy, aminoguanidine and aspirin. Diabetes 50:1636-1642. [DOI] [PubMed] [Google Scholar]
- 28.Kraigsley, A. M., and S. E. Finkel. 2009. Adaptive evolution in single species bacterial biofilms. FEMS Microbiol. Lett. 293:135-140. [DOI] [PubMed] [Google Scholar]
- 29.MacLean, M. J., L. S. Ness, G. P. Ferguson, and I. R. Booth. 1998. The role of glyoxalase I in the detoxification of methylglyoxal and in the activation of the KefB K+ efflux system in Escherichia coli. Mol. Microbiol. 27:563-571. [DOI] [PubMed] [Google Scholar]
- 30.Makita, Z., H. Vlassara, E. Rayfield, K. Cartwright, E. Friedman, R. Rodby, A. Cerami, and R. Bucala. 1992. Hemoglobin-AGE, a circulating marker of advanced glycosylation. Science 258:651-653. [DOI] [PubMed] [Google Scholar]
- 31.Meng, J., N. Sakata, Y. Imanaga, S. Takebayashi, R. Nagai, and S. Horiuchi. 2001. Carboxymethyllysine in dermal tissues of diabetic and nondiabetic patients with chronic renal failure, relevance to glycoxidation damage. Nephron 88:30-35. [DOI] [PubMed] [Google Scholar]
- 32.Mironova, R., T. Niwa, Y. Handzhiyski, A. Sredovska, and I. Ivanov. 2005. Evidence for non-enzymatic glycosylation of Escherichia coli chromosomal DNA. Mol. Microbiol. 55:1801-1811. [DOI] [PubMed] [Google Scholar]
- 33.Mironova, R., T. Niwa, H. Hayashi, R. Dimitrova, and I. Ivanov. 2001. Evidence for non-enzymatic glycosylation in Escherichia coli. Mol. Microbiol. 39:1061-1068. [DOI] [PubMed] [Google Scholar]
- 34.Murata, K., K. Tani, J. Kato, and I. Chibata. 1980. Excretion of glutathione by methylglyoxal-resistant Escherichia coli. J. Gen. Microbiol. 120:545-547. [DOI] [PubMed] [Google Scholar]
- 35.Nystrom, T. 2005. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 24:1311-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poggioli, S., J. Mary, H. Bakala, and B. Friguet. 2004. Evidence of preferential protein targets for age-related modifications in peripheral blood lymphocytes. Ann. N. Y. Acad. Sci. 1019:211-214. [DOI] [PubMed] [Google Scholar]
- 37.Reddy, V. P., M. R. Garrett, G. Perry, and M. A. Smith. 2005. Carnosine, a versatile antioxidant and antiglycating agent. Sci. Aging Knowledge Environ. 18:12. [DOI] [PubMed] [Google Scholar]
- 38.Seiquer, I., J. Diaz-Alguacil, C. Delgado-Andrade, M. Lopez-Frias, A. Munoz Hoyos, G. Galdo, and M. P. Navarro. 2006. Diets rich in Maillard reaction products affect protein digestibility in adolescent males aged 11-14 years. Am. J. Clin. Nutr. 83:1082-1088. [DOI] [PubMed] [Google Scholar]
- 39.Sell, D. R., R. H. Nagaraj, S. K. Grandhee, P. Odetti, A. Lapolla, J. Fogarty, and V. M. Monnier. 1991. Pentosidine, a molecular marker for the cumulative damage to proteins in diabetes, aging, and uremia. Diabetes Metab. Rev. 7:239-251. [DOI] [PubMed] [Google Scholar]
- 40.Singh, R., A. Barden, T. Mori, and L. Beilin. 2001. Advanced glycation end-products: a review. Diabetologia 44:129-146. [DOI] [PubMed] [Google Scholar]
- 41.Stenesh, J., and T. Winnick. 1960. Carnosine-anserine synthetase of muscle. Partial purification of the enzyme and further studies of β-alanyl peptide synthesis. Biochem. J. 77:575-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webster, J., M. Wilke, P. Stahl, R. Kientsch-Engel, and G. Munch. 2005. Maillard reaction products in food as pro-inflammatory and pro-arteriosclerotic factors of degenerative diseases. Z. Gerontol. Geriatr. 38:347-353. [DOI] [PubMed] [Google Scholar]
- 43.Zambrano, M., D. Siegele, M. Almiron, A. Tormo, and R. Kolter. 1993. Microbial competition, Escherichia coli mutants that take over stationary phase cultures. Science 259:1757-1760. [DOI] [PubMed] [Google Scholar]