Abstract
Isobutene is an important commercial chemical used for the synthesis of butyl rubber, terephthalic acid, specialty chemicals, and a gasoline performance additive known as alkylate. Currently, isobutene is produced from petroleum and hence is nonrenewable. Here, we report that the Saccharomyces cerevisiae mevalonate diphosphate decarboxylase (ScMDD) can convert 3-hydroxy-3-methylbutyrate (3-HMB) to isobutene. Whole cells of Escherichia coli producing ScMDD with an N-terminal 6×His tag (His6-ScMDD) formed isobutene from 3-HMB at a rate of 154 pmol h−1 g cells−1. In contrast, no isobutene was detected from control cells lacking ScMDD. His6-ScMDD was purified by nickel affinity chromatography and shown to produce isobutene from 3-HMB at a rate of 1.33 pmol min−1 mg−1 protein. Controls showed that both His6-ScMDD and 3-HMB were required for detectable isobutene formation. Isobutene was identified by gas chromatography (GC) with flame ionization detection as well as by GC-mass spectrometry (MS). ScMDD was subjected to error-prone PCR, and two improved variants were characterized, ScMDD1 (I145F) and ScMDD2 (R74H). Whole cells of E. coli producing ScMDD1 and ScMDD2 produced isobutene from 3-HMB at rates of 3,000 and 5,888 pmol h−1 g cells−1, which are 19- and 38-fold increases compared to rates for cells producing His6-ScMDD. This showed that genetic modifications can be used to increase the rate at which ScMDD converts 3-HMB to isobutene. Because 3-HMB can be produced from l-leucine, ScMDD has a potential application for the production of renewable isobutene. Moreover, isobutene is a gas, which might simplify its purification from a fermentation medium, substantially reducing production costs.
The United States consumed about 360 billion gallons of petroleum products in 2008 while domestically producing only 76 billion gallons (34-35). The U.S. Census Bureau estimates that these imports cost the United States about $252 billion (33). Petroleum is critical for the production of transportation fuels as well as a variety of consumer products, including plastics, medicines, and fertilizers. However, petroleum is nonrenewable, a source of greenhouse gasses when burned, and a national security issue. Hence, renewable petroleum replacements are a national priority. Biofuels, such as ethanol, have displaced some of our gasoline needs, reducing our dependence on foreign oil as well as benefiting our environment (20). However, ethanol has a lower energy density than gasoline, which increases distribution costs, and it is not fully compatible with existing pipelines and engines (1, 2, 8, 26). Longer-chain alcohols and biological hydrocarbons are promising second-generation biofuels with improved characteristics compared to those of bioethanol, but the technology needed to produce such fuels economically is currently unavailable (1, 2, 8, 26). Accordingly, intensive research is under way to improve the production of second-generation biofuels and identify new renewable fuel molecules.
Isobutene is an interesting molecule for the production of renewable fuels and chemicals. Isobutene is a product of petroleum refining that is used for the production of butyl rubber, specialty chemicals, and a gasoline additive known as alkylate (15, 22, 28). Gasoline alkylate is produced by polymerization of a mixture of alkanes and alkenes (such as isobutene) and is usually blended with gasoline at a level of 10 to 15% (22). Alkylate increases octane, improves combustion, reduces tailpipe emissions, and prevents engine knock. Isobutene can also be dimerized and hydrogenated to isooctane, a promising replacement for the gasoline oxygenate methyl tertiary-butyl ether, which is an environmental concern (22). Moreover, isooctane could serve as a direct gasoline replacement that has a high energy density and is fully compatible with the existing infrastructure, although it would need to be blended with a minor fraction of butane to increase its vapor pressure for cold-weather starting.
A major challenge to commercial production of renewable isobutene is that an efficient biological pathway for its formation is unavailable. Prior studies showed that a microsome-associated cytochrome, P450rm, from Rhodotorula produced isobutene from isovalerate at a rate of 4.8 nmol min−1 mg−1 (12). However, this rate is too low for commercial application, and to our knowledge no genetic improvements have been reported for P450rm in the 13 years since its encoding gene was identified (11). Such studies may have been hampered by the fact that P450rm is a poorly understood membrane-associated protein that is difficult to produce in heterologous hosts (29). Consequently, the identification of alternative isobutene-forming enzyme(s) will likely be crucial for the production of renewable isobutene. In this report, we show that mevalonate diphosphate decarboxylase from Saccharomyces cerevisiae (ScMDD) can convert 3-hydroxy-3-methylbutyrate (3-HMB) to isobutene.
MATERIALS AND METHODS
Bacterial strains and growth media.
The bacterial strains and plasmids used in the study are shown in Table 1. Escherichia coli was cultured in lysogeny broth (LB) (Difco, Detroit, MI) at 37°C (3, 4). Kanamycin was used at 50 μg/ml when necessary.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source |
|---|---|---|
| Strains | ||
| BL21(DE3) | F−ompT hsdSB(rB− mB−) gal dcm (DE3) | New England Biolabs |
| DH5α | λ− φ80dlacZΔM15 fhuA2 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK+) phoA glnV44 thi-1 gyrA96 relA1 | New England Biolabs |
| DH10β | araD139 Δ(ara,leu)7697 fhuA lacX74 galK (φ80lacZΔM15) mcrA galU recA1 endA1 nupG rpsL (Strr)Δ(mrr-hsdRMS-mcrBC) | New England Biolabs |
| DG25 | DH5α with pUC57-ScMDD | This study |
| DG26 | DH5α with pTA925-ScMDD | This study |
| DG29 | BL21(DE3) with pTA925 | This study |
| DG30 | BL21(DE3) with pTA925-ScMDD | This study |
| DG62 | BL21(DE3) with pTA925-ScMDD with an I145F mutation (ScMVDC1) | This study |
| DG63 | BL21(DE3) with pTA925-ScMDD with an R74H mutation (ScMVDC2) | This study |
| Plasmids | ||
| pUC57 | Ampr; pUC19 derivative | GenScript |
| pET41a | Kmr; T7 expression vector | Novagen |
| pTA925 | Kmr, pET41a derivative with a 5′ BglII cloning site | 16 |
Chemicals and reagents.
Antibiotics were from Sigma Chemical Company (St. Louis, MO). Isopropyl-β-d-thiogalactopyranoside (IPTG) was from Diagnostic Chemicals Limited (Charlottesville, PEI, Canada). Restriction enzymes and T4 DNA ligase were from New England Biolabs (Beverly, MA). Ethidium bromide, 2-mercaptoethanol, and SDS were from Bio-Rad (Hercules, CA). Other chemicals were from Fisher Scientific (Pittsburgh, PA).
Construction of E. coli strains for production of ScMDD.
The ScMDD gene (ERG19) was synthesized by GenScript optimized for protein production in E. coli with an N-terminal His6 tag and flanking BglII and HindIII sites. The synthetic ScMDD gene was excised from pUC57 by using BglII and HindIII, isolated by agarose gel electrophoresis (27), and purified using a Qiagen QIAquick kit according to the manufacturer's instructions. The gene was ligated into T7 expression vector pTA925 (16) BglII to HindIII by using T4 DNA ligase (New England Biolabs). The ligation mixture was used to transform E. coli DH5α by electroporation with a Bio-Rad gene pulser as previously described (5). The pTA925-His6-ScMDD DNA was isolated using a Qiagen miniprep kit and electroporated into E. coli BL21(DE3) for protein production. The synthetic ScMDD gene sequence was confirmed by DNA sequencing.
Whole-cell assay for isobutene production.
About 300 ml of LB medium was inoculated with 1 ml of an overnight culture and incubated at 37°C until the optical density at 600 nm (OD600) reached 1.2 (about 5 h). Then, protein production was induced with 1 mM IPTG for 17 h at 28°C, and cells were harvested using a Beckman Coulter Avanti J-25 centrifuge at 10,000 × g for 5 min. Harvested cells were resuspended in LB to a concentration of 0.3 g ml−1, and 1 ml of cells was added to 1 ml of LB medium containing 50 μg/ml kanamycin, 1 mM IPTG, and 3-HMB as indicated in the text. Assay components were combined in 10-ml glass gas chromatography (GC) vials that had screw caps with polytetrafluoroethylene (PTFE) (Teflon) inserts from Supelco (Bellefonte, PA). Vials were incubated at 37°C for the time specified in the text. The vial headspace was sampled with a gas-tight syringe and analyzed for isobutene by gas chromatography with a flame ionization detector (GC-FID) or gas chromatography-mass spectrometry (GC-MS) as described below.
Purification of His6-ScMDD and variants for in vitro enzyme assays.
About 300 ml of LB medium was inoculated with 1 ml of an overnight culture and incubated at 37°C until the OD600 reached 1.2. Protein production was induced with 1 mM IPTG for 17 h at 28°C. Cells were pelleted by centrifugation and washed twice with 20 mM Tris-HCl, pH 7.0, containing 300 mM NaCl and 0.5 mM dithiothreitol (DTT). Washed cells were resuspended in 10 ml B-PER II reagent along with 1 mg DNase I, 40 mM Calbiochem protease inhibitor cocktail set I, and 3 mM β-mercaptoethanol. Subsequently, a B-PER His tag purification kit (Thermo Scientific) was used for Ni affinity purification with all manipulations carried out inside an anaerobic chamber (Coy Laboratories) containing an atmosphere of 5% CO2, 5% hydrogen, and 90% nitrogen. Protein concentrations were determined using Bio-Rad's protein assay reagent. Purified enzymes were stored in sealed serum vials under an N2 atmosphere at 4°C. The purification was evaluated by SDS-PAGE using 10 to 20% acrylamide Bio-Rad Ready gels and Bio-Rad Mini-Protean II electrophoresis cells according to the manufacturer's instructions. Gels were stained with Bio-Safe Coomassie (Bio-Rad).
Enzyme assays for isobutene production.
Purified ScMDD and variants were added to 75 mM potassium-phosphate buffer, pH 7.4, with 5 mM magnesium chloride, 20 mM potassium chloride, and ATP and β-HMB in various amounts. Assays were initiated by adding enzyme to assay mixtures in 4-ml vials while using anaerobic precautions. The headspace of assay mixtures was sampled and analyzed by GC-FID.
Isobutene analysis by gas chromatography.
Isobutene was measured by using a Varian 450GC with a CP-Sil 5 CB column fitted with a flame ionization detector. The method used a 10:1 split ratio and a 30-ml/min flow rate with helium as the carrier gas. The oven was held at 30°C for 3 min, which was the duration of the method.
Isobutene analysis by GC-MS.
For confirmation of the isobutene peaks observed during GC-FID screening, an Alliant GC-mass spectrophotometer was used coupled with an Alliant HP-Plot/Q column composed of a polystyrene-divinylbenzene solid phase for monitoring very small chain hydrocarbons. A linear velocity of 40 cm/s was used with a split ratio of 5:1 and a temperature program that held at 40°C for 1 min followed by a ramp at 10°C/min to 200°C.
Random PCR mutagenesis.
ScMDD was subjected to random mutagenesis using Clontech's PCR mutagenesis kit and primers ScMVDC-F (GCCGCCAGATCTATGCATCATCATCATCATCATATGACCGT) and ScMVDC-R (GCCGCCAAGCTTACTCTTTCGGCAGGCCGGTTTT). DNA amplified under mutagenic conditions was digested with BglII and HindIII and then gel purified using a Qiagen QIAquick kit. Next, the purified DNA was ligated into the T7 expression plasmid pTA925 by using T4 DNA ligase and transformed into E. coli BL21(DE3) RIL by electroporation. Transformation mixtures were diluted to obtain about 100 transformants per plate. In some cases, pure cultures were made from selected transformants. Alternatively, plates were then replica printed to two LB kanamycin plates. Then, transformants were pooled by adding 2 ml of LB to the plate and using a spreader to suspend the colonies. The pooled transformants (1 ml) were assayed for isobutene production.
RESULTS
Isobutene production from 3-HMB by whole cells of E. coli producing His6-ScMDD.
Renewable isobutene has substantial commercial potential. Consequently, we tested ScMDD to determine whether it could convert 3-HMB (an analog of its natural substrate) to isobutene (Fig. 1). E. coli strain DG30 was constructed for high-level production of ScMDD with an N-terminal 6×His tag (His6-ScMDD). Approximately 0.3 g of induced DG30 (wet weight) was assayed for isobutene production by GC-FID. Whole cells of this strain formed isobutene at a rate of 155 ± 19 pmol h−1 g cells−1 (mean ± standard deviation). No detectable isobutene was formed when cells were omitted from the assay or when whole cells of the negative control (strain DG29) were used. DG29 is isogenic to DG30 except that it contains the expression plasmid without the insert. In addition, no isobutene was detected when 3-HMB was omitted from assays. For the above assays, isobutene was identified and quantified based on GC retention time and peak area compared to an authentic isobutene standard. These results suggested that ScMDD converts 3-HMB to isobutene.
FIG. 1.
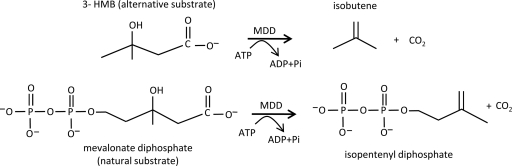
Reactions catalyzed by diphosphomevalonate decarboxylase (MDD). MDD converts diphosphomevalonate to CO2 and isopentenyl-diphosphate, which is a precursor to isoprenoids. In this report, we show that MDD also converts 3-HMB to isobutene and CO2. 3-HMB is a product of leucine degradation. ATP is required to phosphorylate the hydroxyl group at the 3 position, which is thought to leave as the phosphate dianion during decarboxylation.
Mutagenesis of ScMDD for enhanced isobutene production.
The His6-ScMDD gene was mutagenized by error-prone PCR, ligated into the T7 expression plasmid pTA925, and transformed into E. coli BL21(DE3) RIL. Individual transformants (4,000) along with 200 pools, each of which contained about 100 independent transformants (20,000 transformants total), were also tested for isobutene production by the whole-cell assay (see Materials and Methods for a description of how the pools were made). About 20 of these 200 pools showed increased isobutene production. In these cases, the transformants used to make the pools were retested for isobutene production individually. From the above studies, 21 mutants were identified that showed increased isobutene production in the whole-cell assay compared to that of E. coli producing wild-type ScMDD. Of the 21 mutants identified above, two (strains DG62 and DG63) were selected for further study. DG62 and DG63 produced about 18-fold and 38-fold more isobutene than DG30 (produces wild-type ScMDD) in our whole-cell assay using GC-FID detection (Table 2). The His6-ScMDD variants produced by DG62 and DG63 were named His6-ScMDD1 and His6-ScMDD2, respectively. DNA sequence analysis showed that His6-ScMDD1 and His6-ScMDD2 had single-amino-acid changes (I145F and R74H, respectively). Controls showed that ScMDD and 3-HMB were required for isobutene formation by whole cells.
TABLE 2.
Isobutene production by whole cells producing ScMDD and variants
| Strain/condition | Amt of isobutene produced (pmol h−1 g cells−1)a |
|---|---|
| No cells | ND |
| DG29 (no insert) | ND |
| DG30 (wild-type ScMDD) | 154 |
| DG62 (ScMDD1) (I145F) | 3,000 |
| DG63 (ScMDD2) (R74H) | 5,888 |
Isobutene was measured by GC-FID. Assays were performed as described in Materials and Methods and contained approximately 0.3 g of induced whole cells and 10 mM 3-HMB. No isobutene was detected without 3-HMB or cells producing ScMDD. ND, none detected.
Verification of isobutene formation by GC-MS.
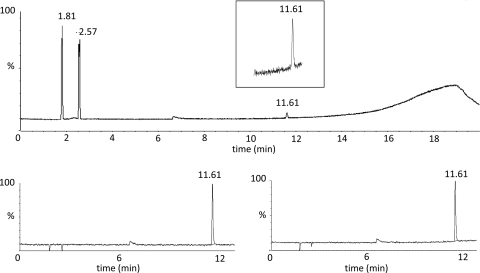
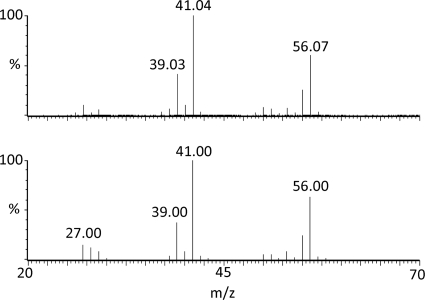
Isobutene formation by whole cells of DG63 was verified by GC-MS. About 0.3 g of DG63 cells (wet weight) was incubated together with 3-HMB in a sealed GC vial for 40 min. Then, 100 μl of the headspace was analyzed by GC-MS (Fig. 2). The total ion chromatogram (TIC) showed a peak at 11.61 min that matched the retention time of the isobutene standard. The mass chromatograms for ions diagnostic of isobutene (56.07 and 41.04 m/z) (Fig. 2) also indicated that isobutene was present in the headspace of assays containing whole cells of DG63. In addition, the mass spectrum of the peak at 11.61 min was used to query the National Institute of Standards and Technology Library. Results showed a near-perfect match to spectra for isobutene (2-methylpropene) (Fig. 3). No isobutene peak was detected in assay mixtures containing control cells (DG29-pTA925-no insert) or in assay mixtures that lacked enzyme or 3-HMB. Cumulatively, these results confirmed that isobutene was formed in assay mixtures containing cells that produce His6-ScMDD2 (DG63).
FIG. 2.
GC-MS of isobutene produced by whole cells of ScMDD variant R74H (strain DG63). The top panel shows the total ion chromatogram obtained from assays of isobutene production by whole cells of DG63. In this method, isobutene had a retention time of about 11.61 min. The inset is a close-up of the isobutene peak. The peaks at 1.81 and 2.57 min in the TIC are air and CO2, respectively. The two smaller figures below are mass chromatograms obtained by monitoring the ions characteristic of isobutene (m/z 56.07 and 41.03).
FIG. 3.
Comparison of the MS spectrum for isobutene produced by ScMDD to library standards. The top trace is the spectrum of the isobutene peak found in the headspace of assays containing DG63 whole cells, which produce ScMDD R74H. This spectrum was used to search the National Institute of Standards and Technology Library, and a near-perfect match to an isobutene standard was found (bottom trace).
Purification of His6-ScMDD and His6-ScMDD2.
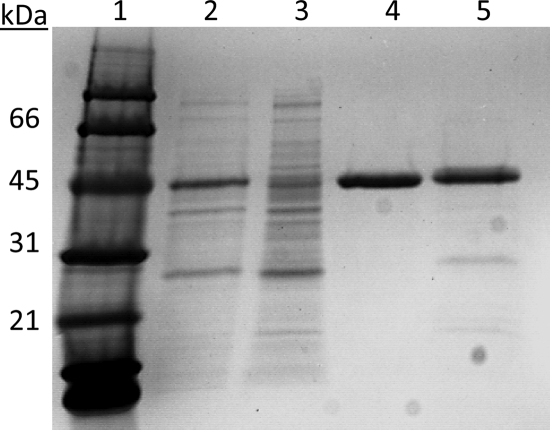
His6- ScMDD (wild-type) and His6-ScMDD2 (R74H, the most active variant) were purified from DG30 and DG63 by nickel affinity chromatography under anaerobic conditions. Based on SDS-PAGE, we estimate that His6-ScMDD and His6-ScMDD2 were 90 to 95% homogenous (Fig. 4). ScMDD is the band near 44 kDa. The yields of purified protein from the 300-ml LB culture were 18.1 and 6.7 mg, respectively, for His6-ScMDD and His6-ScMDD2.
FIG. 4.
SDS-PAGE analysis of His6-ScMDD purification. Lane 1, molecular mass markers; lanes 2 and 3, soluble crude extracts of ScMDD (wild-type) and ScMDD2 (R74H), respectively; lanes 4 and 5, ScMDD and ScMDD2 following purification by Ni-nitrilotriacetic acid (NTA) chromatography. Lanes 2 to 5 contain 2 μg of protein each, and the gel was stained with Coomassie blue.
Isobutene production by purified His6-ScMDD and His6-ScMDD2.
To test whether purified His6-ScMDD and His6-ScMDD2 converted 3-HMB to isobutene, assays were carried out in sealed vials, and the headspace was analyzed by GC-FID. Results showed that His6-ScMDD and His6-ScMDD2 produced isobutene at rates of 1.33 ± 0.27 and 6.44 ± 0.95 pmol min−1 mg−1 protein, respectively (Table 3). Controls showed that detectable isobutene formation required ScMDD, 3-HMB, and ATP as expected (Table 3). The use of anaerobic procedures gave consistently higher levels of activity, possibly due to protection of an oxygen-sensitive thiol group of the enzyme. When enzyme assay mixtures were incubated for longer periods of time, trace amounts of isobutene were produced in negative controls due to chemical reactions as expected (23), but these amounts were much lower than the amount of isobutene produced enzymatically. In addition, GC-MS was used to verify that isobutene was produced by purified His6-ScMDD and His6-ScMDD2. The mass chromatograms obtained had ions diagnostic of isobutene (56.07 and 41.04 m/z) at the expected retention time.
TABLE 3.
Reaction requirements for isobutene formation by purified His6-ScMDD and His6-ScMDD2a
| Assay component | Amt of isobutene (pmol min−1 mg protein−1) produced by: |
|
|---|---|---|
| His6-ScMDD | His6-ScMDD2 | |
| Complete | 1.33 ± 0.27 | 6.44 ± 0.95 |
| −ATP | ND | ND |
| −3-HMB | ND | ND |
| Boiled enzyme | ND | ND |
The complete assay contained 500 μg ScMDD, 333 mM 3-HMB, 250 mM ATP, and standard assay components. No isobutene was detected when ATP or 3-HMB was omitted (−ATP and −3-HMB, respectively) from assay mixtures or when boiled enzyme was used in place of purified ScMDD. ND, none detected.
In the enzyme assays described above, ScMDD2 formed isobutene at a rate 4.8-fold higher than the wild-type enzyme (ScMDD). This is substantially less than the 38-fold increase in isobutene production observed for whole cells expressing ScMDD2 compared to isobutene production observed for cells expressing ScMDD. This was most likely due to instability resulting from the R74H mutation, since unlike the wild-type enzyme, some ScMDD2 precipitated when assay mixtures were heated to 37°C.
Structural mapping.
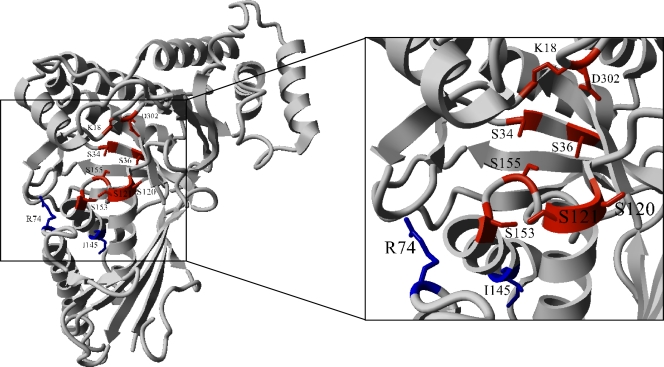
The mutations found in ScMDD1 and ScMDD2 were mapped onto the three-dimensional structure of wild-type ScMDD (Fig. 5). ScMDD1 and ScMDD2 have mutations that map within or close to the proposed active site (36). The I145F mutation in ScMDD1 is positioned close to the active site, near serine 153, which was shown through mutational analysis to be an important contributor in binding and positioning the Mg-ATP complex that serves as a reaction substrate (13, 18, 37). The arginine 74 that is changed in ScMDD2 (R74H) is on the surface of the active site and is thought to contact the phosphate tail of the native substrate, diphosphomevalonate (36). Based on these analyses, we infer that the I145F and R74H mutations alter the active site configuration, thereby improving enzymatic activity with 3-HMB.
FIG. 5.
Structural mapping of ScMDD variants. The left side of the figure shows the structure of the ScMDD monomer, and the right side is an expanded view of the active site. Residues proposed to be part of the active site are red or blue, with blue indicating a mutation that increased the rate of isobutene formation from 3-HMB. Variant ScMDD2, which produces isobutene 38 times faster than wild-type ScMDD, has an R74H mutation. Arginine 74 is thought to form the bottom edge of the active site and interact with the pyrophosphate group of the substrate. Variant ScMDD1, which produces isobutene 18 times faster than the wild type, has an I145F mutation. Although not within the active site, the residue occurs within a helix important for ATP binding and could affect the active site structure. The structure shown corresponds to Protein Data Bank (PDB) accession number 1FI4.
DISCUSSION
Isobutene is an important petroleum-derived chemical, but it is nonrenewable. In this report, we showed that His6-ScMDD catalyzes the conversion of 3-HMB to isobutene. Whole cells producing His6-ScMDD converted 3-HMB to isobutene at a rate of 155 ± 19 pmol h−1 g cells−1, and purified His6-ScMDD produced isobutene at a rate of 1.33 ± 0.27 pmol min−1 mg protein−1. No detectable isobutene was formed without ScMDD enzyme or 3-HMB under the conditions used. Recently, Marliere reported similar results in a European patent application for a number of MDD homologues from various organisms (22a). MDD is now one of two enzymes known to produce isobutene. The other is a cytochrome, P450rm, from Rhodotorula minuta (12). Both enzymes produce isobutene from products of leucine degradation and hence have a potential application to the production of renewable isobutene.
Importantly, the biological production of isobutene might minimize two major costs typically associated with the production of biofuels and green chemicals. A number of renewable chemicals (1-butanol is a notable example) are toxic to producer organisms and inhibit their growth at relatively low concentrations (38). This reduces yield, which dramatically raises product recovery costs. Because isobutene is a gas, it may be relatively simple to pipe it away from the fermentation vessel as it is formed, minimizing toxicity. In addition, gas-phase removal may provide a simple method for the separation of isobutene from the fermentation where it is produced, which can be a major cost in the production of renewable chemicals (14, 26, 38). In the case of bioethanol, distillation accounts for about 9% of its total cost and 58% of the total energy use at an ethanol plant (21), and distillation of 1-butanol would be more expensive due to its higher heat of vaporization (26). Thus, isobutene produced from renewable feedstocks has significant potential for the production of renewable chemicals and second-generation biofuels at lower cost.
In terms of theoretical yields and energy requirements, the production of isobutene from glucose appears to be competitive with other biofuels. The production of one isobutene and two CO2 molecules from one glucose molecule preserves 90% of the heat of combustion of glucose, is redox balanced, and ATP generating (if produced via the metabolic pathway discussed below). When glucose is converted to two ethanol molecules or one butanol molecule (and CO2), the enthalpies of combustion yield are 97% and 95%, respectively, and both processes are redox balanced and ATP generating. Thus, for biofuel applications, isobutene is at a disadvantage compared to ethanol and 1-butanol in terms of energy yield (and polymerization costs). However, these costs might be more than offset by the higher energy density of alkylate (about 48 MJ/kg), its compatibility with existing infrastructure, and the possibility that a gas may be less expensive to purify from fermentation medium. Thus, overall, isobutene appears to have significant potential as a renewable chemical.
Presently, there are two main challenges to the commercial production of renewable isobutene from 3-HMB using the ScMDD enzyme described in this report. First, although biological production of 3-HMB is possible via leucine degradation, the E. coli pathway of leucine biosynthesis is unsuitable. E. coli synthesizes leucine from two molecules of pyruvate plus one molecule of acetyl coenzyme A (acetyl-CoA). This pathway would convert 1.5 molecules of glucose to one isobutene molecule, five CO2 molecules, and 5 excess reducing equivalents. There is no straightforward way of recapturing the reducing equivalents formed; reaction with molecular oxygen to form water would substantially reduce the enthalpy of combustion yield, which would raise feedstock (glucose) costs to prohibitive levels with respect to biofuel applications. However, there are some promising alternatives. One possibility is to produce 3-HMB in a homoacetogen. These organisms can produce two ATP and three acetyl-CoA molecules from one glucose molecule (10). A pathway that converts three acetyl-CoA molecules to methylcrotonyl-CoA has been proposed in Myxococcus (6), and studies of Galactomyces have identified a route for the conversion of methylcrotonyl-CoA to 3-HMB (9). The combination of the Galactomyces and Myxococcus pathways in an acetogen potentially provides a redox-balanced pathway from glucose to isobutene via 3-HMB that would have a high theoretical enthalpy of combustion yield (90%) (Fig. 6). Engineering this pathway into a homoacetogen could likely be possible, although difficult since genetic systems are not as advanced in the homoacetogens as they are in other bacteria (10). Alternatively, a syngas fermentation might be used for isobutene production via 3-HMB (32). Syngas is a mixture primarily of H2, CO, and CO2 that is produced by thermochemical treatment of organic matter, such as lignocellulose. Certain anaerobic bacteria, including Clostridia, can convert syngas to acetyl-CoA, which could then be converted to isobutene via 3-HMB as described above. Lastly, it might also be possible to engineer isobutene production into a photosynthetic organism where carbon and energy are derived by CO2 fixation and sunlight rather than organic feedstocks such as glucose, which are the major production cost.
FIG. 6.
Possible pathway for the production of 3-HMB from glucose. Homoacetogens can degrade one molecule of glucose to three acetyl-CoA molecules, which can be used for the production of 3-hydroxy-3-methylglutaryl-CoA. Enzymes found in Myxococcus are proposed to convert 3-hydroxy-3-methylglutaryl-CoA to methylcrotonyl-CoA (6), which can be converted to 3-HMB by enzyme activities present in cell extracts of Galactomyces species (19). When this pathway is used to produce isobutene from 3-HMB in a homoacetogen, the net result is that one glucose molecule is converted to one isobutene, two CO2, and two H2O molecules with the formation of two ATP molecules.
A second challenge to the production of renewable isobutene is the development of an enzyme that produces isobutene at a high rate. The rates of isobutene formation by ScMDD (described here) and P450rm of Rhodotorula (12) are 1.33 pmol min−1 mg−1 and 4.8 nmol min−1 mg−1, respectively. These levels of activity are too low for commercial application, but genetic improvements may be possible. In the case of ScMDD, the highest rate of isobutene formation observed for whole cells was 5,888 pmol h−1 g cells−1 (0.33 μg h−1 g cells−1). We estimate that this level of activity is about 106-fold below the activity needed for a commercial process (2 to 4 g liter−1 h−1 of product is needed). However, we have shown that the activity of ScMDD with 3-HMB can be improved genetically, and in recent years excellent progress has been made in directed enzyme evolution (24, 25, 31). For example, the activity of glyphosate acetyltransferase was increased about 10,000-fold (7, 30). Moreover, if ScMDD could be engineered to have the same activity with 3-HMB as with its native substrate (about 6 μmol min−1 mg−1), that would be sufficient for commercial application (17). This suggests that the needed level of increase is possible. Importantly, ScMDD offers the opportunity for directed evolution of an isobutene-forming enzyme in a host (E. coli) that is highly amenable to sophisticated genetic manipulations. This will substantially facilitate the development of high-throughput screens and selections crucial for directed enzyme evolution. Thus, the production of renewable isobutene via ScMDD is a possibility, and because of the advantages inherent in the chemical nature of isobutene, we think it has the potential to transform the biofuels industry.
Acknowledgments
We thank the ISU DNA Sequencing and Synthesis Facility for assistance with DNA analyses. We thank Steve Vesey and the ISU Chemical Instrumentation Facility for assistance with mass spectroscopy.
This research was supported by the state of Iowa.
Footnotes
Published ahead of print on 22 October 2010.
REFERENCES
- 1.Alper, H., and G. Stephanopoulos. 2009. Engineering for biofuels: exploiting innate microbial capacity or importing biosynthetic potential? Nat. Rev. Microbiol. 7:715-723. [DOI] [PubMed] [Google Scholar]
- 2.Atsumi, S., and J. C. Liao. 2008. Metabolic engineering for advanced biofuels production from Escherichia coli. Curr. Opin. Biotechnol. 19:414-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertani, G. 2004. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J. Bacteriol. 186:595-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertani, G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobik, T. A., G. D. Havemann, R. J. Busch, D. S. Williams, and H. C. Aldrich. 1999. The propanediol utilization (pdu) operon of Salmonella enterica serovar Typhimurium LT2 includes genes necessary for formation of polyhedral organelles involved in coenzyme B12-dependent 1,2-propanediol degradation. J. Bacteriol. 181:5967-5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bode, H. B., M. W. Ring, G. Schwar, M. O. Altmeyer, C. Kegler, I. R. Jose, M. Singer, and R. Muller. 2009. Identification of additional players in the alternative biosynthesis pathway to isovaleryl-CoA in the myxobacterium Myxococcus xanthus. Chembiochem 10:128-140. [DOI] [PubMed] [Google Scholar]
- 7.Castle, L. A., D. L. Siehl, R. Gorton, P. A. Patten, Y. H. Chen, S. Bertain, H. J. Cho, N. Duck, J. Wong, D. Liu, and M. W. Lassner. 2004. Discovery and directed evolution of a glyphosate tolerance gene. Science 304:1151-1154. [DOI] [PubMed] [Google Scholar]
- 8.Dellomonaco, C., F. Fava, and R. Gonzalez. 2010. The path to next generation biofuels: successes and challenges in the era of synthetic biology. Microb. Cell Fact. 9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhar, A., K. Dhar, and J. P. Rosazza. 2002. Purification and characterization of a Galactomyces reessii hydratase that converts 3-methylcrotonic acid to 3-hydroxy-3-methylbutyric acid. J. Ind. Microbiol. Biotechnol. 28:81-87. [DOI] [PubMed] [Google Scholar]
- 10.Drake, H. L., A. S. Gossner, and S. L. Daniel. 2008. Old acetogens, new light. Ann. N. Y. Acad. Sci. 1125:100-128. [DOI] [PubMed] [Google Scholar]
- 11.Fujii, T., K. Nakamura, K. Shibuya, S. Tanase, O. Gotoh, T. Ogawa, and H. Fukuda. 1997. Structural characterization of the gene and corresponding cDNA for the cytochrome P450rm from Rhodotorula minuta which catalyzes formation of isobutene and 4-hydroxylation of benzoate. Mol. Gen. Genet. 256:115-120. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda, H., T. Fujii, E. Sukita, M. Tazaki, S. Nagahama, and T. Ogawa. 1994. Reconstitution of the isobutene-forming reaction catalyzed by cytochrome P450 and P450 reductase from Rhodotorula minuta: decarboxylation with the formation of isobutene. Biochem. Biophys. Res. Commun. 201:516-522. [DOI] [PubMed] [Google Scholar]
- 13.Hunter, W. N. 2007. The non-mevalonate pathway of isoprenoid precursor biosynthesis. J. Biol. Chem. 282:21573-21577. [DOI] [PubMed] [Google Scholar]
- 14.Jarboe, L. R., X. Zhang, X. Wang, J. C. Moore, K. T. Shanmugam, and L. O. Ingram. 2010. Metabolic engineering for production of biorenewable fuels and chemicals: contributions of synthetic biology. J. Biomed. Biotechnol. 2010:761042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jhung, S. H., and J. S. Chang. 2009. Trimerization of isobutene over solid acid catalysts. Catalysis Surveys from Asia 13:229-236. [Google Scholar]
- 16.Johnson, C. L., E. Pechonick, S. D. Park, G. D. Havemann, N. A. Leal, and T. A. Bobik. 2001. Functional genomic, biochemical, and genetic characterization of the Salmonella pduO gene, an ATP:cob(I)alamin adenosyltransferase gene. J. Bacteriol. 183:1577-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krepkiy, D., and H. M. Miziorko. 2004. Identification of active site residues in mevalonate diphosphate decarboxylase: implications for a family of phosphotransferases. Protein Sci. 13:1875-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krepkiy, D. V., and H. M. Miziorko. 2005. Investigation of the functional contributions of invariant serine residues in yeast mevalonate diphosphate decarboxylase. Biochemistry 44:2671-2677. [DOI] [PubMed] [Google Scholar]
- 19.Lee, I. Y., and J. P. N. Rosazza. 1998. Enzyme analyses demonstrate that beta-methylbutyric acid is converted to beta-hydroxy-beta-methylbutyric acid via the leucine catabolic pathway by Galactomyces reessii. Arch. Microbiol. 169:257-262. [DOI] [PubMed] [Google Scholar]
- 20.Liska, A. J., H. S. Yang, V. R. Bremer, T. J. Klopfenstein, D. T. Walters, G. E. Erickson, and K. G. Cassman. 2009. Improvements in life cycle energy efficiency and greenhouse gas emissions of corn-ethanol. J. Ind. Ecol. 13:58-74. [Google Scholar]
- 21.Louisiana Department of Natural Resources. 2008. Basic ethanol production. Louisiana Department of Natural Resources, Baton Rouge, LA. http://dnr.louisiana.gov/sec/execdiv/techasmt/alternative_fuels/ethanol/fuel_alcohol_1987/015.htm#XI.
- 22.Marchionna, M., M. Di Girolamo, and R. Patrini. 2001. Light olefins dimerization to high-quality gasoline components. Catalysis Today 65:397-403. [Google Scholar]
- 22a.Marliere, P. January 2010. Production of alkenes by enzymatic decarboxylation of 3-hydroxyalkanoic acids. International patent WO/2010/001078.
- 23.Pressman, D., and H. J. Lucas. 1940. The hydration of unsaturated compounds. VIII. The rate of hydration of beta,beta-dimethylacrylic acid; the rates of dehydration and decarboxylation of beta-hydroxyisovaleric acid. J. Am. Chem. Soc. 62:2069-2080. [Google Scholar]
- 24.Romero, P. A., and F. H. Arnold. 2009. Exploring protein fitness landscapes by directed evolution. Nat. Rev. Mol. Cell Biol. 10:866-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin-Pitel, S. B., and H. Zhao. 2006. Recent advances in biocatalysis by directed enzyme evolution. Comb. Chem. High Throughput Screen. 9:247-257. [DOI] [PubMed] [Google Scholar]
- 26.Rude, M. A., and A. Schirmer. 2009. New microbial fuels: a biotech perspective. Curr. Opin. Microbiol. 12:274-281. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook, J., and D. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, NY.
- 28.Sassmannshausen, J. 2009. Cationic and dicationic zirconocene compounds as initiators of carbocationic isobutene polymerisation. Dalton Trans. 2009:9026-9032. [DOI] [PubMed]
- 29.Shiningavamwe, A., G. Obiero, J. Albertyn, J. M. Nicaud, and M. Smit. 2006. Heterologous expression of the benzoate para-hydroxylase encoding gene (CYP53B1) from Rhodotorula minuta by Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 72:323-329. [DOI] [PubMed] [Google Scholar]
- 30.Siehl, D. L., L. A. Castle, R. Gorton, and R. J. Keenan. 2007. The molecular basis of glyphosate resistance by an optimized microbial acetyltransferase. J. Biol. Chem. 282:11446-11455. [DOI] [PubMed] [Google Scholar]
- 31.Tao, H., and V. W. Cornish. 2002. Milestones in directed enzyme evolution. Curr. Opin. Chem. Biol. 6:858-864. [DOI] [PubMed] [Google Scholar]
- 32.Tirado-Acevedo, O., M. S. Chinn, and A. M. Grunden. 2010. Production of biofuels from synthesis gas using microbial catalysts. Adv. Appl. Microbiol. 70:57-92. [DOI] [PubMed] [Google Scholar]
- 33.United States Census Bureau. 2009. Foreign trade statistics. U.S. Census Bureau, Washington, DC. http://www.census.gov/foreign-trade/statistics/highlights/annual.html.
- 34.United States Energy Information Administration. 2008. Crude oil production. U.S. Energy Information Administration, Washington, DC. http://www.eia.doe.gov/dnav/pet/pet_crd_crpdn_adc_mbbl_a.htm.
- 35.United States Energy Information Administration. 2008. U.S. imports by country of origin. U.S. Energy Information Administration, Washington, DC. http://www.eia.doe.gov/energyexplained/index.cfm?page=oil_home#tab2.
- 36.Voynova, N. E., Z. J. Fu, K. P. Battaile, T. J. Herdendorf, J. J. P. Kim, and H. M. Miziorko. 2008. Human mevalonate diphosphate decarboxylase: characterization, investigation of the mevalonate diphosphate binding site, and crystal structure. Arch. Biochem. Biophys. 480:58-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weerasinghe, S., and R. S. Dassanayake. 2010. Simulation of structural and functional properties of mevalonate diphosphate decarboxylase (MVD). J. Mol. Model. 16:489-498. [DOI] [PubMed] [Google Scholar]
- 38.Zheng, Y. N., L. Z. Li, M. Xian, Y. J. Ma, J. M. Yang, X. Xu, and D. Z. He. 2009. Problems with the microbial production of butanol. J. Ind. Microbiol. Biotechnol. 36:1127-1138. [DOI] [PubMed] [Google Scholar]