Abstract
Escherichia coli clonal group A isolates cause infections in people. We investigated 158 phylogroup D E. coli isolates from animals, meat, and humans. Twenty-five of these isolates were of clonal group A, and 15 isolates were shown to cause infection in a mouse urinary tract infection (UTI) model. We conclude that clonal group A isolates are found in both broiler chickens and broiler chicken meat and may cause UTI in humans.
Urinary tract infection (UTI) is one of the most common bacterial infections and is mainly caused by Escherichia coli. Clonal groups and epidemic strains that cause UTI have been identified by many methods, including serotyping, multilocus sequence typing (MLST), and PCR (11, 16, 18, 22). A recently characterized group causing invasive disease in humans, particularly UTI, is clonal group A (CgA). The CgA isolates are closely related to the clonal O15:K52:H1 group. CgA isolates are characterized by belonging to phylogroup D and MLST clonal complex 69 (CC69) and often possess resistance to trimethoprim-sulfonamides (3, 9). In Denmark, the frequency of resistance to sulfonamides—the drugs of choice for treatment of uncomplicated UTI—has remained constant (≈40%) during the past years despite a decline in their use in humans. The level of resistance to trimethoprim, also frequently used for treatment of UTI, has increased significantly, to 34% (1). Recent studies have suggested that animals and food may be the source of antimicrobial-resistant extraintestinal pathogenic E. coli isolates (19). The distribution of CgA isolates has mainly been investigated in North America, although a few CgA isolates have been identified among UTI E. coli isolates from Europe, South America, and the Middle East (2, 3, 10-12, 14-17). Furthermore, the sources of CgA isolates have received little investigation. We therefore examined a temporally and geographically matched collection, obtained in Denmark, of E. coli isolates from animals, meat, community-dwelling humans, and UTI patients for the presence of CgA isolates and studied their clonal relationship, as well as their virulence in a mouse model of UTI, which is representative for UTI (4, 6, 21).
A total of 964 E. coli isolates from Danish healthy broiler chickens (n = 138), Danish (n = 197) and imported (n = 86) broiler chicken meat, Danish healthy pigs (n = 145), Danish (n = 177) and imported (n = 10) pork, community-dwelling humans (n = 109) (all collected in Denmark in 2004 using stratified sampling schemes), and UTI patients (n = 102 total; 21 trimethoprim-sulfonamide resistant) (collected in one region of Denmark in 2005 to 2006) were previously analyzed for phylogroups (A, B1, B2, and D) (8). Sampling of community-dwelling human isolates was approved by the Frederiksberg scientific ethical committee [(KF) 01-006/02]. Of these 964 isolates, 158 isolates from all origins (except imported pork) belonged to phylogroup D (Table 1). These 158 E. coli phylogroup D isolates were screened by PCR for CgA-associated single-nucleotide polymorphisms (SNPs) in fumC and gyrB (11). The presence of these SNPs identified strains belonging to CC69 and excluded strains belonging to the closely related CC394. Among the 158 tested phylogroup D isolates, 25 CgA isolates were detected from broiler chickens, Danish broiler chicken meat, community-dwelling humans, and UTI patients, as shown in Table 1. This suggests that broiler chickens and broiler chicken meat may be the source of CgA isolates in humans. To our knowledge, CgA isolates have been detected only among animal isolates (including cows, turkeys, chickens, dogs, cats, etc.) in one study in the United States (19). Although no CgA isolates were detected among pork and pigs, there was no significant difference between the number of CgA isolates from Danish broiler chickens and broiler chicken meat collectively versus the number from pork and pigs collectively (9 CgA isolates out of 80 versus 0 CgA isolates out of 28, P = 0.1080, Fisher's exact test [two-tailed], GraphPad Prism 5; GraphPad Software, San Diego, CA). Thus, we cannot exclude such isolate origins as possible reservoirs. The detection of CgA isolates from UTI patients also supported previous findings of a widespread global emergence of CgA isolates among uropathogenic E. coli (UPEC) (2, 3, 10-12, 14-17).
TABLE 1.
Occurrence of clonal group A isolates, sulfamethoxazole and trimethoprim resistance, and related PFGE profiles among phylogroup D E. coli isolates from various sources
| Source (no. of E. coli isolates) | No. (%) of CgA isolates | Isolate | Drug resistancea | Related PFGE profilesb |
|---|---|---|---|---|
| Danish broiler chickens (30) | 5 (17) | 7630114-1 | ||
| 7630135-1 | SUL, TRI | |||
| 7630311-1 | ||||
| 7630534-1 | SUL, TRI | |||
| 7630634-1 | SUL, TRI | |||
| Danish broiler chicken meat (40) | 4 (10) | 7633036-3 | ||
| 7633041-7 | ||||
| 7633080-10 | ||||
| 7633083-6 | ||||
| Imported broiler chicken meat (10) | 0 (0) | |||
| Danish pigs (16) | 0 (0) | |||
| Danish pork (12) | 0 (0) | |||
| Community-dwelling humans (27) | 5 (19) | 1944C04 | A | |
| 2171C04 | B | |||
| 2231C04 | ||||
| 2559C04 | A | |||
| 2656C04 | ||||
| UTI patients (23) | 11 (48) | 27-a | SUL, TRI | C |
| 35-a | SUL, TRI | C | ||
| 104-a | SUL | B | ||
| 140-a | SUL | |||
| 173-a | SUL | |||
| 175-a | SUL, TRI | |||
| 182-a | D | |||
| 187-a | SUL, TRI | A | ||
| 195-a | SUL, TRI | B | ||
| 275-a | D | |||
| 303-a | SUL |
SUL, sulfamethoxazole; TRI, trimethoprim.
A, two community-dwelling human isolates and one UTI isolate had closely related PFGE profiles; B, one community-dwelling human isolate and two UTI isolates had PFGE profiles possibly related to that of the ATCC BAA-457 strain; C, two UTI isolates had closely related PFGE profiles; D, two UTI isolates had indistinguishable profiles.
CgA isolates were investigated for phenotypic resistance to sulfamethoxazole and trimethoprim (8). CgA isolates are reported to be responsible for up to 50% of the trimethoprim-sulfonamide-resistant E. coli isolates from U.S. women with acute uncomplicated cystitis and pyelonephritis (10, 15, 16). In our study, five UTI CgA isolates were resistant to both sulfamethoxazole and trimethoprim (Table 1). Thus, the CgA isolates accounted for 24% (5/21) of the sulfamethoxazole-trimethoprim-resistant isolates from the Danish UTI patients. Three broiler chicken isolates were resistant to sulfamethoxazole and trimethoprim (Table 1). Broiler chicken isolates may contribute to the high resistance to these antimicrobials observed in UPEC in Denmark since these antimicrobial agents are used for broiler chickens (1).
The 25 identified CgA isolates and the CgA reference strain ATCC BAA-457 (from a female UTI patient in California) were subjected to pulsed-field gel electrophoresis (PFGE) with XbaI using the protocol made available by the CDC's PulseNet (5, 20). The PFGE patterns were interpreted according to the protocol of Tenover et al. (23). No CgA animal or meat isolates had PFGE profiles related to those of any human isolates. As PFGE is a highly discriminatory method, it would have been surprising to find animal or meat isolates with PFGE profiles possibly or closely clonally related to those of human isolates. However, two community-dwelling-human isolates were closely related to a UTI isolate (differences of 1 to 3 bands), and two sets of two UTI isolates were closely related (a difference of 3 bands) and indistinguishable, respectively (Table 1). Further, two UTI isolates and a community-dwelling human isolate were possibly related to the CgA reference isolate ATCC BAA-457 (Table 1). To our knowledge, the humans were unrelated (e.g., none shared addresses or surnames, and the isolates were obtained >1 month apart except for the UTI isolates 27-a and 35-a). An epidemiological study has previously identified frequent pork and chicken consumption to be associated with multidrug-resistant UTI (13). We speculate that meat (or other food) may have been a common source of such isolates since the meat, produced at Danish slaughterhouses, is distributed nationwide. Direct contact with animals following transmission of isolates may also be possible; however, it was not investigated in this study. The possibility that the PFGE profiles of one community-dwelling human and two UTI isolates are related to the PFGE profile of the ATCC BAA-457 isolate is surprising considering the geographical distance and absence of any apparent epidemiological connections. However, other studies have likewise reported PFGE similarities of UTI isolates obtained in Chicago, IL, and Curitiba and Rio de Janeiro, Brazil, to the ATCC BAA-457 isolate (2, 3, 12). We have no explanation for this finding, but there is a possibility that the BAA-457 isolate belongs to an epidemic, broadly disseminated E. coli lineage. This could be the case since another study also detected PFGE-related CgA isolates from geographical locations as distinct as Montréal, Canada, and California (14).
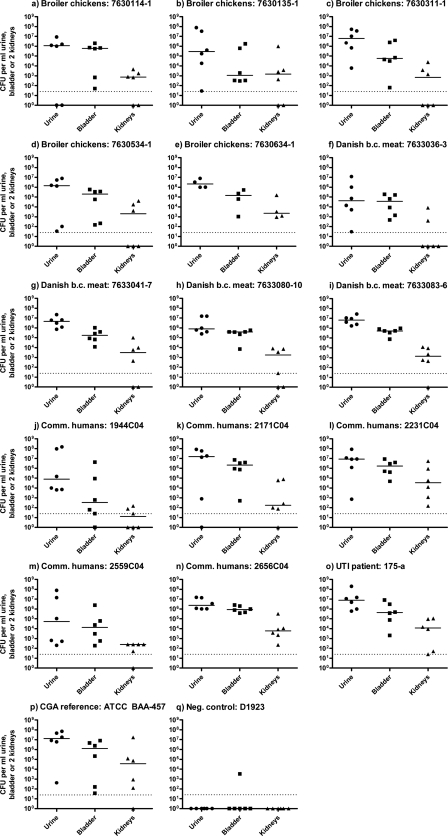
All 14 CgA isolates from broiler chickens, broiler chicken meat, and community-dwelling humans, as well as a UTI CgA isolate, the CgA reference isolate (ATCC BAA-457), and E. coli strain D1923 (O rough:H−; negative control) were investigated for virulence in the UTI mouse model described earlier, with two modifications (animal inspectorate approval no. 2004/561-835) (7). The model is representative for the human UTI (4, 6, 21). In this study, we used OF-1 mice (Charles River Laboratories, France), and urine, bladders, and kidneys were collected after 96 h. To our knowledge, this is the first study to investigate the in vivo virulence of CgA E. coli isolates from animals and meat. Only once before has in vivo virulence in the UTI mouse model of commensal isolates from production animals and meat been demonstrated (7). All of the CgA isolates tested (including UTI isolate 175-a and ATCC BA-457) were virulent, i.e., all isolates yielded positive bladder and urine cultures, and 13 of the isolates gave kidney infection as well (Fig. 1). The community-dwelling human isolate 2171C04 and the ATCC BAA-457 isolate, which were possibly related based on their PFGE profiles, had similar bacterial counts in urine, bladder, and kidneys (P > 0.05, Mann-Whitney test [two-tailed], GraphPad Prism 5). This was likewise found for the closely related community-dwelling-human isolates 1944C04 and 2559C04 (P > 0.05, Mann-Whitney test [two-tailed], GraphPad Prism 5). This may support the finding of relatedness by PFGE. The negative-control strain failed to produce any bacterial counts above the detection limit except for one positive bladder culture. This finding clearly demonstrates that CgA isolates from meat and animals are capable of causing UTIs in humans; hence, UTI is at times a zoonosis. It is especially worrisome that mouse kidneys were also infected, as this suggests that these CgA isolates from broiler chickens and broiler chicken meat are capable of causing severe infections, such as complicated pyelonephritis.
FIG. 1.
Bacterial counts in urine, bladder, and kidneys of mice killed 72 hours after inoculation with the different clonal group A E. coli phylogroup D isolates from healthy broiler chickens (a to e), broiler chicken (b.c.) meat (f to i), community-dwelling (Comm.) humans (j to n), a UTI patient (o), and CgA reference isolate ATCC BAA-457 (p). Each point represents the result for one mouse. The short horizontal lines represent the median bacterial counts. The dotted lines indicate the detection limit of 25 CFU/ml.
In conclusion, detection of CgA isolates among broiler chickens, broiler chicken meat, community-dwelling humans, and UTI patients suggests that there is an animal reservoir of CgA isolates. No CgA animal or meat isolates in this study, though, had PFGE profiles related to those of any human isolates. However, several community-dwelling human and UTI isolates were related, and we speculate that meat may have been a common source of such isolates. Finally, CgA isolates from broiler chickens, broiler chicken meat, and community-dwelling humans caused infection in the urine, bladder, and kidneys of mice, providing important circumstantial evidence that UTI is at times a zoonosis.
Acknowledgments
This study was supported by grant no. 2101-05-001 from The Danish Research Council. This work is part of the Danish Integrated Antimicrobial Resistance Monitoring and Research Programme (DANMAP) and the Marie Curie program TRAINAU (Training Risk-Assessment in Non-human Antibiotic Usage).
We thank Frank Hansen, Karin S. Pedersen, and Rikke Holm Nielsen for excellent technical assistance. Thank you to James R. Johnson, University of Minnesota, for sharing CgA primers prior to publication. Amee R. Manges, McGill University, is thanked for the kind gift of the CgA reference strain BAA-457, and likewise, Flemming Scheutz, Statens Serum Institut, is thanked for the kind gift of the negative-control strain D1923.
Footnotes
Published ahead of print on 29 October 2010.
REFERENCES
- 1.DANMAP. 2008. Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, foods and humans in Denmark. ISSN 1600-2032. http://www.danmap.org/.
- 2.Dias, R., D. Marangoni, L. Riley, and B. Moreira. 2009. Identification of uropathogenic Escherichia coli clonal group A (CgA) in hospitalised patients. Mem. Inst. Oswaldo Cruz 104:787-789. [DOI] [PubMed] [Google Scholar]
- 3.Dias, R. C. S., D. V. Marangoni, S. P. Smith, E. M. Alves, F. L. P. C. Pellegrino, L. W. Riley, and B. M. Moreira. 2009. Clonal composition of Escherichia coli causing community-acquired urinary tract infections in the state of Rio de Janeiro, Brazil. Microb. Drug Resist. 15:303-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagberg, L., I. Engberg, R. Freter, J. Lam, S. Olling, and C. Svanborg Eden. 1983. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect. Immun. 40:273-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter, S. B., P. Vauterin, M. A. Lambert-Fair, M. S. Van Duyne, K. Kubota, L. Graves, D. Wrigley, T. Barrett, and E. Ribot. 2005. Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: converting the national databases to the new size standard. J. Clin. Microbiol. 43:1045-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hvidberg, H., C. Struve, K. A. Krogfelt, N. Christensen, S. N. Rasmussen, and N. Frimodt-Møller. 2000. Development of a long-term ascending urinary tract infection mouse model for antibiotic treatment studies. Antimicrob. Agents Chemother. 44:156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jakobsen, L., A. M. Hammerum, and N. Frimodt-Møller. 2010. Virulence of Escherichia coli B2 isolates from meat and animals in a murine model of ascending urinary tract infection (UTI): evidence that UTI is a zoonosis. J. Clin. Microbiol. 48:2978-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakobsen, L., A. Kurbasic, L. Skjøt-Rasmussen, K. Ejrnæs, L. J. Porsbo, K. Pedersen, L. B. Jensen, H.-D. Emborg, Y. Agersø, K. E. Olsen, F. M. Aarestrup, N. Frimodt-Møller, and A. M. Hammerum. 2010. Escherichia coli isolates from broiler chicken meat, broiler chickens, pork, and pigs share phylogroups and antimicrobial resistance with community-dwelling humans and patients with urinary tract infection. Foodborne Pathog. Dis. 7:537-547. [DOI] [PubMed] [Google Scholar]
- 9.Johnson, J. R., M. A. Kuskowski, K. Owens, A. Gajewski, and P. L. Winokur. 2003. Phylogenetic origin and virulence genotype in relation to resistance to fluoroquinolones and/or extended-spectrum cephalosporins and cephamycins among Escherichia coli isolates from animals and humans. J. Infect. Dis. 188:759-768. [DOI] [PubMed] [Google Scholar]
- 10.Johnson, J. R., A. R. Manges, T. T. O'Bryan, and L. W. Riley. 2002. A disseminated multidrug-resistant clonal group of uropathogenic Escherichia coli in pyelonephritis. Lancet 359:2249-2251. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, J. R., M. Menard, B. Johnston, M. A. Kuskowski, K. Nichol, and G. G. Zhanel. 2009. Epidemic clonal groups of Escherichia coli as a cause of antimicrobial-resistant urinary tract infections in Canada, 2002 to 2004. Antimicrob. Agents Chemother. 53:2733-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, J., A. Murray, M. Kuskowski, S. Schubert, M. Prère, B. Picard, R. Colodner, R. Raz, and Trans-Global Initiative for Antimicrobial Resistance Analysis (TIARA) Investigators. 2005. Distribution and characteristics of Escherichia coli clonal group A. Emerg. Infect. Dis. 11:141-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manges, A. R., P. M. Sherry, B. J. Lau, C. J. Nuval, J. N. S. Eisenberg, P. S. Dietrich, and L. W. Riley. 2007. Retail meat consumption and the acquisition of antimicrobial resistant Escherichia coli causing urinary tract infections: a case-control study. Foodborne Pathog. Dis. 4:419-431. [DOI] [PubMed] [Google Scholar]
- 14.Manges, A. R., H. Tabor, P. Tellis, C. Vincent, and P. Tellier. 2008. Endemic and epidemic lineages of Escherichia coli that cause urinary tract infections. Emerg. Infect. Dis. 14:1575-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manges, A. R., P. S. Dietrich, and L. W. Riley. 2004. Multidrug-resistant Escherichia coli clonal groups causing community-acquired pyelonephritis. Clin. Infect. Dis. 38:329-334. [DOI] [PubMed] [Google Scholar]
- 16.Manges, A. R., J. R. Johnson, B. Foxman, T. T. O'Bryan, K. E. Fullerton, and L. W. Riley. 2001. Widespread distribution of urinary tract infections caused by a multidrug-resistant Escherichia coli clonal group. N. Engl. J. Med. 345:1007-1013. [DOI] [PubMed] [Google Scholar]
- 17.Moreno, E., G. Prats, M. Sabate, T. Perez, J. R. Johnson, and A. Andreu. 2006. Quinolone, fluoroquinolone and trimethoprim/sulfamethoxazole resistance in relation to virulence determinants and phylogenetic background among uropathogenic Escherichia coli. J. Antimicrob. Chemother. 57:204-211. [DOI] [PubMed] [Google Scholar]
- 18.Phillips, I., S. Eykyn, A. King, B. Rowe, J. A. Frost, and R. J. Gross. 1988. Epidemic multiresistant Escherichia coli infection in West Lambeth Health District. Lancet i:1038-1041. [DOI] [PubMed] [Google Scholar]
- 19.Ramchandani, M., A. R. Manges, C. DebRoy, S. P. Smith, J. R. Johnson, and L. W. Riley. 2005. Possible animal origin of human-associated, multidrug-resistant, uropathogenic Escherichia coli. Clin. Infect. Dis. 40:251-257. [DOI] [PubMed] [Google Scholar]
- 20.Ribot, E. M., M. A. Fair, R. Gautom, D. N. Cameron, S. B. Hunter, B. Swaminathan, and T. J. Barrett. 2006. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 3:59-67. [DOI] [PubMed] [Google Scholar]
- 21.Rosen, D., T. Hooton, W. Stamm, P. Humphrey, and S. Hultgren. 2007. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 4:e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tartof, S. Y., O. D. Solberg, A. R. Manges, and L. W. Riley. 2005. Analysis of a uropathogenic Escherichia coli clonal group by multilocus sequence typing. J. Clin. Microbiol. 43:5860-5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]